A Novel Based Synthesis of Silver/Silver Chloride Nanoparticles from Stachys emodi Efficiently Controls Erwinia carotovora, the Causal Agent of Blackleg and Soft Rot of Potato
Abstract
1. Introduction
2. Results
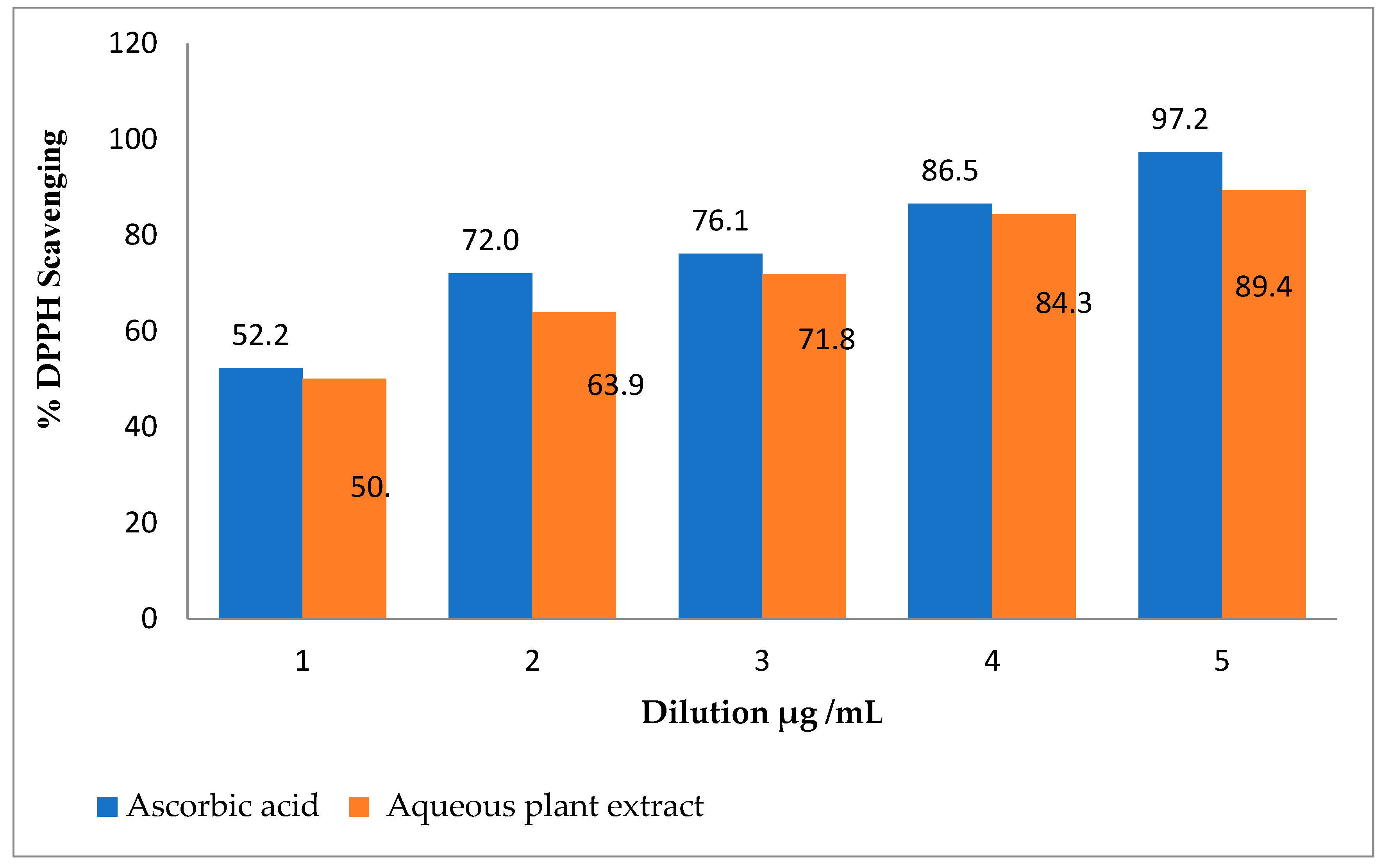
2.1. DPPH Assay
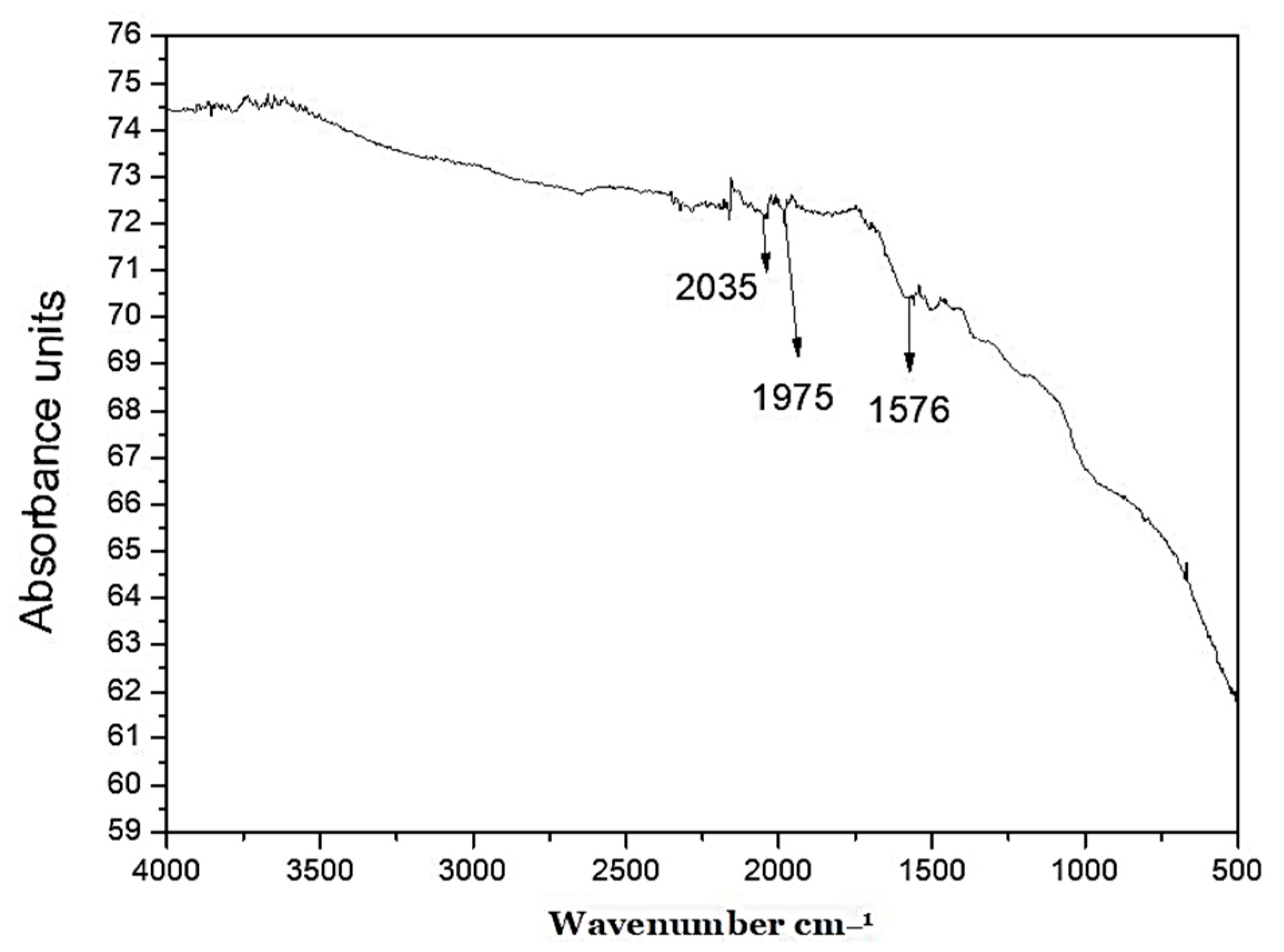
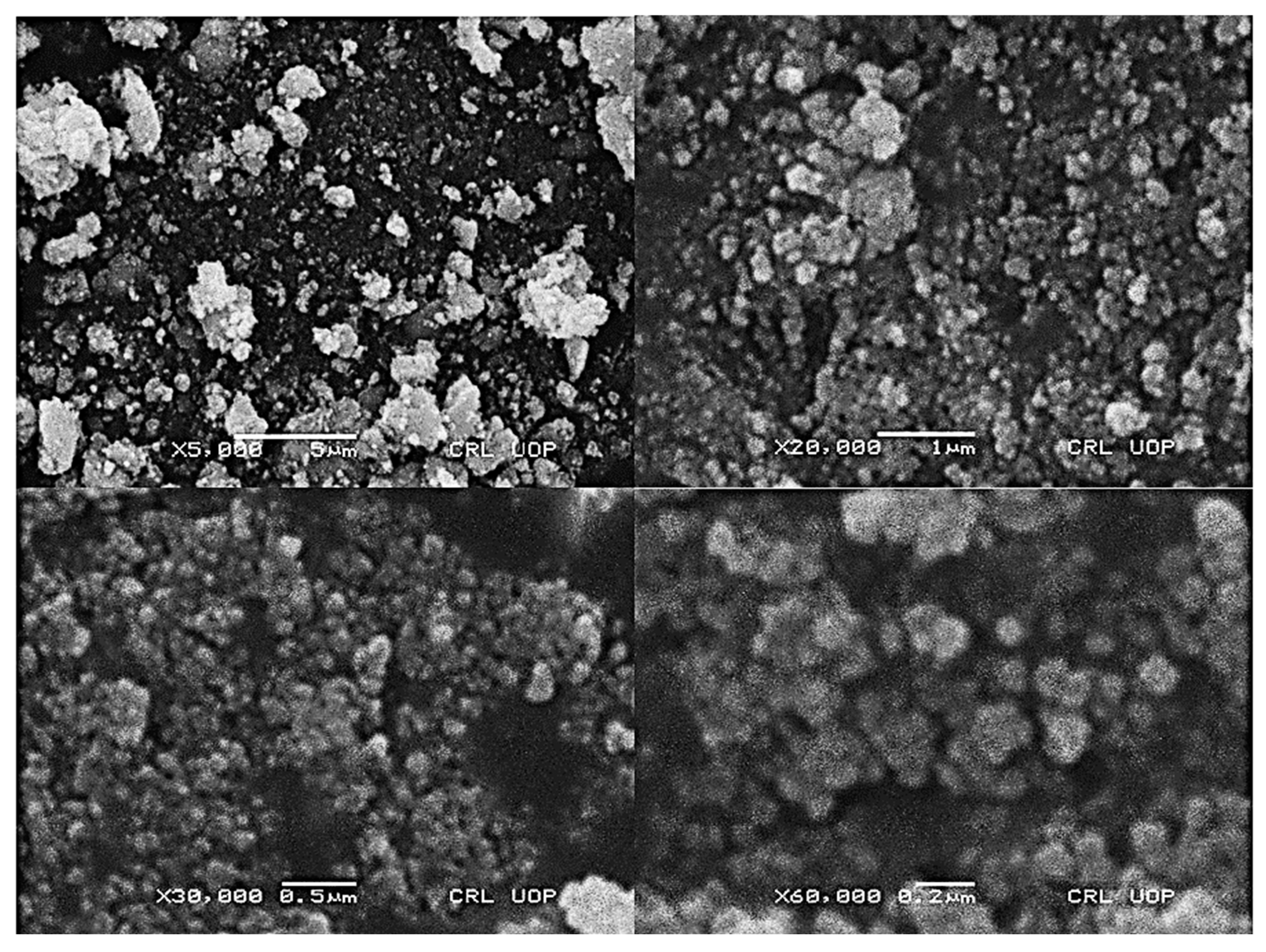
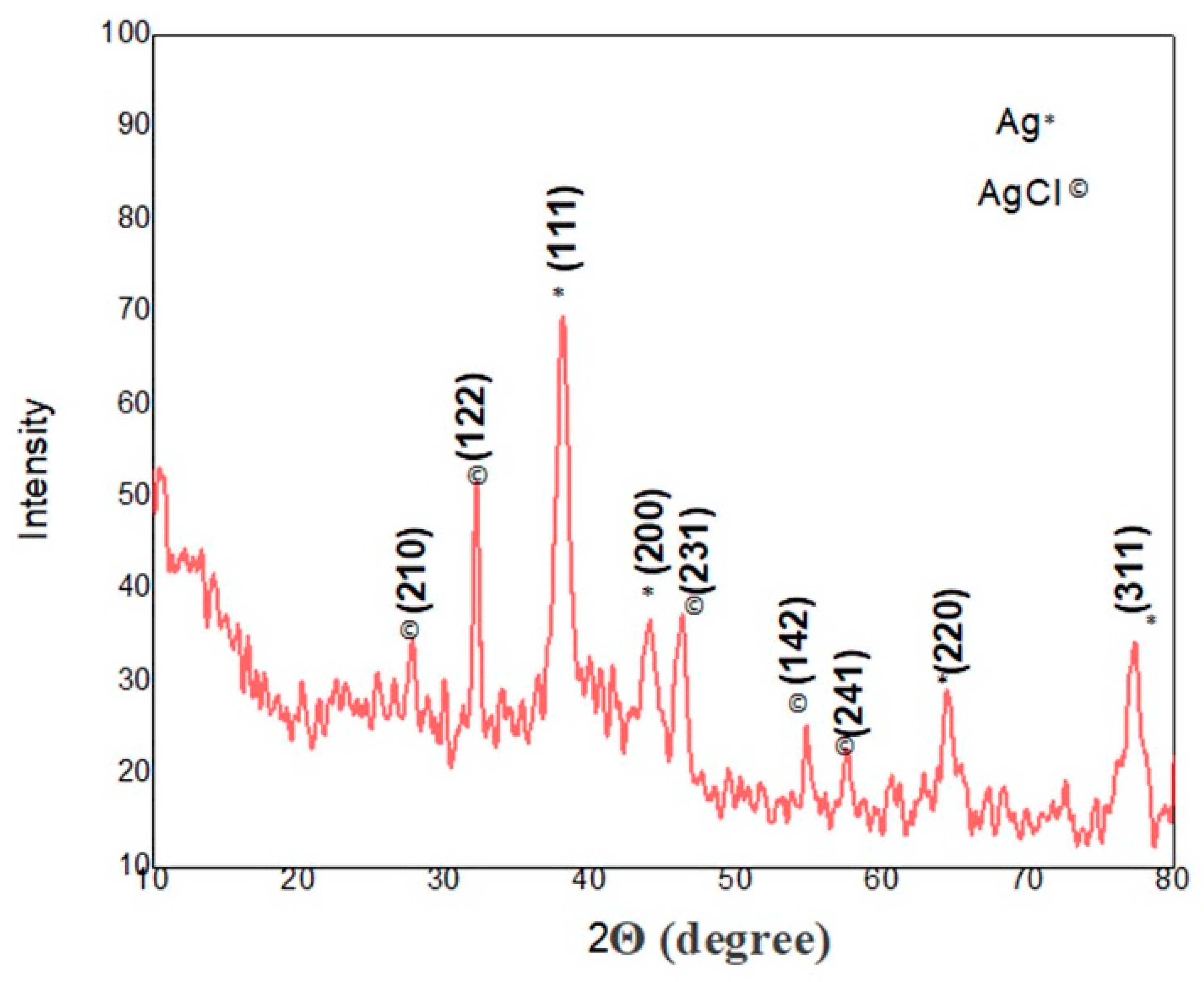
2.2. Characterization of Silver/Silver Chloride Nanoparticles
2.3. Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Preparation of Leaf Extract
4.2. Antioxidant Activity
4.3. Biosynthesis of Silver/Silver Chloride Nanoparticles
4.4. Characterization of Synthesized Particles
4.5. Antibacterial Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ali, M.; Kim, B.; Belfield, K.D.; Norman, D.; Brennan, M.; Ali, G.S. Inhibition of Phytophthora parasitica and P. capsici by silver nanoparticles synthesized using aqueous extract of Artemisia absinthium. Phytopathology 2015, 105, 1183–1190. [Google Scholar] [PubMed]
- Rafique, K.; Rauf, C.A.; Gul, A.; Bux, H.; Memon, R.A.; Ali, A.; Farrakh, S. Evaluation of d-genome synthetic hexaploid wheats and advanced derivatives for powdery mildew resistance. Pak. J. Bot. 2017, 49, 735–743. [Google Scholar]
- Seepe, H.A.; Nxumalo, W.; Amoo, S.O. Natural products from medicinal plants against phytopathogenic Fusarium species: Current research endeavours, challenges and prospects. Molecules 2021, 26, 6539. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Coniglio, M.A.; Laganà, P. Natural Inflammatory Molecules in Fruits and Vegetables; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Singh, D.; Singh, S.K.; Modi, A.; Singh, P.K.; Zhimo, V.Y.; Kumar, A. Impacts of Agrochemicals on Soil Microbiology and Food Quality; Elsevier: Amsterdam, The Netherlands, 2022; pp. 101–116. [Google Scholar]
- Jayanty, S.S.; Diganta, K.; Raven, B. Effects of cooking methods on nutritional content in potato tubers. Am. J. Poatoto Res. 2019, 96, 183–194. [Google Scholar] [CrossRef]
