Expanding the Library of 1,2,4-Oxadiazole Derivatives: Discovery of New Farnesoid X Receptor (FXR) Antagonists/Pregnane X Receptor (PXR) Agonists
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Evaluation of Biological Activity
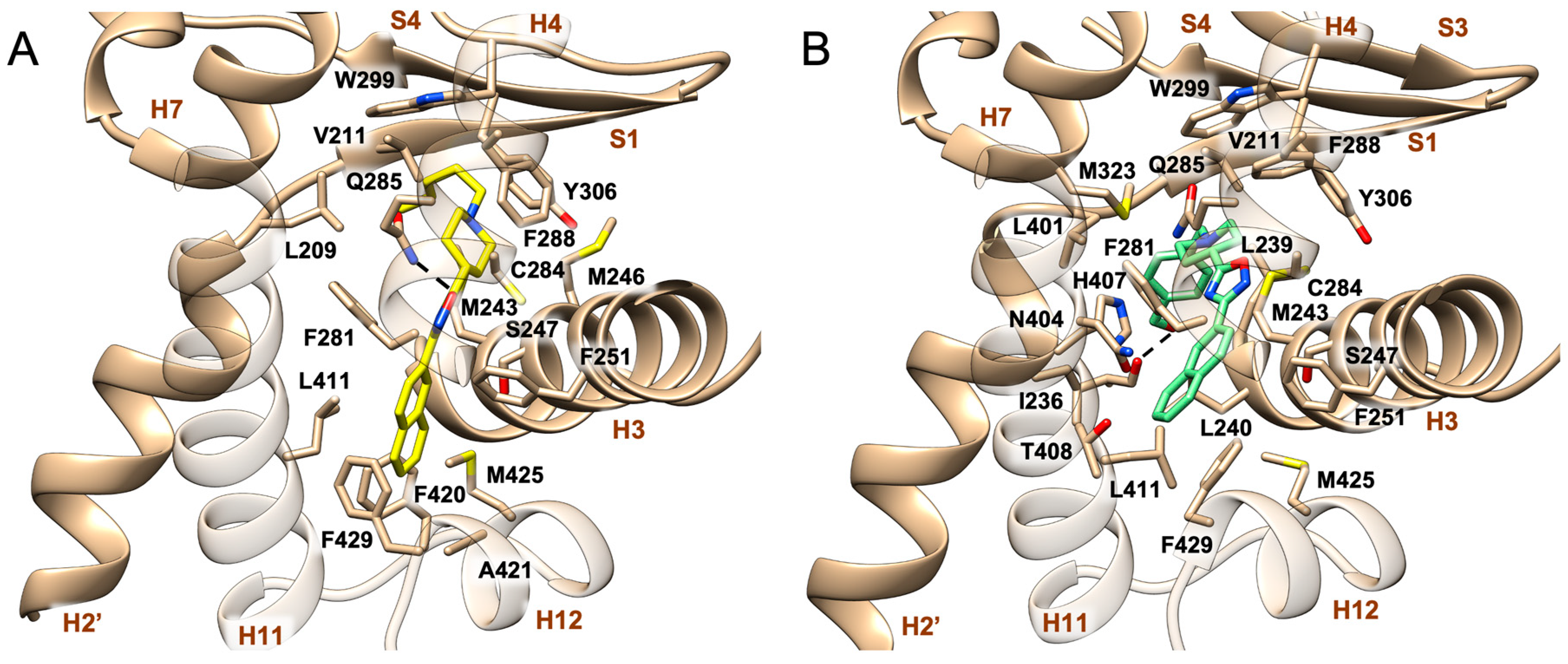
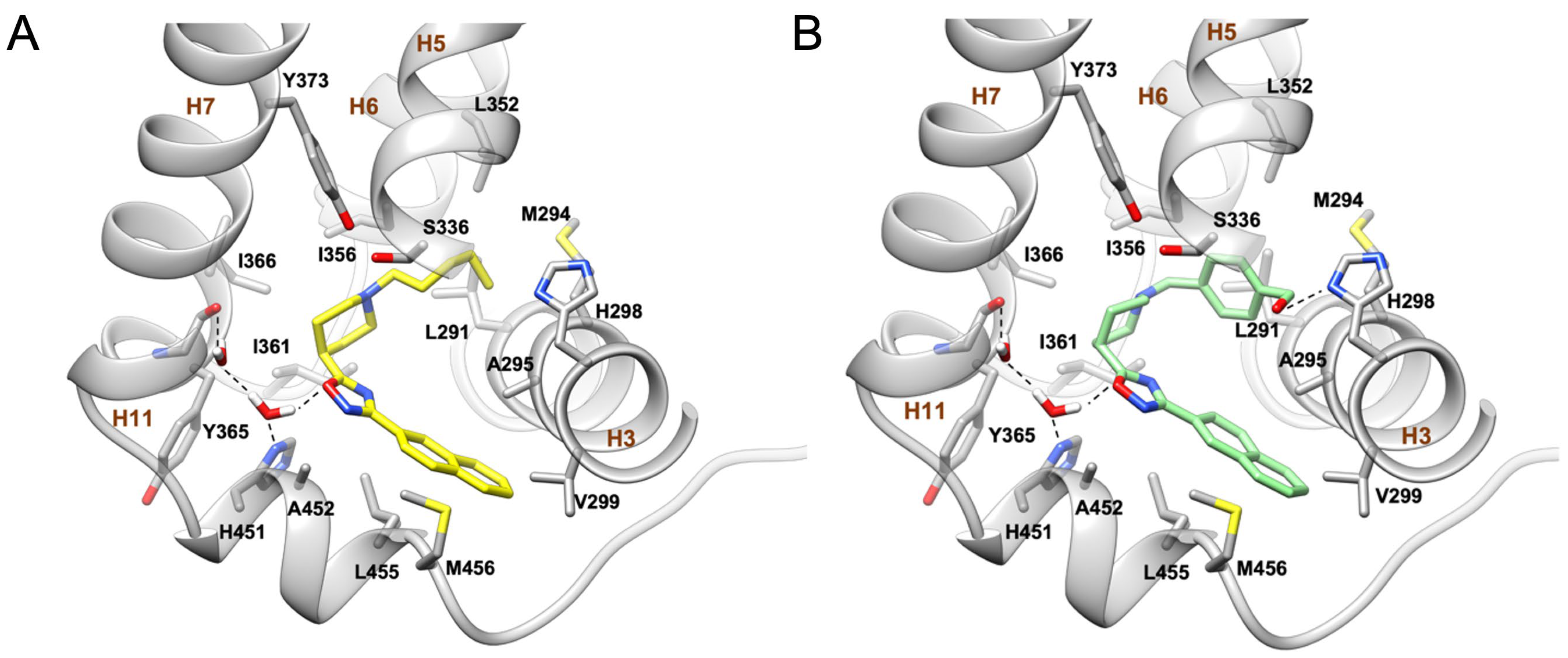
2.2. Molecular Docking
3. Materials and Methods
3.1. Synthesis
3.1.1. General Information
3.1.2. Synthetic Procedures
3.2. Biological Assays
3.2.1. Luciferase Reporter Gene Assay
3.2.2. Cell Culture
3.2.3. Reverse Transcription of mRNA and Real-Time PCR
3.3. Physicochemical Properties and Pharmacokinetic Characterization
3.3.1. S9 Stability of Compounds 5 and 11
3.3.2. LogD Measurement
3.4. Molecular Docking
3.4.1. Receptor Preparation
3.4.2. Ligand Preparation
3.4.3. Docking Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, Y.D.; Chen, W.D.; Moore, D.D.; Huang, W. FXR: A metabolic regulator and cell protector. Cell Res. 2008, 18, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Lia, G.; Guoc, G.L. Farnesoid X receptor, the bile acid sensing nuclear receptor, in liver re-generation. Acta Pharma. Sin. B 2015, 5, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, W.; Huang, W. FXR and liver carcinogenesis. Acta Pharmacol. Sin. 2015, 36, 37–43. [Google Scholar] [CrossRef]
- Moris, D.; Giaginis, C.; Tsourouflis, G.; Theocharis, S. Farnesoid-X Receptor (FXR) as a promising pharmaceutical target in atherosclerosis. Curr. Med. Chem. 2017, 24, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Levi, M. Nuclear receptors in renal disease. Biochim. Biophys. Acta 2011, 1812, 1061–1067. [Google Scholar] [CrossRef]
- Carino, A.; Biagioli, M.; Marchianò, S.; Scarpelli, P.; Zampella, A.; Limongelli, V.; Fiorucci, S. Disruption of TFGβ-SMAD3 pathway by the nuclear receptor SHP mediates the antifibrotic activities of BAR704, a novel highly selective FXR ligand. Pharmacol. Res. 2018, 131, 17–31. [Google Scholar] [CrossRef]
