Upcycling of Acid-Leaching Solutions from Li-Ion Battery Waste Treatment through the Facile Synthesis of Magnetorheological Fluid
Abstract
1. Introduction
2. Results and Discussion
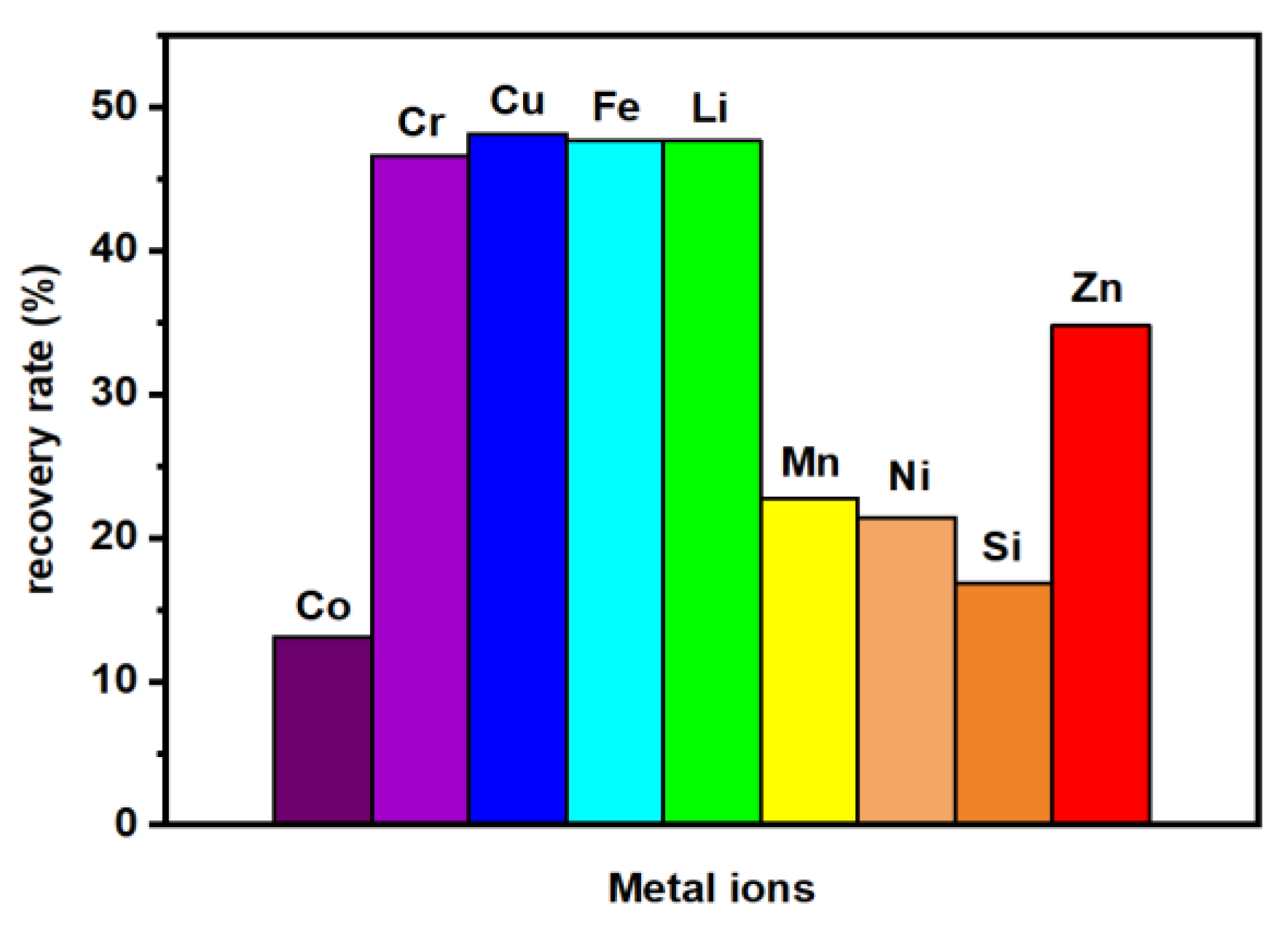
2.1. UV-Vis Analysis of the Post-Synthesis Solution
2.2. Synthesis of Magnetorheological Fluid
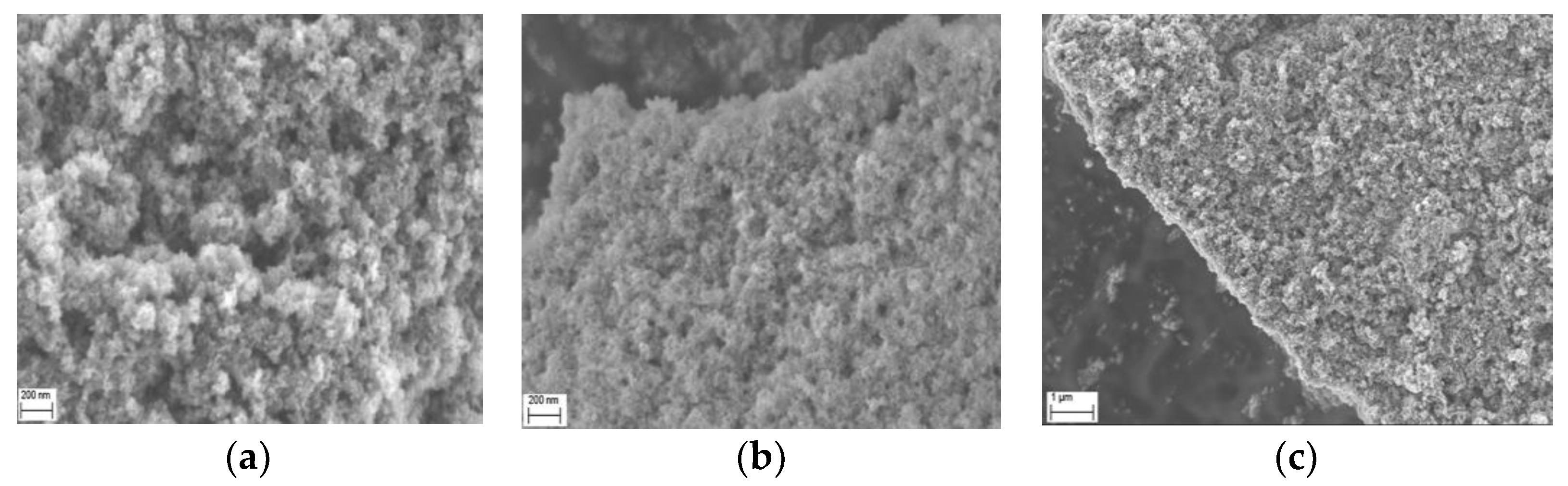
2.3. Morphology Studies
2.4. Magnetic Properties
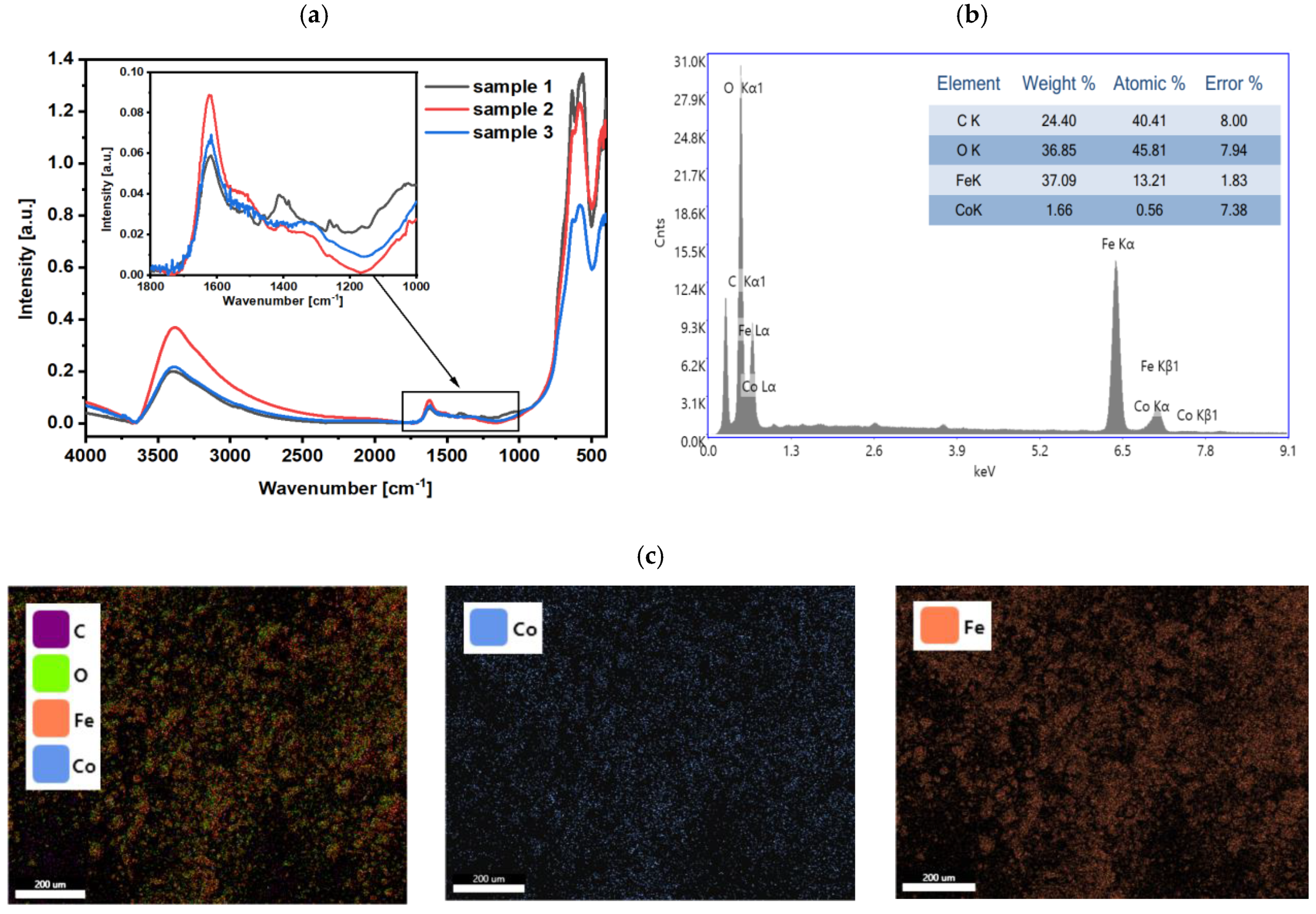
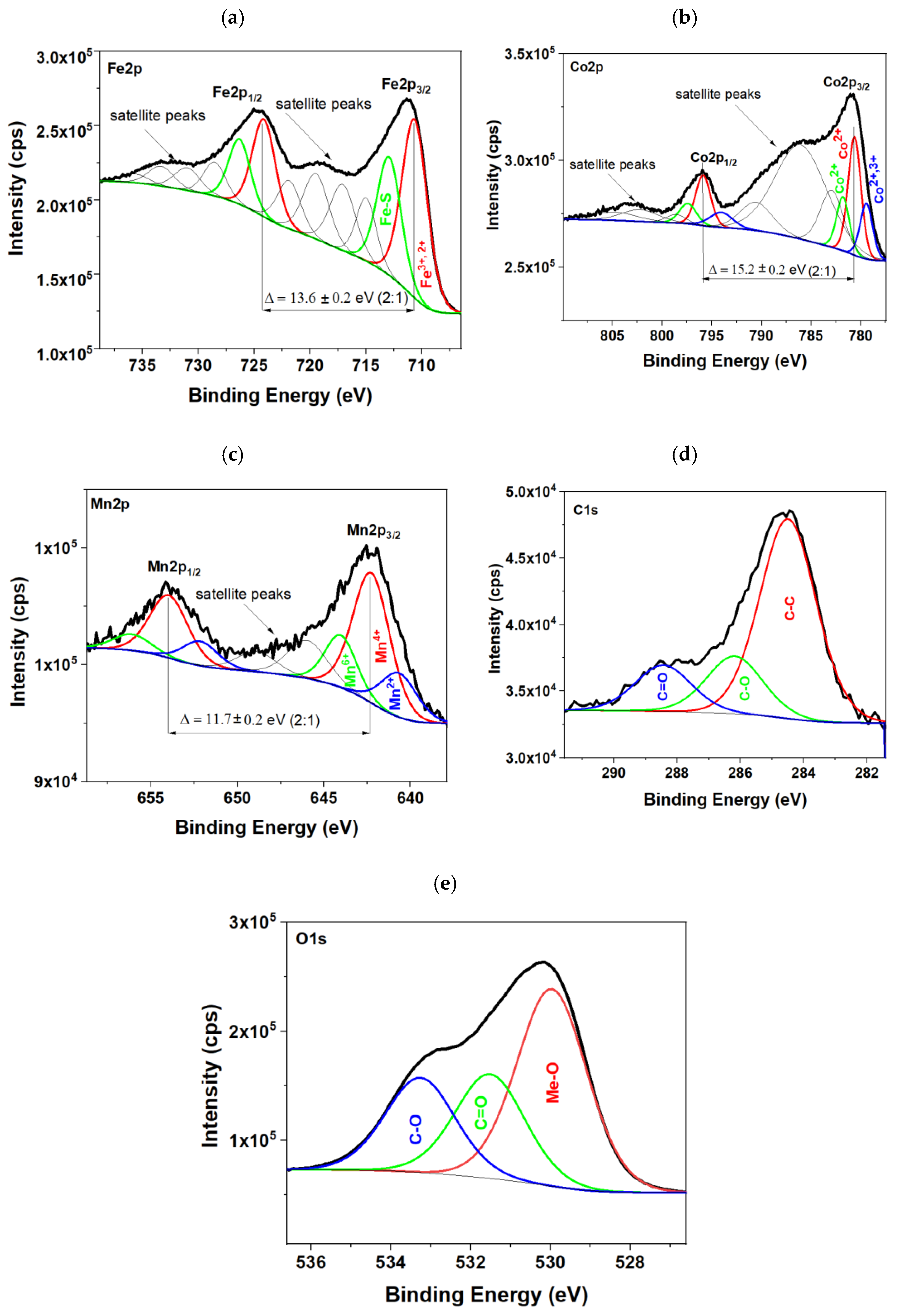
2.5. Chemical Composition Studies
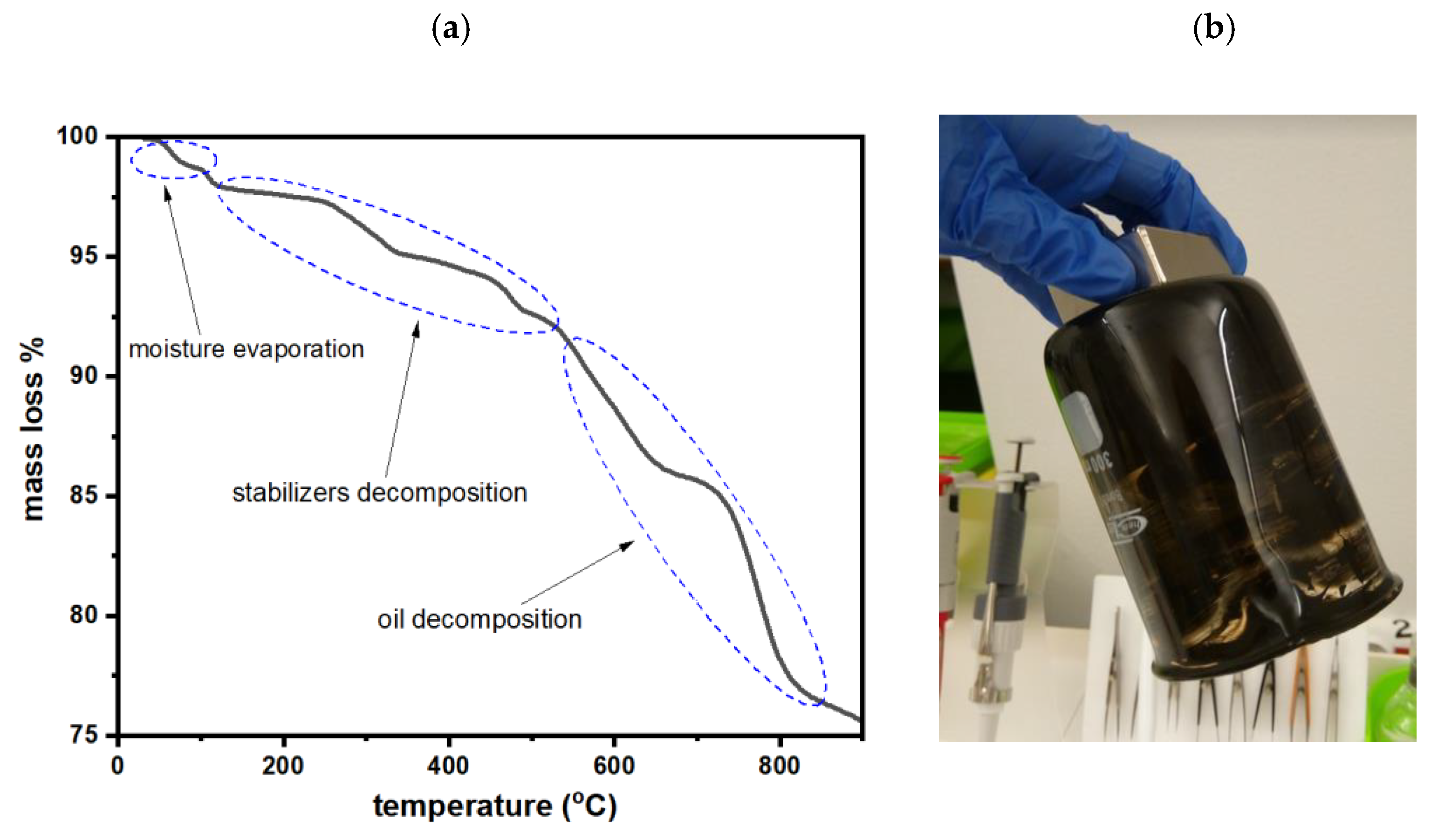
2.6. TGA Analysis of the MRF
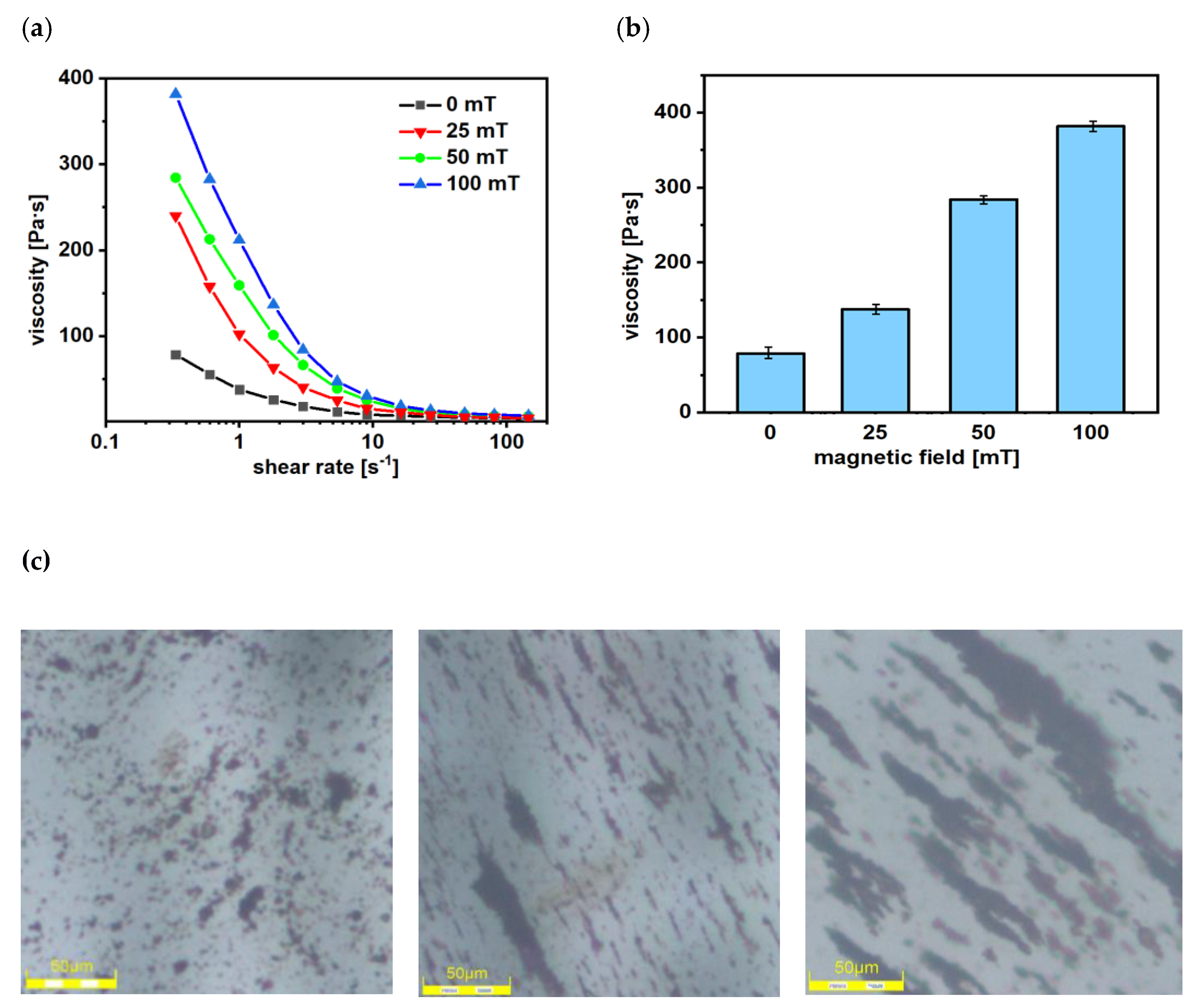
2.7. Viscosity Measurements
3. Materials and Methods
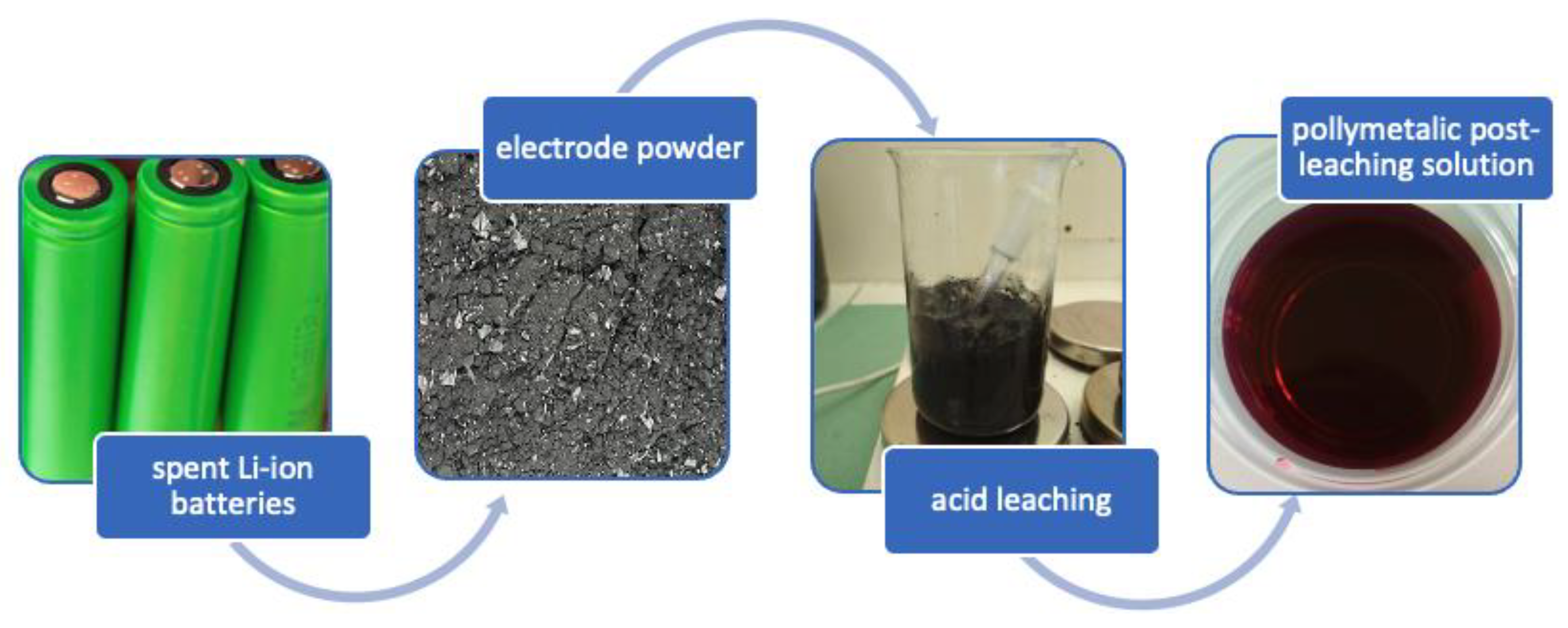
3.1. Acid Leaching of Spent Battery Powder
3.2. The Application of the Spent Post-Leaching Solution to Prepare SPIONs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gao, W.; Song, J.; Cao, H.; Lin, X.; Zhang, X.; Zheng, X.; Zhang, Y.; Sun, Z. Selective recovery of valuable metals from spent lithium-ion batteries–Process development and kinetics evaluation. J. Clean. Prod. 2018, 178, 833–845. [Google Scholar] [CrossRef]
- Wei, Q.; Wu, Y.; Li, S.; Chen, R.; Ding, J.; Zhang, C. Spent lithium ion battery (LIB) recycle from electric vehicles: A mini-review. Sci. Total Environ. 2023, 866, 161380. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Sarkis, J.; Bleischwitz, R. How to globalize the circular economy. Springer Nat. Ltd. 2019, 565, 5–7. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Recovery of valuable metals from cathodic active material of spent lithium ion batteries: Leaching and kinetic aspects. Waste Manag. 2015, 45, 306–313. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy–A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Xu, S.; Sun, W.; He, Y. On the sustainability of lithium ion battery industry–A review and perspective. Energy Storage Mater. 2021, 36, 186–212. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Hu, X.; Geng, Y.; Yang, L.; Shao, P.; Luo, X. Critical strategies for recycling process of graphite from spent lithium-ion batteries: A review. Sci. Total Environ. 2022, 816, 151621. [Google Scholar] [CrossRef] [PubMed]
