Cytotoxic Activity of Amaryllidaceae Plants against Cancer Cells: Biotechnological, In Vitro, and In Silico Approaches
Abstract
1. Introduction
2. Results
2.1. Plant Material
2.2. Establishment of In Vitro Culture
2.3. Cytotoxic Activities of the Different Tested Alkaloid Fractions
2.4. Alkaloid Profile of the Different Alkaloid Fractions
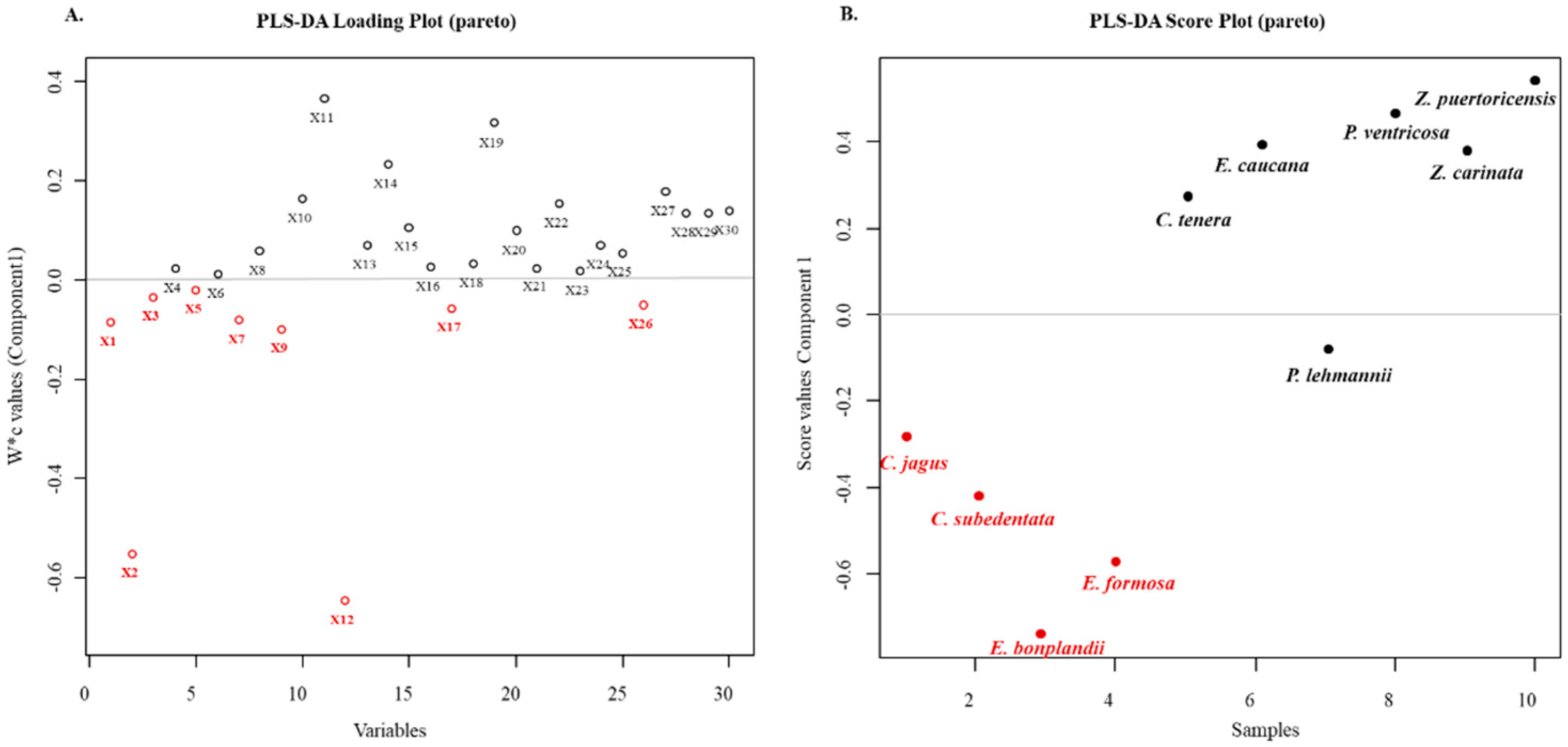
2.5. Multivariate Analyses of the Cytotoxic Activities of the Alkaloid Fractions
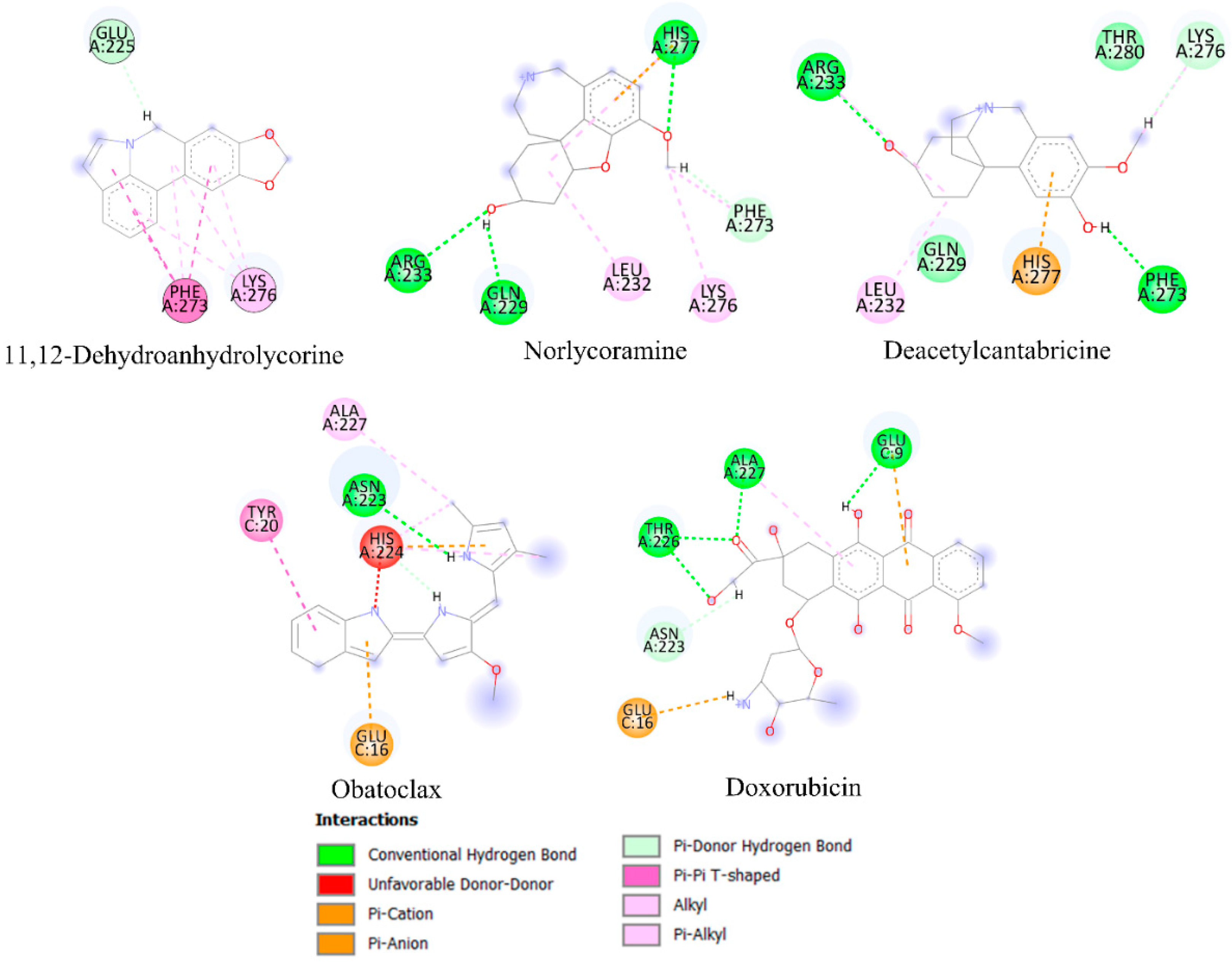
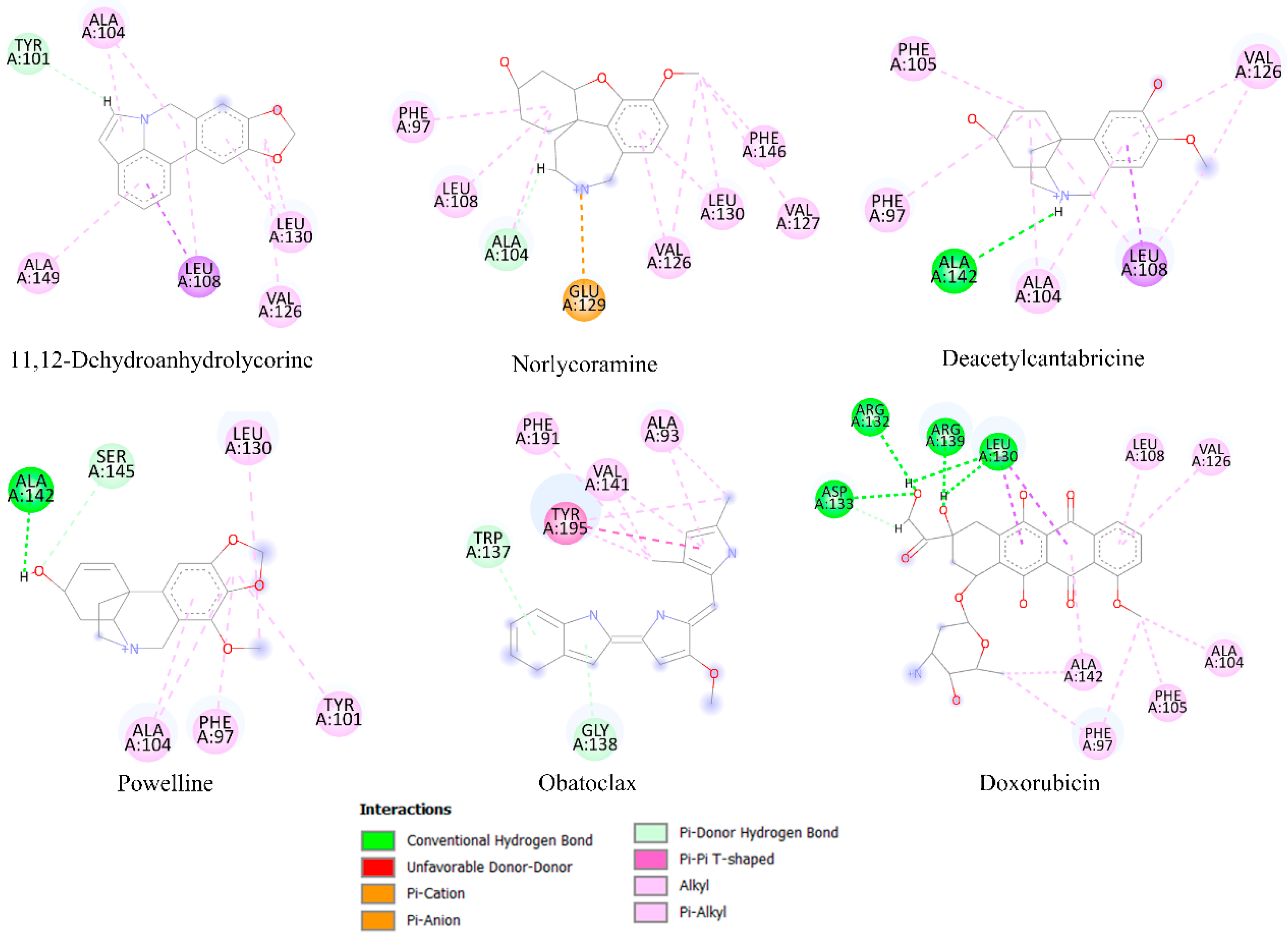
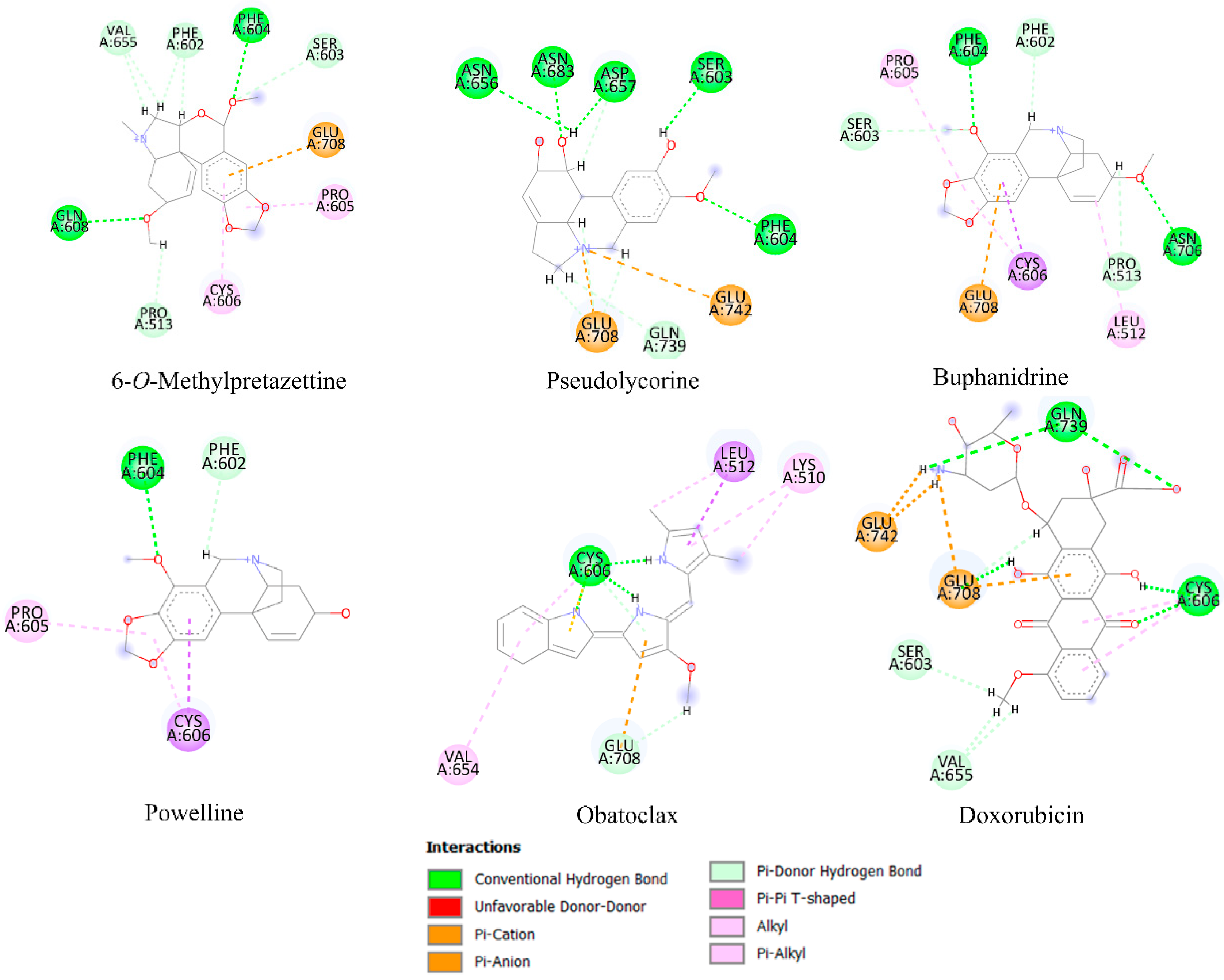
2.6. Molecular Docking Analysis
3. Discussion
3.1. In Vitro Propagation
3.2. Alkaloid Fractions Differed in Alkaloid Profiles and Cytotoxic Activities
4. Materials and Methods
4.1. Plant Material
4.2. Micropropagation of Amaryllidaceae Species
4.3. Extraction of Alkaloids
4.4. Alkaloid Analyses by GC/MS
Data Processing and Analysis
4.5. Cell Viability Screening of Alkaloid Fractions
4.5.1. Cell Culture
4.5.2. ATP Quantification by Bioluminescence
4.5.3. Mitochondrial MTT Reduction
4.6. In-Silico Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, B.D.T.; Coughlin, E.C.; Nair-Shalliker, V.; McCaffery, K.; Smith, D.P. Socioeconomic differences in prostate cancer treatment: A systematic review and meta-analysis. Cancer Epidemiol. 2022, 79, 102164. [Google Scholar] [CrossRef] [PubMed]
- López, M.J.; Carbajal, J.; Alfaro, A.L.; Saravia, L.G.; Zanabria, E.D.; Araujo, J.M.; Quispe, L.; Vizcarra, K.A.; Buleje, J.L.; Choo, C.E.; et al. Characteristics of gastric cancer around the World. Crit. Rev. Oncol. Hematol. 2022, 181, 103841. [Google Scholar] [CrossRef]
- Nyame, Y.A.; Cooperberg, M.R.; Cumberbatch, M.G.; Eggener, S.E.; Etzioni, R.; Gomez, S.L.; Haiman, C.; Huang, F.; Lee, C.T.; Litwin, M.S.; et al. Deconstructing, addressing, and eliminating racial and ethnic inequities in prostate cancer care. Eur. Urol. 2022, 82, 341–351. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Koskas, M.; Amant, F.; Mirza, M.R.; Creutzberg, C.L. Cancer of the corpus uteri: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 45–60. [Google Scholar] [CrossRef]
- Patel, A.; Iyer, P.; Matsuzaki, S.; Matsuo, K.; Sood, A.K.; Fleming, N.D. Emerging trends in neoadjuvant chemotherapy for ovarian cancer. Cancers 2021, 13, 626. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, K.; Pucher, P.H.; Armstrong, T.; Bateman, A.; Hamady, Z. Systemic neoadjuvant chemotherapy in modern pancreatic cancer treatment: A systematic review and meta-analysis. Ann. R. Coll. Surg. Engl. 2019, 101, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Groenewold, M.D.; Olthof, C.G.; Bosch, D.J. Anaesthesia after neoadjuvant chemotherapy, immunotherapy or radiotherapy. BJA Educ. 2022, 22, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mo, H.; He, Z.; Chen, A.; Cheng, P. Extracellular vesicles as an emerging drug delivery system for cancer treatment: Current strategies and recent advances. Biomed. Pharmacother. 2022, 153, 113480. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Singh, A.K.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023; in press. [Google Scholar] [CrossRef]
