Comparing Condensed and Hydrolysable Tannins for Mechanical Foaming of Furanic Foams: Synthesis and Characterization
Abstract
1. Introduction
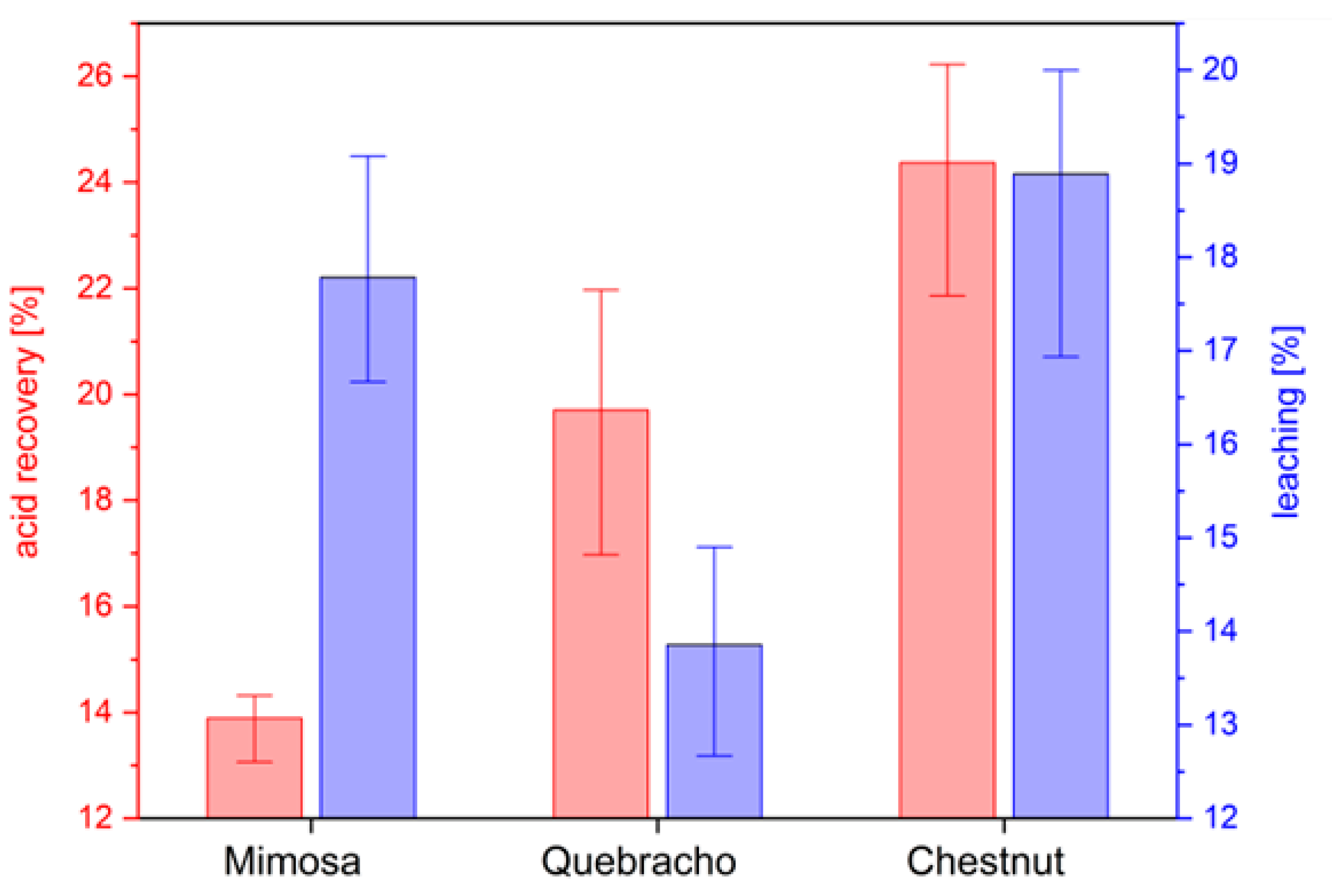
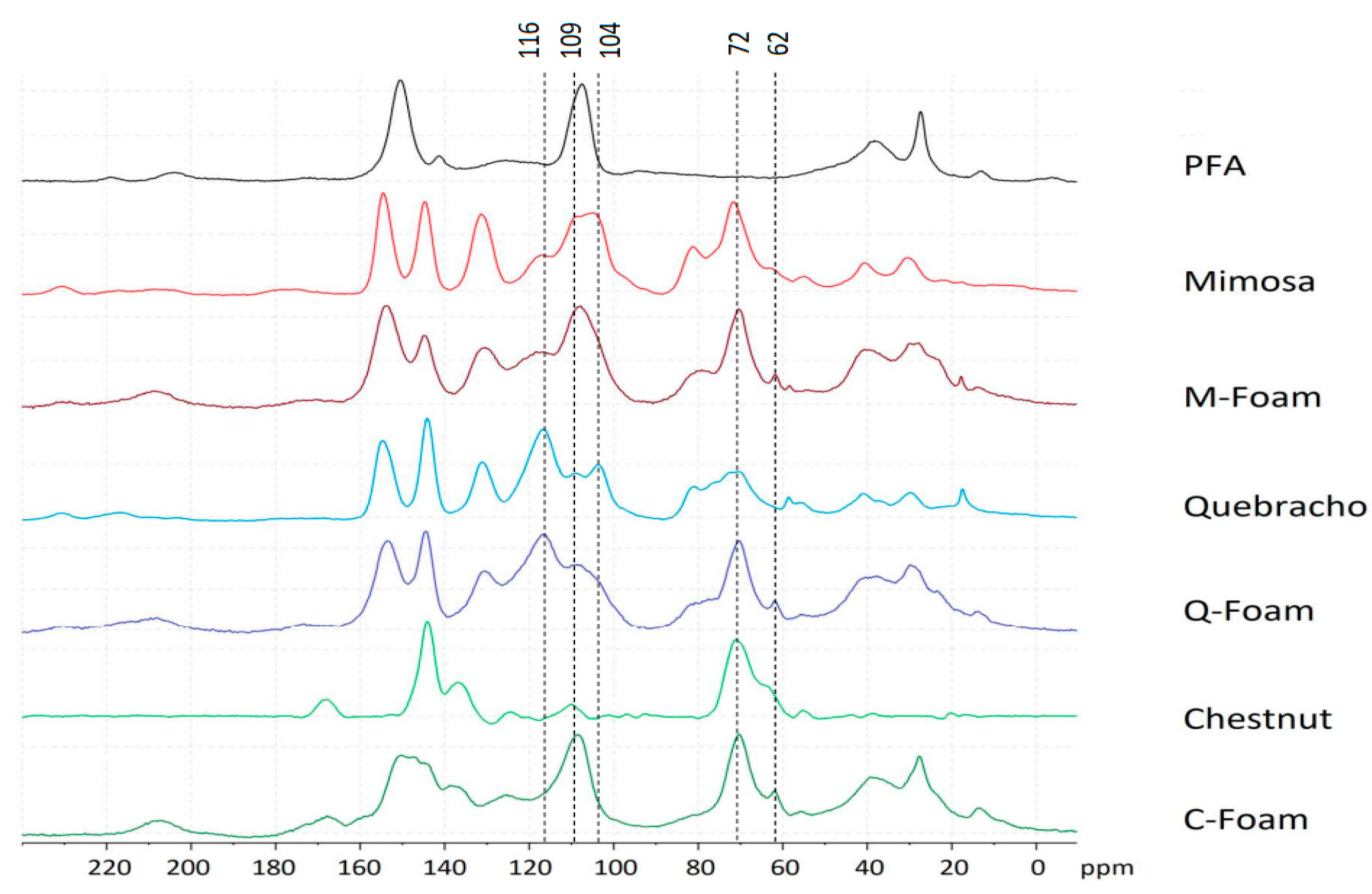
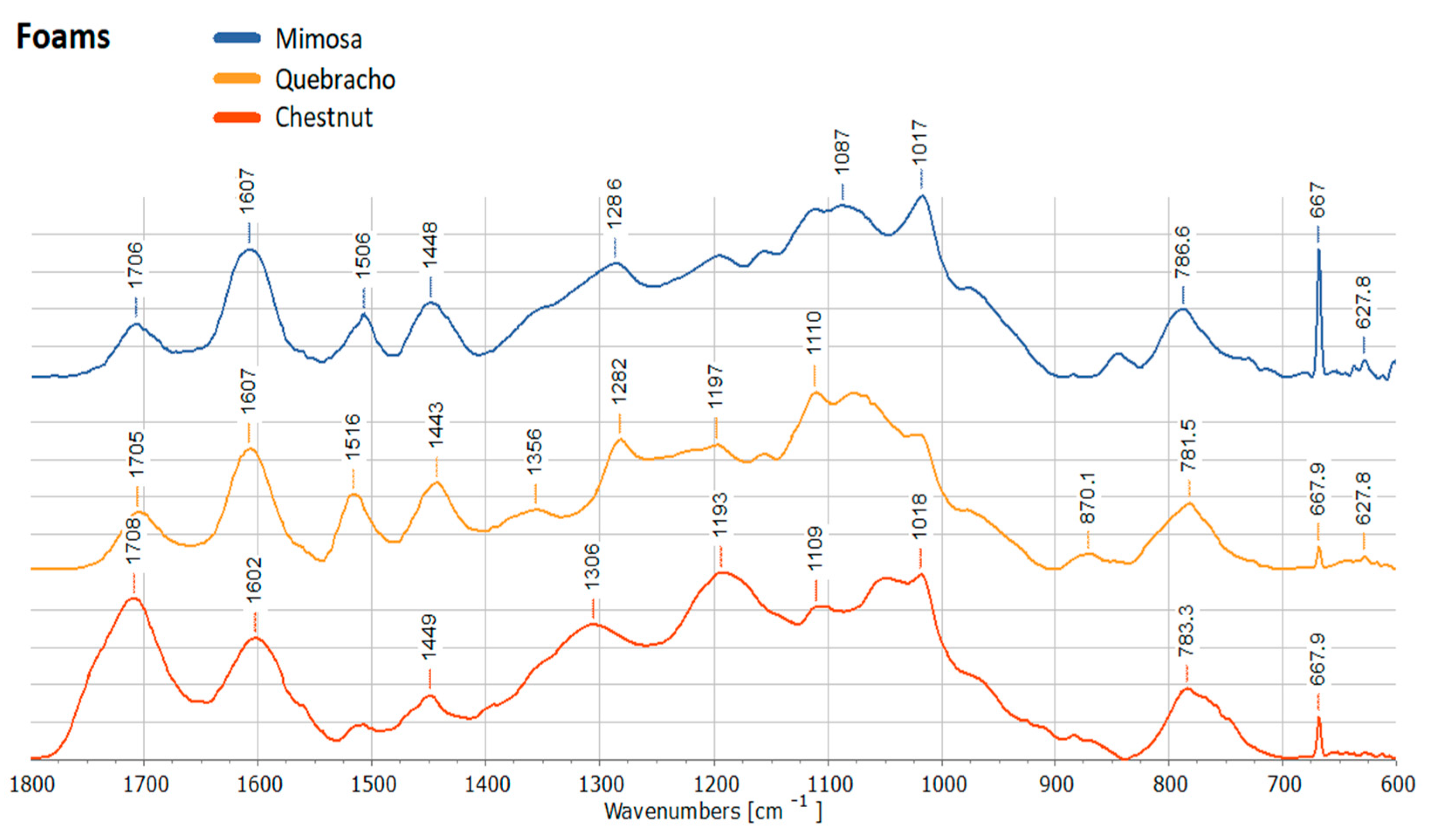
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Method
3.2.1. Foam Synthesis
3.2.2. Bulk Density
3.2.3. Cell Dimensions and Orthotropicity
3.2.4. Thermal Conductivity
3.2.5. Compression Strength
3.2.6. Acid Recovery and Leaching
3.2.7. Attenuated Total Reflectance Fourier-Transform Infrared (ATR FT-IR) Spectroscopy
3.2.8. 13C-NMR Solid State
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission; Joint Research Centre; Pavel, C.; Blagoeva, D. Competitive Landscape of the EU’s Insulation Materials Industry for Energy-Efficient Buildings: Revised Edition; Publications Office: Maastricht, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Kalhor, K.; Emaminejad, N. Qualitative and Quantitative Optimization of Thermal Insulation Materials: Insights from the Market and Energy Codes. J. Build. Eng. 2020, 30, 101275. [Google Scholar] [CrossRef]
- Schiavoni, S.; D′Alessandro, F.; Bianchi, F.; Asdrubali, F. Insulation Materials for the Building Sector: A Review and Comparative Analysis. Renew. Sustain. Energy Rev. 2016, 62, 988–1011. [Google Scholar] [CrossRef]
- Michalak, J.; Czernik, S.; Marcinek, M.; Michałowski, B. Environmental Burdens of External Thermal Insulation Systems. Expanded Polystyrene vs. Mineral Wool: Case Study from Poland. Sustainability 2020, 12, 4532. [Google Scholar] [CrossRef]
- Torres-Rivas, A.; Palumbo, M.; Haddad, A.; Cabeza, L.F.; Jiménez, L.; Boer, D. Multi-Objective Optimisation of Bio-Based Thermal Insulation Materials in Building Envelopes Considering Condensation Risk. Appl. Energy 2018, 224, 602–614. [Google Scholar] [CrossRef]
