Anion-Induced Structural Diversity and Optical Chromism in a Series of Cyano-Bridged Heterometallic 3d-4f Coordination Polymers
Abstract
1. Introduction
2. Results and Discussion
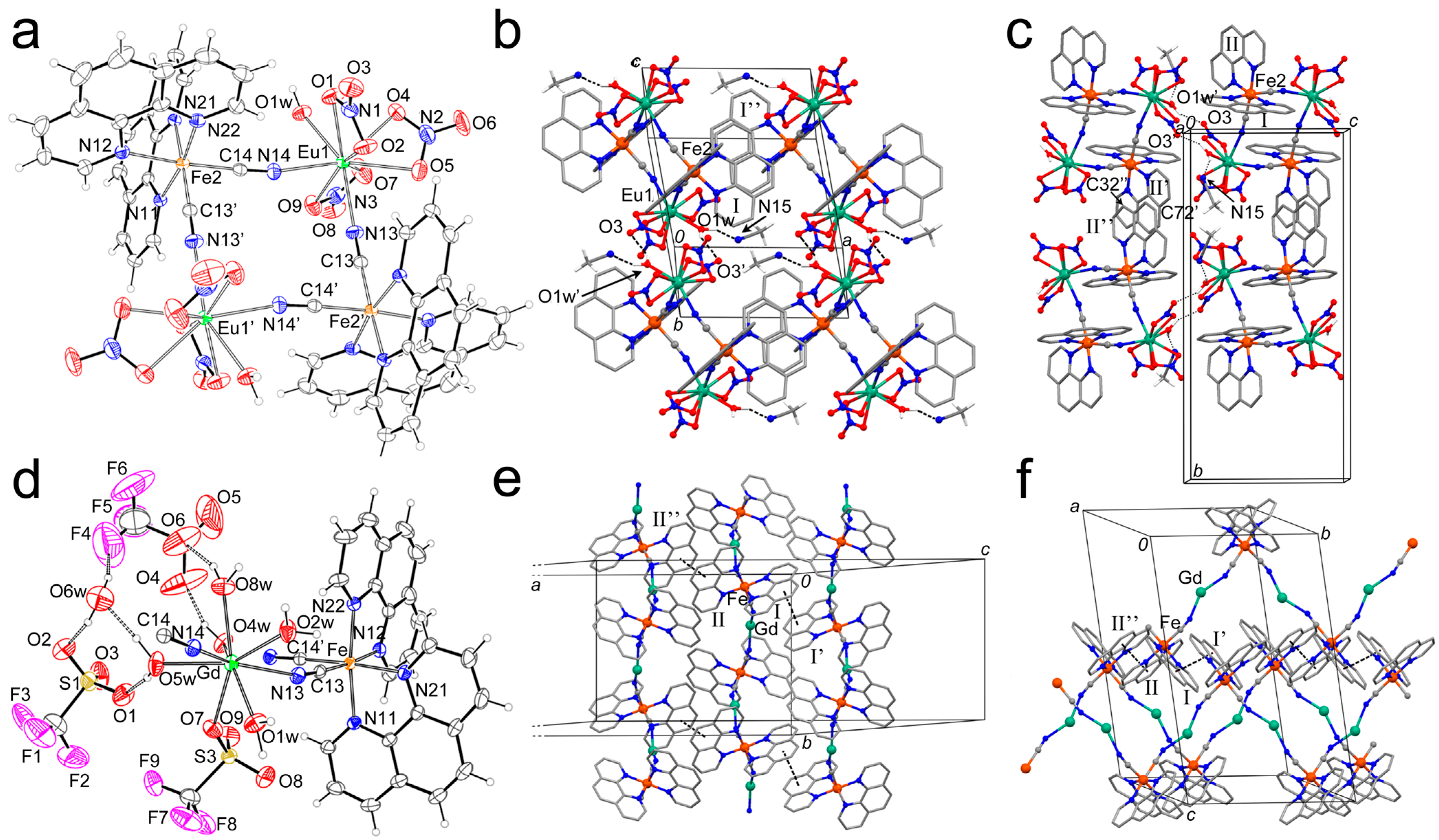
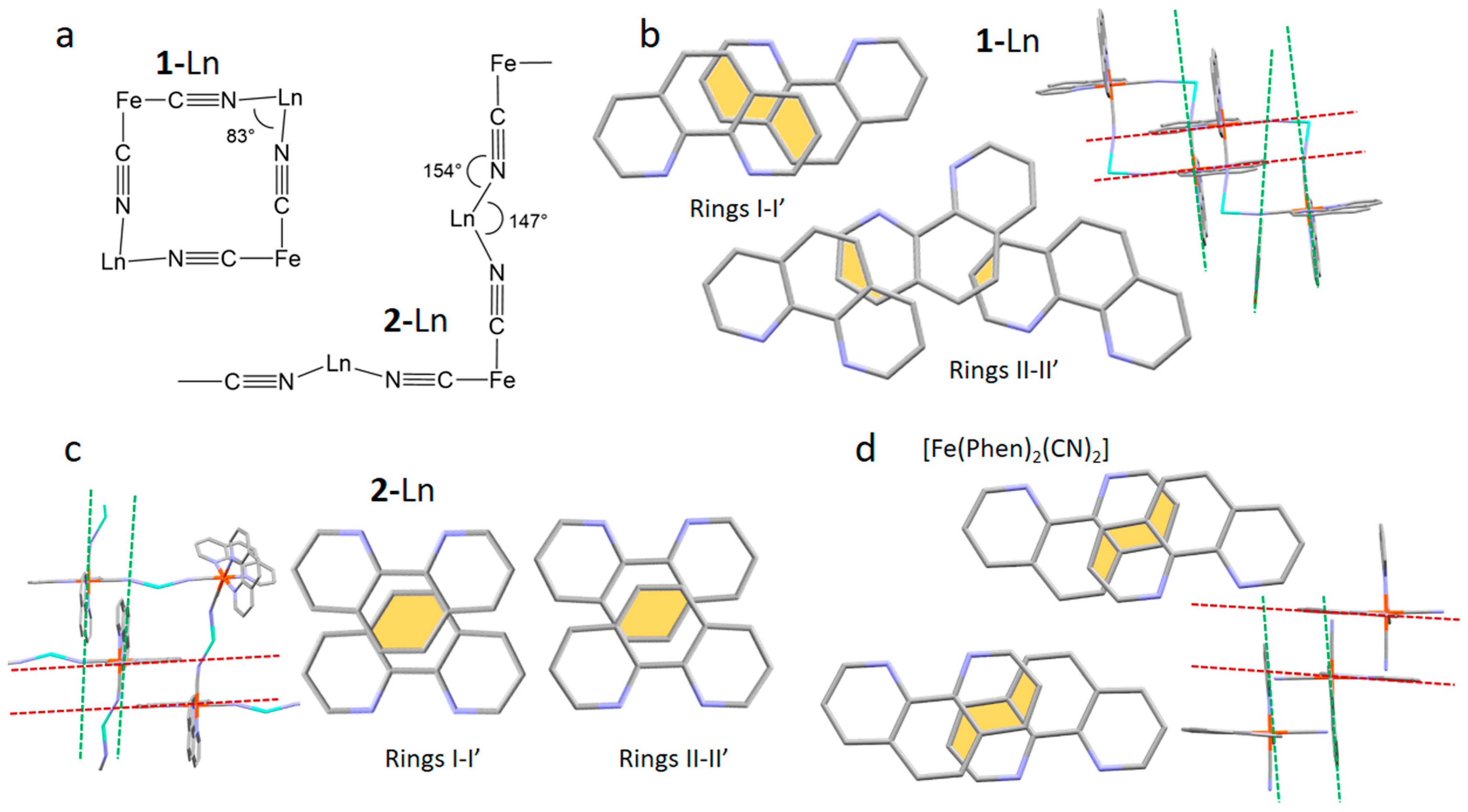
2.1. Synthesis and Crystal Structure Description
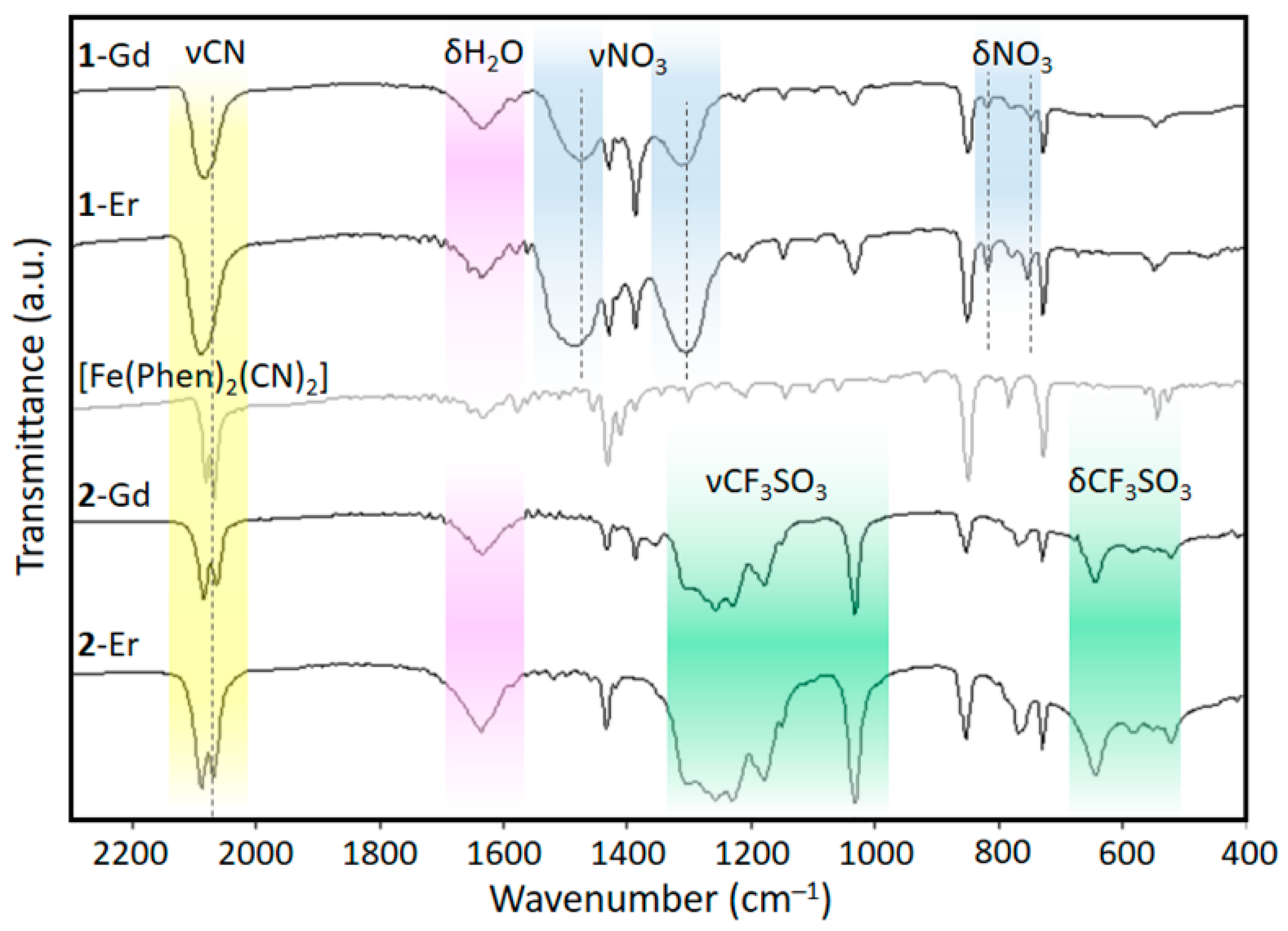
2.2. Vibrational Spectroscopy
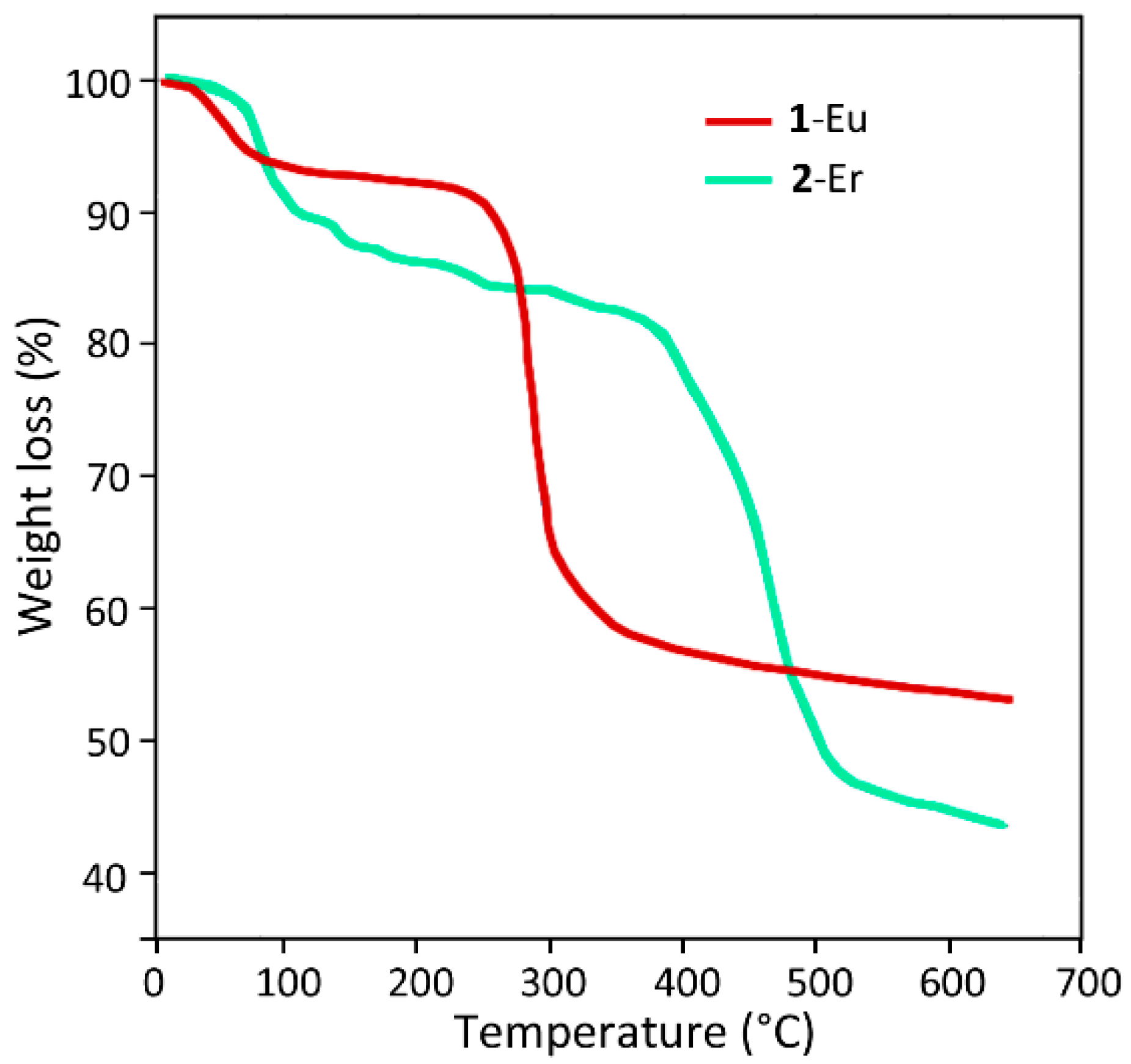
2.3. Thermal Stability
2.4. Optical Properties and Chromism
3. Materials and Methods
3.1. Materials and Analytical Measurements
3.2. Syntheses
3.3. X-ray Crystallography
3.4. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dey, A.; Acharya, J.; Chandrasekhar, V. Heterometallic 3d–4f Complexes as Single-Molecule Magnets. Chem. Asian J. 2019, 14, 4433–4453. [Google Scholar] [CrossRef]
- Tangoulis, V.; Nastopoulos, V.; Panagiotou, N.; Tasiopoulos, A.; Itskos, G.; Athanasiou, M.; Moreno-Pineda, E.; Wernsdorfer, W.; Schulze, M.; Malina, O. High-Performance Luminescence Thermometer with Field-Induced Slow Magnetic Relaxation Based on a Heterometallic Cyanido-Bridged 3d–4f Complex. Inorg. Chem. 2022, 61, 2546–2557. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, J.; Kong, M.; Wang, G.J.; Zhang, Y.Q.; Song, Y. Detailed magnetic properties and theoretical calculation in ferromagnetic coupling DyIII-MII 3d-4f complexes based on a 1,4,7,10-tetraazacyclododecane derivative. Inorg. Chim. Acta 2023, 546, 121301. [Google Scholar] [CrossRef]
