A Facile Method to Fabricate Al2O3-SiO2 Aerogels with Low Shrinkage up to 1200 °C
Abstract
1. Introduction
2. Results
2.1. Synthesis of Al2O3-SiO2 Aerogels
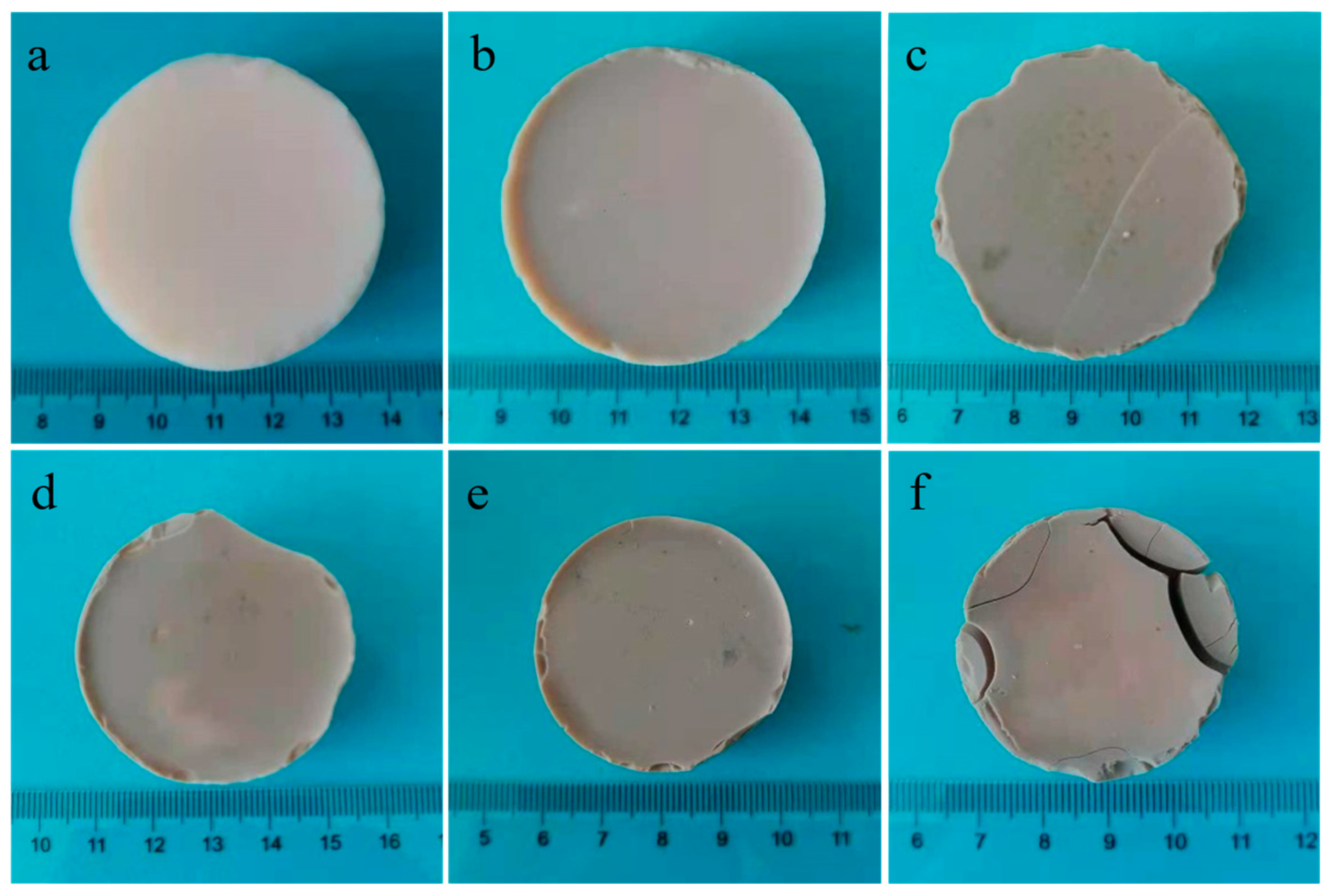
2.2. Macro Morphology and Properties at Room Temperature
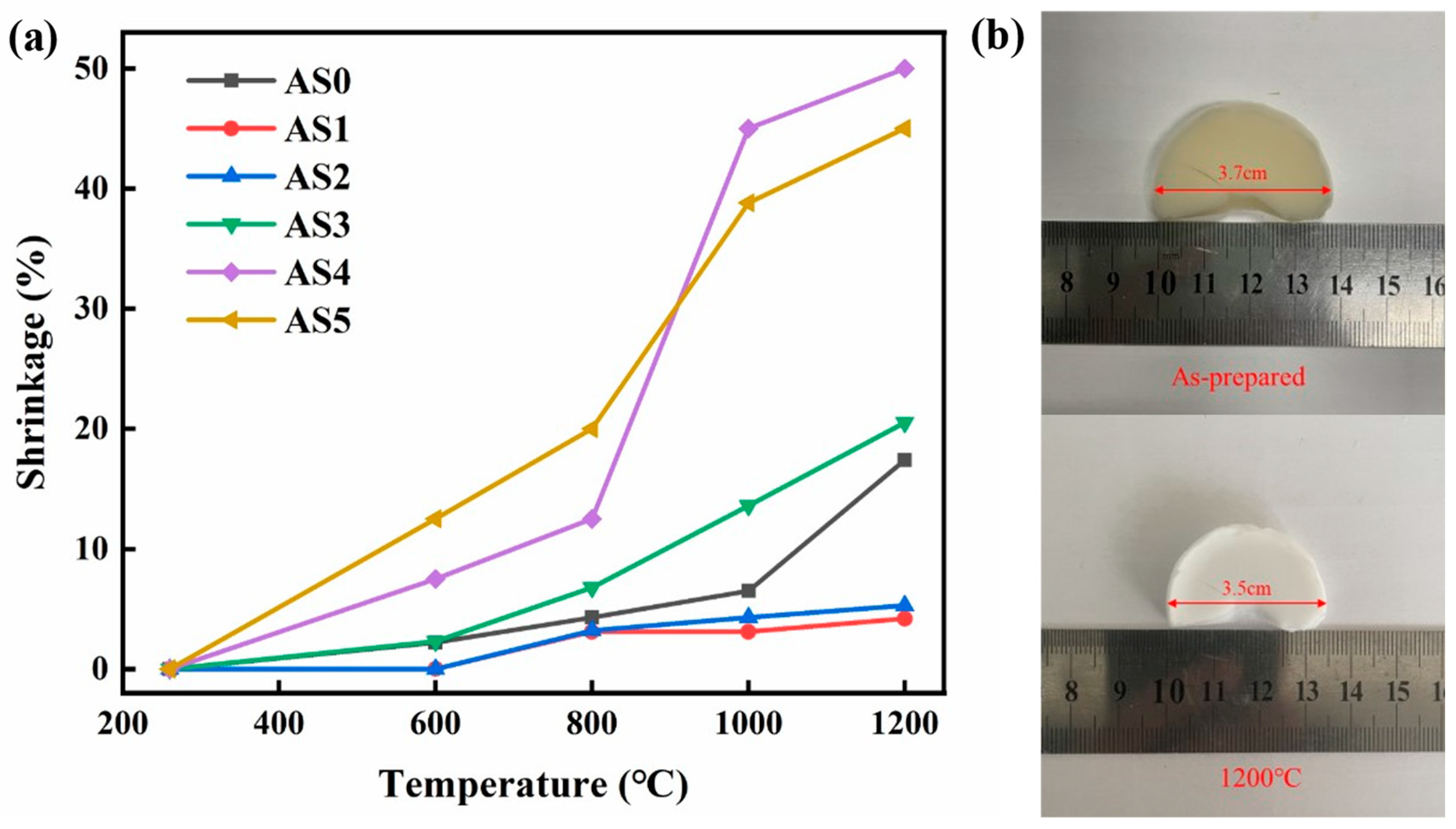
2.3. Shrinkage after Different Heating Temperatures
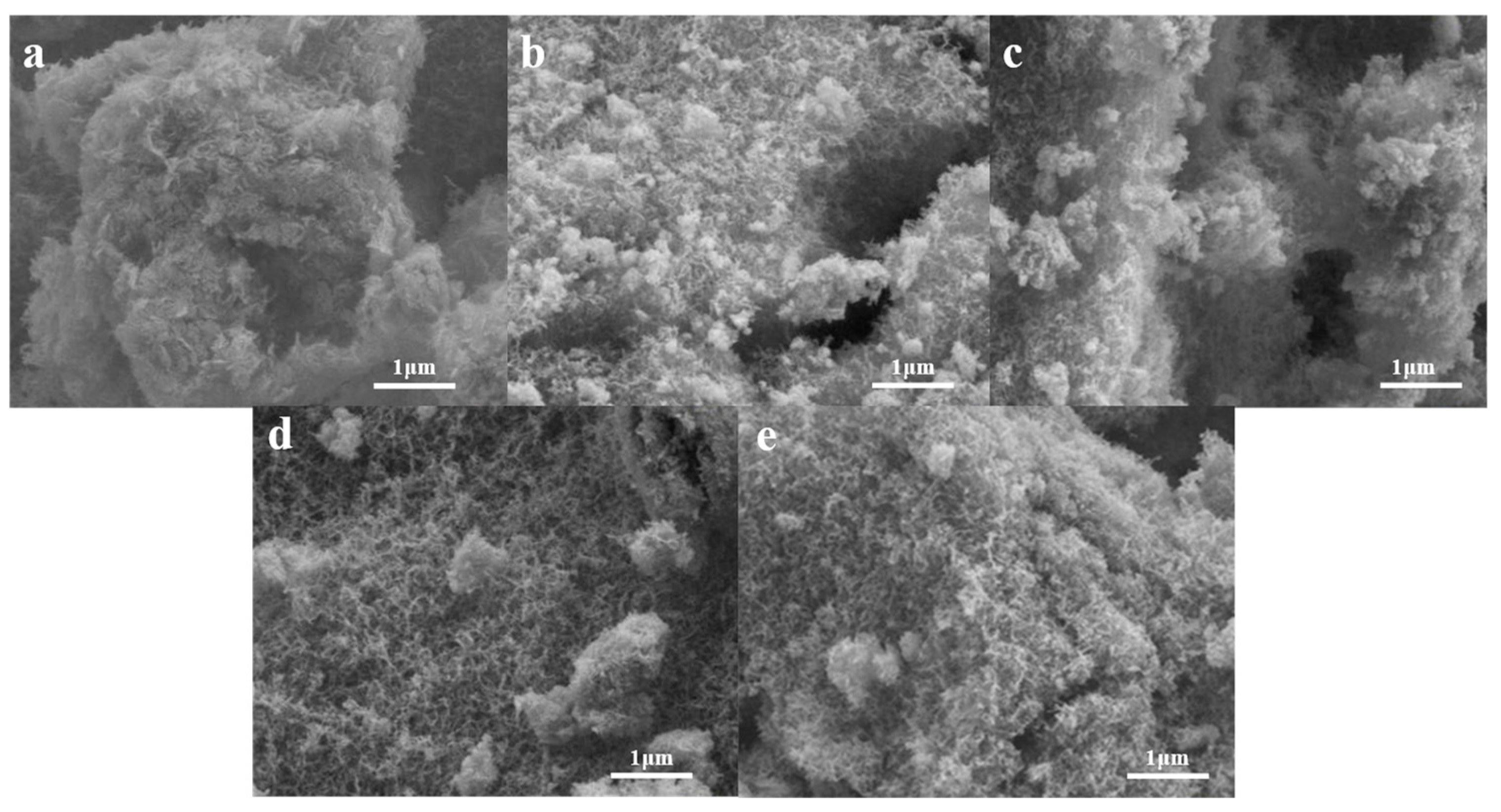
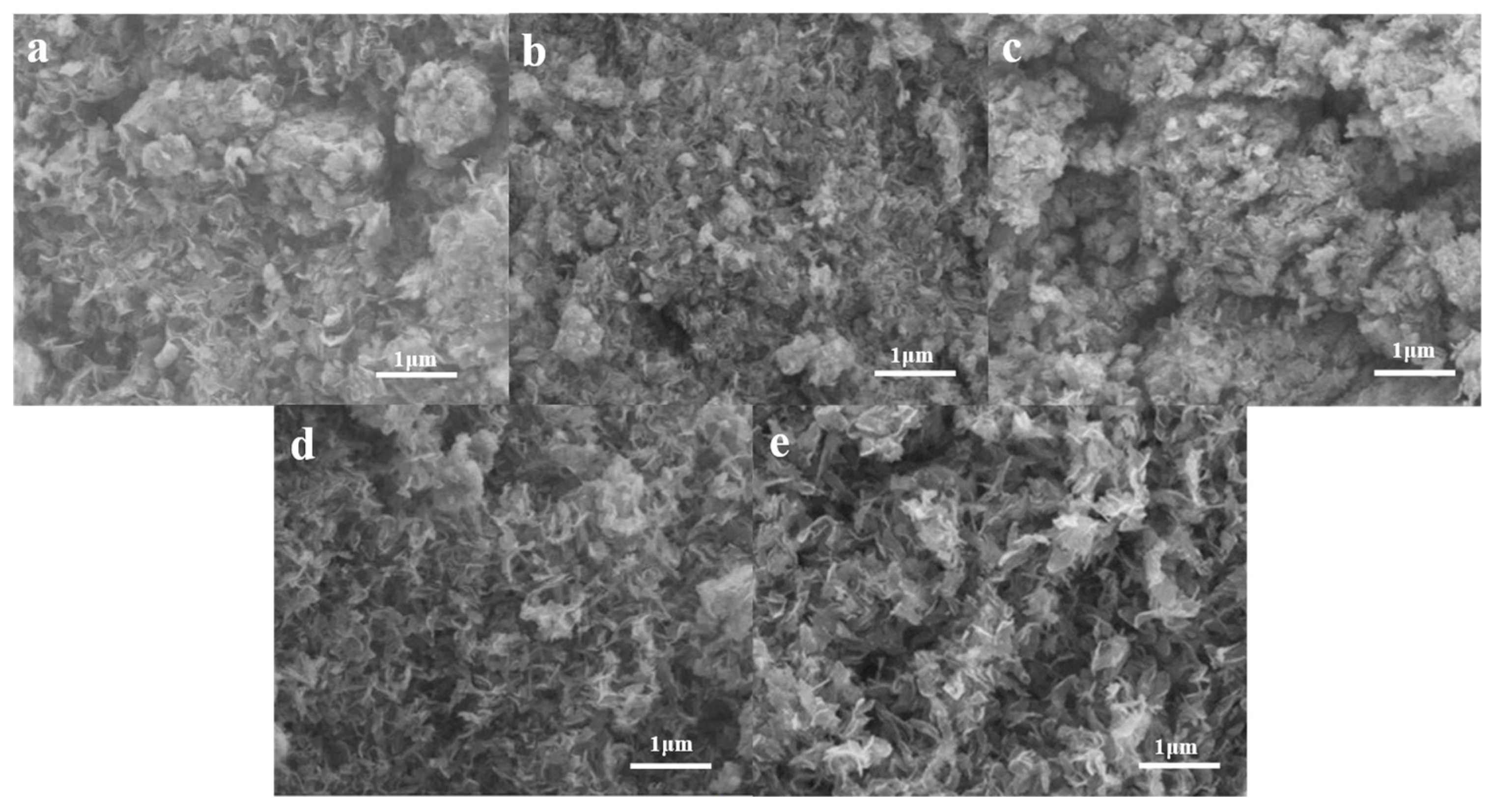
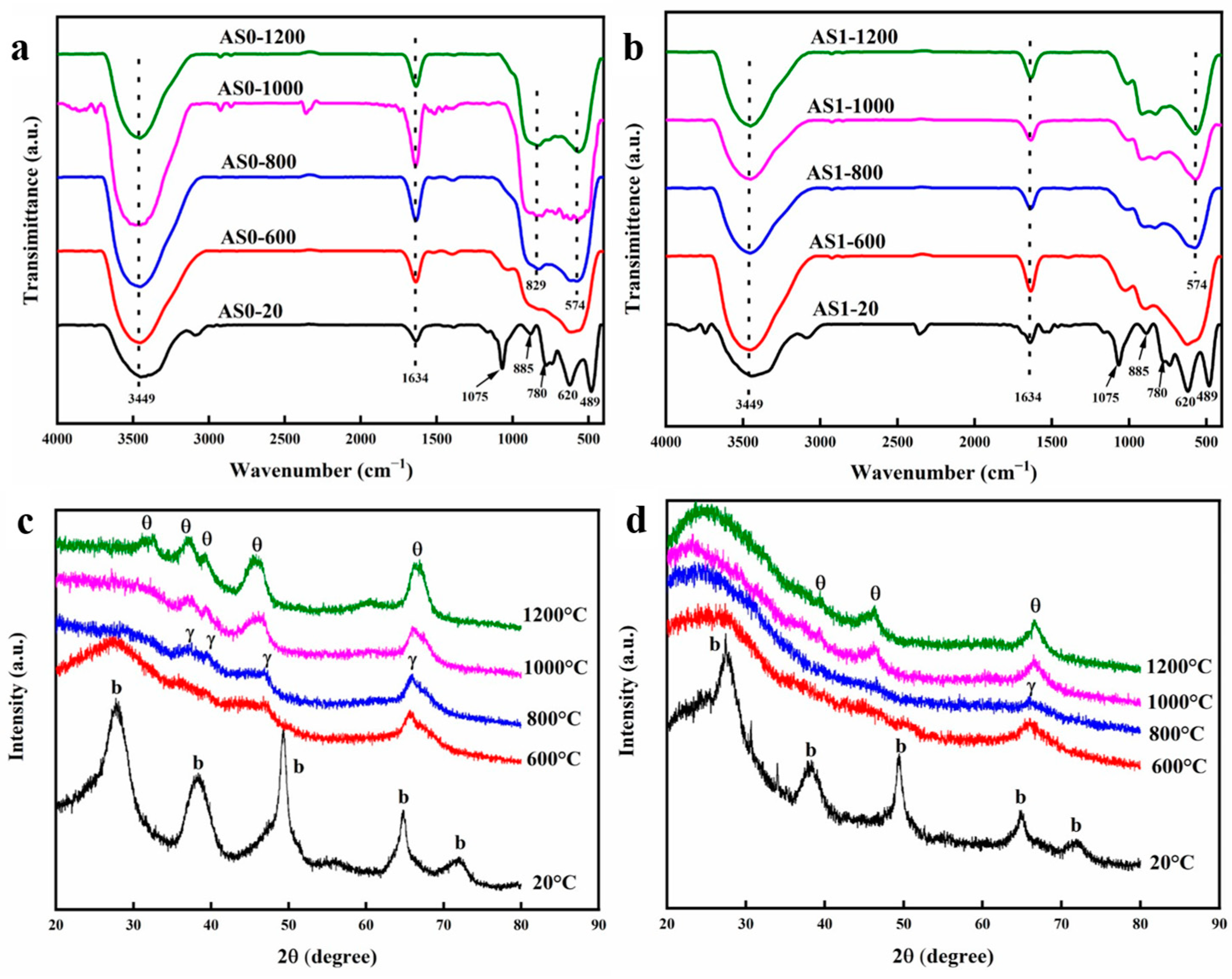
2.4. Micromorphology at Different Temperatures
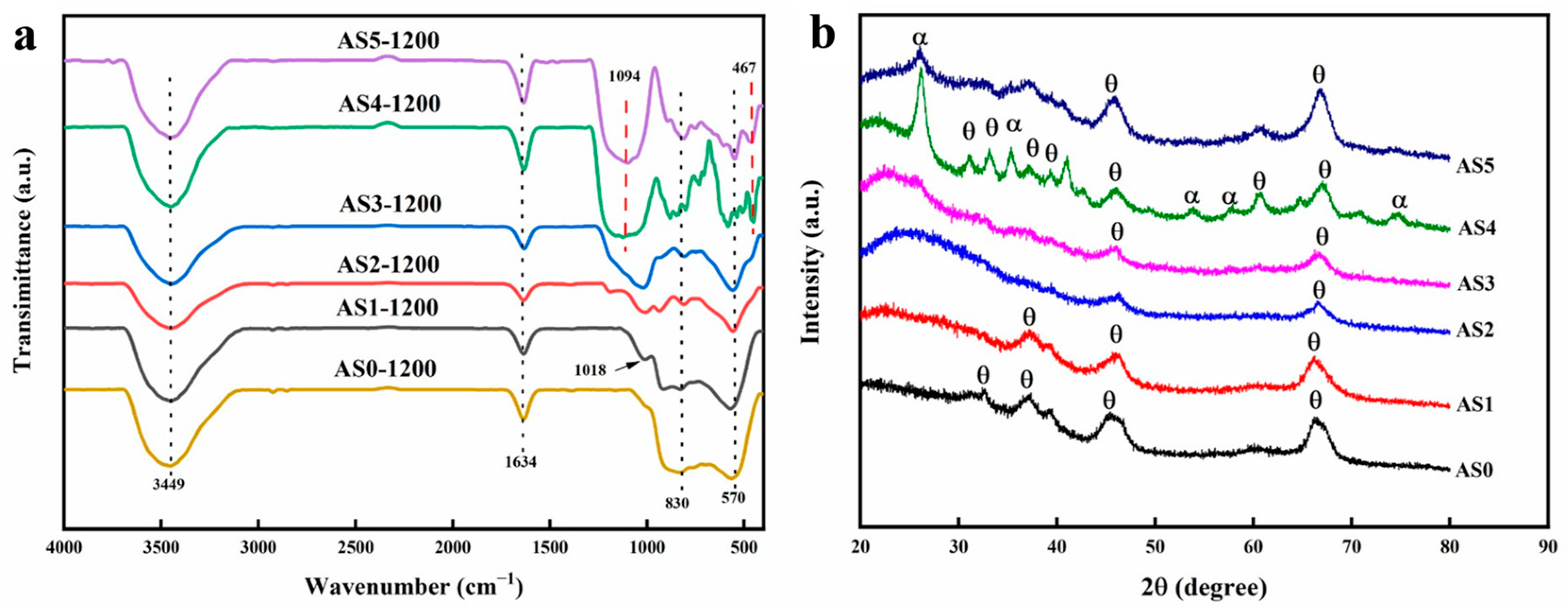
2.5. Variation of Chemical Composition at Different Temperatures
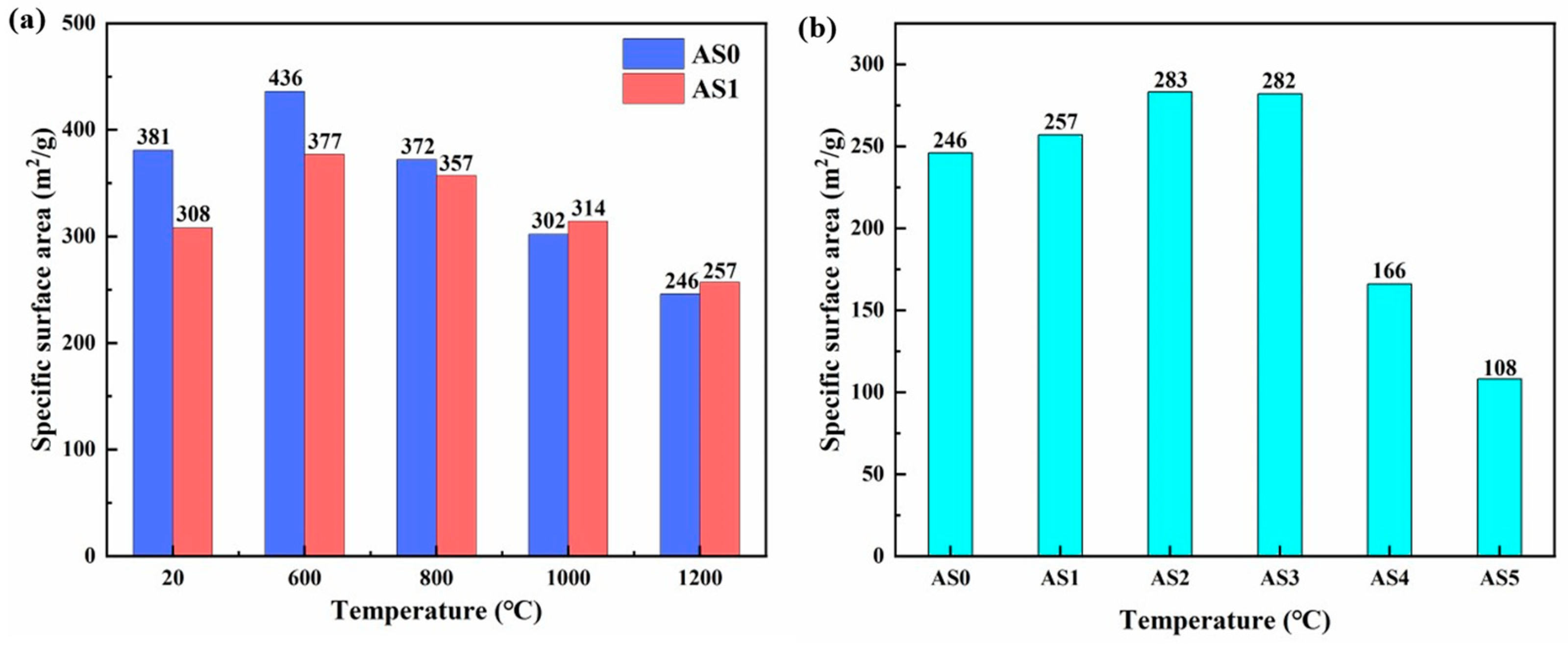
2.6. SSA and Pore Size Distribution at Different Temperatures
3. Materials and methods
3.1. Materials
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Peng, F.; Jiang, Y.G.; Feng, J.; Cai, H.F.; Feng, J.Z.; Li, L.J. Research Progress on Alumina Aerogel Composites for High-temperature Thermal Insulation. J. Inorg. Mater. 2021, 36, 673–684. [Google Scholar] [CrossRef]
- Despetis, F.; Calas-Etienne, S.; Etienne, P. Slow crack growth in silica aerogels: A review. J. Sol-Gel Sci. Technol. 2019, 90, 20–27. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wu, Y.; Zou, W.; Wu, X.; Shen, J. Aerogels and Their Applications—A Short Review. J. Chin. Ceram. Soc. 2018, 46, 1426–1446. [Google Scholar]
