Quality Markers’ Discovery and Quality Evaluation of Jigucao Capsule Using UPLC-MS/MS Method
Abstract
1. Introduction
2. Results
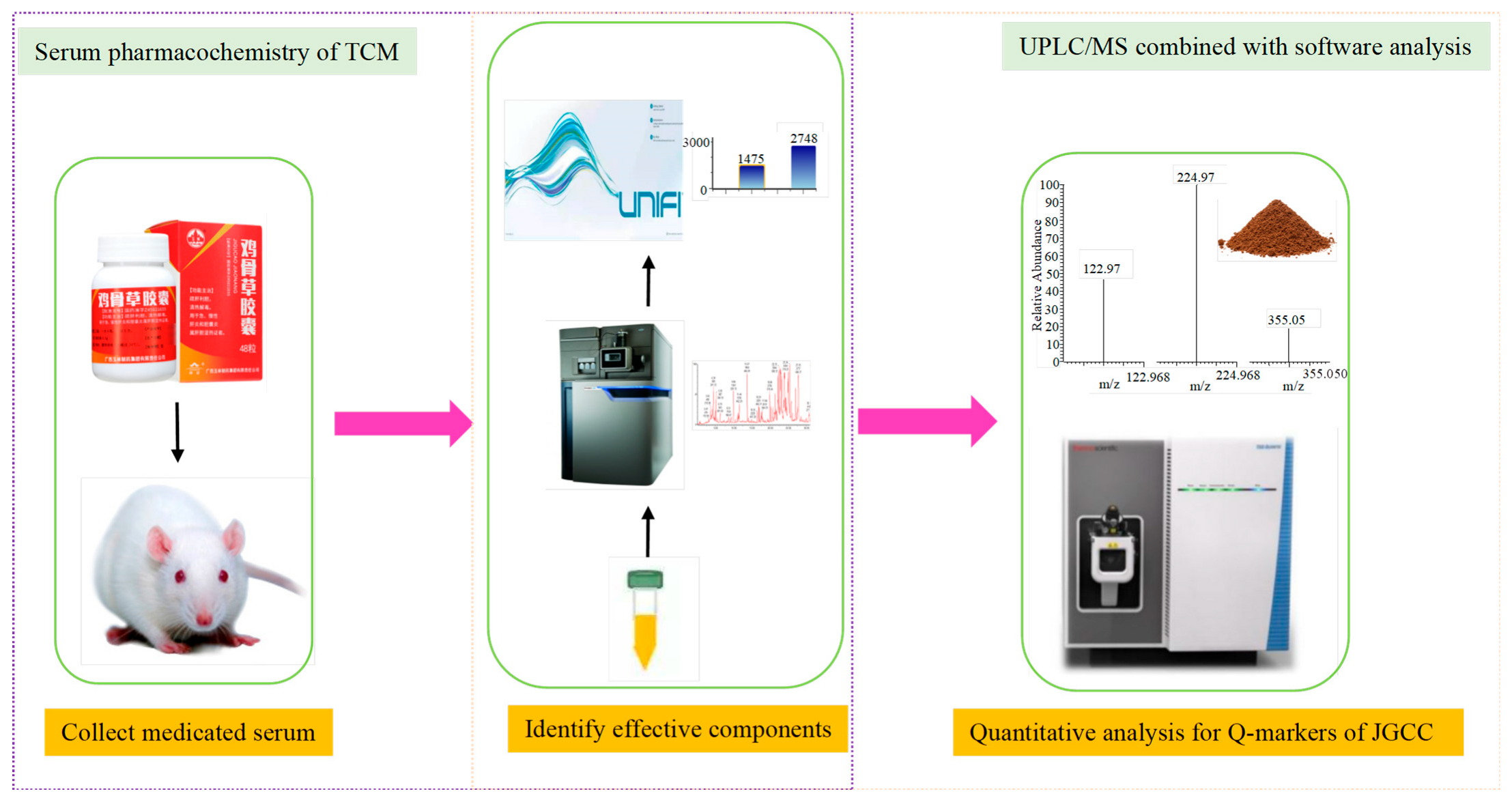
2.1. The Evaluation for Preparation of DHJS Rat Model and Effectiveness of JGCC
2.2. Prototype Components Analysis of JGCC Found in the Blood
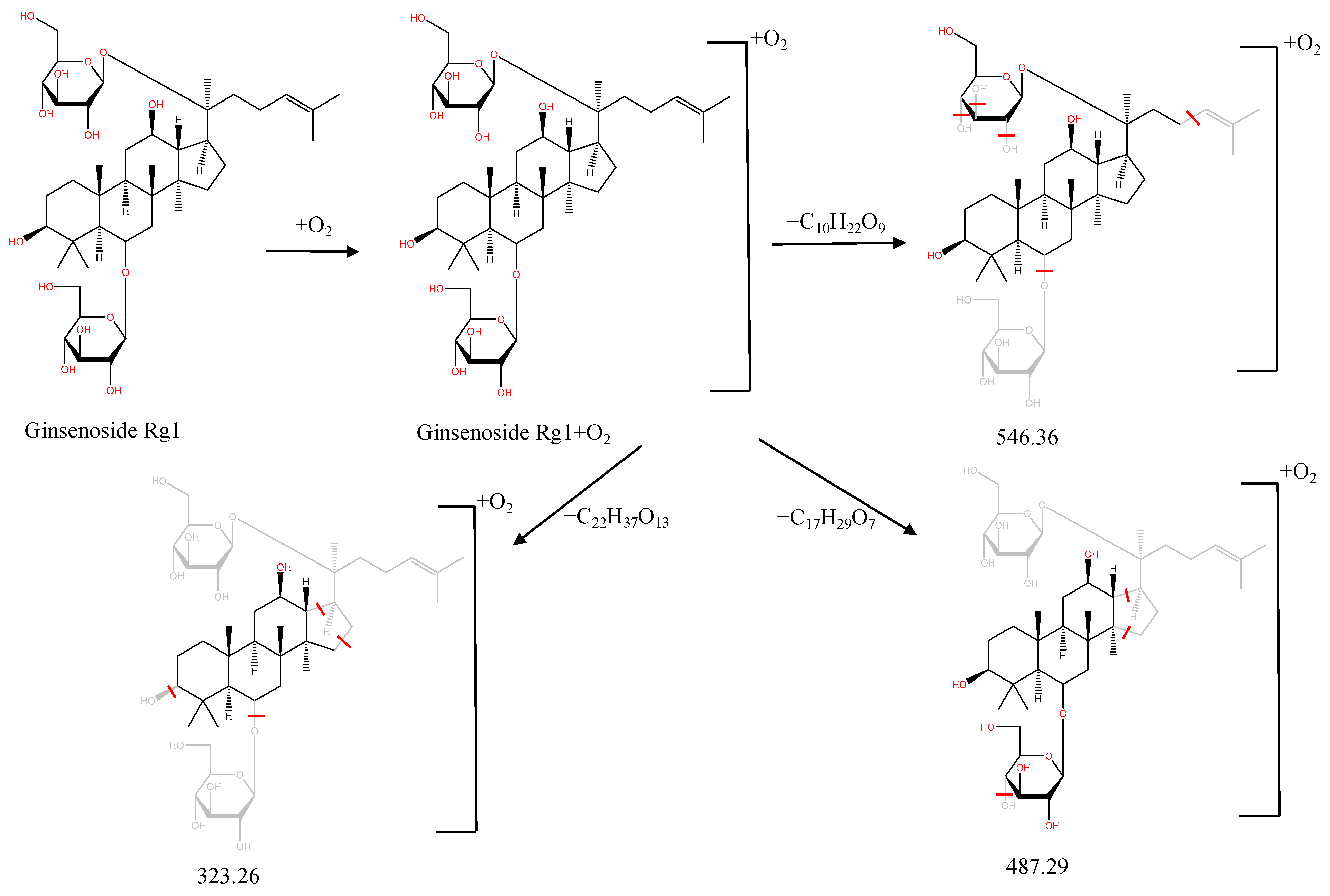
2.3. Metabolite Analysis of JGCC into Blood
2.4. Determination of Q-Markers for JGCC
- (1)
- The pharmacodynamic components of JGCC. Through the literature review, it was found that 27 of the 43 prototype blood components were successfully characterized and identified, and they have obvious hepatoprotective effects. The nine prototype components of metabolites have obvious protective effects on the liver. Finally, trigonelline, 3,4,5-trihydroxybenzoic acid, geniposide acid, abrine, chlorogenic acid, hypaphorine, p-coumaric acid, geniposide, genipin-1-gentiobioside, vicenin-2, albiflorin, isoschaftoside, paeoniflorin, isovitexin, kaempferol, ginsenoside Rg1, luteolin, TCA, ginsenoside Rb1, notoginsenoside Fa, beta-ionone, THDCA, TCDCA, soyasaponin I, ginsenoside Rh4, CDCA, betulonic acid, scoparone, capillarisin, ginsenoside Rd and HDCA are considered to be the pharmacodynamic material basis of JGCC in the treatment of DHJS.
- (2)
- The inherent components of JGCC. The inherent components mean the prototype components of the pharmacodynamic material basis in JGCC, so the 31 components mentioned in (1) are also the inherent components of JGCC.
- (3)
- The unique ingredients in the herbal medicine of JGCC. Among the inherent pharmacodynamic components, chlorogenic acid is a common component of Artemisia capillaris Thunb and Gardenia jasminoides Ellis. Kaempferol is a common component of Paeonia lactiflora Pall, Origanum vulgare L. and Gardenia jasminoides Ellis. HDCA is a common component of Sus scrofadomestica Brisson and Bovis calculus Artifactus. The remaining components are the unique ingredients of each herb.
- (4)
- The measurable components in JGCC. Yan [25] and Liu [26] established a method for the content determination of trigonelline, abrine, hypaphorine, isoschaftoside, isovitexin, luteolin and vicenin-2 in Abri Herba and Abri Mollis Herba by HPLC-MS/MS. The “Chinese Pharmacopoeia” includes the content determination methods for chlorogenic acid and scoparone in Artemisia capillaris Thunb, ginsenoside Rg1 and ginsenoside Rb1 in Panax notoginseng (Burk.) F.H.Chen, THDCA in Sus scrofadomestica Brisson and paeoniflorin in Paeonia lactiflora Pall. In addition, according to the domestic and foreign literature, the following components have been determined: geniposide, geniposidic acid and genipin-1-gentiobioside are present in Gardenia jasminoides Ellis [27], albiflorin is discovered in Paeonia lactiflora Pall [28], TCA and TCDCA are found in snake bile [29], CDCA is discovered in bio-transformed samples [30] and scoparone and capillarisin are found in Artemisia capillaris Thunb and its compound preparations [31]. These have all been previously quantified.
- (5)
- The prescription compatibility properties of Q-markers in JGCC. In JGCC, Abrus cantoniensis Hance is the monarch (jun), Artemisia capillaris Thunb and Gardenia jasminoides Ellis are the ministers (chen) and Paeonia lactiflora Pall, Panax notoginseng (Burk.) F.H.Chen, Origanum vulgare L., Sus scrofadomestica Brisson and Bovis calculus Artifactus are the adjuvants (zuo). Lycium barbarum L. and Ziziphus jujuba Mill are the ambassadors (shi). Therefore, the selection of Q-markers is mainly based on the specific pharmacodynamic components in the monarch medicine Abrus cantoniensis Hance, such as trigonelline, abrine, hypaphorine, isoschaftoside, isovitexin, luteolin, vicenin-2 and soyasaponin I, as well as the specific pharmacodynamic components of other medicinal materials.
