Cytotoxicity against A549 Human Lung Cancer Cell Line via the Mitochondrial Membrane Potential and Nuclear Condensation Effects of Nepeta paulsenii Briq., a Perennial Herb
Abstract
1. Introduction
2. Results and Discussion
2.1. FT-IR Analysis of Extracts
2.2. GC-MS Analysis of Extracts
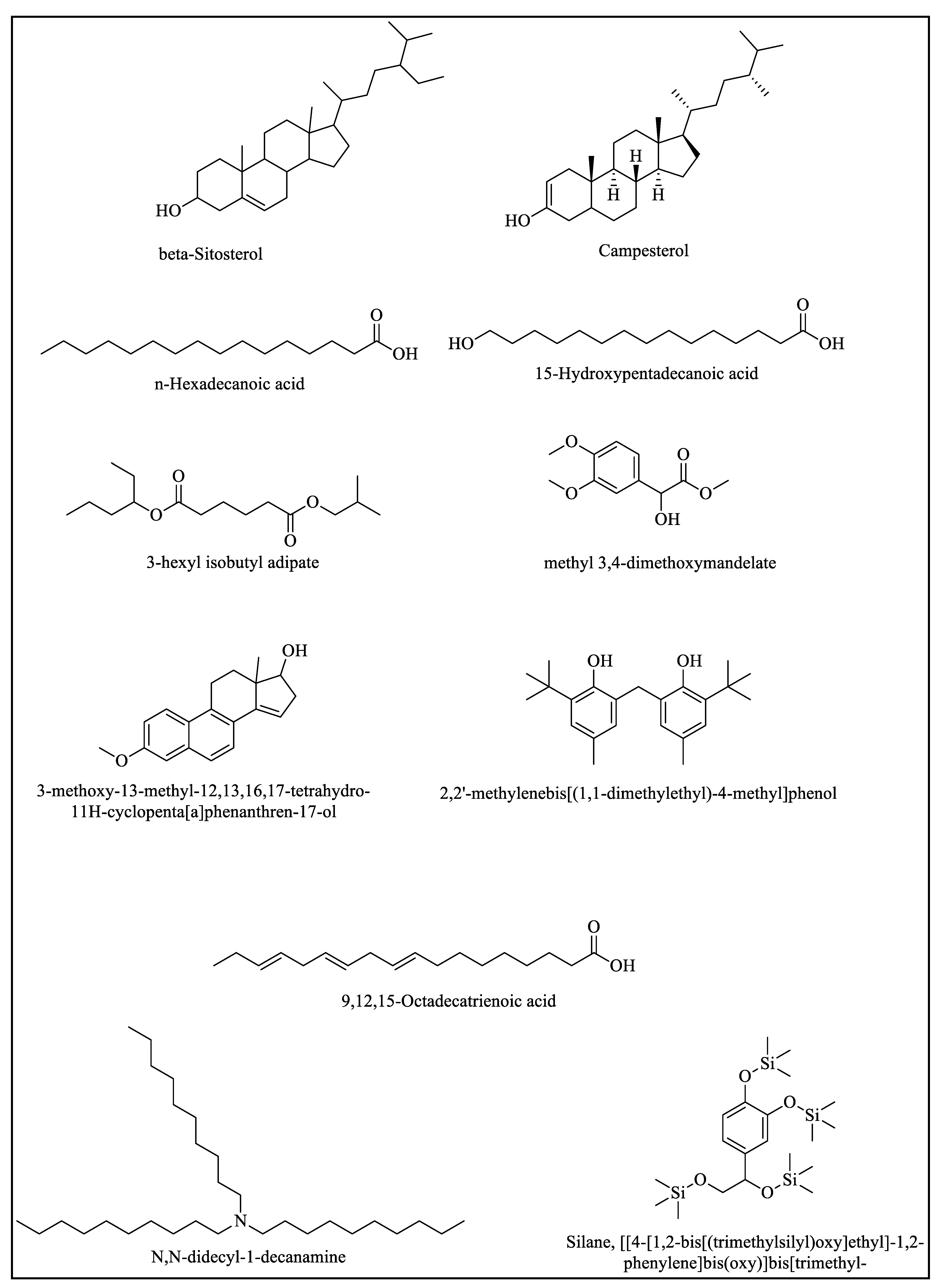
2.2.1. GC-MS Study of Ethyl Acetate and the Ethanolic and Aqueous Extract of the Root
2.2.2. GC-MS Study of the Ethyl Acetate, Aqueous, and Ethanolic Extracts of the Stem
2.2.3. GC-MS Study of the Ethyl Acetate, Ethanolic, and Aqueous Extracts of the Leaf
2.2.4. GC-MS Study of the Ethyl Acetate, Aqueous, and Ethanolic Extracts of the Flower
2.2.5. GC-MS Study of the Ethyl Acetate, Aqueous, and Ethanolic Extracts of the Mix of the Flowers, Stem, Leaves, and Root
3. Cytotoxicity Potential of Nepeta paulsenii Briq.
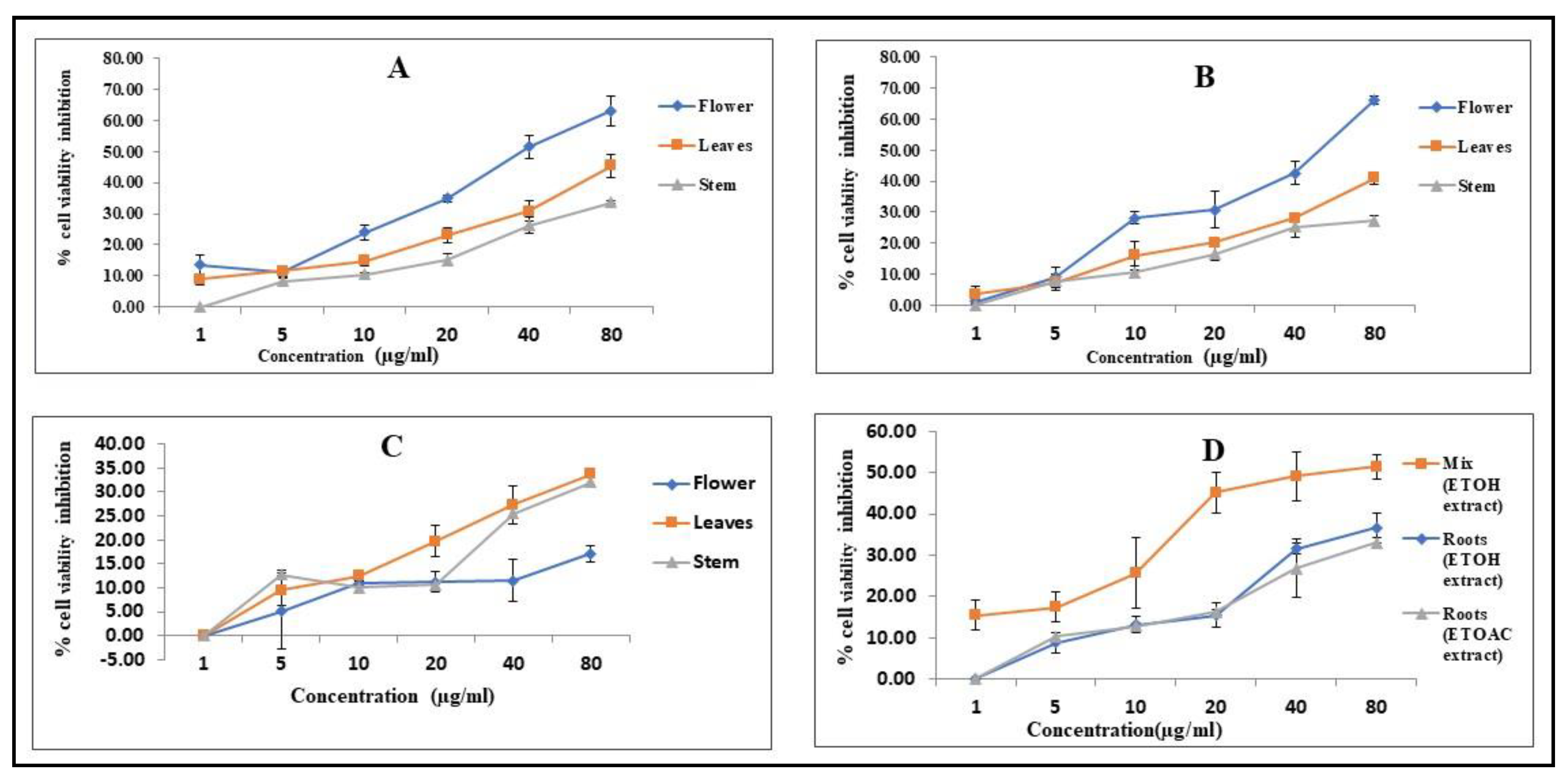
3.1. Cytotoxicity Effect of the Ethyl Acetate Extract of the Flowers, Leaf, and Stem
3.2. Cytotoxicity Effect of the Ethanolic Extract of the Flower, Leaf, and Stem
3.3. Cytotoxicity Effect of Aqueous Extract of Leaves, Flowers and Stem
3.4. Cytotoxicity Activity of the Extracts of the Root, Stem, Leaf and Flower Mix against A549 Lung Cancer Cell Line
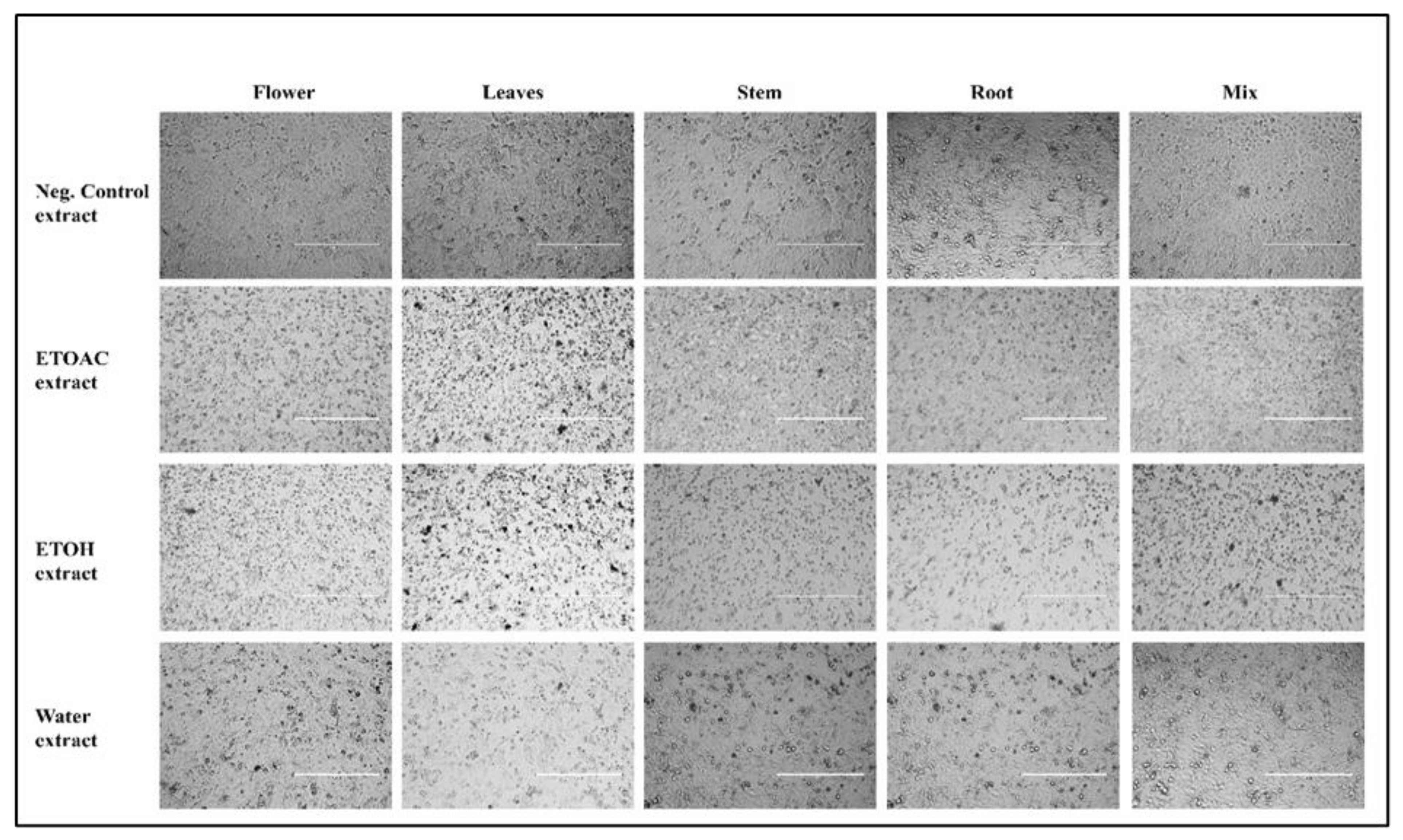
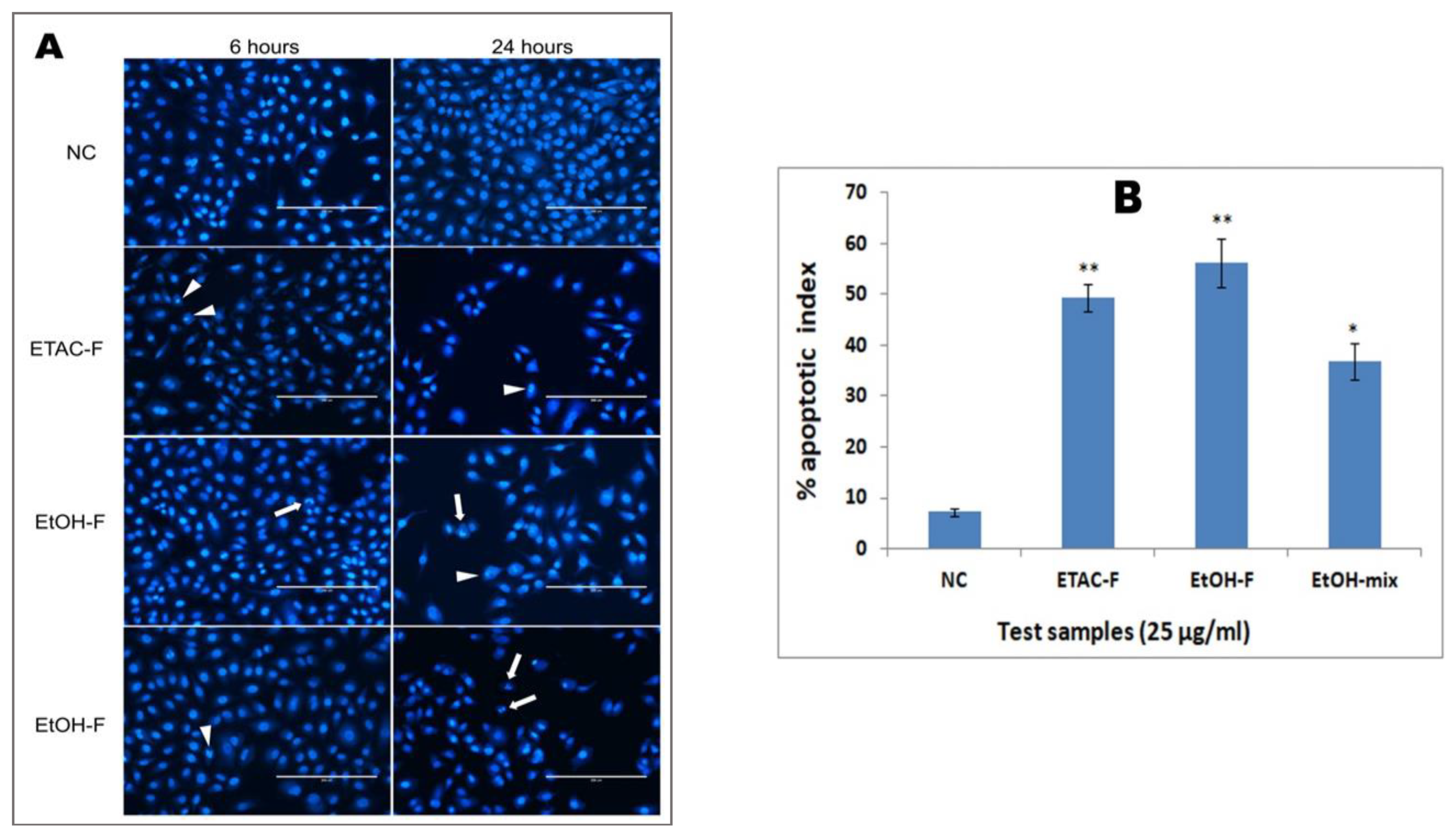
3.5. Effect of Extracts on Nuclear Morphology and Condensation in A549 Cancer Cells
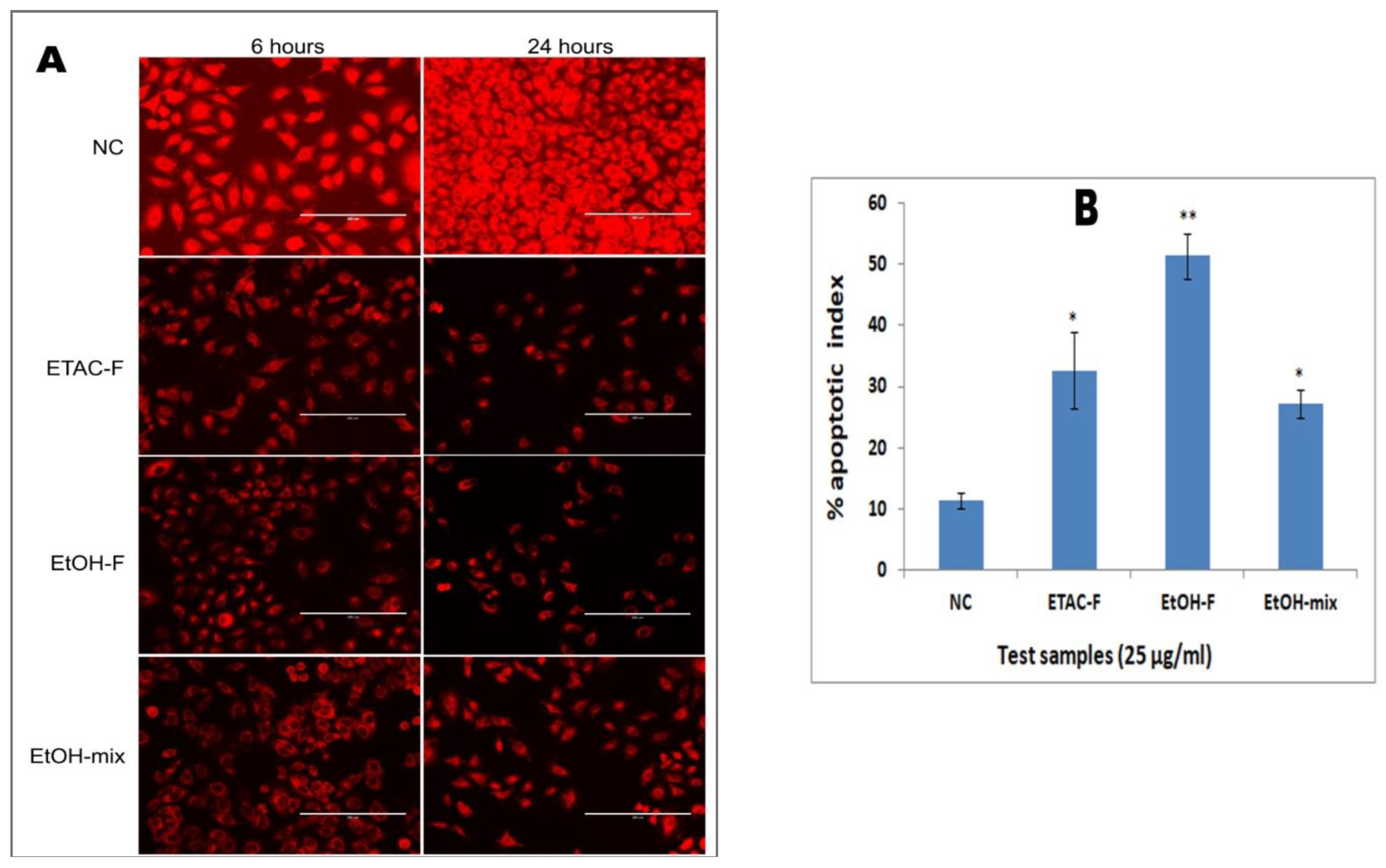
3.6. Effect of the Extracts on Mitochondrial Membrane Potential in Human Lung Cancer (A549) Cells
4. Experimental Section
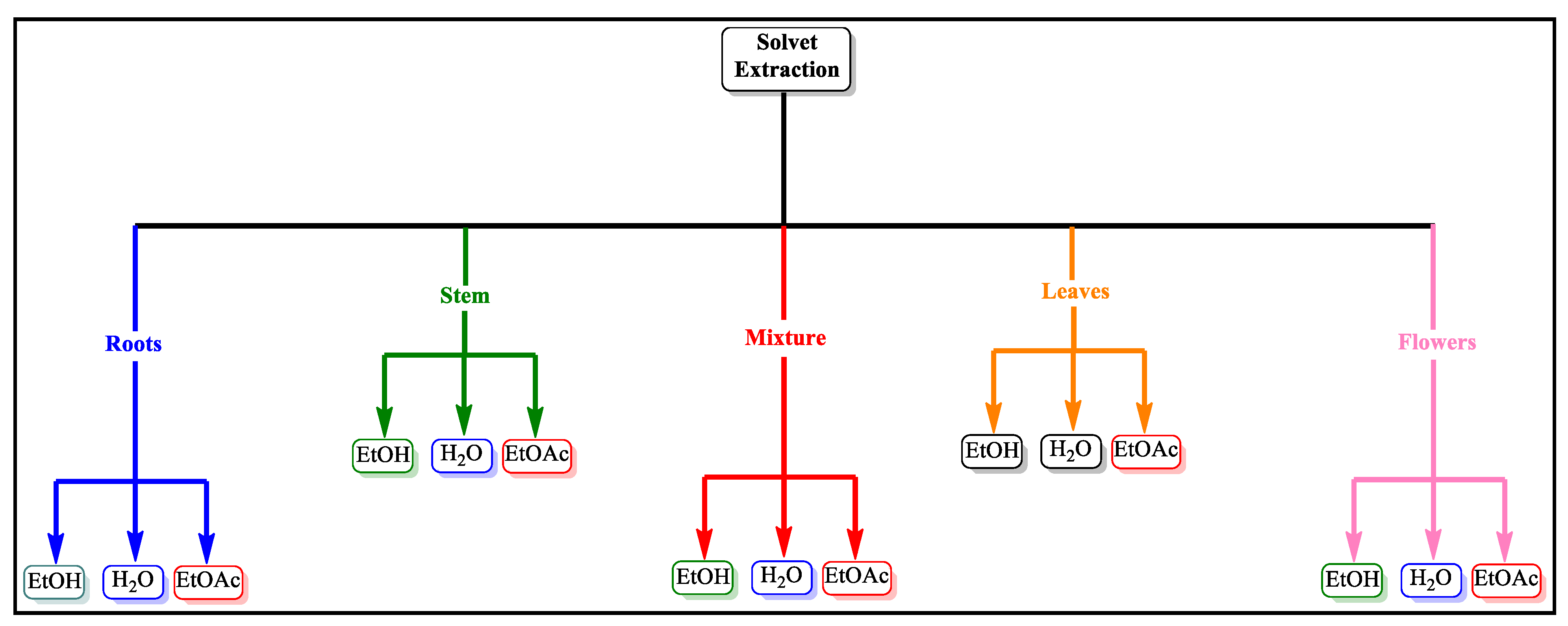
4.1. Material and Methods
4.1.1. Plant Material
4.1.2. Plants Extracts
4.1.3. Characterization of Plant Extracts
4.1.4. Cell Culture
4.1.5. MTT Assay (Colorimetric Assay)
Cell Viability Assay
Cytotoxicity Assay
4.1.6. Hoechst 33342 Stain Assay
4.1.7. Stain of Rhodamine 123 Assay
4.2. Statistical Analysis
5. Conclusions
6. Recommendations for Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Asgarpanah, J.; Sarabian, S.; Ziarati, P. Essential oil of Nepeta genus (Lamiaceae) from Iran: A review. J. Essent. Oil Res. 2014, 26, 1–12. [Google Scholar] [CrossRef]
- Sharma, A.; Cooper, R.; Bhardwaj, G.; Cannoo, D.S. The genus Nepeta: Traditional uses, phytochemicals and pharmacological properties. J. Ethnopharmacol. 2021, 268, 113679. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, S.; Heydari, M.; Ahmadi, K.; Khwarahm, N.R.; Karami, O.; Almasieh, K.; Naderi, B.; Bernard, P.; Mosavi, A. The current and future potential geographical distribution of Nepeta crispa Willd., an endemic, rare and threatened aromatic plant of Iran: Implications for ecological conservation and restoration. Ecol. Indic. 2022, 137, 108752. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Aktumsek, A.; Jekő, J.; Cziáky, Z.; Rodrigues, M.J.; Custodio, L.; Polat, R.; Cakilcioglu, U.; Ayna, A. Chemical profiling and biological evaluation of Nepeta baytopii extracts and essential oil: An endemic plant from Turkey. Plants 2021, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Irfan, S.; Abohashrh, M.; Wahab, S.; Abullais, S.S.; Javali, M.A.; Nisar, N.; Alam, M.M.; Srivastava, S.; Saleem, M. Inhibitory effect of Nepeta deflersiana on climax bacterial community isolated from the oral plaque of patients with periodontal disease. Molecules 2021, 26, 202. [Google Scholar] [CrossRef]