- Saenz-Banos, M.; Latorre-Biel, J.I.; Martínez-Cámara, E.; Jiménez-Macías, E.; Longo, F.; Blanco-Fernández, J. Methodology for energy demand reduction of potato cold storage process. J. Food Process Eng. 2022, 45, e14127. [Google Scholar] [CrossRef]
- Koch, M.; Naumann, M.; Pawelzik, E.; Gransee, A.; Thiel, H. The importance of nutrient management for potato production Part I: Plant nutrition and yield. Potato Res. 2020, 63, 97–119. [Google Scholar] [CrossRef]
- Pato, U.; Riftyan, E.; Jonnaidi, N.N.; Wahyuni, M.S.; Feruni, J.A.; Abdel-Wahhab, M.A. Isolation, characterization, and antimicrobial evaluation of bacteriocin produced by lactic acid bacteria against Erwinia carotovora. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Su, Z.; Liu, X.; Guo, Q.; Xuan, L.; Lu, X.; Dong, L.; Ma, P. Insights into complex infection by two Pectobacterium species causing potato blackleg and soft rot. Microbiol. Res. 2022, 261, 127072. [Google Scholar] [CrossRef]
- Ma, X.; Lofton, L.; Bamberg, J.; Swingle, B. Identification of resistance to Dickeya dianthicola soft rot in Solanum microdontum. Am. J. Potato Res. 2022, 99, 58–68. [Google Scholar] [CrossRef]
- Osei, R.; Yang, C.; Cui, L.; Ma, T.; Li, Z.; Boamah, S. Isolation, identification, and pathogenicity of Lelliottia amnigena causing soft rot of potato tuber in China. Microb. Patho. 2022, 164, 105441. [Google Scholar] [CrossRef]
- Przepiora, T.; Figaj, D.; Bogucka, A.; Fikowicz-Krosko, J.; Czajkowski, R.; Hugouvieux-Cotte-Pattat, N.; Skorko-Glonek, J. The periplasmic oxidoreductase DsbA is required for virulence of the phytopathogen Dickeya solani. Int. J. Mol. Sci. 2020, 23, 697. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.K.; Sharma, S.; Shah, M.A. Sustainable Management of Potato Pests and Diseases; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Munyaneza, J.E.; Bizimungu, B. Management of potato pests and diseases in Africa. In Insect Pests of Potato; Academic Press: San Diego, CA, USA, 2022; pp. 407–426. [Google Scholar]
- Tariq, A.; Shah, G.M.; Zada, A.; Ali, A.; Shah, A.Z.; Fatima, I. Phytochemical analysis and in-vitro anti-bacterial and anti-fungal activity of Verbascum arianthum (Benth). Pure Appl. Biol. 2021, 10, 797–806. [Google Scholar] [CrossRef]