- Nguyen, L.T.S.; Jacob, M.A.C.; Parajón, E.; Robinson, D.N. Cancer as a biophysical disease: Targeting the mechanical-adaptability program. Biophys. J. 2022, 121, 3573–3585. [Google Scholar] [CrossRef]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Pricl, S.; Townley, H.; Thorat, N. Novel epigenetic therapeutic strategies and targets in cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166552. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Lu, J.J.; Wang, Y.T. Identification of anti-cancer compounds from natural products. Chin. J. Nat. Med. 2020, 18, 481–482. [Google Scholar] [CrossRef]
- Qiu, S.; Sun, H.; Zhang, A.H.; Xu, H.Y.; Yan, G.L.; Han, Y.; Wang, X.J. Natural alkaloids: Basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 2014, 12, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.J.R. The evolution of anticancer drug discovery from plants. Lancet Oncol. 2018, 19, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Roussi, F.; Gueritte, F.; Fahy, J. The Vinca alkaloids. In Anticancer Agents from Natural Products, 2nd ed.; Cragg, G.M., Kingston, D.G.I., Newman, D.J., Eds.; CRC/Taylor & Francis: Boca Raton, FL, USA, 2012; pp. 177–198. [Google Scholar]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef]
- Nair, J.J.; Van Staden, J.; Bastida, J. Cytotoxic alkaloid constituents of the Amaryllidaceae. Stud. Nat. Prod. Chem. 2016, 49, 107–156. [Google Scholar]
- Berkov, S.; Osorio, E.; Viladomat, F.; Bastida, J. Chemodiversity, chemotaxonomy and chemoecology of Amaryllidaceae alkaloids. In Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 83, pp. 113–185. [Google Scholar]
- Kornienko, A.; Evidente, A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.J.; Bastida, J.; van Staden, J. In vivo cytotoxicity studies of Amaryllidaceae alkaloids. Nat. Prod. Commun. 2016, 11, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Botteon, C.E.A.; Silva, L.B.; Ccana-Ccapatinta, G.V.; Silva, T.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Marcato, P.D. Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci. Rep. 2021, 11, 1974. [Google Scholar] [CrossRef]
- Omoruyi, S.I.; Kangwa, T.S.; Ibrakaw, A.S.; Cupido, C.N.; Marnewick, J.L.; Ekpo, O.E.; Hussein, A.A. Cytotoxic activities of selected plants of the family Amaryllidaceae on brain tumour cell lines. S. Afr. J Bot. 2021, 136, 118–125. [Google Scholar] [CrossRef]
- Silverstone, P. Los Muertos Vivientes: La Historia Natural de Cuatro Lirios Amazónicos Del Suroccidente de Colombia; Editorial Universidad del Valle: Santiago de Cali, Colombia, 2011; p. 24. [Google Scholar]