- Richa, K.; Babbitt, C.W.; Gaustad, G. Eco-Efficiency Analysis of a Lithium-Ion Battery Waste Hierarchy Inspired by Circular Economy. J. Ind. Ecol. 2017, 21, 715–730. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Pagliaro, M.; Meneguzzo, F. Lithium battery reusing and recycling: A circular economy insight. Heliyon 2019, 5, e01866. [Google Scholar] [CrossRef] [PubMed]
- Porvali, A.; Aaltonen, M.; Ojanen, S.; Velazquez-Martinez, O.; Eronen, E.; Liu, F.; Wilson, B.P.; Serna-Guerrero, R.; Lundström, M. Mechanical and hydrometallurgical processes in HCl media for the recycling of valuable metals from Li-ion battery waste. Resour. Conserv. Recycl. 2019, 142, 257–266. [Google Scholar] [CrossRef]
- Piątek, J.; Budnyak, T.M.; Monti, S.; Barcaro, G.; Gueret, R.; Grape, E.S.; Jaworski, A.; Inge, A.K.; Rodrigues, B.V.M.; Slabon, A. Toward Sustainable Li-Ion Battery Recycling: Green Metal-Organic Framework as a Molecular Sieve for the Selective Separation of Cobalt and Nickel. ACS Sustain. Chem. Eng. 2021, 9, 9770–9778. [Google Scholar] [CrossRef]
- Li, Y.M.; Wang, Y.; Chen, M.J.; Huang, T.Y.; Yang, F.H.; Wang, Z.J. Current status and technological progress in lead recovery from electronic waste. Int. J. Environ. Sci. Technol. 2022, 20, 1037–1052. [Google Scholar] [CrossRef]
- Li, B.; Li, Q.; Wang, Q.; Yan, X.; Shi, M.; Wu, C. Deep eutectic solvent for spent lithium-ion battery recycling: Comparison with inorganic acid leaching. Phys. Chem. Chem. Phys. 2022, 24, 19029–19051. [Google Scholar] [CrossRef]
- Zhu, S.G.; He, W.Z.; Li, G.M.; Zhou, X.; Zhang, X.J.; Huang, J.W. Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation. Trans. Nonferrous Met. Soc. China Engl. Ed. 2012, 22, 2274–2281. [Google Scholar] [CrossRef]
