Catalytic Properties of Zirconocene-Based Systems in 1-Hexene Oligomerization and Structure of Metal Hydride Reaction Centers
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalytic Effect of L2ZrCl2–HAlBui2–Activator Systems in 1-Hexene Oligomerization
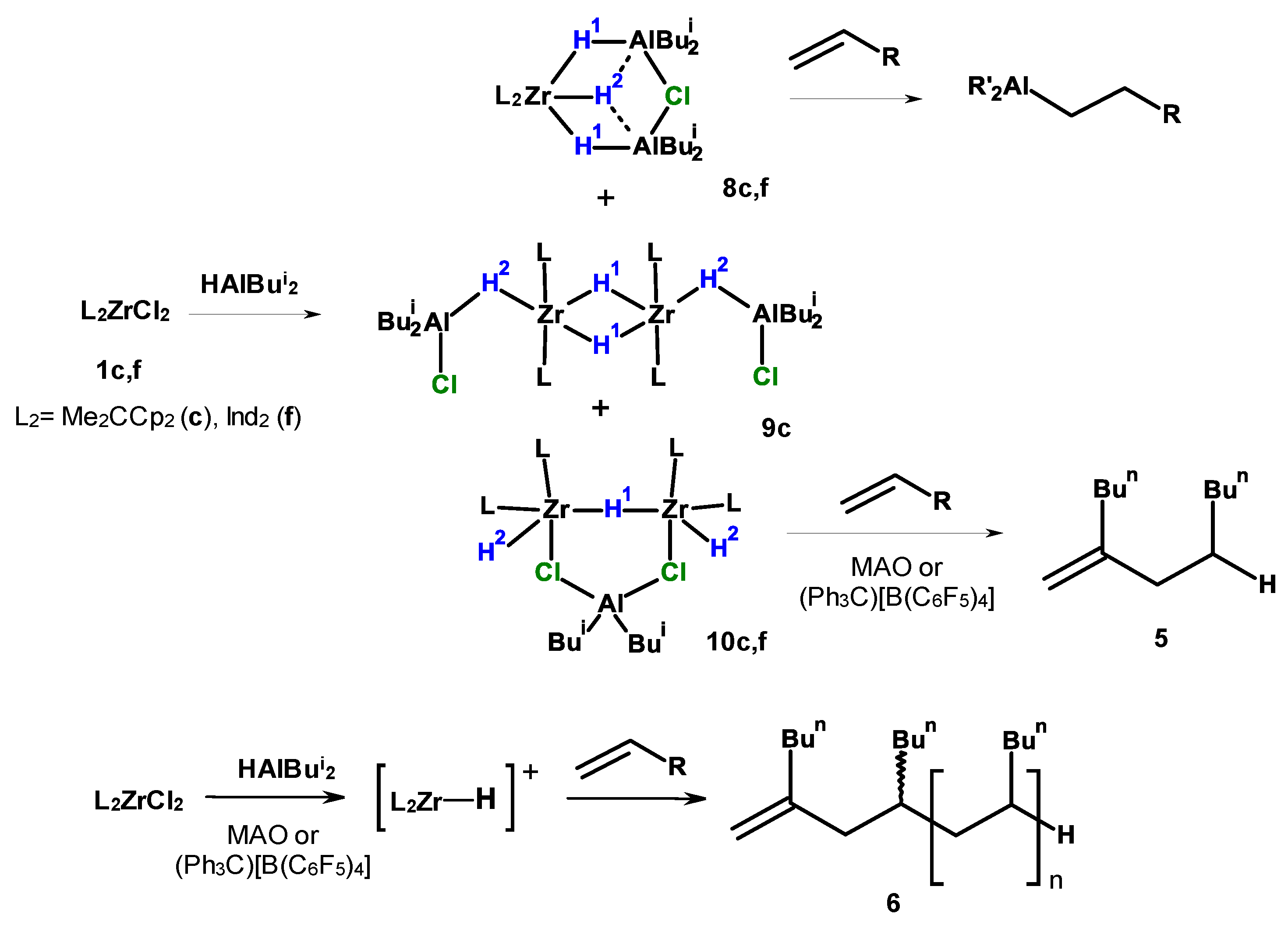
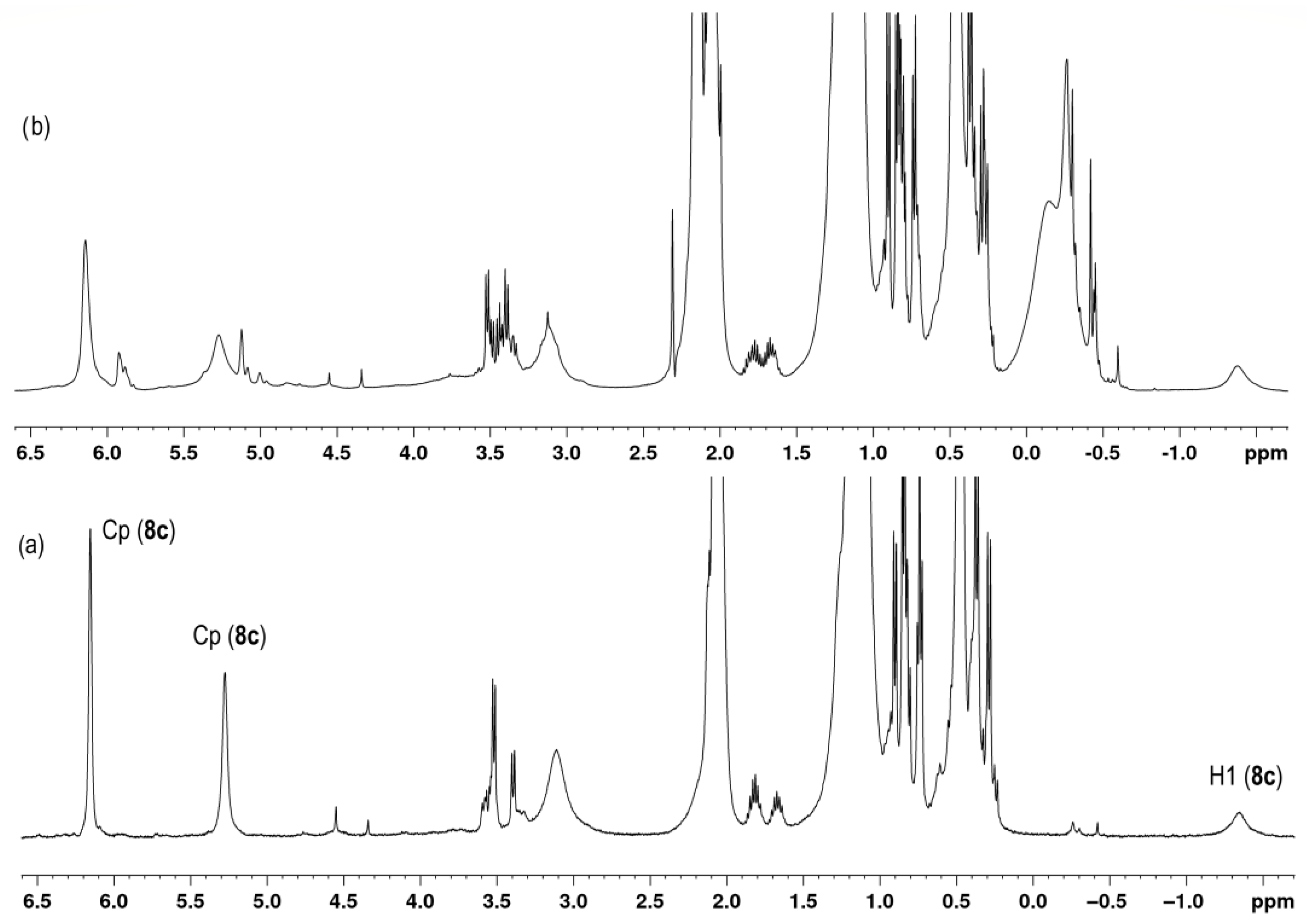
2.2. NMR Study of the L2ZrCl2–HAlBui2–Activator Catalytic Systems
3. Materials and Methods
3.1. General Procedures
3.2. Reaction of L2ZrCl2 (1a-j) with HAlBui2, MMAO-12 or (Ph3C)[B(C6F5)4] and 1-Hexene
3.3. NMR Study of the Reaction of Me2CCp2ZrCl2 or Ind2ZrCl2 with HalBui2 and Activator (MMAO-12, (Ph3C)[B(C6F5)4])
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaminsky, W.; Sinn, H. Methylaluminoxane: Key Component for New Polymerizati on Catalysts, in Polyolefins: 50 Years after Ziegler and Natta II; Advances in Polymer Science; Kaminsky, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–28. [Google Scholar]
- Janiak, C. Metallocene and Related Catalysts for Olefin, Alkyne and Silane Dimerization and Oligomerization. Coord. Chem. Rev. 2006, 250, 66–94. [Google Scholar] [CrossRef]
- Shubkin, R.L.; Kerkemeyer, M.E. Tailor-making polyalphaolefins. J. Synth. Lubr. 1991, 8, 115–134. [Google Scholar] [CrossRef]
- Ray, S.; Rao, P.V.C.; Choudary, N.V. Poly-α-olefin-based synthetic lubricants: A short review on various synthetic routes. Lubr. Sci. 2012, 24, 23–44. [Google Scholar] [CrossRef]
- Whiteley, K.S.; Heggs, T.G.; Koch, H.; Mawer, R.L.; Immel, W. Polyolefins. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Nifant’ev, I.; Ivchenko, P.; Tavtorkin, A.; Vinogradov, A.; Vinogradov, A. Non-traditional Ziegler-Natta catalysis in a-olefin transformations: Reaction mechanisms and product design. Pure Appl. Chem. 2017, 89, 1017–1032. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P. Fair Look at Coordination Oligomerization of Higher α-Olefins. Polymers 2020, 12, 1082. [Google Scholar] [CrossRef]
- Kaminsky, W.; Külper, K.; Brintzinger, H.H.; Wild, F.R.W.P. Polymerization of Propene and Butene with a Chiral Zirconocene and Methylalumoxane as Cocatalyst. Angew. Chem. Int. Ed. Engl. 1985, 24, 507–508. [Google Scholar] [CrossRef]
- Kaminsky, W.; Niedoba, S.; Möller-Lindenhof, N.; Rabe, O. Isotactic Olefin Polymerization with Optically Active Catalysts. In Catalysis in Polymer Synthesis; American Chemical Society: Washington, DC, USA, 1992; pp. 63–71. [Google Scholar]
