Comparative Study on the Volatile Organic Compounds and Characteristic Flavor Fingerprints of Five Varieties of Walnut Oil in Northwest China Using Using Headspace Gas Chromatography-Ion Mobility Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. GC–IMS Spectrum Analysis
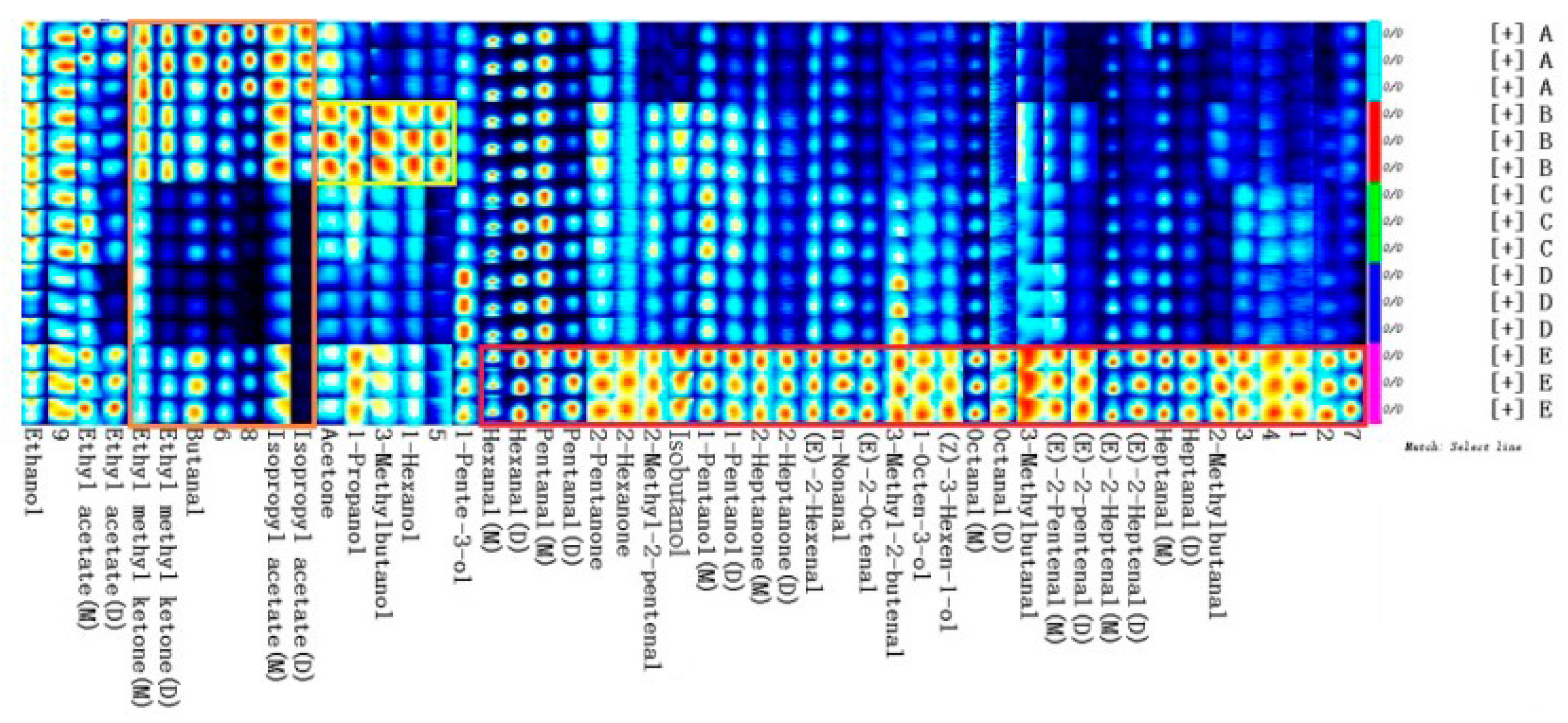
2.2. Comparison of VOCs Fingerprints in Walnut Oil from Five Different Varieties
2.3. Qualitative Analysis of VOCs of Walnut Oil from Five Different Varieties
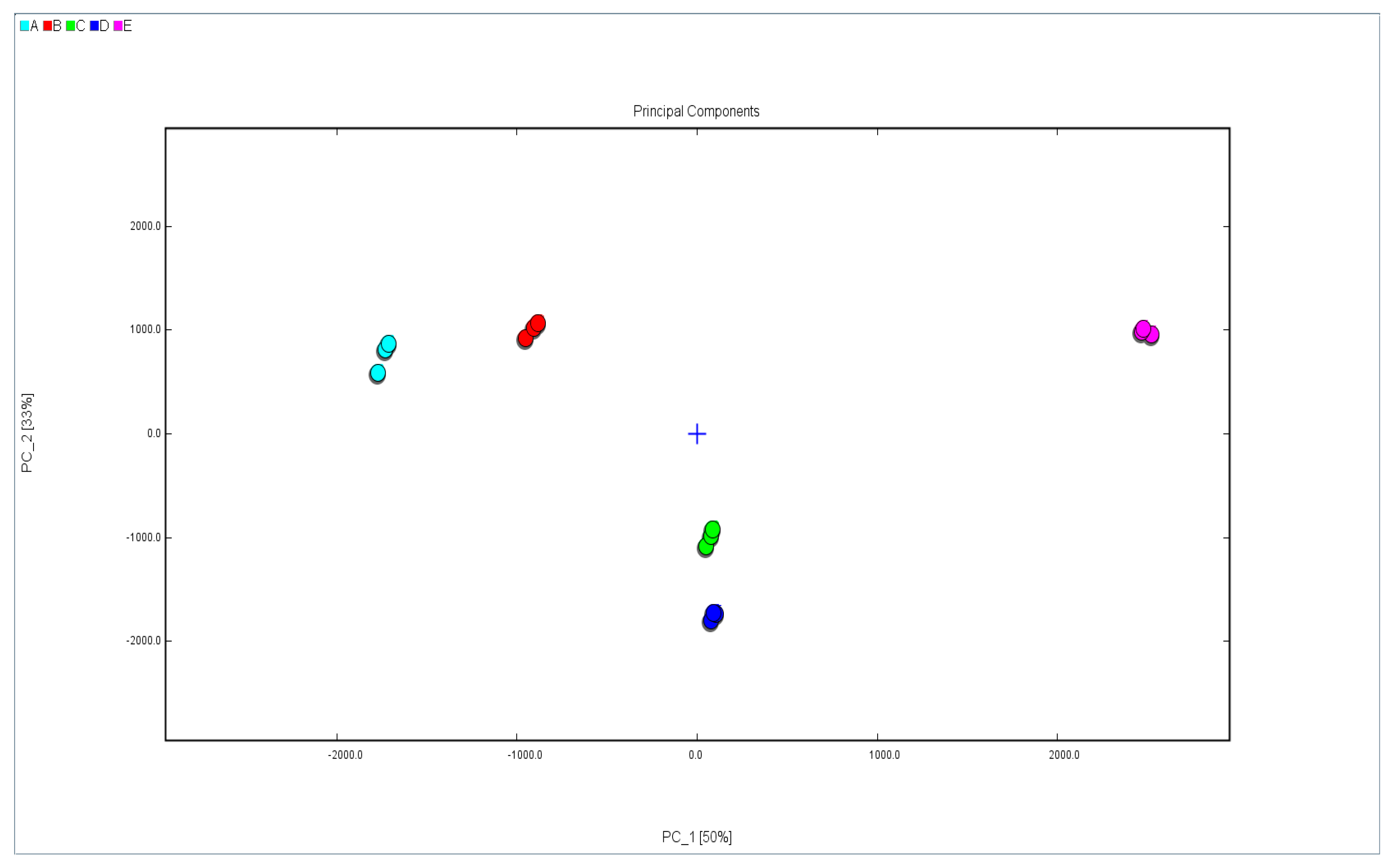
2.4. PCA of Walnut Oil from Five Different Varieties
2.5. Heat Map Analysis
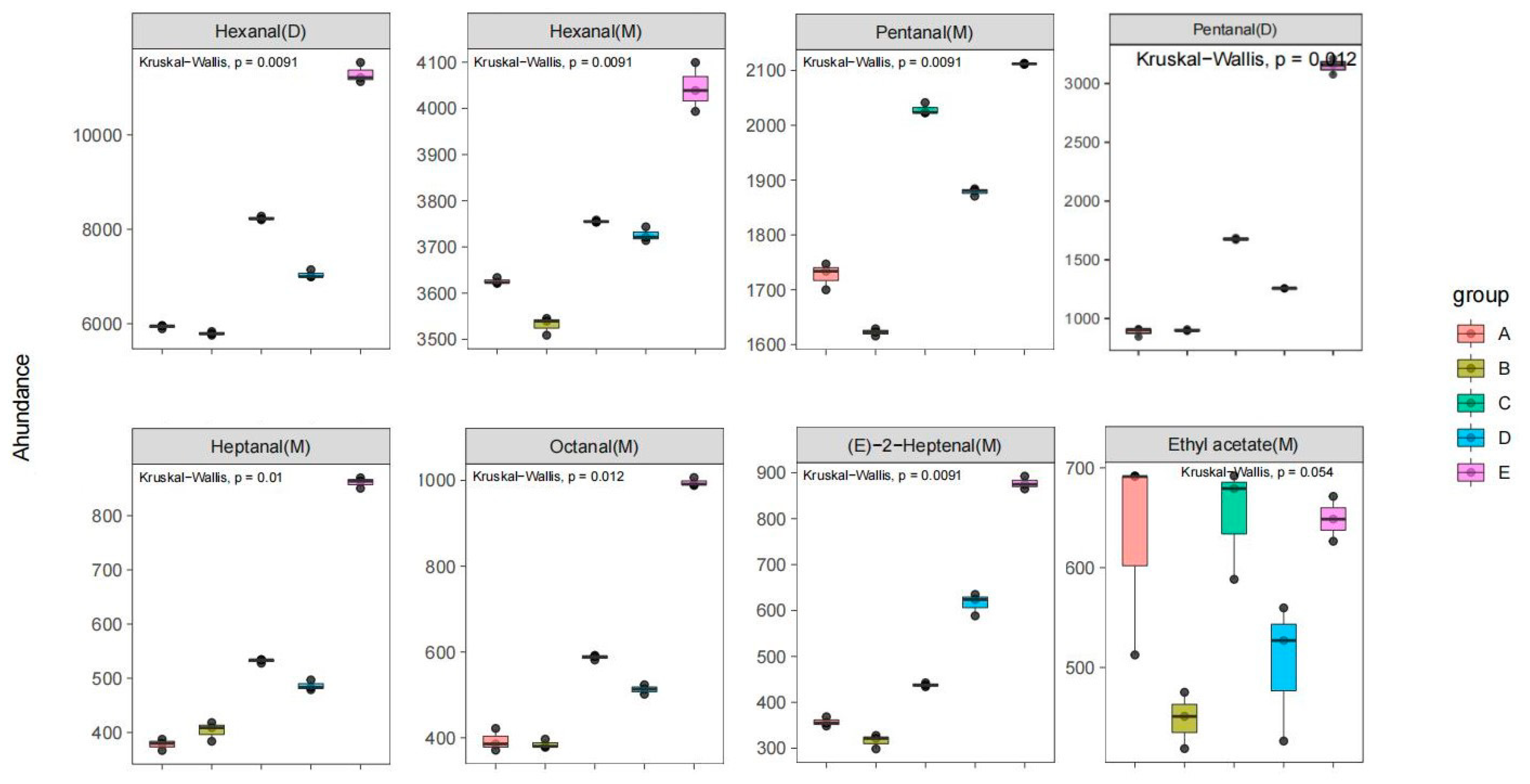
2.6. Analysis of Key Aroma Components of Walnut Oil from Different Varieties
3. Materials and Methods
3.1. Materials
3.2. Preparation of Walnut Oil
3.3. HS–GC–IMS Analysis
3.4. Determination of Key Flavor Compounds
3.5. Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martínez, M.L.; Penci, M.C.; Ixtaina, V.; Ribotta, P.D.; Maestri, D. Effect of natural and synthetic antioxidants on the oxidative stability of walnut oil under different storage conditions. LWT Food Sci. Technol. 2013, 51, 44–50. [Google Scholar] [CrossRef]
- Uzma, N.S.; Mir, J.I.; Ahmed, N.; Khalid, M.F. Assessment of germplasm diversity and genetic relationships among walnut (Juglans regia L.) genotypes through microsatellite markers. J. Saudi Soc. Agric. Sci. 2018, 17, 339–350. [Google Scholar]
- Calvo, P.; Lozano, M.; Espinosa-Mansilla, A.; González-Gómez, D. In-vitro evaluation of the availability of ϖ-3 and ϖ-6 fatty acids and tocopherols from microencapsulated walnut oil. Food Res. Int. 2012, 48, 316–321. [Google Scholar] [CrossRef]
- Sun, H.; Xu, J.; Lu, X.Z.; Xu, Y.Y.; Joe, M.R.; Zhang, Y.; Wang, F.J. Development and characterization of monoglyceride oleogels prepared with crude and refined walnut oil—ScienceDirect. LWT Food Sci. Technol. 2022, 154, 112769. [Google Scholar] [CrossRef]
- Ojeda-Amador, R.M.; Salvador, M.D.; Gómez-Alonso, S.; Fregapane, G. Characterization of virgin walnut oils and their residual cakes produced from different varieties. Food Res. Int. 2018, 108, 396–404. [Google Scholar] [CrossRef]