- Shan, M.; Jiang, Y.; Fu, Y.; Zhou, Y.; Lu, Z.; Yu, S.; Yan, H.; Liu, C.; Chen, P.; Bao, B. A review of the botany, traditional uses, phytochemistry and pharmacology of Nepeta tenuifolia Briq. Phytochem. Rev. 2021, 20, 991–1012. [Google Scholar] [CrossRef]
- Aničić, N.; Gašić, U.; Lu, F.; Ćirić, A.; Ivanov, M.; Jevtić, B.; Dimitrijević, M.; Anđelković, B.; Skorić, M.; Nestorović Živković, J. Antimicrobial and immunomodulating activities of two endemic Nepeta species and their major iridoids isolated from natural sources. Pharmaceuticals 2021, 14, 414. [Google Scholar] [CrossRef]
- Zhang, L.; Bian, Z.; Liu, Q.; Deng, B. Dealing with Stress in Cats: What Is New About the Olfactory Strategy? Front. Vet. Sci. 2022, 9, 928943. [Google Scholar] [CrossRef]
- Mutlu, S.; Atici, Ö. Allelopathic effect of Nepeta meyeri Benth. extracts on seed germination and seedling growth of some crop plants. Acta Physiol. Plant. 2009, 31, 89–93. [Google Scholar] [CrossRef]
- Sharma, A.; Bhardwaj, G.; Cannoo, D.S. Antioxidant potential, GC/MS and headspace GC/MS analysis of essential oils isolated from the roots, stems and aerial parts of Nepeta leucophylla. Biocatal. Agric. Biotechnol. 2021, 32, 101950. [Google Scholar] [CrossRef]
- Samad, A.; Ijaz, M.U.; Ashraf, A.; Sajid, M.; Imran, M.; Abbas, K.; Zafar, S.; Al-Ghanim, K.A.; Al-Misned, F.; Al-Mulahim, N.; et al. Methanolic extract of Nepeta paulsenii as an ameliorative agent against CCl4 induced testicular damage in male albino rats. J. King Saud Univ.-Sci. 2020, 32, 1168–1174. [Google Scholar] [CrossRef]
- Couraud, S.; Zalcman, G.; Milleron, B.; Morin, F.; Souquet, P.-J. Lung cancer in never smokers—A review. Eur. J. Cancer 2012, 48, 1299–1311. [Google Scholar] [CrossRef]
- Karnosky, J.; Dietmaier, W.; Knuettel, H.; Freigang, V.; Koch, M.; Koll, F.; Zeman, F.; Schulz, C. HPV and lung cancer: A systematic review and meta-analysis. Cancer Rep. 2021, 4, e1350. [Google Scholar] [CrossRef]
- Yorifuji, T.; Kashima, S.; Tsuda, T.; Ishikawa-Takata, K.; Ohta, T.; Tsuruta, K.-i.; Doi, H. Long-term exposure to traffic-related air pollution and the risk of death from hemorrhagic stroke and lung cancer in Shizuoka, Japan. Sci. Total Environ. 2013, 443, 397–402. [Google Scholar] [CrossRef]
- Archontogeorgis, K.; Steiropoulos, P.; Tzouvelekis, A.; Nena, E.; Bouros, D. Lung Cancer and Interstitial Lung Diseases: A Systematic Review. Pulm. Med. 2012, 2012, 315918. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Petridis, D.; Ritzoulis, C.; Darwiche, K.; Spyratos, D.; Huang, H.; Goldberg, E.P.; Yarmus, L.; Li, Q.; Freitag, L. Establishing the optimal nebulization system for paclitaxel, docetaxel, cisplatin, carboplatin and gemcitabine: Back to drawing the residual cup. Int. J. Pharm. 2013, 453, 480–487. [Google Scholar] [CrossRef]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Rajeswaran, A.; Trojan, A.; Burnand, B.; Giannelli, M. Efficacy and side effects of cisplatin-and carboplatin-based doublet chemotherapeutic regimens versus non-platinum-based doublet chemotherapeutic regimens as first line treatment of metastatic non-small cell lung carcinoma: A systematic review of randomized controlled trials. Lung Cancer 2008, 59, 1–11. [Google Scholar]
- Baker, J.; Ajani, J.; Scotté, F.; Winther, D.; Martin, M.; Aapro, M.S.; von Minckwitz, G. Docetaxel-related side effects and their management. Eur. J. Oncol. Nurs. 2009, 13, 49–59. [Google Scholar] [CrossRef]
