Mechanical Stimulation Decreases Auxin and Gibberellic Acid Synthesis but Does Not Affect Auxin Transport in Axillary Buds; It Also Stimulates Peroxidase Activity in Petunia × atkinsiana
Abstract
1. Introduction
2. Results
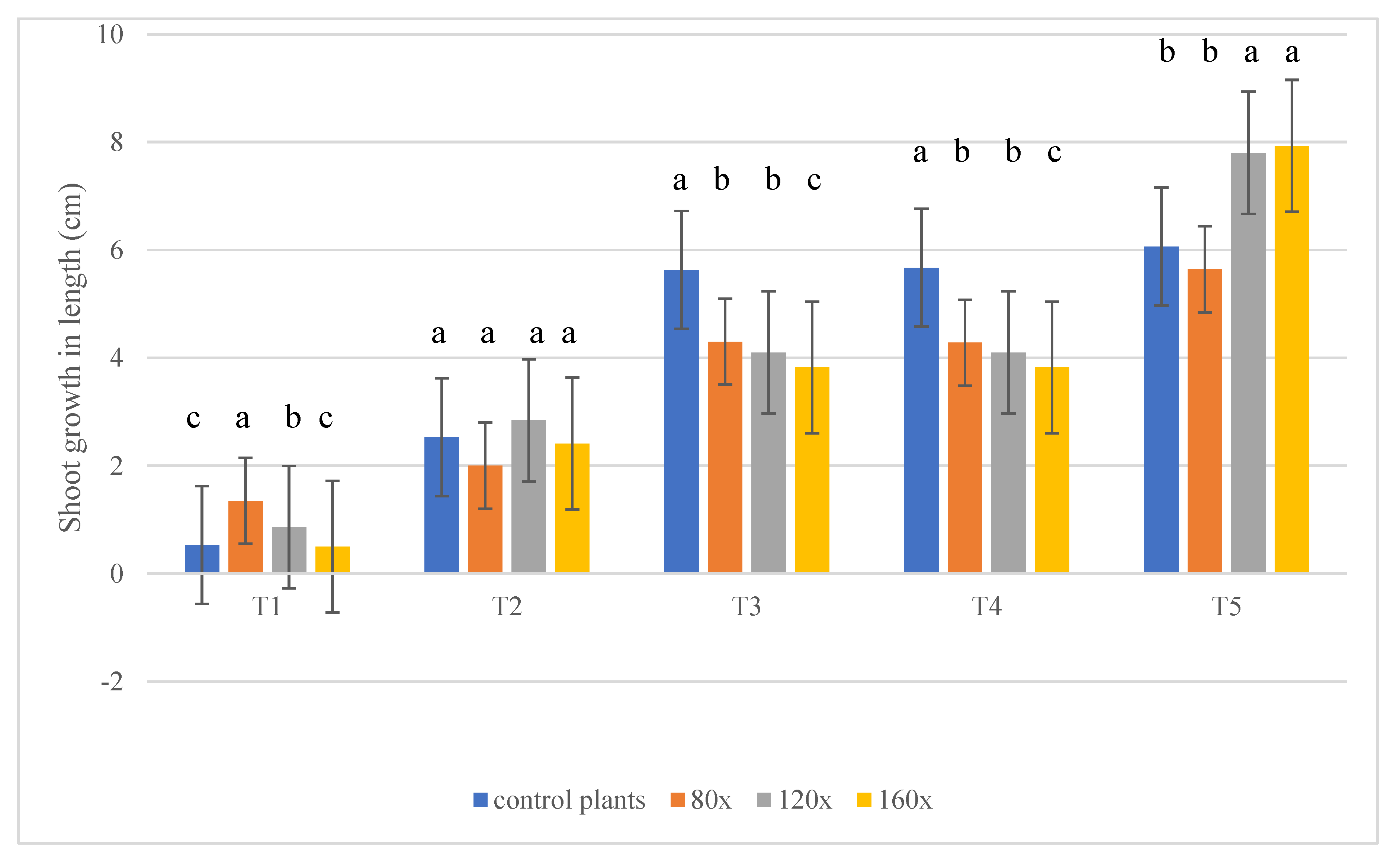
2.1. Shoot Growth
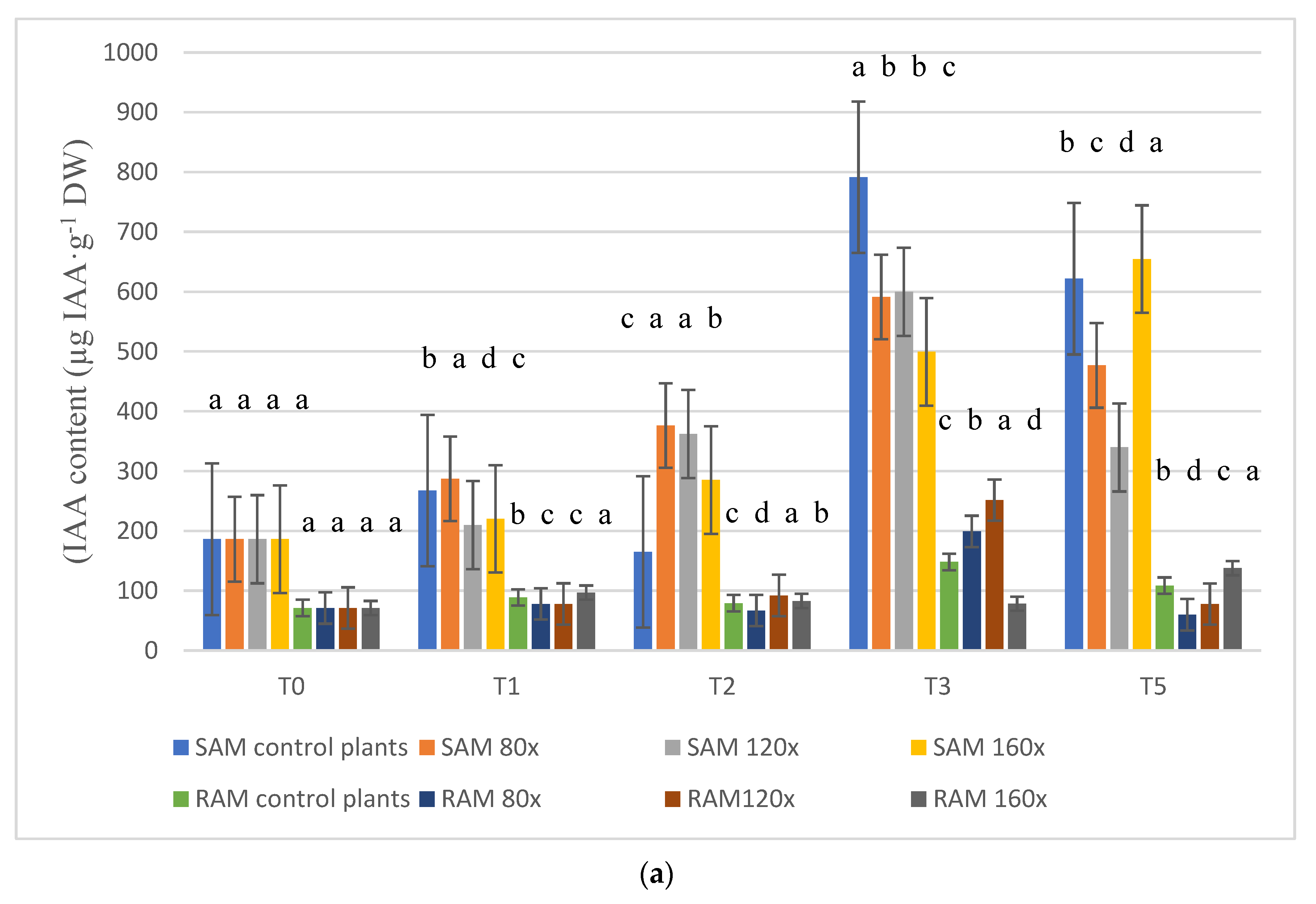
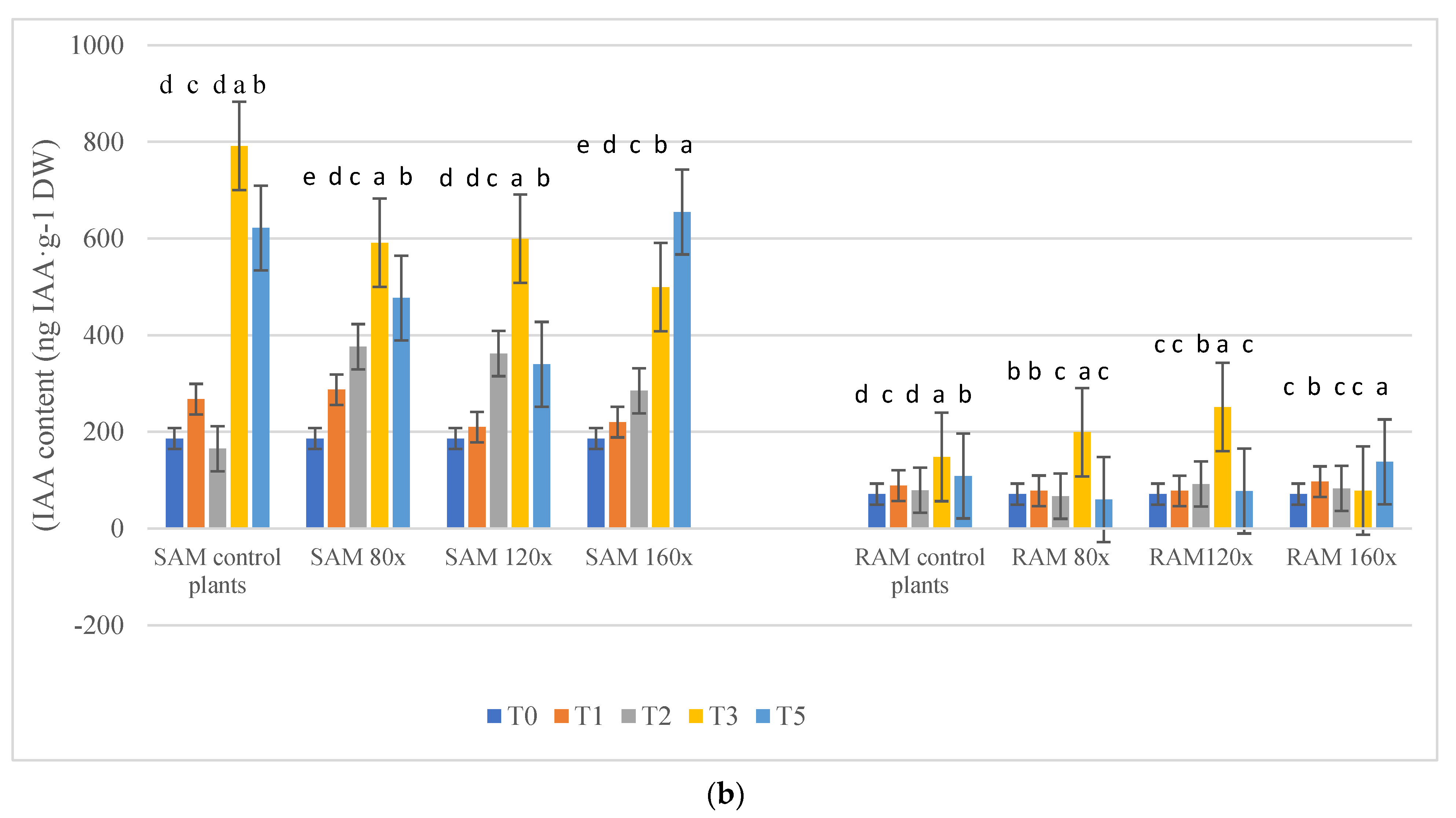
2.2. IAA Content
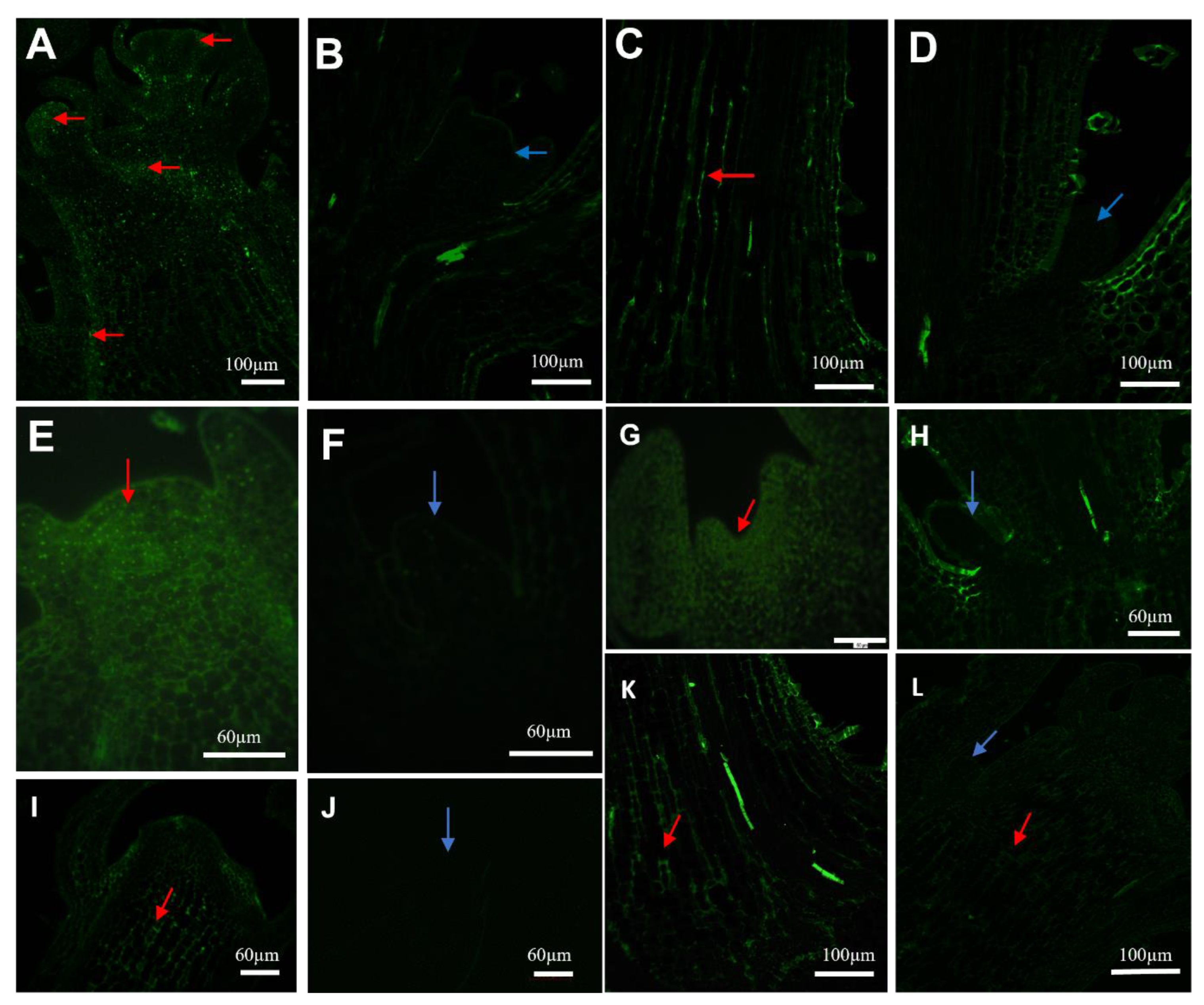
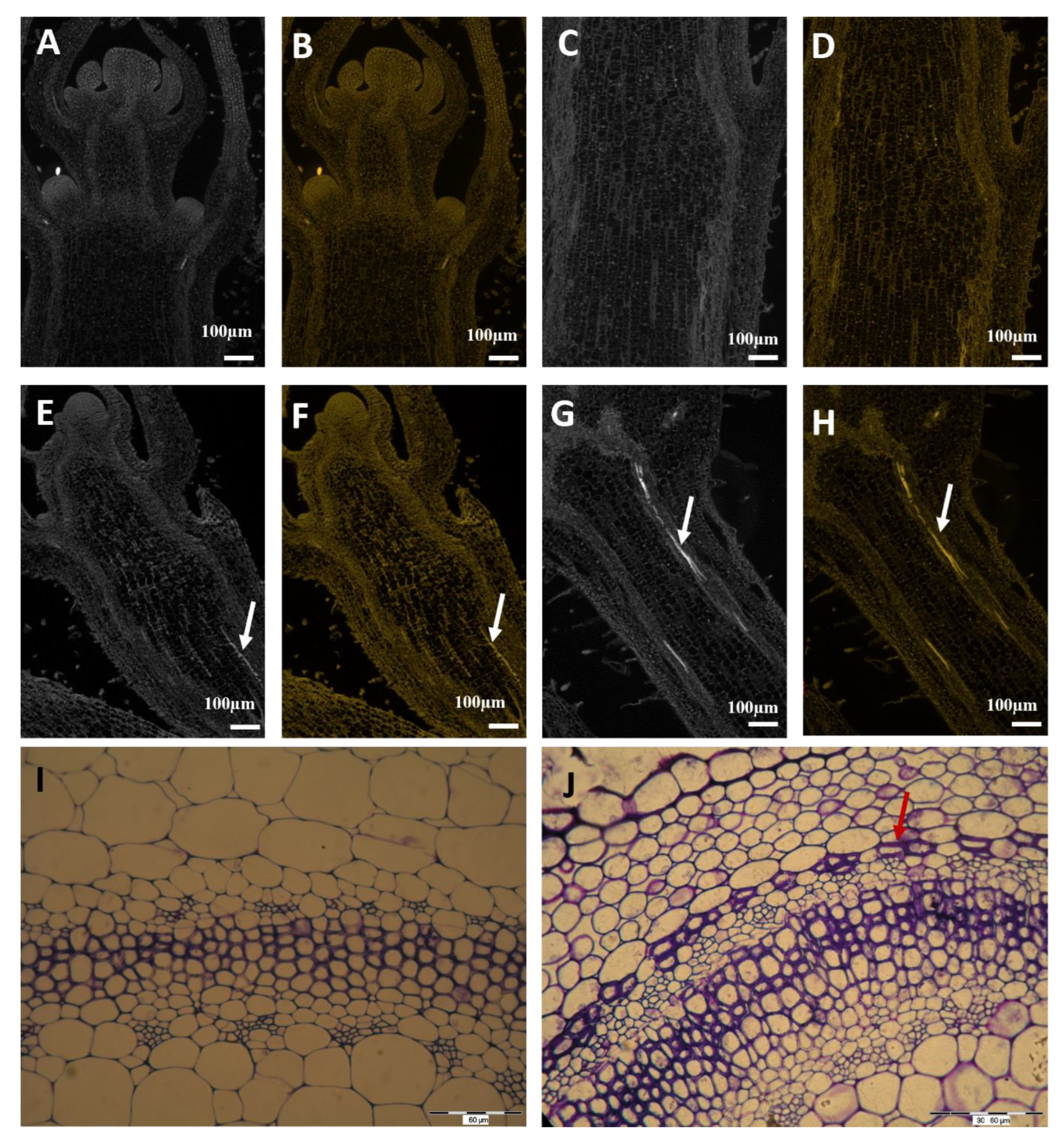