- Abdallah, I.B.; Tlili, N.; Martinez-Force, E.; Rubio, A.G.P.; Perez-Camino, M.C.; Albouchi, A.; Boukhchina, S. Content of carotenoids, tocopherols, sterols, triterpenic and aliphatic alcohols, and volatile compounds in six walnuts (Juglans regia L.)varieties. Food Chem. 2015, 173, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; XU, X.L.; Jin, F.; Regenstein, J.M.; Wang, F.J. HS-SPME GC–MS characterization of volatiles in processed walnuts and their oxidative stability. J. Food Sci. Technol. 2020, 43, 9–21. [Google Scholar] [CrossRef]
- Xu, Y.; Bi, S.; Niu, X.Y.; Chen, Y.M.; Liu, Y.; Zhou, Q. Comparison of aroma active compounds in cold- and hot-pressed walnut oil by comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry and headspace-gas chromatography-ion mobility spectrometry. Food Res. Int. 2023, 163, 112208. [Google Scholar] [CrossRef]
- Hurtado, C.; Parastar, H.; Matamoros, V.; Piña, B.; Tauler, R.; Bayona, J.M. Linking the morphological and metabolomic response of Lactuca sativa L exposed to emerging contaminants using GC×GC-MS and chemometric tools. Sci. Rep. 2017, 7, 6546. [Google Scholar] [CrossRef] [PubMed]
- Prebihalo, S.E.; Berrier, K.L.; Freye, C.E.; Bahaghighat, H.D.; Moore, N.R.; Pinkerton, D.K.; Synovec, R.E. Multidimensional Gas Chromatography: Advances in Instrumentation, Chemometrics, and Applications. Anal. Chem. 2017, 90, 505–532. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.; Nicolae, B.; Udo, W. Electronic nose: Current status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar]
- Gu, S.; Zhang, J.; Wang, J.; Wang, X.Y.; Du, D.D. Recent development of HS-GC-IMS technology in rapid and nondestructive detection of quality and contamination in agri-food products. Trends Anal. Chem. 2021, 144, 116435. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; Dobao-Prieto, M.M.; Arce, L.; Aguilar, J.; Cumplido, J.L.; Valcárcel, M. Ion mobility spectrometry versus classical physico-chemical analysis for assessing the shelf life of extra virgin olive oil according to container type and storage conditions. J. Agric. Food Chem. 2015, 63, 2179–2188. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Martín-Gómez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace-gas chromatography-ion mobility spectrometry. Food Chem. 2017, 246, 65–73. [Google Scholar] [CrossRef]
- Karpas, Z. Applications of ion mobility spectrometry (IMS) in the field of foodomics. Food Res. Int. 2013, 54, 1146–1151. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; García-Nicolás, M.; Castell, A.; Campillo, N.; Viñas, P.; López-García, I.; Hernández-Córdoba, M. Untargeted headspace gas chromatography—Ion mobility spectrometry analysis for detection of adulterated honey. Talanta 2019, 205, 120123. [Google Scholar] [CrossRef]
- Gerhardt, N.; Birkenmeier, M.; Sanders, D.; Rohn, S.; Weller, P. Resolution-optimized headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) for non-targeted olive oil profiling. Anal. Bioanal. Chem. 2017, 409, 3933–3942. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.V.; Beegle, L.W.; Kim, H.I.; Eiceman, G.A.; Kanik, I. Ion mobility spectrometry in space exploration. Int. J. Mass Spectrom. 2007, 262, 1–15. [Google Scholar] [CrossRef]
- Campuzano, I.D.; Lippens, J.L. Ion mobility in the pharmaceutical industry: An established biophysical technique or still niche? Curr. Opin. Chem. Biol. 2018, 42, 147–159. [Google Scholar] [CrossRef]
- Mairinger, T.; Causon, T.J.; Hann, S. The potential of ion mobility–mass spectrometry for non-targeted metabolomics. Curr. Opin. Chem. Biol. 2018, 42, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Puton, J.; Namieśnik, J. Ion mobility spectrometry: Current status and application for chemical warfare agents detection. TrAC Trends Anal. Chem. 2016, 85, 10–20. [Google Scholar] [CrossRef]