- Erstad, D.J.; Farrar, C.T.; Ghoshal, S.; Masia, R.; Ferreira, D.S.; Chen, Y.I.; Choi, J.K.; Wei, L.; Waghorn, P.A.; Rotile, N.J.; et al. Molecular magnetic resonance imaging accurately measures the antifibrotic effect of EDP-305, a novel farnesoid X recep-tor agonist. Hepatol. Commun. 2018, 2, 821–835. [Google Scholar] [CrossRef]
- Akwabi-Ameyaw, A.; Bass, J.Y.; Caldwell, R.D.; Caravella, J.A.; Chen, L.; Creech, K.L.; Deaton, D.N.; Madauss, K.P.; Marr, H.B.; Miller, A.B.; et al. Conformationally constrained farnesoid X receptor (FXR) agonists: Naphthoic acid-based analogs of GW 4064. Bioorg. Med. Chem. Lett. 2008, 18, 4339–4343. [Google Scholar] [CrossRef]
- Han, C.Y. Update on FXR Biology: Promising Therapeutic Target? Int. J. Mol. Sci. 2018, 19, 2069. [Google Scholar] [CrossRef]
- Gonzalez, F.J.; Jiang, C.; Patterson, A.D. An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 2016, 151, 845–859. [Google Scholar] [CrossRef]
- Jiang, C.; Xie, C.; Li, F.; Zhang, L.; Nichols, R.G.; Krausz, K.W.; Cai, J.; Qi, Y.; Fang, Z.Z.; Takahashi, S.; et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Investig. 2015, 125, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xie, C.; Lv, Y.; Li, J.; Krausz, K.W.; Shi, J.; Brocker, C.N.; Desai, D.; Amin, S.G.; Bisson, W.H.; et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 2015, 6, 10166. [Google Scholar] [CrossRef]
- Xie, C.; Jiang, C.; Shi, J.; Gao, X.; Sun, D.; Sun, L.; Wang, T.; Takahashi, S.; Anitha, M.; Krausz, K.W.; et al. An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 2017, 66, 613–626. [Google Scholar] [CrossRef]
