Abstract
Two series of novel steroidal[17,16-d]pyrimidines derived from natural epiandrosterone and androsterone were designed and synthesized, and these compounds were screened for their potential anticancer activities. The preliminary bioassay indicated that some of these prepared compounds exhibited significantly good cytotoxic activities against human gastric cancer (SGC-7901), lung cancer (A549), and hepatocellular liver carcinoma (HepG2) cell lines compared with 5-fluorouracil (5-FU), epiandrosterone, and androsterone. Especially the respective pairs from epiandrosterone and androsterone showed significantly different inhibitory activities, and the possible configuration-activity relationships have also been summarized and discussed based on kinase assay and molecular docking, which indicated that the inhibition activities of these steroidal[17,16-d]pyrimidines might obviously be affected by the configuration of the hydroxyl group in the part of the steroidal scaffold.
1. Introduction
Cancer is a disorder that rigorously affects the human population worldwide despite significant improvements in new treatment options [1,2], which is the second leading cause of death in the world. The increasing multidrug resistance and side effects have become an important cause of clinical death in the twenty-first century. Although there are many effective therapies for cancer control, chemotherapy remains the main option to treat cancer disease.
It is well known that pyrimidine is a class of aromatic heterocyclic compounds that contain two nitrogen atoms at positions 1 and 3 of the six-membered ring. Heterocyclic compounds bearing the pyrimidine core are of tremendous interest because the pyrimidine structure, which is an important part of many endogenous substances, can easily interact with enzymes, genetic materials, and bio-components within the cell [3,4]. Up to now, many natural and synthetic pyrimidines derivatives have been of enormous importance and demonstrate a variety of pharmacological activities, including anticancer [5,6,7,8,9,10,11], antiviral [12,13,14,15], antifungal [16,17,18], antioxidant [19,20,21,22,23], antibacterial [24,25,26], antituberculosis [27,28], anticonvulsant [29,30,31,32,33,34], antimalarial [35,36,37,38,39,40], antihypertensive [41,42,43,44,45], and anti-inflammatory [46,47,48,49,50,51]. Pyrimidine scaffolds are privileged heterocycles in drug discovery because they have considerable pharmacological and chemical significance and are also easily soluble in water [41]. Moreover, many disubstituted pyrimidines are also used as successful moieties to construct novel functional molecules [4]. Many scientists have focused on the discovery and structural optimization of pyrimidine derivatives, and so many novel pyrimidine derivatives shown in Figure 1 have been discovered [2,3,52,53,54,55,56]. Pyrimidine derivatives have gained a great deal of attention, especially for their considerable antiproliferative activity [2,57,58,59].
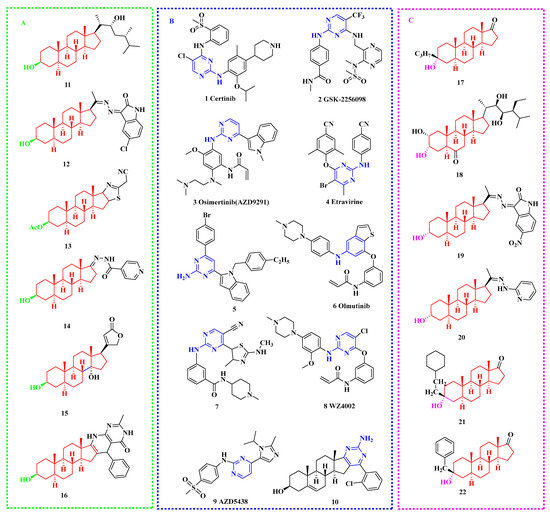
Figure 1.
Chemical structures of typical compounds with antiproliferative activity (A) epiandrosterone derivatives; (B) 2-aminopyrimidine derivatives; (C) androsterone derivatives.
In addition, natural steroids from animal and plant metabolites are types of important secondary metabolites that are widely distributed in marine environments and are extremely important biomarkers in the field of marine chemistry. In the marine sedimentary environment, the growth and reproduction of in-situ plankton and the input of terrestrial higher plant debris are the main sources of steroids. In the past few years, many steroids have been used as prototype scaffolds for constructing diverse molecules with extensive pharmaceutical activities, especially for dehydroepiandrosterone (DHEA), epiandrosterone (EPIA), and androsterone (AND) steroids [60,61,62]. Some of these novel steroidal derivatives showed significant inhibitory activities on human tumor cells in culture, suggesting that the natural four fused rings scaffold of steroids might be closely bound up with the cytotoxic activity [63,64,65,66,67]. Some of the EPIA and AND derivatives [67,68,69,70,71,72,73,74,75] that exhibited significant inhibition on cancer cell lines are shown in Figure 1A and Figure 1C, respectively.
Based on these observations, two series of steroidal[17,16-d]pyrimidines derived from epiandrosterone and androsterone were also designed and synthesized, which integrate the structural features of pyrimidines and EPIA/AND unit to a core molecule as indicated in Figure 2, and their cytotoxic effects on tumor cell lines (HepG2, A549, SGC-7901) were fully investigated by MTT colorimetric method, and the possible configuration-activity relationships have also been summarized and discussed based on the activity data, kinase assay, and molecular docking. These results may provide much useful information for the discovery of novel cytotoxic agents from natural steroids.

Figure 2.
Design strategy of steroidal[17,16-d]pyrimidines.
2. Results and Discussion
2.1. Chemistry
In this work, two series of heterocyclic steroidal[17,16-d]pyrimidines derived from epiandrosterone and androsterone were conveniently prepared, and the general method for the preparation of these steroidal[17,16-d]pyrimidines derivatives 3a–l and 6a–l is described in Scheme 1.
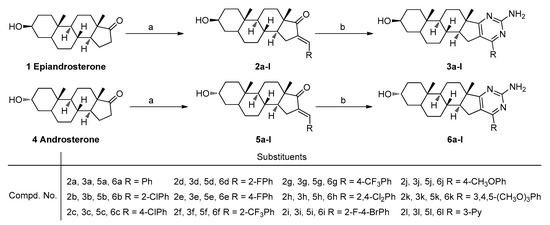
Scheme 1.
Synthetic route for steroidal[17,16-d]pyrimidine derivatives. Reagents and conditions: a. RCHO, NaOH, MeOH, rt, yield 83–94%; b. Guanidine nitrate, tBuOK, tBuOH, reflux, yield 61–91%.
According to Scheme 1, these steroidal[17,16-d]pyrimidines derivatives 3a–l and 6a–l have been conveniently synthesized via a two-step transformation from epiandrosterone or androsterone, respectively. First, the various substituted aromatic aldehydes were treated with epiandrosterone or androsterone via aldol condensation to obtain the intermediates 2a–l and 5a–l. Subsequently, the reaction of intermediates 2a–l and 5a–l with guanidine nitrate in the presence of potassium tert-butoxide have been conveniently processed to obtain the target molecules.
All the newly synthesized steroidal[17,16-d]pyrimidines 3a–l and 6a–l gave satisfactory chemical analyses, including 1H NMR, 13C NMR, and ESI-MS spectra analyses, and the chemical structures and physiochemical properties of these compounds were summarized in the experimental (Some of them have been collected in the Supplementary Materials). For NMR analyses, the assignments of different signals are based on the chemical shifts and intensity patterns. All 1H NMR spectra of molecules 3a–l and 6a–l presented distinctive signals of methine proton attached to the hydroxyl group, which always indicated a multiplet or broad singlet at about 3.29–3.55 ppm and 3.77–3.82 ppm, respectively. The signal for protons of the hydroxyl group showed doublet peaks at about 4.43–4.48 ppm in compounds 3a–l, but at 4.16–4.19 ppm in compounds 6a–l. The signal for protons of the 2-amino group attached to the pyrimidine ring resonated almost the same as a singlet between 5.36 ppm and 6.58 ppm. The other set of signals that appeared in their 1H NMR spectra in the ranges 2.79–0.69 ppm belonged to the protons of epiandrosterone or androsterone scaffold, and the signals at lower fields were assigned to the signals of aromatic protons as described in general structures in Scheme 1. The 13C NMR analysis of molecules 3a–l and 6a–l display obvious peaks in the alkyl region, indicating the presence of the epiandrosterone or androsterone scaffold, respectively. Other peaks appearing at lower fields were assigned to the carbon signals of the aromatic and heterocyclic moieties. The electron spray impact mass spectra (ESI-MS) for compounds 2a–l and 6a–l were measured on a WATERS ACQUITY UPLC® H-CLASS PDA (Waters®) instrument, and the ESI-MS of target molecules exhibited obvious molecular peak [M + H]+ in the positive ion mode. All the characteristic peaks observed within the 1H NMR and 13C NMR spectra for title compounds are given in the experimental section.
2.2. Pharmacology Evaluation
2.2.1. Inhibitory Effects of the Target Compounds
All newly synthesized steroidal[17,16-d]pyrimidines derivatives 3a–l, 6a–l and the intermediates 2a–l, 5a–l were evaluated for their potential in vitro cytotoxic effects on human gastric cancer (SGC-7901), lung cancer (A549), and hepatocellular liver carcinoma (HepG2) cell lines by the standard MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay [76] using 5-FU (5-Fluorouracil) as a positive control. The preliminary screening results are summarized in the following Figure 3. Generally, as shown in Figure 3, we can find most of these steroidal derivatives displayed well in vitro cytotoxic activities against three human cancer cell lines except compounds 2l, 5g, 5h, 5i, 5j, 5l, 6g, and 6l. Notably, the compounds 3a–l exhibited obviously inhibitory activities against all tested cell lines with 75.0–84.1% growth inhibition at the concentration of 40 µg/mL. From Figure 3, we also can observe that the steroidal[17,16-d]pyrimidines (3a–l) derived from epiandrosterone presented significantly better inhibitory activities than that of steroidal[17,16-d]pyrimidines (6a–l) derived from androsterone.
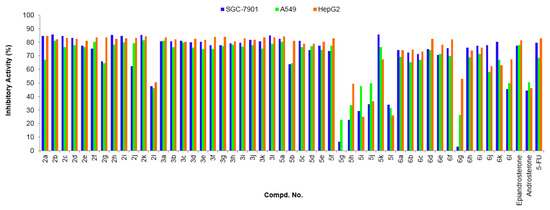
Figure 3.
Antitumor activities of compounds 2a–l, 3a–l, 5a–l, and 6a–l at 40 µg/mL. Abbreviations: SGC-7901—Human gastric cancer cell line, A549—Human lung adenocarcinoma cell line; HepG2—human hepatocellular liver carcinoma cell line; 5-FU—5-Fluorouracil, used as a positive control.
The preliminary assay demonstrated that many of these novel steroidal derivatives displayed good inhibitory activities (Figure 3), so in order to further clarify the potential activities, the IC50 values for all molecules were fully evaluated. The cytotoxic activities expressed as IC50 values for all molecules are described in Table 1, which further confirmed that all the steroidal[17,16-d]pyrimidine derivatives 3a–l exhibited higher inhibition activity than that of compounds 6a–l and the commercial 5-FU under the same conditions, respectively.

Table 1.
Cytotoxic activity of the steroidal derivatives against human tumor cells.
As indicated in Table 1, compounds 2b, 2d, 2h, 2i, 2k, 3a–l, 5c–e, and 6b have higher cytotoxicity activities (Entries 2, 4, 8, 9, 13–24, 27–29, and 38) against all tested cell lines. In particular, the compounds 3a, 3b, 3d, and 3k derived from epiandrosterone exhibited significant inhibition (Entries 13, 14, 16, and 23) on all cancer cell lines compared to the positive control 5-FU, the activities of which were also more successful than those of the corresponding 6a, 6b, 6d, and 6k derived from androsterone. On the whole, the activities of molecules 3a–l are superior to that of molecules 6a–l, which confirms that the configuration of the molecule might have a significant effect on the inhibition and that the compounds with epiandrosterone scaffold are favorable for inhibition activities.
