Abstract
Dopamine, adrenaline and octopamine are small polar molecules that play a vital role in regulatory systems. In this paper, phthalylglycyl chloride was proposed as a derivatization agent for octopamine, adrenaline and dopamine determination in urine for the first time. The derivatization procedure facilitated the use of reversed-phase liquid chromatography with positive electrospray ionization–high-resolution mass spectrometry. An LC-HRMS method was developed that provided quantification limits of 5 ng/mL and detection limits of 1.5 ng/mL for all analytes. The 95–97% yield of derivates was observed after a 10 min derivatization with phthalylglycyl chloride at pH 6.5 and 30 °C. The proposed method was successfully applied to the analysis of human urine samples. The obtained results were compared with those of conventional derivatization procedures with 9-fluorenyl-methoxycarbonyl chloride and dansyl chloride.
1. Introduction
Dopamine, adrenaline and octopamine are important catecholamines that play a vital role in regulatory functions and can be used in Alzheimer’s disease diagnostics. They are also involved in mediating a number of cognitive functions, spatial, recognition and working memory [,,]. According to published research [,,,], their concentration below 5 ng/mL could be associated with functional changes and neuropathology, which makes their accurate and precise determination an actual task.
The studied compounds contain phenol- or catechol- groups and a side primary or secondary amino- group (Figure 1). Due to their high hydrophilicity and basicity (see the properties in Table 1), these compounds can be effectively separated by various techniques including ion chromatography (IC) [], hydrophilic chromatography (HILIC) [] or ion-pair reversed-phase HPLC using the addition of anionic surfactants to the eluent [,]. It should be noted that native catecholamines are not retained on common chromatographic columns for reversed-phase HPLC, so derivatization can be used to increase their hydrophobic properties.
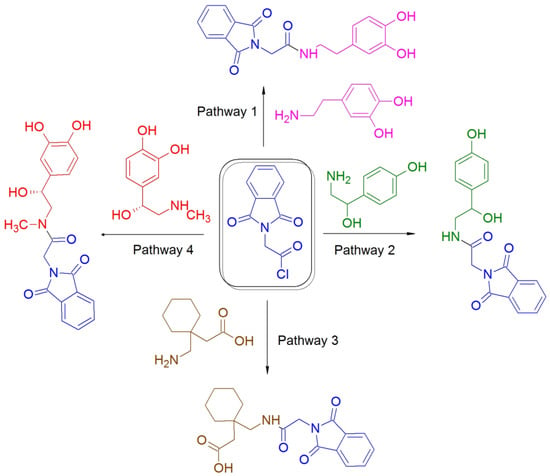
Figure 1.
Scheme of catecholamines derivatization with phthalylglycyl chloride (pathway 1—dopamine, pathway 2—octopamine, pathway 4—adrenaline) and pathway 3—gabapentin.

Table 1.
Hydrophobic and acid-base properties of studied biogenic amines.
The most used methods for their determination are liquid chromatography with tandem mass spectrometry (LC-MS/MS) [,,,], liquid chromatography with electrochemical detection (LC-ECD) [,,] and liquid chromatography with ultraviolet (LC-UV) or fluorometric detection (LC-FLD) [,,,,].
However, the recent advances in the synthesis of highly efficient sorbents make possible the usage of so-called “polar” C18 columns with a sub-3 µm particle size, which provides greater retention for small polar molecules []. At the same time, mixed-mode C18 columns with the same particle size available on the market nowadays could be a suitable solution []. They also provide a significant increase in the retention of some polar compounds because of the combination of the conventional reversed-phase and weak cation-exchange separation mechanisms []. They are also suitable for the separation of some critical pairs for conventional C18 sorbents. The main limitation for such columns is the prevalence of the conventional columns for multicomponent screening in most routine laboratories, and application of the novel sorbents requires at least method revalidation.
In the study [], possible ways of using a mixed-mode sulfonate-modified sorbent for catecholamines’ separation were demonstrated. According to their conclusions, due to the coexistence of reversed-phase and strong coulombic interactions, polar cationic compounds have significantly higher retention and much better separation through a “hydrophobically assisted cation-exchange mechanism”. The most interesting part of the research is the comparison of the conventional C18 and silica-based hyper-crosslinked sulfonate-modified reversed phase and the clear demonstration of its advantages. Significant differences in the selectivity and efficiency of other compounds’ separation were also noticed based on the results of 47 drugs’ separation.