- Fennell, C.; Crouch, N.; van Staden, J. Micropropagation of the River Lily, Crinum variabile (Amaryllidaceae). S. Afr. J. Bot. 2001, 67, 74–77. [Google Scholar] [CrossRef]
- Guerrero-Valencia, F.A.; Rodríguez-de la O, J.L.; De, J.; Juárez-Hernández, M.; Ayala-Arreola, J.; Ramírez-González, G. Micropropagation of Amazon Lily (Eucharis Grandiflora Planch. & Linden) Through Direct Organogenesis. Polibotánica 2021, 51, 141–153. [Google Scholar]
- Akinyele, S.T.; Elusiyan, C.A.; Omisore, N.O.; Adewunmi, C.O. Antimalarial activities and alkaloids from Crinum jagus (Thomps) DANDY. J. Ethnopharmacol. 2022, 296, 115359. [Google Scholar] [CrossRef] [PubMed]
- Cortes, N.; Posada-Duque, R.A.; Alvarez, R.; Alzate, F.; Berkov, S.; Cardona-Gómez, G.P.; Osorio, E. Neuroprotective activity and acetylcholinesterase inhibition of five Amaryllidaceae species: A comparative study. Life Sci. 2015, 122, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Cortes, N.; Castañeda, C.; Osorio, E.H.; Cardona-Gomez, G.P.; Osorio, E. Amaryllidaceae alkaloids as agents with protective effects against oxidative neural cell injury. Life Sci. 2018, 203, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Ka, S.; Masi, M.; Merindol, N.; Di Lecce, R.; Plourde, M.B.; Seck, M.; Górecki, M.; Pescitelli, G.; Desgagne-Penix, I.; Evidente, A. Gigantelline, gigantellinine and gigancrinine, cherylline- and crinine-type alkaloids isolated from Crinum jagus with anti-acetylcholinesterase activity. Phytochemistry 2020, 175, 112390. [Google Scholar] [CrossRef]
- Cortes, N.; Posada-Duque, R.; Cardona-Gómez, G.P.; Bastida, J.; Osorio, E. Chapter 13—Amaryllidaceae alkaloids and neuronal cell protection. In Pathology, Oxidative Stress and Dietary Antioxidants; Preedy, V.R., Ed.; Academic Press: London, UK, 2020; pp. 135–144. [Google Scholar]
- Trujillo-Chacón, L.M.; Alarcón-Enos, J.E.; Céspedes-Acuña, C.L.; Bustamante, L.; Baeza, M.; López, M.G.; Fernández-Mendívil, C.; Cabezas, F.; Pastene-Navarrete, E.R. Neuroprotective activity of isoquinoline alkaloids from of Chilean Amaryllidaceae plants against oxidative stress-induced cytotoxicity on human neuroblastoma SH-SY5Y cells and mouse hippocampal slice culture. Food Chem. Toxicol. 2019, 132, 110665. [Google Scholar] [CrossRef]
- Cortes, N.; Sabogal-Guaqueta, A.M.; Cardona-Gomez, G.P.; Osorio, E. Neuroprotection and improvement of the histopathological and behavioral impairments in a murine Alzheimer’s model treated with Zephyranthes carinata alkaloids. Biomed. Pharmacother. 2019, 110, 482–492. [Google Scholar] [CrossRef]
- Rojas-Vera, J.; Buitrago-Díaz, A.A.; Possamai, L.M.; Timmers, L.F.S.M.; Tallini, L.R.; Bastida, J. Alkaloid profile and cholinesterase inhibition activity of five species of Amaryllidaceae family collected from Mérida state-Venezuela. S. Afr. J. Bot. 2021, 136, 126–136. [Google Scholar] [CrossRef]