Moreover, the dose-response analysis of cell growth inhibition activities for high potential compounds 3a, 3b, 3d, 3k, and 5-FU have been displayed in Figure 4, which indicated that the target compounds significantly inhibited SGC-7901, A549, and HepG2 cell proliferation in a concentration-dependent manner. In particular, compound 3a containing a phenyl unit exhibited the highest potential inhibitory activities against all tested cell lines with the IC50 values of 1.07 ± 0.22, 0.61 ± 0.19, and 0.51 ± 0.13 µg·mL−1 (Entry 13 in Table 1), respectively, which was significantly better than that of the control 5-FU and epiandrosterone.
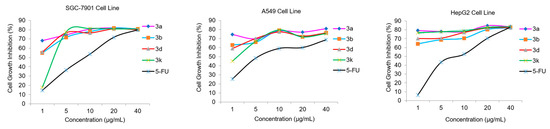
Figure 4.
Dose-response analysis of cell growth inhibition activity for the potential compounds 3a, 3b, 3d, 3k, and 5-FU (positive control) against SGC-7901, A549, and HepG2 cell lines.
2.2.2. Selectivity Profiling of Compound 3a
Compound 3a was selected as a representative to further investigate the kinase selectivity profile against a panel of tyrosine kinases (Table 2). The kinase inhibitory activities of compound 3a (at the concentration of 10 μM) were determined by ADP-Glo Protocol or LANCE Protocol. As shown in Table 2, compounds 3a displayed some inhibitory effect on the kinases of CDK1/CyclinA2, ALK, FGFR1, and FAK with inhibition rates of 22.51%, 17.36%, 11.82%, and 10.52%, respectively.

Table 2.
Selectivity profiling of compound 3a against a panel of tyrosine kinases.
2.2.3. Structure and Activity Relationships (SARs) and Molecular Docking
The structure evolution here was to modify epiandrosterone or androsterone scaffold with a pyrimidine ring system (3a–1, 6a–l) and aromatic enones (2a–l, 5a–l), respectively. According to the aforementioned results indicated in Table 1, we can obtain the general structure-activity profile for these novel steroidal[17,16-d]pyrimidine derivatives (Figure 5).
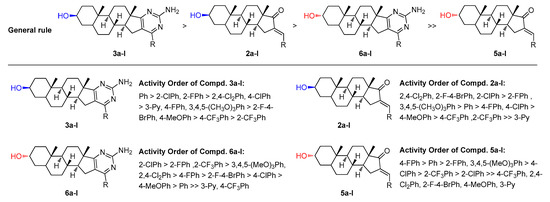
Figure 5.
General structure-activity profile for the steroidal derivatives used in this study.
Generally, as indicated in Figure 5, the compounds containing pyrimidine ring systems exhibit better inhibitory activity than the compounds modified by aromatic enones, which testifies to the potential importance of a pyrimidine core. We also can find that compounds 3a–l exhibited higher inhibition activity than the derivatives 6a–l. In addition, for the compounds containing a pyrimidine ring system, the compounds bearing 2-ClPh, 2-FPh, 2-CF3Ph, 2,4-Cl2Ph, 4-ClPh, 4-FPh, 3,4,5-(MeO)3Ph and 2-F-4-BrPh group present the higher potential activities. However, when the substituents R are Ph and 4-CH3OPh, there is a significant difference in efficacy between heterocyclic steroidal[17,16-d]pyrimidines derived from epiandrosterone and androsterone. With substituents R are 3-Py and 4-CF3Ph especially, the compounds derived from androsterone obviously decreased the cytotoxic activity. On the other hand, for the compounds containing aromatic enone moiety, the results showed that compounds containing 2-FPh, Ph, 4-FPh, 4-ClPh, and 3,4,5-(MeO)3Ph group exhibited higher activity than the compounds bearing other substituents. However, the two series of substituted benzylidene derived from epiandrosterone and androsterone showed a significant difference in efficacy when the substituents R are 4-CF3Ph, 2,4-Cl2Ph, 2-F-4-BrPh, 4-CH3OPh and 3-Py. From Table 1, we also can find that, within the series of halogen derivatives, it is clear that the ortho-substituted compounds 2b, 2d, 3b, 3d, 6b, and 6d perform better than the para-substituted compounds 2c, 2e, 3c, 3e, 6c, 6e, respectively. In particular, most of the compounds containing 4-ClPh, 2-FPh, 4-FPh, and 3,4,5-(MeO)3Ph group (2c, 2d, 2e, 2k, 3c, 3d, 3e, 3k, 5c, 5d, 5e, 5k, 6c, 6d, 6e and 6k) present good inhibitory activities in these four systems, which may be due to the steric size of substituted groups are favourable for the binding to the potential receptor. Compared with steroidal[17,16-d]pyrimidines derived from dehydroepiandrosterone, we also can find that the double bond between C5 and C6 in the B ring decreases the cytotoxic activity [65].
As indicated in Table 2, compound 3a displayed some inhibitory effect on the kinases of CDK1/CyclinA2, ALK, FGFR1, and FAK. In addition, it is widely known that the cyclin-dependent protein kinase (CDK) has diverse cellular roles, including regulation of the cell cycle and transcription and differentiation. It also plays a crucial role in regulation of the growth, proliferation, and differentiation of cancer cells. Blocking the CDK-driven pathway by inhibiting the intracellular tyrosine kinase domain of CDK has resulted in considerable improvements in tumor therapy. In particular, a variety of pyrimidine derivatives were synthesized and evaluated for their abilities to target CDK tyrosine kinases, such as AZD5438 (shown in Figure 1B) and dinaciciclib. In order to find a deeper explanation of the structure and inhibitory activities of these steroidal[17,16-d]pyrimidine derivatives, the possible docking modes of compounds 3a, 3b, 3l, 6a, 6b, and 6l with CDK1 (PDB code: 6GU6) were modeled (Figure 6, Figure 7, Figure 8 and Figure 9).
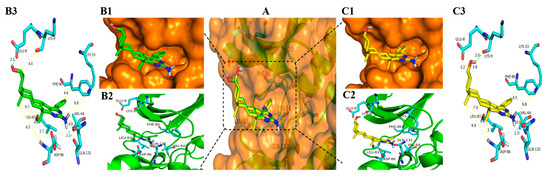
Figure 6.
Docking modes of 3a and 6a with CDK1 (PDB code: 6GU6). (A) Overview of the binding site of 3a (green stick) and 6a (yellow stick) with CDK1 (orange transparent surface structure fused with a sticks structure of white hydrogen atoms, cyan carbon atoms, blue nitrogen atoms, and red oxygen atoms); (B1) Position of 3a in the orange surface structure of CDK1; (B2) Position of 3a in cartoon structure of CDK1; (B3) Binding model for 3a bond to CDK1 (Hydrogen bonds are drawn as green dashed lines with distances labelled in Å while the yellow dashed lines just display the distance between the atoms); (C1) Position of 6a in the orange surface structure of CDK1; (C2) Position of 6a in cartoon structure of CDK1; (C3) Binding model for 6a bond to CDK1 (Hydrogen bonds are drawn as green dashed lines with distances labelled in Å while the yellow dashed lines just display the distance between the atoms). Figures were drawn with Pymol (Schrödinger).
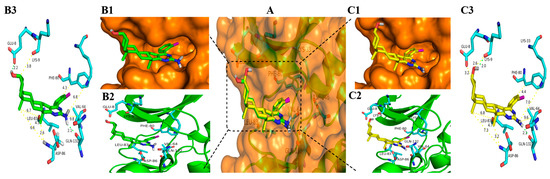
Figure 7.
Docking modes of 3b and 6b with CDK1 (PDB code: 6GU6). (A) Overview of the binding site of 3b (green stick) and 6b (yellow stick) with CDK1 (orange transparent surface structure fused with a sticks structure of white hydrogen atoms, cyan carbon atoms, blue nitrogen atoms, red oxygen atoms, and purple chlorine atoms); (B1) Position of 3b in the orange surface structure of CDK1; (B2) Position of 3b in cartoon structure of CDK1; (B3) Binding model for the 3b bond to CDK1 (Hydrogen bonds are drawn as green dashed lines with distances labelled in Å while the yellow dashed lines just display the distance between the atoms); (C1) Position of 6b in the orange surface structure of CDK1; (C2) Position of 6b in cartoon structure of CDK1; (C3) Binding model for the 6b bond to CDK1 (Hydrogen bonds are drawn as green dashed lines with distances labelled in Å while the yellow dashed lines just display the distance between the atoms). Figures were drawn with Pymol (Schrödinger).
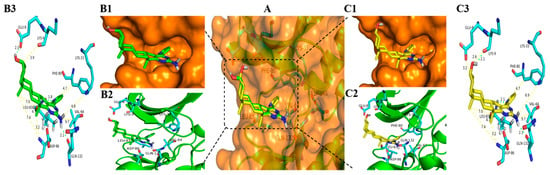
Figure 8.
Docking modes of 3l and 6l with CDK1 (PDB code: 6GU6). (A) Overview of the binding site of 3l (green stick) and 6l (yellow stick) with CDK1 (orange transparent surface structure fused with a sticks structure of white hydrogen atoms, cyan carbon atoms, blue nitrogen atoms, and red oxygen atoms); (B1) Position of 3l in the orange surface structure of CDK1; (B2) Position of 3l in cartoon structure of CDK1; (B3) Binding model for 3l bond to CDK1 (Hydrogen bonds are drawn as green dashed lines with distances labelled in Å while the yellow dashed lines just display the distance between the atoms); (C1) Position of 6l in the orange surface structure of CDK1; (C2) Position of 6l in cartoon structure of CDK1; (C3) Binding model for 6l bond to CDK1 (Hydrogen bonds are drawn as green dashed lines with distances labelled in Å while the yellow dashed lines just display the distance between the atoms). Figures were drawn with Pymol (Schrödinger).
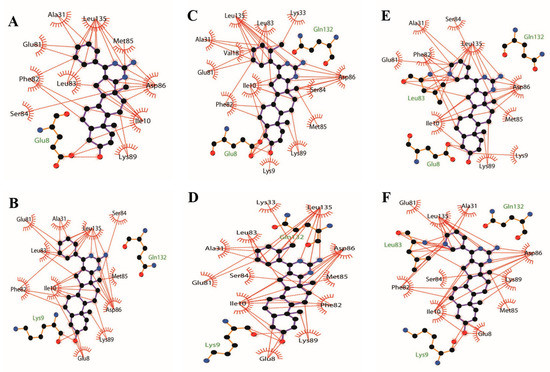
Figure 9.
The 2D docked model of compounds 3a, 3b, 3l, 6a, 6b, and 6l into the binding pocket of CDK1 kinase (PDB code: 6GU6, black carbon atoms, red oxygen atoms, yellow sulphur atoms, and blue nitrogen atoms. Brick red dashed lines just display the hydrophobic interaction between the atoms and the amino acids of CDK1). (A) Hydrophobic interaction between the atoms of 3a and the amino acids of CDK1; (B) Hydrophobic interaction between the atoms of 6a and the amino acids of CDK1; (C) Hydrophobic interaction between the atoms of 3b and the amino acids of CDK1; (D) Hydrophobic interaction between the atoms of 6b and the amino acids of CDK1; (E) Hydrophobic interaction between the atoms of 3l and the amino acids of CDK1; (F) Hydrophobic interaction between the atoms of 6l and the amino acids of CDK1. Figures were drawn with LigPlot+.
As shown in Figure 6B1,C1, Figure 7B1,C1 and Figure 8B1,C1, the six compounds 3a, 3b, 3l, 6a, 6b, and 6l almost bound to the outer edge of the ATP-binding pocket sandwiched between the P-loop (Gly 11-Glu 12-Gly 13-Thr 14-Phe 15-Gly 16) and active loop with the protein backbone from Gln 132 to Gly 145, but were far from the active segment spans with the protein backbone from Asp 146 to Glu 173 that can form a platform recognizing the CDK1 substrate residues to either side of the site phosphotransfer. In addition, the Lys 33-Glu12-Asp 146 triad in the bottom of the binding pocket of CDK1 also impacts the binding of the ATP adenine ring and phosphate moieties [77]. The hydrogen atom from the amidogen of the pyrimidine ring of compounds 3a, 3b, 3l, 6a, 6b, and 6l was far from Lys 33 with a different distance from 6.8 Å to 7.0 Å. These may be the reasons that compounds displayed lower inhibitory effects on the kinases of CDK1/CyclinA2.