- Song, W.; Tang, Z.; Li, M.; Lv, S.; Sun, H.; Deng, M.; Liu, H.; Chen, X. Polypeptide-based combination of paclitaxel and cisplatin for enhanced chemotherapy efficacy and reduced side-effects. Acta Biomater. 2014, 10, 1392–1402. [Google Scholar] [CrossRef]
- Moysan, E.; Bastiat, G.; Benoit, J.-P. Gemcitabine versus Modified Gemcitabine: A Review of Several Promising Chemical Modifications. Mol. Pharm. 2013, 10, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.A.; Saad, A.A.E.-M. Evaluation of the cytotoxic anticancer effect of polysaccharide of Nepeta septemcrenata. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 53. [Google Scholar] [CrossRef]
- Akhter, S.; Hossain, M.W.; Sultana, S.; Ferdous Jharna, J.; Sultana Meghla, N.; Alam, R.; Anis-Ul-Haque, K.M.; Mashiar Rahman, M. Ruellia prostrata Poir. activity evaluated by phytoconstituents, antioxidant, anti-inflammatory, antibacterial activity, and in silico molecular functions. J. Saudi Chem. Soc. 2022, 26, 101401. [Google Scholar] [CrossRef]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Anticancer antioxidant regulatory functions of phytochemicals. Curr. Med. Chem. 2011, 18, 2315–2338. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.F.; Carneiro, C.N.; de Sousa, C.B.d.C.; Gomez, F.J.; Espino, M.; Boiteux, J.; Fernández, M.d.l.Á.; Silva, M.F.; Dias, F.d.S. Sustainable extraction bioactive compounds procedures in medicinal Plants based on the principles of Green Analytical Chemistry: A review. Microchem. J. 2022, 175, 107184. [Google Scholar] [CrossRef]
- Kaewkod, T.; Songkhakul, W.; Tragoolpua, Y. Inhibitory Effects of Tea Leaf and Medicinal Plant Extracts on Enteric Pathogenic Bacteria Growth, Oxidation and Epithelial Cell Adhesion. Pharmacogn. Res. 2022, 14, 71–81. [Google Scholar] [CrossRef]
- Siddique, M.H.; Ashraf, A.; Hayat, S.; Aslam, B.; Fakhar-e-Alam, M.; Muzammil, S.; Atif, M.; Shahid, M.; Shafeeq, S.; Afzal, M.; et al. Antidiabetic and antioxidant potentials of Abelmoschus esculentus: In vitro combined with molecular docking approach. J. Saudi Chem. Soc. 2022, 26, 101418. [Google Scholar] [CrossRef]
- Noori, S.; Kiasat, A.R.; Kolahi, M.; Mirzajani, R.; Seyyed Nejad, S.M. Determination of secondary metabolites including curcumin in Rheum ribes L. and surveying of its antioxidant and anticancer activity. J. Saudi Chem. Soc. 2022, 26, 101479. [Google Scholar] [CrossRef]
- Ouasri, A.; Rhandour, A.; Saadi, M.; El Ammari, L. X-Ray, DSC, TGA-dTGA, and Vibrational Studies of the Propylenediammonium Hexafluorosilicate NH3 (CH2) 3NH3SiF6. Biointerface Res. Appl. Chem. 2021, 11, 12618–12632. [Google Scholar]
- Pakkirisamy, M.; Kalakandan, S.K.; Ravichandran, K. Phytochemical screening, GC-MS, FT-IR analysis of methanolic extract of Curcuma caesia Roxb (Black Turmeric). Pharmacogn. J. 2017, 9, 952–956. [Google Scholar] [CrossRef]
- Hugar, A.L.; Londonkar, R.L. GC-MS Profiling of bioactive components from aqueous extract of pterocarpus Marsupium. Int. J. ChemTech Res. 2017, 10, 557–564. [Google Scholar]
- Awad, A.B.; Chan, K.C.; Downie, A.C.; Fink, C.S. Peanuts as a source of β-sitosterol, a sterol with anticancer properties. Nutr. Cancer 2000, 36, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Novotny, L.; Abdel-Hamid, M.; Hunakova, L. Anticancer potential of β-sitosterol. Int. J. Clin. Pharmacol. Pharmacother. 2017, 2, 10.15344. [Google Scholar] [CrossRef]
- Woyengo, T.; Ramprasath, V.; Jones, P. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009, 63, 813–820. [Google Scholar] [CrossRef]
