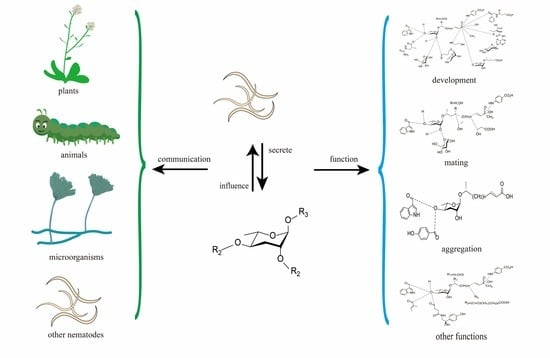
Nematode Pheromones: Structures and Functions
Abstract
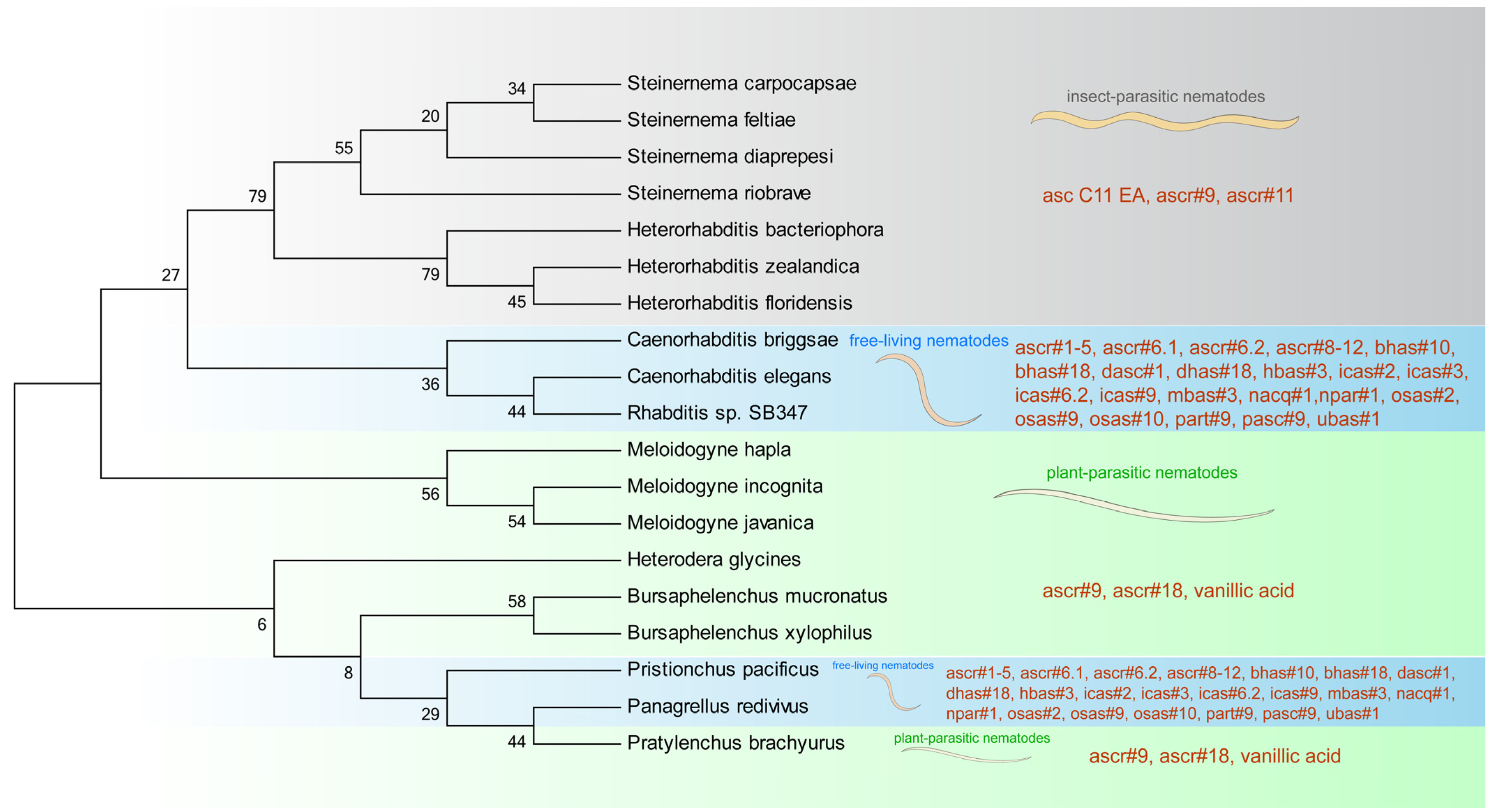
1. Introduction
2. Synthesis and Regulation of Ascarosides
3. Pheromones Secreted by Nematodes
3.1. Development-Related Pheromones Secreted by Nematodes
3.2. Sex Pheromones Secreted by Nematodes
3.3. Aggregation of Pheromones Secreted by Nematodes
3.4. Pheromones with Other Functions Secreted by Nematodes
| Name | Chemical Constitution | Function | Organism | Reference | |
|---|---|---|---|---|---|
| Asc C11 EA |  | Development | C. elegans | [66] | |
| Ascr#1 |  | Development; mating | C. elegans, P. pacificus, P. redivivus, and Rhabditis sp. SB347 | [47,48,59,61,62,74,83] | |
| Ascr#2 | 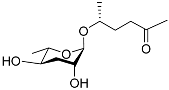 | Development, mating, foraging, and dispersal | C. elegans; C. briggsae | [48,49,71,72,97] | |
| Ascr#3 | 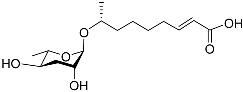 | Development, mating, foraging, and dispersal | C. elegans | [36,48,49,50,71,72,90,91,92,93,95,97,98,99,100] | |
| Ascr#4 | 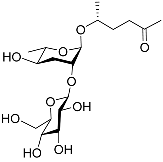 | Development; mating | C. elegans | [50,71,72] | |
| Ascr#5 | 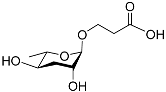 | Development; foraging | C. elegans | [25,26,51,97] | |
| Ascr#6.1 | 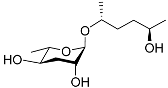 | Mating | C. elegans | [71] | |
| Ascr#6.2 |  | Mating | C. elegans | [71] | |
| Ascr#8 | 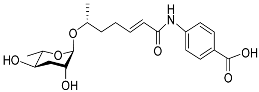 | Development, mating, foraging, and dispersal | C. elegans | [36,50,52,71,72,97] | |
| Ascr#9 | 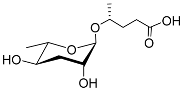 | Mating; dispersal | C. elegans, P. pacificus, Rhabditis sp. SB347, B. xylophilus, B. mucronatus H. bacteriophora, H.zealandica, H. floridensis, S. carpocapsae, S. riobrave, S. diaprepesi, and S. feltiae | [36,59,83,102,103,104,108,109] | |
| Ascr#10 | 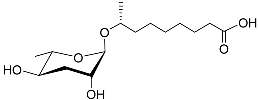 | Mating | C. elegans; P. redivivus | [73,74,75,76,77,78,79,101] | |
| Ascr#11 | 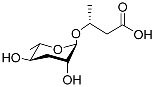 | Dispersal | C. elegans, S. carpocapsae, S. riobrave, S. diaprepesi, and S. feltiae | [102,103,104,106] | |
| Ascr#12 | 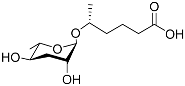 | Development | C. elegans; P. pacificus | [36,59,105,109] | |
| Ascr#18 | 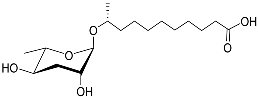 | Avoidance | M. incognita, M. javanica, M. hapla, H. glycines, and P. brachyurus | [19,101] | |
| Bhas#10 | 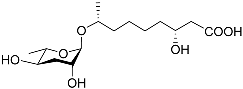 | Mating | C. elegans; P. redivivus | [74] | |