It is clear that hydrogen bonds play an important role in the inhibition activities of compounds. The hydrogen atom from the amidogen of the pyrimidine ring of compounds 3a, 3b, 3l, 6a, 6b, and 6l can form one hydrogen bond with the oxygen atom of Gln 132 with the same distance of 2.1 Å shown in Figure 6B3,C3, Figure 7B3,C3 and Figure 8B3,C3. As indicated in Figure 6, Figure 7 and Figure 8, the hydroxyl group of compounds 3a, 3b, and 3l was oriented toward the Glu8 of CDK1, but the hydroxyl group of compounds 6a, 6b, and 6l was oriented toward the Lys 9 of CDK1. As a result, the hydrogen atom of the hydroxyl group of compounds 3a, 3b, and 3l from epiandrosterone and compounds 6a, 6b, and 6l from androsterone can form one different hydrogen bond with the oxygen atom of Glu 8 and Lys 9 with different distances, respectively. The hydrogen bond of the hydroxyl group from compound 3a is stronger than 3b and 3l with a shorter length of 2.1 Å, 2.2 Å, and 2.3 Å, respectively. Meanwhile, the significant difference in efficacy of compound 6l was mainly due to the longest distance (2.1 Å) of the hydrogen bond in the hydroxyl group, while the length of the hydrogen bond of the hydroxyl group of compounds 6a and 6b was 2.0 Å.
Additionally, hydrophobic forces also influence the interaction of inhibitors with kinases. A hydrophobic interaction between the non-polar residues of CDK1 and the atoms of steroidal[17,16-d]pyrimidine derivatives is shown in Figure 9. In this example, Ile 10, Phe 82, Lys 89, Asp 86, and Leu 83 interact with A, B, C, and D, four fused ring scaffolds of steroids. The methyl of C19 only interacts with Lys 89, while the methyl of C18 interacts with Ser 84, Met 85, and Asp 86. Both the methyls are oriented toward the hinge region of CDK1 with the protein backbone of Phe 80, Glu 81, Phe 82, Leu 83, Ser 84, Met 85, and Asp 86 (shown in Figure 6B2,C2, Figure 7B2,C2 and Figure 8B2,C2). The pyrimidine ring interacted with Ile10, Asp 86, and Leu 135, while the R-substituent group interacted with Ala 31, Glu 81, Phe 82, Leu 83, and Leu 135.
The analysis from above helps to explain that all the steroidal[17,16-d]pyrimidine derivatives 3a–l exhibited higher inhibition activity than that of compounds 6a–l, respectively. On the one hand, it occupied more space in the binding pocket when the hydroxyl group was oriented towards Glu 8 (shown in Figure 6B1,C1, Figure 7B1,C1 and Figure 8B1,C1). On the other hand, it had stronger hydrophobic force due to the skeleton of steroidal[17,16-d]pyrimidines near the hinge region of CDK1, especially the methyl of C18 (shown in Figure 6B3,C3, Figure 7B3,C3 and Figure 8B3,C3 and Figure 9). The above analysis also helped to explain why compound 3a exhibited the highest potent inhibitory activities on all tested cell lines (Entry 13 in Table 1).
The analysis indicated that the configuration of hydroxy in the C3 position might be a key influence on the inhibitory activities of compounds. The molecular docking analyses were helpful in identifying the target steroidal[17,16-d]pyrimidines derived from epiandrosterone that could serve as potential lead compounds for the discovery of anticancer agents.
3. Conclusions
Twenty-four steroidal[17,16-d]pyrimidines derived from epiandrosterone(3a–l) and androsterone(6a–l) were designed and synthesized, and their in vitro inhibition activities on three cell lines were investigated. All the steroidal[17,16-d]pyrimidine derivatives 3a–l exhibited higher inhibition activities against SGC-7901, A549, and HepG2 cell lines than that of compounds 6a–l with the same substituents, respectively. Compound 3a, containing a phenyl unit, exhibited the highest potential inhibitory activities against all tested cell lines with the IC50 values of 1.07 ± 0.22, 0.61 ± 0.19, and 0.51 ± 0.13 µg·mL−1. In addition, the detailed SARs analysis based on the inhibition activities, kinase assay, and molecular docking model demonstrated that the configuration of the hydroxyl group in the C3 position of A ring of steroidal scaffold might obviously affect the potential activities of these steroidal[17,16-d]pyrimidines. The β-configuration of the hydroxyl group of steroidal[17,16-d]pyrimidines performed better than an α-configuration of the hydroxyl group with the reason that it occupied more space in the binding pocket when the hydroxyl group was oriented towards Glu 8, and it also had stronger hydrophobic force due to the proximity of the skeleton of steroidal[17,16-d]pyrimidines to the hinge region of CDK1, and especially to the methyl of C18, which will provide key evidence for further structural optimization for the discovery of novel anticancer agents.
4. Experimental
4.1. Instrumentation and Chemicals
All starting materials and reagents are commercially available and were used without further purification unless otherwise specified. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance III 600 MHz FT-NMR spectrometer using DMSO-d6 or CD3OD as the solvent and tetramethylsilane (TMS) as the internal standard. Chemical shifts were reported in δ (parts per million) values, and coupling constants nJ were reported in Hz. Mass spectra were performed on a WATERS ACQUITY UPLC® H-CLASS PDA (Waters®) instrument. Analytical thin-layer chromatography was carried out on precoated silica gel plates GF254 (Qindao Haiyang Chemical, China), and spots were visualized with ultraviolet light. The calculated logP values (logP), which are the logarithms of the partition coefficients for octan-1-ol/water, were determined using the CS ChemOffice Ultra program (version 12.0, Cambridge-Soft, Cambridge, MA, USA).
4.2. Chemical Synthesis
4.2.1. General Synthetic Procedure for Intermediates 2a–l and 5a–l
The intermediates 2a–l and 5a–l were synthesized via the classical aldol reaction. Generally, a solution of epiandrosterone or androsterone (1 mmol) in methanol (15 mL) was added to appropriate aldehyde (1.1 mmol) and sodium hydroxide (0.4 g, 10 mmol), respectively. The mixture was stirred at room temperature and detected by thin-layer chromatography. After the completion of this reaction, the mixture was poured into 40 mL of ice water with stirring. Then the precipitate was filtered and dried to obtain the corresponding powder 2a–l and 5a–l. Their basic physico-chemical properties and spectra data are as follows:
16-Benzylidene-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 2a
This compound was obtained following the above method as a white powder, yield 93%. MS (ESI) m/z 379.5 (M + H)+, calcd. for C26H34O2 m/z = 378.2.
16-(2-Chlorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 2b
This compound was obtained following the above method as a white powder, yield 92%. MS (ESI) m/z 413.4 (M + H)+, calcd. for C26H33ClO2 m/z = 412.2.
16-(4-Chlorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 2c
This compound was obtained following the above method as a white powder, yield 89%. MS (ESI) m/z 413.4 (M + H)+, calcd. for C26H33ClO2 m/z = 412.2.
16-(2-Fluorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 2d
This compound was obtained following the above method as a white powder, yield 94%. MS (ESI) m/z 397.4 (M + H)+, calcd. for C26H33FO2 m/z = 396.2.
16-(4-Fluorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 2e
This compound was obtained following the above method as a white powder, yield 92%. MS (ESI) m/z 397.4 (M + H)+, calcd. for C26H33FO2 m/z = 396.2.
16-(2-Trifluorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 2f
This compound was obtained following the above method as a white powder, yield 86%. MS (ESI) m/z 447.4 (M + H)+, calcd. for C27H33F3O2 m/z = 446.2.
16-(4-Trifluorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 2g
This compound was obtained following the above method as a white powder, yield 83%. MS (ESI) m/z 447.5 (M + H)+, calcd. for C27H33F3O2 m/z = 446.2.
16-(2,4-Dichlorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 2h
This compound was obtained following the above method as a white powder, yield 83%. MS (ESI) m/z 447.4 (M + H)+, calcd. for C26H32Cl2O2 m/z = 446.2.
16-(2-Fluoro-4-bromobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 2i
This compound was obtained following the above method as a white powder, yield 90%. MS (ESI) m/z 475.4 (M + H)+, calcd. for C26H32BrFO2 m/z = 474.2.
16-(4-Methoxybenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 2j
This compound was obtained following the above method as a white powder, yield 93%. MS (ESI) m/z 409.5 (M + H)+, calcd. for C27H36O3 m/z = 408.3.
16-(3,4,5-Trimethoxybenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 2k
This compound was obtained following the above method as a white powder, yield 87%. MS (ESI) m/z 469.5 (M + H)+, calcd. for C29H40O5 m/z = 468.3.
16-(3-Pyridinmethylene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 2l
This compound was obtained following the above method as a white powder, yield 85%. MS (ESI) m/z 380.3 (M + H)+, calcd. for C25H33NO2 m/z = 379.3.
16-Benzylidene-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 5a
This compound was obtained following the above method as a white powder, yield 89%. MS (ESI) m/z 379.5 (M + H)+, calcd. for C26H34O2 m/z = 378.2.
16-(2-Chlorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 5b
This compound was obtained following the above method as a white powder, yield 91%. MS (ESI) m/z 413.4 (M + H)+, calcd. for C26H33ClO2 m/z = 412.2.
16-(4-Chlorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 5c
This compound was obtained following the above method as a white powder, yield 86%. MS (ESI) m/z 413.4 (M + H)+, calcd. for C26H33ClO2 m/z = 412.2.
16-(2-Fluorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 5d
This compound was obtained following the above method as a white powder, yield 90%. MS (ESI) m/z 397.4 (M + H)+, calcd. for C26H33FO2 m/z = 396.2.
16-(4-Fluorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 5e
This compound was obtained following the above method as a white powder, yield 93%. MS (ESI) m/z 397.4 (M + H)+, calcd. for C26H33FO2 m/z = 396.2.
16-(2-Trifluorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 5f
This compound was obtained following the above method as a white powder, yield 92%. MS (ESI) m/z 447.4 (M + H)+, calcd. for C27H33F3O2 m/z = 446.2.
16-(4-Trifluorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 5g
This compound was obtained following the above method as a white powder, yield 88%. MS (ESI) m/z 447.5 (M + H)+, calcd. for C27H33F3O2 m/z = 446.2.
16-(2,4-Dichlorobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 5h
This compound was obtained following the above method as a white powder, yield 91%. MS (ESI) m/z 447.4 (M + H)+, calcd. for C26H32Cl2O2 m/z = 446.2.
16-(2-Fluoro-4-bromobenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 5i
This compound was obtained following the above method as a white powder, yield 94%. MS (ESI) m/z 475.4 (M + H)+, calcd. for C26H32BrFO2 m/z = 474.2.
16-(4-Methoxybenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 5j
This compound was obtained following the above method as a white powder, yield 84%. MS (ESI) m/z 409.5 (M + H)+, calcd. for C27H36O3 m/z = 408.3.
16-(3,4,5-Trimethoxybenzylidene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 5k
This compound was obtained following the above method as a white powder, yield 86%. MS (ESI) m/z 469.5 (M + H)+, calcd. for C29H40O5 m/z = 468.3.
16-(3-Pyridinmethylene)-3-hydroxy-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one 5l
This compound was obtained following the above method as a white powder, yield 83%. MS (ESI) m/z 380.3 (M + H)+, calcd. for C25H33NO2 m/z = 379.3.