Another important methodological aspect is that a lot of different combinations for mobile phase compositions and ion-pair reagents are applicable for UV or FLD detection, which could be used to increase analytes’ retention in the RP-HPLC separation mode with conventional columns. In the case of MS detection, the number of possible mobile phases and their modifications is extremely limited [].
In the case of MS detection, a possibility of strong matrix effects and high chemical noise level could be observed and should be evaluated. Prior to analysis, derivatization is usually carried out for several reasons: firstly, it allows their polarity to be decreased making possible separation by reversed-phase liquid chromatography; secondly, it results in an increase in their molecular weight, which reduces the signal-to-noise ratio in mass spectrometric detection; and thirdly, it can make fluorescent detection possible due to the addition of the respective functional groups. Typically, 9-fluorenyl-methoxycarbonyl chloride (FMOC-Cl), (DNS-Cl) [] and benzoyl-chloride [] are used for these purposes.
Overall, derivatization [,] is preferable for the determination of such polar and small molecules as catecholamines. The above-mentioned reagents, namely, FMOC-Cl and dansyl chloride, require at least 30 min for reaction and the presence of an alkali [,]. It leads to a high degradation speed of some analytes (including catecholamines) and requires neutralization of the alkali in a final step of the sample preparation procedure.
The most used reagents for derivatization and methods for catecholamines’ determination are shown in Table 2.

Table 2.
An overview of some methods for the determination of catecholamines.
Due to their limitations, the synthesis of novel inexpensive derivatization agents that could provide sufficient sensitivity, selectivity, chromatographic peak shape, quick derivatization at close to neutral pH value and specific MS fragmentation has become an actual task.
Phthalylglycyl chloride (PG-Cl) is a commonly used reagent for amido-alkylation for the synthesis of benzofuran derivatives, remote C-H bond functionalization and photoinduced single electron transfer cyclization for the preparation of cyclic peptides [,,,]. At the same time, it could be used as a derivatization reagent for amines. It features a phthalimide fragment, which improves retention on reversed-phase columns, and the presence of additional nitrogen in the structure could positively affect ionization efficiency of the analytes. Moreover, it should be noticed that the PG-fragment in derivatives is not as hydrophobic and small in comparison with FMOC-Cl and DNS-Cl, and even minor differences in the native analytes make it easier to separate them.
Low stability of these reagents in aqueous solutions limits their application. To prevent it, sample dilution with acetonitrile is required. It could also be used for protein precipitation as a step in the sample preparation procedure [].
The aim of this research is the investigation of the selectivity and efficiency of PG-Cl as a derivatization agent for catecholamines’ determination in urine.
2. Results
2.1. Phthalylglycyl Chloride (PG-Cl) Synthesis
N-phthaloylglycine was prepared in 95% yield by the known procedure [], based on the reaction of glycine with phthalic anhydride in a hot DMF solution for 6 h (Figure 2). The spectral data are in accordance with those reported in [].
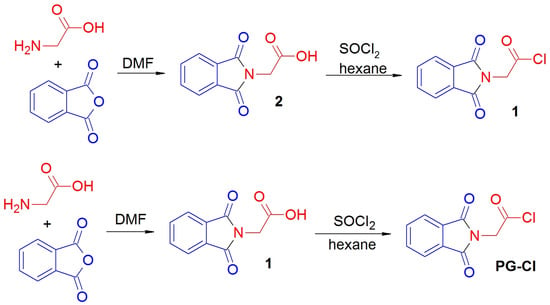
Figure 2.
Scheme of N-phthaloylglycine chloride synthesis.
N-phthaloylglycine chloride (1) was prepared by a slightly modified procedure [] as follows: N-phthaloylglycine (2.052 g, 0.01 mol) was treated with an excess of SOCl2 (5.0 mL) in a moisture-free atmosphere. The resulting mixture was stirred until the evolution of gases ceased (about 3 h) and then allowed to stand overnight. The excessive thionyl chloride was then evaporated in a vacuum, and the solid residue was treated with hexane. Hexane was re-evaporated, and this process was repeated once more to remove trace amounts of SOCl2. The resulting white crystalline solid was filtered off and washed on a filter with petroleum ether, and dried in a vacuum to afford pure N-phthaloylglycine chloride PG-Cl. The yield was 89%. 1H NMR (400 MHz, CDCl3), δ 4.85 (s, 2H, CH2), 7.77–7.80 (m, 2H, H5, H6), 7.90–7.94 (m, 2H, H4, H7). 13C NMR (101 MHz, CDCl3), δ 44.5 (CH2), 124.0 (2 CH), 131.5 (2 CH), 134.8 (2C), 167.0 (2C = O), 169.0 (C = O).