2.3. Immunohistochemistry of Auxin Carriers
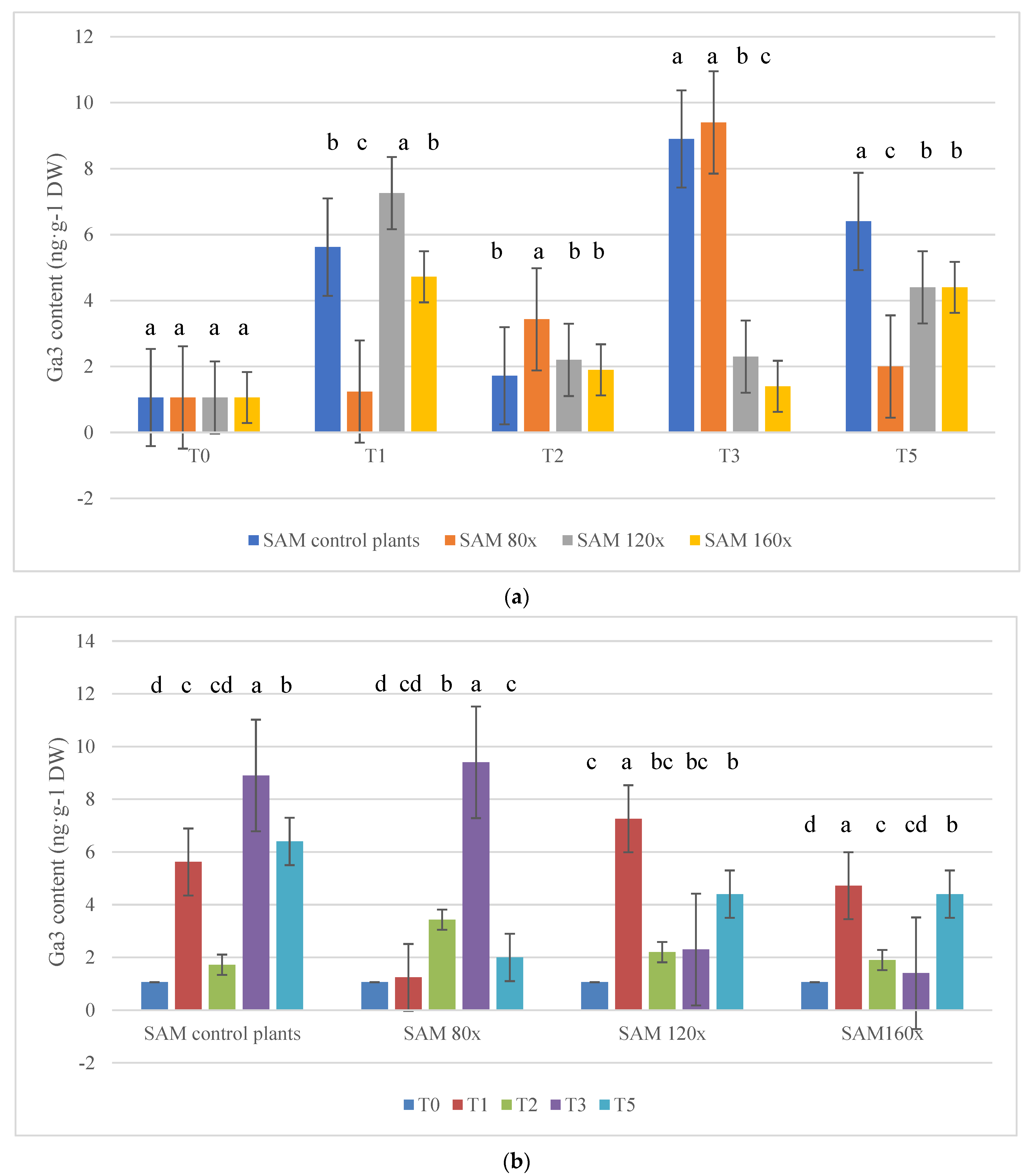
2.4. Gibberellic Acid (GA3) Content
2.5. Peroxidase Activity
2.6. Histological Determination of Cell Wall Lignification in Vascular Bundles of Stems and Roots
3. Discussion
4. Conclusions
- In the current research, we confirmed, that over a longer period of time, mechanical stimulation arrests growth dynamics and auxin as well as gibberellins synthesis in petunia.