- Sierra, K.; de Andrade, J.P.; Tallini, L.R.; Osorio, E.H.; Yañéz, O.; Osorio, M.I.; Oleas, N.H.; García-Beltrán, O.; Borges, W.; Bastida, J.; et al. In vitro and in silico analysis of galanthine from Zephyranthes carinata as an inhibitor of acetylcholinesterase. Biomed. Pharmacother. 2022, 150, 113016. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Sig. Transduct. Target Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Nair, J.J.; Bastida, J.; Viladomat, F.; van Staden, J. Cytotoxic agents of the crinane series of Amaryllidaceae alkaloids. Nat. Prod. Commun. 2012, 7, 1677–1688. [Google Scholar] [CrossRef]
- Nair, J.J.; van Staden, J. Cytotoxicity studies of lycorine alkaloids of the Amaryllidaceae. Nat. Prod. Commun. 2014, 9, 1193–1210. [Google Scholar] [CrossRef] [PubMed]
- D’Aguanno, S.; Del Bufalo, D. Inhibition of anti-apoptotic Bcl-2 proteins in preclinical and clinical studies: Current Overview in Cancer. Cells 2020, 9, 1287. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Mei, S.; Ding, Q.; Wang, Q.; Yu, L.; Zi, F. A pan-cancer analysis of the role of hexokinase II (HK2) in human tumors. Sci. Rep. 2022, 12, 18807. [Google Scholar] [CrossRef] [PubMed]
- Basu, A. The interplay between apoptosis and cellular senescence: Bcl-2 family proteins as targets for cancer therapy. Pharmacol. Ther. 2022, 230, 107943. [Google Scholar] [CrossRef] [PubMed]
- Gryko, M.; Pryczynicz, A.; Guzińska-Ustymowicz, K.; Kamocki, Z.; Zaręba, K.; Kemona, A.; Kędra, B. Immunohistochemical assessment of apoptosis-associated proteins: p53, Bcl-xL, Bax and Bak in gastric cancer cells in correlation with clinical and pathomorphological factors. Adv. Med. Sci. 2012, 57, 77–83. [Google Scholar] [CrossRef]
- Yuan, L.W.; Yamashita, H.; Seto, Y. Glucose metabolism in gastric cancer: The cutting-edge. World J. Gastroenterol. 2016, 22, 2046–2059. [Google Scholar] [CrossRef]
- Chen, L.; Guo, L.; Sun, Z.; Yang, G.; Guo, J.; Chen, K.; Xiao, R.; Yang, X.; Sheng, L. Monoamine oxidase a is a major mediator of mitochondrial homeostasis and glycolysis in gastric cancer progression. Cancer Manag. Res. 2020, 12, 8023–8035. [Google Scholar] [CrossRef]
- Nguyen, M.; Marcellus, R.C.; Roulston, A.; Watson, M.; Serfass, L.; Murthy Madiraju, S.R.; Goulet, D.; Viallet, J.; Bélec, L.; Billot, X.; et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc. Natl. Acad. Sci. USA 2007, 104, 19512–19517. [Google Scholar] [CrossRef]
- Rahmani, M.; Aust, M.M.; Attkisson, E.; Williams, D.C.; Ferreira-Gonzalez, A.; Grant, S. Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood 2012, 119, 6089–6098. [Google Scholar] [CrossRef]
- Sebola, T.E.; Uche-Okereafor, N.C.; Mekuto, L.; Makatini, M.M.; Green, E.; Mavumengwana, V. Antibacterial and anticancer activity and untargeted secondary metabolite profiling of crude bacterial endophyte extracts from Crinum macowanii Baker Leaves. Int. J. Microbiol. 2020, 2020, 8839490. [Google Scholar] [CrossRef]