2.5. Specificity, Linear Range, Limit of Detection (LOD), Limit of Quantification (LOQ)
2.6. Precision, Stability and Accuracy
2.7. Determination Results of Multiple Batches of JGCC
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chromatographic Analysis
4.3. UPLC-Q-TOF-MS Conditions
4.4. UPLC-QQQ-MS Conditions and Optimization
4.5. Preparation of the Modeling Solution
4.6. Source and Processing of Serum Samples
4.7. ”Five Principles” for Determining Q-Markers
4.8. Preparation of JGCC for Quantitative Analysis
4.9. Preparation of Mixed Standard Solutions
4.10. Specificity, LOD, LOQ
4.11. Precision
4.12. Stability
4.13. Accuracy
4.14. Content Determination of the Multiple Samples
4.15. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xiao, B.D. Lingnan Herbs Collection; Guangdong Science and Technology Press: Guangzhou, China, 1970; p. 114. [Google Scholar]
- Wang, S.; Huang, H.; Liu, X.; Wang, J.; Yan, Z. Preliminary observation on the treatment of infectious hepatitis with Abrus cantoniensis. Peoples Mil. Surg. 1958, 11, 829–830. [Google Scholar]
- Bai, B.; Yu, W.; Zhang, Y.; Zhang, L.; Liu, J.; Qiu, J.; Wu, Y.; Chen, X.; Shen, Y.; Deng, Z. A preliminary report on curativeeffect of Jigucao powder on 57 cases of infectious hepatitis. Peoples Mil. Surg. 1959, 9, 715–723.1. [Google Scholar]
- Sun, H.; Yang, L.; Li, M.X.; Fang, H.; Zhang, A.H.; Song, Q.; Liu, X.Y.; Su, J.; Yu, M.D.; Makino, T.; et al. UPLC-G2Si-HDMS untargeted metabolomics for identification of metabolic targets of Yin-Chen-Hao-Tang used as a therapeutic agent of dampness-heat jaundice syndrome. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1081–1082, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.F.; Sun, H.; Liu, S.B.; Lu, S.W.; Zhang, A.H.; Wang, W.Y.; Chai, W.J.; Wu, F.F.; Yan, G.L.; Guan, Y.; et al. Targets and Effective Constituents of ZhiziBaipi Decoction for Treating Damp-Heat Jaundice Syndrome Based on Chinmedomics Coupled with UPLC-MS/MS. Front. Pharm. 2022, 13, 857361. [Google Scholar] [CrossRef]
- He, Y.; Zhang, M.; Li, T.; Tan, Z.; Zhang, A.; Ou, M.; Huang, D.; Wu, F.; Wang, X. Metabolomics Analysis Coupled With UPLC/MS on Therapeutic Effect of Jigucao Capsule Against Dampness-Heat Jaundice Syndrome. Front. Pharm. 2022, 13, 822193. [Google Scholar] [CrossRef]
- Li, T.; He, Y.; Zhang, M.; Tan, Z.; Zhang, A.; Ou, M.; Huang, D.; Wu, F.; Wang, X. High throughput metabolomics explores the mechanism of Jigucao capsules in treating Yanghuang syndrome rats using ultra-performance liquid chromatography quadrupole time of flight coupled with mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1194, 123185. [Google Scholar] [CrossRef]
- Lei, Q. Discuss the efficacy of Jigucao capsule combined with antiviral treatment of chronic hepatitis B and its effect on liver function, serum TGF-β1 and ECM levels. Mod. Diagn. Treat. 2018, 29, 2036–2038. [Google Scholar]
- Qin, J.; Huang, W.; Li, D. Jigucao capsule combined with entecavir in the treatment of 24 cases of chronic hepatitis B. Guid. J. Tradit. Chin. Med. Pharm. 2013, 19, 116–117. [Google Scholar]
- Lei, Q. The effect of Jigucao capsule adjuvant therapy on liver fibrosis in patients with non-alcoholic fatty liver disease. Shenzhen J. Integr. Med. 2018, 28, 37–39. [Google Scholar]
- Lin, X.; Zhang, Q.; Hao, G. Jigucao Capsule in the treatment of 30 cases of chronic cholecystitis. Shanxi J. Tradit. Chin. Med. 2007, 28, 24–25. [Google Scholar]
- Sui, F.; Jiang, X.; Jiang, X.; Chen, L.; Zhao, T. Clinical observation of Jigucao capsule in the treatment of acute hepatitis. Chin. Youjiang Med. J. 2002, 30, 247–248. [Google Scholar]
- Wu, Q. Therapeutic effect of Jigucao capsule on immune hepatic fibrosis in rats. Guide Chin. Med. 2010, 8, 46–48. [Google Scholar]
- Zhao, P.; Ye, Z.W.; He, D.X.; Pan, L.; Lin, J. Study on the protective effect of Jigucao capsule on liver fibrosis in rats. Chin. J. Exp. Tradit. Med. Formulae 2009, 15, 99–101. [Google Scholar]
- Wang, X.J. Study on Serum Medicinal Chemistry of Traditional Chinese Medicine and Chinese Medicine Compounds. World Sci. Technol. 2002, 4, 1–4,+78. [Google Scholar]
- Wang, X.J. A systematic methodology for basic research on pharmacodynamics of traditional Chinese medicine—Chinmedomics. China J. Chin. Mater. Med. 2015, 40, 13–17. [Google Scholar]
- Liu, C.; Chen, S.L.; Xiao, X.H.; Zhang, T.J.; Hou, W.B.; Liao, M.L. Traditional Chinese medicine quality marker (Q-marker): A new concept for quality control of traditional Chinese medicine Products. Chin. Tradit. Herb. Drugs 2016, 47, 1443–1457. [Google Scholar]