- Mechanical stimulation does not arrest basipetal auxin transport.
- In the current research, we proved that one of the factors affecting the growth of petunia may be peroxidase activity, which is responsible for cell wall lignification and suberization in stems.
- In the current research, we proved that petunia plants subjected to mechanical stress 160 times a day clearly reduced their growth while they increased their diameter, which is an asset for the production of bedding plants such as petunia.
- The increase in growth dynamics in petunias after the cessation of mechanical stress is a clear physiological response of plants to return to a state of homeostasis.
- Besides POX activity, AGPs may also play an important role in cell wall modifications of MS-subjected plants.
- In the current study, we did not study the activity of AGPs in the SAM or RAM of petunia. In any case, there are no data in the literature on whether MS may increase AGPs production and has the same effect on cell wall modifications in plants, so it could be the next step to understand the physiological reaction of plants to MS.
5. Material and Methods
5.1. Experimental Design
5.2. Biometric Measurements and Biochemical Analyses
5.3. IAA Content
5.4. GA3 Content
5.5. Peroxidase (POD) Activity
5.6. Immunohistochemistry of Auxin Carriers
5.7. Safranin and Crystal Violet Staining
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaffe, M.J. Thigmomorphogenesis: The response of plant growth and development to mechanical stimulation. Planta 1973, 114, 143–157. [Google Scholar] [CrossRef]
- Chehab, E.W.; Eich, E.; Braam, J. Thigmomorphogenesis: A complex plant response to mechano-stimulation. J. Exp. Bot. 2009, 60, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Bauerle, W.L. Internode elongation and strobili production of Humulus lupulus cultivars in response to local strain sensing. Sci. Rep. 2021, 11, 9017. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.B.; Dun, E.A.; Ferguson, B.J.; Rameau, C.; Beveridge, C.A. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009, 150, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Gong, M. Mechanical stimulation-induced cross-adaptation in plants: An overview. J. Plant Biol. 2011, 54, 358–364. [Google Scholar] [CrossRef]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, C.; Wu, Z.; Jia, Y.; Wang, H.; Sun, S.; Wang, X. Abscisic acid regulates auxin homeostasis in rice root tips to promote root hair elongation. Front. Plant Sci. 2017, 8, 1121. [Google Scholar] [CrossRef]
- Lin, C.; Sauter, M. Polar auxin transport determines adventitious root emergence and growth in rice. Front. Plant Sci. 2019, 10, 444. [Google Scholar] [CrossRef]
- Cipollini, D.F., Jr. The induction of soluble peroxidase activity in bean leaves by wind-induced mechanical perturbation. Am. J. Bot. 1998, 85, 1586–1591. [Google Scholar] [CrossRef]
- Saidi, I.; Ammar, S.; Demont-Caulet, N.; Thévenin, J.; Lapierre, C.; Bouzid, S.; Jouanin, L. Thigmomorphogenesis in Solanum lycopersicum: Morphological and biochemical responses in stem after mechanical stimulation. Plant Signal. Behav. 2010, 5, 122–125. [Google Scholar] [CrossRef]
- Jędrzejuk, A.; Kuźma, N.; Nawrot, K.; Budzyński, R.; Orłowski, A. Mechanical stimulation affects growth dynamics, IAA content and activity of POD and IAA oxidase in Petunia × atkinsiana. Sci. Hortic. 2020, 274, 109661. [Google Scholar] [CrossRef]
- Prusinkiewicz, P.; Crawford, S.; Smith, R.S.; Ljung, K.; Bennett, T.; Ongaro, V.; Leyser, O. Control of bud activation by an auxin transport switch. Proc. Natl. Acad. Sci. USA 2009, 106, 17431–17436. [Google Scholar] [CrossRef] [PubMed]
- Balla, J.; Kalousek, P.; Reinöhl, V.; Friml, J.; Procházka, S. Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J. 2011, 65, 571–577. [Google Scholar] [CrossRef]
- Balla, J.; Medveďová, Z.; Kalousek, P.; Matiješčuková, N.; Friml, J.; Reinöhl, V.; Procházka, S. Auxin flow-mediated competition between axillary buds to restore apical dominance. Sci. Rep. 2016, 6, 35955. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Jiao, Y. A systems approach to understand shoot branching. Curr. Plant Biol. 2015, 3, 13–19. [Google Scholar] [CrossRef]
- Leyser, O. Plant hormones: Ins and outs of auxin transport. Curr. Biol. 1999, 9, 8–10. [Google Scholar] [CrossRef]
- Vieten, A.; Sauer, M.; Brewer, P.B.; Friml, J. Molecular and cellular aspects of auxin-transport-mediated development. LTWA 2007, 12, 160–168. [Google Scholar] [CrossRef]
- Telewski, F.W. Mechanosensing and plant growth regulators elicited during the thigmomorphogenetic response. Front. For. Glob. 2021, 3, 147. [Google Scholar] [CrossRef]
- Suge, H. Growth and gibberellin production in Phaseolus vulgaris as affected by mechanical stress. Plant Cell Physiol. 1978, 19, 1557–1560. [Google Scholar]
- Takahashi, H.; Suge, H. Sex expression in cucumber plants as affected by mechanical stress. Plant Cell Physiol. 1980, 21, 303–310. [Google Scholar] [CrossRef]