- Nair, J.J.; van Staden, J. Cytotoxic tazettine alkaloids of the plant family Amaryllidaceae. S. Afr. J. Bot. 2021, 136, 147–156. [Google Scholar] [CrossRef]
- Rice, L.J.; Finnie, J.F.; Van Staden, J. In vitro bulblet production of Brunsvigia undulata from twin-scales. S. Afr. J. Bot. 2011, 77, 305–312. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Dartier, J.; Lemaitre, E.; Chourpa, I.; Goupille, C.; Servais, S.; Chevalier, S.; Mahéo, K.; Dumas, J.-F. ATP-dependent activity and mitochondrial localization of drug efflux pumps in doxorubicin-resistant breast cancer cells. Biochim. Biophys. Acta 2017, 5, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Park, K.S.; Lee, S.H. Curcumin targets both apoptosis and necroptosis in acidity-tolerant prostate carcinoma cells. BioMed Res. Int. 2021, 2021, 8859181. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Porter, J.; Payne, A.; de Candole, B.; Ford, D.; Hutchinson, B.; Trevitt, G.; Turner, J.; Edwards, C.; Watkins, C.; Whitcombe, I.; et al. Crystal Structure of Chimaeric Bcl2-xL and Phenyl Tetrahydroisoquinoline Amide Complex. Protein Data Bank 2008, 2W3L. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lee, E.F.; Smith, B.J.; Deshayes, K.; Zobel, K.; Fairlie, W.D.; Colman, P.M. Crystal structure of Bcl-xL in complex with ABT-737. Protein Data Bank 2007, 2YXJ. [Google Scholar] [CrossRef]
- Dutta, S.; Fire, E.; Grant, R.A.; Sauer, R.T.; Keating, A.E. MCL-1 complex with MCL-1-specific selected peptide. Protein Data Bank 2017, 3KZ0. [Google Scholar] [CrossRef]
- Lin, H.; Zeng, J.; Xie, R.; Schulz, M.J.; Tedesco, R.; Qu, J.; Erhard, K.F.; Mack, J.F.; Raha, K.; Rendina, A.R.; et al. Discovery of a novel 2,6-disubstituted glucosamine series of potent and selective Hexokinase 2 Inhibitors. ACS Med. Chem. Lett. 2016, 7, 217–222. [Google Scholar] [CrossRef]
- Sathishkumar, N.; Sathiyamoorthy, S.; Ramya, M.; Yang, D.-U.; Lee, H.N.; Yang, D.-C. Molecular docking studies of anti-apoptotic BCL-2, BCL-XL, and MCL-1 proteins with ginsenosides from Panax ginseng. J. Enzyme Inhib. Med. Chem. 2012, 27, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Swargiary, G.; Mani, S. Molecular docking and simulation studies of phytocompounds derived from Centella asiatica and Andrographis paniculata against hexokinase II as mitocan agents. Mitochondrion 2021, 61, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Kazi, A.; Sun, J.; Doi, K.; Sung, S.S.; Takahashi, Y.; Yin, H.; Rodriguez, J.M.; Becerril, J.; Berndt, N.; Hamilton, A.D.; et al. The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner. J. Biol. Chem. 2011, 286, 9382–9392. [Google Scholar] [CrossRef]
- Dalafave, D.S.; Prisco, G. Inhibition of antiapoptotic BCL-XL, BCL-2, and MCL-1 proteins by small molecule mimetics. Cancer Inform. 2010, 9, CIN.S5065-177. [Google Scholar] [CrossRef]