- Liberka, M.; Zychowicz, M.; Zychowicz, W.; Chorazy, S. Neutral dicyanidoferrate(II) metalloligands for the rational design of dysprosium(III) single-molecule magnets. Chem. Commun. 2022, 58, 6381–6384. [Google Scholar] [CrossRef] [PubMed]
- Liberka, M.; Boidachenko, K.; Zakrzewski, J.J.; Zychowicz, M.; Wang, J.; Ohkoshi, S.-I.; Chorazy, S. Near-Infrared Emissive Cyanido-Bridged {YbFe2} Molecular Nanomagnets Sensitive to the Nitrile Solvents of Crystallization. Magnetochemistry 2021, 7, 79. [Google Scholar] [CrossRef]
- Yan, H.; Wang, C.M.; Chen, P.; Zhang, Y.Q.; Sun, W.B. Schiff base tetranuclear Zn2Ln2 single-molecule magnets bridged by hydroxamic acid in association with near-infrared luminescence. Dalton Trans. 2022, 51, 6918–6926. [Google Scholar] [CrossRef] [PubMed]
- Artizzu, F.; Quochi, F.; Marchiò, L.; Sessini, E.; Saba, M.; Serpe, A.; Mura, A.; Mercuri, M.L.; Bongiovanni, G.; Deplano, P. Fully Efficient Direct Yb-to-Er Energy Trnsfer at Molecular Level in a Near-Infrared Emitting Heterometallic Trinuclear Quinolinolato Complex. J. Phys. Chem. Lett. 2013, 4, 3062–3066. [Google Scholar] [CrossRef]
- Mara, D.; Pilia, L.; Van de Steen, M.; Miletto, I.; Zeng, M.; Van Hecke, K.; Serpe, A.; Deplano, P.; Van Deun, R.; Artizzu, F. Single-component panchromatic white light generation, and tuneable excimer-like visible orange and NIR emission in a Dy quinolinolate complex. J. Mater. Chem. C 2021, 9, 15641–15648. [Google Scholar] [CrossRef]
- Yang, C.; Artizzu, F.; Folens, K.; Du Laing, G.; Van Deun, R. Excitation dependent multicolour luminescence and colour blueshifted afterglow at room-temperature of europium incorporated hydrogen-bonded multicomponent frameworks. J. Mater. Chem. C 2021, 9, 7154–7162. [Google Scholar] [CrossRef]
