Advances in Semiconductor-Based Nanocomposite Photo(electro)catalysts for Nitrogen Reduction to Ammonia
Abstract
1. Introduction
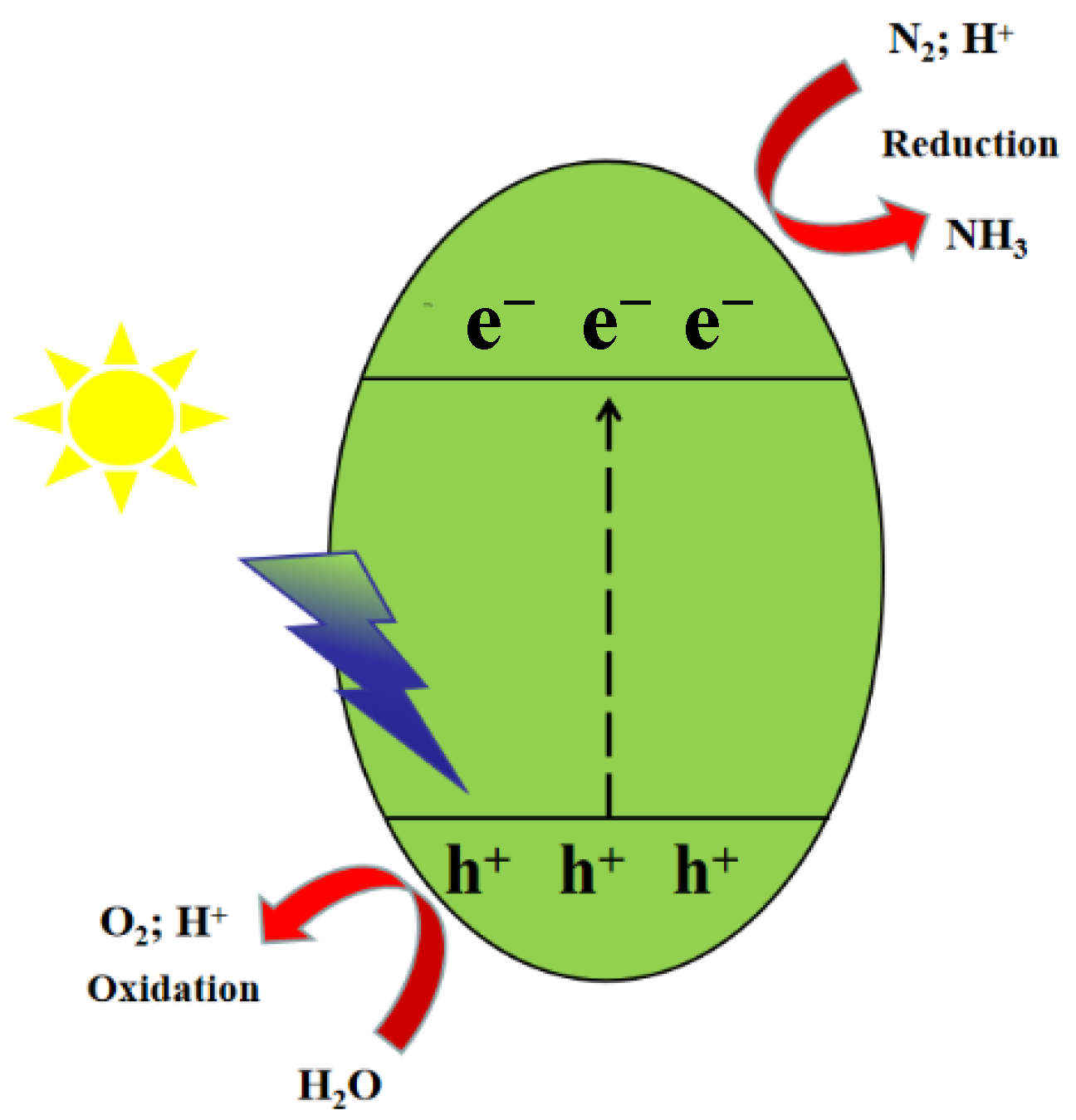
2. Nitrogen Reduction Reaction
3. Challenges of NRR
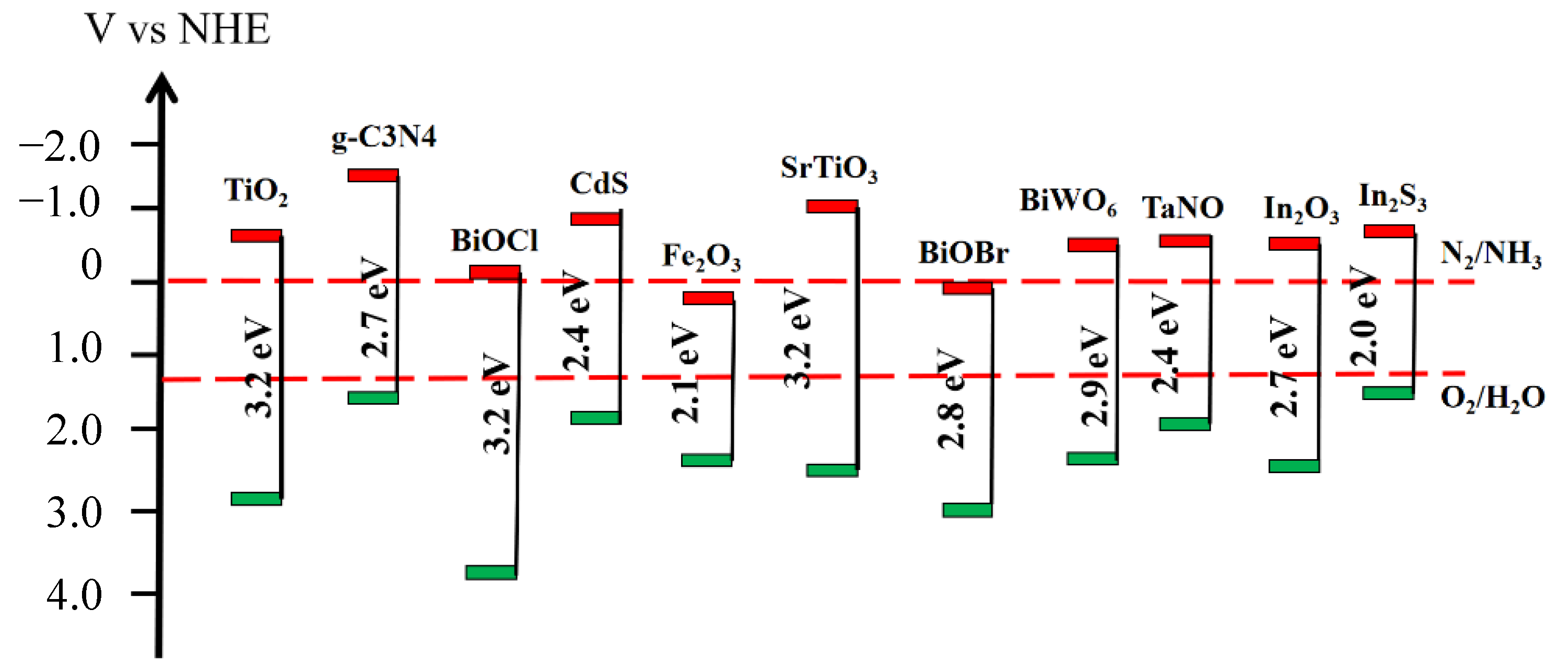
3.1. Low Utilization of Light Energy
3.2. Low Separation Rate of Photogenerated Carriers
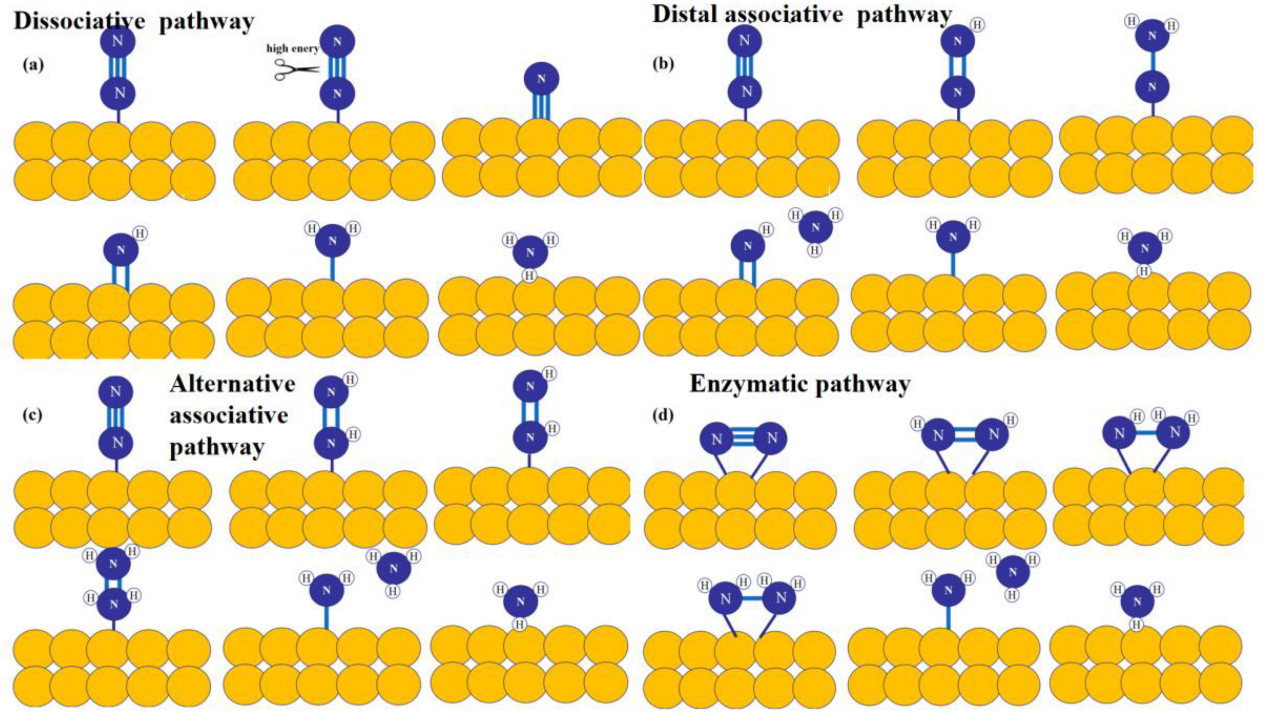
3.3. N2 Adsorption and Activation Difficulties
3.4. Hydrogen Analysis Competition
3.5. Other Problems in NRR
4. Modification Strategies for the Photo(electro)catalysts
4.1. Morphology Modulation
4.1.1. Photocatalyst
4.1.2. Electrocatalyst
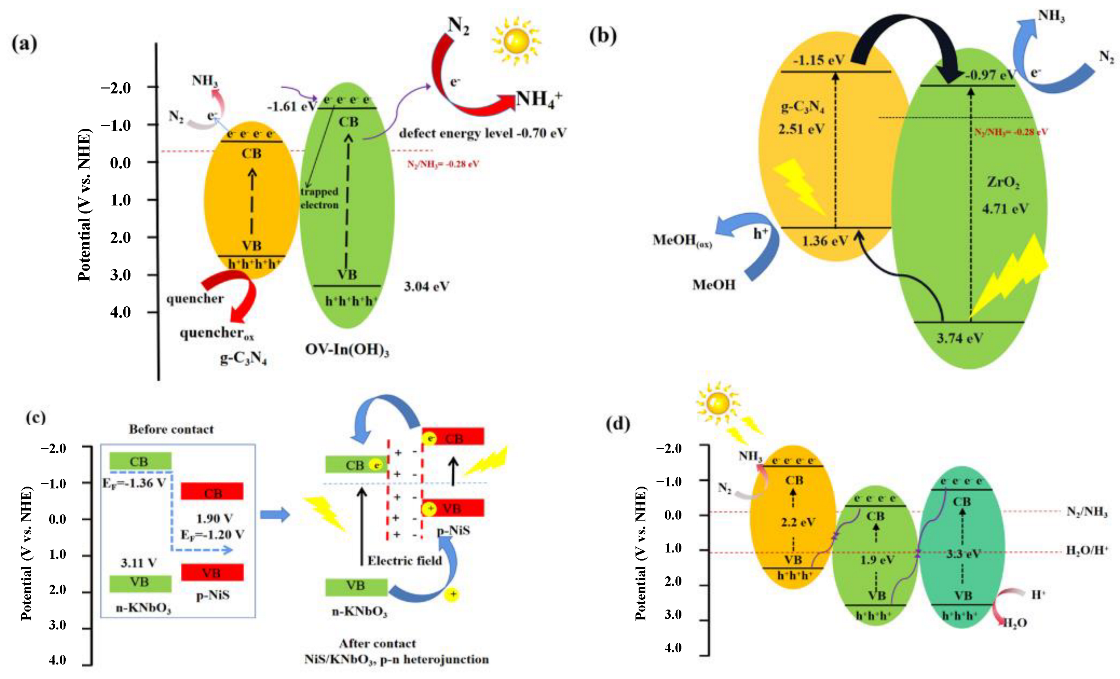
4.2. Heterostructure Construction
4.3. Introduction of Vacancies
4.3.1. Photocatalyst
4.3.2. Electrocatalyst
4.4. Cocatalyst Addition
4.5. Computational Modeling
5. Conclusions and Prospects
- Formulate a unified evaluation standard for nitrogen fixation systems. Various factors, including reaction equipment, reaction temperature and pressure, light source and intensity, and product detection methods, influenced the stability of photo(electro)catalytic efficiency. In addition, the evaluation standard of the photocatalytic NRR reaction is usually based on the absolute yield and evolution rate of ammonia production (μmol·gcat−1 h−1 or μmol·h−1). With the innovation and development of the photocatalytic reaction system, reactors’ design types have been enriched [92]. The application form of photocatalyst is no longer a single suspension type, and the supported catalyst that is convenient for recovery and utilization has also begun to receive attention [93]. How to make a uniform and fair comparison of the performance of these different types of photocatalysis systems has become a thorny problem. In addition to specifying various parameters in detail in the report, the number of substances that mainly play a catalytic role and the corresponding active sites are discussed. Therefore, a reliable and strict evaluation standard for the photo(electro)catalytic nitrogen fixation must be established to ensure the reliability and comparability of the experimental data.