- Hazarika, A.; Yadav, M.; Yadav, D.K.; Yadav, H.S. An overview of the role of nanoparticles in sustainable agriculture. Biocatal. Agric. Biotechnol. 2022, 43, 102399. [Google Scholar] [CrossRef]
- Ningthoujam, R.; Jena, B.; Pattanayak, S.; Dash, S.; Panda, M.K.; Behera, R.K.; Singh, Y.D. Nanotechnology in food science. In Bio-Nano Interface; Springer: Singapore, 2022; pp. 59–73. [Google Scholar]
- Ghazal, B.; Saif, S.; Farid, K.; Khan, A.; Rehman, S.; Reshma, A.; Fazal, H.; Ali, M.; Ahmad, A.; Rahman, L.; et al. Stimulation of secondary metabolites by copper and gold nanoparticles in submerge adventitious root cultures of Stevia rebaudiana (Bert.). IET Nanobiotechnol. 2018, 12, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Sintes, J.R.; Rauscher, H. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H.M. The current application of nanotechnology in food and agriculture. J. Food. Drug. Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef]
- Elmer, W.; White, J.C. The future of nanotechnology in plant pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef]
- Worrall, E.A.; Hamid, A.; Mody, K.T.; Mitter, N.; Pappu, H.R. Nanotechnology for plant disease management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef]
- Ali, I.; Khan, A.; Ali, A.; Ullah, Z.; Dai, D.-Q.; Khan, N.; Khan, A.; Al-Tawaha, A.R.; Sher, H. Iron and zinc micronutrients and soil inoculation of Trichoderma harzianum enhance wheat grain quality and yield. Front. Plant Sci. 2022, 13, 960948. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-Álvarez, J.; Vega-Fernández, L.; Montes de Oca-Vásquez, G.; Vega-Baudrit, J.R. Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Bharathi, D.; Diviya Josebin, M.; Vasantharaj, S.; Bhuvaneshwari, V. Biosynthesis of silver nanoparticles using stem bark extracts of Diospyros montana and their antioxidant and antibacterial activities. J. Nanostruct. Chem. 2018, 8, 83–92. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. 2022, 19, 674. [Google Scholar] [CrossRef]