- Yang, C.; Folens, K.; Du Laing, G.; Artizzu, F.; Van Deun, R. Improved Quantum Yield and Excellent Luminescence Stability of Europium-Incorporated Polymeric Hydrogen-Bonded Heptazine Frameworks Due to an Efficient Hydrogen-Bonding Effect. Adv. Funct. Mater. 2020, 30, 2003656. [Google Scholar] [CrossRef]
- Yang, C.; Mara, D.; Goura, J.; Artizzu, F.; Van Deun, R. Photophysical and Primary Self-Referencing Themometric Properties of Europium Hydrogen-Bonded Triazine Frameworks. Molecules 2022, 27, 6687. [Google Scholar] [CrossRef]
- Aguilà, D.; Velasco, V.; Barrios, L.A.; Gonzalez-Fabra, J.; Bo, C.; Teat, S.J.; Roubeau, O.; Aromí, G. Selective Lanthanide Distribution within a Comprehensive Series of Heterometallic [LnPr] Complexes. Inorg. Chem. 2018, 57, 8429–8439. [Google Scholar] [CrossRef] [PubMed]
- Artizzu, F.; Quochi, F.; Marchiò, L.; Fonseca Correia, R.; Saba, M.; Serpe, A.; Mura, A.; Mercuri, M.L.; Bongiovanni, G.; Deplano, P. Ln3Q9 as a Molecular Framework for Ion-Size-Driven Assembly of Heterolanthanide (Nd, Er, Yb) Multiple Near-Infrared Emitters. Chem. A Eur. J. 2015, 21, 3882–3885. [Google Scholar] [CrossRef]
- Artizzu, F.; Atzori, M.; Liu, J.; Mara, D.; Van Hecke, K.; Van Deun, R. Solution-processable Yb/Er 2D-layered metallorganic frameworks with high NIR-emission quantum yields. J. Mater. Chem. C 2019, 7, 11207–11214. [Google Scholar] [CrossRef]