2.2. Optimization of Derivatization Conditions
In previous studies [,], different approaches utilizing FMOC-Cl and DNS-Cl were discussed, and optimal conditions for derivatization were established. However, PG-Cl was not previously described as a derivatization reagent, and its selectivity and reaction speed as well as the analytical characteristics of its derivatives are unknown.
The main aim of acetonitrile usage as a solvent for PG-Cl was its high hydrolysis speed. Dry acetonitrile is a commonly used and available solvent suitable for further reversed-phase separation. In our study, parameters such as pH value (established optimum value was 6.5), temperature (Figure 3) and reaction time (Figure 4) were optimized for dopamine and octopamine in synthetic urine, prepared according to []. As can be seen from the presented figures, the maximum yield of derivatization was observed at 30 °C after 10 min of the reaction. A further increase in the temperature leads to degradation of the derivatives, which could not be inhibited by changing the pH value or the addition of such reagents as methylamine.
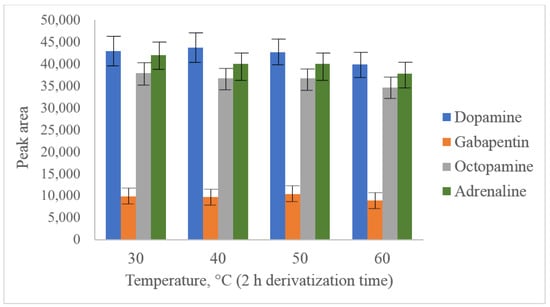
Figure 3.
Optimization of derivatization temperature with PG-Cl at 100 ng/mL dopamine, octopamine, adrenaline and 50 ng/mL gabapentin.
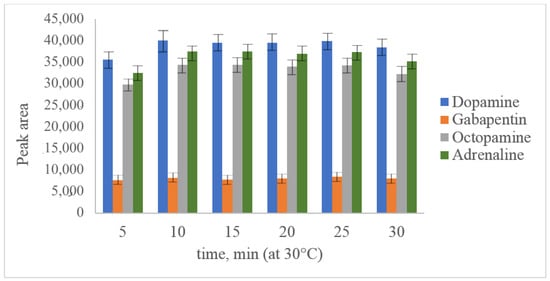
Figure 4.
Optimization of derivatization reaction time with PG-Cl at 100 ng/mL dopamine, octopamine, adrenaline and 50 ng/mL gabapentin.
The stability of the analytes at 30 °C is an important part of the investigation because it shows a possibility for further adaptation for routine analysis. When the reaction was finished, samples were transferred into the glass vials and stored at 5 °C in an autosampler tray prior to analysis, and they were found to be stable up to 24 h after their preparation (RSD < 15%).
During the method optimization, the high speed of the reagent hydrolysis in an aqueous solution was established, and its low stability requires the usage of a freshly prepared solution before the experiments. An important factor determining the stability of the obtained derivatives was the pH value. The derivatization reaction was inhibited in an acidic solution, leading to the prevalence of the hydrolysis process of the derivatization agent, while in an alkaline solution, the hydrolysis speed predominated over the formation of derivatives. The derivatives were stable when the reaction was carried out at pH 6.5.
2.3. MS Detection
The investigation of PG-Cl suitability for LC-MS/MS and LC-HRMS was conducted with two major points: informative fragments produced in the collision cell of the mass spectrometer and the influence of the derivative fragment on retention parameters.
Typically, one of the most abundant product ions observed in the MS/MS spectrum is a part of the derivatization agent (Figure 5). In this case, the same result was observed. A phthaloylglycyl moiety of the molecule was easily eliminated at low collision energies (up to 7 eV for the investigated compounds) producing the abundant fragment ion with m/z 160.0393. All the other fragments corresponded to typically observed fragments for native catecholamines.
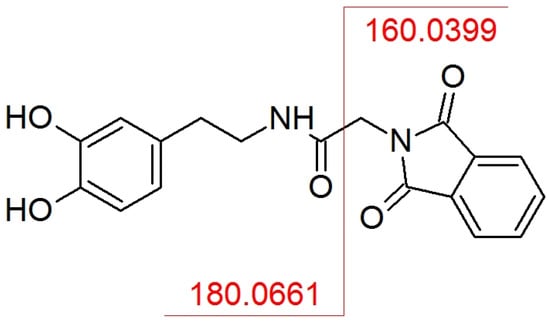
Figure 5.