4.2.2. General Synthetic Procedure for Target Compounds 3a–l and 6a–l
Guanidine nitrate (0.488 g, 4 mmol) and potassium t-butoxide (0.336 g, 3 mmol) were added to a solution of different intermediates 2a–l or 5a–l (1 mmol) in t-butanol (15 mL), which were heated under reflux overnight. After the completion of the reaction, the mixtures were poured into 50 mL of ice water with stirring, the pH value was tuned to 7–8, and then the precipitate was filtered and washed with water and dried under reduced pressure to obtain the crude compounds 3a–l and 5a–l, which were further purified by recrystallization or silica column chromatography. All compounds were fully characterized by the ESI-MS, 1H NMR, and 13C NMR spectrums, and their physico-chemical properties and spectra data are as follows:
10-Amino-6a,8a-dimethyl-12-phenyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 3a
This compound was obtained following the above method as a white powder, yield 63%. 1H NMR (600 MHz, DMSO-d6): δ 7.80 (dd, J = 8.1, 1.4 Hz, 2H), 7.45–7.41 (m, 3H), 6.46 (s, 2H, NH2), 4.46 (d, J = 4.7 Hz, 1H, C3-βOH), 3.34–3.30 (m, 1H, C3-αH), 2.66–2.62 (m, 1H), 2.58–2.53 (m, 1H), 1.98 (dd, J = 6.8, 4.1 Hz, 1H), 1.73–1.68 (m, 1H), 1.65–1.57 (m, 4H), 1.44–1.36 (m, 4H), 1.28–1.21 (m, 5H), 1.18–1.12 (m, 1H), 1.05–1.01 (m, 1H), 0.94 (s, 3H, CH3), 0.81 (s, 3H, CH3), 0.74–0.70 (m, 1H); 13C NMR (150 MHz, DMSO-d6): δ 183.45, 163.00, 158.98, 138.04, 129.34, 129.34, 128.25, 128.18, 117.92, 69.30, 54.95, 54.25, 45.45, 44.48, 38.18, 36.47, 35.41, 33.86, 33.06, 31.42, 31.14, 29.45, 28.29, 20.49, 17.11, 12.14; MS (ESI) m/z 418.5 (M + H)+, calcd. for C27H35N3O m/z = 417.3.
10-Amino-12-(2-chlorophenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 3b
This compound was obtained following the above method as yellowish powder, yield 71%. 1H NMR (600 MHz, CD3OD): δ 7.52 (dd, J = 7.7, 1.4 Hz, 1H), 7.44 (m, 1H), 7.38 (dd, J = 7.4, 1.9 Hz, 1H), 3.55–3.48 (m, 1H, C3-αH), 2.47–2.29 (m, 1H), 2.22–2.01 (m, 1H), 1.83–1.66 (m, 5H), 1.64–1.49 (m, 4H), 1.46–1.25 (m, 8H), 1.19–1.14 (m, 1H), 1.03 (s, 3H, CH3), 0.91 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 182.46, 162.86, 159.54, 157.96, 137.38, 130.97, 130.56, 129.42, 127.14, 119.87, 69.28, 54.58, 54.22, 50.66, 45.74, 44.46, 38.16, 36.46, 35.40, 33.80, 32.90, 31.33, 28.24, 20.46, 17.10, 13.46, 12.12; MS (ESI) m/z 452.4 (M + H)+, calcd. for C27H34ClN3O m/z = 451.2.
10-Amino-12-(4-chlorophenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 3c
This compound was obtained following the above method as a pale white powder, yield 81%. 1H NMR (600 MHz, DMSO-d6): δ 7.83 (d, J = 8.6 Hz, 2H), 7.54 (d, J = 8.6 Hz, 2H), 6.54 (s, 1H, NH2), 4.48 (d, J = 4.1 Hz, 1H, C3-βOH), 3.34–3.30 (m, 1H, C3-αH), 2.79–2.53 (m, 2H), 2.00–1.97 (m, 1H), 1.74–1.71 (m, 1H), 1.69–1.57 (m, 5H), 1.47–1.38 (m, 4H), 1.27–1.24 (m, 4H), 1.19–1.13 (m, 1H), 1.08–1.04 (m, 1H), 0.94 (s, 3H, CH3), 0.81 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 183.73, 162.93, 157.60, 136.82, 134.10, 129.94, 128.34, 117.95, 69.27, 54.86, 54.22, 45.42, 44.47, 38.14, 36.44, 35.39, 33.86, 33.00, 31.34, 29.31, 28.24, 20.44, 17.07, 14.14, 12.12; MS (ESI) m/z 452.4 (M + H)+, calcd. for C27H34ClN3O m/z = 451.2.
10-Amino-12-(2-fluorophenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 3d
This compound was obtained following the above method as a white powder, yield 83%. 1H NMR (600 MHz, DMSO-d6): δ 7.56–7.47 (m, 2H), 7.33–7.28 (m, 2H), 6.58 (s, 2H, NH2), 4.47 (d, J = 4.6 Hz, 1H, C3-βOH), 3.34–3.30 (m, 1H, C3-αH), 2.38–2.26 (m, 2H), 2.00–1.95 (m, 1H), 1.67–1.57 (m, 6H), 1.45–1.38 (m, 4H), 1.27–1.20 (m, 4H), 1.17–1.11 (m, 2H), 1.06–1.03 (m, 1H), 0.94 (s, 3H, CH3), 0.80 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 182.73, 163.01, 156.21, 131.07 (dd, J = 12.9, 6.9 Hz), 126.04, 124.42, 120.12, 115.92, 115.78, 99.52, 69.26, 54.54, 54.20, 50.65, 45.71, 44.46, 38.14, 36.44, 35.39, 33.80, 32.94, 31.34, 31.07, 28.23, 20.43, 17.07, 12.10; MS (ESI) m/z 436.5 (M + H)+, calcd. for C27H34FN3O m/z = 435.3.
10-Amino-12-(4-fluorophenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 3e
This compound was obtained following the above method as a white powder, yield 75%. 1H NMR (600 MHz, DMSO-d6): δ 7.90–7.84 (m, 2H), 7.31 (t, J = 8.9 Hz, 2H), 6.51 (s, 2H, NH2), 4.48 (d, J = 4.4 Hz, 1H, C3-βOH), 3.34–3.30 (m, 1H, C3-αH), 2.69–2.55 (m, 2H), 2.01–1.94 (m, 1H), 1.75–1.70 (m, 1H), 1.69–1.57 (m, 5H), 1.44–1.37 (m, 4H), 1.29–1.24 (m, 4H), 1.19–1.13 (m, 2H), 1.08–1.02 (m, 1H), 0.95 (s, 3H, CH3), 0.82 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 184.06, 163.38, 158.29, 130.88(d, J = 8.6 Hz), 118.20, 115.72, 115.58, 69.75, 55.37, 54.71, 45.89, 44.95, 38.62, 36.92, 35.87, 34.33, 33.49, 31.86, 31.59, 29.83, 28.72, 20.93, 17.55, 12.60; MS (ESI) m/z 436.5 (M + H)+, calcd. for C27H34FN3O m/z = 435.3.
10-Amino-12-(2-trifluoromethylphenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 3f
This compound was obtained following the above method as a white powder, yield 82%. 1H NMR (600 MHz, DMSO-d6): δ 7.87–7.36 (m, 4H), 6.50 (s, 2H, NH2), 4.43 (s, 1H, C3-βOH), 3.36–3.34 (m, 1H, C3-αH), 2.39–2.35 (m, 1H), 2.13 (d, J = 9.2 Hz, 1H), 2.00–1.96 (m, 1H), 1.86–1.79 (m, 1H), 1.75–1.71 (m, 1H), 1.60–1.56 (m, 6H), 1.48–1.38 (m, 5H), 1.26–1.21 (m, 4H), 0.90 (s, 3H, CH3), 0.79 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 182.42, 161.70, 160.03, 132.83 (d, J = 2.2 Hz), 130.70, 129.27, 126.83, 126.75, 119.63, 69.73, 54.35, 51.13, 47.56, 44.86, 38.60, 37.07, 35.77, 35.74, 35.00, 31.82, 30.98, 28.63, 21.84, 20.56, 17.49, 13.91, 12.56; MS (ESI) m/z 486.4 (M + H)+, calcd. for C28H34F3N3O m/z = 485.3.
10-Amino-12-(4-trifluoromethylphenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 3g
This compound was obtained following the above method as a pale white powder, yield 82%. 1H NMR (600 MHz, DMSO-d6): δ 8.01 (d, J = 8.1 Hz, 2H), 7.84 (d, J = 8.3 Hz, 2H), 6.57 (s, 2H, NH2), 4.43 (d, J = 4.5 Hz, 1H, C3-βOH), 3.37–3.34 (m, 1H, C3-αH), 2.71–2.67 (m, 1H), 2.62–2.58 (m, 1H), 1.99 (d, J = 8.3 Hz, 1H), 1.74–1.72 (m, 1H), 1.68–1.60 (m, 5H), 1.45–1.41 (m, 4H), 1.30–1.24 (m 4H), 1.17–1.04 (m, 3H), 0.96 (s, 3H, CH3), 0.82 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 183.96, 163.02, 157.36, 141.94, 130.74, 128.90, 125.19 (d, J = 3.6 Hz), 118.53, 69.27, 54.84, 54.21, 45.49, 44.47, 38.14, 36.44, 35.39, 33.86, 32.97, 31.38, 31.11, 29.19, 28.23, 20.44, 17.08, 12.11; MS (ESI) m/z 486.3 (M + H)+, calcd. for C28H34F3N3O m/z = 485.3.
10-Amino-12-(2,4-dichlorophenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 3h
This compound was obtained following the above method as a pale white powder, yield 74%. 1H NMR (600 MHz, DMSO-d6): δ 7.72 (d, J = 2.0 Hz, 1H), 7.50 (dd, J = 8.3, 2.1 Hz, 1H), 7.43 (d, J = 8.3 Hz, 1H), 6.58 (s, 2H, NH2), 4.43 (d, J = 3.7 Hz, 1H, C3-βOH), 3.37–3.34 (m, 1H, C3-αH), 2.31–2.26 (m, 1H), 2.20–2.17 (m, 1H), 2.00–1.96 (m, 1H), 1.67–1.58 (m, 6H), 1.46–1.40 (m, 4H), 1.27–1.20 (m, 4H), 1.15–1.03 (m, 3H), 0.92 (s, 3H, CH3), 0.80 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 182.70, 162.85, 158.43, 136.30, 133.90, 132.10, 131.92, 128.96, 127.41, 119.89, 69.26, 54.54, 54.20, 45.74, 44.45, 38.14, 36.44, 35.38, 33.78, 32.85, 31.37, 31.06, 28.22, 27.59, 20.43, 17.07, 12.09; MS (ESI) m/z 486.3 (M + H)+, calcd. for C27H33Cl2N3O m/z = 485.2.
10-Amino-12-(2-fluoro-4-bromophenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 3i
This compound was obtained following the above method as a pale white powder, yield 82%. 1H NMR (600 MHz, DMSO-d6): δ 7.66 (dd, J = 9.9, 1.6 Hz, 1H), 7.56–7.46 (m, 2H), 6.58 (s, 2H, NH2), 4.43 (d, J = 4.4 Hz, 1H, C3-βOH), 3.36–3.33 (m, 1H, C3-αH), 2.37–2.27 (m, 2H), 2.00–1.97 (m 1H), 1.64–1.60(m, 6H), 1.44–1.40 (m, 4H), 1.27–1.21 (m, 4H), 1.15–1.03 (m, 3H), 0.93 (s, 3H, CH3), 0.80 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 183.49, 163.49, 160.33, 158.59, 155.62, 133.01, 128.22, 123.14,128.22, 123.14, 120.58, 119.88, 69.74, 54.98, 54.66, 46.20, 44.93, 38.62, 36.91, 35.86, 34.27, 33.39, 31.85, 31.54, 28.70, 28.35, 20.90, 17.52, 12.58; MS (ESI) m/z 514.3 (M + H)+, calcd. for C27H33BrFN3O m/z = 513.2.
10-Amino-12-(4-methoxyphenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 3j
This compound was obtained following the above method as a white powder, yield 89%. 1H NMR (600 MHz, DMSO-d6): δ 7.78 (d, J = 8.7 Hz, 2H), 7.00 (d, J = 8.7 Hz, 2H), 4.45 (d, J = 4.5 Hz, 1H, C3-βOH), 3.79 (s, 3H, OCH3), 3.37–3.31 (m, 1H, C3-αH), 2.66–2.54 (m, 2H), 1.97–1.90 (m, 1H), 1.70 (dd, J = 8.2, 4.0 Hz, 1H), 1.66–1.57 (m, 4H), 1.43–1.34 (m, 4H), 1.27–1.20 (m, 4H), 1.17–0.99 (m, 3H), 0.91 (s, 3H, CH3), 0.79 (s, 3H, CH3), 0.73–0.67 (m, 1H); 13C NMR (150 MHz, DMSO-d6): δ 183.19, 162.87, 160.22, 158.56, 130.41, 129.71, 117.16, 113.60, 69.29, 55.22, 54.94, 54.26, 45.34, 44.46, 38.19, 36.46, 35.40, 33.85, 33.07, 31.42, 31.13, 29.67, 28.30, 20.80, 17.10, 12.12; MS (ESI) m/z 448.4 (M + H)+, calcd. for C28H37N3O2 m/z = 447.3.