- Barley, R.; Waites, R. Plant meristems: The interplay of KNOX and gibberellins. Curr. Biol. 2002, 12, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Lee, S.C.; Shin, D.H.; Jang, S.W.; Nam, J.W.; Park, T.S.; Lee, I.J. Quantification of endogenous gibberellins in leaves and tubers of Chinese yam, Dioscorea opposita Thunb. cv. Tsukune during tuber enlargement. Plant Growth Regul. 2003, 39, 125–130. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef]
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal. Behav. 2013, 8, 25504. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Wang, X.; Fang, G.; Yang, J.; Li, Y. A thioredoxin-dependent glutathione peroxidase (OsGPX5) is required for rice normal development and salt stress tolerance. Plant Mol. Biol. Rep. 2017, 35, 333–342. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef]
- Mohan, R.; Vijayan, P.; Kolattukudy, P.E. Developmental and tissue-specific expression of a tomato anionic peroxidase (tap1) gene by a minimal promoter, with wound and pathogen induction by an additional 5′-flanking region. Plant Mol. Biol. 1993, 22, 475–490. [Google Scholar] [CrossRef]
- Quiroga, M.; Guerrero, C.; Botella, M.A.; Barceló, A.; Amaya, I.; Medina, M.I.; Valpuesta, V. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol. 2000, 122, 1119–1128. [Google Scholar] [CrossRef]
- Gladala-Kostarz, A. The Impact of Wind and Mechanical Stress on Growth and Development of Brachypodium Distachyon Stems. Ph.D. Thesis, Aberystwyth University, Aberystwyth, UK, 2019. [Google Scholar]
- Green, T.R.; Ryan, C.A. Wound-induced proteinase inhibitor in plant leaves: A possible defense mechanism against insects. Science 1972, 175, 776–777. [Google Scholar] [CrossRef]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 1997; p. 319. [Google Scholar]
- Braam, J. In touch: Plant responses to mechanical stimuli. New Phytol. 2005, 165, 373–389. [Google Scholar] [CrossRef]
- Chen, H.; Wilkerson, C.G.; Kuchar, J.A.; Phinney, B.S.; Howe, G.A. Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc. Natl. Acad. Sci. USA 2005, 102, 19237–19242. [Google Scholar] [CrossRef] [PubMed]
- Chehab, E.W.; Kaspi, R.; Savchenko, T.; Rowe, H.; Negre-Zakharov, F.; Kliebenstein, D.; Dehesh, K. Distinct roles of jasmonates and aldehydes in plant-defense responses. PLoS ONE 2008, 3, e1904. [Google Scholar] [CrossRef] [PubMed]
- Chehab, E.W.; Raman, G.; Walley, J.W.; Perea, J.V.; Banu, G.; Theg, S.; Dehesh, K. Rice Hydroperoxide lyases with unique expression patterns generate distinct aldehyde signatures in Arabidopsis. Plant Physiol. 2006, 141, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Biddington, N.L. The effects of mechanically-induced stress in plants—A review. J. Plant Growth Regul. 1986, 4, 103–123. [Google Scholar] [CrossRef]
- Telewski, F.W.; Jaffe, M.J. Thigmomorphogenesis: Anatomical, morphological and mechanical analysis of genetically different sibs of Pinus taeda in response to mechanical perturbation. Physiol. Plant. 1986, 66, 219–226. [Google Scholar] [CrossRef]
- Komatsu, K.; Maekawa, M.; Ujiie, S.; Satake, Y.; Furutani, I.; Okamoto, H.; Kyozuka, J. LAX and SPA: Major regulators of shoot branching in rice. Proc. Natl. Acad. Sci. USA 2003, 100, 11765–11770. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin–cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009, 69, 429–435. [Google Scholar] [CrossRef]
- Péret, B.; Swarup, K.; Ferguson, A.; Seth, M.; Yang, Y.; Dhondt, S.; Swarup, R. AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell Rep. 2012, 24, 2874–2885. [Google Scholar] [CrossRef]
- Telewski, F.W. Thigmomorphogenesis: The response of plants to mechanical perturbation. Italus Hortus 2016, 23, 1–16. [Google Scholar]
- Swarup, R.; Bhosale, R. Developmental roles of AUX1/LAX auxin influx carriers in plants. Front. Plant Sci. 2019, 10, 1306. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Victor, T.S.; Vanderhoef, L.N. Mechanical inhibition of hypocotyl elongation induces radial enlargement: Implications for cytokinin action. Plant Physiol. 1975, 56, 845–846. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.A. Influence of mechanical stress on auxin-stimulated growth of excised pea stem sections. Physiol. Plant. 1977, 41, 129–134. [Google Scholar] [CrossRef]
- Hofinger, M.; Chapelle, B.; Boyer, N.; Gaspar, T. GCMS identification and titration of IAA in mechanically perturbed Bryonia dioica. Plant Physiol. 1979, 63, 52. [Google Scholar]
- Reinhardt, D.; Pesce, E.R.; Stieger, P.; Mandel, T.; Baltensperger, K.; Bennett, M.; Kuhlemeier, C. Regulation of phyllotaxis by polar auxin transport. Nature 2003, 426, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Leyser, O. Dynamic integration of auxin transport and signalling. Curr. Biol. 2006, 16, 424–433. [Google Scholar] [CrossRef]
- Sieburth, L.E.; Deyholos, M.K. Vascular development: The long and winding road. Curr. Opin. Plant Biol. 2006, 9, 48–54. [Google Scholar] [CrossRef]