- Vaghasiya, T.P.; Kumar, A.; Nakum, K. A Review on Wide Range Application of Nanoparticles in Agriculture and its Implications in Plant Disease Management. J. Nanoworld. 2022, 8, 55–65. [Google Scholar]
- Tundis, R.; Peruzzi, L.; Menichini, F. Phytochemical and biological studies of Stachys species in relation to chemotaxonomy: A review. Phytochemistry 2014, 102, 7–39. [Google Scholar] [CrossRef]
- Tomou, E.M.; Barda, C.; Skaltsa, H. Genus Stachys: A review of traditional uses, phytochemistry and bioactivity. Med. Mater. Lett. 2020, 7, 63. [Google Scholar] [CrossRef]
- Rahman, S.U.; Ullah, Z.; Ali, A.; Aziz, M.A.; Alam, N.; Sher, H.; Ali, I. Traditional knowledge of medicinal flora among tribal communities of Buner Pakistan. Phytomedicine 2022, 2, 100277. [Google Scholar] [CrossRef]
- Sher, H.; Ali, A.; Ullah, Z.; Sher, H. Alleviation of Poverty through Sustainable Management and Market Promotion of Medicinal and Aromatic Plants in Swat, Pakistan: Alleviation of Poverty through Sustainable Management. Ethnobot. Res. Appl. 2022, 23, 1–19. [Google Scholar]
- Bahadori, M.B.; Zengin, G.; Dinparast, L.; Eskandani, M. The health benefits of three Hedgenettle herbal teas (Stachys byzantina, Stachys inflata, and Stachys lavandulifolia)-profiling phenolic and antioxidant activities. Eur. J. Integr. Med. 2022, 36, 101134. [Google Scholar] [CrossRef]
- Ullah, Z.; Ali, U.; Ali, S.; Ali, A.; Alam, N.; Sher, H. Medicinal Flora and Cultural Values of Arkot-Biakand Valley Hindu Kush Region Swat, Pakistan; Springer: Berlin/Heidelberg, Germany, 2021; pp. 327–380. [Google Scholar]
- Hedge, I.C. Labiatae in Flora of Pakistan. Univ. Karachi Karachi 1990, 192, 310. [Google Scholar]
- Arif, M.; Ullah, R.; Ahmad, M.; Ali, A.; Ullah, Z.; Ali, M.; Sher, H. Green synthesis of silver nanoparticles using Euphorbia wallichii leaf extract: Its antibacterial action against citrus canker causal agent and antioxidant potential. Molecules 2022, 27, 3525. [Google Scholar] [CrossRef]