- Randall, J.P.; Meador MA, B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Inter. 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wang, Y.; Malfait, W.J.; Zhao, S.; Tian, Y.; Liu, T.; Zhang, X.; Du, A.; Shen, J. Flexible, high-temperature-resistant silica-polymer aerogel hybrids by templating polymethylsilsesquioxane microstructure with trace polyimide. Adv. Compos. Hybrid Mater. 2023, 6, 32. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Y.; Yu, C.; Liu, L.; Zhang, Z.; Wu, Y.; Shen, J. Organic/inorganic double-precursor cross-linked alumina aerogel with high specific surface area and high-temperature resistance. Ceram. Int. 2022, 48, 17261–17269. [Google Scholar] [CrossRef]
- Bono, M.S.; Anderson, A.M.; Carroll, M.K. Alumina aerogels prepared via rapid supercritical extraction. J. Sol-Gel Sci. Technol. 2010, 53, 216–226. [Google Scholar] [CrossRef]
- Juhl, S.J.; Dunn NJ, H.; Carroll, M.K.; Anderson, A.M.; Bruno, B.A.; Madero, J.E.; Bono, M.S. Epoxide-assisted alumina aerogels by rapid supercritical extraction. J. Non-Cryst. Solids 2015, 426, 141–149. [Google Scholar] [CrossRef]