- Celzard, A.; Fierro, V.; Amaral-Labat, G.; Pizzi, A.; Torero, J. Flammability Assessment of Tannin-Based Cellular Materials. Polym. Degrad. Stab. 2011, 96, 477–482. [Google Scholar] [CrossRef]
- Chen, X.; Xi, X.; Pizzi, A.; Fredon, E.; Zhou, X.; Li, J.; Gerardin, C.; Du, G. Preparation and Characterization of Condensed Tannin Non-Isocyanate Polyurethane (NIPU) Rigid Foams by Ambient Temperature Blowing. Polymers 2020, 12, 750. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A. Tannin-Based Biofoams—A Review. J. Renew. Mater. 2019, 7, 477–492. [Google Scholar] [CrossRef]
- Delgado-Sánchez, C.; Santiago-Medina, F.; Fierro, V.; Pizzi, A.; Celzard, A. Optimisation of “Green” Tannin-Furanic Foams for Thermal Insulation by Experimental Design. Mater. Des. 2018, 139, 7–15. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Laborie, M.-P.; Celzard, A.; Fierro, V. Pine Tannin-Based Rigid Foams: Mechanical and Thermal Properties. Ind. Crops Prod. 2013, 43, 245–250. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Delgado-Regaña, A.; Rodríguez-González, M.A.; Rubio-Alonso, F. Optimization of Tannin Rigid Foam as Adsorbents for Wastewater Treatment. Ind. Crops Prod. 2013, 49, 507–514. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.-C.; Pizzi, A.; Celzard, A.; Ella Ebang, E.; Gallon, N.; Charrier, B. Pine (P. pinaster) and Quebracho (S. lorentzii) Tannin-Based Foams as Green Acoustic Absorbers. Ind. Crops Prod. 2015, 67, 70–73. [Google Scholar] [CrossRef]
- Meikleham, N.E.; Pizzi, A. Acid-and Alkali-Catalyzed Tannin-Based Rigid Foams. J. Appl. Polym. Sci. 1994, 53, 1547–1556. [Google Scholar] [CrossRef]
- Tondi, G.; Link, M.; Oo, C.W.; Petutschnigg, A. A Simple Approach to Distinguish Classic and Formaldehyde-Free Tannin Based Rigid Foams by ATR FT-IR. J. Spectrosc. 2015, 2015, 902340. [Google Scholar] [CrossRef]
- Basso, M.C.; Li, X.; Fierro, V.; Pizzi, A.; Giovando, S.; Celzard, A. Green, Formaldehyde-Free, Foams for Thermal Insulation. Adv. Mater. Lett. 2011, 2, 378–382. [Google Scholar] [CrossRef]