- Panzitt, K.; Zollner, G.; Marschall, H.U.; Wagner, M. Recent advances on FXR-targeting therapeutics. Mol. Cell. Endocrinol. 2022, 552, 111678. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Thorell, A.; Claudel, T.; Jha, P.; Koefeler, H.; Lackner, C.; Hoesel, B.; Fauler, G.; Stojakovic, T.; Einarsson, C.; et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J. Hepatol. 2015, 62, 1398–1404. [Google Scholar] [CrossRef]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Okamoto, A.Y.; Shan, B. FXR, a bile acid receptor and biological sensor. Trends Cardiovasc. Med. 2000, 10, 30–35. [Google Scholar] [CrossRef]
- Festa, C.; Finamore, C.; Marchianò, S.; Di Leva, F.S.; Carino, A.; Monti, M.C.; del Gaudio, F.; Ceccacci, S.; Limongelli, V.; Zampella, A.; et al. Investigation around the oxadiazole core in the discovery of a new chemotype of potent and selective FXR antagonists. ACS Med. Chem. Lett. 2019, 10, 504–510. [Google Scholar] [CrossRef]
- Oladimeji, P.O.; Chen, T. PXR: More Than Just a Master Xenobiotic Receptor. Mol. Pharmacol. 2018, 93, 119–127. [Google Scholar] [CrossRef]
- Fiorucci, S.; Zampella, A.; Distrutti, E. Development of FXR, PXR and CAR agonists and antagonists for treatment of liver disorders. Curr. Top. Med. Chem. 2012, 12, 605–624. [Google Scholar] [CrossRef]
- De Marino, S.; Ummarino, R.; D’Auria, M.V.; Chini, M.G.; Bifulco, G.; Renga, B.; D’Amore, C.; Fiorucci, S.; Debitus, C.; Zampella, A. Theonellasterols and Conicasterols from Theonella swinhoei. Novel marine natural ligands for human nuclear receptors. J. Med. Chem. 2011, 54, 3065–3075. [Google Scholar] [CrossRef]
- Mihorianu, M.; Franz, M.H.; Jones, P.G.; Freytag, M.; Kelter, G.; Fiebig, H.-H.; Tamm, M.; Neda, I. N-Heterocyclic carbenes (NHC) derived from imidazo [1,5-a]pyridines related to natural products: Synthesis, structure and potential biological activity of some corresponding gold(I) and silver(I) complexes. Appl. Organometal. Chem. 2016, 30, 581–589. [Google Scholar] [CrossRef]