- Nayl, A.A.; Elkhashab, R.A.; Badawy, S.M.; El-Khateeb, M.A. Acid leaching of mixed spent Li-ion batteries. Arab. J. Chem. 2017, 10, S3632–S3639. [Google Scholar] [CrossRef]
- Almeida, J.R.; Moura, M.N.; Barrada, R.V.; Barbieri, E.M.S.; Carneiro, M.T.W.D.; Ferreira, S.A.D.; Lelis, M.F.F.; de Freitas, M.B.J.G.; Brandão, G.P. Composition analysis of the cathode active material of spent Li-ion batteries leached in citric acid solution: A study to monitor and assist recycling processes. Sci. Total Environ. 2019, 685, 589–595. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Nakamura, T.; Shibata, E.; Iizuka, A. Adaptation of minerals processing operations for lithium-ion (LiBs) and nickel metal hydride (NiMH) batteries recycling: Critical review. Miner. Eng. 2013, 45, 4–17. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials for Strategic Technologies and Sectors in the EU—A Foresight Study; The Reuse Policy of European Commission Documents. European Commission. 2020. Available online: https://rmis.jrc.ec.europa.eu/uploads/CRMs_for_Strategic_Technologies_and_Sectors_in_the_EU_2020.pdf (accessed on 20 October 2022).
- Ghassa, S.; Farzanegan, A.; Gharabaghi, M.; Abdollahi, H. The reductive leaching of waste lithium ion batteries in presence of iron ions: Process optimization and kinetics modelling. J. Clean. Prod. 2020, 262, 121312. [Google Scholar] [CrossRef]
- Song, D.; Wang, T.; Liu, Z.; Zhao, S.; Quan, J.; Li, G.; Zhu, H.; Huang, J.; He, W. Characteristic comparison of leaching valuable metals from spent power Li-ion batteries for vehicles using the inorganic and organic acid system. J. Environ. Chem. Eng. 2022, 10, 107102. [Google Scholar] [CrossRef]