- Zou, W.; Wang, X.; Wu, Y.; Zu, G.; Zou, L.; Zhang, R.; Yao, X.; Shen, J. Highly thermally stable alumina-based aerogels modified by partially hydrolyzed aluminum tri-sec-butoxide. J. Sol-Gel Sci. Technol. 2017, 84, 507–514. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Ghica, M.E.; Durães, L. An overview on alumina-silica-based aerogels. Adv. Colloid Interface Sci. 2020, 282, 102189. [Google Scholar] [CrossRef]
- Lebedev, A.E.; Menshutina, N.V.; Khudeev, I.I.; Kamyshinsky, R.A. Investigation of alumina aerogel structural characteristics at different «precursor-water-ethanol» ratio. J. Non-Cryst. Solids 2021, 553, 120475. [Google Scholar] [CrossRef]
- Poco, J.F.; Satcher, J.H.; Hrubesh, L.W. Synthesis of high porosity, monolithic alumina aerogels. J. Non-Cryst. Solids 2001, 285, 57–63. [Google Scholar] [CrossRef]
- Li, F.B.; Li, M.; Xu, X.; Yang, Z.C.; Xu, H.; Jia, C.K.; Li, K.; He, J.; Li, B.; Wang, H. Understanding colossal barocaloric effects in plastic crystals. Nat. Commun. 2020, 11, 4190. [Google Scholar] [CrossRef]
- Horiuchi, T.; Chen, L.; Osaki, T.; Sugiyama, T.; Suzuki, K.; Mori, T. A novel alumina catalyst support with high thermal stability derived from silica-modified alumina aerogel. Catal. Lett. 1999, 58, 89–92. [Google Scholar] [CrossRef]