- Evans, B.E.; Rittle, K.E.; Bock, M.G.; DiPardo, R.M.; Freidinger, R.M.; Whitter, W.L.; Lundell, G.F.; Veber, D.F.; Anderson, P.S.; Chang, R.S.L.; et al. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chaudhry, S.T.; Mei, J. Chapter Five—Heterocyclic building blocks for organic semiconductors. Adv. Heterocycl. Chem. 2017, 121, 133–171. [Google Scholar]
- Shiri, P. Novel hybrid molecules based on triazole-β-lactam as potential biological agents. Mini Rev. Med. Chem. 2021, 21, 536–553. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Uzzaman, M. A review on biological and medicinal impact of heterocyclic compounds. Results Chem. 2022, 4, 100606. [Google Scholar] [CrossRef]
- Pace, A.; Pierro, P. The new era of 1,2,4-oxadiazoles. Org. Biomol. Chem. 2009, 7, 4337–4348. [Google Scholar] [CrossRef]
- Salassa, G.; Terenzi, A. Metal complexes of oxadiazole ligands: An overview. Int. J. Mol. Sci. 2019, 20, 3483. [Google Scholar] [CrossRef]
- Maftei, C.V.; Fodor, E.; Jones, P.G.; Daniliuc, C.G.; Franz, M.H.; Kelter, G.; Fiebig, H.-H.; Tamm, M. Novel 1,2,4-oxadiazoles and trifluoromethylpyridines related to natural products: Synthesis, structural analysis and investigation of their antitumor activity. Tetrahedron 2016, 72, 1185–1199. [Google Scholar] [CrossRef]
- Anzini, M.; Valenti, S.; Braile, C.; Cappelli, A.; Vomero, S.; Alcaro, S.; Ortuso, F.; Marinelli, L.; Limongelli, V.; Novellino, E.; et al. New insight into the central benzodiazepine receptor-ligand interactions: Design, synthesis, biological evalua-tion, and molecular modeling of 3-substituted 6-phenyl-4H-imidazo [1,5-a][1,4]benzodiazepines and related compounds. J. Med. Chem. 2011, 54, 5694–5711. [Google Scholar] [CrossRef]
- Limongelli, V. Ligand binding free energy and kinetics calculation in 2020. Comput. Mol. Sci. 2020, 10, e1455. [Google Scholar] [CrossRef]
- Deplano, A.; Karlsson, J.; Svensson, M.; Moraca, F.; Catalanotti, B.; Fowler, C.J.; Onnis, V. Exploring the fatty acid amide hydrolase and cyclooxygenase inhibitory properties of novel amide derivatives of ibuprofen. J. Enzyme Inhib. Med. Chem. 2020, 35, 815–823. [Google Scholar] [CrossRef]