- Van Dong, P.; Ha, C.H.; Binh, L.T.; Kasbohm, J. Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int. Nano Lett. 2012, 2, 9. [Google Scholar] [CrossRef]
- Kiumarzi, F.; Morshedloo, M.R.; Zahedi, S.M.; Mumivand, H.; Behtash, F.; Hano, C.; Lorenzo, J.M. Selenium Nanoparticles (Se-NPs) Alleviates Salinity Damages and Improves Phytochemical Characteristics of Pineapple Mint (Mentha suaveolens Ehrh.). Plants 2022, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Phatik, T.; Das, J.; Boruah, P. Antifungal activity of Polygonum hydropiper and Solanum melongena against plant pathogenic fungi. Plant Arch. 2014, 14, 15–17. [Google Scholar]
- Olfati, A.; Kahrizi, D.; Balaky, S.T.J.; Sharifi, R.; Tahir, M.B.; Darvishi, E. Green synthesis of nanoparticles using Calendula officinalis extract from silver sulfate and their antibacterial effects on Pectobacterium caratovorum. Inorg. Chem. Commun. 2021, 125, 108439. [Google Scholar] [CrossRef]
- Dada, A.O.; Adekola, F.A.; Dada, F.E.; Adelani-Akande, A.T.; Bello, M.O.; Okonkwo, C.R.; Adetunji, C.O. Silver nanoparticle synthesis by Acalypha wilkesiana extract: Phytochemical screening, characterization, influence of operational parameters, and preliminary antibacterial testing. Heliyon 2019, 5, e02517. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Shin, H.S. Facile green biosynthesis of silver nanoparticles using Pisum sativum L. outer peel aqueous extract and its antidiabetic, cytotoxicity, antioxidant, and antibacterial activity. Int. J. Nanomed. 2019, 14, 6679. [Google Scholar] [CrossRef]
- Kup, F.Ö.; Çoşkunçay, S.; Duman, F. Biosynthesis of silver nanoparticles using leaf extract of Aesculus hippocastanum (horse chestnut): Evaluation of their antibacterial, antioxidant and drug release system activities. Mater. Sci. Eng. 2020, 107, 110207. [Google Scholar] [CrossRef]
- Ionita, P. The chemistry of DPPH· free radical and congeners. Int. J. Mol. Sci. 2021, 22, 1545. [Google Scholar] [CrossRef]