- Because of the low reaction efficiency caused by the poor solubility of the N2 molecule, it is considered that the nitrogen source used in the photo(electro)catalytic reaction can be replaced. Nitrogen-based compounds such as nitrate, nitrite and nitrogen oxide are readily soluble in water. Therefore, the problem of N≡N cracking and activation could be avoided, and the hydrogen evolution reaction could be inhibited. Similarly, water vapor can also be used as the proton source. Simplifying the traditional gas–liquid–solid three-phase reaction into a gas–solid two-phase reaction is a potential method to improve the efficiency of the NRR reaction.
- Recently, a first-principle calculation combined with kinetic analysis has been widely used to predict the reaction potential barrier of the rate-determining step. However, the precise reaction kinetics theory has not been determined and is still developing. It is necessary to conduct more in-depth thermodynamic and kinetic studies to understand the catalytic performance of ammonia synthesis more practically. For example, the photo(electro)catalytic nitrogen reduction process was studied at the molecular or atomic level by combining experiments and theoretical calculations. Although some of the studies combine theory and experiment, most theoretical calculations focus on the free–energy change in the active intermediate—the energy barrier of the rate-limiting step. These calculations of the adsorption energy of N2 are assumed to be performed under vacuum conditions, ignoring the influence of the determinants of the electrochemical system (e.g., temperature, pH, mass transfer rate, proton supply, N2 solubility), which are different from the actual experimental conditions. Given these problems, in-situ experimental techniques can be used to capture and identify reaction intermediates and monitor the microscopic changes of catalysts to assist theoretical research. Therefore, it is necessary to further improve the calculation method and model of NRR on the surface of non-homogeneous catalysts to combine theoretical calculations and experiments and to provide further guidance for designing electrocatalyst structures.
- Currently, the catalytic activity and selectivity of catalysts for ammonia synthesis in aqueous solutions are extremely low. One of the main factors is the presence of competition from side reactions. Therefore, the catalytic activity and selectivity can be significantly improved by suppressing the occurrence of side reactions. Specifically, optimizing the size and morphology of catalysts can generate favorable coordination sites, influence the binding strength of reactants or key intermediates on the catalyst surface and construct defect engineering of catalysts. It was necessary to capture photoelectrons and sub-stable electrons through vacancies and transfer them into the antibonding orbitals of adsorbed N2 to promote the breakage of N≡N bonds. The construction of stress engineering to regulate the atomic surface spacing and bond length also can change the catalyst’s electronic structure, thus facilitating the nitrogen reduction reaction. In addition, while exploring effective catalysts and constructing excellent photocatalytic systems for future research, we should also pay attention to the efficiency and stability issues.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Foster, S.L.; Bakovic, S.I.P.; Duda, R.D.; Maheshwari, S.; Lauren, F.G. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 2018, 1, 490–500. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Guth, T.D. Photolysis of water and photoreduction of nitrogen on Titanium Dioxide. J. Am. Chem. Soc. 1977, 99, 7189–7193. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Strampach, N.; Hui, L.N.; Palmer, M.R.; Salehi, J. Nitrogen photoreduction on desert sands under sterile conditions. Proc. Natl. Acad. Sci. USA 1983, 80, 3873–3876. [Google Scholar] [CrossRef]
- Tennakone, K.; Bandara, J.M.S.; Thaminimulla, C.T.K.; Jayatilake, W.D.W.; Ketipearachchi, U.S.; Ileperuma, O.A.; Priyadarshana, M.K.A. Photoreduction of dinitrogen to ammonia by ultrafine particles of Fe(O)OH formed by photohydrolysis of iron(Ⅱ) bicarbonate. Langmuir 1991, 7, 2166–2168. [Google Scholar] [CrossRef]
- Faria, J.A. Renaissance of ammonia synthesis for sustainable production of energy and fertilizers. Curr. Opin. Green Sustain. Chem. 2021, 29, 100466. [Google Scholar] [CrossRef]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of Earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef]