- Delfosse, V.; Huet, T.; Harrus, D.; Granell, M.; Bourguet, M.; Gardia-Parège, C.; Chiavarina, B.; Grimaldi, M.; Le Mével, S.; Blanc, P.; et al. Mechanistic insights into the synergistic activation of the RXR-PXR heterodimer by endocrine disruptor mixtures. Proc. Natl. Acad. Sci USA 2021, 118, e2020551118. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Prosise, W.W.; Chen, J.; Taremi, S.S.; Le, H.V.; Madison, V.; Cui, X.; Thomas, A.; Cheng, K.C.; Lesburg, C.A. Construction and characterization of a fully active PXR/SRC-1 tethered protein with increased stability. Protein Eng. Des. Sel. 2008, 21, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, P.; Zhu, Z.Y.; Chen, J.; Fu, H.A.; Chen, L.L.; Hu, L.H.; Shen, X. Structural basis for small molecule NDB (N-benzyl-N-(3-(tert-butyl)-4-hydroxyphenyl)-2,6-dichloro-4-(dimethylamino) benzamide) as a selective antagonist of Farnesoid X Receptor α (FXRα) in stabilizing the homodimerization of the receptor. J. Biol. Chem. 2015, 290, 19888–19899. [Google Scholar] [CrossRef] [PubMed]
- De Marino, S.; Finamore, C.; Biagioli, M.; Carino, A.; Marchianò, S.; Roselli, R.; Giorgio, C.D.; Bordoni, M.; Di Leva, F.S.; Novellino, E.; et al. GPBAR1 activation by C6-substituted hyodeoxycholane analogues protect against colitis. ACS Med. Chem. Lett. 2020, 11, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.R.; Calkins, D.; Sullivan, A.P.; Shelley, J.C. Towards the comprehensive, rapid, and accurate prediction of the favorable tautomeric states of drug-like molecules in aqueous solution. J. Comput. Aided Mol. Des. 2010, 24, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.L.; Beard, H.S.; Cao, Y.; Cho, A.E.; Damm, W.; Farid, R.; Felts, A.K.; Halgren, T.A.; Mainz, D.T.; Maple, J.R.; et al. Integrated modeling program, applied chemical theory (IMPACT). J. Comput. Chem. 2005, 26, 1752–1780. [Google Scholar] [CrossRef]