Possible fragmentation method of the most abundant product ion (m/z 160.0393) for investigated analytes on dopamine example.
2.4. Chromatographic Conditions
As for chromatographic properties, the proposed derivatization agent significantly increased the retention of the catecholamines on a reversed-phase column (Figure 6) and allowed the use of a conventional screening gradient elution program with a high speed of mobile phase composition change.
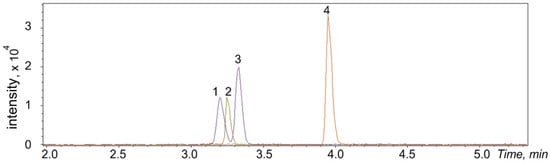
Figure 6.
LC-HRMS chromatogram of catecholamines and gabapentin derivatives in synthetic urine at quality control (QC) low (10 ng/mL) concentration: 1—PG-octopamine, 2—PG-adrenaline, 3—PG-dopamine, 4—PG-gabapentin (IS).
A significant difference in the retention times between analytes and the hydrolyzed form of the derivatization reagent makes it possible to use data-dependent and data-independent acquisition modes with low collision energies without warnings about overlapping of the product ions in non-targeted screening applications. As can be seen from Figure 6, octopamine and dopamine peaks overlapped by less than 10% under the described gradient elution conditions, which does not lead to a higher standard deviation in quantitative analysis.
Another important aspect of the proposed method is establishing some metrological characteristics, which could be obtained with PG-Cl for catecholamines’ determination (Table 3).

Table 3.
Accuracy and precision results established on synthetic urine samples.
Calibration solutions were used to establish linear ranges, while quality control solutions were analyzed to evaluate inter- and intra-day accuracy and precision. Accuracy was assessed as the bias between the nominal and observed concentrations within one day and on different days. Precision was expressed as the relative standard deviation of the results.
3. Discussion
3.1. Comparison of PG-Cl with FMOC-Cl and DNS-Cl
In comparison to conventional derivatizing agents such as FMOC-Cl or DNS-Cl, PG-Cl is not a fluorescent marker. However, the presence of a secondary nitrogen atom promotes its use as a derivatization agent for LC-MS since it provides a higher ionization yield in comparison to FMOC-Cl and DNS-Cl and improves the detection and quantification limits obtained using synthetic urine samples (Table 4).

Table 4.
Comparison of sensitivities obtained with different derivatization agents.
As can be seen from Table 4, PG-Cl provides up to a two times higher quantification limit for all analytes and demonstrates a notable difference in the detection limit for dopamine, which can be due to a significant difference in the ionization efficiency of the applied derivatization reagents and, as a result, in the derivatives.
3.2. Urine Samples Analysis
Real urine samples were prepared according to Section 2.4; the results obtained using FMOC-Cl and PG-Cl as derivatization reagents are provided in Table 5. The good convergence of the results suggests that the proposed approach may be promising for further research. This section may be divided by subheadings. It should provide a concise and precise description of the experimental results and their interpretation, as well as the experimental conclusions that can be drawn.

Table 5.
Analysis of real urine samples using FMOC-Cl and PG-Cl as derivatization reagents.
4. Materials and Methods
4.1. Chemicals
Standards of dopamine (≥98%), octopamine (≥98%), adrenaline (epinephrine) (≥99%), 9-fluorenyl-methoxycarbonyl chloride (≥99%), dansyl chloride (≥99%) were purchased from Sigma-Aldrich (St. Louis, MO, USA), gabapentin (IS) (≥75%) was obtained from Pfizer (New York, NY, USA). HPLC-grade acetonitrile (“Biosolve”, Jerusalem, Israel), 18.2 MΩ water (Milli-Q, Millipore, Molsheim, France) and formic acid (98%, Acros Organics, Geel, Belgium) were used as the mobile phase. Methanol of HPLC grade was purchased from Vecton (Saint-Petersburg, Russia). Potassium carbonate (≥99%, Vecton, Saint-Petersburg, Russia), potassium bicarbonate (≥99%, Vecton, Saint-Petersburg, Russia), sodium hydroxide (≥99%, Reactive, Saint-Petersburg, Russia), sodium tetraborate (≥99%, Vecton, Saint-Petersburg, Russia), sodium hydrogen phosphate (≥99%, Vecton, Saint-Petersburg, Russia), potassium dihydrogen phosphate (≥99%, Vecton, Saint-Petersburg, Russia), ammonium acetate (≥99%, Vecton, Saint-Petersburg, Russia) and acetic acid were used for the preparation of buffer solutions with pH 10.5, 9.5, 6.5 and 4.5, respectively. Glycine (HPLC grade, Vecton, Saint-Petersburg, Russia), phthalic anhydride (99%, Vecton, Saint-Petersburg, Russia), dimethylformamide (HPLC grade, Vecton, Saint-Petersburg, Russia) were used for PG-Cl synthesis.