- Khan, A.; Mohammad, T.; Shamsi, A.; Hussain, A.; Alajmi, M.F.; Husain, S.A.; Iqbal, M.A.; Hassan, M.I. Identification of plant-based hexokinase 2 inhibitors: Combined molecular docking and dynamics simulation studies. J. Biomol. Struct. Dyn. 2022, 40, 10319–10331. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]


| Species | Origin Georeference | Voucher |
|---|---|---|
| C. jagus 1 | 6°9′33.58″ N, 75°21′32.75″ W 2120 m; Marinilla-Antioquia | 5270 Alzate |
| C. subedentata 2 | 2°32′27″ N, 76°37′44″ O. 1692 m; Popayan-Cauca | 5400 Alzate |
| C. tenera 2 | 2°32′27″ N, 76°37′44″ O. 1692 m; Popayan-Cauca | 5400 Alzate |
| E. bonplandii 1 | 4°35′18.47″ N, 75°51′19.78″ W. 1150 m; Armenia-Quindío | 5107 Alzate |
| E. caucana 2 | 5°29′54″ N, 76°32′29″ O. 69 m; Lloró, Chocó | 5168 Alzate |
| E. formosa 2 | 2°32′27″ N, 76°37′44″ O. 1692 m; Popayan-Cauca | 5401 Alzate |
| P. lehmannii 1 | 2°4′27″ N, 76º 54′1′′ O, 1610 m; Rosas-Cauca | 5106 Alzate |
| P. ventricosa 1 | 1°12′29″ N,77°27′57″ O, 1663 m; Consaca-Nariño | 5402 Alzate |
| Z. carinata 1 | 6°4′30.37″ N, 75°22′46.00″ W, 2150 m; Carmen del Viboral-Antioquia | 5307 Alzate |
| Z. puertoricensis 1 | 4°26′54″ N 75°11′56″ O; Ibagué-Tolima | 5308 Alzate |
| Species | Cell Viability (%) 1 | ||||||
|---|---|---|---|---|---|---|---|
| AGS | PC3 | MCF-7 | MDA-MB 231 | BT-549 | HEC-1B | HaCat | |
| C. jagus | 48.06 ± 3.35 | 73.42 ± 3.90 | 83.91 ± 1.15 | 58.88 ± 6.15 | 73.64 ± 3.38 | 87.65 ± 6.52 | 91.94 ± 0.83 |
| C. subedentata | 52.15 ± 2.10 | 84.29 ± 3.48 | 98.05 ± 5.53 | 56.51 ± 0.62 | 77.67 ± 3.12 | 88.09 ± 5.90 | 99.49 ± 0.09 |
| C. tenera | 73.72 ± 5.90 | 86.41 ± 2.28 | 73.94 ± 8.31 | 58.26 ± 8.12 | 65.28 ± 2.17 | 84.54 ± 0.88 | 99.11 ± 2.76 |
| E. bonplandii | 45.79 ± 3.05 | 77.92 ± 3.91 | 90.61 ± 4.94 | 57.56 ± 2.99 | 69.43 ± 5.76 | 88.25 ± 4.38 | 94.27 ± 1.28 |
| E. caucana | 61.46 ± 5.30 | 80.43 ± 3.79 | 52.82 ± 2.36 | 62.35 ± 4.35 | 65.51 ± 4.24 | 87.88 ± 4.91 | 99.57 ± 1.63 |
| E. formosa | 54.18 ± 2.56 | 74.02 ± 5.60 | 94.93 ± 3.41 | 54.81 ± 4.61 | 62.36 ± 6.46 | 80.38 ± 8.46 | 88.26 ± 0.86 |
| P. lehmannii | 71.90 ± 11.74 | 88.54 ± 2.34 | 73.28 ± 3.75 | 70.87 ± 4.27 | 76.40 ± 7.43 | 87.41 ± 1.49 | 98.32 ± 2.94 |
| P. ventricosa | 80.41 ± 6.28 | 88.91 ± 4.49 | 98.38 ± 4.28 | 73.69 ± 4.58 | 86.11 ± 2.75 | 83.78 ± 6.63 | 98.34 ± 2.97 |
| Z. carinata | 70.33 ± 8.91 | 88.40 ± 2.10 | 75.29 ± 7.44 | 72.46 ± 4.32 | 62.84 ± 5.37 | 78.29 ± 1.13 | 99.55 ± 4.78 |
| Z. puertoricensis | 67.25 ± 1.90 | 74.76 ± 5.88 | 69.86 ± 3.58 | 56.02 ± 5.63 | 51.40 ± 1.42 | 75.95 ± 2.18 | 97.33 ± 6.69 |
| Species 1 | Alkaloids Type (mg/gDW) 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Crinane | Galanthamine | Lycorine | Miscellaneous | |||||
| C. jagus | Crinine (X1) Crinane acetate (X3) Buphanidrine (X5) Crinamine (X7) Powelline (X9) | 0.073 0.006 0.002 0.033 0.050 | 5,6-Dihydrobicolorine (X13) Anhydrolycorine (X15) 11,12-Dehydroanhydrolycorine (X19) Lycorine (X31) | 0.009 0.090 0.022 0.295 | Trisphaeridine (X22) | 0.026 | ||