- Schrödinger Release 2022-3: Maestro; Schrödinger LLC: New York, NY, USA, 2021.
- Schrödinger Release 2022-3: LigPrep; Schrödinger LLC: New York, NY, USA, 2021.
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pK(a) pre-diction and protonation state generation for drug-like molecules. J. Comput. Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef]
- Schrödinger Release 2022-3: Epik; Schrödinger LLC: New York, NY, USA, 2021.
- Grippo, L.; Lucidi, S. A globally convergent version of the Polak-Ribiere conjugate gradient method. Math. Program. 1997, 78, 375–391. [Google Scholar] [CrossRef]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef]
- Schrödinger Release 2022-3; Schrödinger LLC: New York, NY, USA, 2021.
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all-atom protein loop prediction. Proteins 2004, 55, 351–367. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Friesner, R.A.; Xiang, Z.; Honig, B. On the role of the crystal environment in determining protein side-chain conformations. J. Mol. Biol. 2002, 320, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2022-3: Glide; Schrödinger LLC: New York, NY, USA, 2021.
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera visalization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
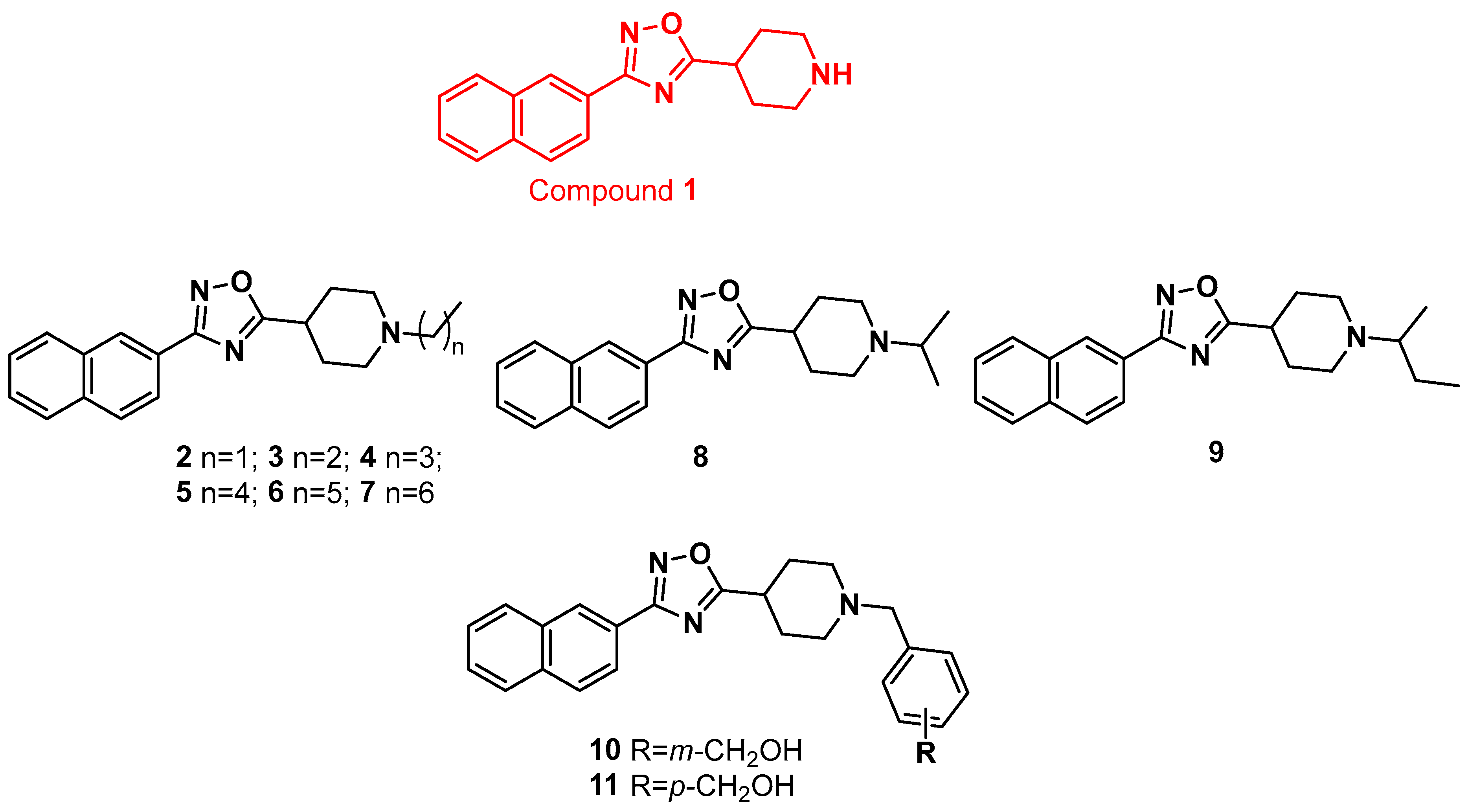
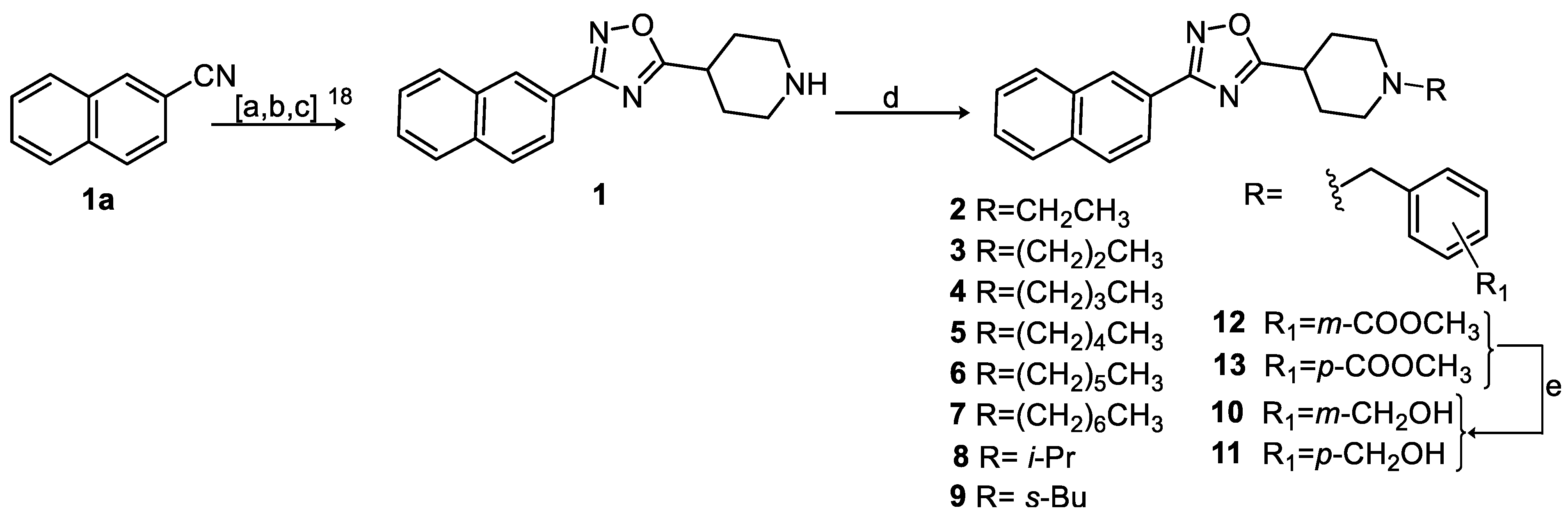

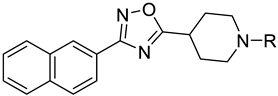 | |||
|---|---|---|---|
| Compound | R | FXR a Antagonism Eff (%) b | PXR a Agonism Eff (%) c |
| 2 | Et | Toxic | 2 ± 0.3 |
| 3 | n-Pr | Toxic | 15 ± 0.5 |
| 4 | n-Bu | 38 ± 5 | 68 ± 8 |
| 5 | n-Pe | 66 ± 1 | 71 ± 4 |
| 6 | n-Hx | NA | 23 ± 0.7 |
| 7 | n-Hp | Toxic | 28 ± 3 |
| 8 | i-Pr | NA | 50 ± 7 |
| 9 | Sec-Bu | Toxic | 3 ± 0.2 |
| 10 | 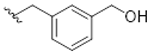 | 66 ± 13 | 20 ± 3 |