- Vautz, W.; Franzke, J.; Zampolli, S.; Elmi, I.; Liedtke, S. On the potential of ion mobility spectrometry coupled to GC pre-separation—A tutorial. Anal. Chim. Acta 2018, 1024, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, P.; Liao, L.; Qin, Y.; Jiang, L.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.J.; Li, H.Z.; Hou, T.Y.; Zhao, Y.N.; Li, H. Effects of ethanol, activated carbon, and activated kaolin on perilla seed oil: Volatile organic compounds, physicochemical characteristics, and fatty acid composition. J. Food Sci. 2021, 86, 4393–4404. [Google Scholar] [CrossRef]
- Elmore, J.S.; Nisyrios, I.; Mottram, D.S. Analysis of the headspace aroma compounds of walnuts (Juglans regia L.). Flavour Fragr. J. 2005, 20, 501–506. [Google Scholar] [CrossRef]
- Martínez, M.L.; Maestri, D.M. Oil chemical variation in walnut (Juglans regia L.) genotypes grown in Argentina. Eur. J. Lipid Sci. Technol. 2008, 110, 1183–1189. [Google Scholar] [CrossRef]
- Torres, M.M.; Martínez, M.L.; Maestri, D.M. A Multivariate Study of the Relationship Between Fatty Acids and Volatile Flavor Components in Olive and Walnut Oils. J. Am. Oil Chem. Soc. 2005, 82, 105–110. [Google Scholar] [CrossRef]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Melucci, D.; Bendini, A.; Tesini, F.; Barbieri, S.; Zappi, A.; Vichi, S.; Conte, L.; Toschi, T.G. Rapid direct analysis to discriminate geographic origin of extra virgin olive oils by flash gas chromatography electronic nose and chemometrics. Food Chem. 2016, 204, 263–273. [Google Scholar] [CrossRef]
- Xiao, L.; Lee, J.; Zhang, G.; Ebeler, S.E.; Wickramasinghe, N.; Seiber, J.; Mitchell, A.E. HS-SPME GC/MS characterization of volatiles in raw and dry-roasted almonds (Prunus dulcis). Food Chem. 2014, 151, 31–39. [Google Scholar] [CrossRef]
- Giovanelli, S.; Giusti, G.; Cioni, P.L.; Minissale, P.; Ciccarelli, D.; Pistelli, L. Aroma profile and essential oil composition of Rhus coriaria fruits from four Sicilian sites of collection. Ind. Crops Prod. 2017, 97, 166–174. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Manousi, N.; Rosenberg, E.; Zachariadis, G.A.; Paraskevopoulou, A.; Samanidou, V. Exploring the volatile metabolome of conventional and organic walnut oils by solid-phase microextraction and analysis by GC-MS combined with chemometrics. Food Chem. 2021, 363, 130331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fan, W.; Chu, F.X.; Wang, C.Z.; Pei, D. Identifification of Volatile Oxidation Compounds as Potential Markers of Walnut Oil Quality. J. Food Sci. 2018, 83, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.P.; Zhang, Y.X.; Wang, Y.W.; Ju, H.M.; Niu, C.; Song, Z.H.; Yuan, Y.H.; Yue, T.L. Assessment of chemical composition and sensorial properties of ciders fermented with different non-Saccharomyces yeasts in pure and mixed fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef] [PubMed]



| Number | Compounds | CAS# | Molecular Formula | Retention Index | Retention Time (s) | Relative Content (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | ||||||
| 1 | n-Nonanal | C124196 | C9H18O | 1103.7 | 767.133 | 0.80 | 0.82 | 1.18 | 1.16 | 1.18 |
| 2 | (E)-2-Octenal | C2548870 | C8H14O | 1070 | 690.61 | 0.51 | 0.54 | 0.84 | 0.87 | 1.25 |
| 3 | Octanal(M) | C124130 | C8H16O | 1013 | 578.335 | 1.41 | 1.38 | 2.07 | 2.06 | 2.50 |
| 4 | Octanal(D) | C124130 | C8H16O | 1011.6 | 575.826 | 0.19 | 0.18 | 0.22 | 0.22 | 0.26 |
| 5 | (E)-2-Heptenal(M) | C18829555 | C7H12O | 962 | 481.114 | 1.28 | 1.13 | 1.55 | 2.48 | 2.20 |
| 6 | (E)-2-Heptenal(D) | C18829555 | C7H12O | 960.6 | 478.605 | 0.26 | 0.18 | 0.22 | 0.37 | 0.45 |
| 7 | 1-Octen-3-ol | C3391864 | C8H16O | 993.2 | 542.583 | 0.37 | 0.45 | 0.52 | 0.55 | 0.61 |
| 8 | Heptanal(M) | C111717 | C7H14O | 900.7 | 380.185 | 1.36 | 1.45 | 1.88 | 1.95 | 2.16 |
| 9 | Heptanal(D) | C111717 | C7H14O | 901.3 | 381.061 | 0.13 | 0.10 | 0.17 | 0.16 | 0.30 |
| 10 | 2-Heptanone(M) | C110430 | C7H14O | 892.5 | 368.356 | 0.50 | 0.70 | 0.60 | 0.67 | 0.70 |
| 11 | 2-Heptanone(D) | C110430 | C7H14O | 891.2 | 366.603 | 0.09 | 0.12 | 0.12 | 0.14 | 0.23 |
| 12 | 1-Hexanol | C111273 | C6H14O | 881.4 | 353.897 | 0.22 | 0.53 | 0.31 | 0.32 | 0.24 |
| 13 | (E)-2-Hexenal | C6728263 | C6H10O | 852.7 | 319.285 | 0.48 | 0.50 | 0.63 | 0.74 | 0.58 |
| 14 | Hexanal(M) | C66251 | C6H12O | 794.1 | 258.823 | 13.03 | 12.65 | 13.25 | 14.98 | 10.16 |