| Bhas#18 |  | Unknown | P. redivivus | [74] | |
| Dasc#1 | 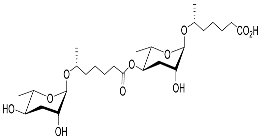 | Development | P. pacificus | [59,61,62] | |
| Dhas#18 | 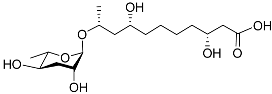 | Mating | P. redivivus | [74] | |
| Hbas#3 |  | Aggregation | C. elegans | [19] | |
| Icas#2 | 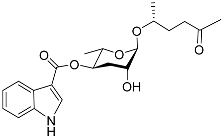 | Mating | C. briggsae | [80] | |
| Icas#3 | 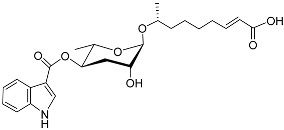 | Aggregation | C. elegans | [15,89] | |
| Icas#6.2 | 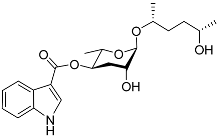 | Mating | C. briggsae | [80] | |
| Icas#9 |  | Development, aggregation, foraging, and dispersal | C. elegans | [15,19,53,72,97] | |
| Mbas#3 |  | Dispersal | C. elegans | [19,95] | |
| Nacq#1 |  | Development | C. elegans | [54] | |
| Nacq#2 | 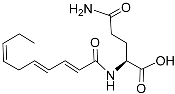 | Unknown | C. elegans | [54] | |
| Npar#1 | 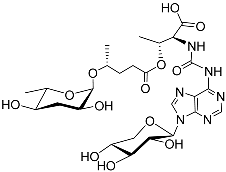 | Development | P. pacificus | [59,61,62] | |
| Osas#2 | 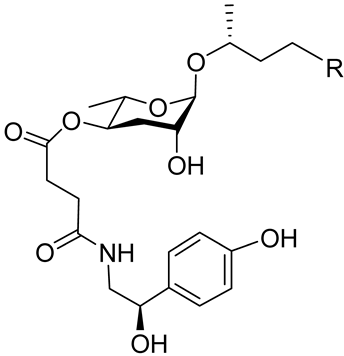 | R=(C=O)CH3 | Dispersal | C. elegans | [72] |
| Osas#10 | R=(CH2)4COOH | Dispersal | C. elegans | [72] | |
| Osas#9 | 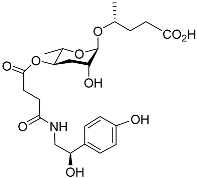 | Dispersal | C. elegans | [34,72] | |
| Part#9 | 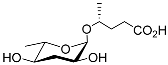 | Development | P. pacificus | [59,61,62] | |
| Pasc#9 | 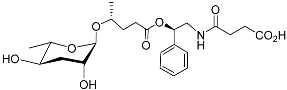 | Development | P. pacificus | [59,61,62] | |
| Ubas#1 | 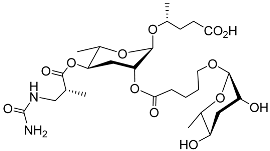 | Development | P. pacificus | [59,61,62] | |
| Vanillic acid | 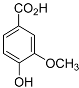 | Mating | H. glycines | [38,84] | |
4. Nematode Pheromone Communication with Other Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van den Hoogen, J.; Geisen, S.; Wall, D.H.; Wardle, D.A.; Traunspurger, W.; de Goede, R.G.; Adams, B.J.; Ahmad, W.; Ferris, H.; Bardgett, R.D.; et al. A Global Database of Soil Nematode Abundance and Functional Group Composition. Sci. Data 2020, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome Sequence of the Metazoan Plant-Parasitic Nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef]
- Kenney, E.; Eleftherianos, I. Entomopathogenic and Plant Pathogenic Nematodes as Opposing Forces in Agriculture. Int. J. Parasitol. 2016, 46, 13–19. [Google Scholar] [CrossRef]