- Möhring, P.C.; Coville, N.J. Homogeneous group 4 metallocene ziegler-natta catalysts: The influence of cyclopentadienyl-ring substituents. J. Organomet. Chem. 1994, 479, 1–29. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in Propene Polymerization with Metallocene Catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef]
- Xiao, A.; Wang, L.; Liu, Q.; Dong, X. Recent research progress in influence of the ansa-zirconcene catalytic system on the polypropylene microstructure. Des. Monomers Polym. 2007, 10, 281–295. [Google Scholar] [CrossRef]
- Chen, E.Y.-X.; Marks, T.J. Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure—Activity Relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef]
- Dong, S.Q.; Mi, P.K.; Xu, S.; Zhang, J.; Zhao, R.D. Preparation and Characterization of Single-Component Poly-α-olefin Oil Base Stocks. Energy Fuels 2019, 33, 9796–9804. [Google Scholar] [CrossRef]
- Zhao, R.; Mi, P.; Xu, S.; Dong, S. Structure and Properties of Poly-α-olefins Containing Quaternary Carbon Centers. ACS Omega 2020, 5, 9142–9150. [Google Scholar] [CrossRef] [PubMed]
- Desert, X.; Carpentier, J.-F.; Kirillov, E. Quantification of active sites in single-site group 4 metal olefin polymerization catalysis. Coord. Chem. Rev. 2019, 386, 50–68. [Google Scholar] [CrossRef]
- Desert, X.; Proutiere, F.; Welle, A.; Den Dauw, K.; Vantomme, A.; Miserque, O.; Brusson, J.-M.; Carpentier, J.-F.; Kirillov, E. Zirconocene-Catalyzed Polymerization of α-Olefins: When Intrinsic Higher Activity Is Flawed by Rapid Deactivation. Organometallics 2019, 38, 2664–2673. [Google Scholar] [CrossRef]
- Moscato, B.M.; Zhu, B.; Landis, C.R. Mechanistic Investigations into the Behavior of a Labeled Zirconocene Polymerization Catalyst. Organometallics 2012, 31, 2097–2107. [Google Scholar] [CrossRef]
- Sillars, D.R.; Landis, C.R. Catalytic Propene Polymerization: Determination of Propagation, Termination, and Epimerization Kinetics by Direct NMR Observation of the (EBI)Zr(MeB(C6F5)3)propenyl Catalyst Species. J. Am. Chem. Soc. 2003, 125, 9894–9895. [Google Scholar] [CrossRef] [PubMed]
- Christianson, M.D.; Tan, E.H.P.; Landis, C.R. Stopped-Flow NMR: Determining the Kinetics of [rac-(C2H4(1-indenyl)2)ZrMe][MeB(C6F5)3]-Catalyzed Polymerization of 1-Hexene by Direct Observation. J. Am. Chem. Soc. 2010, 132, 11461–11463. [Google Scholar] [CrossRef] [PubMed]
- Novstrup, K.A.; Travia, N.E.; Medvedev, G.A.; Stanciu, C.; Switzer, J.M.; Thomson, K.T.; Delgass, W.N.; Abu-Omar, M.M.; Caruthers, J.M. Mechanistic Detail Revealed via Comprehensive Kinetic Modeling of [rac-C2H4(1-indenyl)2ZrMe2]-Catalyzed 1-Hexene Polymerization. J. Am. Chem. Soc. 2010, 132, 558–566. [Google Scholar] [CrossRef]