- Kime, M.B.; Makgoale, D. Characterization of Copper–Cobalt Ores and Quantification of Cu2+, Co2+, Co3+, and Fe3+ in Aqueous Leachates Using UV/Visible Spectrophotometry. Chem. Eng. Commun. 2016, 203, 1648–1655. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yang, L.; Li, L.; Guo, Y.Q.; Pang, X.X.; Li, P.; Ye, F.; Fu, Y. A new fluorescent chemosensor for cobalt(II) ions in living cells based on 1,8-naphthalimide. Molecules 2019, 24, 3093. [Google Scholar] [CrossRef]
- Uchikoshi, M. Determination of the Distribution of Cobalt-Chloro Complexes in Hydrochloric Acid Solutions at 298 K. J. Solut. Chem. 2018, 47, 2021–2038. [Google Scholar] [CrossRef]
- Jia, H.R.; Chang, J.; Zhang, H.J.; Li, J.; Sun, Y.X. Three polyhydroxyl-bridged defective dicubane tetranuclear MnIII complexes: Synthesis, crystal structures, and spectroscopic properties. Crystals 2018, 8, 272. [Google Scholar] [CrossRef]
- Olusegun, S.J.; Larrea, G.; Osial, M.; Jackowska, K.; Krysinski, P. Photocatalytic Degradation of Antibiotics by Superparamagnetic Iron Oxide Nanoparticles. Tetracycline Case. Catalysts 2021, 11, 1243. [Google Scholar] [CrossRef]
- Giersig, M.; Hilgendorff, M. Magnetic nanoparticle superstructures. Eur. J. Inorg. Chem. 2005, 5, 3571–3583. [Google Scholar] [CrossRef]
- Dun, C.; Xi, G.; Zhang, Y.; Heng, X.; Liu, Y.; Xing, X.; Liang, R. Magnetic and magnetostrictive properties of non-stoichiometric cobalt ferrite synthesized from spent Li-ion batteries. J. Magn. Magn. Mater. 2020, 513, 167185. [Google Scholar] [CrossRef]
- Moura, M.N.; Barrada, R.V.; Almeida, J.R.; Moreira, T.F.M.; Schettino, M.A.; Freitas, J.C.C.; Ferreira, S.A.D.; Lelis, M.F.F.; Freitas, M.B.J.G. Synthesis, characterization and photocatalytic properties of nanostructured CoFe2O4 recycled from spent Li-ion batteries. Chemosphere 2017, 182, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xi, G.; Lou, T.; Wang, X.; Wang, J.; He, Y. Preparation and magnetic performance of Co0.8Fe2.2O4 by a sol-gel method using cathode materials of spent Li-ion batteries. Ceram. Int. 2016, 42, 1897–1902. [Google Scholar] [CrossRef]