- Liu, J.Q.; Luo, Z.D.; Pan, Y.; Kumar Singh, A.; Trivedi, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Demakov, P.A.; Vasileva, A.A.; Volynkin, S.S.; Ryadun, A.A.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate. Molecules 2021, 26, 5145. [Google Scholar] [CrossRef]
- Demakov, P.A.; Vasileva, A.A.; Lazarenko, V.A.; Ryadun, A.A.; Fedin, V.P. Crystal Structures, Thermal and Luminescent Properties of Gadolinium(III) Trans-1,4-cyclohexanedicarboxylate Metal-Organic Frameworks. Crystals 2021, 11, 1375. [Google Scholar] [CrossRef]
- Schilt, A.A. Mixed Ligand Complexes of Iron(II) and (III) with Cyanide and Aromatic Di-imines. J. Am. Chem. Soc. 1960, 82, 3000–3005. [Google Scholar] [CrossRef]
- Zhan, S.; Meng, Q.; You, X.; Wang, G.; Zheng, P.J. The properties, crystal and molecular structure of cis-dicyano-bis(1,10-phenanthroline)iron(II) trihydrate. Polyhedron 1996, 15, 2655–2658. [Google Scholar] [CrossRef]
- Jin, J.; Xu, X.T.; Li, D.; Han, X.; Li, L.; Chi, Y.X.; Niu, S.Y.; Zhang, G.N. Preparation and surface photoelectric properties of Fe(II/III) complexes. Solid State Sci. 2013, 19, 73–79. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Butterworth-Heinmann: Oxford, UK, 1998. [Google Scholar]
- Harada, K.; Yuzurihara, J.; Ishii, Y.; Sato, N.; Kambayashi, H.; Fukuda, Y. d-f Metal-Ion Interaction Through Fe-CN-Ln Bridge Between Dicyanobis(1,10-Phenanthroline)Iron(II) Complex and LnCl3. Chem. Lett. 1995, 24, 887–888. [Google Scholar] [CrossRef]
- Song, X.J.; Xu, J.J.; Chen, Y.; Muddassir, M.; Cao, F.; Wei, R.M.; Song, Y.; You, X.Z. Synthesis, structures and magnetic properties of cyano-bridged 3d–4f rectangular tetranuclear [FeIII2LnIII2] (Ln = Y, Tb, Dy) compounds containing [FeIII(bpy)(CN)4] unit. Polyhedron 2013, 66, 212–217. [Google Scholar] [CrossRef]