- Sian, L.; Dall’Anese, A.; Macchioni, A.; Tensi, L.; Busico, V.; Cipullo, R.; Goryunov, G.P.; Uborsky, D.; Voskoboynikov, A.Z.; Ehm, C.; et al. Role of Solvent Coordination on the Structure and Dynamics of ansa-Zirconocenium Ion Pairs in Aromatic Hydrocarbons. Organometallics 2022, 41, 547–560. [Google Scholar] [CrossRef]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Alkylaluminum-Complexed Zirconocene Hydrides: Identification of Hydride-Bridged Species by NMR Spectroscopy. J. Am. Chem. Soc. 2008, 130, 17423–17433. [Google Scholar] [CrossRef]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Cationic Alkylaluminum-Complexed Zirconocene Hydrides as Participants in Olefin Polymerization Catalysis. J. Am. Chem. Soc. 2010, 132, 13969–13971. [Google Scholar] [CrossRef] [PubMed]
- Kissin, Y.V. Oligomerization reactions of 1-hexene with metallocene catalysts: Detailed data on reaction chemistry and kinetics. Mol. Catal. 2019, 463, 87–93. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Zirconocene-Catalyzed Dimerization of 1-Hexene: Two-stage Activation and Structure–Catalytic Performance Relationship. Cat. Commun. 2016, 79, 6–10. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sedov, I.V.; Dorokhov, V.G.; Lyadov, A.S.; Ivchenko, P.V. Structurally Uniform 1-Hexene, 1-Octene, and 1-Decene Oligomers: Zirconocene/MAO-Catalyzed Preparation, Characterization, and Prospects of Their Use as low-viscosity Low-temperature Oil Base Stocks. Appl. Cat. A Gen. 2018, 549, 40–50. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Churakov, A.V.; Bagrov, V.V.; Kashulin, I.A.; Roznyatovsky, V.A.; Grishin, Y.K.; Ivchenko, P.V. The Catalytic Behavior of Heterocenes Activated by TIBA and MMAO under a Low Al/Zr Ratios in 1-Octene Polymerization. Appl. Cat. A Gen. 2019, 571, 12–24. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Zirconocene-Catalyzed Dimerization of α-Olefins: DFT Modeling of the Zr-Al Binuclear Reaction Mechanism. Molecules 2019, 24, 3565. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Experimental and Theoretical Study of Zirconocene-Catalyzed Oligomerization of 1-Octene. Polymers 2020, 12, 1590. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Bagrov, V.V.; Churakov, A.V.; Minyaev, M.E.; Kiselev, A.V.; Salakhov, I.I.; Ivchenko, P.V. A competetive way to low-viscosity PAO base stocks via heterocene-catalyzed oligomerization of dec-1-ene. Mol. Catal. 