- Horiuchi, T.; Osaki, T.; Sugiyama, T.; Suzuki, K.; Mori, T. Maintenance of large surface area of alumina heated at elevated temperatures above 1300 °C by preparing silica-containing pseudoboehmite aerogel. J. Non-Cryst. Solids 2001, 291, 187–198. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Zou, L.; Wang, W.; Lian, Y.; Zhang, Z.; Du, A. Nanoengineering Super Heat-Resistant, Strong Alumina Aerogels. Chem. Mater. 2013, 25, 4757–4764. [Google Scholar] [CrossRef]
- Xu, X.; Fu, S.; Guo, J.; Li, H.; Huang, Y.; Duan, X. Elastic ceramic aerogels for thermal superinsulation under extreme conditions. Mater. Today 2021, 42, 162–177. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Q.; Wang, T.; Liang, Y. Facile one-step precursor-to-aerogel synthesis of silica-doped alumina aerogels with high specific surface area at elevated temperatures. J. Porous Mater. 2017, 24, 889–897. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.; Zu, G.; Shen, J.; Zou, L.; Lian, Y.; Liu, B.; Zhang, F. Trimethylethoxysilane-modified super heat-resistant alumina aerogels for high-temperature thermal insulation and adsorption applications. RSC Adv. 2014, 4, 54864–54871. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Jin, S.; Hu, Z.; Liu, Y.; Jin, M. Synthesis of alumina aerogels from AlCl3·6H2O with an aid of acetoacetic-grafted polyvinyl alcohol. J. Sol-Gel Sci. Technol. 2018, 87, 486–495. [Google Scholar] [CrossRef]