10-Amino-12-(3,4,5-trimethoxyphenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 3k
This compound was obtained following the above method as a white powder, yield 91%. 1H NMR (600 MHz, DMSO-d6): δ 7.08 (s, 2H), 6.41 (s, 2H, NH2), 4.44 (s, 1H, C3-βOH), 3.81 (s, 6H, OCH3), 3.70 (s, 3H, OCH3), 3.34–3.29 (m, 1H, C3-αH), 2.71 (t, J = 13.0 Hz, 1H), 2.60 (m, 1H), 1.97 (d, J = 9.9 Hz, 1H), 1.74 (dd, J = 8.2, 4.0 Hz, 1H), 1.67–1.56 (m, 4H), 1.45–1.35 (m, 4H), 1.32–1.19 (m, 4H), 1.18–0.99 (m, 3H), 0.94 (s, 3H, CH3), 0.79 (s, 3H, CH3), 0.75–0.69 (m, 1H); 13C NMR (150 MHz, DMSO-d6): δ 183.44, 162.84, 158.80, 152.62, 138.56, 133.50, 117.83, 105.65, 69.31, 60.09, 55.91, 55.03, 54.25, 45.47, 44.50, 38.18, 36.46, 35.39, 33.84, 33.07, 31.39, 31.07, 29.59, 28.28, 20.48, 17.06, 12.09; MS (ESI) m/z 508.5 (M + H)+, calcd. for C30H41N3O4 m/z = 507.3.
10-Amino-12-(3-pyridinyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 3l
This compound was obtained following the above method as a white powder, yield 81%. 1H NMR (600 MHz, DMSO-d6): δ 8.99 (d, J = 1.6 Hz, 1H), 8.64 (dd, J = 4.8, 1.6 Hz, 1H), 8.17 (dt, J = 7.9, 1.9 Hz, 1H), 7.53–7.49 (m, 1H), 6.56 (s, 2H, NH2), 4.44 (d, J = 4.6 Hz, 1H, C3-βOH), 3.38–3.34 (m, 1H, C3-αH), 2.73–2.69 (m, 1H), 2.61–2.58 (m, 1H), 1.99 (d, J = 8.5 Hz, 1H), 1.77–1.73 (m, 1H), 1.68–1.61 (m, 5H), 1.47–1.41 (m, 4H), 1.28–1.25 (m, 4H), 1.18–1.04 (m, 3H), 0.97 (s, 3H, CH3), 0.83 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 184.23, 163.49, 157.02, 150.54, 149.46, 135.92, 134.02, 123.93, 118.92, 69.75, 55.37, 54.70, 45.96, 44.95, 38.62, 36.92, 35.87, 34.33, 33.44, 31.86, 31.55, 29.47, 28.72, 20.92, 17.56, 12.59; MS (ESI) m/z 419.4 (M + H)+, calcd. for C26H34N4O m/z = 418.3.
10-Amino-6a,8a-dimethyl-12-phenyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 6a
This compound was obtained following the above method as a yellowish powder, yield 82%. 1H NMR (600 MHz, DMSO-d6): δ 7.81 (dd, J = 8.0, 1.4 Hz, 2H), 7.46 (m, 3H), 6.44 (s, 2H, NH2), 4.16 (d, J = 2.4 Hz, 1H, C3-αOH), 3.82–3.79 (m, 1H, C3-βH), 2.70–2.56 (m, 2H), 2.03–1.96 (m, 1H), 1.77–1.72 (m, 1H), 1.70–1.63 (m, 2H), 1.56–1.49 (m, 3H), 1.48–1.37 (m, 4H), 1.35–1.28 (m, 3H), 1.25–1.14 (m, 4H), 0.96 (s, 3H, CH3), 0.81 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 183.98, 163.43, 159.42, 138.52, 129.76, 129.76, 128.69, 128.62, 118.46, 64.52, 55.50, 54.88, 45.90, 39.10, 36.39, 36.22, 34.34, 33.53, 32.26, 31.68, 29.83, 29.13, 28.61, 20.51, 17.56, 11.63; MS (ESI) m/z 418.4 (M + H)+, calcd. for C27H35N3O m/z = 417.3.
10-Amino-12-(2-chlorophenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 6b
This compound was obtained following the above method as a white powder, yield 81%. 1H NMR (600 MHz, DMSO-d6): δ 7.60–7.14 (m, 4H), 6.53 (s, 2H, NH2), 4.16 (d, J = 2.4 Hz, 1H, C3-αOH), 2–3.76 (m, 1H, C3-βH), 2.71–2.51 (m, 1H), 2.40–2.16 (m, 1H), 2.02–1.86 (m, 1H), 1.84–1.72 (m, 1H), 1.67–1.59 (m, 2H), 1.58–1.43 (m, 5H), 1.38–1.22 (m, 6H), 1.19–1.06 (m, 3H), 0.93 (s, 3H, CH3), 0.78 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 182.51, 159.51, 137.37, 130.97, 130.15, 129.40, 128.24, 119.89, 99.53, 64.03, 54.37, 48.65, 45.73, 44.40, 35.91, 33.79, 32.84, 31.17, 28.62, 28.04, 20.01, 17.09, 15.58, 14.08, 13.45, 11.13; MS (ESI) m/z 452.6 (M + H)+, calcd. for C27H34ClN3O m/z = 451.2.
10-Amino-12-(4-chlorophenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno1,2-d]pyrimidin-4-ol 6c
This compound was obtained following the above method as a white powder, yield 88%. 1H NMR (600 MHz, DMSO-d6): δ 7.84 (d, J = 8.6 Hz, 1H), 7.65 (d, J = 8.6 Hz, 1H), 7.52 (dd, J = 19.8, 8.6 Hz, 2H), 6.49 (s, 2H, NH2), 4.17 (d, J = 6.6, 3.7 Hz, 1H, C3-αOH), 3.82–3.79 (m, 1H, C3-βH), 2.79–2.59 (m, 1H), 2.02–1.81 (m, 1H), 1.78–1.71 (m, 1H), 1.67–1.58 (m, 2H), 1.56–1.45 (m, 4H), 1.42–1.23 (m, 8H), 1.20–1.15 (m, 2H), 1.04–0.97 (m, 1H), 0.86 (s, 3H, CH3), 0.79 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 208.47, 137.22, 134.06, 131.93, 130.36, 129.94, 128.82, 128.34, 64.03, 54.00, 48.71, 47.01, 45.42, 38.55, 35.87, 35.70, 34.18, 31.85, 31.31, 30.68, 28.62, 28.02, 19.70, 14.15, 11.14; MS (ESI) m/z 452.6 (M + H)+, calcd. for C27H34ClN3O m/z = 451.2.
10-Amino-12-(2-fluorophenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 6d
This compound was obtained following the above method as a white powder, yield 79%. 1H NMR (600 MHz, DMSO-d6): δ 7.74–7.08 (m, 4H), 6.52 (s, 2H, NH2), 4.19 (s, 1H, C3-αOH), 3.82–3.79 (m, 1H, C3-βH), 2.45–2.25 (m, 2H), 2.02–1.70 (m, 2H), 1.68–1.56 (m, 3H), 1.55–1.37 (m, 6H), 1.35–1.21 (m, 5H), 1.18–1.12 (m, 2H), 0.94 (s, 3H, CH3), 0.79 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 182.79, 163.03, 159.97, 158.33, 156.21, 131.08(dd, J = 16.4, 5.9Hz), 126.06, 124.44, 120.16, 115.94, 64.05, 54.37, 54.06, 50.73, 47.12, 45.74, 35.93, 34.54, 33.82, 31.38, 28.64, 28.06, 21.36, 19.66, 17.09, 13.47, 11.16; MS (ESI) m/z 436.6 (M + H)+, calcd. for C27H34FN3O m/z = 435.3.
10-Amino-12-(4-fluorophenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 6e
This compound was obtained following the above method as a white powder, yield 83%. 1H NMR (600 MHz, DMSO-d6): δ 7.88 (dd, J = 8.8, 5.6 Hz, 1H), 7.69 (dd, J = 8.6, 5.6 Hz, 1H), 7.32–7.27 (m, 2H), 6.46 (s, 2H, NH2), 4.17 (d, J = 3.0 Hz, 1H, C3-αOH), 3.82–3.79 (m, 1H, C3-βH), 2.77–2.58 (m, 1H), 2.01–1.80 (m, 1H), 1.78–1.72 (m, 1H), 1.69–1.61 (m, 2H), 1.60–1.43 (m, 4H), 1.41–1.22 (m, 7H), 1.20–1.14 (m, 2H), 1.03–0.94 (m, 2H), 0.86 (s, 3H, CH3), 0.79 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 208.51, 162.90, 136.16, 132.55 (d, J = 8.5 Hz), 130.56, 115.88, 115.73, 64.03, 54.02, 48.80, 46.97, 38.54, 35.87, 35.70, 31.85, 31.85, 31.35, 31.30, 30.67, 28.65, 28.02, 19.71, 17.08, 14.16, 11.14; MS (ESI) m/z 436.6 (M + H)+, calcd. for C27H34FN3O m/z = 435.3.
10-Amino-12-(2-trifluoromethylphenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 6f
This compound was obtained following the above method as a white powder, yield 81%. 1H NMR (600 MHz, DMSO-d6): δ 7.81 (d, J = 7.8 Hz, 1H), 7.72 (t, J = 7.5 Hz, 1H), 7.63 (t, J = 7.7 Hz, 1H), 7.41 (d, J = 7.6 Hz, 1H), 6.48 (s, 2H, NH2), 4.18 (d, J = 3.0 Hz, 1H, C3-αOH), 3.82–3.77 (d, J = 2.6 Hz, 1H, C3-βH), 2.17–2.08 (m, 2H), 2.01–1.97 (m, 1H), 1.69–1.63 (m, 1H), 1.60–1.44 (m, 7H), 1.39–1.21 (m, 6H), 1.14–1.06 (m, 2H), 0.90 (s, 3H, CH3), 0.85–0.80 (m, 1H), 0.77 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 182.73, 162.77, 160.90, 137.59, 132.81, 130.50, 129.32, 126.72 (dd, J = 8.8, 4.7 Hz), 123.51, 119.80, 64.52, 55.16, 54.85, 46.27, 39.07, 36.37, 36.17, 34.24, 33.33, 32.25, 31.64, 29.10, 28.55, 27.68, 20.47, 17.52, 11.58; MS (ESI) m/z 486.6 (M + H)+, calcd. for C28H34F3N3O m/z = 485.3.
10-Amino-12-(4-trifluoromethylphenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 6g
This compound was obtained following the above method as a white powder, yield 75%. 1H NMR (600 MHz, DMSO-d6): δ 8.02 (d, J = 8.1 Hz, 2H), 7.84 (d, J = 8.3 Hz, 2H), 6.57 (s, 2H, NH2), 4.17 (d, J = 2.9 Hz, 1H, C3-αOH), 3.82–3.80 (m, C3-βH), 2.71–2.66 (m, 1H), 2.63–2.59 (m, 1H), 2.00 (d, J = 9.6 Hz, 1H), 1.76–1.72 (m, 1H), 1.68–1.63 (m, 2H), 1.59–1.53 (m, 2H), 1.50–1.42 (m, 4H), 1.37–1.28 (m, 4H), 1.26–1.22 (m, 2H), 1.19–1.14 (m, 2H), 0.97 (s, 3H, CH3), 0.80 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 184.49, 163.50, 157.83, 142.43, 129.41, 125.59 (d, J = 5.3 Hz), 123.77, 119.04, 64.72, 55.29, 54.83, 45.97, 36.39, 36.18, 34.62, 32.26, 31.94, 29.59, 29.38, 29.09, 28.59, 17.60, 17.52, 11.62, 11.58; MS (ESI) m/z 486.4 (M + H)+, calcd. for C28H34F3N3O m/z = 485.3.