- Luporini, P.; Pedrini, B.; Alimenti, C.; Vallesi, A. Revisiting Fifty Years of Research on Pheromone Signaling in Ciliates. Eur. J. Protistol. 2016, 55, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Alimenti, C.; Buonanno, F.; Di Giuseppe, G.; Guella, G.; Luporini, P.; Ortenzi, C.; Vallesi, A. Bioactive Molecules from Ciliates: Structure, Activity, and Applicative Potential. J. Eukaryot. Microbiol. 2022, 69, e12887. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.J.; Mo, B.T.; Guo, H.; Yang, J.; Tang, R.; Wang, C.Z. Revisiting the Sex Pheromone of the Fall Armyworm Spodoptera frugiperda, a New Invasive Pest in South China. Insect Sci. 2022, 29, 865–878. [Google Scholar] [CrossRef]
- Chen, D.; Hou, L.; Wei, J.; Guo, S.; Cui, W.; Yang, P.; Kang, L.; Wang, X. Aggregation Pheromone 4-Vinylanisole Promotes the Synchrony of Sexual Maturation in Female Locusts. Elife 2022, 11, e74581. [Google Scholar] [CrossRef]
- Tamrakar, S.; Huerta, B.; Chung-Davidson, Y.-W.; Li, W. Plasma Metabolomic Profiles Reveal Sex- and Maturation-Dependent Metabolic Strategies in Sea Lamprey (Petromyzon marinus). Metabolomics 2022, 18, 90. [Google Scholar] [CrossRef]
- Zaremska, V.; Renzone, G.; Arena, S.; Ciaravolo, V.; Buberl, A.; Balfanz, F.; Scaloni, A.; Knoll, W.; Pelosi, P. An Odorant-Binding Protein in the Elephant’s Trunk is Finely Tuned to Sex Pheromone (Z)-7-Dodecenyl Acetate. Sci. Rep. 2022, 12, 19982. [Google Scholar] [CrossRef]
- Mayer, M.G.; Rödelsperger, C.; Witte, H.; Riebesell, M.; Sommer, R.J. The Orphan Gene Dauerless Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation. PLoS Genet. 2015, 11, e1005146. [Google Scholar] [CrossRef]
- Edison, A.S. Caenorhabditis elegans Pheromones Regulate Multiple Complex Behaviors. Curr. Opin. Neurobiol. 2009, 19, 378–388. [Google Scholar] [CrossRef] [PubMed]
- von Reuss, S.H.; Schroeder, F.C. Combinatorial Chemistry in Nematodes: Modular Assembly of Primary Metabolism-Derived Building Blocks. Nat. Prod. Rep. 2015, 32, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Manohar, M.; Tenjo-Castano, F.; Chen, S.; Zhang, Y.K.; Kumari, A.; Williamson, V.M.; Wang, X.; Klessig, D.F.; Schroeder, F.C. Plant Metabolism of Nematode Pheromones Mediates Plant-Nematode Interactions. Nat. Commun. 2020, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, A.H.; Schroeder, F.C. Ascaroside Signaling in C. elegans. WormBook 2013, 18, 1–22. [Google Scholar] [CrossRef]