| C. subedentata | Crinine (X1) 8-O-Demethylmaritidine (X2) 6-O-Methylpretazettine (X4) Haemantamine (X6) Tazettine (X8) Hamayne (X10) | 0.216 1.700 0.022 1.005 0.211 0.771 | Galanthamine (X12) | 1.824 | 5,6-Dihydrobicolorine (X13) Anhydrolycorine (X15) Assoanine (X20) 11,12-Dehydroanhydrolycorine (X19) Lycorine (X31) | 0.069 0.279 0.038 0.210 0.629 | Ismine (X21) Trisphaeridine (X22) | 0.032 0.140 |
| C. tenera | Crinine (X1) 8-O-Demethylmaritidine (X2) Haemantamine (X6) Tazettine (X8) Hamayne (X10) | 0.133 0.282 1.114 0.010 0.377 | Galanthamine (X12) | 0.055 | 5,6-Dihydrobicolorine (X13) 11,12-Dehydrolycorene (X16) 11,12-Dehydroanhydrolycorine (X19) Lycorine (X31) Pseudolycorine (X26) | 0.372 0.020 0.312 0.167 0.009 | Trisphaeridine (X22) Demethylismine (X23) | 0.371 0.018 |
| E. bonplandii | Crinine (X1) 8-O-Demethylmaritidine (X2) Hamayne (X10) | 0.018 0.339 0.066 | Galanthamine (X12) Sanguinine (X14) Epinorgalanthamine (X17) | 0.280 0.004 0.006 | 5,6-Dihydrobicolorine (X13) Anhydrolycorine (X15) 11,12-Dehydroanhydrolycorine (X19) Lycorine (X31) Pseudolycorine (X26) | 0.003 0.056 0.008 0.581 0.004 | Trisphaeridine (X22) | 0.008 |
| E. caucana | Crinine (X1) 8-O-Demethylmaritidine (X2) 6-O-Methylpretazettine (X4) Tazettine (X8) Hamayne (X10) | 0.013 0.015 0.012 0.045 0.324 | Galanthamine (X12) Sanguinine (X14) Narwedine (X18) N-formylnorgalanthamine (X25) | 0.181 0.434 0.010 0.027 | Anhydrolycorine (X15) 11,12-Dehydroanhydrolycorine (X19) Lycorine (X31) | 0.025 0.065 0.135 | Ismine (X21) Galanthindole (X24) | 0.008 0.016 |
| E. formosa | 8-O-Demethylmaritidine (X2) Haemantamine (X6) Tazettine (X8) Hamayne (X10) | 0.068 0.315 0.019 0.247 | Galanthamine (X12) Sanguinine (X14) | 0.921 0.012 | 5,6-Dihydrobicolorine (X13) Lycorine (X31) | 0.010 2.390 | Ismine (X21) Trisphaeridine (X22) | 0.004 0.032 |
| P. lehmannii | 8-O-Demethylmaritidine (X2) | 0.015 | Galanthamine (X12) Sanguinine (X14) | 0.015 0.004 | Lycorine (X31) Pseudolycorine (X26) | 0.090 0.029 | ||
| P. ventricosa | Hamayne (X10) Deacetylcantabricine (X11) | 0.036 0.018 | Galanthamine (X12) | 0.015 | 11,12-Dehydroanhydrolycorine (X19) | 0.013 | Trisphaeridine (X22) | 0.017 |
| Z. carinata | Crinine (X1) 8-O-Demethylmaritidine (X2) Haemantamine (X6) Tazettine (X8) | 0.718 0.138 1.707 0.417 | Galanthamine (X12) Lycoramine (X27) | 0.354 2.274 | 5,6-Dihydrobicolorine (X13) 11,12-Dehydrolycorene (X16) Anhydrolycorine (X15) 11,12-Dehydroanhydrolycorine (X19) Galanthine (X30) | 0.269 0.011 0.197 0.450 1.380 | Ismine (X21) Galanthindole (X24) Trisphaeridine (X22) | 0.075 0.105 0302 |
| Z. puertoricensis | Deacetylcantabricine (X11) Aulicine (X28) | 0.021 0.006 | Sanguinine (X14) Norlycoramine (X29) | 0.003 0.006 | Anhydrolycorine (X15) Assoanine (X20) 11,12-Dehydroanhydrolycorine (X19) Lycorine (X31) | 0.030 0.004 0.006 0.099 | ||
| Estimated Free Energy of Binding 1 | |||||
|---|---|---|---|---|---|