- Stein, L.Y.; Klotz, M.G. The nitrogen cycle. Curr. Biol. 2016, 26, 94–98. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber-Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Wang, Y.; Meyer, T.J. A route to renewable energy triggered by the Haber-Bosch process. Chem 2019, 5, 496–497. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, Y.; You, Y.; Zeng, X.; Ma, T.; Huang, H. Bifunctionalydrogen Production and Storage on 0D-1DHeterojunctin Cd0.5Zn0.5S@Halloysites. Adv. Funct. Mater. 2019, 29, 1903825. [Google Scholar] [CrossRef]
- Guo, J.; Chen, P. Ammonia history in the making. Nat. Catal. 2021, 4, 734–735. [Google Scholar] [CrossRef]
- Lu, W.; Xia, M.; Hong, W.; Huang, K.; Ozin, G.A. Greening ammonia toward the solar ammonia refinery. Joule 2018, 2, 1055–1074. [Google Scholar]
- Prasidha, W.; Widyatama, A.; Aziz, M. Energy-saving and environmentally-benign integrated ammonia production system. Energy 2021, 235, 121400. [Google Scholar]
- Wang, Q.; Guo, J.; Chen, P. Recent progress towards mild-condition ammonia synthesis. J. Energy Chem. 2019, 36, 25–36. [Google Scholar] [CrossRef]
- Ali, H.; Masar, M.; Guler, A.C.; Urbanek, M.; Machovsky, M.; Kuritka, I. Heterojunction-based photocatalytic nitrogen fixation: Principles and current progress. Nanoscale Adv. 2021, 3, 6358–6372. [Google Scholar] [CrossRef]
- Van der Ham, C.J.; Koper, M.T.; Hetterscheid, D.G. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef]
- Hao, D.; Liu, Y.; Gao, S.; Arandiyan, H.; Bai, X.; Kong, Q.; Wei, W.; Shen, P.K.; Ni, B.J. Emerging artificial nitrogen cycle processes through novel electrochemical and photochemical synthesis. Mater. Today 2021, 46, 212–233. [Google Scholar] [CrossRef]
- Wang, L.; Xia, Y.; Yu, J. Hydrogen-bond activation of N2 molecules and photocatalytic nitrogen fixation. Chem 2021, 7, 1983–1985. [Google Scholar] [CrossRef]
- Xue, X.; Chen, R.; Yan, C.; Zhao, P.Y.; Hu, Y.; Zhang, W.J.; Yang, S.Y.; Zhong, J. Review on photocatalytic and electrocatalytic artificial nitrogen fixation for ammonia synthesis at mild conditions: Advances, challenges and perspectives. Nano Res. 2019, 12, 1229–1249. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibanez, P.; DiSomma, I. Solar photocatalysis: Materials, Reactors, Some Commer-cial, and Pre-industrialized Applications. A Comprehensive Approach. Appl. Catal. B: Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Vu, M.H.; Sakar, M.; Hassanzadeh-Tabrizi, S.A.; Do, T.-O. Photo(electro)catalytic nitrogen fixation: Problems and possibilities. Adv. Mater. Interfaces 2019, 6, 1900091. [Google Scholar] [CrossRef]
- Luo, J.; Bai, X.; Li, Q.; Liu, Z.H. Band structure engineering of bioinspired Fe doped SrMoO4 for enhanced photocatalytic nitrogen reduction performance. Nano Energy 2019, 66, 104187. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Shi, R.; Waterhouse, G.I.N.; Zhang, T. Photocatalytic ammonia synthesis: Recent progress and future. Energy Chem. 2019, 1, 100013. [Google Scholar] [CrossRef]
- Niu, H.; Wang, X.; Shao, C.; Zhang, Z.; Guo, Y. Computational screening single-atom catalysts supported on g–CN for N2 reduction: High activity and selectivity. ACS Sustainable Chem. Eng. 2020, 8, 13749–13758. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Zhao, Z.J.; Yang, W.; Gong, J. Promoted fixation of molecular nitrogen with surface oxygen vacancies on plasmon-enhanced TiO2 photoelectrodes. Angew. Chem. Int. Ed. 2018, 57, 5278–5282. [Google Scholar] [CrossRef]
- Mehta, P.; Barboun, P.; Herrera, F.A. Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis. Nat. Catal. 2018, 1, 269–275. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.-i.; Moon, G.-h.; Choi, W. Photoinduced Charge Transfer Processes in Solar Photocatalysis Based on Modified TiO2. Energy Environ. Sci. 2016, 9, 411–433. [Google Scholar] [CrossRef]
- Ojha, N.; Kumar, S. Tri-phase photocatalysis for CO2 reduction and N2 fixation with efficient electron transfer on a hydrophilic surface of transition-metal-doped MIL-88A (Fe). Appl. Catal. B Environ. 2021, 292, 120166. [Google Scholar] [CrossRef]
- Chalkley, M.J.; Drover, M.W.; Peters, J.C. Catalytic N2-to-NH3 (or -N2H4) conversion by well-defined molecular coordination complexes. Chem. Rev. 2020, 120, 5582–5636. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Yu, J.; Parkin, I.P.; Fujishima, A.; Nakata, K. Intrinsic Intermediate Gap States of TiO2 Materials and Their Roles in Charge Carrier Kinetics. J. Photochem. Photobio. C: Photochem. Rev. 2019, 39, 1–57. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, X.; Waterhouse, G.I.N.; Zhao, Y.; Zhang, T. The journey toward low temperature, low pressure catalytic nitrogen fixation. Adv. Energy Mater. 2020, 10, 2000659. [Google Scholar] [CrossRef]