- Tatarczak-Michalewska, M.; Flieger, J. Application of High-Performance Liquid Chromatography with Diode Array Detection to Simultaneous Analysis of Reference Antioxidants and 1, 1-Diphenyl-2-picrylhydrazyl (DPPH) in Free Radical Scavenging Test. Int. J. Environ. Res. 2022, 19, 8288. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Khan, Q.U.; Tahir, K.; Ullah, S.; Arooj, A.; Li, B.; Ullah, I. A Tagetes minuta based eco-benign synthesis of multifunctional Au/MgO nanocomposite with enhanced photocatalytic, antibacterial and DPPH scavenging activities. Mater. Sci. Eng. 2021, 126, 112146. [Google Scholar] [CrossRef] [PubMed]
- Tosun, R.B.; Hamaloğlu, K.Ö.; Kavaklı, C.; Kavaklı, P.A.; Tuncel, A. Reusable water oxidation catalyst with dual active center for enhanced water oxidation: Iridium oxide nanoparticles immobilized on monodisperse-porous Mn5O8 microspheres. Int. J. Hydrogen Energy 2021, 46, 15482–15496. [Google Scholar] [CrossRef]
- Mohamed, J.M.M.; Alqahtani, A.; Kumar, T.V.A.; Fatease, A.A.; Alqahtani, T.; Krishnaraju, V.; Vijaya, R. Superfast synthesis of stabilized silver nanoparticles using aqueous Allium sativum (garlic) extract and isoniazid hydrazide conjugates: Molecular docking and in-vitro characterizations. Molecules 2021, 27, 110. [Google Scholar] [CrossRef]
- Khan, S.; Bibi, G.; Dilbar, S.; Iqbal, A.; Ahmad, M.; Ali, A.; Ullah, Z.; Jaremko, M.; Iqbal, J.; Ali, M.; et al. Biosynthesis and characterization of iron oxide nanoparticles from Mentha spicata and screening its combating potential against Phytophthora infestans. Front. Plant Sci. 2022, 13, 1001499. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, H.; Mehmood, A.; Ahmad, K.S.; Raffi, M. Green synthesis of silver nanoparticles and their applications as an alternative antibacterial and antioxidant agent. Mater. Sci. Eng. 2020, 112, 110901. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, Z.; Mohammadyan, M.; Naderi, S.; Fakhar, M.; Biparva, P.; Akhtari, J.; Ebrahimzadeh, M.A. Green synthesis of silver nanoparticles using Ferula persica extract (Fp-NPs): Characterization, antibacterial, antileishmanial, and in vitro anticancer activities. Mater. Today. Commun. 2021, 27, 102264. [Google Scholar] [CrossRef]