- Sankaran, K.J.; Suman, S.; Sahaw, A.; Balaji, U.; Sakthivel, R. Improved LPG sensing properties of nickel doped cobalt ferrites derived from metallurgical wastes. J. Magn. Magn. Mater. 2021, 537, 168231. [Google Scholar] [CrossRef]
- Xi, G.; Xu, H.; Yao, L. Study on preparation of NiCo ferrite using spent lithium-ion and nickel-metal hydride batteries. Sep. Purif. Technol. 2015, 145, 50–55. [Google Scholar] [CrossRef]
- Szczygieł, I.; Winiarska, K.; Sobianowska-Turek, A. The study of thermal, microstructural and magnetic properties of manganese–zinc ferrite prepared by co-precipitation method using different precipitants. J. Therm. Anal. Calorim. 2018, 134, 51–57. [Google Scholar] [CrossRef]
- Kim, T.H.; Kang, J.G.; Sohn, J.S.; Rhee, K.I.; Lee, S.W.; Shin, S.M. Preparation of Mn-Zn ferrite from spent zinc-carbon batteries by alkali leaching, acid leaching and co-precipitation. Met. Mater. Int. 2008, 14, 655–658. [Google Scholar] [CrossRef]
- Song, Y.; Huang, Q.; Niu, Z.; Ma, J.; Xin, B.; Chen, S.; Dai, J.; Wang, R. Preparation of Zn-Mn ferrite from spent Zn-Mn batteries using a novel multi-step process of bioleaching and co-precipitation and boiling reflux. Hydrometallurgy 2015, 153, 66–73. [Google Scholar] [CrossRef]
- Dun, C.; Xi, G.; Heng, X.; Zhang, Y.; Liu, Y.; Xing, X. Comparative study on the magnetostrictive property of cobalt ferrite synthesized by different methods from spent Li-ion batteries. Ceram. Int. 2019, 45, 8539–8545. [Google Scholar] [CrossRef]
- Osial, M.; Nowicki, M.; Klejman, E.; Frąś, L. Investigation of the well-dispersed magnetorheological oil-based suspension with superparamagnetic nanoparticles using modified split Hopkinson pressure bar. Rheol. Acta 2022, 61, 111–122. [Google Scholar] [CrossRef]
- Thanh, D.T.M.; Phuong, N.T.; Hai, D.T.; Giang, H.N.; Thom, N.T.; Nam, P.T.; Dung, N.T.; Giersig, M.; Osial, M. Influence of Experimental Conditions during Synthesis on the Physicochemical Properties of the SPION/Hydroxyapatite Nanocomposite for Magnetic Hyperthermia Application. Magnetochemistry 2022, 8, 90. [Google Scholar] [CrossRef]