- Vanneste, S.; Friml, J. Auxin: A trigger for change in plant development. Cell 2009, 136, 1005–1016. [Google Scholar] [CrossRef]
- Wang, R.; Estelle, M. Diversity and specificity: Auxin perception and signaling through the TIR1/AFB pathway. Curr. Opin. Plant Biol. 2014, 21, 51–58. [Google Scholar] [CrossRef]
- Lavy, M.; Estelle, M. Mechanisms of auxin signaling. Development 2016, 143, 3226–3229. [Google Scholar] [CrossRef]
- Mason, M.G.; Ross, J.J.; Babst, B.A.; Wienclaw, B.N.; Beveridge, C.A. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. USA 2014, 111, 6092–6097. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Guan, S.C.; Wen, C.; Li, P.; Gao, Z.; Chen, X. Auxin and cytokinin coordinate the dormancy and outgrowth of axillary bud in strawberry runner. BMC Plant Biol. 2019, 19, 528. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nishimura, T.; Koshiba, T. Vigorous synthesis of indole-3-acetic acid in the apical very tip leads to a constant basipetal flow of the hormone in maize coleoptiles. Plant Sci. 2005, 168, 467–473. [Google Scholar] [CrossRef]
- Sun, T.P. Gibberellin metabolism, perception and signaling pathways in Arabidopsis. Arab. Book/Am. Soc. Plant Biol. 2008, 6, e01003. [Google Scholar] [CrossRef]
- Rinne, P.L.; Paul, L.K.; Vahala, J.; Kangasjärvi, J.; van der Schoot, C. Axillary buds are dwarfed shoots that tightly regulate GA pathway and GA-inducible 1,3-β-glucanase genes during branching in hybrid aspen. J. Exp. Bot. 2016, 67, 5975–5991. [Google Scholar] [CrossRef]
- Serova, T.A.; Tsyganova, A.V.; Tikhonovich, I.A.; Tsyganov, V.E. Gibberellins inhibit nodule senescence and stimulate nodule meristem bifurcation in pea (Pisum sativum L.). Front. Plant Sci. 2019, 10, 285. [Google Scholar] [CrossRef]
- Tanimoto, M.; Jowett, J.; Stirnberg, P.; Rouse, D.; Leyser, O. pax1-1 partially suppresses gain-of-function mutations in Arabidopsis AXR3/IAA17. BMC Plant Biol. 2007, 7, 20. [Google Scholar] [CrossRef]
- Ikeda, A.; Ueguchi-Tanaka, M.; Sonoda, Y.; Kitano, H.; Koshioka, M.; Futsuhara, Y.; Yamaguchi, J. Slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell Rep. 2001, 13, 999–1010. [Google Scholar] [CrossRef]
- Richards, D.E.; King, K.E.; Ait-Ali, T.; Harberd, N.P. How gibberellin regulates plant growth and development: A molecular genetic analysis of gibberellin signaling. Annu. Rev. Plant Biol. 2001, 52, 67–88. [Google Scholar] [CrossRef]
- Fu, X.; Harberd, N.P. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 2003, 421, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Richards, D.E.; Ait-Ali, T.; Hynes, L.W.; Ougham, H.; Peng, J.; Harberd, N.P. Gibberellin-mediated proteasome-dependent degradation of the barley DELLA protein SLN1 repressor. Plant Cell Rep. 2002, 14, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M. Gibberellin signaling: How do plant cells respond to GA signals? J. Plant Growth Regul. 2003, 22, 123–125. [Google Scholar] [CrossRef]
- Moore, R.; Dickey, K. Growth and graviresponsiveness of primary roots of Zea mays seedlings deficient in abscisic acid and gibberellic acid. J. Exp. Bot. 1985, 36, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Alvey, L.; Harberd, N.P. DELLA proteins: Integrators of multiple plant growth regulatory inputs? Physiol. Plant. 2005, 123, 153–160. [Google Scholar] [CrossRef]
- Normanly, J.; Grisafi, P.; Fink, G.R.; Bartel, B. Arabidopsis mutants resistant to the auxin effects of indole-3-acetonitrile are defective in the nitrilase encoded by the NIT1 gene. Plant Cell Rep. 1997, 9, 1781–1790. [Google Scholar]
- Ostin, A.; Kowalyczk, M.; Bhalerao, R.P.; Sandberg, G. Metabolism of indole-3-acetic acid in Arabidopsis. Plant Physiol. 1998, 118, 285–296. [Google Scholar] [CrossRef]
- Boyer, N.; Gaspar, T. Redistribution cellulaire des peroxydases de la Bryone à la suite d’une irritation tactile et d’un traitement. C. R. Acad. Sci. D 1980, 291, 577–580. [Google Scholar]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Potocka, I.; Szymanowska-Pułka, J. Morphological responses of plant roots to mechanical stress. Ann. Bot. 2018, 122, 711–723. [Google Scholar] [CrossRef]
- Jaffe, M.J.; Leopold, A.C. Callose deposition during gravitropism of Zea mays and Pisum sativum and its inhibition by 2-deoxy-D-glucose. Planta 1985, 161, 20–26. [Google Scholar] [CrossRef]
- Patterson, D.T. Effect of temperature and photoperiod on growth and reproductive development of goatsrue. J. Range Manag. Arch. 1992, 45, 449–453. [Google Scholar] [CrossRef]
- Beier, M.P.; Tsugawa, S.; Demura, T.; Fujiwara, T. Root shape adaptation to mechanical stress derived from unidirectional vibrations in Populus nigra. Plant Biotechnol. 2020, 37, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Takatani, S.; Motose, H.; Iida, H.; Takahashi, T. The root growth reduction in response to mechanical stress involves ethylene-mediated microtubule reorganization and transmembrane receptor-mediated signal transduction in Arabidopsis. Plant Cell Rep. 2021, 40, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Kouhen, M.; Dimitrova, A.; Scippa, G.S.; Trupiano, D. The Course of Mechanical Stress: Types, Perception, and Plant Response. Biology 2023, 12, 217. [Google Scholar] [CrossRef]