- Ren, J.L.; Zhang, A.H.; Kong, L.; Han, Y.; Yan, G.L.; Sun, H.; Wang, X.J. Analytical strategies for the discovery and validation of quality-markers of traditional Chinese medicine. Phytomedicine 2020, 67, 153165. [Google Scholar] [CrossRef]
- Liu, S.-B.; Lu, S.-W.; Sun, H.; Zhang, A.-H.; Wang, H.; Wei, W.-F.; Han, J.-R.; Guo, Y.-J.; Wang, X.-J. Deciphering the Q-markers of nourishing kidney-yin of Cortex Phellodendri amurense from ZhibaiDihuang pill based on Chinmedomics strategy. Phytomedicine 2021, 91, 153690. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhu, J.; Yang, Z.; Shao, Q.; Fan, X.; Cheng, Y. Q-marker based strategy for CMC research of Chinese medicine: A case study of Panax Notoginseng saponins. Phytomedicine 2018, 44, 129–137. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, A.-H.; Zhao, Q.-Q.; Yan, G.-L.; Sun, H.; Wang, X.-J. Discovery of quality-marker ingredients of Panax quinquefolius driven by high-throughput chinmedomics approach. Phytomedicine 2020, 74, 152928. [Google Scholar] [CrossRef]
- Li, W.; Polachi, N.; Wang, X.; Chu, Y.; Wang, Y.; Tian, M.; Li, D.; Zhou, D.; Zhou, S.; Ju, A.; et al. A quality marker study on salvianolic acids for injection. Phytomedicine 2018, 44, 138–147. [Google Scholar] [CrossRef] [PubMed]
- WS3-B-3420-98; Formulated Preparation of Traditional Chinese Medicine. Ministry of Health of the People’s Republic of China: Shanghai, China, 1998.
- Li, T.; Zhang, M.; Tan, Z.; Miao, J.; He, Y.; Zhang, A.; Ou, M.; Huang, D.; Wu, F.; Wang, X. Rapid characterization of the constituents in Jigucao capsule using ultra high performance liquid chromatography with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2022, 45, 677–696. [Google Scholar] [CrossRef]
- Yan, W.; Han, Q.; Guo, P.; Wang, C.; Zhang, Z. Simultaneous Detection of Flavonoids, Phenolic Acids and Alkaloids in Abri Herba and Abri Mollis Herba using Liquid Chromatography Tandem Mass Spectrometry. Phytochem. Anal. PCA 2016, 27, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Yan, W.Y.; Wang, X.; Fan, X.Y.; Lv, T.; Wang, C.Y. Determination of the concentrations and excretion kinetics of the main components in the urine and bile of rats by HPLC-MS/MS. Chin. J. Pharm. Anal. 2018, 38, 406–417. [Google Scholar]
- Oshima, T.; Sagara, K.; Yoshida, T.; Tong, Y.Y.; Zhang, G.D.; Chen, Y.H. Determination of geniposide, gardenoside, geniposidic acid and genipin-1-beta- gentiobioside in Gardenia jasminoides by high-performance liquid chromatography. J. Chromatogr. 1988, 455, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, R.Y.; Li, J.; Yan, J.; Zhao, Y.L.; Wang, X.Z.; Dai, L.L. Simultaneous Determination of 15 Components in Medicinal and Ornamental Peony by UPLC-MS/MS. Chin. J. Pharm. Anal. 2022, 42, 484–493. [Google Scholar]
- Xiang, Y.; Shi, C.Z.; Mei, H.S.; Peng, H. Determination of taurocholic acid, taurochenodeoxycholic acid and taurodeoxycholic acid in artificial snake bile by reversed-phase high performance liquid chromatography. Univ. Sci. Technol. Huazhong 2000, 29, 220–222. [Google Scholar]
- Zhao, J.; Guan, L.H.; Zhu, L.C. Simultaneous determination of five components including tauroursodeoxycholic acid in biotransformation samples by HPLC-ELSD. China Pharm. 2014, 25, 4291–4293. [Google Scholar]
- Wang, X.J.; Sun, H.; Liu, Z.S. Quantitative study of 6, 7-dimethoxy-coumarin and capillarisin and its compound preparation. China J. Chin. Mater. Med. 1994, 19, 667–670, 702. [Google Scholar]
- Huang, P.; Mo, H.; Ma, W.F.; Wei, S.X. RP-HPLC method was used for simultaneous determination of abrine and hypaphorine. Chin. J. Pharm. Anal. 2009, 29, 1702–1704. [Google Scholar]
- Zhang, S. Abrine Elicits Liver Carcinoma Immunity and Enhances Antitumor Efficacy of Immune Checkpoint Blockade by Modulating PD-L1 Signaling. J. Oncol. 2022, 2022, 7609676. [Google Scholar] [CrossRef]
- Sharma, L.; Lone, N.A.; Knott, R.M.; Hassan, A.; Abdullah, T. Trigonelline prevents high cholesterol and high fat diet induced hepatic lipid accumulation and lipo-toxicity in C57BL/6J mice, via restoration of hepatic autophagy. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 121, 283–296. [Google Scholar] [CrossRef]
- Su, Y.; Kang, Y.; Yi, J.; Lin, Q.; Zhang, C.; Lin, Z.; Yan, Z.; Qu, J.; Liu, J. Isoschaftoside Reverses Nonalcoholic Fatty Liver Disease via Activating Autophagy In Vivo and In Vitro. Evid. Based Complement. Altern. Med. 2022, 2022, 2122563. [Google Scholar] [CrossRef]