- Pietrzyk, P.; Thu Phuong, N.; Joseph Olusegun, S.; Hong Nam, N.; Thi Mai Thanh, D.; Giersig, M.; Krysínski, P.; Osial, M. Titan Yellow and Congo Red Removal with Superparamagnetic Iron-Oxide-Based Nanoparticles Doped with Zinc. Magnetochemistry 2022, 8, 91. [Google Scholar] [CrossRef]
- Dagdelen, S.; Mackiewicz, M.; Osial, M.; Waleka-Bargiel, E.; Romanski, J.; Krysinski, P.; Karbarz, M. Redox-responsive degradable microgel modified with superparamagnetic nanoparticles exhibiting controlled, hyperthermia-enhanced drug release. J. Mater. Sci. 2023, 58, 4094–4114. [Google Scholar] [CrossRef]
- Dehghanpour, H.R. The Effects of Surfactant Changing on Physical Properties of Fe3O4 Nanoparticles Produced in Coprecipitation Method. Russ. J. Inorg. Chem. 2020, 65, 1282–1286. [Google Scholar] [CrossRef]
- Rezayan, A.H.; Mosavi, M.; Kheirjou, S.; Amoabediny, G.; Ardestani, M.S.; Mohammadnejad, J. Monodisperse magnetite (Fe3O4) nanoparticles modified with water soluble polymers for the diagnosis of breast cancer by MRI method. J. Magn. Magn. Mater. 2016, 420, 210–217. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, W.; Qin, F.; Fang, H.; Hadjiev, V.G.; Litvinov, D.; Bao, J. Identification of Cobalt Oxides with Raman Scattering and Fourier Transform Infrared Spectroscopy. J. Phys. Chem. C 2016, 120, 4511–4516. [Google Scholar] [CrossRef]
- Rahmani, R.; Gharanfoli, M.; Gholamin, M.; Darroudi, M.; Chamani, J.; Sadri, K.; Hashemzadeh, A. Plant-mediated synthesis of superparamagnetic iron oxide nanoparticles (SPIONs) using aloe vera and flaxseed extracts and evaluation of their cellular toxicities. Ceram. Int. 2020, 46, 3051–3058. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Lan, Y.; Butler, E.C. Iron-Sulfide-Associated Products Formed during Reductive Dechlorination of Carbon Tetrachloride. Environ. Sci. Technol. 2016, 50, 5489–5497. [Google Scholar] [CrossRef]