- Singh, R.; Hano, C.; Nath, G.; Sharma, B. Green biosynthesis of silver nanoparticles using leaf extract of Carissa carandas L. and their antioxidant and antimicrobial activity against human pathogenic bacteria. Biomolecules 2021, 11, 299. [Google Scholar] [CrossRef]
- Shakeri, A.D.; Urso, G.; Taghizadeh, S.F.; Piacente, S.; Norouzi, S.; Soheili, V.; Salarbashi, D. LC-ESI/LTQOrbitrap/MS/MS and GC–MS profiling of Stachys parviflora L. and evaluation of its biological activities. J. Pharm. Biomed. Anal. 2019, 168, 209–216. [Google Scholar] [CrossRef]
- Jan, S.; Khan, H.; Hamayun, M.; Mehmood, A.; Ahmad, N.; Lee, I.J. Chemical Composition, Mineral Profile and Antimicrobial Activity of Stachys parviflora and Calotropis procera. J. Pure Appl. Microbiol. 2015, 9, 1103–1110. [Google Scholar]
- Vorobyova, V.I.; Vasyliev, G.S.; Pylypenko, I.V.; Khrokalo, L.A. Preparation, characterization, and antibacterial properties of “green” synthesis of Ag nanoparticles and AgNPs/kaolin composite. Appl. Nanosci. 2022, 12, 889–896. [Google Scholar] [CrossRef]
- Naligama, K.N.; Halmillawewa, A.P. Pectobacterium spp. isolated from rotting carrots obtained from markets in Gampaha district, Sri Lanka exhibit the potential of having broad host ranges. Eur. J. Plant Pathol. 2022, 163, 841–852. [Google Scholar] [CrossRef]
- Benada, M.H.; Boumaaza, B.; Boudalia, S.; Khaladi, O.; Guessas, B. Variability of aggressiveness and virulence of Erwinia carotovora subsp. carotovorum causing the soft rot on potato tubers in the western of Algeria. Int. J. Plant Bio. 2018, 9, 7568. [Google Scholar]
- Balachandar, R.; Navaneethan, R.; Biruntha, M.; Kumar, K.K.A.; Govarthanan, M.; Karmegam, N. Antibacterial activity of silver nanoparticles phytosynthesized from Glochidion candolleanum leaves. Mater. Lett. 2022, 311, 131572. [Google Scholar] [CrossRef]