- Alexandru, M.G.; Visinescu, D.; Shova, S.; Oliveira, W.N.X.C.; Lloret, F.; Julve, M. Design of 3d–4f molecular squares through the [Fe{(HB(pz)3)}(CN)3]-metalloligand. Dalton Trans. 2018, 47, 6005–6017. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, M.G.; Visinescu, D.; Cula, B.; Shova, S.; Rabelo, R.; Moliner, N.; Lloret, F.; Cano, J.; Julve, M. A rare isostructural series of 3d–4f cyanide bridged heterometallic squares obtained by assembling [FeIII{HB(pz)3}(CN)3] − and LnIII ions: Synthesis, X-ray structure and cryomagnetic study. Dalton Trans. 2021, 50, 14640–14652. [Google Scholar] [CrossRef] [PubMed]
- Figuerola, A.; Diaz, C.; Ribas, J.; Tangoulis, V.; Sangregorio, C.; Gatteschi, D.; Maestro, M.; Mahia, J. Magnetism of cyano-bridged hetero-one-dimensional Ln3+-M3+ complexes (Ln3+ = Sm, Gd, Yb; M3+ = Fe-LS, Co). Inorg. Chem. 2003, 42, 5274–5281. [Google Scholar] [CrossRef]
- Koner, R.; Drew, M.G.B.; Figuerola, A.; Diaz, C.; Mohanta, S. A new cyano-bridged one-dimensional GdIIIFeIII coordination polymer with o-phenanthroline as the blocking ligand: Synthesis, structure, and magnetic properties. Inorg. Chim. Acta 2005, 358, 3041–3047. [Google Scholar] [CrossRef]
- Zhao, H.H.; Lopez, N.; Prosvirin, A.; Chifotides, H.T.; Dunbar, K.R. Lanthanide-3d cyanometalate chains Ln(III)-M(III) (Ln = Pr, Nd, Sm, Eu, Gd, Tb; M = Fe) with the tridentate ligand 2,4,6-tri(2-pyridyl)-1,3,5-triazine (tptz): Evidence of ferromagnetic interactions for the Sm(III)-M(III) compounds (M = Fe, Cr). Dalton Trans. 2007, 8, 878–888. [Google Scholar] [CrossRef]