4.2. Instrumentation
A Bruker MaXis Impact (Bruker Daltonik GmbH, Bremen, Germany) quadrupole-time-of-flight mass spectrometer (Q-TOF) equipped with an electrospray ionization (ESI) source coupled with an ultra-high performance liquid chromatography Bruker Elute system (UHPLC) with a Phenomenex Kinetex C18 (100 mm × 2.1 mm, 1.7 μm) column and an appropriate guard column was used for the chromatographic separation. A two-component system of methanol (A)–0.1% formic acid in water was (B) used as the mobile phase. The gradient elution program was as follows: 0.0–1.0 min 5% A; 2.7–4.0 min 60% A; 5.0–7.5 min 90% A; 7.51–9.0 min 5% A. The injection volume was 10 μL. The flow rate was held constant at 0.45 mL/min, and the column thermostat temperature was 40 °C. The voltage at the ionization source was 3.5 kV, drying gas flow rate was 8 L/min, spray gas pressure was 2 bar, temperature of the ionization source was 250 °C, mass scanning range (m/z) was 50–600, scanning speed was 3 Hz. Data acquisition and analysis were performed with Bruker Compass HyStar 4.1 and Bruker DataAnalysis 4.4 software, respectively.
4.3. Urine Samples
Urine samples obtained from volunteers (males and females aged between 20 and 45) were used to prepare calibration curves and validate the procedure. The samples were preserved with sodium azide and then stored at −20 °C prior to analysis.
4.4. Urine Sample Preparation
Due to high concentrations of the analytes in urine samples, 50 µL of urine was transferred into a 1.5 mL Eppendorf tube followed by the addition of 850 µL of water–acetonitrile (30:70, v/v) mixture containing gabapentin as the internal standard and 100 µL of 250 μg/mL PG-Cl solution in acetonitrile for analytes’ derivatization. After vortex mixing for 2 min, the samples were incubated at 30 °C for 10 min, diluted by 500 µL of water–methanol mixture (1:1, v/v), centrifuged at 10,000 rpm for 10 min, and 500 µL of the supernatant was transferred into the glass vial. The final concentration of IS in samples was 50 ng/mL.
4.5. Preparation of Standard and Stock Solutions
Stock standard solutions of catecholamines (adrenaline, dopamine, octopamine) with the concentrations of 1 mg/mL were prepared by dissolving accurately weighted reagents in acetate, phosphate, borate and carbonate buffers and were further diluted with acetonitrile to obtain working solutions. Working solutions of the derivatization agents (FMOC-Cl, PG-Cl, DNS-Cl at 1 mg/mL) were obtained by dissolving appropriate reagent weights in acetonitrile. Quality control (QC) solutions containing catecholamines at high (250 ng/mL), medium (100 ng/mL) and low (10 ng/mL) concentrations were prepared independently from the working solutions.
5. Conclusions
According to the obtained results, PG-Cl can provide greater sensitivity in comparison to FMOC-Cl and DNS-Cl. Considering the high concentrations of catecholamines in real samples, it can be assumed that the sensitivity will be excessive, which makes it possible for a significant sample dilution to increase the robustness and decrease the signal suppression caused by matrix compounds. At the same time, matrices such as plasma and serum contain large amounts of amines, peptides and proteins, which may cause competitive reactions and lead to unstable results of the quantification.