- Zein, R.; Alghoraibi, I.; Soukkarieh, C.; Ismail, M.T.; Alahmad, A. Influence of polyvinylpyrrolidone concentration on properties and anti-bacterial activity of green synthesized silver nanoparticles. Micromachines 2022, 13, 777. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol.Sci. 2022, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.K.; Rai, V.R.; Ramachandra, Y.L. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Ul Haq, M.N.; Shah, G.M.; Menaa, F.; Khan, R.A.; Althobaiti, N.A.; Albalawi, A.E.; Alkreathy, H.M. Green Silver Nanoparticles Synthesized from Taverniera couneifolia Elicits Effective Anti-Diabetic Effect in Alloxan-Induced Diabetic Wistar Rats. Nanomaterials 2022, 12, 1035. [Google Scholar] [CrossRef]
- Ahmad, M.; Ali, A.; Ullah, Z.; Sher, H.; Dai, D.-Q.; Ali, M.; Iqbal, J.; Zahoor, M.; Ali, I. Biosynthesized silver nanoparticles using Polygonatum germiniflorum efficiently controls Fusarium wilt disease of tomato. Front. Bioeng. Biotechnol. 2022, 10, 988607. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dilbar, S.; Sher, H.; Binjawhar, D.N.; Ali, A.; Ali, I. A Novel Based Synthesis of Silver/Silver Chloride Nanoparticles from Stachys emodi Efficiently Controls Erwinia carotovora, the Causal Agent of Blackleg and Soft Rot of Potato. Molecules 2023, 28, 2500. https://doi.org/10.3390/molecules28062500
Dilbar S, Sher H, Binjawhar DN, Ali A, Ali I. A Novel Based Synthesis of Silver/Silver Chloride Nanoparticles from Stachys emodi Efficiently Controls Erwinia carotovora, the Causal Agent of Blackleg and Soft Rot of Potato. Molecules. 2023; 28(6):2500. https://doi.org/10.3390/molecules28062500
Chicago/Turabian StyleDilbar, Shazia, Hassan Sher, Dalal Nasser Binjawhar, Ahmad Ali, and Iftikhar Ali. 2023. "A Novel Based Synthesis of Silver/Silver Chloride Nanoparticles from Stachys emodi Efficiently Controls Erwinia carotovora, the Causal Agent of Blackleg and Soft Rot of Potato" Molecules 28, no. 6: 2500. https://doi.org/10.3390/molecules28062500
APA StyleDilbar, S., Sher, H., Binjawhar, D. N., Ali, A., & Ali, I. (2023). A Novel Based Synthesis of Silver/Silver Chloride Nanoparticles from Stachys emodi Efficiently Controls Erwinia carotovora, the Causal Agent of Blackleg and Soft Rot of Potato. Molecules, 28(6), 2500. https://doi.org/10.3390/molecules28062500