- Figuerola, A.; Ribas, J.; Llunell, M.; Casanova, D.; Maestro, M.; Alvarez, S.; Diaz, C. Magnetic properties of cyano-bridged Ln3+-M3+ complexes. Part I: Trinuclear complexes (Ln3+ = La, Ce, Pr, Nd, Sm; M3+ = Fe-LS, Co) with bpy as blocking ligand. Inorg. Chem. 2005, 44, 6939–6948. [Google Scholar] [CrossRef]
- Schilt, A.A. Cyanide Stretching Frequencies of Some Mixed Ligand Complexes of Iron, Ruthenium and Osmium. Inorg. Chem. 1964, 3, 1323–1325. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Hu, S.; Wen, Y.; Xue, Z.; Zhu, X.; Zhang, X.; Sheng, T.; Wu, X. Syntheses, crystal structures, MMCT and magnetic properties of four one-dimensional cyanide-bridged complexes comprised of MII–CN–FeIII (M = Fe, Ru, Os). Dalton Trans. 2014, 43, 17453–17462. [Google Scholar] [CrossRef]
- Al-Aousy, A.; Burgess, J. Bis(1,10-phenanthroline) dicyanoiron(II): An almost universal inorganic solvent polarity indicator. Inorg. Chim. Acta 1990, 169, 167–170. [Google Scholar] [CrossRef]
- Tłaczała, T.; Bartecki, A. Studies on the solvatochromism of Fe(CN)2(phen)2. Monatsch. Chem. 1997, 128, 225–234. [Google Scholar] [CrossRef]
- Taha, A.; Mahmoud, M.M. Lewis acidity parameter for binary solvent mixtures and adduct formation studies using the solvatochromic dicyanobis(1,10-phenanthroline)iron(II) complex. New J. Chem. 2002, 26, 953–957. [Google Scholar] [CrossRef]