- Shi, W.; Guo, F.; Wang, H.; Han, M.; Li, H.; Yuan, S.; Huang, H.; Liu, Y.; Kang, Z. Carbon dots decorated the exposing high-reactive (111) facets CoO octahedrons with enhanced photocatalytic activity and stability for tetracycline degradation under visible light irradiation. Appl. Catal. B Environ. 2017, 219, 36–44. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Havlica, J.; Kalina, L.; Urbánek, P.; Machovsky, M.; Masař, M.; Holek, M. Influence of La3+ on structural, magnetic, dielectric, electrical and modulus spectroscopic characteristics of single phase CoFe2−xLaxO4 nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 9139–9154. [Google Scholar] [CrossRef]
- Stevie, F.A.; Donley, C.L. Introduction to x-ray photoelectron spectroscopy. J. Vac. Sci. Technol. A 2020, 38, 063204. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, L.; Shi, J.; Zhao, J.; Gao, J.; Yan, D. A simple template-free strategy to synthesize nanoporous manganese and nickel oxides with narrow pore size distribution, and their electrochemical properties. Adv. Funct. Mater. 2008, 18, 1544–1554. [Google Scholar] [CrossRef]
- Stranick, M.A.; Stranick, M.A. MnO2 by XPS. Surf. Sci. Spectra 1999, 6, 31–38. [Google Scholar] [CrossRef]
- Morgan, D.J. Comments on the XPS Analysis of Carbon Materials. C—J. Carbon Res. 2021, 7, 51. [Google Scholar] [CrossRef]
- Charles, S. The Preparation of Magnetic Fluids, Ferrofluids: Magnetically Controllable Fluids and Their Applications; Odenbach, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 3-540-43978-1. [Google Scholar]
- Urbanska, W.; Osial, M. Investigation of the physico-chemical properties of the products obtained after mixed organic-inorganic leaching of spent li-ion batteries. Energies 2020, 13, 6732. [Google Scholar] [CrossRef]
- Mantuano, D.P.; Dorella, G.; Elias, R.C.A.; Mansur, M.B. Analysis of a hydrometallurgical route to recover base metals from spent rechargeable batteries by liquid-liquid extraction with Cyanex 272. J. Power Sources 2006, 159, 1510–1518. [Google Scholar] [CrossRef]