- Srinivasan, J.; von Reuss, S.H.; Bose, N.; Zaslaver, A.; Mahanti, P.; Ho, M.C.; O’Doherty, O.G.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A Modular Library of Small Molecule Signals Regulates Social Behaviors in Caenorhabditis elegans. PLoS Biol. 2012, 10, e1001237. [Google Scholar] [CrossRef]
- McGrath, P.T.; Ruvinsky, I. A Primer on Pheromone Signaling in Caenorhabditis elegans for Systems Biologists. Curr. Opin. Syst. Biol. 2019, 13, 23–30. [Google Scholar] [CrossRef]
- Park, J.Y.; Joo, H.J.; Park, S.; Paik, Y.K. Ascaroside Pheromones: Chemical Biology and Pleiotropic Neuronal Functions. Int. J. Mol. Sci. 2019, 20, 3898. [Google Scholar] [CrossRef]
- Wang, J.; Kim, S.K. Global Analysis of Dauer Gene Expression in Caenorhabditis elegans. Development 2003, 130, 1621–1634. [Google Scholar] [CrossRef]
- von Reuss, S.H.; Bose, N.; Srinivasan, J.; Yim, J.J.; Judkins, J.C.; Sternberg, P.W.; Schroeder, F.C. Comparative Metabolomics Reveals Biogenesis of Ascarosides, a Modular Library of Small-Molecule Signals in C. elegans. J. Am. Chem. Soc. 2012, 134, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Huang, X.; Chen, X.; Yang, Y.; Wang, Z.; Yang, Y.; Wu, F.; Zhou, J.; Yao, C.; Ma, G.; et al. Acyl-CoA Oxidase ACOX-1 Interacts with a Peroxin PEX-5 to Play Roles in Larval Development of Haemonchus contortus. PLoS Pathog. 2021, 17, e1009767. [Google Scholar] [CrossRef]
- Gao, C.; Li, Q.; Yu, J.; Li, S.; Cui, Q.; Hu, X.; Chen, L.; Zhang, S.O. Endocrine Pheromones Couple Fat Rationing to Dauer Diapause Through HNF4α Nuclear Receptors. Sci. China Life Sci. 2021, 64, 2153–2174. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Shi, H.; Shi, Y.; Guo, X.; Zheng, X.; Chen, X.; Zhou, Q.; Yang, Y.; Du, A. Characterization and Function Analysis of a Novel Gene, Hc-maoc-1, in the Parasitic Nematode Haemonochus contortus. Parasit. Vectors 2017, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Markov, G.V.; Meyer, J.M.; Panda, O.; Artyukhin, A.B.; Claassen, M.; Witte, H.; Schroeder, F.C.; Sommer, R.J. Functional Conservation and Divergence of daf-22 Paralogs in Pristionchus pacificus Dauer Development. Mol. Biol. Evol. 2016, 33, 2506–2514. [Google Scholar] [CrossRef]
- Rebecca, A.; Butchera, J.R.R.; Weiqing, L.; Ruvkunc, J.C.G.; Mak, H.Y. Biosynthesis of the Caenorhabditis elegans Dauer Pheromone. Proc. Natl. Acad. Sci. USA 2009, 106, 1875–1879. [Google Scholar]