- Linert, W.; Jaeson, R.F.; Bauer, G.; Taha, A. Estimation of the acceptor numbers of cations by means of an acid-base indicator. J. Coord. Chem. 1997, 42, 211–229. [Google Scholar] [CrossRef]
- Georgieva, I.; Aquino, A.J.A.; Trendafilova, N.; Santos, P.S.; Lischka, H. Solvatochromic and Ionochromic Effects of Iron(II)bis(1,10-phenanhroline)dicyano: A Theoretical Study. Inorg. Chem. 2010, 49, 1634–1646. [Google Scholar] [CrossRef] [PubMed]
- SMART (Control) and SAINT (Integration) Software for CCD Systems; Bruker AXS: Madison, WI, USA, 2008.
- Area-Detector Absorption Correction; Siemens Industrial Automation, Inc.: Madison, WI, USA, 1996.
- Walker, N.; Stuart, D. An Empirical Method for Correcting Diffractometer Data for Absorption Effects. Acta Cryst. Sect. A 1983, 39, 158–166. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. App. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX97. Programs for Crystal Structure Analysis; 1997 (Release 97-2); University of Göttingen: Göttingen, Germany, 2008. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Van der Sluis, P.; Spek, A.L. BYPASS: An effective method for the refinement of crystal structures containing disordered solvent regions. Acta Cryst. 1990, A46, 194–201. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565–568. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01, Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Andrae, D.; Haussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Thompson, M.A. ArgusLab 4.0.1, Planaria Software LLC: Seattle, WA, USA, 2021. Available online: http://www.arguslab.com/arguslab.com/ArgusLab.html/ (accessed on 6 March 2020).