- Jacobsen, A.G.; Jervis, G.; Xu, J.; Topping, J.F.; Lindsey, K. Root growth responses to mechanical impedance are regulated by a network of ROS, ethylene and auxin signalling in Arabidopsis. New Phytol. 2021, 231, 225–242. [Google Scholar] [CrossRef]
- Singroha, G.; Sharma, P.; Sunkur, R. Current status of microRNA-mediated regulation of drought stress responses in cereals. Physiol. Plant. 2021, 172, 1808–1821. [Google Scholar] [CrossRef]
- Ellis, M.; Egelund, J.; Schultz, C.J.; Bacic, A. Arabinogalactan-proteins: Key regulators at the cell surface? Plant Physiol. 2010, 153, 403–419. [Google Scholar] [CrossRef]
- Showalter, A.M. Arabinogalactan-proteins: Structure, expression and function. Cell. Mol. Life Sci. 2001, 58, 1399–1417. [Google Scholar] [CrossRef]
- Dolan, L.; Roberts, K. The development of cell pattern in the root epidermis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1995, 350, 95–99. [Google Scholar]
- Itoh, J.I.; Nonomura, K.I.; Ikeda, K.; Yamaki, S.; Inukai, Y.; Yamagishi, H.; Nagato, Y. Rice plant development: From zygote to spikelet. Plant Cell Physiol. 2005, 46, 23–47. [Google Scholar] [CrossRef] [PubMed]
- Bossy, A.; Blaschek, W.; Classen, B. Characterization and immunolocalization of arabinogalactan-proteins in roots of Echinacea purpurea. Planta Med. 2009, 75, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, C.P.; Mansfield, S.D.; Stachurski, Z.H.; Evans, R.; Southerton, S.G. Fasciclin-like arabinogalactan proteins: Specialization for stem biomechanics and cell wall architecture in Arabidopsis and Eucalyptus. Plant J. 2010, 62, 689–703. [Google Scholar] [CrossRef]
- Nguema-Ona, E.; Coimbra, S.; Vicré-Gibouin, M.; Mollet, J.C.; Driouich, A. Arabinogalactan proteins in root and pollen-tube cells: Distribution and functional aspects. Ann. Bot. 2012, 110, 383–404. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.J. Fascinating fasciclins: A surprisingly widespread family of proteins that mediate interactions between the cell exterior and the cell surface. Int. J. Mol. Sci. 2018, 19, 1628. [Google Scholar] [CrossRef]
- Seifert, J.; Kirchhelle, C.; Moore, I.; Contera, S. Mapping cellular nanoscale viscoelasticity and relaxation times relevant to growth of living Arabidopsis thaliana plants using multifrequency AFM. Acta Biomater. 2021, 121, 371–382. [Google Scholar] [CrossRef]
- Tucker, M.R.; Lou, H.; Aubert, M.K.; Wilkinson, L.G.; Little, A.; Houston, K.; Shirley, N.J. Exploring the role of cell wall-related genes and polysaccharides during plant development. Plants 2018, 7, 42. [Google Scholar] [CrossRef]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef]
- Shi, H.; Kim, Y.S.; Guo, Y.; Stevenson, B.; Zhu, J.K. The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell Rep. 2003, 15, 19–32. [Google Scholar] [CrossRef]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of indole-3-acetic acid (IAA) production in Klebsiella by LC-MS/MS and the Salkowski method. Bio-Protocol 2019, 9, 3230. [Google Scholar] [CrossRef]
- Graham, H.D.; Thomas, L.B. Rapid, simple colorimetrie method for the determination of micro quantities of gibberellic acid. J. Pharm. Sci. 1961, 50, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Rorison, I.H. Root hairs and plant growth at low nitrogen availabilities. New Phytol. 1987, 107, 681–693. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrzejuk, A.; Kuźma, N.; Orłowski, A.; Budzyński, R.; Gehl, C.; Serek, M. Mechanical Stimulation Decreases Auxin and Gibberellic Acid Synthesis but Does Not Affect Auxin Transport in Axillary Buds; It Also Stimulates Peroxidase Activity in Petunia × atkinsiana. Molecules 2023, 28, 2714. https://doi.org/10.3390/molecules28062714
Jędrzejuk A, Kuźma N, Orłowski A, Budzyński R, Gehl C, Serek M. Mechanical Stimulation Decreases Auxin and Gibberellic Acid Synthesis but Does Not Affect Auxin Transport in Axillary Buds; It Also Stimulates Peroxidase Activity in Petunia × atkinsiana. Molecules. 2023; 28(6):2714. https://doi.org/10.3390/molecules28062714
Chicago/Turabian StyleJędrzejuk, Agata, Natalia Kuźma, Arkadiusz Orłowski, Robert Budzyński, Christian Gehl, and Margrethe Serek. 2023. "Mechanical Stimulation Decreases Auxin and Gibberellic Acid Synthesis but Does Not Affect Auxin Transport in Axillary Buds; It Also Stimulates Peroxidase Activity in Petunia × atkinsiana" Molecules 28, no. 6: 2714. https://doi.org/10.3390/molecules28062714
APA StyleJędrzejuk, A., Kuźma, N., Orłowski, A., Budzyński, R., Gehl, C., & Serek, M. (2023). Mechanical Stimulation Decreases Auxin and Gibberellic Acid Synthesis but Does Not Affect Auxin Transport in Axillary Buds; It Also Stimulates Peroxidase Activity in Petunia × atkinsiana. Molecules, 28(6), 2714. https://doi.org/10.3390/molecules28062714