10-Amino-12-(2,4-dichlorophenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 6h
This compound was obtained following the above method as a white powder, yield 81%. 1H NMR (600 MHz, DMSO-d6): δ 7.72 (d, J = 2.1 Hz, 1H), 7.54–7.49 (m, 1H), 7.45–7.39 (m, 1H), 6.58 (s, 2H, NH2), 4.17 (d, J = 7.9 Hz, 1H, C3-αOH), 3.81–3.78 (m, 1H, C3-βH), 2.31–2.17 (m, 1H), 2.03–1.70 (m, 2H), 1.68–1.61 (m, 2H), 1.55–1.43 (m, 6H), 1.37–1.20 (m, 7H), 1.18–1.12 (m, 2H), 0.92 (s, 3H, CH3), 0.78 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 182.77, 162.86, 158.43, 152.40, 136.38, 136.31, 133.93, 133.90, 132.12, 119.93, 75.80, 64.26, 54.39, 45.75, 35.72 (dd, J = 37.8, 26.5 Hz), 31.76, 31.53, 28.25, 28.12, 17.11, 17.05, 11.18, 11.09, 10.99; MS (ESI) m/z 486.3 (M + H)+, calcd. for C27H33Cl2N3O m/z = 485.2.
10-Amino-12-(2-fluoro-4-bromophenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 6i
This compound was obtained following the above method as a yellowish powder, yield 71%. 1H NMR (600 MHz, DMSO-d6): δ 7.69–7.64 (m, 1H), 7.55–7.47 (m, 2H), 6.58 (s, 2H, NH2), 4.17 (d, J = 1.8 Hz, 1H, C3-αOH), 3.81–3.79 (m, 1H, C3-βH), 2.41–2.24 (m, 2H), 2.02–1.96 (m, 1H), 1.66–1.63 (m, 2H), 1.55–1.41 (m, 7H), 1.35–1.22 (m, 6H), 1.18–1.13 (m, 2H), 0.94 (s, 3H, CH3), 0.79 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 183.09, 163.03, 155.15, 132.57, 127.77, 125.46, 122.78, 120.15, 119.42, 119.25, 64.05, 54.36, 50.73, 45.75, 35.93, 35.32, 34.54, 33.82, 32.94, 31.79, 31.20, 28.63, 28.13, 19.66, 17.07, 13.47, 11.16; MS (ESI) m/z 514.3 (M + H)+, calcd. for C27H33BrFN3O m/z = 513.2.
10-Amino-12-(4-methoxyphenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 6j
This compound was obtained following the above method as a yellowish powder, yield 70%. 1H NMR (600 MHz, DMSO-d6): δ 7.69 (dd, J = 8.8 Hz, 2H), 7.01 (dd, J = 8.7, 6.0 Hz, 2H), 6.36 (s, 2H, NH2), 4.17 (d, J = 4.7 Hz, 1H, C3-αOH), 3.81 (s, 3H, OCH3), 3.80–3.79 (m, 1H, C3-βH), 2.62 (dd, J = 14.6, 9.2 Hz, 1H), 1.99–1.81 (m, 1H), 1.75 (d, J = 11.3 Hz, 1H), 1.64 (dd, J = 20.8, 9.5 Hz, 2H), 1.59–1.45 (m, 4H), 1.44–1.37 (m, 3H), 1.35–1.23 (m, 5H), 1.22–1.16 (m, 2H), 1.03–0.98 (m, 1H), 0.94 (s, 3H, CH3), 0.80 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 183.73, 162.89, 160.61, 159.06, 130.56, 130.10, 117.92, 114.01, 64.48, 55.57, 55.45, 54.73, 45.69, 36.21, 35.90, 34.14, 33.33, 32.11, 31.52, 29.88, 28.78, 28.43, 20.34, 17.34, 11.46; MS (ESI) m/z 448.4 (M + H)+, calcd. for C28H37N3O2 m/z = 447.3.
10-Amino-12-(3,4,5-trimethoxyphenyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 6k
This compound was obtained following the above method as a white powder, yield 72%. 1H NMR (600 MHz, DMSO-d6): δ 7.08 (s, 2H), 6.42 (s, 2H, NH2), 4.17 (d, J = 2.4 Hz, 1H, C3-αOH), 3.82 (s, 6H, OCH3), 3.81–3.79 (s, 1H, C3-βH), 3.71 (s, 3H, OCH3), 2.75–2.70 (m, 1H), 2.64–2.58 (m, 1H), 2.02–1.94 (m, 1H), 1.80–1.75 (m, 1H), 1.72–1.63 (m, 2H), 1.62–1.45 (m, 4H), 1.44–1.38 (m, 3H), 1.37–1.27 (m, 3H), 1.26–1.20 (m, 2H), 1.19–1.10 (m, 2H), 0.97 (s, 3H, CH3), 0.80 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 183.50, 162.84, 158.80, 152.62, 138.49, 133.52, 117.91, 105.67, 64.30, 60.16, 59.61, 57.01, 56.52, 56.17, 55.68, 55.32, 54.40, 45.48, 35.93, 31.82, 31.41, 28.15, 17.10, 17.04, 11.18, 11.10; MS (ESI) m/z 508.4 (M + H)+, calcd. for C30H41N3O4 m/z = 507.3.
10-Amino-12-(3-pyridinyl)-6a,8a-dimethyl-tetradecahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-d]pyrimidin-4-ol 6l
This compound was obtained following the above method as a white powder, yield 61%. 1H NMR (600 MHz, DMSO-d6): δ 8.98 (d, J = 1.6 Hz, 1H), 8.64 (dd, J = 4.8, 1.6 Hz, 1H), 8.16 (dt, J = 7.9, 1.9 Hz, 1H), 7.51 (dd, J = 7.7, 5.1 Hz, 1H), 6.55 (s, 2H, NH2), 4.17 (d, J = 2.2 Hz, 1H, C3-αOH), 3.82–3.79 (m, 1H, C3-βH), 2.73–2.68 (m, 1H), 2.63–2.58 (m, 1H), 2.03–1.96 (m, 1H), 1.77–1.73 (m, 1H), 1.70–1.64 (m, 2H), 1.59–1.52 (m, 2H), 1.50–1.42 (m, 4H), 1.40–1.28 (m, 4H), 1.26–1.20 (m, 2H), 1.19–1.12 (m, 2H), 0.96 (s, 3H, CH3), 0.80 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 183.82, 163.03, 156.55, 150.09, 149.10, 135.46, 133.57, 123.53, 118.49, 64.27, 54.43, 54.40, 45.49, 35.92, 35.80, 35.78, 31.80, 31.43, 30.94, 30.94, 28.91, 28.70, 28.13, 17.11, 17.06, 11.20, 11.12; MS (ESI) m/z 419.5 (M + H)+, calcd. for C26H34N4O m/z = 418.3.
4.3. In Vitro Cytotoxicity Assay
The in vitro cytotoxicity of the synthesized compounds against various human cancer cell lines was measured by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide] colorimetric method [76], and the general procedures were reported in previous publications [63,64,67,78]. All the data of the experiment were analyzed according to SPSS software, and the 50% inhibitory concentrations (IC50) of each compound for the different cell lines were determined. All assays were performed in triplicate on three independent experiments, and measurement data were expressed as the mean ± S.D.
4.4. Kinase Activity Assay
The kinase inhibitory activities of compounds were determined by ADP-Glo Protocol or LANCE Protocol. Enzymes, substrate, ATP, and inhibitors were diluted in the kinase buffer, which contained 40 mM Tris, pH 7.5; 20 mM MgCl2; 0.1 mg/mL BSA; and 50 μM DTT.
ADP-Glo protocol: (1) Add to the wells of 384 low volume plate: 1 μL of inhibitor or (5% DMSO), 2 μL of enzymes, and 2 μL of substrate/ATP mix; (2) Incubate at 25 °C; (3) Add 5 μL of ADP-Glo™ Reagent; (4) Incubate at 25 °C for 40 min; (5) Add 10 μL of Kinase Detection Reagent; (6) Incubate at 25 °C for 30 min; (7) Record luminescence (Integration time 0.5 s, RLU); (8) Calculate the enzyme Inhibition rate: Inhibition% = (RLU(Sample) ™ RLU(1%DMSO))/(RLU(Blank) ™ RLU(1%DMSO)) × 100%.
LANCE protocol: (1) Add to the wells of a white Optiplate-384: 5 µL of enzyme, 2.5 µL of inhibitor or kinase buffer, 2.5 µL of Ulight-4E-BP1 or Ulight- CREB/ATP mix; (2) Incubate at 25 °C for 2 h; (3) Stop kinase reactions by adding 5 µL of 40 mM EDTA prepared in 1X detection buffer (stop solution); (4) Add 5 µL of 4X detection mix (Eu-anti-phospho-tyrosine antibody at a final concentration of 2 nM); (5) Cover with TopSeal-A and incubate for 1 h at 25 °C; (6) Remove TopSeal-A and read signal with the Nivo Reader in TR-FRET mode (excitation at 320 nm and emission at 665 and 615 nm); (7) Calculate enzyme inhibition rate: Ratio = 665/615, Inhibition% = (RatioSample ™ RatioDMSO)/(RatioBlank ™ RatioDMSO) × 100%. Dilute the kinase, ATP, inhibitors, and Ulight-4E-BP1 or Ulight-CREB peptide in kinase buffer. Prepare a 4X detection mix by diluting the Eu-anti-phospho-tyrosine antibody to 8 nM in 1X LANCE detection buffer.
4.5. Molecular Docking Study
All the molecular docking simulations were carried out by the AutoDock 4.2 software [79]. The docking tutorial we used and the detailed AutoDock basic operation methods can be found at: https://autodock.scripps.edu/ (accessed on 6 January 2023). The protein preparation process of flexible docking mainly includes fixing the exact residues, removing irrelevant water molecules, adding hydrogen atoms and adding charges, etc. The crystal structure (PDB: 6GU6, https://www.rcsb.org/structure/6GU6, accessed on 6 January 2023) of the CDK1 bond to 3a, 3b, 3l, 6a, 6b, and 6l were used in the docking studies. We first removed inhibitor dinaciclib from the crystal structure, then put the target molecules 3a, 3b, 3l, 6a, 6b, and 6l in the binding site, and the energy was optimized using a genetic algorithm. Only the best-scoring ligand-protein complex was used for the binding site analysis. All the docking results were processed and modified in PyMOL 1.7.4.5 software (https://pymol.org, accessed on 6 January 2023) and LigPlot+ v.2.2.7 software (https://www.ebi.ac.uk/thornton-srv/software/LigPlus/, accessed on 6 January 2023).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules28062691/s1, Figures S1–S48. all the 1H NMR and 13C NMR spectroscopy for target compounds 3a–l and 6a–l.