| Alkaloid Type | Compound | Bcl-2 | Mcl-1 | Bcl-xL | HK2 |
| Lycorine | 5,6-dihydrobicolorine | −5.65 | −4.18 | −6.46 | −6.69 |
| Anhydrolycorine | −5.79 | −5.01 | −6.23 | −6.83 | |
| Assoanine | −5.63 | −5.02 | −5.66 | −6.62 | |
| Lycorine | −5.75 | −3.85 | −4.77 | −8.25 | |
| 11,12-Dehydroanhydrolycorine | −5.57 | −5.58 | −6.79 | −6.69 | |
| Pseudolycorine | −5.79 | −3.51 | −4.24 | −8.48 | |
| Crinane | 8-O-demethylmaritidine | −6.40 | −3.62 | −4.71 | −7.61 |
| Buphanidrine | −6.35 | −4.05 | −5.04 | −8.59 | |
| Crinamin | −5.80 | −4.32 | −5.95 | −7.64 | |
| Crinane acetate | −6.55 | −4.34 | −5.15 | −8.01 | |
| Crinine | −6.00 | −4.78 | −5.33 | −8.26 | |
| Haemanthamine | −5.70 | −4.71 | −5.50 | −7.75 | |
| Hamayne | −6.02 | −3.57 | −4.83 | −7.66 | |
| Powelline | −6.10 | −3.98 | −6.93 | −8.80 | |
| Tazettine | −6.53 | −4.74 | −4.74 | −7.76 | |
| 6-O-Methylpretazettine | −6.53 | −3.31 | −4.64 | −8.38 | |
| Deacetylcantabricine | −5.88 | −5.82 | −6.76 | −6.90 | |
| Aulicine | −5.46 | −4.95 | −6.20 | −7.72 | |
| Galanthamine | Galanthamine | −5.78 | −3.67 | −4.91 | −7.23 |
| Sanguinine | −5.87 | −3.84 | −5.16 | −7.54 | |
| Epinorgalanthamine | −6.56 | −4.63 | −6.64 | −7.87 | |
| Narwedine | −6.34 | −4.16 | −5.46 | −7.65 | |
| N-formylnorgalanthamine | −5.64 | −4.83 | −6.23 | −6.01 | |
| Norlycoramine | −6.27 | −5.39 | −6.99 | −7.63 | |
| Miscellaneous | Ismine | −5.48 | −3.67 | −4.01 | −6.43 |
| Trisphaeridine | −5.69 | −4.57 | −5.55 | −6.41 | |
| Galanthindole | −5.57 | −4.54 | −4.89 | −7.03 | |
| Control | Obatoclax 2 | −6.76 | −4.62 | −5.77 | −7.64 |
| Doxorubicin 2 | −5.66 | −3.48 | −6.74 | −8.35 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trujillo, L.; Bedoya, J.; Cortés, N.; Osorio, E.H.; Gallego, J.-C.; Leiva, H.; Castro, D.; Osorio, E. Cytotoxic Activity of Amaryllidaceae Plants against Cancer Cells: Biotechnological, In Vitro, and In Silico Approaches. Molecules 2023, 28, 2601. https://doi.org/10.3390/molecules28062601
Trujillo L, Bedoya J, Cortés N, Osorio EH, Gallego J-C, Leiva H, Castro D, Osorio E. Cytotoxic Activity of Amaryllidaceae Plants against Cancer Cells: Biotechnological, In Vitro, and In Silico Approaches. Molecules. 2023; 28(6):2601. https://doi.org/10.3390/molecules28062601
Chicago/Turabian StyleTrujillo, Lina, Janeth Bedoya, Natalie Cortés, Edison H. Osorio, Juan-Carlos Gallego, Hawer Leiva, Dagoberto Castro, and Edison Osorio. 2023. "Cytotoxic Activity of Amaryllidaceae Plants against Cancer Cells: Biotechnological, In Vitro, and In Silico Approaches" Molecules 28, no. 6: 2601. https://doi.org/10.3390/molecules28062601
APA StyleTrujillo, L., Bedoya, J., Cortés, N., Osorio, E. H., Gallego, J.-C., Leiva, H., Castro, D., & Osorio, E. (2023). Cytotoxic Activity of Amaryllidaceae Plants against Cancer Cells: Biotechnological, In Vitro, and In Silico Approaches. Molecules, 28(6), 2601. https://doi.org/10.3390/molecules28062601