- Liu, X.; Pan, Y.; Shen, Y.; Liu, H.; Zhao, X.; Li, J.; Ma, N. Protective Effects of Abrus cantoniensis Hance on the Fatty Liver Hemorrhagic Syndrome in Laying Hens Based on Liver Metabolomics and Gut Microbiota. Front. Vet. Sci. 2022, 9, 862006. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, Y.H.; Song, L.; Chen, T.F.; Zhang, G.P.; Ye, Z.G.; Gao, Y.; Huo, W. Ginsenoside Rg1 protects mice against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced liver injury by inhibiting CYP1A1 through the aryl hydrocarbon receptor. J. Ethnopharmacol. 2022, 294, 115394. [Google Scholar] [CrossRef]
- Li, G.; Xie, H.; Cao, X.; Ma, C.; Li, Y.; Chen, L. Ginsenoside Rg1 exerts anti-apoptotic effects on non-alcoholic fatty liver cells by downregulating the expression of SGPL1. Mol. Med. Rep. 2022, 25, 178. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.L.; Xu, Q.M.; Li, X.R.; Yang, S.L. The protective effect of ginsenoside Rb1 on cantharidin-induced hepatotoxicity in SD rats. Asia-Pac. Tradit. Med. 2022, 18, 8–11. [Google Scholar]
- Wang, G.E.; Yang, F.; Liu, X.Y.; Ye, Y.J.; Wang, R.H.; Lv, Q.T.; Liu, X.T. Study on the mechanism of ginsenoside Rb1 in improving restraint stress and lipopolysaccharide-induced immune liver injury in mice. Chin. Tradit. Herb. Drugs 2022, 53, 4028–4034. [Google Scholar]
- Zhu, H.; Jiang, W.; Liu, C.; Wang, C.; Hu, B.; Guo, Y.; Cheng, Y.; Qian, H. Ameliorative effects of chlorogenic acid on alcoholic liver injury in mice via gut microbiota informatics. Eur. J. Pharmacol. 2022, 928, 175096. [Google Scholar] [CrossRef]
- Miao, H.; Ouyang, H.; Guo, Q.; Wei, M.; Lu, B.; Kai, G.; Ji, L. Chlorogenic acid alleviated liver fibrosis in methionine and choline deficient diet-induced nonalcoholic steatohepatitis in mice and its mechanism. J. Nutr. Biochem. 2022, 106, 109020. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, A.; Zhang, T.; Sun, H.; Guan, Y.; Yan, G.; Wang, X. Dissect new mechanistic insights for geniposide efficacy on the hepatoprotection using multiomics approach. Oncotarget 2017, 8, 108760–108770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, A.; Qiu, S.; Sun, H.; Han, Y.; Guan, Y.; Wang, X. High-throughput metabolomics approach reveals new mechanistic insights for drug response of phenotypes of geniposide towards alcohol-induced liver injury by using liquid chromatography coupled to high resolution mass spectrometry. Mol. Biosyst. 2016, 13, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Seo, K.O.; Yun, K.W.; Kim, M.J.; Lee, M.K. Comparative study of the hepatoprotective efficacy of Artemisia iwayomogi and Artemisia capillaris on ethanol-administered mice. J. Food Sci. 2011, 76, T207-11. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhang, A.; Yu, J.; Wang, L.; Liu, C.; Zhou, X.; Sun, H.; Song, Q.; Wang, X. Insight into the metabolic mechanism of scoparone on biomarkers for inhibiting Yanghuang syndrome. Sci. Rep. 2016, 6, 37519. [Google Scholar] [CrossRef]
- Kang, J.W.; Kim, D.W.; Choi, J.S.; Kim, Y.S.; Lee, S.M. Scoparone attenuates D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure through inhibition of toll-like receptor 4 signaling in mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 57, 132–139. [Google Scholar] [CrossRef]
- Farooqui, N.; Elhence, A.; Shalimar, A. Current Understanding of Bile Acids in Chronic Liver Disease. J. Clin. Exp. Hepatol. 2022, 12, 155–173. [Google Scholar] [CrossRef]
- Greimel, T.; Jahnel, J.; Pohl, S.; Strini, T.; Tischitz, M.; Meier-Allard, N.; Holasek, S.; Meinel, K.; Aguiriano-Moser, V.; Zobel, J.; et al. Bile acid-induced tissue factor activity in hepatocytes correlates with activation of farnesoid X receptor. Lab. Investig. 2021, 101, 1394–1402. [Google Scholar] [CrossRef]
- Carubbi, F.; Guicciardi, M.E.; Concari, M.; Loria, P.; Bertolotti, M.; Carulli, N. Comparative cytotoxic and cytoprotective effects of taurohyodeoxycholic acid (THDCA) and tauroursodeoxycholic acid (TUDCA) in HepG2 cell line. Biochim. Biophys. Acta 2002, 1580, 31–39. [Google Scholar] [CrossRef]
- Loria, P.; Bozzoli, M.; Concari, M.; Guicciardi, M.E.; Carubbi, F.; Bertolotti, M.; Piani, D.; Nistri, A.; Angelico, M.; Romani, M.; et al. Effect of taurohyodeoxycholic acid on biliary lipid secretion in humans. Hepatology 1997, 25, 1306–1314. [Google Scholar] [CrossRef]
- Zhang, T.J.; Bai, G.; Chen, C.Q.; Xu, J.; Han, Y.Q.; Gong, S.X.; Zhang, H.B.; Liu, C.X. Research path of compound traditional Chinese medicine quality marker (Q-marker) based on “five principles”. Chin. Tradit. Herb. Drugs 2018, 49, 1–13. [Google Scholar]
- Wang, Y.H.; Chen, X. Determination of geniposide in Jigucao Capsules. Chin. J. Inf. Tradit. Chin. Med. 2007, 14, 41–42. [Google Scholar]
- Li, G.; Zhou, X.; Zhao, Y.; Zhang, M.M.; Shen, J.X. Determination of abrine in Jigucao Capsules by High Performance Liquid Chromatography. J. Guangdong Pharm. Univ. 2011, 27, 271–273. [Google Scholar]