- Wu, X.; Shao, G.; Shen, X.; Cui, S.; Wang, L. Novel Al2O3–SiO2 composite aerogels with high specific surface area at elevated temperatures with different alumina/silica molar ratios prepared by a non-alkoxide sol–gel method. RSC Adv. 2016, 6, 5611–5620. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Wang, G.; Liu, Y.; Kang, W.; Deng, N.; Zhuang, X.; Zhou, X. Research progress of ultrafine alumina fiber prepared by sol-gel method: A review. Chem. Eng. J. 2021, 421, 127744. [Google Scholar] [CrossRef]
- Yu, H.; Tong, Z.; Yue, S.; Li, X.; Su, D.; Ji, H. Effect of SiO2 deposition on thermal stability of Al2O3-SiO2 aerogel. J. Eur. Ceram. Soc. 2021, 41, 580–589. [Google Scholar] [CrossRef]
- Baumann, T.F.; Gash, A.E.; Chinn, S.C.; Sawvel, A.M.; Maxwell, R.S.; Satcher, J.H. Synthesis of High-Surface-Area Alumina Aerogels without the Use of Alkoxide Precursors. Chem. Mater. 2005, 17, 395–401. [Google Scholar] [CrossRef]
- Wu, X.; Shao, G.; Cui, S.; Wang, L.; Shen, X. Synthesis of a novel Al2O3–SiO2 composite aerogel with high specific surface area at elevated temperatures using inexpensive inorganic salt of aluminum. Ceram. Int. 2016, 42, 874–882. [Google Scholar] [CrossRef]
- Osaki, T.; Nagashima, K.; Watari, K.; Tajiri, K. Silica-doped alumina cryogels with high thermal stability. J. Non-Cryst. Solids 2007, 353, 2436–2442. [Google Scholar] [CrossRef]