Author Contributions
Conceptualization, A.T., P.N.N. and S.N.A.; methodology, A.T., V.V.D. and Y.-Q.F.; software, M.Z.; validation, M.Z., E.D. and V.V.D.; formal analysis, M.Z. and E.D.; investigation, A.T.; resources, A.T.; data curation, P.N.N. and S.N.A.; writing—original draft preparation, A.T., Y.-Q.F., V.V.D. and S.N.A.; writing—review and editing, E.D. and S.N.A.; visualization, M.Z.; supervision, P.N.N.; project administration, A.T.; funding acquisition, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
The study was carried out with the financial support of the Russian Science Foundation and Kuban Scientific Foundation (project No. 22-13-20018) using the scientific equipment of the Center for Environmental Analysis at the Kuban State University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by State budgetary healthcare institution “Research Institute–Regional Clinical Hospital No 1 named after Professor S.V. Ochapovsky”.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds dopamine, octopamine and adrenaline are available from the authors.
References
- Shi, N.; Bu, X.; Zhang, M.; Wang, B.; Xu, X.; Shi, X.; Hussain, D.; Xu, X.; Chen, D. Current Sample Preparation Methodologies for Determination of Catecholamines and Their Metabolites. Molecules 2022, 27, 2702. [Google Scholar] [CrossRef]
- Adaway, J.E.; Peitzsch, M.; Keevil, B.G. A novel method for the measurement of plasma metanephrines using online solid phase extraction-liquid chromatography tandem mass spectrometry. Ann. Clin. Biochem. 2015, 52, 361–369. [Google Scholar] [CrossRef]
- He, H.; Zhou, Z.; Dong, C.; Wang, X.; Yu, Q.; Lei, Y.; Luo, L.; Feng, Y. Facile synthesis of a boronate affinity sorbent from mesoporous nanomagnetic polyhedral oligomeric silsesquioxanes composite and its application for enrichment of catecholamines in human urine. Anal. Chim. Acta 2016, 944, 1–13. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, X.-E.; Zhu, S.; Tao, Y.; Ji, W.; Geng, Y.; Wang, X.; Chen, G.; You, J. A new combined method of stable isotope-labeling derivatization-ultrasound-assisted dispersive liquid–liquid microextraction for the determination of neurotransmitters in rat brain microdialysates by ultra high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2017, 1054, 64–72. [Google Scholar]
- Li, Y.; Nesterenko, P.N.; Stanley, R.; Paull, B.; Macka, M. Comparison of cation-exchange capillary columns used for ion chromatographic separation of biogenic amines. J. Chromatogr. A 2018, 1571, 193–200. [Google Scholar] [CrossRef]
- Vilhena, R.O.; Pontes, F.L.D.; Marson, B.M.; Ribeiro, R.P.; Teixeira de Carvalho, K.A.; Cardoso, M.A.; Pontarolo, R. A new HILIC-MS/MS method for the simultaneous analysis of carbidopa, levodopa, and its metabolites in human plasma. J. Chromatogr. B 2014, 967, 41–49. [Google Scholar] [CrossRef]
- Kumar, A.; Hart, J.P.; McCalley, D.V. Determination of catecholamines in urine using hydrophilic interaction chromatography with electrochemical detection. J. Chromatogr. A 2011, 1218, 3854–3861. [Google Scholar] [CrossRef]
- Alsaeedi, M.; Alghamdi, H.; Hayes, P.; Hogan, A.; Glennon, J. Efficient Sub-1 Minute Analysis of Selected Biomarker Catecholamines by Core-Shell Hydrophilic Interaction Liquid Chromatography (HILIC) with Nanomolar Detection at a Boron-Doped Diamond (BDD) Electrode. Separations 2021, 8, 124. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. KOWWIN Ver. 1.68. EPI Suite™-Estimation Program Interface. Available online: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface (accessed on 2 March 2023).