2022, 529, 112542. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Tyumkina, T.V.; Islamov, D.N.; Lyapina, A.R.; Karchevsky, S.G.; Ivchenko, P.V. Reactions of bimetallic Zr,Al- hydride complexes with methylaluminoxane: NMR and DFT study. J. Organomet. Chem. 2017, 851, 30–39. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K. Bimetallic Zr,Zr-Hydride Complexes in Zirconocene Catalyzed Alkene Dimerization. Molecules 2020, 25, 2216. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K.; Palatov, E.R. Catalytic Systems Based on Cp2ZrX2 (X = Cl, H), Organoaluminum Compounds and Perfluorophenylboranes: Role of Zr,Zr- and Zr,Al-Hydride Intermediates in Alkene Dimerization and Oligomerization. Catalysts 2021, 11, 39. [Google Scholar] [CrossRef]
- Kovyazin, P.V.; Bikmeeva, A.K.; Islamov, D.N.; Yanybin, V.M.; Tyumkina, T.V.; Parfenova, L.V. Ti Group Metallocene-Catalyzed Synthesis of 1-Hexene Dimers and Tetramers. Molecules 2021, 26, 2775. [Google Scholar] [CrossRef] [PubMed]
- Chirik, P.J.; Bercaw, J.E. Cyclopentadienyl and olefin substituent effects on insertion and β-hydrogen elimination with group 4 metallocenes. kinetics, mechanism, and thermodynamics for zirconocene and hafnocene alkyl hydride derivatives. Organometallics 2005, 24, 5407–5423. [Google Scholar] [CrossRef]
- Asakura, T.; Demura, M.; Nishiyama, Y. Carbon-13 NMR spectral assignment of five polyolefins determined from the chemical shift calculation and the polymerization mechanism. Macromolecules 1991, 24, 2334–2340. [Google Scholar] [CrossRef]
- Babu, G.N.; Newmark, R.A.; Chien, J.C.W. Microstructure of Poly(1-hexene) Produced by ansa-Zirconocenium Catalysis. Macromolecules 1994, 27, 3383–3388. [Google Scholar] [CrossRef]
- Ahmadjo, S. Preparation of ultra high molecular weight amorphous poly(1-hexene) by a Ziegler–Natta catalyst. Polym. Adv. Technol. 2016, 27, 1523–1529. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, D.; He, S.; Ren, H.; Xu, Y. Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications. e-Polymers 2019, 19, 511–518. [Google Scholar] [CrossRef]
- Shoer, L.I.; Gell, K.I.; Schwartz, J. Mixed-metal hydride complexes containing Zr-H-Al Bridges. Synthesis and Relation to Transition-Metal-Catalyzed Reactions of Aluminum Hydrides. J. Organomet. Chem. 1977, 136, c19–c22. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Pechatkina, S.V.; Khalilov, L.M.; Dzhemilev, U.M. Mechanism of Cp2ZrCl2-Catalyzed Olefin Hydroalumination by Alkylalanes. Russ. Chem. Bull. 2005, 54, 316–327. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Nifant’ev, I.E.; Khalilov, L.M.; Dzhemilev, U.M. Role of Zr,Al Hydride Intermediate Structure and Dynamics in Alkene Hydroalumination with XAlBui2 (X = H, Cl, Bui), Catalyzed by Zr η5-Complexes. Organometallics 2015, 34, 3559–3570. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Vil’danova, R.F.; Pechatkina, S.V.; Khalilov, L.M.; Dzhemilev, U.M. New effective reagent [Cp2ZrH2·ClAlEt2]2 for alkene hydrometallation. J. Organomet. Chem. 2007, 692, 3424–3429. [Google Scholar] [CrossRef]
- Tyumkina, T.V.; Islamov, D.N.; Kovyazin, P.V.; Parfenova, L.V. Chain and cluster models of methylaluminoxane as activators of zirconocene hydride, alkyl and metallacyclopropane intermediates in alkene transformations. Mol. Catal. 2021, 512, 111768. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Panchenko, V.N.; Timofeeva, M.N.; Zakharov, V.A.; Brintzinger, H.H. Novel Zirconocene Hydride Complexes in Homogeneous and in SiO2-Supported Olefin-Polymerization Catalysts Modified with Diisobutylaluminum Hydride or Triisobutylaluminum. Macromol. Chem. Phys. 2008, 209, 1210–1219. [Google Scholar] [CrossRef]
- Freidlina, R.K.; Brainina, E.M.; Nesmeyanov, A.N. The Synthesis of Mixed Pincerlike Cyclopentadienyl Compounds of Zirconium. Dokl. Acad. Nauk SSSR 1961, 138, 1369–1372. [Google Scholar]
- Koch, T.; Blaurock, S.; Somoza, F.B.; Voigt, A.; Kirmse, R.; Hey-Hawkins, E. Unexpected P−Si or P−C Bond Cleavage in the Reaction of Li2[(C5Me4)SiMe2PR] (R = Cyclohexyl, 2,4,6-Me3C6H2) and Li[(C5H4)CMe2PHR] (R = Ph, tBu) with ZrCl4 or [TiCl3(thf)3]: Formation and Molecular Structure of the ansa-Metallocenes [{(η-C5Me4)2SiMe2}ZrCl2] and [{(η-C5H4)2CMe2}MCl2] (M = Ti, Zr). Organometallics 2000, 19, 2556–2563. [Google Scholar]
- Bajgur, C.S.; Tikkanen, W.; Petersen, J.L. Synthesis, structural characterization, and electrochemistry of [1]metallocenophane complexes, [Si(alkyl)2(C5H4)2]MCl2, M = Ti, Zr. Inorg. Chem. 1985, 24, 2539–2546. [Google Scholar] [CrossRef]
- Schwemlein, H.; Brintzinger, H.H. ansa-Metallocene derivatives: V. Synthesis of tetramethylethylene-bridged titanocene and zirconocene derivatives via reductive fulvene coupling. J. Organomet. Chem. 1983, 254, 69–73. [Google Scholar] [CrossRef]