- Shahzad, N.; Khan, W.; Shadab, M.; Ali, A.; Saluja, S.S.; Sharma, S.; Al-Allaf, F.A.; Abduljaleel, Z.; Ibrahim, I.A.A.; Abdel-Wahab, A.F. Phytosterols as a natural anticancer agent: Current status and future perspective. Biomed. Pharmacother. 2017, 88, 786–794. [Google Scholar] [CrossRef]
- Bae, H.; Park, S.; Yang, C.; Song, G.; Lim, W. Disruption of endoplasmic reticulum and ROS production in human ovarian cancer by campesterol. Antioxidants 2021, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Lee, E.O.; Lee, H.J.; Kim, K.H.; Ahn, K.S.; Shim, B.S.; Kim, N.I.; Song, M.C.; Baek, N.I.; Kim, S.H. Identification of campesterol from Chrysanthemum coronarium L. and its antiangiogenic activities. Phytother. Res. 2007, 21, 954–959. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, L.; Peng, Y.; Zhu, X.; Liu, F.; Yang, X.; Li, H. Physicochemical, Antioxidant and Anticancer Characteristics of Seed Oil from Three Chenopodium quinoa Genotypes. Molecules 2022, 27, 2453. [Google Scholar] [CrossRef]
- Oanh, D.T.K.; Van Hai, H.; Park, S.H.; Kim, H.-J.; Han, B.-W.; Kim, H.-S.; Hong, J.-T.; Han, S.-B.; Nam, N.-H. Benzothiazole-containing hydroxamic acids as histone deacetylase inhibitors and antitumor agents. Bioorg. Med. Chem. Lett. 2011, 21, 7509–7512. [Google Scholar] [CrossRef]
- Pantelić, N.; Zmejkovski, B.B.; Marković, D.D.; Vujić, J.M.; Stanojković, T.P.; Sabo, T.J.; Kaluđerović, G.N. Synthesis, characterization, and cytotoxicity of a Novel Gold (III) complex with O, O′-diethyl ester of ethylenediamine-N, N′-Di-2-(4-Methyl) Pentanoic acid. Metals 2016, 6, 226. [Google Scholar] [CrossRef]
- Rehana, D.; Haleel, A.K.; Rahiman, A.K. Hydroxy, carboxylic and amino acid functionalized superparamagnetic iron oxide nanoparticles: Synthesis, characterization and in vitro anti-cancer studies. J. Chem. Sci. 2015, 127, 1155–1166. [Google Scholar] [CrossRef]
- Mustafa, J.; Khan, S.I.; Ma, G.; Walker, L.A.; Khan, I.A. Synthesis and anticancer activities of fatty acid analogs of podophyllotoxin. Lipids 2004, 39, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Sivakumaran, G.; Prabhu, K.; Rao, M.; Jones, S.; Sundaram, R.L.; Ulhas, V.R.; Vijayalakshmi, N. Gas chromatography-mass spectrometry analysis of one Ayurvedic oil, Ksheerabala Thailam. Drug Invent Today 2019, 11, 2661–2665. [Google Scholar]
- Elsayed, E.A.; Sharaf-Eldin, M.A.; Wadaan, M. In vitro evaluation of cytotoxic activities of essential oil from Moringa oleifera seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 cell lines. Asian Pac. J. Cancer Prev. 2015, 16, 4671–4675. [Google Scholar] [CrossRef] [PubMed]
- Nath, M.; Roy, P.; Mishra, R.; Thakur, M. Structure-cytotoxicity relationship for apoptotic inducers organotin (IV) derivatives of mandelic acid and L-proline and their mixed ligand complexes having enhanced cytotoxicity. Appl. Organomet. Chem. 2019, 33, e4663. [Google Scholar] [CrossRef]
- Kaur, G.; Shamim, M.; Bhardwaj, V.; Gupta, V.K.; Banerjee, B. Mandelic acid catalyzed one-pot three-component synthesis of α-aminonitriles and α-aminophosphonates under solvent-free conditions at room temperature. Synth. Commun. 2020, 50, 1545–1560. [Google Scholar] [CrossRef]
- Zou, X.G.; Xu, M.T.; Dong, X.L.; Ying, Y.M.; Guan, R.F.; Wu, W.C.; Yang, K.; Sun, P.L. Solid-state-cultured mycelium of Antrodia camphorata exerts potential neuroprotective activities against 6-hydroxydopamine-induced toxicity in PC12 cells. J. Food Biochem. 2022, 46, e14208. [Google Scholar] [CrossRef]