- Basso, M.C.; Giovando, S.; Pizzi, A.; Celzard, A.; Fierro, V. Tannin/Furanic Foams without Blowing Agents and Formaldehyde. Ind. Crops Prod. 2013, 49, 17–22. [Google Scholar] [CrossRef]
- Li, X.; Essawy, H.A.; Pizzi, A.; Delmotte, L.; Rode, K.; Le Nouen, D.; Fierro, V.; Celzard, A. Modification of Tannin Based Rigid Foams Using Oligomers of a Hyperbranched Poly(Amine-Ester). J. Polym. Res. 2012, 19, 21. [Google Scholar] [CrossRef]
- Zhou, X.; Li, B.; Xu, Y.; Essawy, H.; Wu, Z.; Du, G. Tannin-Furanic Resin Foam Reinforced with Cellulose Nanofibers (CNF). Ind. Crops Prod. 2019, 134, 107–112. [Google Scholar] [CrossRef]
- Li, X.; Basso, M.C.; Fierro, V.; Pizzi, A.; Celzard, A. Chemical Modification of Tannin/Furanic Rigid Foams by Isocyanates and Polyurethanes. Maderas Cienc. Tecnol. 2012, 14, 257–265. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Pizzi, A.; Fredon, E.; Gerardin, C.; Zhou, X.; Du, G. Tannin-Furanic Foams Modified by Soybean Protein Isolate (SPI) and Industrial Lignin Substituting Formaldehyde Addition. Ind. Crops Prod. 2021, 168, 113607. [Google Scholar] [CrossRef]
- Eckardt, J.; Neubauer, J.; Sepperer, T.; Donato, S.; Zanetti, M.; Cefarin, N.; Vaccari, L.; Lippert, M.; Wind, M.; Schnabel, T.; et al. Synthesis and Characterization of High-Performing Sulfur-Free Tannin Foams. Polymers 2020, 12, 564. [Google Scholar] [CrossRef]
- Santiago-Medina, F.J.; Delgado-Sánchez, C.; Basso, M.C.; Pizzi, A.; Fierro, V.; Celzard, A. Mechanically Blown Wall-Projected Tannin-Based Foams. Ind. Crops Prod. 2018, 113, 316–323. [Google Scholar] [CrossRef]
- Santiago-Medina, F.J.; Tenorio-Alfonso, A.; Delgado-Sánchez, C.; Basso, M.C.; Pizzi, A.; Celzard, A.; Fierro, V.; Sánchez, M.C.; Franco, J.M. Projectable Tannin Foams by Mechanical and Chemical Expansion. Ind. Crops Prod. 2018, 120, 90–96. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a Sustainable Raw Material for Green Chemistry: A Review. Ind. Crops Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and Actual Industrial Applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Arbenz, A.; Avérous, L. Chemical Modification of Tannins to Elaborate Aromatic Biobased Macromolecular Architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Basso, M.C.; Lacoste, C.; Pizzi, A.; Fredon, E.; Delmotte, L. MALDI-TOF and 13C NMR Analysis of Flexible Films and Lacquers Derived from Tannin. Ind. Crops Prod. 2014, 61, 352–360. [Google Scholar] [CrossRef]
- Pizzi, A.; Stephanou, A. A Comparative C13 NMR Study of Polyflavonoid Tannin Extracts for Phenolic Polycondensates. J. Appl. Polym. Sci. 1993, 50, 2105–2113. [Google Scholar] [CrossRef]
- Pasch, H.; Pizzi, A. Considerations on the Macromolecular Structure of Chestnut Ellagitannins by Matrix-Assisted Laser Desorption/Ionization-Time-of-Flight Mass Spectrometry. J. Appl. Polym. Sci. 2002, 85, 429–437. [Google Scholar] [CrossRef]
- Spina, S.; Zhou, X.; Segovia, C.; Pizzi, A.; Romagnoli, M.; Giovando, S.; Pasch, H.; Rode, K.; Delmotte, L. Phenolic Resin Adhesives Based on Chestnut (Castanea sativa) Hydrolysable Tannins. J. Adhes. Sci. Technol. 2013, 27, 2103–2111. [Google Scholar] [CrossRef]
- Čop, M.; Lacoste, C.; Conradi, M.; Laborie, M.-P.; Pizzi, A.; Sernek, M. The Effect of the Composition of Spruce and Pine Tannin-Based Foams on Their Physical, Morphological and Compression Properties. Ind. Crops Prod. 2015, 74, 158–164. [Google Scholar] [CrossRef]
- Marie, Z.; Nicolas, V.; Celzard, A.; Fierro, V. Experimental Investigation of the Physical Foaming of Tannin-Based Thermoset Foams. Ind. Crops Prod. 2019, 138, 111424. [Google Scholar] [CrossRef]
- Azadeh, E.; Chen, X.; Pizzi, A.; Gérardin, C.; Gérardin, P.; Essawy, H. Self-Blowing Non-Isocyanate Polyurethane Foams Based on Hydrolysable Tannins. J. Renew. Mater. 2022, 10, 3217–3227. [Google Scholar] [CrossRef]