- Piccolrovazzi, N.; Pino, P.; Consiglio, G.; Sironi, A.; Moret, M. Electronic effects in homogeneous indenylzirconium Ziegler-Natta catalysts. Organometallics 1990, 9, 3098–3105. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Ivchenko, P.V. Synthesis of Zirconium and Hafnium ansa-Metallocenes via Transmetalation of Dielement-Substituted Bis(cyclopentadienyl) and Bis(indenyl) Ligands. Organometallics 1997, 16, 713–715. [Google Scholar] [CrossRef]
- Ellis, W.W.; Hollis, T.K.; Odenkirk, W.; Whelan, J.; Ostrander, R.; Rheingold, A.L.; Bosnich, B. Synthesis, structure, and properties of chiral titanium and zirconium complexes bearing biaryl strapped substituted cyclopentadienyl ligands. Organometallics 1993, 12, 4391–4401. [Google Scholar] [CrossRef]
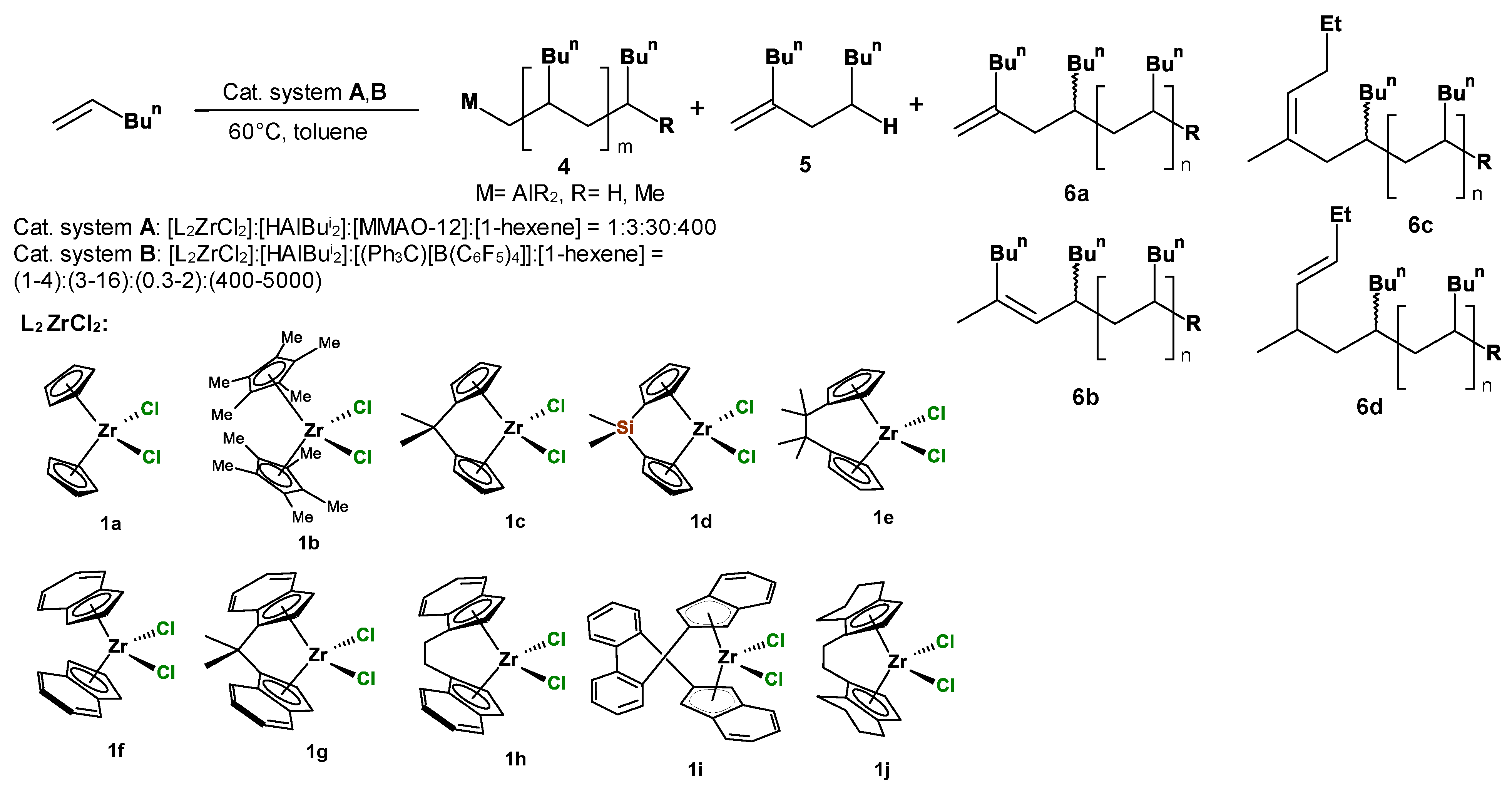


| Entry | Catalytic Systems | Mole Ratio [Zr]: [HAlBui2]: [Activator]: [1-Alkene] | Time, Min | Alkene Conver-sion, % | Light Fraction Yield, wt.% | Light Fraction Product Composition, % (GC-MS) | Heavy Fraction Yield, wt.% (GPC) | MW, Da | MN, Da | MW/ MN | Tacticity, mmmm% | Oligomer Structural Type | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complex | Activator | 5 | 6 | |||||||||||||||
| n = 1 | n = 2 | n = 3 | n = 4 | n = 5 | ||||||||||||||
| 1 | Cp2ZrCl2 (1a) | (Ph3C)[B(C6F5)4] | 1:3:0.3:400 | 60 | 95 | 98 | 61 | 21 | 10 | 6 | 2 | - | 2 | 6211 | 6129 | 1.01 | atactic | 6b–d |
| 2 | 1:3:1:400 | >99 | 99 | 30 | 28 | 20 | 10 | 8 | 4 | - | ||||||||
| 3 | 1:3:2:400 | >99 a | 33 | 5277 | 5208 | 1.01 | ||||||||||||
| 4 | 4:16:1:400 | >99 | 88 | 61 | 5 | 12 | 8 | 8 | 5 | 2 | 5571 | 5459 | 1.02 | |||||
| 10 | 1578 | 1211 | 1.30 | |||||||||||||||
| 5 | MMAO-12 [31] | 1:3:30:400 | 5 | 98 | 97 | - | ||||||||||||
| 6 | (C5Me5)2ZrCl2 (1b) | (Ph3C)[B(C6F5)4] | 4:16:1:400 | 180 | 99 | 44 | 0 | 27 | 18 | 30 | 24 | 55 | 1109 | 933 | 1.19 | atactic | 6a | |
| 7 | MMAO-12 | 1:3:30:400 | 60 | 99 | 3 | 28 | 23 | 17 | 16 | 16 | 71 | 5605 | 5906 | 1.05 | atactic | 4, 6a d | ||
| 26 | 861 | 798 | 1.08 | |||||||||||||||
| 8 | Me2CCp2ZrCl2 (1c) | (Ph3C)[B(C6F5)4] | 4:16:1:400 | 60 | >99 | 95 | 4 | - | ||||||||||
| 9 | 1:1:0.1:400 | 180 | 91 | 86 | 5 | |||||||||||||
| 10 | MMAO-12 | 1:3:30:400 | 60 | >99 | 94 | 6 | - | |||||||||||
| 11 | 1:1:11:400 | 180 | 77 | 75 | 2 | |||||||||||||
| 12 | Me2SiCp2ZrCl2 (1d) | (Ph3C)[B(C6F5)4] | 4:16:1:400 | 180 | >99 | 5 | 17 | 28 | 15 | 21 | 18 | 89 | 6130 | 6041 | 1.02 | atactic | 6a, 6d | |
| 6 | 2769 | 2672 | 1.04 | |||||||||||||||
| 13 | MMAO-12 | 1:3:30:400 | 60 | >99 | 99 | 80 | 16 | 4 | - | |||||||||
| 14 | (Me2C)2Cp2ZrCl2 (1e) | (Ph3C)[B(C6F5)4] | 4:16:1:400 | 60 | >99 | 99 | 98 | 1 | - | |||||||||
| 15 | MMAO-12 | 1:3:30:400 | 60 | 85 | 78 | 7 | - | |||||||||||