| 15 | Hexanal(D) | C66251 | C6H12O | 796 | 260.575 | 21.33 | 20.75 | 29.05 | 28.32 | 28.36 |
| 16 | 1-Pentanol(M) | C71410 | C5H12O | 771.2 | 237.626 | 1.52 | 2.21 | 2.58 | 2.51 | 1.88 |
| 17 | 1-Pentanol(D) | C71410 | C5H12O | 772.1 | 238.462 | 0.19 | 0.44 | 0.43 | 0.37 | 0.39 |
| 18 | (E)-2-Pentenal | C1576870 | C5H8O | 741.5 | 211.989 | 0.49 | 0.58 | 0.72 | 1.01 | 0.90 |
| 19 | 3-Methylbutanol | C1576870 | C5H8O | 741.8 | 212.268 | 0.23 | 0.70 | 0.38 | 0.36 | 0.36 |
| 20 | (E)-2-pentenal | C123513 | C5H12O | 739.1 | 210.038 | 0.05 | 0.13 | 0.08 | 0.12 | 0.30 |
| 21 | 1-Penten-3-ol | C616251 | C5H10O | 689 | 173.255 | 0.98 | 0.93 | 1.09 | 1.68 | 1.05 |
| 22 | 2-Pentanone | C107879 | C5H10O | 684.4 | 170.925 | 0.17 | 0.25 | 0.21 | 0.23 | 0.16 |
| 23 | Pentanal(M) | C110623 | C5H10O | 697.2 | 178.808 | 6.20 | 5.81 | 7.16 | 7.55 | 5.31 |
| 24 | Pentanal(D) | C110623 | C5H10O | 695.9 | 177.956 | 3.19 | 3.23 | 5.92 | 5.05 | 7.91 |
| 25 | Isopropyl acetate(M) | C108214 | C5H10O2 | 657.2 | 157.714 | 1.56 | 1.68 | 0.76 | 0.88 | 1.02 |
| 26 | Isopropyl acetate(D) | C108214 | C5H10O2 | 656.2 | 157.288 | 1.06 | 0.68 | 0.06 | 0.08 | 0.06 |
| 27 | 3-Methylbutanal | C590863 | C5H10O | 651.3 | 155.001 | 0.67 | 1.12 | 0.95 | 1.21 | 1.47 |
| 28 | 2-Methylbutanal | C96173 | C5H10O | 656.9 | 157.608 | 0.16 | 0.43 | 0.20 | 0.23 | 0.72 |
| 29 | Ethyl acetate(M) | C141786 | C4H8O2 | 613.8 | 138.799 | 2.27 | 1.61 | 2.30 | 2.03 | 1.63 |
| 30 | Ethyl acetate(D) | C141786 | C4H8O2 | 614.9 | 139.227 | 1.32 | 1.02 | 0.83 | 0.43 | 1.28 |
| 31 | Ethyl methyl ketone(M) | C78933 | C4H8O | 597.2 | 132.173 | 4.88 | 4.61 | 3.46 | 4.33 | 2.39 |
| 32 | Ethyl methyl ketone(D) | C78933 | C4H8O | 593.3 | 130.677 | 8.74 | 7.89 | 1.86 | 1.87 | 3.94 |
| 33 | Butanal | C123728 | C4H8O | 602.1 | 134.097 | 2.81 | 2.35 | 1.12 | 0.78 | 1.83 |
| 34 | 1-Propanol | C71238 | C3H8O | 568 | 121.273 | 0.97 | 2.03 | 1.75 | 1.13 | 1.34 |
| 35 | Acetone | C67641 | C3H6O | 515.7 | 103.954 | 2.87 | 3.55 | 2.32 | 2.07 | 1.89 |
| 36 | Ethanol | C64175 | C2H6O | 486.7 | 95.438 | 5.53 | 6.37 | 5.69 | 4.09 | 3.72 |