- Kiani, F.; Abbaszadeh, M.; Pousti, M.; Koohyar, F. Ab initio and DFT studies on ionization of octopamine and 6-aminopenicillanic acid in aqueous solution. Ind. J. Chem.—Sec. A Inorg. Phys. Theor. Anal. Chem. 2015, 5, 619–626. [Google Scholar]
- Jameson, R.F.; Kiss, T. The oxovanadyl(IV) catalysed oxidation of adrenaline by molecular oxygen. J. Chem. Soc. Dalton Trans. 1986, 9, 1833–1838. [Google Scholar] [CrossRef]
- Armstrong, J.; Barlow, R.B. The ionization of phenolic amines, including apomorphine, dopamine and catecholamines and an assessment of zwitterion constants. British J. Pharm. 1976, 57, 501–516. [Google Scholar] [CrossRef]
- Bockbrader, H.N.; Wesche, D.; Miller, R.; Chapel, S.; Janiczek, N.; Burger, P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin. Pharm. 2010, 49, 661–669. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Luo, Y.; Shang, J.; Jiang, X. Simultaneous determination of eleven compounds related to metabolism of bioamines in rat cortex and hippocampus by HPLC-ECD with boron-doped diamond working electrode. J. Pharm. Biomed. Anal. 2016, 118, 41–51. [Google Scholar] [CrossRef]
- Brodnik, Z.D.; Jaskiw, G.E. Effect of Mobile Phase pH on the Function of Other Optimization Parameters in an HPLC–ECD Assay of Biogenic Amines and Their Metabolites. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 467–471. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, X.; Shen, J.; Zhang, W. Selective solid-phase extraction of catecholamines from plasma using nanofibers doped with crown ether and their quantitation by HPLC with electrochemical detection. Anal. Bioanal. Chem. 2016, 408, 4987–4994. [Google Scholar] [CrossRef]
- Huang, X.; Guo, X.-F.; Wang, H.; Zhang, H.-S. Analysis of catecholamines and related compounds in one whole metabolic pathway with high performance liquid chromatography based on derivatization. Arab. J. Chem. 2019, 12, 1159–1167. [Google Scholar] [CrossRef]
- Cao, L.; Wu, L.; Zhong, H.; Wu, H.; Zhang, S.; Meng, J.; Li, F. Analysis of neurotransmitter catecholamines and related amines in human urine and serum by chromatography and capillary electrophoresis with 1, 3, 5, 7-tetramethyl-8-(N-hydroxysuccinimidyl propionic ester)-difluoro-boradiaza-s-indacene. AChrom 2022, 34, 276–286. [Google Scholar] [CrossRef]
- Gu, Q.; Shi, X.; Yin, P.; Gao, P.; Lu, X.; Xu, G. Analysis of catecholamines and their metabolites in adrenal gland by liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2008, 609, 192–200. [Google Scholar] [CrossRef]
- Fernández, E.; Vårdal, L.; Vidal, L.; Canals, A.; Gjelstad, A.; Pedersen-Bjergaard, S. Complexation-mediated electromembrane extraction of highly polar basic drugs—A fundamental study with catecholamines in urine as model system. Anal. Bioanal. Chem. 2017, 409, 4215–4223. [Google Scholar] [CrossRef]
- Chen, F.; Fang, B.; Wang, S. A Fast and Validated HPLC Method for Simultaneous Determination of Dopamine, Dobutamine, Phentolamine, Furosemide, and Aminophylline in Infusion Samples and Injection Formulations. J. Anal. Methods Chem. 2021, 2021, 8821126. [Google Scholar] [CrossRef]
- Wang, L.; Wei, W.; Xia, Z.; Jie, X.; Xia, Z.Z. Recent advances in materials for stationary phases of mixed-mode high-performance liquid chromatography. TrAC—Trends Anal. Chem. 2016, 80, 495–506. [Google Scholar] [CrossRef]
- Luo, H.; Ma, L.; Paek, C.; Carr, P.W. Application of silica-based hyper-crosslinked sulfonate-modified reversed stationary phases for separating highly hydrophilic basic compounds. J. Chromatogr. A 2008, 1202, 8–18. [Google Scholar] [CrossRef]
- Tesoro, C.; Lelario, F.; Ciriello, R.; Bianco, G.; Di Capua, A.; Acquavia, M.A. An Overview of Methods for L-Dopa Extraction and Analytical Determination in Plant Matrices. Separations 2022, 9, 224. [Google Scholar] [CrossRef]
- Zhu, X.; Shaw, P.N.; Barrett, D.A. Catecholamines derivatized with 4-fluoro-7-nitro-2,1,3-benzoxadiazole: Characterization of chemical structure and fluorescence properties. Anal. Chim. Acta 2003, 478, 259–269. [Google Scholar] [CrossRef]