- Li, H.; Mao, C.; Shang, H.; Yang, Z.; Ai, Z.; Zhang, L. New opportunities for efficient N2 fixation by nanosheet photocatalysts. Nanoscale 2018, 10, 15429–15435. [Google Scholar] [CrossRef]
- Rohilla, S.; Gupta, A.; Kumar, V.; Kumari, S.; Petru, M.; Amor, N.; Noman, M.T.; Dalal, J. Excellent UV-Light Triggered Photocatalytic Performance of ZnO.SiO2 Nanocomposite for Water Pollutant Compound Methyl Orange Dye. Nanomaterials 2021, 11, 2548. [Google Scholar] [CrossRef]
- Shimi, A.K.; Parvathiraj, C.; Kumari, S. Green synthesis of SrO nanoparticles using leaf extract of Albizia julibrissin and its recyclable photocatalytic activity: An eco-friendly approach for treatment of industrial wastewater. Environ. Sci. Adv. 2022, 1, 849. [Google Scholar] [CrossRef]
- Zhao, T.Y.; Zhao, Y.; Jiang, L. Nano-/microstructure improved photocatalytic activities of semiconductors. Phil. Trans. R. Soc. A 2013, 371. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.D.; Xie, Y. Recent progress in ultmicrostructurerathin two-dimensional semiconductors for photocatalysis. Mater. Sci. Eng. R Rep. 2018, 130, 1–39. [Google Scholar]
- Gao, X.M.; Shang, Y.Y.; Liu, L.B.; Fu, F. Chemisorption-enhanced photocatalytic nitrogen fixation via 2D ultrathin p-n heterojunction AgCl/δ-Bi2O3 nanosheets. J. Catal. 2019, 371, 71–80. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Z.; Zhao, K.; Lin, S.L.; Li, H.; Gao, X. Photocatalytic nitrogen fixation: Oxygen vacancy modified novel micro-nanosheet structure Bi2O2CO3 with band gap engineering. J. Colloid Interface Sci. 2021, 583, 499–509. [Google Scholar] [CrossRef]
- Vu, M.H.; Quach, T.A.; Do, T.O. The construction of Ru-doped In2O3 hollow peanut-like structure for an enhanced photocatalytic nitrogen reduction under solar light irradiation. Sustain. Energy Fuels 2021, 5, 2528–2536. [Google Scholar] [CrossRef]
- Xiong, J.; Song, P.; Di, J.; Li, H.M. Atomic-level active sites steering in ultrathin photocatalysts to trigger high efficiency nitrogen fixation. Chem. Eng. J. 2020, 402, 126208. [Google Scholar] [CrossRef]
- Guo, X.L.; Duan, J.H.; Li, C.J.; Zhang, Z.S.; Wang, W.W. Enhanced photocatalytic nitrogen fixation of etched Ag-doped PM-CdS catalyst under visible light irradiation. Opt. Mater. 2022, 125, 112137. [Google Scholar] [CrossRef]
- Zhang, G.H.; Meng, Y.; Xie, B. Precise location and regulation of active sites for highly efficient photocatalytic synthesis of ammonia by facet-dependent BiVO4 single crystals. Appl. Catal. B Environ. 2021, 296, 120379. [Google Scholar] [CrossRef]
- Guo, C.; Ran, J.; Vasileff, A. Rational design of electrocatalysts and photo(electro)catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions. Energy Environ. Sci. 2018, 11, 45–56. [Google Scholar] [CrossRef]
- de Sá, I.F.; Carvalho, P.H.; Centurion, H.A.; Gonçalves, R.V.; Scholten, J.D. Sustainable Nitrogen Photofixation Promoted by Carbon Nitride Supported Bimetallic RuPd Nanoparticles under Mild Conditions. ACS Sustain. Chem. Eng. 2021, 9, 8721–8730. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, X.; Zhang, W.; Kheradmand, A.; Jiang, Y. Near-infrared-triggered nitrogen fixation over upconversion nanoparticles assembled carbon nitride nanotubes with nitrogen vacancies. ACS Appl. Mater. Interfaces 2021, 13, 32937–32947. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, J.; Wu, D.; Bai, H.; Yang, B.C. Au nanoparticle-embedded, nitrogen-deficient hollow mesoporous carbon nitride spheres for nitrogen photofixation. J. Mater. Chem. A 2020, 8, 16218. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Wang, Z.; Liu, S.; Xu, Y.; Li, X.; Wang, L.; Wang, H. One-step synthesis of self-standing porous palladium-ruthenium nanosheet array on Ni foam for ambient electrosynthesis of ammonia. Int. J. Hydrogen Energy 2020, 45, 5997–6005. [Google Scholar] [CrossRef]
- Shi, M.M.; Bao, D.; Wulan, B.R.; Li, Y.H.; Zhang, Y.F.; Yan, J.M.; Jiang, Q. Au Sub-Nanoclusters on TiO2 toward Highly Efficient and Selective Electrocatalyst for N2 Conversion to NH3 at Ambient Conditions. Adv. Mater. 2017, 29, 1606550. [Google Scholar] [CrossRef]