| 16 | Ind2ZrCl2 (1f) | (Ph3C)[B(C6F5)4] | 1:3:0.3:400 | 180 | >99 | 96 | 7 | 23 | 32 | 21 | 17 | 3 | 6307 | 6200 | 1.02 | atactic | 6a–d | |
| 17 | 4:16:1:400 | 60 | >99 | 63 | 0 | 22 | 20 | 26 | 31 | 36 | 988 | 869 | 1.14 | atactic | 6a | |||
| 18 e | 1:3:0.2:5 | 10 | 74 f | 12 | 16 | 16 | ||||||||||||
| 19 e | 1:8:0.2:80 | 10 | >99 g | 13 | 16 | 16 | 10 | 9 | 8 | |||||||||
| 20 | MMAO-12 | 1:3:30:400 | 60 | >99 | 27 | 6 | 15 | 17 | 19 | 43 | 72 | 2182 | 1599 | 1.36 | isotactic (67%) | 4, 6a,d | ||
| 21 e | 1:3:7:3 | 10 | >99 h | 46 | 8 | |||||||||||||
| 22 e | 1:8:10:80 | 10 | >99 i | 27 | 22 | 17 | 8 | 4 | ||||||||||
| 23 | rac-Me2CInd2ZrCl2 (1g) | (Ph3C)[B(C6F5)4] | 1:3:0.3:400 | 60 | 97 | 70 | 22 | 21 | 20 | 19 | 18 | 27 | 6359 | 6291 | 1.01 | isotactic (93%) | 6d | |
| 24 | 4:16:1:400 | 60 | >99 | 72 | 79 | 10 | 5 | 4 | 2 | 24 | 5585 | 5144 | 1.08 | isotactic (76%) | 6a–d | |||
| 3 | 903 | 871 | 1.04 | |||||||||||||||
| 25 | MMAO-12 | 1:3:30:400 | 60 | >99 | 55 | 4 | 33 | 33 | 18 | 12 | 44 | 1081 | 927 | 1.17 | atactic | 4, 6a–d d | ||
| 26 | rac-H4C2Ind2ZrCl2 (1h) | (Ph3C)[B(C6F5)4] | 4:16:1:400 | 60 | >99 | 99 | 6396 | 6285 | 1.02 | isotactic (71%) | 6a–d | |||||||
| 27 | MMAO-12 | 1:3:30:400 | 60 | >99 | 5 | 7 | 22 | 22 | 24 | 25 | 94 | 6347 | 6287 | 1.01 | isotactic (61%) | 4, 6a,d | ||
| 28 | BIPh(Ind)2ZrCl2 (1i) | (Ph3C)[B(C6F5)4] | 1:3:0.3:400 | 180 | 47 b | 25 | 13 | 49 | 23 | 15 | 22 | 6037 | 6125 | 1.01 | ||||
| 29 | 4:16:1:400 | 60 | >99 b | 59 | 7 | 21 | 26 | 14 | 32 | 40 | 1096 | 1003 | 1.09 | isotactic (33%) | 6a | |||
| 30 | MMAO-12 | 1:3:30:400 | 60 | >99 b | 9 | 3 | 20 | 25 | 23 | 29 | 20 | 5742 | 5657 | 1.02 | isotactic (45%) | 4, 6a d | ||
| 70 | 2159 | 1532 | 1.4 | |||||||||||||||
| 31 | rac-H4C2[THInd]2ZrCl2 (1j) | (Ph3C)[B(C6F5)4] | 1:3:0.3:400 | 180 | 89 c | 69 | 13 | 18 | 20 | 15 | 14 | 12 | 10 | 6131 | 6048 | 1.02 | ||
| 10 | 960 | 909 | 1.06 | |||||||||||||||
| 32 | 4:16:1:400 | 180 | >99 | 11 | 29 | 19 | 18 | 17 | 17 | 88 | 5530 | 5033 | 1.10 | isotactic (38%) | 6a–d | |||
| 33 | MMAO-12 | 1:3:30:400 | 180 | 95 | 8 | 11 | 24 | 24 | 23 | 18 | 38 | 6159 | 6103 | 1.01 | isotactic (58%) | 6a–d | ||
| 19 | 3145 | 3114 | 1.01 | |||||||||||||||
| 30 | 2074 | 1971 | 1.06 | |||||||||||||||
| Complex | Activator | T, K | δH Cp | δC Cp | δH H1 | δH H2 |
|---|---|---|---|---|---|---|
| 8c | 250 K | 6.11 (s, 4H) 5.19 (s, 4H) 1.03 (s, 12H) | 110.9 110.3 100.8 36.8 22.3 | −1.44 (br.s, 2H) | 1.29–1.43 (br.s, 1H) | |