| 1-Eu | 1-Er | |
|---|---|---|
| Empirical formula | C56H42Eu2Fe2N20O20 | C56H42Er2Fe2N20O20 |
| Formula weight | 1730.72 | 1761.32 |
| Color, habit | Prism, red | Prism, red |
| Crystal size, mm | 0.40 × 0.30 × 0.18 | 0.20 × 0.10 × 0.05 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/a | P21/a |
| a, Å | 10.730(1) | 10.74(7) |
| b, Å | 25.623(3) | 25.59(9) |
| c, Å | 11.789(2) | 11.74(8) |
| α, deg. | 90 | 90 |
| β, deg. | 100.63(2) | 100.26(3) |
| γ, deg. | 90 | 90 |
| V, Å3 | 3181.6(7) | 3174(3) |
| Z | 2 | 2 |
| T, K | 293(2) | 293(2) |
| ρ (calc), Mg/m3 | 1.804 | 1.843 |
| μ, mm−1 | 2.479 | 3.256 |
| θ range, deg. | 1.59 to 28.02 | 3.07 to 25.00 |
| No. of rflcn/obsv | 39,388/5692 | 6741/2582 |
| GooF | 1.002 | 0.768 |
| R1 | 0.0299 | 0.0482 |
| wR2 | 0.0528 | 0.0535 |
| 2-Gd | 2-Eu | 2-Er | |
|---|---|---|---|
| Empirical formula | C30H29Cl3F9FeGdN6O15S3 | C30H29Cl3EuF9FeN6O15S3 | C30H29Cl3ErF9FeN6O15S3 |
| Formula weight | 1300.22 | 1294.93 | 1310.23 |
| Color, habit | Plate, orange | Plate, orange | Plate, orange |
| Crystal size, mm | 0.45 × 0.22 × 0.10 | 0.15 × 0.08 × 0.05 | 0.12 × 0.07 × 0.05 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | C2/c | C2/c |
| a, Å | 33.191(2) | 33.158(4) | 33.247(8) |
| b, Å | 13.790(1) | 13.809(2) | 13.716(3) |
| c, Å | 27.296(1) | 27.281(3) | 27.173(5) |
| α, deg. | 90 | 90 | 90 |
| β, deg. | 131.359(1) | 131.197(2) | 131.16(3) |
| γ, deg. | 90 | 90 | 90 |
| V, Å3 | 9378.8(9) | 9399(2) | 9329(3) |
| Z | 8 | 8 | 8 |
| T, K | 293(2) | 293(2) | 293(2) |
| ρ (calc), Mg/m3 | 1.842 | 1.830 | 1.866 |
| μ, mm−1 | 2.120 | 2.039 | 2.508 |
| θ range, deg. | 1.53 to 31.10 | 1.53 to 25.09 | 1.53 to 24.90 |
| No. of rflcn/obsv | 76,817/11,455 | 50,971/5560 | 48,135/5383 |
| GooF | 1.020 | 1.051 | 1.015 |
| R1 | 0.0433 | 0.0556 | 0.0677 |
| wR2 | 0.1322 | 0.1299 | 0.1663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artizzu, F.; Pilia, L.; Serpe, A.; Mara, D.; Casula, M.F.; Marchiò, L.; Deplano, P. Anion-Induced Structural Diversity and Optical Chromism in a Series of Cyano-Bridged Heterometallic 3d-4f Coordination Polymers. Molecules 2023, 28, 2871. https://doi.org/10.3390/molecules28062871
Artizzu F, Pilia L, Serpe A, Mara D, Casula MF, Marchiò L, Deplano P. Anion-Induced Structural Diversity and Optical Chromism in a Series of Cyano-Bridged Heterometallic 3d-4f Coordination Polymers. Molecules. 2023; 28(6):2871. https://doi.org/10.3390/molecules28062871
Chicago/Turabian StyleArtizzu, Flavia, Luca Pilia, Angela Serpe, Dimitrije Mara, Maria Francesca Casula, Luciano Marchiò, and Paola Deplano. 2023. "Anion-Induced Structural Diversity and Optical Chromism in a Series of Cyano-Bridged Heterometallic 3d-4f Coordination Polymers" Molecules 28, no. 6: 2871. https://doi.org/10.3390/molecules28062871
APA StyleArtizzu, F., Pilia, L., Serpe, A., Mara, D., Casula, M. F., Marchiò, L., & Deplano, P. (2023). Anion-Induced Structural Diversity and Optical Chromism in a Series of Cyano-Bridged Heterometallic 3d-4f Coordination Polymers. Molecules, 28(6), 2871. https://doi.org/10.3390/molecules28062871