| 37 | Isobutanol | C78831 | C4H10O | 632 | 146.463 | 0.37 | 1.21 | 0.81 | 0.84 | 1.10 |
| 38 | 3-Methyl-2-butenal | C107868 | C5H8O | 755.4 | 223.599 | 0.45 | 0.50 | 0.72 | 1.11 | 0.73 |
| 39 | 1 | unidentified | - | 1099.5 | 757.097 | 0.23 | 0.22 | 0.38 | 0.31 | 0.41 |
| 40 | 2 | unidentified | - | 945.3 | 451.217 | 0.17 | 0.22 | 0.22 | 0.29 | 0.39 |
| 41 | 3 | unidentified | - | 905.5 | 387.195 | 0.19 | 0.18 | 0.29 | 0.26 | 0.30 |
| 42 | 4 | unidentified | - | 905.2 | 386.757 | 0.14 | 0.16 | 0.20 | 0.22 | 0.24 |
| 43 | 5 | unidentified | - | 882.7 | 355.65 | 0.18 | 0.47 | 0.12 | 0.17 | 0.17 |
| 44 | 6 | unidentified | - | 752.9 | 221.463 | 4.02 | 2.99 | 1.30 | 0.80 | 1.75 |
| 45 | 7 | unidentified | - | 752.9 | 221.463 | 0.74 | 0.71 | 0.56 | 0.54 | 1.16 |
| 46 | 8 | unidentified | - | 751.6 | 220.349 | 3.39 | 1.69 | 0.41 | 0.24 | 1.19 |
| 47 | 9 | unidentified | - | 517.2 | 104.419 | 1.99 | 2.14 | 2.08 | 1.75 | 1.39 |
| 48 | 2-Methyl-2-pentenal | C623369 | C6H10O | 827.2 | 291.404 | 0.08 | 0.22 | 0.20 | 0.16 | 0.16 |
| 49 | (Z)-3-Hexen-1-ol | C928961 | C6H12O | 852.9 | 319.566 | 0.13 | 0.11 | 0.14 | 0.20 | 0.20 |
| 50 | 2-Hexanone | C591786 | C6H12O | 782 | 247.67 | 0.08 | 0.08 | 0.08 | 0.09 | 0.07 |
| Number | Compounds | Threshold value (μg/kg) | ROAV | ||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||
| 1 | Octanal (M) | 0.7 | 2.17 | 2.18 | 2.34 | 2.39 | 2.90 |
| 2 | (E)-2-Heptenal (M) | 3 | 0.46 | 0.42 | 0.41 | 0.67 | 0.60 |
| 3 | (E)-2-Heptenal (D) | 3 | 0.09 | 0.06 | 0.06 | 0.10 | 0.12 |
| 4 | Heptanal (M) | 0.26 | 5.63 | 6.16 | 5.72 | 6.11 | 6.75 |
| 5 | Hexanal (M) | 0.23 | 61.08 | 60.95 | 45.61 | 52.89 | 35.81 |
| 6 | Hexanal (D) | 0.23 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| 7 | Pentanal (M) | 0.85 | 7.87 | 7.58 | 6.67 | 7.21 | 5.06 |
| 8 | Pentanal (D) | 0.85 | 4.05 | 4.21 | 5.51 | 4.83 | 7.55 |
| 9 | Ethyl acetate (M) | 5 | 0.49 | 0.36 | 0.36 | 0.33 | 0.26 |
| 10 | Ethyl acetate (D) | 5 | 0.29 | 0.23 | 0.13 | 0.07 | 0.21 |