Author Contributions
Conceptualization, S.K.; Methodology, F.Y.; Validation, F.L.; Formal analysis, F.Y.; Investigation, Y.M.; Resources, K.W.; Data curation, L.S.; Writing—original draft, F.Y.; Writing—review & editing, S.K. and Y.G.; Visualization, M.L.; Supervision, S.K., Y.G. and Z.Y.; Project administration, Z.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Program for Leading Talents of Hubei Academy of Agricultural Sciences (L2018031) and the Innovation and Application of Key Technologies of Quality improving and Efficiency-increasing for the Fengtou Ginger Industry (2020-620-002-06). The authors also gratefully acknowledge the partial support from Hubei Agricultural Science Innovation Center (2021-620-000-001-027) and Hubei Biopesticide Engineering Research Centre (HBERC-RC-202002, HBERC-RC-202104).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Mazumder, K.; Aktar, A.; Roy, P.; Biswas, B.; Hossain, M.; Sarkar, K.; Bachar, S.; Ahmed, F.; Monjur-Al-Hossain, A.; Fukase, K. A Review on Mechanistic Insight of Plant Derived Anticancer Bioactive Phytocompounds and Their Structure Activity Relationship. Molecules 2022, 27, 3036. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Moghimi, S.; Toolabi, M.; Foroumadi, A. Pyrimidine-based EGFR TK inhibitors in targeted cancer therapy. Eur. J. Med. Chem. 2021, 221, 113523. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.; Khan, A. Pyrimidine: An elite heterocyclic leitmotif in drug discovery-synthesis and biological activity. Chem. Biol. Drug Des. 2021, 100, 818–842. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Ma, S. Recent Development of Pyrimidine-Containing Antimicrobial Agents. ChemMedChem 2020, 15, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Song, X.J.; Shao, Y.; Dong, X.G. Microwave-assisted synthesis of some novel fluorinated pyrazolo [3, 4-d] pyrimidine derivatives containing 1, 3, 4-thiadiazole as potential antitumor agents. Chin. Chem. Lett. 2011, 22, 1036–1038. [Google Scholar] [CrossRef]
- Kurumurthy, C.; Rao, P.S.; Rao, P.S.; Narsaiah, B.; Velatooru, L.; Pamanji, R.; Rao, J.V. Synthesis of novel alkyltriazole tagged pyrido [2, 3-d] pyrimidine derivatives and their anticancer activity. Eur. J. Med. Chem. 2011, 46, 3462–3468. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Lin, H.; Zuo, D.; Wang, L.; Zhao, Y.; Gong, P. Design, synthesis and biological evaluation of novel thieno [3, 2-d] pyrimidine derivatives containing diaryl urea moiety as potent antitumor agents. Eur. J. Med. Chem. 2014, 85, 215–227. [Google Scholar] [CrossRef]
- Zhu, W.F.; Zhai, X.; Li, S.; Cao, Y.Y.; Gong, P.; Liu, Y.J. Synthesis and cytotoxic activity of novel 2, 6-disubstituted-4-mor-pholinothieno [3, 2-d] pyrimidines as potent anti-tumor agents. Chin. Chem. Lett. 2012, 23, 703–706. [Google Scholar] [CrossRef]
- Al-Issa, S. Synthesis and anticancer activity of some fused pyrimidines and related heterocycles. Saudi Pharm. J. 2013, 21, 305–316. [Google Scholar] [CrossRef]
- Kumar, R.N.; Dev, G.J.; Ravikumar, N.; Swaroop, D.K.; Debanjan, B.; Bharath, G.; Narsaiah, B.; Jain, S.N.; Rao, A.G. Synthesis of novel triazole/isoxazole functionalized 7-(trifluoromethyl) pyrido [2, 3-d] pyrimidine derivatives as promising anticancer and antibacterial agents. Bioorg. Med. Chem. Lett. 2016, 26, 2927–2930. [Google Scholar] [CrossRef]
- Lv, N.; Sun, M.; Liu, C.; Li, J. Design and synthesis of 2-phenylpyrimidine coumarin derivatives as anticancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 4578–4581. [Google Scholar] [CrossRef] [PubMed]
- Meneghesso, S.; Vanderlinden, E.; Stevaert, A.; McGuigan, C.; Balzarini, J.; Naesens, L. Synthesis and biological evaluation of pyrimidine nucleoside monophosphate prodrugs targeted against influenza virus. Antivir. Res. 2012, 94, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Yao, Q. Synthesis and quantitative structure–activity relationship (QSAR) analysis of some novel oxadiazolo [3, 4-d] pyrimidine nucleosides derivatives as antiviral agents. Bioorg. Med. Chem. Lett. 2015, 25, 241–244. [Google Scholar] [CrossRef]
- Amblard, F.; Aucagne, V.; Guenot, P.; Schinazi, R.F.; Agrofoglio, L.A. Synthesis and antiviral activity of novel acyclic nucleosides in the 5-alkynyl-and 6-alkylfuro [2, 3-d] pyrimidine series. Bioorg. Med. Chem. 2005, 13, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Sari, O.; Roy, V.; Métifiot, M.; Marchand, C.; Pommier, Y.; Bourg, S.; Bonnet, P.; Schinazi, R.F.; Agrofoglio, L.A. Synthesis of dihydropyrimidine α, γ-diketobutanoic acid derivatives targeting HIV integrase. Eur. J. Med. Chem. 2015, 104, 127–138. [Google Scholar] [CrossRef]
- El-Gaby, M.; Gaber, A.; Atalla, A.; Abd Al-Wahab, K. Novel synthesis and antifungal activity of pyrrole and pyrrolo [2, 3-d] pyrimidine derivatives containing sulfonamido moieties. Il Farm. 2002, 57, 613–617. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, X.-L.; Jiang, L.-L.; Liu, Z.-M.; Yang, G.-F. Synthesis, antifungal activity and CoMFA analysis of novel 1, 2, 4-triazolo [1, 5-a] pyrimidine derivatives. Eur. J. Med. Chem. 2008, 43, 595–603. [Google Scholar] [CrossRef]
- Maddila, S.; Gorle, S.; Seshadri, N.; Lavanya, P.; Jonnalagadda, S.B. Synthesis, antibacterial and antifungal activity of novel benzothiazole pyrimidine derivatives. Arab. J. Chem. 2016, 9, 681–687. [Google Scholar] [CrossRef]
- Bhalgat, C.M.; Ali, M.I.; Ramesh, B.; Ramu, G. Novel pyrimidine and its triazole fused derivatives: Synthesis and investigation of antioxidant and anti-inflammatory activity. Arab. J. Chem. 2014, 7, 986–993. [Google Scholar] [CrossRef]
- Quiroga, J.; Romo, P.E.; Ortiz, A.; Isaza, J.H.; Insuasty, B.; Abonia, R.; Nogueras, M.; Cobo, J. Synthesis, structures, electrochemical studies and antioxidant activity of 5-aryl-4-oxo-3, 4, 5, 8-tetrahydropyrido [2, 3-d] pyrimidine-7-carboxylic acids. J. Mol. Struct. 2016, 1120, 294–301. [Google Scholar] [CrossRef]
- Malik, N.; Dhiman, P.; Verma, P.K.; Khatkar, A. Design, synthesis, and biological evaluation of thiourea and guanidine derivatives of pyrimidine-6-carboxylate. Res. Chem. Intermed. 2015, 41, 7981–7993. [Google Scholar] [CrossRef]
- Kotaiah, Y.; Nagaraju, K.; Harikrishna, N.; Rao, C.V.; Yamini, L.; Vijjulatha, M. Synthesis, docking and evaluation of antioxidant and antimicrobial activities of novel 1, 2, 4-triazolo [3, 4-b][1, 3, 4] thiadiazol-6-yl) selenopheno [2, 3-d] pyrimidines. Eur. J. Med. Chem. 2014, 75, 195–202. [Google Scholar] [CrossRef]
- Vartale, S.P.; Halikar, N.K.; Pawar, Y.D.; Tawde, K.V. Synthesis and evaluation of 3-cyano-4-imino-2-methylthio-4H-pyrido[1,2-a]pyrimidine derivatives as potent antioxidant agents. Arab. J. Chem. 2016, 9, S1117–S1124. [Google Scholar] [CrossRef]
- Marepu, N.; Yeturu, S.; Pal, M. 1,2,3-Triazole fused with pyridine/pyrimidine as new template for antimicrobial agents: Regioselective synthesis and identification of potent N-heteroarenes. Bioorg. Med. Chem. Lett. 2018, 28, 3302–3306. [Google Scholar] [CrossRef]
- AlNeyadi, S.S.; Salem, A.A.; Ghattas, M.A.; Atatreh, N.; Abdou, I.M. Antibacterial activity and mechanism of action of the benzazole acrylonitrile-based compounds: In vitro, spectroscopic, and docking studies. Eur. J. Med. Chem. 2017, 136, 270–282. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xia, H.; Xia, Q.; Ren, Y.; He, H. Design and optimization of N-acylhydrazone pyrimidine derivatives as E. coli PDHc E1 inhibitors: Structure-activity relationship analysis, biological evaluation and molecular docking study. Bioorg. Med. Chem. 2017, 25, 5652–5661. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.K.; Pathak, V.; Seitz, L.E.; Suling, W.J.; Reynolds, R.C. Antimycobacterial agents. 1. Thio analogues of purine. J. Med. Chem. 2004, 47, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Johar, M.; Manning, T.; Tse, C.; Desroches, N.; Agrawal, B.; Kunimoto, D.Y.; Kumar, R. Growth Inhibition of Mycobacterium bovis, Mycobacterium tuberculosis and Mycobacterium avium In Vitro: Effect of 1-β-d-2 ‘-Arabinofuranosyl and 1-(2 ′-Deoxy-2 ′-fluoro-β-d-2 ′-ribofuranosyl) Pyrimidine Nucleoside Analogs. J. Med. Chem. 2007, 50, 3696–3705. [Google Scholar] [CrossRef]
- Ashour, H.M.; Shaaban, O.G.; Rizk, O.H.; El-Ashmawy, I.M. Synthesis and biological evaluation of thieno [2′, 3′: 4, 5] pyrimido [1, 2-b][1, 2, 4] triazines and thieno [2, 3-d][1, 2, 4] triazolo [1, 5-a] pyrimidines as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2013, 62, 341–351. [Google Scholar] [CrossRef]
- Sahu, M.; Siddiqui, N.; Sharma, V.; Wakode, S. 5, 6-Dihydropyrimidine-1 (2H)-carbothioamides: Synthesis, in vitro GABA-AT screening, anticonvulsant activity and molecular modelling study. Bioorg. Chem. 2018, 77, 56–67. [Google Scholar] [CrossRef]
- Huang, L.; Ding, J.; Li, M.; Hou, Z.; Geng, Y.; Li, X.; Yu, H. Discovery of [1, 2, 4]-triazolo [1, 5-a] pyrimidine-7 (4H)-one derivatives as positive modulators of GABAA1 receptor with potent anticonvulsant activity and low toxicity. Eur. J. Med. Chem. 2020, 185, 111824. [Google Scholar] [CrossRef] [PubMed]
- Severina, H.I.; Skupa, O.O.; Voloshchuk, N.I.; Suleiman, M.M.; Georgiyants, V.A. Synthesis and anticonvulsant activity of 6-methyl-2-((2-oxo-2-arylethyl) thio) pyrimidin-4 (3 H)-one derivatives and products of their cyclization. Pharmacia 2019, 66, 141–146. [Google Scholar] [CrossRef]
- Sahu, M.; Siddiqui, N.; Iqbal, R.; Sharma, V.; Wakode, S. Design, synthesis and evaluation of newer 5, 6-dihydropyrimidine-2 (1H)-thiones as GABA-AT inhibitors for anticonvulsant potential. Bioorg. Chem. 2017, 74, 166–178. [Google Scholar] [CrossRef]
- Sahin, Z.; Ertas, M.; Berk, B.; Biltekin, S.N.; Yurttas, L.; Demirayak, S. Studies on non-steroidal inhibitors of aromatase enzyme; 4-(aryl/heteroaryl)-2-(pyrimidin-2-yl) thiazole derivatives. Bioorg. Med. Chem. Res. 2018, 26, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Khan, S.I.; Thakur, A.; Ponnan, P.; Rawat, D.S. 4-Aminoquinoline-pyrimidine-aminoalkanols: Synthesis, in vitro antimalarial activity, docking studies and ADME predictions. New J. Chem. 2015, 39, 3474–3483. [Google Scholar] [CrossRef]
- Agarwal, A.; Srivastava, K.; Puri, S.; Chauhan, P.M. Synthesis of 2, 4, 6-trisubstituted pyrimidines as antimalarial agents. Bioorg. Med. Chem. 2005, 13, 4645–4650. [Google Scholar] [CrossRef]
- Kumar, D.; Khan, S.I.; Tekwani, B.L.; Ponnan, P.; Rawat, D.S. Synthesis, antimalarial activity, heme binding and docking studies of 4-aminoquinoline–pyrimidine based molecular hybrids. RSC Adv. 2014, 4, 63655–63669. [Google Scholar] [CrossRef]