- Chaubey, A.; Dubey, A.K. Chemistry and Antioxidant Potential of Phytoconstituents from Aegle Marmelos Fruit-Shell. Curr. Drug Metab. 2020, 21, 525–533. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, Y.; Zhang, T.; He, J.; Luo, X.; Bian, X.; Wu, J.; Zou, C.; Wang, Y.; Fu, L. Identifying potential serum biomarkers of breast cancer through targeted free fatty acid profiles screening based on a GC–MS platform. Biomed. Chromatogr. 2020, 34, e4922. [Google Scholar] [CrossRef]
- Banday, A.H.; Akram, S.; Shameem, S.A. Benzylidine pregnenolones and their oximes as potential anticancer agents: Synthesis and biological evaluation. Steroids 2014, 84, 64–69. [Google Scholar] [CrossRef]
- Marrez, D.A.; Naguib, M.M.; Sultan, Y.Y.; Higazy, A.M. Antimicrobial and anticancer activities of Scenedesmus obliquus metabolites. Heliyon 2019, 5, e01404. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-M.; Yue, G.G.-L.; Li, P.; Wong, E.C.-W.; Lee, J.K.-M.; Kennelly, E.J.; Bik-San Lau, C. Screening and analysis of potential anti-tumor components from the stipe of Ganoderma sinense using high-performance liquid chromatography/time-of-flight mass spectrometry with multivariate statistical tool. J. Chromatogr. A 2017, 1487, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Rusli, I.S.; Salim, N.; Faujan, N.H.; Kassim, N.K.; Abd Rahman, M.B. Phytochemical investigation and cytotoxicity study of Indigofera zollingeriana crude extract. Mater. Today Proc. 2022, 60, 1074–1081. [Google Scholar] [CrossRef]
- Saleem, A.; Saleem, M.; Akhtar, M.F.; Rasul, A.; Baig, M.M.F.A. Chemical characterisation, in vitro antioxidant, cytotoxicity and safety evaluation of Polystichum braunii (Spenn.) fee roots. Nat. Prod. Res. 2021, 35, 6223–6228. [Google Scholar] [CrossRef]
- Uthirasamy, S.; Chitra, T.; Murugan, A.; Manjula, G.; Arulmanickam, P.; Kavitha, T.; Thinakaran, M. Identification of Bioactive Constituents in Calotropis gigantea Leaves by GC-MS, HPLC and FTIR Techniques. Asian J. Adv. Res. 2021, 7, 1–8. [Google Scholar]
- Uthirasamy, S.; Chitra, T.; Murugan, A.; Manjula, G.; Arulmanickam, P.; Kavitha, T.; Thinakaran, M. Determining the Bioactive Constituents in Calotropis gigantea Leaves by GC-MS, HPLC and FTIR Techniques. New Vis. Biol. Sci. 2021, 1, 1–11. [Google Scholar]
- Roy, R.N. Bioactive natural derivatives of phthalate ester. Crit. Rev. Biotechnol. 2020, 40, 913–929. [Google Scholar] [CrossRef]
- Arshad, N.; Ishtiaq, S.; Khan, F.Z.; Danish, Z.; Rashid, A.J.; Ijaz, B.; Tariq, S. GC-MS analysis, anticancer and anti-inflammatory activities of Saussurea hypoleuca spreng. Root. Pak. J. Pharm. Sci. 2021, 34, 291–300. [Google Scholar]
- Sathya, R.; Devi, S.N.; Ramamurth, V. Pphtochemical Screening and GC-MS Profiling of Ethanolic Fuits Extracts of Solanum torvum. Infokara Res. 2019, 8, 287–294. [Google Scholar]
- Chou, C.-K.; Yang, Y.-T.; Yang, H.-C.; Liang, S.-S.; Wang, T.-N.; Kuo, P.-L.; Wang, H.-M.D.; Tsai, E.-M.; Chiu, C.-C. The impact of di (2-ethylhexyl) phthalate on cancer progression. Arch. Immunol. Et Ther. Exp. 2018, 66, 183–197. [Google Scholar] [CrossRef]
- Abou Baker, D.H. Achillea millefolium L. ethyl acetate fraction induces apoptosis and cell cycle arrest in human cervical cancer (HeLa) cells. Ann. Agric. Sci. 2020, 65, 42–48. [Google Scholar] [CrossRef]