- Lagel, M.C.; Pizzi, A.; Giovando, S.; Celzard, A. Development and Characterisation of Phenolic Foams with Phenol-Formaldehyde-Chestnut Tannins Resin. J. Renew. Mater. 2014, 2, 220–229. [Google Scholar] [CrossRef]
- Varila, T.; Romar, H.; Luukkonen, T.; Lassi, U. Physical Activation and Characterization of Tannin-Based Foams Enforced with Boric Acid and Zinc Chloride. AIMS Mater. Sci. 2019, 6, 301–314. [Google Scholar] [CrossRef]
- Čop, M.; Gospodarič, B.; Kemppainen, K.; Giovando, S.; Laborie, M.-P.; Pizzi, A.; Sernek, M. Characterization of the Curing Process of Mixed Pine and Spruce Tannin-Based Foams by Different Methods. Eur. Polym. J. 2015, 69, 29–37. [Google Scholar] [CrossRef]
- Čop, M.; Laborie, M.P.; Pizzi, A.; Sernek, M. Curing Characterisation of Spruce Tannin-Based Foams Using the Advanced Isoconversional Method. BioResources 2014, 9, 4643–4655. [Google Scholar] [CrossRef]
- Petkova, B.; Tcholakova, S.; Chenkova, M.; Golemanov, K.; Denkov, N.; Thorley, D.; Stoyanov, S. Foamability of Aqueous Solutions: Role of Surfactant Type and Concentration. Adv. Colloid Interface Sci. 2020, 276, 102084. [Google Scholar] [CrossRef]
- Politova, N.; Tcholakova, S.; Valkova, Z.; Golemanov, K.; Denkov, N.D. Self-Regulation of Foam Volume and Bubble Size during Foaming via Shear Mixing. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 18–28. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, W.; Li, C.; Wang, G.; Lin, X.; Liu, Z. Effects of Surfactants on the Mechanical Properties, Microstructure, and Flame Resistance of Phenol–Urea–Formaldehyde Foam. Polym. Bull. 2016, 73, 1–20. [Google Scholar] [CrossRef]
- Jalalian, M.; Jiang, Q.; Coulon, A.; Storb, M.; Woodward, R.; Bismarck, A. Mechanically Whipped Phenolic Froths as Versatile Templates for Manufacturing Phenolic and Carbon Foams. Mater. Des. 2019, 168, 107658. [Google Scholar] [CrossRef]
- Sepperer, T.; Šket, P.; Petutschnigg, A.; Hüsing, N. Tannin-Furanic Foams Formed by Mechanical Agitation: Influence of Surfactant and Ingredient Ratios. Polymers 2021, 13, 3058. [Google Scholar] [CrossRef]