- Pretorius, S.I.; Breytenbach, W.J.; De Kock, C.; Smith, P.J.; N’Da, D.D. Synthesis, characterization and antimalarial activity of quinoline–pyrimidine hybrids. Bioorg. Med. Chem. 2013, 21, 269–277. [Google Scholar]
- Azeredo, L.F.S.; Coutinho, J.P.; Jabor, V.A.; Feliciano, P.R.; Nonato, M.C.; Kaiser, C.R.; Menezes, C.M.S.; Hammes, A.S.; Caffarena, E.R.; Hoelz, L.V. Evaluation of 7-arylaminopyrazolo [1, 5-a] pyrimidines as anti-Plasmodium falciparum, antimalarial, and Pf-dihydroorotate dehydrogenase inhibitors. Eur. J. Med. Chem. 2017, 126, 72–83. [Google Scholar] [CrossRef]
- Yadav, R.R.; Khan, S.I.; Singh, S.; Khan, I.A.; Vishwakarma, R.A.; Bharate, S.B. Synthesis, antimalarial and antitubercular activities of meridianin derivatives. Eur. J. Med. Chem. 2015, 98, 160–169. [Google Scholar] [CrossRef]
- Rani, J.; Kumar, S.; Saini, M.; Mundlia, J.; Verma, P.K. Biological potential of pyrimidine derivatives in a new era. Res. Chem. Intermed. 2016, 42, 6777–6804. [Google Scholar] [CrossRef]
- Bukhari, S.; Butt, A.; Amjad, M.; Ahmad, W.; Shah, V.; Trivedi, A. Synthesis and evaluation of chalcone analogues based pyrimidines as angiotensin converting enzyme inhibitors. Pak. J. Biol. Sci. 2013, 16, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Marvaniya, H.M.; Parikh, P.K.; Sen, D.J. Synthesis and in-vitro screening of 3, 4-dihydropyrimidin-2 (1H)-one derivatives for antihypertensive and calcium channel blocking activity. J. Appl. Pharm. Sci. 2011, 01, 109–113. [Google Scholar]
- Alam, O.; Khan, S.A.; Siddiqui, N.; Ahsan, W.; Verma, S.P.; Gilani, S.J. Antihypertensive activity of newer 1, 4-dihydro-5-pyrimidine carboxamides: Synthesis and pharmacological evaluation. Eur. J. Med. Chem. 2010, 45, 5113–5119. [Google Scholar] [CrossRef]
- Katouah, H.A.; Gaffer, H.E. Synthesis and docking study of pyrimidine derivatives scaffold for anti-hypertension application. ChemistrySelect 2019, 4, 6250–6255. [Google Scholar] [CrossRef]
- Tozkoparan, B.; Ertan, M.; Kelicen, P.; Demirdamar, R. Synthesis and anti-inflammatory activities of some thiazolo [3, 2-a] pyrimidine derivatives. Il Farm. 1999, 54, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Yejella, R.P.; Atla, S.R. A study of anti-inflammatory and analgesic activity of new 2, 4, 6-trisubstituted pyrimidines. Chem. Pharm. Bull. 2011, 59, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.P.; Ding, Y.W.; Zhang, H.B.; Xu, L.; Dai, Y. Synthesis and anti-inflammatory activity of imidazo [1, 2-a] pyrimidine derivatives. Chin. Chem. Lett. 2008, 19, 669–672. [Google Scholar] [CrossRef]
- Keche, A.P.; Hatnapure, G.D.; Tale, R.H.; Rodge, A.H.; Birajdar, S.S.; Kamble, V.M. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: Synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 3445–3448. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Kamel, R.; Fatahala, S.S. Synthesis and biological evaluation of some thio containing pyrrolo [2, 3-d] pyrimidine derivatives for their anti-inflammatory and anti-microbial activities. Eur. J. Med. Chem. 2010, 45, 2994–3004. [Google Scholar] [CrossRef]
- Sondhi, S.M.; Jain, S.; Dinodia, M.; Shukla, R.; Raghubir, R. One pot synthesis of pyrimidine and bispyrimidine derivatives and their evaluation for anti-inflammatory and analgesic activities. Bioorg. Med. Chem. 2007, 15, 3334–3344. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Liu, X.; Liu, Y.; Liu, W.; Li, Y.; Yu, G.; Tian, X.; Zhang, Y.; Song, J.; Jin, C.; et al. Drug Discovery Targeting Focal Adhesion Kinase (FAK) as a Promising Cancer Therapy. Molecules 2021, 26, 4250. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Martines, M.; Duarte, A.; Jorge, J.; Rasool, S.; Muhammad, R.; Ahmad, N.; Umar, M. Research developments in the syntheses, anti-inflammatory activities and structure-activity relationships of pyrimidines. RSC Adv. 2021, 11, 6060–6098. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Narasimhan, B. Therapeutic potential of heterocyclic pyrimidine scaffolds. Chem. Cent. J. 2018, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Iikubo, K.; Kondoh, Y.; Shimada, I.; Matsuya, T.; Mori, K.; Ueno, Y.; Okada, M. Discovery of N-{2-Methoxy-4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]phenyl}-N′-[2-(propane-2-sulfonyl)phenyl]-1,3,5-triazine-2,4-diamine (ASP3026), a Potent and Selective Anaplastic Lymphoma Kinase (ALK) Inhibitor. Chem. Pharm. Bull. 2018, 66, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Vagiannis, D.; Novotna, E.; Skarka, A.; Kammerer, S.; Küpper, J.; Chen, S.; Guo, L.; Staud, F.; Hofman, J. Ensartinib (X-396) Effectively Modulates Pharmacokinetic Resistance Mediated by ABCB1 and ABCG2 Drug Efflux Transporters and CYP3A4 Biotransformation Enzyme. Cancers 2020, 12, 813. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Wang, L.-Y.; Chang, C.-H.; Kuo, Y.-H.; Kaneko, K.; Takayama, H.; Kimura, M.; Juang, S.-H.; Wong, F.F. One-pot synthesis and antiproliferative evaluation of pyrazolo [3, 4-d] pyrimidine derivatives. Tetrahedron 2012, 68, 9658–9664. [Google Scholar] [CrossRef]
- Ibrahim, D.A.; Ismail, N.S. Design, synthesis and biological study of novel pyrido [2, 3-d] pyrimidine as anti-proliferative CDK2 inhibitors. Eur. J. Med. Chem. 2011, 46, 5825–5832. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, M.; Li, H.; Chen, L. Entrectinib, a new multi-target inhibitor for cancer therapy. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 150, 112974. [Google Scholar] [CrossRef]
- Tutka, P.; Mróz, K.; Mróz, T.; Buszewicz, G.; Aebisher, D.; Bartusik-Aebisher, D.; Kołodziejczyk, P.; Łuszczki, J.J. Effects of androsterone on the protective action of various antiepileptic drugs against maximal electroshock-induced seizures in mice. Psychoneuroendocrinology 2019, 101, 27–34. [Google Scholar] [CrossRef]
- El Kihel, L. Oxidative metabolism of dehydroepiandrosterone (DHEA) and biologically active oxygenated metabolites of DHEA and epiandrosterone (EpiA)--recent reports. Steroids 2012, 77, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Honda, A.; Matsuzaki, Y.; Fukushima, S.; Tanaka, N.; Takagiwa, A.; Fujimoto, Y.; Miyazaki, H.; Salen, G. Anti-proliferative action of endogenous dehydroepiandrosterone metabolites on human cancer cell lines. Steroids 2003, 68, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Wei, Y.; Shi, L.; Yang, Q.; Yang, Z. Synthesis of novel steroid derivatives derived from dehydroepiandrosterone as potential anticancer agents. Anti-Cancer Agents Med. Chem. 2013, 13, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Shi, L.; Zhang, Z.; Yang, Z. Steroidal[17,16-d]pyrimidines derived from dehydroepiandrosterone: A convenient synthesis, antiproliferation activity, structure-activity relationships, and role of heterocyclic moiety. Sci. Rep. 2017, 7, 44439. [Google Scholar] [CrossRef]
- Ke, S.; Shi, L.; Yang, Z. Discovery of novel isatin-dehydroepiandrosterone conjugates as potential anticancer agents. Bioorg. Med. Chem. Lett. 2015, 25, 4628–4631. [Google Scholar] [CrossRef]
- Ke, S.; Li, N.; Ke, T.; Shi, L.; Zhang, Z.; Fang, W.; Zhang, Y.; Wang, K.; Zhou, R.; Wan, Z.; et al. Synthesis and evaluation of steroidal thiazoline conjugates as potential antiviral agents. Future Med. Chem. 2018, 10, 2589–2605. [Google Scholar] [CrossRef]
- Ke, S.; Zhang, Z.; Liu, M.; Fang, W.; Huang, D.; Wan, Z.; Zhou, R.; Wang, K.; Shi, L. Synthesis and bioevaluation of novel steroidal isatin conjugates derived from epiandrosterone/androsterone. J. Enzym. Inhib. Med. Chem. 2019, 34, 1607–1614. [Google Scholar] [CrossRef]
- Tchédam Ngatcha, B.; Luu-The, V.; Labrie, F.; Poirier, D. Androsterone 3α-Ether-3β-Substituted and Androsterone 3β-Substituted Derivatives as Inhibitors of Type 3 17β-Hydroxysteroid Dehydrogenase: Chemical Synthesis and Structure-Activity Relationship. J. Med. Chem. 2005, 48, 5257–5268. [Google Scholar] [CrossRef]
- Malikova, J.; Swaczynova, J.; Kolar, Z.; Strnad, M. Anticancer and antiproliferative activity of natural brassinosteroids. Phytochemistry 2008, 69, 418–426. [Google Scholar] [CrossRef]
- Steigerová, J.; Rárová, L.; Oklešt’ková, J.; Křížová, K.; Levková, M.; Šváchová, M.; Kolář, Z.; Strnad, M. Mechanisms of natural brassinosteroid-induced apoptosis of prostate cancer cells. Food Chem. Toxicol. 2012, 50, 4068–4076. [Google Scholar] [CrossRef]
- Elmegeed, G.A.; Khalil, W.K.B.; Mohareb, R.M.; Ahmed, H.H.; Abd-Elhalim, M.M.; Elsayed, G.H. Cytotoxicity and gene expression profiles of novel synthesized steroid derivatives as chemotherapeutic anti-breast cancer agents. Bioorg. Med. Chem. 2011, 19, 6860–6872. [Google Scholar] [CrossRef] [PubMed]
- Rarova, L.; Zahler, S.; Liebl, J.; Krystof, V.; Sedlak, D.; Bartunek, P.; Kohout, L.; Strnad, M. Brassinosteroids inhibit in vitro angiogenesis in human endothelial cells. Steroids 2012, 77, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, L.; Zhao, D.; Gan, C.; Huang, X.; Xiao, Q.; Qi, B.; Yang, L.; Huang, Y. Synthesis, characterization and antitumor activities of some steroidal derivatives with side chain of 17-hydrazone aromatic heterocycle. Steroids 2015, 95, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.; Schmidt, S.; Fedosova, N.U.; Mollenhauer, J.; Jensen, H.H. Synthesis and evaluation of cardiac glycoside mimics as potential anticancer drugs. Bioorg. Med. Chem. 2011, 19, 2407–2417. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Tang, J.; Qiao, A.; Wang, G.; Jiang, M.; Liu, R.H.; Yao, X. Cytotoxic biotransformed products from cinobufagin by Mucor spinosus and Aspergillus Niger. Steroids 2006, 71, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Alley, M.; Scudiero, D.; Monks, A.; Hursey, M.; Czerwinski, M.; Fine, D.; Abbott, B.; Mayo, J.; Shoemaker, R.; Boyd, M. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Brown, N.R.; Korolchuk, S.; Martin, M.P.; Stanley, W.A.; Moukhametzianov, R.; Noble, M.E.; Endicott, J.A. CDK1 structures reveal conserved and unique features of the essential cell cycle CDK. Nat. Commun. 2015, 6, 6769. [Google Scholar] [CrossRef]
- Ke, S.; Shi, L.; Cao, X.; Yang, Q.; Liang, Y.; Yang, Z. Heterocycle-functional gramine analogues: Solvent- and catalyst-free synthesis and their inhibition activities against cell proliferation. Eur. J. Med. Chem. 2012, 54, 248–254. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