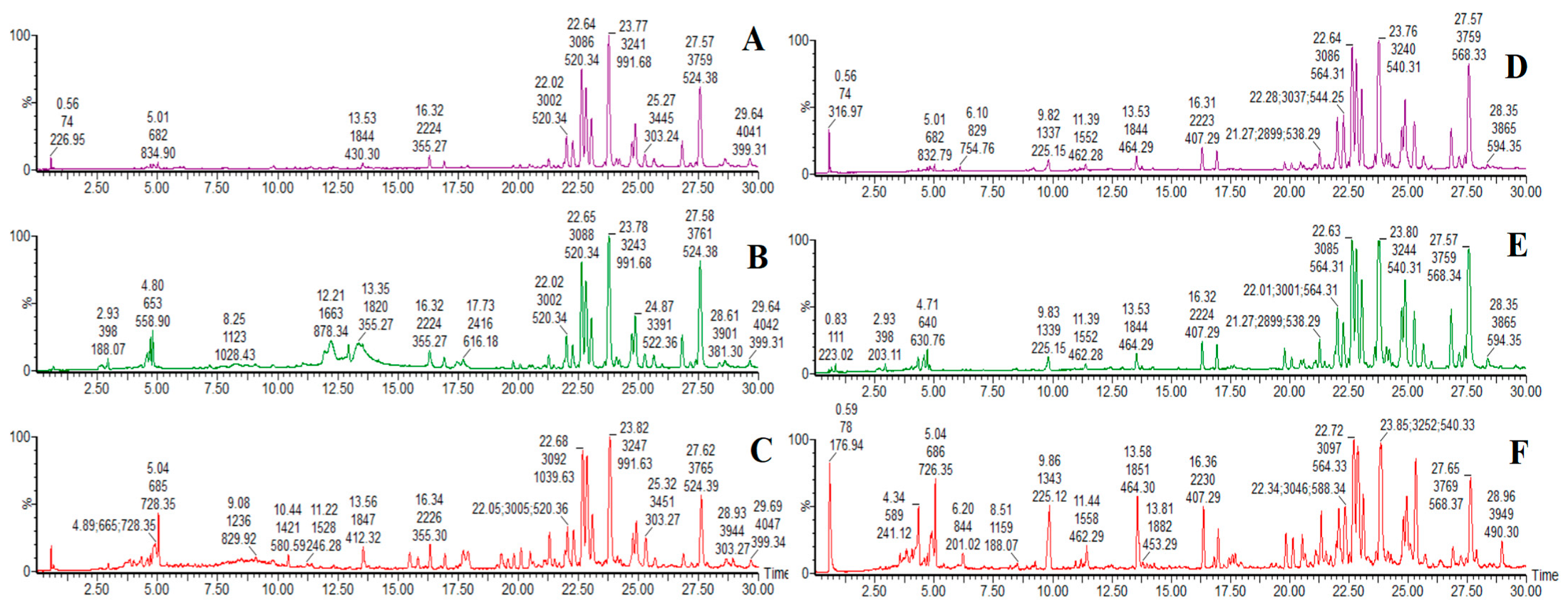
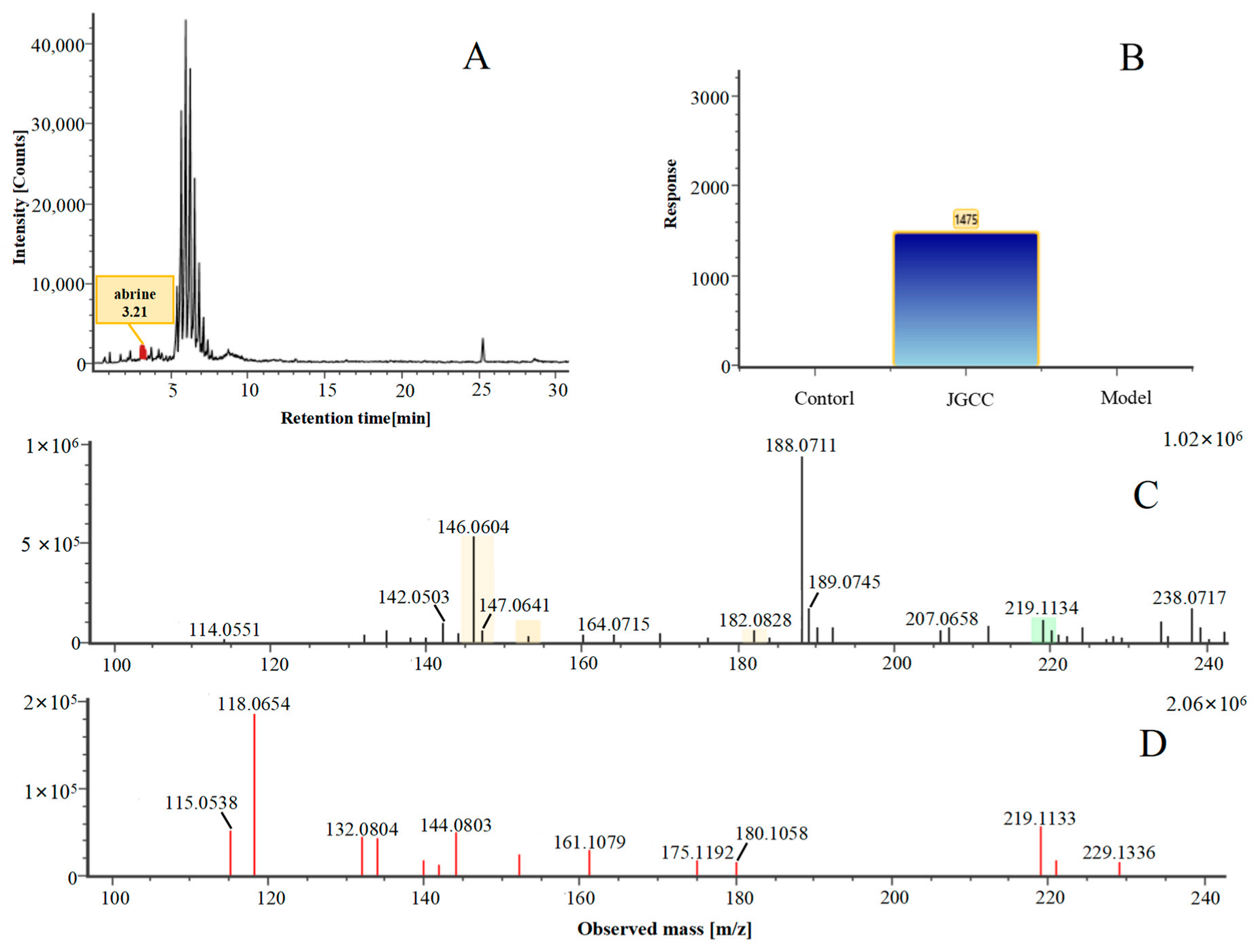
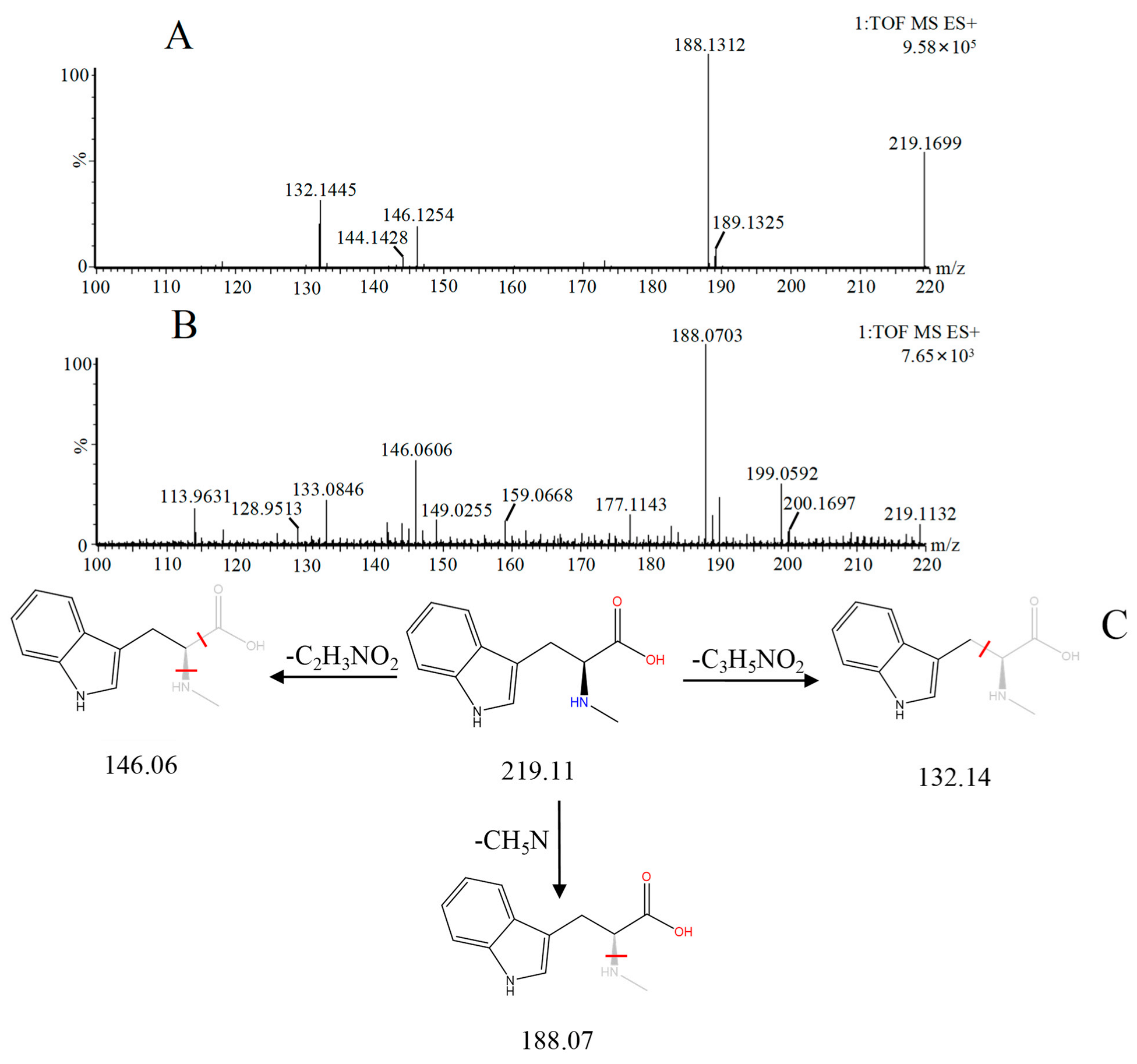

| NO | Compound | Rt | Observed m/z | Molecular Formula | MS/MS | References | Structural Formula |
|---|---|---|---|---|---|---|---|
| 1 | Trigonelline *,# | 0.66 | 138.10 | C7H7NO2 | 138.10[M+H]+,120.13[M+H−H2O]+ | Abrus cantoniensis Hance |  |
| 2 | Citric acid | 1.12 | 215.02 | C6H8O7 | 215.012[M+Na]+,166.08[M+Na−CH5O2]+, 149.02[M+Na−CH6O3]+ | Lycium barbarum L. | 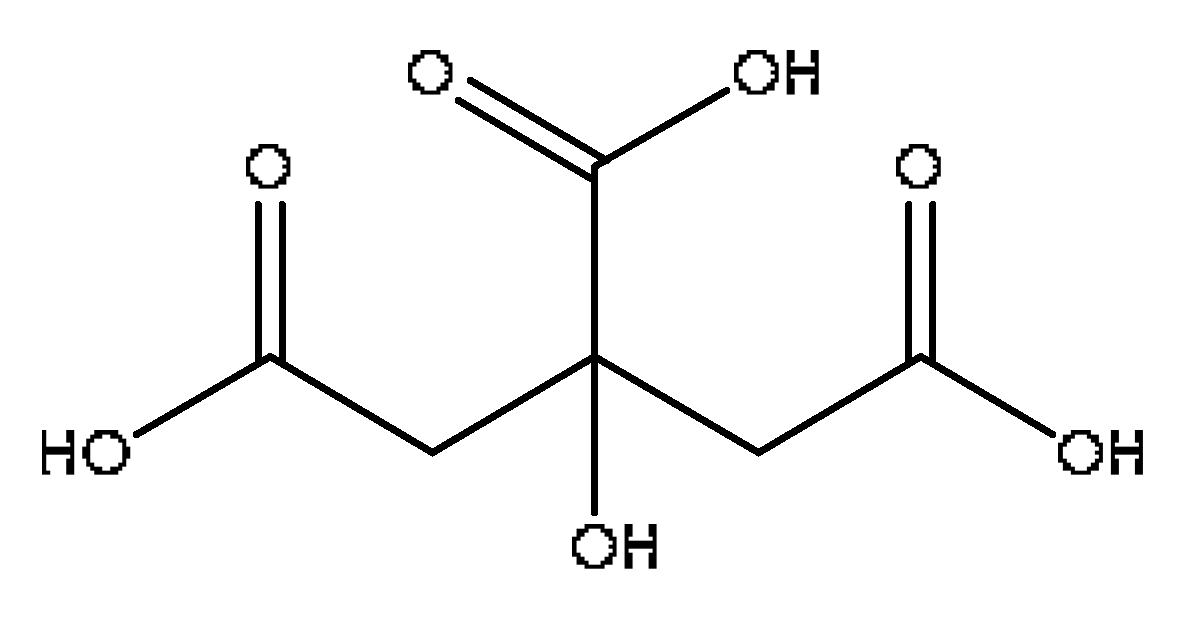 |
| 3 | 3,4,5-trihydroxybenzoic acid # | 1.80 | 169.02 | C7H6O5 | 169.02[M−H]−,141.87[M-H−CO]−, 125.02[M-H−CO2]− | Paeonia lactiflora Pall | 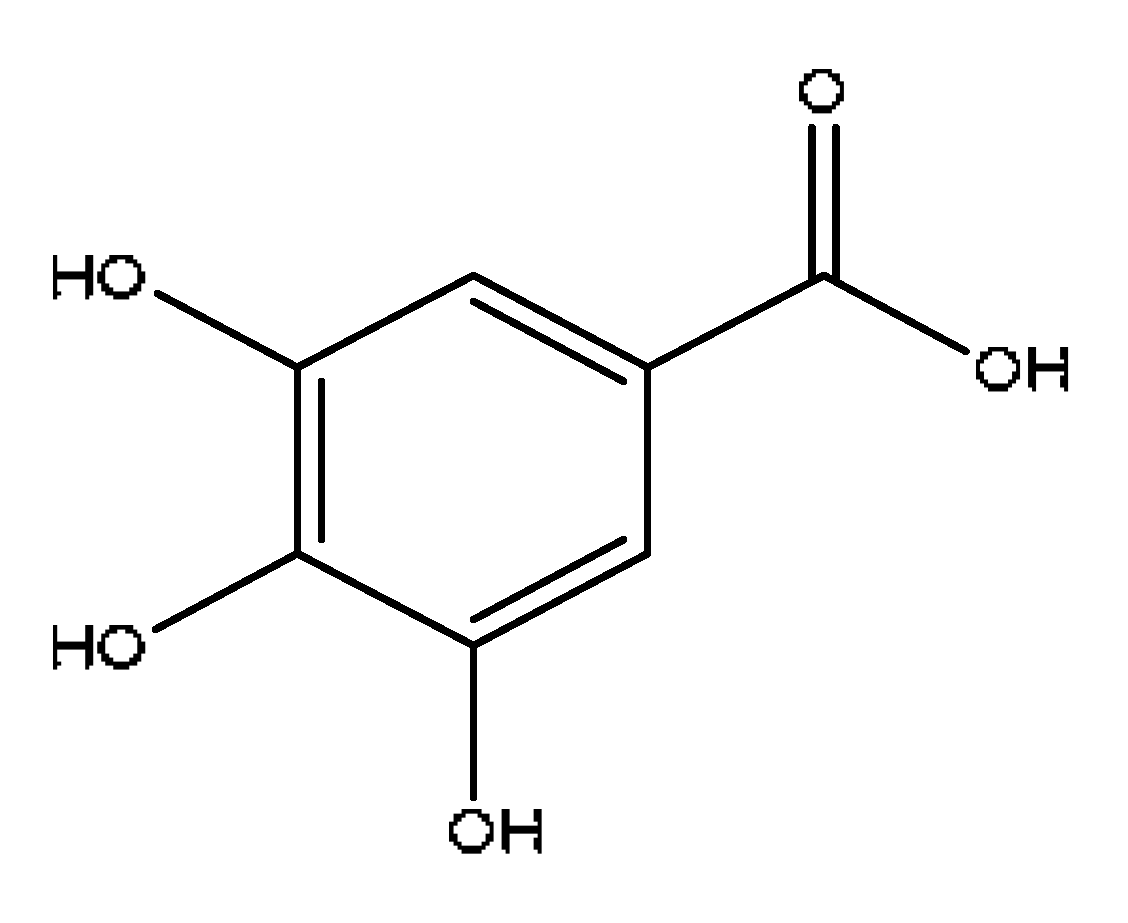 |
| 4 | Progallin A | 2.38 | 197.05 | C9H10O5 | 197.05[M−H]−,180.91[M−H−OH]−, 168.03[M-H−C2H5]−,124.04[M-H-C3H5O2]− | Paeonia lactiflora Pall | 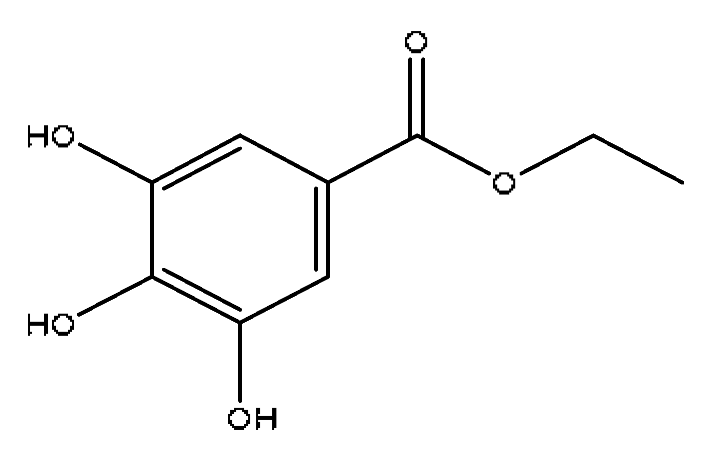 |
| 5 | 7-Methoxycoumarin | 2.46 | 177.05 | C10H8O3 | 177.05[M+H]+,159.09[M+H−H2O]+, 149.06[M+H−C2H4]+,146.06[M+H−CH3O]+ | Artemisia capillaris Thunb | 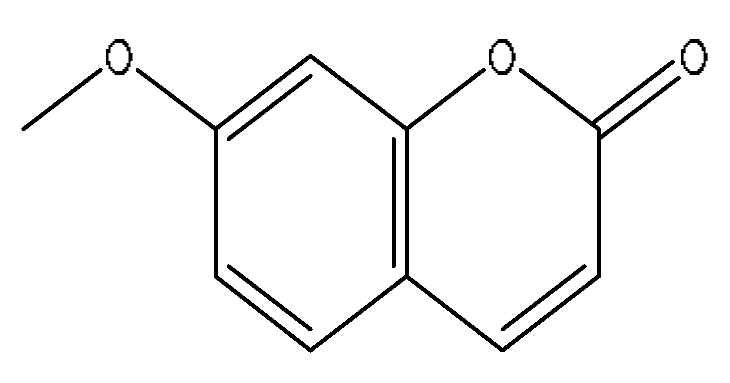 |
| 6 | Scandoside methyl ester | 2.65 | 449.13 | C17H24O11 | 449.13[M+FA−H]−,354.11[M+FA−H−C2H7O4]− | Gardenia jasminoides Ellis | 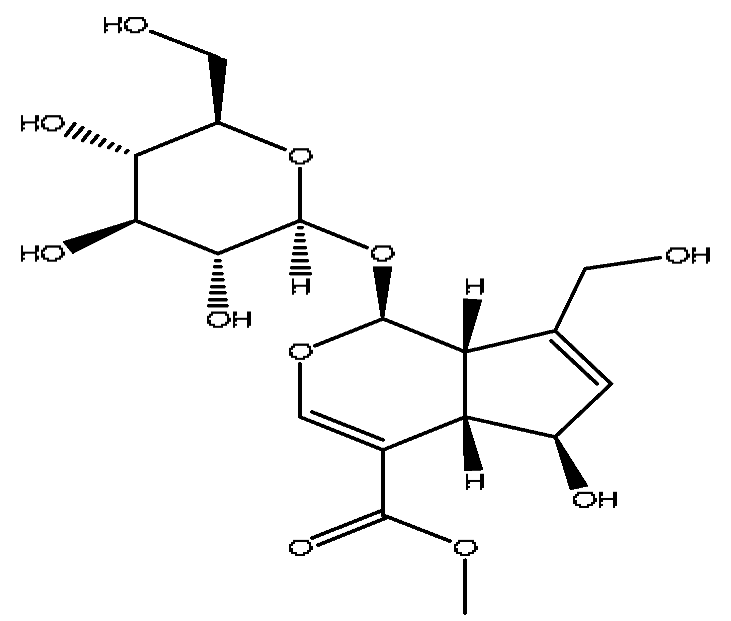 |
| 7 | Geniposidic acid *,# | 2.89 | 373.11 | C16H22O10 | 373.11[M−H]−, 271.07[M−H−C4H6O3]− | Gardenia jasminoides Ellis | 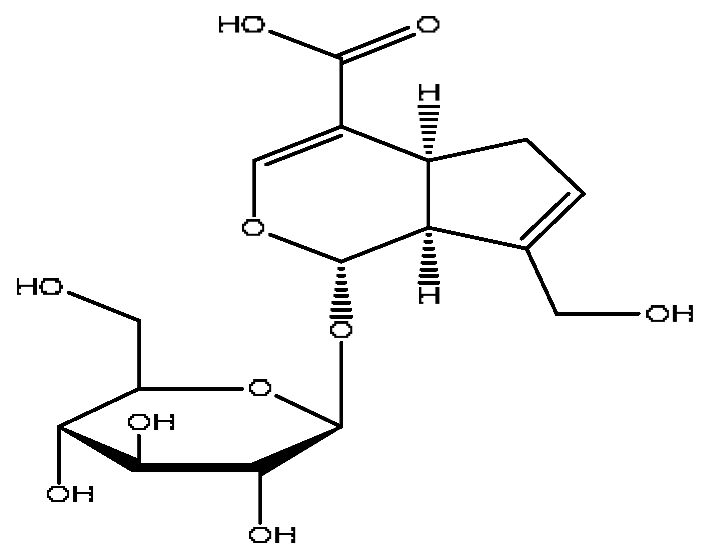 |
| 8 | Scopolin | 3.16 | 353.09 | C16H18O9 | 353.08[M−H]−,228.09[M−H−C6H5O3]−, 205.07[M−H−C5H8O5]− | Lycium barbarum L. | 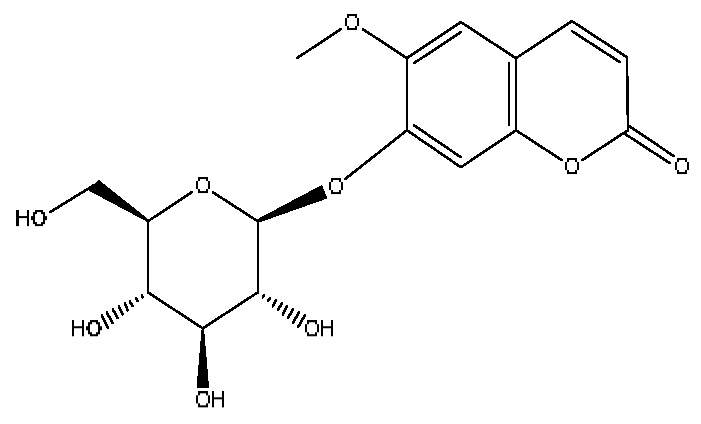 |
| 9 | Abrine *,# | 3.21 | 219.11 | C12H14N2O2 | 219.11[M+H]+,188.07[M+H−CH5N]+, 146.06[M+H−C2H3NO2]+ | Abrus cantoniensis Hance | 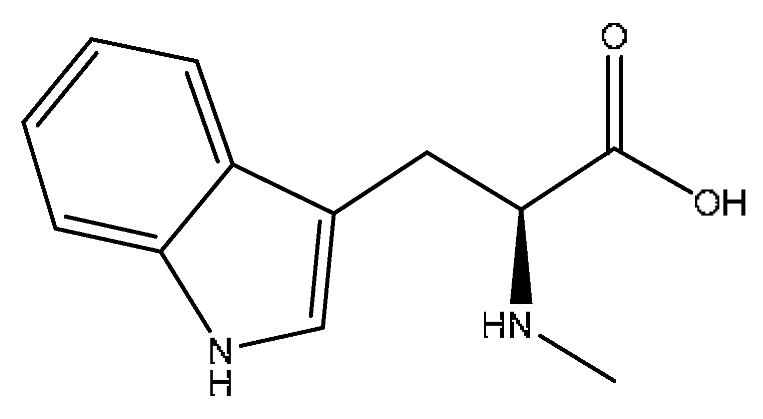 |
| 10 | Chlorogenic acid *,# | 3.40 | 353.08 | C16H18O9 | 353.08[M−H]−,336.07[M−H−OH]−, 212.00[M−H−C3H9O6]− | Gardenia jasminoides Ellis, Artemisia capillaris Thunb | 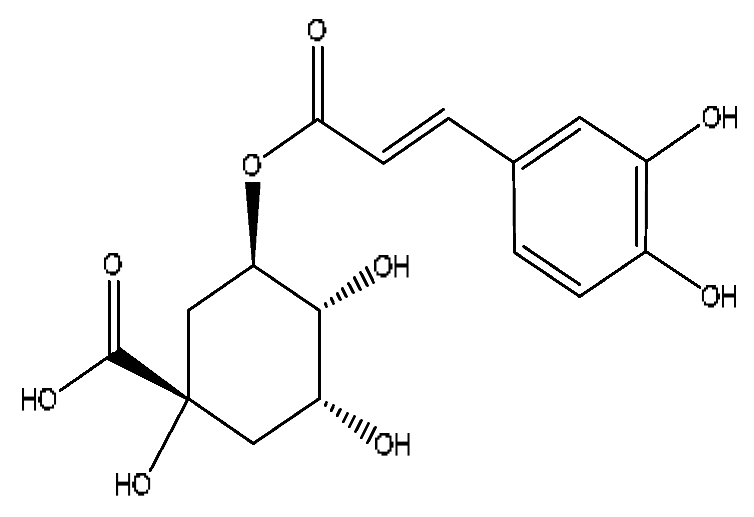 |
| 11 | Hypaphorine *,# | 3.42 | 247.18 | C14H18N2O2 | 247.18[M+H]+,188.1142[M+H−C3H9N]+, 144.1232[M+H−C4H9NO2]+ | Abrus cantoniensis Hance | 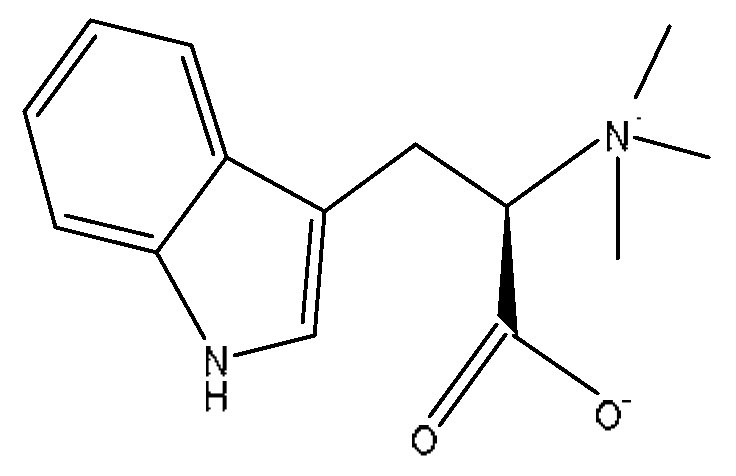 |
| 12 | p-coumaric acid *,# | 3.51 | 163.04 | C9H8O3 | 163.04[M−H]−,146.96[M−H−OH]−, 119.05[M−H−CO2]− | Ziziphus jujuba Mill | 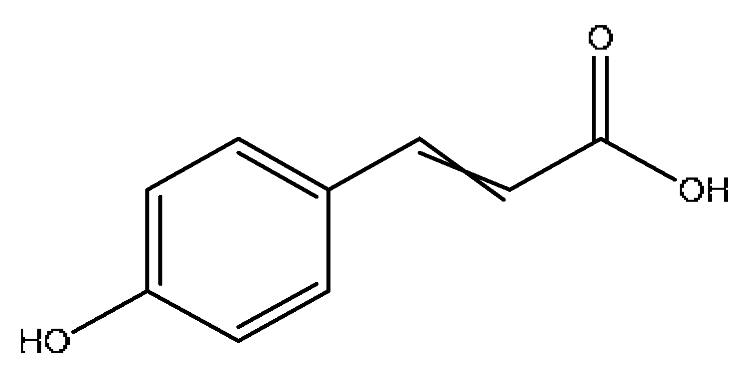 |
| 13 | Geniposide *,# | 3.56 | 387.13 | C17H24O10 | 387.13[M−H]−,353.08[M−H−H2O2]−, 212.00[M−H−C7H11O5]− | Gardenia jasminoides Ellis | 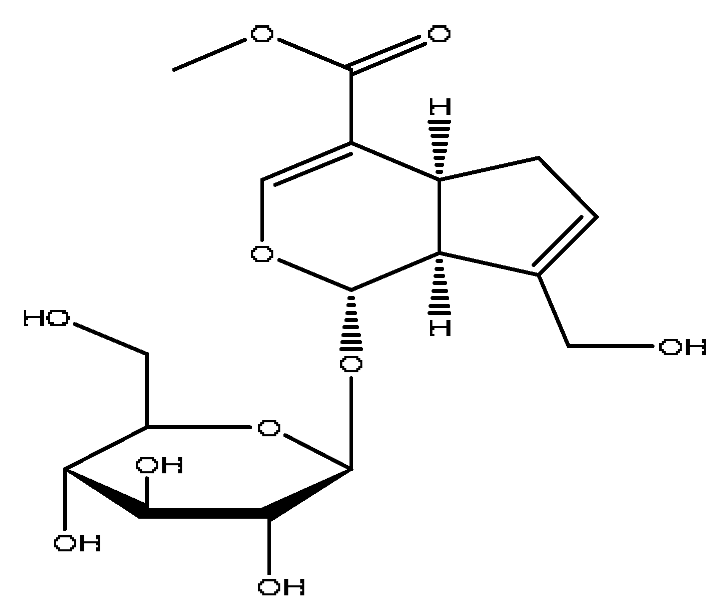 |
| 14 | Ethyl caffeate * | 4.01 | 209.08 | C11H12O4 | 209.08[M+H]+,191.07[M+H−H2O]+, 177.11[M+H−CH4O]+ | Origanum vulgare L. |  |
| 15 | Safrol | 4.03 | 207.07 | C10H10O2 | 207.07[M+FA−H]−,180.91[M+FA−H−C2H3]−, 168.10[M+FA−H−C3H3]−, 153.02[M+FA−H−C4H6]− | Lycium barbarum L. |  |
| 16 | Genipin-1-gentiobioside *,# | 4.03 | 549.15 | C23H34O15 | 549.15[M−H]−,533.19[M−H−O]−, 505.22[M−H−CO2]−,255.10[M−H−C8H22O11]− | Gardenia jasminoides Ellis | 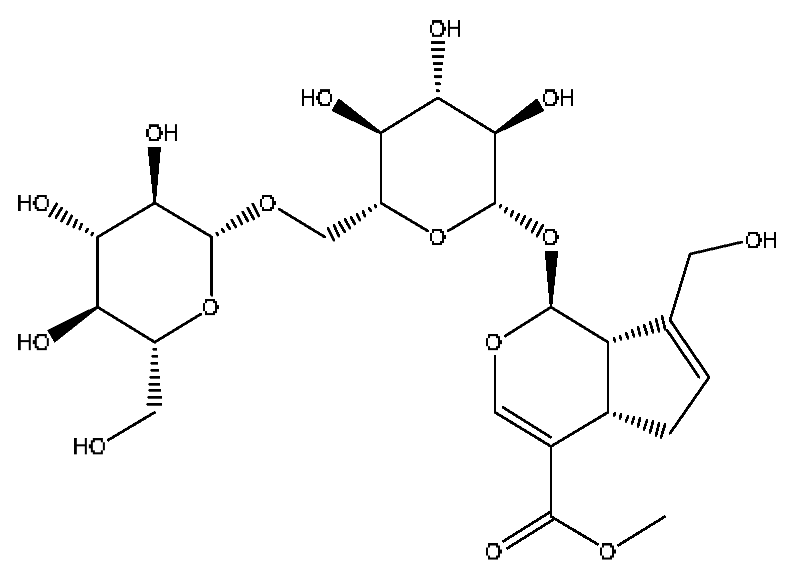 |
| 17 | Vicenin-2 *,# | 4.05 | 595.16 | C27H30O15 | 595.16[M+H]+,523.22[M+H−H8O4]+ | Abrus cantoniensis Hance |  |
| 18 | Albiflorin *,# | 4.32 | 503.15 | C23H28O11 | 503.15[M+Na]+,472.28[M+Na−CH3O]+, 455.26[M+Na−CH4O2]+,437.24[M+Na−CH6O3]+ | Paeonia lactiflora Pall | 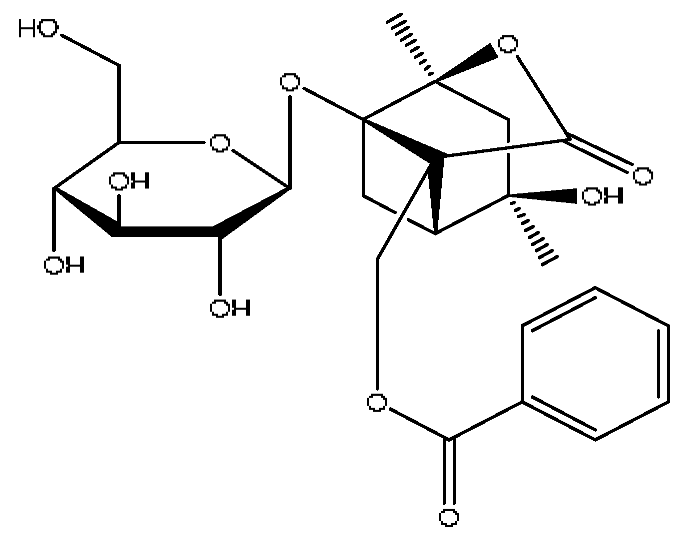 |
| 19 | Isoschaftoside *,# | 4.51 | 563.14 | C26H28O14 | 563.14[M−H]−,427.23[M−H−C8H8O2]−, 283.08[M−H−C11H20O8]− | Abrus cantoniensis Hance | 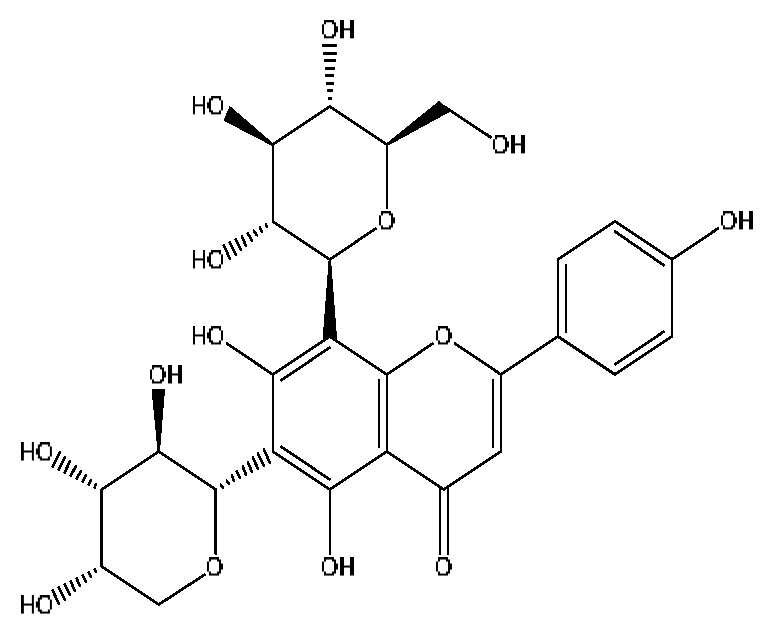 |
| 20 | Paeoniflorin *,# | 4.59 | 525.16 | C23H28O11 | 525.16[M+FA−H]−,447.21[M+FA−H−C6H6]−, 283.08[M+FA−H−C11H14O6]− | Paeonia lactiflora Pall | 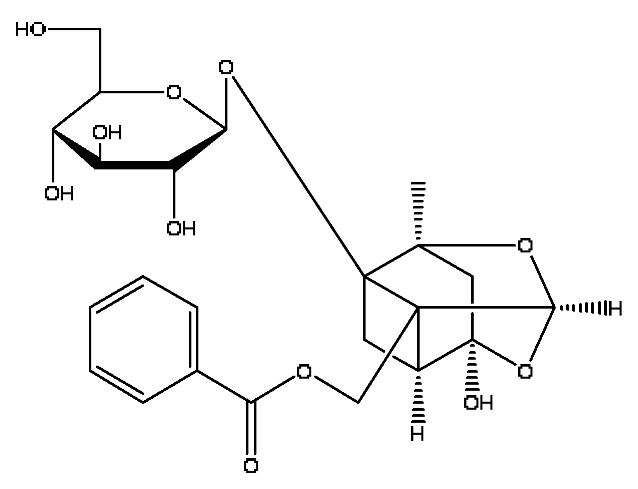 |
| 21 | Isovitexin *,# | 5.45 | 433.27 | C21H20O10 | 433.27[M+H]+,414.27[M+H−H3O]+, 396.23[M+H−H5O2]+ | Abrus cantoniensis Hance | 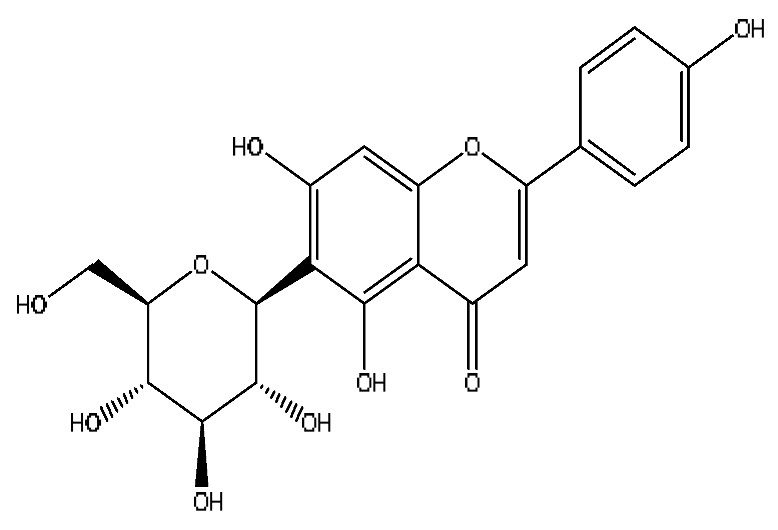 |
| 22 | Kaempferol *,# | 5.74 | 573.10 | C15H10O6 | 573.10[2M+H]+,414.27[2M+H−C7H11O4]+, 382.22[2M+H−C7H11O6]+ | Paeonia lactiflora Pall, Gardenia jasminoides Ellis, Origanum vulgare L. | 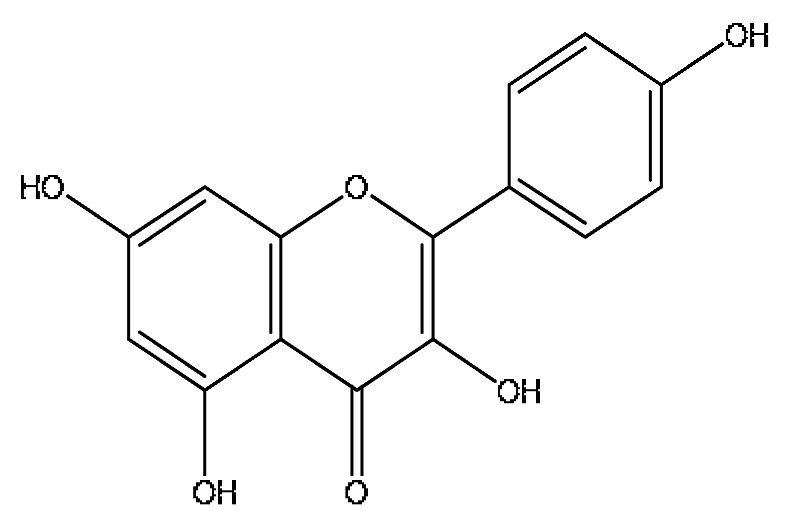 |
| 23 | Ginsenoside Rg1 *,# | 6.27 | 799.47 | C42H72O14 | 799.40[M−H]−,767.43[M−H−O2]−, 417.12[M−H−C14H22O12]− | Panax notoginseng (Burk.) F.H.Chen | 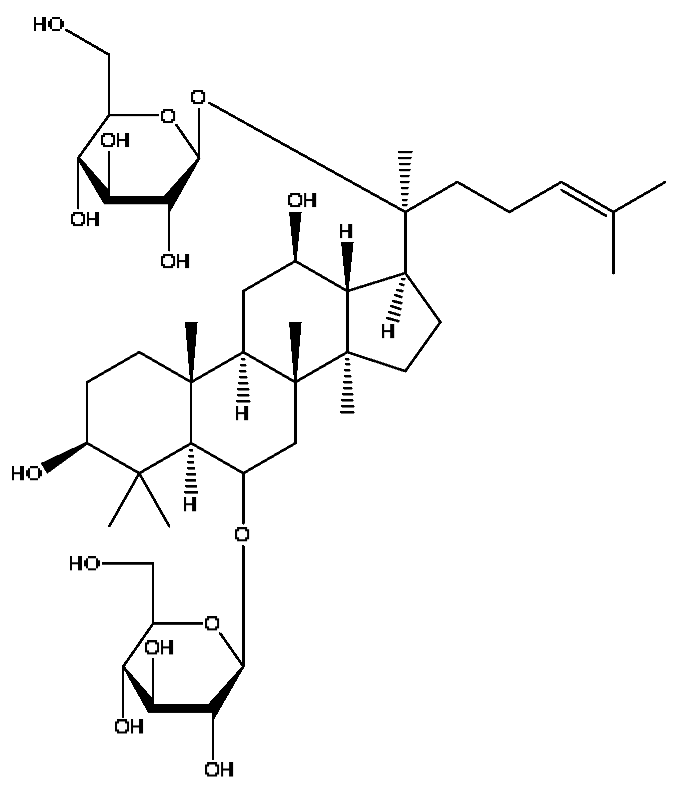 |
| 24 | Mauritine A | 7.45 | 576.32 | C32H41N5O5 | 576.31[M+H]+,306.30[M+H−C16H16NO3]+, 262.27[M+H−C18H22N2O3]+ | Ziziphus jujuba Mill | 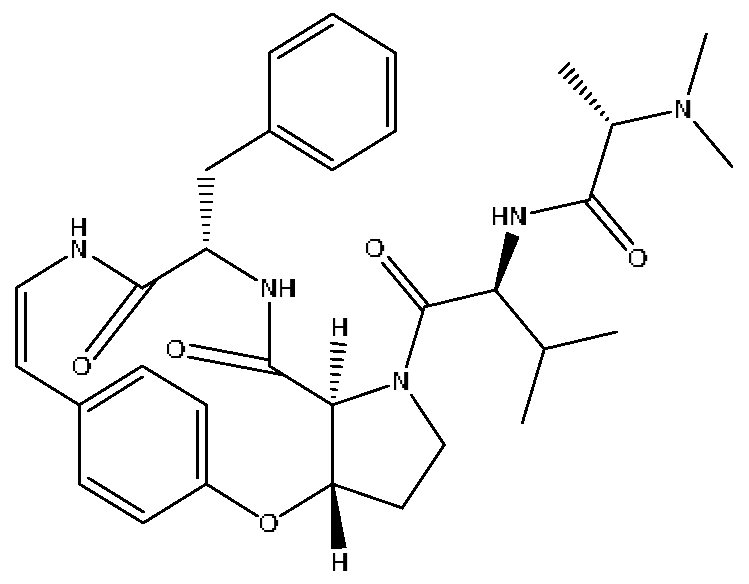 |
| 25 | 3′,4′,7-trihydroxyflavone | 7.84 | 541.11 | C15H10O5 | 541.11[2M+H]+,188.11[2M+H−C19H13O7]+, 170.10[2M+H−C19H15O8]+ | Abrus cantoniensis Hance | 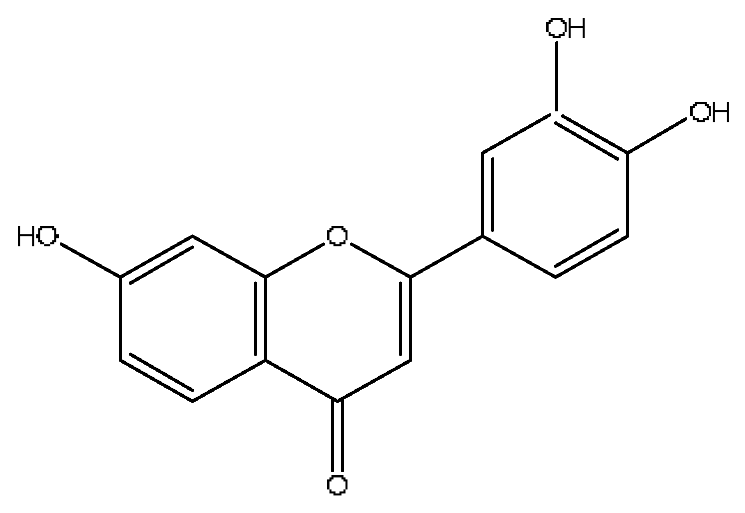 |