- Pakharukova, V.P.; Shalygin, A.S.; Gerasimov, E.Y.; Tsybulya, S.V.; Martyanov, O.N. Structure and morphology evolution of silica-modified pseudoboehmite aerogels during heat treatment. J. Solid State Chem. 2016, 233, 294–302. [Google Scholar] [CrossRef]
- Levin, I.; Brandon, D. Metastable alumina polymorphs: Crystal structures and transition sequences. J. AM. Ceram. Soc. 1998, 81, 1995–2012. [Google Scholar] [CrossRef]
- Tsukada, T.; Segawa, H.; Yasumori, A.; Okada, K. Crystallinity of boehmite and its effect on the phase transition temperature of alumina. J. Mater. Chem. 1999, 9, 549–553. [Google Scholar] [CrossRef]
- Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Berthet, P.; Huntz, A.M.; Roy, P.; Tétot, R. Transition alumina phases induced by heat treatment of boehmite: An X-ray diffraction and infrared spectroscopy study. J. Solid State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- Li, G.; Liu, Y.; Liu, D.; Liu, L.; Liu, C. Synthesis of flower-like Boehmite (AlOOH) via a simple solvothermal process without surfactant. Mater. Res. Bull. 2010, 45, 1487–1491. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Y.; Feng, J.; Li, L.; Cai, H.; Feng, J. A facile method to fabricate monolithic alumina–silica aerogels with high surface areas and good mechanical properties. J. Eur. Ceram. Soc. 2020, 40, 2480–2488. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Wei, X.; Ni, X.; Zhang, Z.; Wang, J.; Liu, G. Preparation and characterization of monolithic alumina aerogels. J. Non-Cryst. Solids 2011, 357, 2903–2906. [Google Scholar] [CrossRef]
- Carnes, C.L.; Kapoor, P.N.; Klabunde, K.J.; Bonevich, J. Synthesis, Characterization, and Adsorption Studies of Nanocrystalline Aluminum Oxide and a Bimetallic Nanocrystalline Aluminum Oxide/Magnesium Oxide. Chem. Mater. 2002, 14, 2922–2929. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, G.; Bai, J.; Ma, Y.; Ren, L. One-pot synthesis of bimetallic catalyst loaded on alumina aerogel as green heterogeneous catalyst: Efficiency, stability, and mechanism. J. Taiwan Inst. Chem. Eng. 2019, 101, 41–49. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]

| Sample | Density (mg/cm3) | Liner Shrinkage (%) | Thermal Conductivity (W/m∙K) |
|---|---|---|---|
| AS0 | 32 | 12 | 0.033 |
| AS1 | 42 | 8 | 0.035 |
| AS2 | 40 | 10 | 0.036 |
| AS3 | 62 | 15 | 0.035 |
| AS4 | 83 | 23 | 0.037 |
| AS5 | 96 | 23 | 0.036 |
| Sample | Pore Volume (cm3/g) | Pore Diameter (nm) | Sample | Pore Volume (cm3/g) | Pore Diameter (nm) | Sample | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|---|---|---|---|---|
| AS0-20 | 3.31 | 31.0 | AS1-20 | 2.15 | 28.3 | AS0-1200 | 2.30 | 33.0 |