- Ihsanullah, I. MXenes as next-generation materials for the photocatalytic degradation of pharmaceuticals in water. J. Environ. Chem. Eng. 2022, 10, 107381. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Li, Y.; Guo, X.; Jin, Z. Lotus-leaf-like Bi2O2CO3 nanosheet combined with Mo2S3 for higher photocatalytic hydrogen evolution. Sep. Purif. Technol. 2022, 288, 120588. [Google Scholar] [CrossRef]
- Fan, J.; Zuo, M.; Ding, Z.; Zhao, Z.; Liu, J.; Sun, B. A readily synthesis of oxygen vacancy-induced In(OH)3/carbon nitride 0D/2D heterojunction for enhanced visible-light-driven nitrogen fixation. Chem. Eng. J. 2020, 396, 125263. [Google Scholar] [CrossRef]
- Mou, H.; Wang, J.; Yu, D.; Zhao, Z.; Liu, J.; Sun, B. Fabricating amorphous g–C3N4/ZrO2 photocatalyst by one-step pyrolysis for solar-driven ambient ammonia synthesis. ACS Appl. Mater. Interfaces 2019, 11, 44360–44365. [Google Scholar] [CrossRef]
- Lee, J.; Tan, L.L.; Chai, S.P. Heterojunction photocatalysts for artificial nitrogen fixation: Fundamentals, latest advances and future perspectives. Nanoscale 2021, 13, 7011–7033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xing, P.; Zhang, J.; Chen, L.; Yang, J.; Hu, X.; Zhao, L.; Wu, Y.; He, Y. Facile preparation of novel nickel sulfide modified KNbO3 heterojunction composite and its enhanced performance in photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2021, 590, 548–560. [Google Scholar] [CrossRef]
- Su, Q.; Wang, W.; Zhang, Z.; Duan, J. Enhanced photocatalytic performance of Cu2O/MoS2/ZnO composites on Cu mesh substrate for nitrogen reduction. Nanotechnology 2021, 32, 285706. [Google Scholar] [CrossRef]
- Hu, K.Q.; Huang, Z.W.; Zeng, L.W.; Zhang, Z.H.; Mei, L.; Chai, Z.F.; Shi, W.Q. Recent advances in MOF-based materials for photocatalytic nitrogen fixation. Eur. J. Inorg. Chem. 2022, 2022, 202100748. [Google Scholar] [CrossRef]
- Ye, H.; Gong, N.; Cao, Y.; Fan, X.; Song, X.; Li, H.; Wang, C.; Mei, Y.; Zhu, Y. Insights into the role of protonation in covalent triazine framework-based photocatalytic hydrogen evolution. Chem. Mater. 2022, 34, 1481–1490. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, S.; Li, F.; Fan, Z.; Bai, J.; Lu, G.; Wang, Q. Photofixation of atmospheric nitrogen to ammonia with a novel ternary metal sulfide catalyst under visible light. RSC Adv. 2016, 6, 49862–49867. [Google Scholar] [CrossRef]
- Dong, G.; Ho, W.; Wang, C. Selective photocatalytic N2 fixation dependent on g–C3N4 induced by nitrogen vacancies. J. Mater. Chem. A 2015, 3, 23435–23441. [Google Scholar] [CrossRef]
- Cao, S.; Fan, B.; Feng, Y.; Chen, H.; Jiang, F.; Wang, X. Sulfur-doped g–C3N4 nanosheets with carbon vacancies: General synthesis and improved activity for simulated solar-light photocatalytic nitrogen fixation. Chem. Eng. J. 2018, 353, 147–156. [Google Scholar] [CrossRef]
- Yao, J.X.; Bao, D.; Zhang, Q.; Shi, M.M.; Wang, Y.; Gao, R.; Yan, J.M.; Jiang, Q. Tailoring Oxygen Vacancies of BiVO4 toward Highly Efficient Noble-Metal-Free Electrocatalyst for Artificial N2 Fixation under Ambient Conditions. Small Methods 2019, 3, 1800333. [Google Scholar] [CrossRef]
- Fang, Y.F.; Liu, Z.C.; Han, J.R.; Jin, Z.Y.; Han, Y.Q.; Wang, F.X.; Niu, Y.S.; Wu, Y.P.; Xu, Y.H. High-Performance Electrocatalytic Conversion of N2 to NH3 Using Oxygen-Vacancy-Rich TiO2 In Situ Grown on Ti3C2Tx MXene. Adv. Energy Mater. 2019, 9, 1803406. [Google Scholar] [CrossRef]
- Mukherjee, S.; Cullen, D.A.; Karakalos, S.; Liu, K.X.; Zhang, H.; Zhao, S.; Xu, H.; More, K.L.; Wang, G.F.; Wu, G. Metal-organic frameworkderived nitrogen-doped highly disordered carbon for electrochemical ammonia synthesis using N2 and H2O in alkaline electrolytes. Nano Energy 2018, 48, 217–226. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, Z.; Hao, S.; Cai, S.; Luo, Z.; Wolverton, C.; Dravid, V.; Yang, J.; Yan, Q.; Kanatzidis, M. Thermoelectric performance of the 2D Bi2Si2Te6 semiconductor. J. Am. Chem. Soc. 2022, 144, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, W.; Jiang, D.; Sun, S.; Zhang, L.; Sun, X. Efficient solar-driven nitrogen fixation over Carbon-Tungstic-Acid hybrids. Chem.-A Eur. J. 2016, 22, 13819–13822. [Google Scholar] [CrossRef] [PubMed]
- Janet, C.M.; Navaladian, S.; Viswanathan, B.; Varadarajan, T.K.; Viswanath, R.P. Heterogeneous wet chemical synthesis of superlattice-type hierarchical ZnO architectures for concurrent H2 production and N2 reduction. J. Phys. Chem. C 2010, 114, 2622–2632. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Mousavi, M.; Ghasemi, J.B.; Van Le, Q.; Delbari, S.A.; Asl, M.S.; Mohammadi, M.; Shokouhimehr, M.; Namini, A.S. High-impressive separation of photoinduced charge carriers on step-scheme ZnO/ZnSnO3/Carbon Dots heterojunction with efficient activity in photocatalytic NH3 production. J. Taiwan Inst. Chem. Eng. 2021, 118, 140–151. [Google Scholar] [CrossRef]