- Dorella, G.; Mansur, M.B. A study of the separation of cobalt from spent Li-ion battery residues. J. Power Sources 2007, 170, 210–215. [Google Scholar] [CrossRef]
- Urbańska, W.; Osial, M.; Wilczewski, S. Application of the Chemical Leaching Method for the Recovery of Li and Co Contained in Spent Li-Ion Batteries. Environ. Sci. Proc. 2022, 18, 12. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramowicz, M.; Osial, M.; Urbańska, W.; Walicki, M.; Wilczewski, S.; Pregowska, A.; Skórczewska, K.; Jenczyk, P.; Warczak, M.; Pisarek, M.; et al. Upcycling of Acid-Leaching Solutions from Li-Ion Battery Waste Treatment through the Facile Synthesis of Magnetorheological Fluid. Molecules 2023, 28, 2558. https://doi.org/10.3390/molecules28062558
Abramowicz M, Osial M, Urbańska W, Walicki M, Wilczewski S, Pregowska A, Skórczewska K, Jenczyk P, Warczak M, Pisarek M, et al. Upcycling of Acid-Leaching Solutions from Li-Ion Battery Waste Treatment through the Facile Synthesis of Magnetorheological Fluid. Molecules. 2023; 28(6):2558. https://doi.org/10.3390/molecules28062558
Chicago/Turabian StyleAbramowicz, Magdalena, Magdalena Osial, Weronika Urbańska, Mikołaj Walicki, Sławomir Wilczewski, Agnieszka Pregowska, Katarzyna Skórczewska, Piotr Jenczyk, Magdalena Warczak, Marcin Pisarek, and et al. 2023. "Upcycling of Acid-Leaching Solutions from Li-Ion Battery Waste Treatment through the Facile Synthesis of Magnetorheological Fluid" Molecules 28, no. 6: 2558. https://doi.org/10.3390/molecules28062558
APA StyleAbramowicz, M., Osial, M., Urbańska, W., Walicki, M., Wilczewski, S., Pregowska, A., Skórczewska, K., Jenczyk, P., Warczak, M., Pisarek, M., & Giersig, M. (2023). Upcycling of Acid-Leaching Solutions from Li-Ion Battery Waste Treatment through the Facile Synthesis of Magnetorheological Fluid. Molecules, 28(6), 2558. https://doi.org/10.3390/molecules28062558