- Olugbuyiro, J.; Bamidele, J.; Fatokun, A. Phytochemical Screening of Citrullus colocynthis (L.) Schrad and its Cytotoxic Activity Against Cervical Cancer Cells. In Bioenergy and Biochemical Processing Technologies: Recent Advances and Future Demands; Springer International Publishing: Cham, Switzerland, 2022; pp. 331–338. [Google Scholar]
- Lee, J.J.; Saiful Yazan, L.; Kassim, N.K.; Che Abdullah, C.A.; Esa, N.; Lim, P.C.; Tan, D.C. Cytotoxic activity of Christia vespertilionis root and leaf extracts and fractions against breast cancer cell lines. Molecules 2020, 25, 2610. [Google Scholar] [CrossRef]
- Tilaoui, M.; Jaafari, A.; Ait Mouse, H.; Zyad, A. Studies on the dual cytotoxicity and antioxidant properties of Berberis vulgaris extracts and its main constituent berberine. Adv. Pharmacol. Sci. 2018, 2018, 3018498. [Google Scholar]
- Burci, L.M.; da Silva, C.B.; Rondon, J.N.; da Silva, L.M.; de Andrade, S.F.; Miguel, O.G.; de Fátima Gaspari Dias, J.; Miguel, M.D. Acute and subacute (28 days) toxicity, hemolytic and cytotoxic effect of Artocarpus heterophyllus seed extracts. Toxicol. Rep. 2019, 6, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Bin Sayeed, M.S.; Ameen, S.S. Beta-sitosterol: A promising but orphan nutraceutical to fight against cancer. Nutr. Cancer 2015, 67, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Chen, Y.-C.; Fink, C.; Hennessey, T. beta-Sitosterol inhibits HT-29 human colon cancer cell growth and alters membrane lipids. Anticancer Res. 1996, 16, 2797–2804. [Google Scholar]
- Mendilaharsu, M.; De Stefani, E.; Deneo-Pellegrini, H.; Carzoglio, J.; Ronco, A. Phytosterols and risk of lung cancer: A case-control study in Uruguay. Lung Cancer 1998, 21, 37–45. [Google Scholar] [CrossRef]
- Rajavel, T.; Mohankumar, R.; Archunan, G.; Ruckmani, K.; Devi, K.P. Beta sitosterol and Daucosterol (phytosterols identified in Grewia tiliaefolia) perturbs cell cycle and induces apoptotic cell death in A549 cells. Sci. Rep. 2017, 7, 3418. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The story of beta-sitosterol-a review. Eur. J. Med. Plants 2014, 4, 590–609. [Google Scholar] [CrossRef]
- Karthik, L.; Vijayakumar, B. Screening of Anti-Cancer Properties of Beta-Sitosterol and its Derivatives against Microtubules: Molecular Modeling Approach. Int. J. Pharm. Phytopharm. Res. (EIJPPR) 2020, 10, 8–21. [Google Scholar]
- Rivero-Cruz, J.F.; Granados-Pineda, J.; Pedraza-Chaverri, J.; Pérez-Rojas, J.M.; Kumar-Passari, A.; Diaz-Ruiz, G.; Rivero-Cruz, B.E. Phytochemical constituents, antioxidant, cytotoxic, and antimicrobial activities of the ethanolic extract of Mexican brown propolis. Antioxidants 2020, 9, 70. [Google Scholar] [CrossRef]
- Rezadoost, M.H.; Kumleh, H.H.; Ghasempour, A. Cytotoxicity and apoptosis induction in breast cancer, skin cancer and glioblastoma cells by plant extracts. Mol. Biol. Rep. 2019, 46, 5131–5142. [Google Scholar] [CrossRef]
- Moacă, E.-A.; Farcaş, C.; Ghiţu, A.; Coricovac, D.; Popovici, R.; Cărăba-Meiţă, N.-L.; Ardelean, F.; Antal, D.S.; Dehelean, C.; Avram, Ş. A comparative study of Melissa officinalis leaves and stems ethanolic extracts in terms of antioxidant, cytotoxic, and antiproliferative potential. Evid.-Based Complement. Altern. Med. 2018, 2018, 7860456. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Epifano, F.; Fiorito, S.; Álvarez-Suarez, J.M. Phytochemical analysis and biological investigation of Nepeta juncea Benth. different extracts. Plants 2020, 9, 646. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheddi, E.S.; Farshori, N.N.; Al-Oqail, M.M.; Al-Massarani, S.M.; Saquib, Q.; Wahab, R.