| 11 | 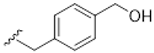 | 72 ± 6 | 105 ± 15 |
| Compound | IC50 a (FXR) | EC50 a (PXR) | t1/2 (min) | LogD | Clint b |
|---|---|---|---|---|---|
| 5 | 8.4 ± 2 | 6.4 ± 0.1 | 69 | 4.4 | 33.7 |
| 11 | 4.4 ± 0.4 | 7.5 ± 1 | 204 | 3.9 | 11.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finamore, C.; Festa, C.; Fiorillo, B.; Leva, F.S.D.; Roselli, R.; Marchianò, S.; Biagioli, M.; Spinelli, L.; Fiorucci, S.; Limongelli, V.; et al. Expanding the Library of 1,2,4-Oxadiazole Derivatives: Discovery of New Farnesoid X Receptor (FXR) Antagonists/Pregnane X Receptor (PXR) Agonists. Molecules 2023, 28, 2840. https://doi.org/10.3390/molecules28062840
Finamore C, Festa C, Fiorillo B, Leva FSD, Roselli R, Marchianò S, Biagioli M, Spinelli L, Fiorucci S, Limongelli V, et al. Expanding the Library of 1,2,4-Oxadiazole Derivatives: Discovery of New Farnesoid X Receptor (FXR) Antagonists/Pregnane X Receptor (PXR) Agonists. Molecules. 2023; 28(6):2840. https://doi.org/10.3390/molecules28062840
Chicago/Turabian StyleFinamore, Claudia, Carmen Festa, Bianca Fiorillo, Francesco Saverio Di Leva, Rosalinda Roselli, Silvia Marchianò, Michele Biagioli, Lucio Spinelli, Stefano Fiorucci, Vittorio Limongelli, and et al. 2023. "Expanding the Library of 1,2,4-Oxadiazole Derivatives: Discovery of New Farnesoid X Receptor (FXR) Antagonists/Pregnane X Receptor (PXR) Agonists" Molecules 28, no. 6: 2840. https://doi.org/10.3390/molecules28062840
APA StyleFinamore, C., Festa, C., Fiorillo, B., Leva, F. S. D., Roselli, R., Marchianò, S., Biagioli, M., Spinelli, L., Fiorucci, S., Limongelli, V., Zampella, A., & De Marino, S. (2023). Expanding the Library of 1,2,4-Oxadiazole Derivatives: Discovery of New Farnesoid X Receptor (FXR) Antagonists/Pregnane X Receptor (PXR) Agonists. Molecules, 28(6), 2840. https://doi.org/10.3390/molecules28062840