| Identification | Varieties | Place of Origin | Harvest Time | Single Fruit Weight/g | Shape | Evaluation of Surface Texture |
|---|---|---|---|---|---|---|
| A | Xin2 | Kashgar, Xinjiang | 15 September 2021 | 15.90–18.40 | Ovoid nut | Smooth shell surface, tight chink |
| B | 185 | Aksur, Xinjiang | 22 September 2021 | 11.20–14.20 | Round nut, has a spike | Smooth shell surface, loose chink |
| C | Xinfeng | Kashgar, Xinjiang | 18 September 2021 | 12.60–14.90 | Short oval nut, has a spike | Obvious surface gullies, tight chink |
| D | Xin’guang | Kashgar, Xinjiang | 18 September 2021 | 16.80–17.50 | Round nut | Smooth shell surface, tight chink |
| E | Zha343 | Kashgar, Xinjiang | 12 September 2021 | 12.40–15.30 | Ovoid nut | Smooth shell surface, tight chink |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Qi, Y.; Meng, M.; Cui, K. Comparative Study on the Volatile Organic Compounds and Characteristic Flavor Fingerprints of Five Varieties of Walnut Oil in Northwest China Using Using Headspace Gas Chromatography-Ion Mobility Spectrometry. Molecules 2023, 28, 2949. https://doi.org/10.3390/molecules28072949
Sun L, Qi Y, Meng M, Cui K. Comparative Study on the Volatile Organic Compounds and Characteristic Flavor Fingerprints of Five Varieties of Walnut Oil in Northwest China Using Using Headspace Gas Chromatography-Ion Mobility Spectrometry. Molecules. 2023; 28(7):2949. https://doi.org/10.3390/molecules28072949
Chicago/Turabian StyleSun, Lina, Yanlong Qi, Meng Meng, and Kuanbo Cui. 2023. "Comparative Study on the Volatile Organic Compounds and Characteristic Flavor Fingerprints of Five Varieties of Walnut Oil in Northwest China Using Using Headspace Gas Chromatography-Ion Mobility Spectrometry" Molecules 28, no. 7: 2949. https://doi.org/10.3390/molecules28072949
APA StyleSun, L., Qi, Y., Meng, M., & Cui, K. (2023). Comparative Study on the Volatile Organic Compounds and Characteristic Flavor Fingerprints of Five Varieties of Walnut Oil in Northwest China Using Using Headspace Gas Chromatography-Ion Mobility Spectrometry. Molecules, 28(7), 2949. https://doi.org/10.3390/molecules28072949