| 9c | 6.28 (s, 2H) 6.23 (s, 2H) 5.66 (s, 2H) 5.37 (s, 2H) 1.15 (NOESY) 1.12 (NOESY) | −1.10 (br.s, 1H) | −0.81 (br.s, 1H) | |||
| 10c | 298 K | −3.58 (t, 17.9 Hz, 1H) | −1.34 (d, 17.9 Hz, 2H) | |||
| 8c∙MAO | MMAO−12 | 298 K | 6.14 (NOESY) 4.93–5.69 (br.m) | −1.62− −1.22 (br.s) | 1.25–1.48 (COSY HH) −0.38 − −0.02 (MAO) 3.26–4.11 (H-MAO) | |
| 10c∙MAO | MMAO−12 | 298 K | 6.12 (NOESY) 5.67 (NOESY) 5.42 (NOESY) 4.85 (NOESY) 0.98 (NOESY) | 119.6 108.7 101.2 98.2 21.1 | −3.57 (t, 17.6 Hz, 2/3H) −3.71 (t, 18.0 Hz, 1/3H) | −1.32 (d, 17.6 Hz, 2H) −0.56 − 0.15 (MAO) |
| 10c a | [Ph3C][B(C6F5)4] | 298 K | −3.59 (t, 18.3 Hz, 2H) | −1.34 (d, 18.3 Hz, 2H) | ||
| 8f [43] | 220 K | 7.37 (m, 4H) 6.87 (m, 4H) 6.32 (m, 2H) 5.19 (m, 4H) | −1.11 (d, 5.6 Hz, 2H) | 0.07 (t, 7.6 Hz, 1H) | ||
| 8f | 292 K | 7.43 (m, 4H) 6.89 (m, 4H) 6.38 (m, 2H) 5.36 (m, 4H) | −0.92 (br.s, 2H) | 0.07 (NOESY) | ||
| 10f∙MAO | MMAO−12 | 298 K | 5.92 (m, 4H) 5.08 (m, 4H) 4.62 (m, 4H) | −6.11 (t, 18.5 Hz, 1H) | 0.05 (COSY HH) | |
| 10f a | [Ph3C][B(C6F5)4] | 298 K | 7.49 (d, 8.3 Hz, 4H) 7.19 (d, 8.3 Hz, 4H) 6.99–7.16 (m, 8H) 5.82 (br.t, 3.0 Hz, 4H) 5.02 (m, 4H) 4.57 (m, 4H) | 127.9 126.6 119.0 117.3 99.1 96.2 | −6.00 (t, 18.1 Hz, 1H) | −0.04 (d, 18.1 Hz, 2H) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K.; Palatov, E.R.; Ivchenko, P.V.; Nifant’ev, I.E.; Khalilov, L.M. Catalytic Properties of Zirconocene-Based Systems in 1-Hexene Oligomerization and Structure of Metal Hydride Reaction Centers. Molecules 2023, 28, 2420. https://doi.org/10.3390/molecules28062420
Parfenova LV, Kovyazin PV, Bikmeeva AK, Palatov ER, Ivchenko PV, Nifant’ev IE, Khalilov LM. Catalytic Properties of Zirconocene-Based Systems in 1-Hexene Oligomerization and Structure of Metal Hydride Reaction Centers. Molecules. 2023; 28(6):2420. https://doi.org/10.3390/molecules28062420
Chicago/Turabian StyleParfenova, Lyudmila V., Pavel V. Kovyazin, Almira Kh. Bikmeeva, Eldar R. Palatov, Pavel V. Ivchenko, Ilya E. Nifant’ev, and Leonard M. Khalilov. 2023. "Catalytic Properties of Zirconocene-Based Systems in 1-Hexene Oligomerization and Structure of Metal Hydride Reaction Centers" Molecules 28, no. 6: 2420. https://doi.org/10.3390/molecules28062420
APA StyleParfenova, L. V., Kovyazin, P. V., Bikmeeva, A. K., Palatov, E. R., Ivchenko, P. V., Nifant’ev, I. E., & Khalilov, L. M. (2023). Catalytic Properties of Zirconocene-Based Systems in 1-Hexene Oligomerization and Structure of Metal Hydride Reaction Centers. Molecules, 28(6), 2420. https://doi.org/10.3390/molecules28062420