- Kothekar, S.C.; Ware, A.M.; Waghmare, J.T.; Momin, S.A. Comparative Analysis of the Properties of Tween-20, Tween-60, Tween-80, Arlacel-60, and Arlacel-80. J. Dispers. Sci. Technol. 2007, 28, 477–484. [Google Scholar] [CrossRef]
- Ziarati, H.B.; Fasihi, M.; Omranpour, H. The Effect of Resin Formulation on the Cellular Morphology and Mechanical Properties of Phenolic Foams. J. Appl. Polym. Sci. 2020, 137, 48331. [Google Scholar] [CrossRef]
- Tondi, G.; Link, M.; Kolbitsch, C.; Lesacher, R.; Petutschnigg, A. Pilot Plant Up-Scaling of Tannin Foams. Ind. Crops Prod. 2016, 79, 211–218. [Google Scholar] [CrossRef]
- Tondi, G.; Zhao, W.; Pizzi, A.; Du, G.; Fierro, V.; Celzard, A. Tannin-Based Rigid Foams: A Survey of Chemical and Physical Properties. Bioresour. Technol. 2009, 100, 5162–5169. [Google Scholar] [CrossRef]
- Tondi, G.; Pizzi, A. Tannin-Based Rigid Foams: Characterization and Modification. Ind. Crops Prod. 2009, 29, 356–363. [Google Scholar] [CrossRef]
- de Yuso, A.M.; Lagel, M.C.; Pizzi, A.; Fierro, V.; Celzard, A. Structure and Properties of Rigid Foams Derived from Quebracho Tannin. Mater. Des. 2014, 63, 208–212. [Google Scholar] [CrossRef]
- Cesprini, E.; Šket, P.; Causin, V.; Zanetti, M.; Tondi, G. Development of Quebracho (Schinopsis balansae) Tannin-Based Thermoset Resins. Polymers 2021, 13, 4412. [Google Scholar] [CrossRef]
- Kolbitsch, C.; Link, M.; Petutschnigg, A.; Wieland, S.; Tondi, G. Microwave Produced Tannin-Furanic Foams. J. Mater. Sci. Res. 2012, 1, 84. [Google Scholar] [CrossRef]
- Li, X.; Nicollin, A.; Pizzi, A.; Zhou, X.; Sauget, A.; Delmotte, L. Natural Tannin–Furanic Thermosetting Moulding Plastics. RSC Adv. 2013, 3, 17732. [Google Scholar] [CrossRef]
- ISO 844: 2007; Rigid Cellular Plastics: Determination of Compression Properties. ISO International Standards: Geneva, Switzerland, 2007.