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Anticancer potential of green synthesized silver nanoparticles using extract of Nepeta deflersiana against human cervical cancer cells (HeLA). Bioinorg. Chem. Appl. 2018, 2018, 9390784. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, M.; Hou, Y.; Fan, C.; Wei, H.; Shi, L.; Ma, G.; Zhang, J. Inflammatory and Cytotoxic Activities of Abietane Terpenoids from Nepeta bracteata Benth. Molecules 2021, 26, 5603. [Google Scholar] [CrossRef]
- Kaska, A.; Deniz, N.; Çiçek, M.; Mammadov, R. Evaluation of antioxidant properties, phenolic compounds, anthelmintic, and cytotoxic activities of various extracts isolated from Nepeta cadmea: An endemic plant for Turkey. J. Food Sci. 2018, 83, 1552–1559. [Google Scholar] [CrossRef]
- Xia, M.; Yang, M.; Wang, Y.; Tian, F.; Hu, J.; Yang, W.; Tao, S.; Lu, L.; Ding, X.; Jiang, S. dl-Mandelic acid exhibits high sperm-immobilizing activity and low vaginal irritation: A potential non-surfactant spermicide for contraception. Biomed. Pharmacother. 2020, 126, 110104. [Google Scholar] [CrossRef] [PubMed]
- Darling, D.A.; Joema, S. Antibacterial activity, optical, mechanical, thermal, and dielectric properties of L-phenylalanine fumaric acid single crystals for biomedical, optoelectronic, and photonic applications. J. Mater. Sci. Mater. Electron. 2020, 31, 22427–22441. [Google Scholar] [CrossRef]
- Zahoor, M.; Shafiq, S.; Ullah, H.; Sadiq, A.; Ullah, F. Isolation of quercetin and mandelic acid from Aesculus indica fruit and their biological activities. BMC Biochem. 2018, 19, 5. [Google Scholar] [CrossRef]
- Koleva, V.; Dragoeva, A.; Stoyanova, Z.; Yordanova, Z.; Ali, S.; Uzunov, N.M.; Melendez-Alafort, L.; Rosato, A.; Enchev, D.D. In vitro cytotoxicity of allelopathic plants L. ssp. L. and subsp. Acta Sci. Nat. 2018, 5, 64–69. [Google Scholar]
- Fattahi, N.; Shahbazi, M.-A.; Maleki, A.; Hamidi, M.; Ramazani, A.; Santos, H.A. Emerging insights on drug delivery by fatty acid mediated synthesis of lipophilic prodrugs as novel nanomedicines. J. Control. Release 2020, 326, 556–598. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wang, C.; Li, B. Metabolomic analysis using liquid chromatography/mass spectrometry for gastric cancer. Appl. Biochem. Biotechnol. 2015, 176, 2170–2184. [Google Scholar] [CrossRef] [PubMed]
- Marques, G.; Rencoret, J.; Gutiérrez, A.; José, C. Lipophilic compounds from maize fiber and rice husk residues—An abundant and inexpensive source of valuable phytochemicals. Ind. Crops Prod. 2020, 146, 112203. [Google Scholar] [CrossRef]
- Ukwubile, C.A.; Ahmed, A.; Katsayal, U.A.; Ya’u, J.; Mejida, S. GC–MS analysis of bioactive compounds from Melastomastrum capitatum (Vahl) Fern. leaf methanol extract: An anticancer plant. Sci. Afr. 2019, 3, e00059. [Google Scholar] [CrossRef]
- Bold, R.J.; Termuhlen, P.M.; McConkey, D.J. Apoptosis, cancer and cancer therapy. Surg. Oncol. 1997, 6, 133–142. [Google Scholar] [CrossRef]
- Al Fayi, M.S. Anti-cancer effects of Nepeta Deflersiana Extract (NDE) in estrogen positive and negative forms of breast cancer. Pharmacogn. Mag. 2021, 17, 287. [Google Scholar] [CrossRef]
- Gu, W.; Li, H.; Niu, X.; Zhou, J. Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer. Green Process. Synth. 2022, 11, 316–326. [Google Scholar] [CrossRef]
- Castedo, M.; Ferri, K.; Roumier, T.; Métivier, D.; Zamzami, N.; Kroemer, G. Quantitation of mitochondrial alterations associated with apoptosis. J. Immunol. Methods 2002, 265, 39–47. [Google Scholar] [CrossRef]