| 26 | Luteolin *,# | 8.61 | 285.04 | C15H10O6 | 285.04[M−H]−,174.96[M−H−C6H7O2]− | Abrus cantoniensis Hance |  |
| 27 | Apigenin 7-glucoside | 8.62 | 477.11 | C21H20O10 | 477.11[M+FA−H]−,461.10[M+FA−H−O]−, 188.07[M+FA−H−C12H17O8]− | Origanum vulgare L. | 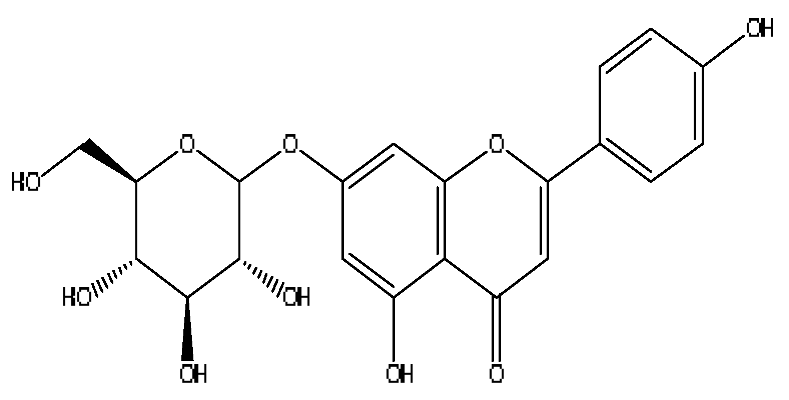 |
| 28 | Taurohyocholic acid | 10.22 | 514.28 | C26H45NO7S | 514.28[M−H]−,498.29[M−H−O]−, 464.30[M−H−CH6O2]−, 304.92[M−H−C7H16NO4S]− | Sus scrofadomestica Brisson, Bovis calculus Artifactus | 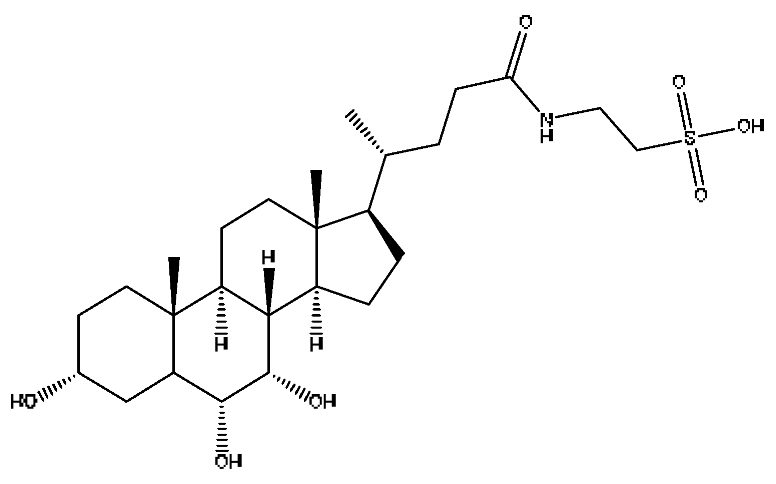 |
| 29 | Taurocholic acid # | 11.46 | 514.28 | C26H45NO7S | 514.28[M−H]−,462.29[M−H−H4O3]−, 369.23[M−H−C2H9O5S]− | Bovis calculus Artifactus | 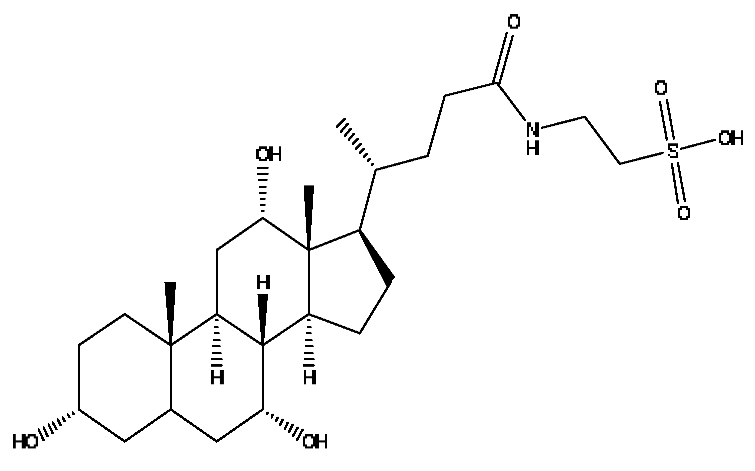 |
| 30 | Glycohyocholic acid | 12.39 | 464.30 | C26H43NO6 | 464.30[M−H]−,405.17[M−H−C2H3O2]−, 369.2292[M−H−C2H7O4]− | Sus scrofadomestica Brisson |  |
| 31 | Ginsenoside Rb1 *,# | 12.69 | 1109.61 | C54H92O23 | 1109.61[M+H]+,874.44[M+H−C10H19O6]+, 786.62[M+H−C12H19O10]+ | Panax notoginseng (Burk.) F.H.Chen |  |
| 32 | Notoginsenoside Fa *,# | 12.97 | 1239.55 | C59H100O27 | 1239.54[M−H]−,1163.57[M−H−C3H8O2]−, 219.84[M−H−C51H88O20]− | Panax notoginseng (Burk.) F.H.Chen |  |
| 33 | Glycocholic acid * | 13.54 | 466.32 | C26H43NO6 | 466.32[M+H]+,448.31[M+H−H2O]+, 430.30[M+H−H4O2]+,412.29[M+H−H6O3]+ | Sus scrofadomestica Brisson, Bovis calculus Artifactus | 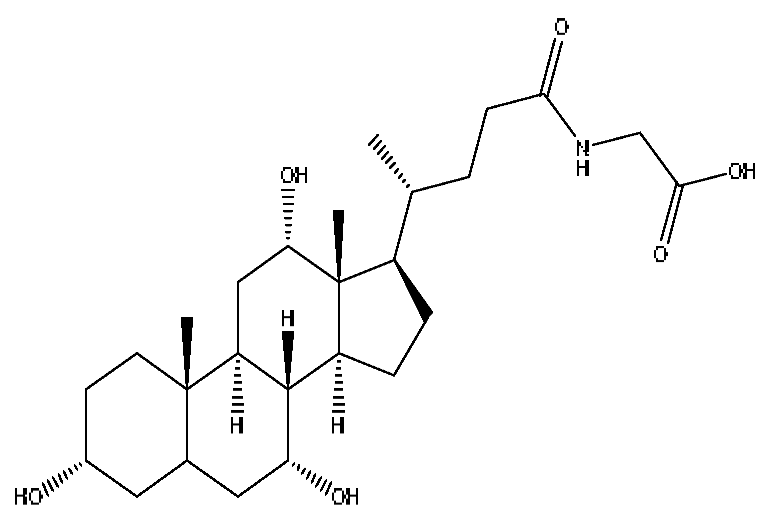 |
| 34 | Beta-Ionone# | 13.85 | 237.15 | C13H20O | 237.15[M+FA−H]−,221.84[M+FA−H−O]−, 195.81[M+FA−H−C2H2O]− | Lycium barbarum L. | 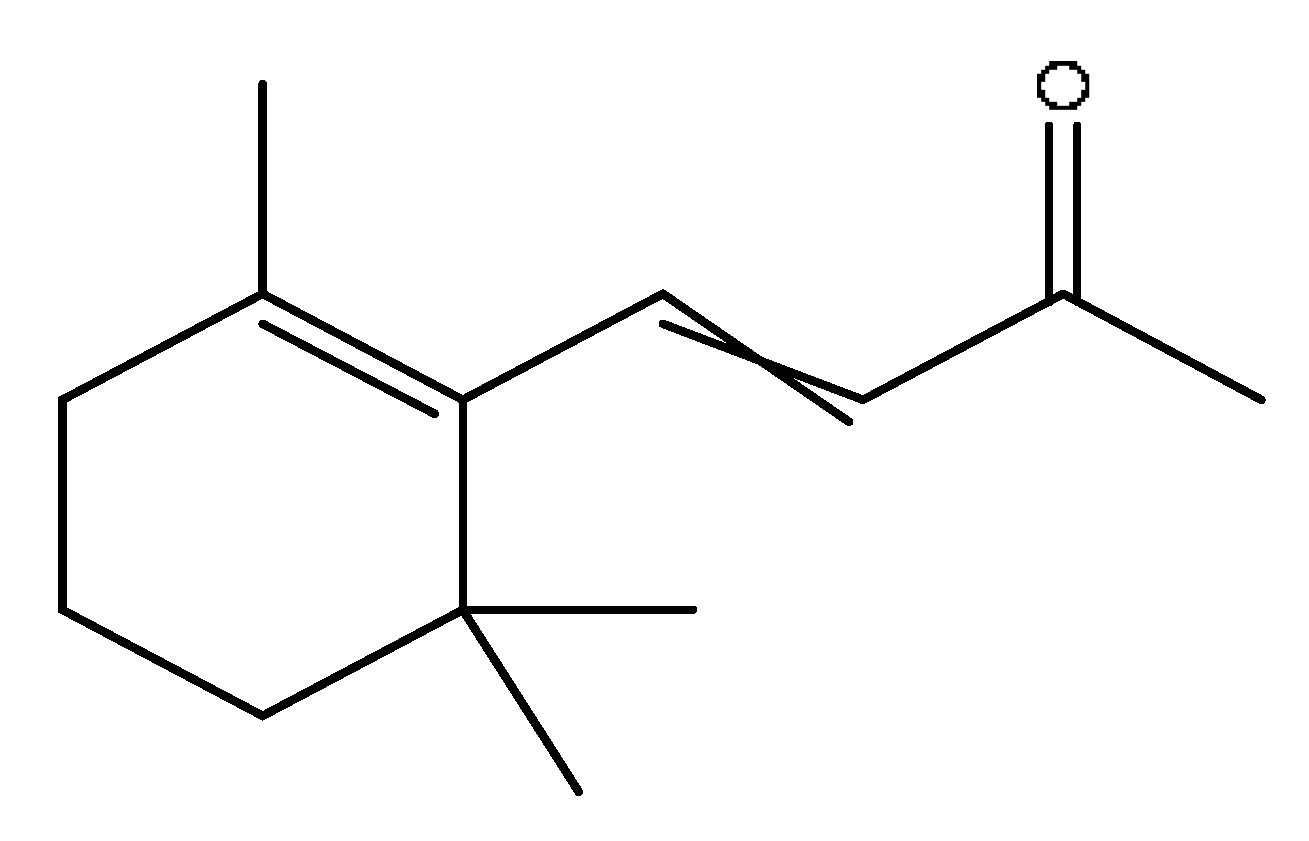 |
| 35 | Taurohyodeoxycholic acid sodium salt | 13.94 | 522.29 | C26H44NNaO6S | 522.29[M+H]+,343.30[M+H−C3H10O4NNaS]+ | Sus scrofadomestica Brisson, Bovis calculus Artifactus |  |
| 36 | Taurohyodeoxycholic acid *,# | 13.97 | 498.29 | C26H45NO6S | 498.29[M−H]−,400.23[M−H−H2SO4]−, 329.23[M−H−C5H13O4S]− | Bovis calculus Artifactus | 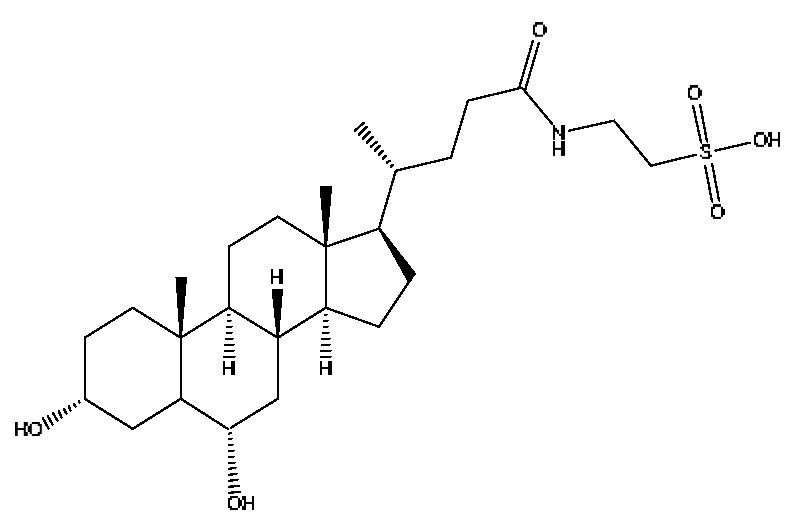 |
| 37 | Taurochenodeoxycholic acid # | 14.59 | 498.29 | C26H45NO6S | 498.29[M−H]−,465.33[M−H−CH5O]−, 448.31[M−H−CH6O2]−, 255.82[M−H−C7H17NO6S]− | Sus scrofadomestica Brisson |  |
| 38 | Hyocholic acid | 15.35 | 453.29 | C24H40O5 | 453.29[M+FA−H]−,407.28[M−H]−, 359.19[M−H−H6O3]−,311.22[M−H−C2H8O4]− | Sus scrofadomestica Brisson, Bovis calculus Artifactus | 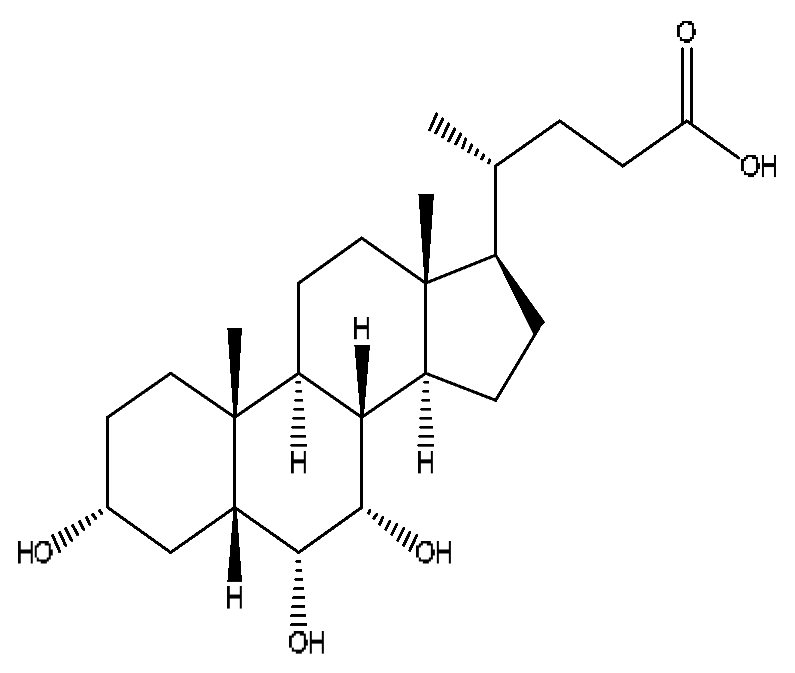 |
| 39 | Soyasaponin I # | 15.75 | 987.52 | C48H78O18 | 987.52[M+FA−H]−,473.32[M+FA−H−C20H34O15]−, 437.29[M+FA−H−C20H38O17]− | Abrus cantoniensis Hance | 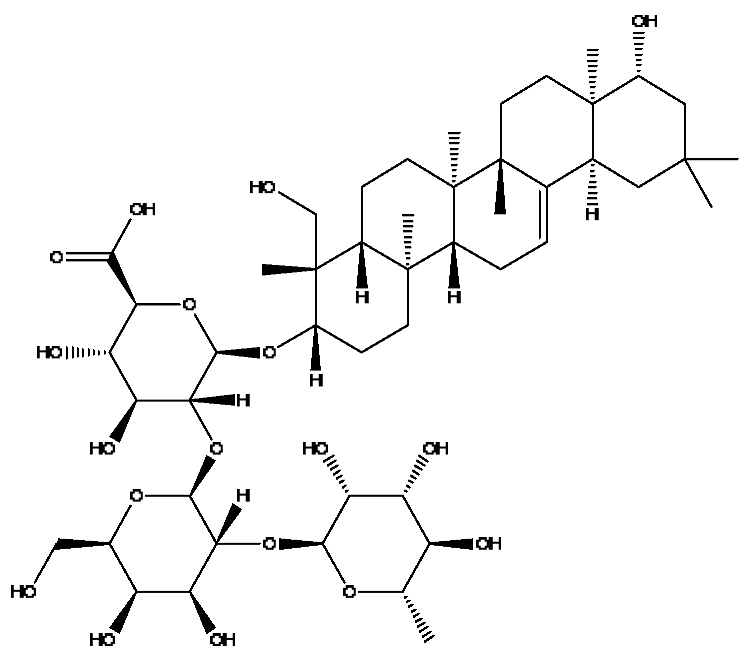 |
| 40 | Ginsenoside Rh4 *,# | 18.14 | 665.43 | C36H60O8 | 665.43[M+FA−H]−,489.34[M+FA−H−C7H12O5]− | Panax notoginseng (Burk.) F.H.Chen | 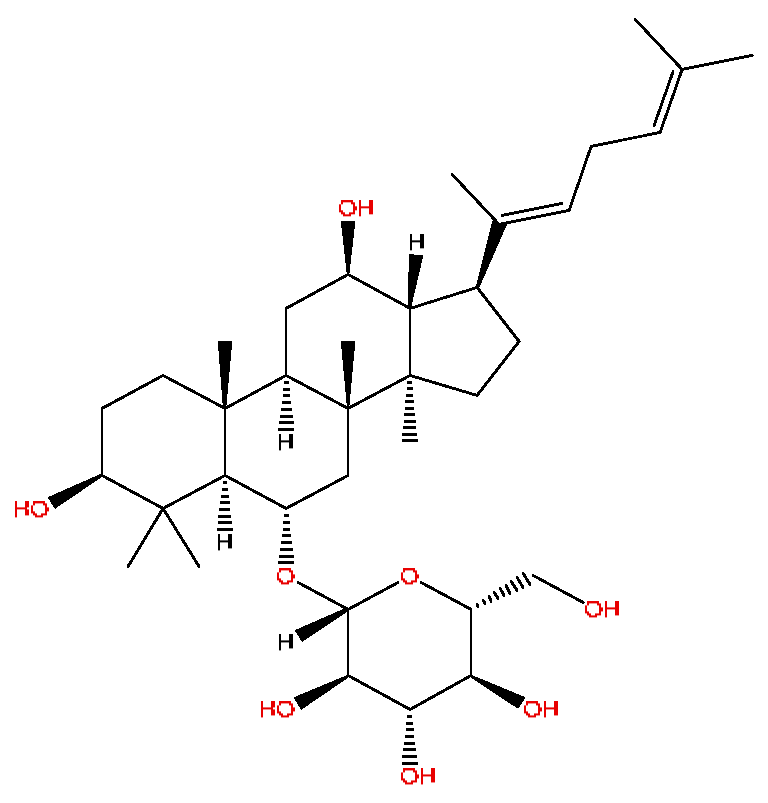 |
| 41 | Chenodeoxycholic acid *,# | 20.51 | 391.29 | C24H40O4 | 391.29[M−H]−,297.15[M−H−C2H6O4]−, 279.20[M−H−C6H8O2]−,261.18[M−H−C6H10O3]− | Sus scrofadomestica Brisson |  |
| 42 | Deoxycholic acid * | 21.04 | 391.29 | C24H40O4 | 391.29[M−H]−,337.24[M−H−H6O3]−, 297.15[M−H−C2H6O4]−,279.20[M−H−C6H8O2]− | Sus scrofadomestica Brisson | 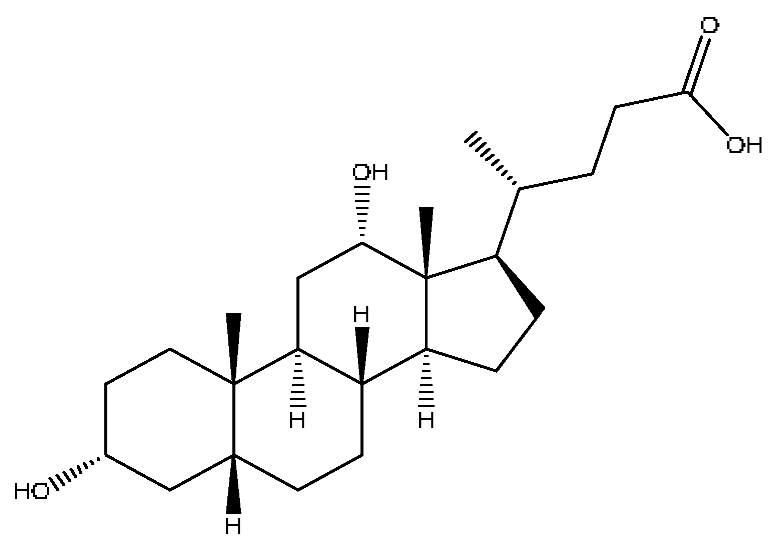 |
| 43 | Betulonic acid # | 24.41 | 477.34 | C30H46O3 | 477.34[M+Na]+,459.25[M+Na−H2O]+, 441.32[M+Na−H4O2]+ | Ziziphus jujuba Mill | 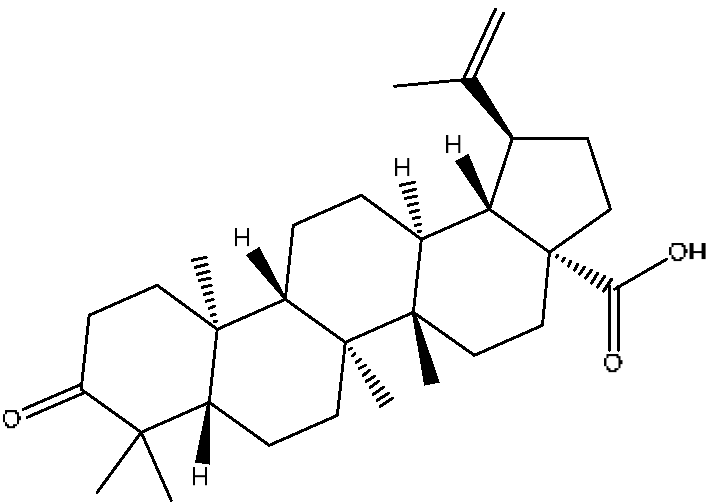 |
| NO | Metabolite Name | Metabolic Way | Rt | Observed m/z | Molecular Formula | Ion Form | MS/MS |
|---|---|---|---|---|---|---|---|
| M1 | Abrine deoxidized and hydrogenated metabolites | Abrine−O+H2 | 3.44 | 205.13 | C12H16N2O | [M+H]+ | 188.11[M−O]+, 146.11[[M−C2H4NO]+ |
| M2 | Abrine hydroglucuronic acid conjugate | Abrine+H2+H2O+C6H8O6 | 3.91 | 437.15 | C18H26N2O9 | [M+Na]+ | 417.14[M+Na−H4O]+, 262.15[M−C8H10NO6]+ |
| M3 | Geniposide oxidized metabolite | Geniposide+2x(+O) | 2.00 | 443.12 | C17H24O12 | [M+Na]+ | 401.48[M−H3O]+, 340.14[M−C2H8O3]+ |
| M4 | Geniposide deglycosylated and desaturated metabolite | Geniposide−C6H10O5−H2 | 9.83 | 225.07 | C11H12O5 | [M+H]+ | 179.08[M−CHO2]+ |
| M5 | Geniposide sulfated metabolite | Geniposide+SO3 | 5.40 | 513.09 | C17H24O13S | [M+FA−H]− | 245.05[M−C7H11O8]−, 165.09[M−C7H11O11S]− |
| M6 | Geniposide desaturated glucuronic acid conjugate | Geniposide−CH2O+2x(−H2) +C6H8O6 | 2.66 | 529.12 | C22H26O15 | [M−H]− | 233.04[M−C10H16O10]− |
| M7 | Geniposide oxidative hydrogenated glycosylation metabolite | Geniposide+O+H2+C6H10O5 | 3.91 | 567.20 | C23H36O16 | [M−H]− | 241.12[M−C13H27O9]− |
| M8 | Afrormosin oxidized and desaturated metabolites | Afrormosin+O−H2 | 14.07 | 357.06 | C17H12O6 | [M+FA−H]− | 283.17[M−C2H5]− |
| M9 | Afrormosin desaturated metabolite | Afrormosin+2x(−H2) | 9.44 | 339.05 | C17H10O5 | [M+FA−H]− | 257.82[M−H5O2]−, 146.96[M−C10H12O]− |