- Wong, J.M.T.; Malec, P.A.; Mabrouk, O.S.; Ro, J.; Dus, M.; Kennedy, R.T. Benzoyl chloride derivatization with liquid chromatography-mass spectrometry for targeted metabolomics of neurochemicals in biological samples. J. Chromatogr. A 2016, 1446, 78–90. [Google Scholar] [CrossRef]
- Woo, H.I.; Yang, J.S.; Oh, H.J.; Cho, Y.Y.; Kim, J.H.; Park, H.-D.; Lee, S.-Y. A simple and rapid analytical method based on solid-phase extraction and liquid chromatography–tandem mass spectrometry for the simultaneous determination of free catecholamines and metanephrines in urine and its application to routine clinical analysis. Clin. Biochem. 2016, 49, 573–579. [Google Scholar] [CrossRef]
- Roiffé, R.R.; Ribeiro, W.D.; Sardela, V.F.; de la Cruz, M.N.S.; de Souza, K.R.; Pereira, H.M.G.; Aquino Neto, F.R. Development of a sensitive and fast method for detection of catecholamines and metabolites by HRMS. Microchem. J. 2019, 150, 104173. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, L.; Guan, Q.; Li, H.; Lu, J.; Wang, X. Development and validation of a liquid chromatography tandem mass spectrometry method for the measurement of urinary catecholamines in diagnosis of pheochromocytoma. Biomed. Chromatogr. 2017, 31, e4003. [Google Scholar] [CrossRef]
- Hou, X.; Huang, W.; Tong, Y.; Tian, M. Hollow dummy template imprinted boronate-modified polymers for extraction of norepinephrine, epinephrine and dopamine prior to quantitation by HPLC. Microchim. Acta. 2019, 186, 686. [Google Scholar] [CrossRef]
- Xing, Y.; Li, J.; Chen, M.; Wang, X.; Hou, X. Tannic acid-directed synthesis of magnetic and boronic acid-functionalized metal-organic frameworks for selective extraction and quantification of catecholamines in human urine. Microchim. Acta 2021, 188, 225. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, J.-X.; Cui, W.-Q.; Zhang, J.-W.; Wu, D.-Q.; Yu, X.-R.; Luo, Y.-B.; Jiang, X.-Y.; Zhu, F.-P.; Hussain, D.; et al. A simultaneous extraction/derivatization strategy coupled with liquid chromatography–tandem mass spectrometry for the determination of free catecholamines in biological fluids. J. Chromatogr. A 2021, 1654, 462474. [Google Scholar] [CrossRef]
- Le, T.H.; Lee, D.H.; Kim, J.H.; Park, S.J. Synthesis of enhanced fluorescent graphene quantum dots for catecholamine neurotransmitter sensing. Korean J. Chem. Eng. 2020, 37, 1000–1007. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, Z.; Zhang, H.; Zhao, L.; Chang, Q.; Zhang, X.; Yan, R.; Wu, X.; Jin, Y. Photoinduced Synthesis of Methylated Marine Cyclopeptide Galaxamide Analogs with Isoindolinone as Anticancer Agents. Mar. Drugs 2022, 20, 379. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, J.; Bao, Y.; Jiang, S.; Wang, Z.; Jin, Y.; Qu, F. The conventional turns rather than irregular γ-/β-turn secondary structures accounting for the antitumor activities of cyclic peptide Phakellistatin 6 analogs. Tetrahedron 2020, 76, 130881. [Google Scholar] [CrossRef]
- Joshi, D.K.; Betancourt, F.; McAdorey, A.; Yalagala, R.S.; Poupon, A.; Yan, H. BODIPY quaternary ammonium salt as photosensitizers. J. Photochem. Photobiol. A Chem. 2023, 434, 114213. [Google Scholar] [CrossRef]
- Azaryan, A.; Ligor, T.; Buszewski, B.; Temerdashev, A.; Dmitrieva, E.; Gashimova, E. LC–MS/MS Determination of Catecholamines in Urine Using FMOC-Cl Derivatization on Solid-Phase Extraction Cartridge. Chromatographia 2018, 81, 1487–1494. [Google Scholar] [CrossRef]
- Yakubov, L.A.; Galanin, N.E.; Shaposhnikov, G.P. 5,15-diaminotetrabenzoporphyrins: Synthesis and spectral properties. Russ. J. Gen. Chem. 2012, 82, 482–487. [Google Scholar] [CrossRef]
- Azaryan, A.A.; Dmitrieva, E.V.; Temerdashev, A.Z. UHPLC-HRMS determination of adrenaline and dopamine dansyl derivatives in human saliva. Analyt. Contr. 2020, 24, 298–304. [Google Scholar] [CrossRef]
- Sarigul, N.; Korkmaz, F.; Kurultak, İ. A New Artificial Urine Protocol to Better Imitate Human Urine. Sci. Rep. 2019, 9, 20159. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).