| AS0-600 | 4.20 | 34.2 | AS1-600 | 3.17 | 33.4 | AS1-1200 | 1.89 | 29.9 |
| AS0-800 | 3.09 | 33.4 | AS1-800 | 2.75 | 33.6 | AS2-1200 | 2.05 | 28.8 |
| AS0-1000 | 2.57 | 35.6 | AS1-1000 | 2.31 | 31.8 | AS3-1200 | 2.33 | 31.5 |
| AS0-1200 | 2.30 | 33.0 | AS1-1200 | 1.89 | 29.9 | AS4-1200 | 1.44 | 24.6 |
| AS5-1200 | 0.75 | 21.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Wang, X.; Wu, Y.; Zhang, X.; Li, C.; Wang, Y.; Shen, J. A Facile Method to Fabricate Al2O3-SiO2 Aerogels with Low Shrinkage up to 1200 °C. Molecules 2023, 28, 2743. https://doi.org/10.3390/molecules28062743
Tian Y, Wang X, Wu Y, Zhang X, Li C, Wang Y, Shen J. A Facile Method to Fabricate Al2O3-SiO2 Aerogels with Low Shrinkage up to 1200 °C. Molecules. 2023; 28(6):2743. https://doi.org/10.3390/molecules28062743
Chicago/Turabian StyleTian, Yulin, Xiaodong Wang, Yu Wu, Xiaoxue Zhang, Chun Li, Yijun Wang, and Jun Shen. 2023. "A Facile Method to Fabricate Al2O3-SiO2 Aerogels with Low Shrinkage up to 1200 °C" Molecules 28, no. 6: 2743. https://doi.org/10.3390/molecules28062743
APA StyleTian, Y., Wang, X., Wu, Y., Zhang, X., Li, C., Wang, Y., & Shen, J. (2023). A Facile Method to Fabricate Al2O3-SiO2 Aerogels with Low Shrinkage up to 1200 °C. Molecules, 28(6), 2743. https://doi.org/10.3390/molecules28062743