| M10 | Afrormosin acetylated metabolite | Afrormosin−CH2O+2x(+H2) +C2H2O | 17.92 | 337.10 | C18H18O5 | [M+Na]+ | 253.18[M−C2H5O2]+, 191.22[M−C7H7O2]+ |
| M11 | Ginsenoside Rg3 | Ginsenoside Rb1−C12H20O10 | 25.65 | 807.49 | C42H72O13 | [M+Na]+ | 572.38[M−C8H20O6]+, 510.37[M−C13H22O6]+ |
| M12 | Ginsenoside Rd deoxymetabolite | Ginsenoside Rb1−C6H10O6 | 21.91 | 975.55 | C48H82O17 | [M+FA−H]− | 476.28[M−C21H42O10]−, 279.23[M−C30H51O15]− |
| M13 | Hyodeoxycholic acid deoxysulfate metabolite | Hyodeoxycholic acid−O+SO3 | 12.81 | 479.24 | C24H40O6S | [M+Na]+ | 409.22[M−C2H7O]+, 393.24[M−CH3O3]+ |
| M14 | Hyodeoxycholic acid oxidized metabolites | Hyodeoxycholic acid+2x(+O) | 15.63 | 423.28 | C24H40O6 | [M−H]− | 405.27[M−H3O]−, 335.23[M−C4H9O2]− |
| M15 | Hyodeoxycholic acid hydroglucuronized conjugate | Hyodeoxycholic acid−O+H2O+C6H8O6 | 5.08 | 615.34 | C30H50O10 | [M+FA−H]− | 348.19[M−C10H22O5]−, 200.13[M−C19H30O7]− |
| M16 | Cholic acid desaturated oxidation metabolite | Cholic acid−H2+O | 26.82 | 423.27 | C24H38O6 | [M+H]+ | 323.26[M−C4H3O3]+, 240.14[M−C11H18O2]+, 184.12[M−C14H22O3]+ |
| M17 | Cholic acid dehydrated glucuronic acid conjugate | Cholic acid−H2O+C6H8O6 | 10.95 | 589.30 | C30H46O10 | [M+Na]+ | 504.27[M−C2H6O2]+, 488.31[M−CH2O4]+ |
| M18 | Cholic acid desaturated glucuronic acid conjugate | Cholic acid−H2+C6H8O6 | 21.61 | 627.31 | C30H46O11 | [M+FA−H]− | 526.31[M−C2O2]−, 466.30[M−C4H4O4]− |
| M19 | Chenodeoxycholic acid dehydrated and sulfated metabolite | Chenodeoxycholic acid−H2O+SO3 | 3.94 | 455.25 | C24H38O6S | [M+H]+ | 281.13[M−C9H17O3]+, 262.15[M−C9H20O4]+, 195.05[M−C14H27O4]+ |
| M20 | Ginsenoside Rg1 desaturated metabolites | Ginsenoside Rg1+2x(−H2) | 24.93 | 797.46 | C42H68O14 | [M+H]+ | 522.37[M−C12H18O7]+, 504.36[M−C12H20O8]+, 184.12[M−C32H52O11]+ |
| M21 | Ginsenoside Rg1 oxidized metabolite | Ginsenoside Rg1+2x(+O) | 27.51 | 855.48 | C42H72O16 | [M+Na]+ | 546.36[M−C10H22O9]+, 487.29[M−C17H29O7]+, 323.26[M−C22H37O13]+ |
| M22 | Ginsenoside Rg1 oxidative sulfated metabolite | Ginsenoside Rg1+O+SO3 | 4.87 | 919.42 | C42H72O18S | [M+Na]+ | 728.35[M−C6H16O5]+, 547.30[M−C15H25O9]+, 327.15[M−C21H45O15S]+ |
| M23 | Ginsenoside Rg1 oxidized glucuronic acid conjugate | Ginsenoside Rg1+O+C6H8O6 | 22.55 | 1037.52 | C48H80O21 | [M+FA−H]− | 476.28[M−C21H38O14]−, 396.09[M−C34H60O8]−, 279.23[M−C31H49O19]− |
| M24 | Ginsenoside Rd oxidized metabolite | Ginsenoside Rd+O2 | 22.16 | 995.54 | C48H82O21 | [M+H]+ | 522.29[M−C24H40O9]+, 494.34[M−C20H36O14]+ |
| M25 | Ginsenoside Rd glucuronic acid conjugate | Ginsenoside Rd+C6H8O6 | 23.25 | 1139.58 | C54H90O25 | [M+H]+ | 570.37[M−C20H40O18]+, 544.34[M−C22H42O18]+, 481.32[M−C27H45O18]+ |
| M26 | Ginsenoside Rd deglycosylated oxidation metabolite | Ginsenoside Rd−C12H20O10+2x(+O) | 13.62 | 693.42 | C32H62O11 | [M+Na]+ | 472.32[M−C7H18O6]+, 432.33[M−C9H18O7]+, 414.33[M−C9H20O8]+, 339.30[M−C15H23O8]+ |
| M27 | Ginsenoside Rb1 dehydrated metabolite | Ginsenoside Rd−H2O+C6H8O6 | 22.27 | 1165.57 | C54H88O24 | [M+FA−H]− | 588.33[M−C27H48O10]−, 544.27[M−C28H48O12]−, 524.28[M−C30H44O12]− |
| M28 | Notoginsenoside T5 desaturated metabolite | Notoginsenoside T5−H2 | 27.41 | 751.46 | C41H66O12 | [M+H]+ | 482.35[M−C14H20O5]+, 464.34[M−C10H22O9]+, 381.33[M−C14H25O11]+ |
| M29 | Notoginsenoside T5 oxidized glucuronic acid conjugate | Notoginsenoside T5−C5H8O5+2x(+O)+C6H8O6 | 27.19 | 813.47 | C42H68O15 | [M+H]+ | 546.36[M−C10H18O8]+, 524.39[M−C12H16O8]+, 481.33[M−C15H23O8]+, 381.33[M−C15H27O14]+ |
| M30 | Notoginsenoside T5 dehydrated glucuronic acid conjugate | Notoginsenoside T5−C5H8O5+2x(−H2O)+C6H8O6 | 4.44 | 743.43 | C42H64O11 | [M−H]− | 245.05[M−C29H55O6]−, 165.09[M−C32H64O12]− |
| M31 | Scoparone hydrosulfated metabolite | Scoparone−CH2+H2+SO3 | 5.56 | 273.01 | C10H10O7S | [M−H]− | 257.82[M−OH]−, 193.03[M−HSO3]− |
| M32 | Scoparone hydrogenated hydroxylation metabolite | Scoparone−CH2O+2x(+H2O) | 2.13 | 211.06 | C10H12O5 | [M−H]− | 197.81[M−CH3]−, 123.04[M−C3H5O3]− |
| M33 | Capillarisin hydrogenated metabolite | Capillarisin+H2 | 2.94 | 317.07 | C16H14O7 | [M−H]− | 203.08[M−C6H11O2]−, 172.99[M−C7H14O3]− |
| Number | Compounds | Regression Equation | R2 | Linear Range (ng/mL) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|---|
| 1 | Trigonelline | Y = 2227X + 781,700 | 0.9993 | 12.18~24,360 | 0.12 | 0.61 |
| 2 | Abrine | Y = 132X − 4611 | 1.0000 | 9.98~99,800 | 0.50 | 2.50 |
| 3 | Hypaphorine | Y = 8061X + 2,141,000 | 0.9997 | 15.28~30,560 | 0.15 | 0.76 |
| 4 | Genipin-1-gentiobioside | Y = 587.9X + 40,930 | 0.9999 | 10.22~20,440 | 0.10 | 0.51 |