- Zhao, W.; Xi, H.; Zhang, M.; Li, Y.; Chen, J.; Zhang, J.; Zhu, X. Enhanced quantum yield of nitrogen fixation for hydrogen storage with in situ-formed carbonaceous radicals. Chem. Commun. 2015, 51, 4785–4788. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, N.; Gao, F.; Chen, H.; Jiang, F. All-solid-state Z-scheme 3, 4-dihydroxybenzaldehyde-functionalized Ga2O3/graphitic carbon nitride photocatalyst with aromatic rings as electron mediators for visible-light photocatalytic nitrogen fixation. Appl. Catal. B Environ. 2017, 218, 600–610. [Google Scholar] [CrossRef]
- Xiao, J.; Lv, J.; Lu, Q. Building Fe2O3/MoO3 nanorod heterojunction enables better tetracycline photocatalysis. Mater. Lett. 2022, 311, 131580. [Google Scholar] [CrossRef]
- Hao, Y.; Dong, X.; Zhai, S.; Ma, H.; Wang, X.; Zhang, X. Hydrogenated bismuth molybdate nanoframe for efficient sunlight-driven nitrogen fixation from air. Chem. -A Eur. J. 2016, 22, 18722–18728. [Google Scholar] [CrossRef]
- Khan, S.; Choi, H.; Kim, D.; Lee, S.; Zhu, Q.; Zhang, J.; Kim, S.; Cho, S. Self-assembled heterojunction of metal sulfides for improved photocatalysis. Chem. Eng. J. 2020, 395, 125092. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Yuan, X.; Wu, Y.; Wang, H.; Tan, Y.; Chew, J. Roles of sulfur-edge sites, metal-edge sites, terrace sites, and defects in metal sulfides for photocatalysis. Chem Catal. 2021, 1, 44–68. [Google Scholar] [CrossRef]
- Khan, M.; Bhardwaj, R.C.; Bhardwaj, C. Catalytic fixation of nitrogen by the photocatalytic CdS/Pt/RuO2 particulate system in the presence of aqueous [Ru(Hedta)N2] complex. Angew. Chem. Int. Ed. 1988, 27, 923–925. [Google Scholar] [CrossRef]
- Wang, W.; Yue, J.; Chu, Y.; Ma, Z.; He, X.; Zhao, H.; Duan, J. Co-doped amorphous MoSx supported on CuO/CM (Cu mesh) with enhanced photocatalytic activity for ammonia synthesis. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128787. [Google Scholar] [CrossRef]
- Skulason, E.; Bligaard, T.; Gudmundsdóttir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jónssonac, H.; Nørskov, J. A theoretical evaluation of possible transition metal electrocatalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012, 14, 1235–1245. [Google Scholar] [CrossRef]
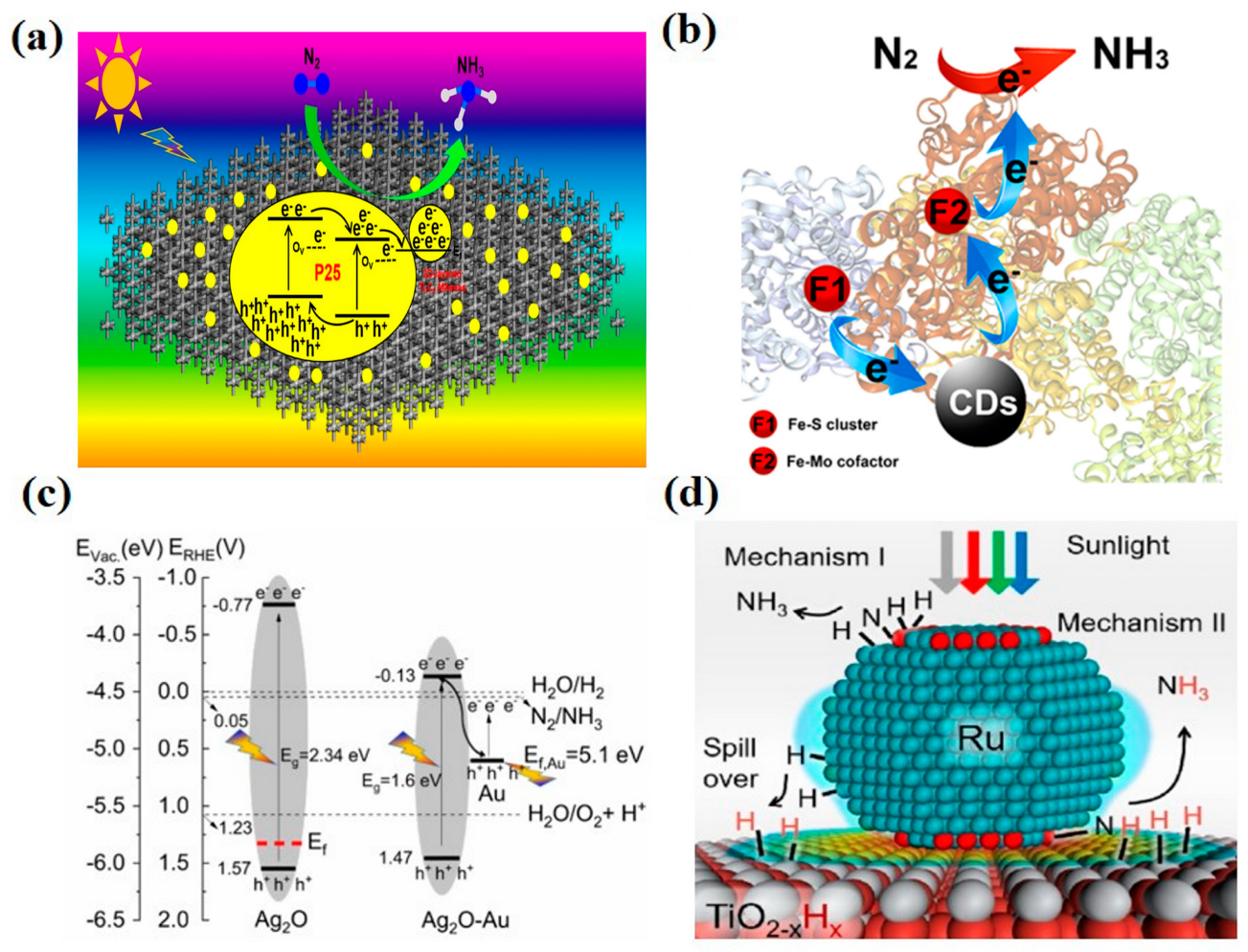
- Liao, Y.; Qian, J.; Xie, G.; Han, Q.; Dang, W.; Wang, Y.; Lv, L.; Zhao, S.; Luo, L.; Zhang, W.; et al. 2D-layered Ti3C2 MXenes for promoted synthesis of NH3 on P25 photocatalysts. Appl. Catal. B Environ. 2020, 273, 119054. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Zhang, M.; Song, Y.; Huang, J.; Huang, H.; Shao, M. Carbon dots enhance the nitrogen fixation activity of azotobacter chroococcum. ACS Appl. Mater. Interfaces 2018, 10, 16308–16314. [Google Scholar] [CrossRef]
- Nazemi, M.; El-Sayed, M.A. Plasmon-enhanced photo(electro)chemical nitrogen fixation under ambient conditions using visible light responsive hybrid hollow Au-Ag2O nanocages. Nano Energy 2019, 63, 103886. [Google Scholar] [CrossRef]
- Mao, C.; Yu, L.; Li, J.; Zhao, J.; Zhang, L. Energy-confined solar thermal ammonia synthesis with K/Ru/TiO2-xHx. Appl. Catal. B Environ. 2018, 224, 612–620. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, K.; Nie, S.; Zhao, X.; Yu, H.; Yu, J.; Fujishima, A.; Nakata, K. Ice-water Quenching Induced Ti3+ Self-doped TiO2 with Surface Lattice Distortion and the Increased Photocatalytic Activity. J. Phys. Chem. C. 2017, 121, 19836–19848. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Black Titanium Dioxide (TiO2) Nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Wang, S.; You, M.; Hong, S.; Wu, T.S.; Soo, Y.L.; Zhao, Z.; Jiang, G.; Jieshan, Q.; et al. Photocatalytic fixation of nitrogen to ammonia by single Ru atom decorated TiO2 nanosheets. ACS Sustain. Chem. Eng. 2019, 7, 6813–6820. [Google Scholar] [CrossRef]
- Lim, J.; Fernández, C.A.; Lee, S.W.; Hatzell, M.C. Ammonia and nitric acid demands for fertilizer use in 2050. ACS Energy Lett. 2021, 6, 3676–3685. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO, Photocatalysis:Mechanisms and Materials. Chem.Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Oshikiri, T.; Ueno, K.; Misawa, H. Plasmon-Induced Ammonia Synthesis through Nitrogen Photofixation with Visible Light Irradiation. Angew. Chem. Int. Ed. 2014, 53, 9802. [Google Scholar] [CrossRef]