| Tannin Type | Surfactant [%] | Density [kg/m3] | Cell Ø [µm] | Orthotropicity | Comp. Strength σc [MPa] |
|---|---|---|---|---|---|
| Mimosa | 1 | 225.5 (10) | 129 (64) | 1.17 (0.09) | 2.250 (0.218) |
| 5 | 175.4 (12.2) | 162 (99) | 1.19 (0.12) | 1.334 (0.255) | |
| 10 | 143.3 (5.3) | 178 (113) | 1.22 (0.13) | 0.734 (0.112) | |
| Quebracho | 5 | 370.8 (26.7) | 60 (28) | 1.17 (0.09) | 4.060 (0.687) |
| 10 | 244.1 (18.2) | 117 (72) | 1.14 (0.08) | 1.214 (0.276) | |
| Chestnut | 5 | 271.6 (7.6) | 103 (44) | 1.11 (0.07) | 1.239 (0.143) |
| 10 | 258.4 (6.8) | 105 (58) | 1.14 (0.09) | 1.019 (0.083) |
| Foam Formulation | Compression Resistance [MPa] | Density [kg/m3] | Reference |
|---|---|---|---|
| Mimosa-FOH-CH2O | 1.04 | 136 | [46] |
| 1.38 | 161 | ||
| 3.97 | 306 | ||
| Pine-FOH-CH2O | 1.03 | 140 | [10] |
| 1.75 | 190 | ||
| Mimosa-FOH | 0.15 | 140 | [45] |
| 0.5 | 200 | ||
| Mimosa-NIPU | 0.15 | 150 | [7] |
| 0.57 | 260 | ||
| Chestnut-NIPU | 0.91 | 350 | [33] |
| Tannin acid-FOH | 0.1 | 150 | [35] |
| Quebracho-FOH-CH2O | 0.24 | 90 | [47] |
| Tannin-Type | Tannin [%] | FOH [%] | DEG [%] | H2O [%] | H2SO4 [%] | Tween80 [%] | S/T-F [%] |
|---|---|---|---|---|---|---|---|
| Mimosa, Quebracho, Chestnut | 37.93 | 23.52 | 11.38 | 11.38 | 15.17 | 0.61 | 1 |
| 37.02 | 22.95 | 11.11 | 11.11 | 14.81 | 3.00 | 5 | |
| 35.95 | 22.29 | 10.78 | 10.78 | 14.38 | 5.82 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckardt, J.; Sepperer, T.; Cesprini, E.; Šket, P.; Tondi, G. Comparing Condensed and Hydrolysable Tannins for Mechanical Foaming of Furanic Foams: Synthesis and Characterization. Molecules 2023, 28, 2799. https://doi.org/10.3390/molecules28062799
Eckardt J, Sepperer T, Cesprini E, Šket P, Tondi G. Comparing Condensed and Hydrolysable Tannins for Mechanical Foaming of Furanic Foams: Synthesis and Characterization. Molecules. 2023; 28(6):2799. https://doi.org/10.3390/molecules28062799
Chicago/Turabian StyleEckardt, Jonas, Thomas Sepperer, Emanuele Cesprini, Primož Šket, and Gianluca Tondi. 2023. "Comparing Condensed and Hydrolysable Tannins for Mechanical Foaming of Furanic Foams: Synthesis and Characterization" Molecules 28, no. 6: 2799. https://doi.org/10.3390/molecules28062799
APA StyleEckardt, J., Sepperer, T., Cesprini, E., Šket, P., & Tondi, G. (2023). Comparing Condensed and Hydrolysable Tannins for Mechanical Foaming of Furanic Foams: Synthesis and Characterization. Molecules, 28(6), 2799. https://doi.org/10.3390/molecules28062799