| 5 | Geniposide | Y = 25.5X + 2832 | 0.9999 | 13.89~138,900 | 0.14 | 0.69 |
| 6 | Vicenin-2 | Y = 399.6X + 10,760 | 1.0000 | 7.21~72,100 | 0.07 | 0.36 |
| 7 | Albiforin | Y = 66.1X + 799.2 | 1.0000 | 10.31~103,100 | 0.10 | 0.52 |
| 8 | Paeoniflorin | Y = 2.5X + 178.7 | 0.9999 | 115.1~115,100 | 2.88 | 115.10 |
| 9 | Isoschaftoside | Y = 384.1X + 59,040 | 0.9997 | 10.93~109,300 | 0.11 | 0.55 |
| 10 | Isovitexin | Y = 1147X − 1774 | 0.9999 | 10.76~2690 | 0.11 | 0.54 |
| 11 | Ginsenoside Rg1 | Y = 254.5X + 19,910 | 0.9999 | 10.93~21,860 | 0.11 | 0.55 |
| 12 | Luteolin | Y = 1607X + 10,180 | 0.9998 | 11.16~2790 | 0.11 | 0.56 |
| 13 | Taurohyodeoxycholic acid | Y = 614.9X − 635 | 1.0000 | 10.98~21,960 | 0.11 | 0.55 |
| 14 | Notoginsenoside Fa | Y = 28.1X + 491 | 0.9999 | 105.1~21,020 | 10.51 | 105.10 |
| 15 | Ginsenoside Rb1 | Y = 7.3X + 1187 | 0.9998 | 11.02~22,040 | 2.76 | 11.02 |
| 16 | Chenodeoxycholic acid | Y = 9.4X − 916.9 | 0.9999 | 10.50~21,000 | 0.53 | 2.63 |
| Compounds | 2111095 | 2105033 | 2111092 | 2111093 | 2111094 | 2102006 | 2102007 | 2102008 | 2101005 | 2101003 | 2111091 | 2111099 | 2108070 | 2108069 | Average (mg/g) | SD (mg/g) | RSD (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trigonelline (1) | 2.04 | 1.39 | 1.67 | 1.72 | 2.10 | 1.85 | 1.80 | 1.81 | 1.51 | 1.80 | 2.31 | 2.08 | 1.68 | 1.54 | 1.81 | 0.25 | 14.09 |
| Abrine (2) | 2.72 | 2.64 | 5.24 | 4.07 | 3.87 | 3.88 | 1.17 | 5.09 | 3.72 | 5.87 | 4.39 | 3.48 | 6.48 | 6.42 | 4.22 | 1.51 | 35.78 |
| Hypaphorine (3) | 0.31 | 0.30 | 0.26 | 0.26 | 0.36 | 0.27 | 0.25 | 0.30 | 0.26 | 0.29 | 0.37 | 0.36 | 0.35 | 0.25 | 0.30 | 0.04 | 14.57 |
| Genipin-1-gentiobioside (4) | 2.58 | 2.79 | 3.01 | 2.79 | 2.66 | 2.70 | 2.41 | 2.69 | 2.47 | 2.40 | 2.72 | 2.66 | 2.79 | 2.91 | 2.68 | 0.18 | 6.60 |
| Geniposide (5) | 5.17 | 5.18 | 5.91 | 5.88 | 5.31 | 5.40 | 4.99 | 5.28 | 5.08 | 5.32 | 5.34 | 5.63 | 5.41 | 5.64 | 5.40 | 0.28 | 5.15 |
| Vicenin-2 (6) | 1.70 | 1.45 | 1.55 | 1.62 | 1.23 | 1.64 | 1.44 | 1.36 | 1.77 | 1.51 | 1.28 | 1.42 | 1.35 | 2.01 | 1.52 | 0.21 | 13.89 |
| Albiforin (7) | 1.34 | 1.70 | 1.67 | 1.60 | 1.76 | 1.74 | 1.70 | 1.88 | 1.72 | 1.70 | 1.79 | 1.72 | 1.69 | 1.70 | 1.69 | 0.12 | 7.09 |
| Paeoniflorin (8) | 2.62 | 2.59 | 2.95 | 2.92 | 2.59 | 3.09 | 2.71 | 3.34 | 2.82 | 2.77 | 2.89 | 2.74 | 3.43 | 3.49 | 2.93 | 0.31 | 10.44 |
| Isoschaftoside (9) | 4.31 | 3.71 | 4.11 | 3.73 | 3.60 | 4.35 | 4.20 | 3.76 | 4.80 | 4.16 | 3.65 | 4.17 | 3.06 | 4.84 | 4.03 | 0.48 | 11.98 |
| Isovitexi (10) | 0.07 | 0.06 | 0.07 | 0.06 | 0.05 | 0.07 | 0.07 | 0.06 | 0.09 | 0.07 | 0.05 | 0.06 | 0.05 | 0.08 | 0.07 | 0.01 | 17.18 |
| Ginsenoside Rg1 (11) | 1.51 | 2.10 | 1.90 | 1.60 | 1.89 | 2.03 | 1.54 | 1.92 | 1.69 | 1.80 | 1.76 | 1.77 | 2.10 | 2.10 | 1.84 | 0.20 | 11.14 |
| Luteolin (12) | 0.09 | 0.11 | 0.08 | 0.07 | 0.07 | 0.10 | 0.11 | 0.10 | 0.09 | 0.08 | 0.07 | 0.07 | 0.06 | 0.10 | 0.08 | 0.02 | 18.17 |
| Taurohyodeoxycholic acid (13) | 0.42 | 0.52 | 0.46 | 0.44 | 0.48 | 0.55 | 0.50 | 0.58 | 0.53 | 0.56 | 0.46 | 0.47 | 0.43 | 0.47 | 0.49 | 0.05 | 10.33 |
| Notoginsenoside Fa (14) | 0.06 | 0.09 | 0.06 | 0.04 | 0.04 | 0.06 | 0.04 | 0.05 | 0.06 | 0.06 | 0.07 | 0.06 | 0.06 | 0.08 | 0.06 | 0.01 | 24.52 |
| Ginsenoside Rb1 (15) | 1.24 | 1.95 | 1.45 | 1.34 | 1.32 | 1.60 | 1.12 | 1.36 | 1.31 | 1.21 | 1.14 | 1.23 | 1.45 | 1.37 | 1.36 | 0.21 | 15.57 |
| Chenodeoxycholic acid (16) | 0.84 | 1.74 | 0.90 | 0.80 | 0.85 | 1.45 | 1.32 | 0.66 | 1.31 | 1.27 | 0.90 | 0.78 | 2.15 | 1.88 | 1.20 | 0.46 | 38.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Wu, F.; Tan, Z.; Zhang, M.; Li, T.; Zhang, A.; Miao, J.; Ou, M.; Long, L.; Sun, H.; et al. Quality Markers’ Discovery and Quality Evaluation of Jigucao Capsule Using UPLC-MS/MS Method. Molecules 2023, 28, 2494. https://doi.org/10.3390/molecules28062494
He Y, Wu F, Tan Z, Zhang M, Li T, Zhang A, Miao J, Ou M, Long L, Sun H, et al. Quality Markers’ Discovery and Quality Evaluation of Jigucao Capsule Using UPLC-MS/MS Method. Molecules. 2023; 28(6):2494. https://doi.org/10.3390/molecules28062494
Chicago/Turabian StyleHe, Yanmei, Fangfang Wu, Zhien Tan, Mengli Zhang, Taiping Li, Aihua Zhang, Jianhua Miao, Min Ou, Lihuo Long, Hui Sun, and et al. 2023. "Quality Markers’ Discovery and Quality Evaluation of Jigucao Capsule Using UPLC-MS/MS Method" Molecules 28, no. 6: 2494. https://doi.org/10.3390/molecules28062494
APA StyleHe, Y., Wu, F., Tan, Z., Zhang, M., Li, T., Zhang, A., Miao, J., Ou, M., Long, L., Sun, H., & Wang, X. (2023). Quality Markers’ Discovery and Quality Evaluation of Jigucao Capsule Using UPLC-MS/MS Method. Molecules, 28(6), 2494. https://doi.org/10.3390/molecules28062494