- Liu, W.; Sun, M.; Ding, Z.; Gao, B.; Ding, W. Ti3C2 MXene embellished g–C3N4 nanosheets for improving photocatalytic redox capacity. J. Alloys Compd. 2021, 877, 160223. [Google Scholar] [CrossRef]
- Gao, G.; Jiao, Y.; Waclawik, E.R. Single atom (Pd/Pt) supported on graphitic carbon nitride as efficient photocatalyst for visible-light reduction of carbon dioxide. J. Am. Chem. Soc. 2016, 138, 6292. [Google Scholar] [CrossRef]
- Chu, K.; Liu, Y.P.; Li, Y.B.; Guo, Y.L.; Tian, Y.; Zhang, H. Multi-functional Mo-doping in MnO2 nanoflowers toward efficient and robust electrocatalytic nitrogen fixation. Appl. Catal. B Environ. 2020, 264, 118525. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.; Li, F.; Fan, Z.; Ma, H.; Li, W.; Kang, X. Construction of g–C3N4/Zn0.11Sn0.12Cd0.88S1.12 Hybrid Heterojunction Catalyst with Outstanding Nitrogen Photofixation Performance Induced by Sulfur Vacancies. ACS Sustain. Chem. Eng. 2016, 4, 2269. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, X.; Liu, M.; Guo, H.; Zhou, J.; Fang, Q.; Liu, Z.; Wu, Q.; Lou, J. Cobalt-Modulated Molybdenum–Dinitrogen Interaction in MoS 2 for Catalyzing Ammonia Synthesis. J. Am. Chem. Soc. 2019, 141, 19269. [Google Scholar] [CrossRef]
- Morawski, A.W.; Ćmielewska, K.; Ekiert, E.; Kusiak-Nejman, E.; Lech, I.P.; Staciwa, P.; Sibera, D.; Wanag, A.; Kapica-Kozar, J.; Gano, M.; et al. Effective green ammonia synthesis from gaseous nitrogen and CO2 saturated-water vapour utilizing a novel photocatalytic reactor. Chem. Eng. J. 2022, 446, 137030. [Google Scholar] [CrossRef]
- Zuo, C.; Tai, X.S.; Su, Q.; Jiang, Z.Y.; Guo, Q.J. S-scheme OV–TiO2@Cu7S4 heterojunction on copper mesh for boosting visible-light nitrogen fixation. Opt. Mater. 2023, 137, 113560. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Bicer, Y. The search for efficient and stable metal-organic frameworks for photocatalysis: Atmospheric fixation of nitrogen. Appl. Surf. Sci. 2022, 583, 152376. [Google Scholar] [CrossRef]
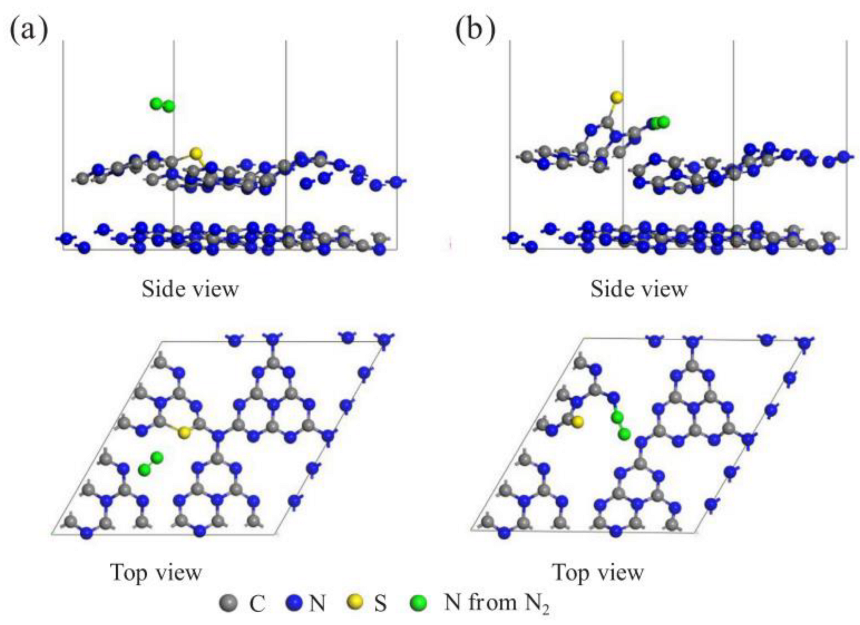
| Reaction | Reduction Potential (V) | Equation |
|---|---|---|
| H2O → 1/2O2 + 2H+ + 2e− | 0.81 | 1a |
| 2H+ + 2e− → H2 | −0.42 | 1b |
| N2 + e− → N2- | −4.16 | 1c |
| N2 + H+ + e− → N2H | −3.2 | 1d |
| N2 + 2H+ + 2e− → N2H2 | −1.10 | 1e |
| N2 + 4H+ + 4e− → N2H4 | −0.36 | 1f |
| N2 + 5H+ + 4e− → N2H5+ | −0.23 | 1g |
| N2 + 6H+ + 6e− → 2N2H3 | 0.55 | 1h |
| N2 + 8H+ + 8e− → 2N2H4 | 0.27 | 1i |
| Photocatalyst | Morphological Characteristics | Preparation Method | Nitrogen Source | Sacrificial Agent | Light Source | Ammonia Yield | References |
|---|---|---|---|---|---|---|---|
| Ag/PM-CdS(e) | Nanospheres (diameter of about 14.2 nm) | Hydrothermal–Etching | N2 | - | λ > 420 nm | 0.343 μg·h−1·mg−1 | [41] |
| AgCl/δ-Bi2O3 | Nanosheets (thickness of about 2.7 nm) | Hydrothermal precipitation method | N2 | - | λ > 420 nm | 606 μmol·h−1·g−1 | [42] |
| BOC/OV3 | Micro-nanosheets (<10 × 10 nm) | Room temperature reaction-reduction | N2 | Na2SO3 | λ > 400 nm | 1178 μmol·L−1·g−1·h−1 | [43] |
| Ru-In2O3 | Hollow peanut structure | Air Calcination | N2 | Methanol | UV-vis | 44.5 μmol·g−1·h−1 | [40] |
| NYF/NV-CNNTs | Nanotubes | Solvothermal method | N2 | Ethanol | λ~980 nm or > 420 nm | 1.72 mmol·L−1·gcat−1 or 5.30 mmol·L−1·gcat−1 | [44] |
| Au/HCNS-NV | Mesoporous hollow spheres | Templating agent calcination-reduction | N2 | Methanol | λ > 420 nm | 783.4 μmol·h−1·gcat−1 | [45] |
| 1T’-MoS2/CNNC | Nanocages | Hydrothermal method | N2 | Methanol | UV-vis | 9.8 mmol·L−1·h−1·g−1 | [46] |
| Nv&Od-CN | Porous hollow prisms | Low-temperature hydrothermal–calcination | N2 | Methanol | λ > 420 nm | 118.8 mg·L−1·h−1·gcat−1 | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, C.; Su, Q. Advances in Semiconductor-Based Nanocomposite Photo(electro)catalysts for Nitrogen Reduction to Ammonia. Molecules 2023, 28, 2666. https://doi.org/10.3390/molecules28062666
Zuo C, Su Q. Advances in Semiconductor-Based Nanocomposite Photo(electro)catalysts for Nitrogen Reduction to Ammonia. Molecules. 2023; 28(6):2666. https://doi.org/10.3390/molecules28062666
Chicago/Turabian StyleZuo, Cheng, and Qian Su. 2023. "Advances in Semiconductor-Based Nanocomposite Photo(electro)catalysts for Nitrogen Reduction to Ammonia" Molecules 28, no. 6: 2666. https://doi.org/10.3390/molecules28062666
APA StyleZuo, C., & Su, Q. (2023). Advances in Semiconductor-Based Nanocomposite Photo(electro)catalysts for Nitrogen Reduction to Ammonia. Molecules, 28(6), 2666. https://doi.org/10.3390/molecules28062666