- Skommer, J.; Wlodkowic, D.; Deptala, A. Larger than life: Mitochondria and the Bcl-2 family. Leuk. Res. 2007, 31, 277–286. [Google Scholar] [CrossRef]
- Hema, R.; Kumaravel, S.; Alagusundaram, K. GC/MS determination of bioactive components of Murraya koenigii. J. Am. Sci. 2011, 7, 80–83. [Google Scholar]
- Yin, X.; Zhou, J.; Jie, C.; Xing, D.; Zhang, Y. Anticancer activity and mechanism of Scutellaria barbata extract on human lung cancer cell line A549. Life Sci. 2004, 75, 2233–2244. [Google Scholar] [CrossRef]
- Nam, G.-H.; Jo, K.-J.; Park, Y.-S.; Kawk, H.W.; Kim, S.-Y.; Kim, Y.-M. In vitro and in vivo Induction of p53-Dependent Apoptosis by Extract of Euryale ferox Salisb in A549 Human Caucasian Lung Carcinoma Cancer Cells Is Mediated Through Akt Signaling Pathway. Front. Oncol. 2019, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Nassar, Z.D.; Aisha, A.F.; Idris, N.; Khadeer Ahamed, M.B.; Ismail, Z.; Abu-Salah, K.M.; Alrokayan, S.A.; Abdul Majid, A.M.S. Koetjapic acid, a natural triterpenoid, induces apoptosis in colon cancer cells. Oncol. Rep. 2012, 27, 727–733. [Google Scholar]
- Abe, H.; Matsubara, I.; Doi, Y. Physical properties and enzymic degradability of polymer blends of bacterial poly [(R)-3-hydroxybutyrate] and poly [(R, S)-3-hydroxybutyrate] stereoisomers. Macromolecules 1995, 28, 844–853. [Google Scholar] [CrossRef]
- Cigremis, Y.; Ulukanli, Z.; Ilcim, A.; Akgoz, M. In vitro antioxidant and antimicrobial assays of acetone extracts from Nepeta meyeri Bentham. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 661–668. [Google Scholar] [PubMed]
| IC50 Values (µg/mL) | |||||
|---|---|---|---|---|---|
| Flower | Leaves | Stem | Root | Mix | |
| ETOAc extract | 51.57 | 86.25 | 113.80 | >1000 | ND |
| ETOH extract | 50.58 | 95.01 | >1000 | 559.88 | 62.82 |
| H2O extract | >1000 | 847.8 | 123.8 | ND | ND |
| 5-FU | 83.62 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanif, A.; Ibrahim, A.H.; Ismail, S.; Al-Rawi, S.S.; Ahmad, J.N.; Hameed, M.; Mustufa, G.; Tanwir, S. Cytotoxicity against A549 Human Lung Cancer Cell Line via the Mitochondrial Membrane Potential and Nuclear Condensation Effects of Nepeta paulsenii Briq., a Perennial Herb. Molecules 2023, 28, 2812. https://doi.org/10.3390/molecules28062812
Hanif A, Ibrahim AH, Ismail S, Al-Rawi SS, Ahmad JN, Hameed M, Mustufa G, Tanwir S. Cytotoxicity against A549 Human Lung Cancer Cell Line via the Mitochondrial Membrane Potential and Nuclear Condensation Effects of Nepeta paulsenii Briq., a Perennial Herb. Molecules. 2023; 28(6):2812. https://doi.org/10.3390/molecules28062812
Chicago/Turabian StyleHanif, Aqsa, Ahmad H. Ibrahim, Sidra Ismail, Sawsan S. Al-Rawi, Jam Nazeer Ahmad, Mansoor Hameed, Ghulam Mustufa, and Samina Tanwir. 2023. "Cytotoxicity against A549 Human Lung Cancer Cell Line via the Mitochondrial Membrane Potential and Nuclear Condensation Effects of Nepeta paulsenii Briq., a Perennial Herb" Molecules 28, no. 6: 2812. https://doi.org/10.3390/molecules28062812
APA StyleHanif, A., Ibrahim, A. H., Ismail, S., Al-Rawi, S. S., Ahmad, J. N., Hameed, M., Mustufa, G., & Tanwir, S. (2023). Cytotoxicity against A549 Human Lung Cancer Cell Line via the Mitochondrial Membrane Potential and Nuclear Condensation Effects of Nepeta paulsenii Briq., a Perennial Herb. Molecules, 28(6), 2812. https://doi.org/10.3390/molecules28062812