- Shimizu, T.; Sugiura, K.; Sakai, Y.; Dar, A.R.; Butcher, R.A.; Matsumoto, K.; Hisamoto, N. Chemical Signaling Regulates Axon Regeneration via the GPCR-Gqα Pathway in Caenorhabditis elegans. J. Neurosci. 2022, 42, 720–730. [Google Scholar] [CrossRef]
- Park, J.; Oh, H.; Kim, D.Y.; Cheon, Y.; Park, Y.J.; Hwang, H.; Neal, S.J.; Dar, A.R.; Butcher, R.A.; Sengupta, P.; et al. CREB Mediates the C. elegans Dauer Polyphenism Through Direct and Cell-Autonomous Regulation of TGF-β Expression. PLoS Genet. 2021, 17, e1009678. [Google Scholar] [CrossRef]
- Chen, S.A.; Lin, H.C.; Schroeder, F.C.; Hsueh, Y.P. Prey Sensing and Response in a Nematode-Trapping Fungus is Governed by the MAPK Pheromone Response Pathway. Genetics 2021, 217, iyaa008. [Google Scholar] [CrossRef] [PubMed]
- Ilbay, O.; Ambros, V. Pheromones and Nutritional Signals Regulate the Developmental Reliance on let-7 Family MicroRNAs in C. elegans. Curr. Biol. 2019, 29, 1735–1745.e4. [Google Scholar] [CrossRef]
- Chute, C.D.; Srinivasan, J. Chemical Mating Cues in C. elegans. Semin. Cell Dev. Biol. 2014, 33, 18–24. [Google Scholar] [CrossRef]
- Cheon, Y.; Hwang, H.; Kim, K. Plasticity of Pheromone-Mediated Avoidance Behavior in C. elegans. J. Neurogenet. 2020, 34, 420–426. [Google Scholar] [CrossRef]
- Wu, T.; Ge, M.; Wu, M.; Duan, F.; Liang, J.; Chen, M.; Gracida, X.; Liu, H.; Yang, W.; Dar, A.R.; et al. Pathogenic Bacteria Modulate Pheromone Response to Promote Mating. Nature 2023, 613, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Aprison EZ, R.I. The Roles of Several Sensory Neurons and the Feedback From Egg Laying in Regulating the Germline Response to a Sex Pheromone in C. elegans Hermaphrodites. MicroPubl. Biol. 2022, 2022, 523. [Google Scholar] [CrossRef]
- Wong, S.S.; Yu, J.; Schroeder, F.C.; Kim, D.H. Population Density Modulates the Duration of Reproduction of C. elegans. Curr. Biol. 2020, 30, 2602–2607.e2. [Google Scholar] [CrossRef] [PubMed]
- Chute, C.D.; DiLoreto, E.M.; Zhang, Y.K.; Reilly, D.K.; Rayes, D.; Coyle, V.L.; Choi, H.J.; Alkema, M.J.; Schroeder, F.C.; Srinivasan, J. Co-option of Neurotransmitter Signaling for Inter-Organismal Communication in C. elegans. Nat. Commun. 2019, 10, 3186. [Google Scholar] [CrossRef]
- Park, J.; Choi, W.; Dar, A.R.; Butcher, R.A.; Kim, K. Neuropeptide Signaling Regulates Pheromone-Mediated Gene Expression of a Chemoreceptor Gene in C. elegans. Mol. Cells 2019, 42, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Choe, A.; von Reuss, S.H.; Kogan, D.; Gasser, R.B.; Platzer, E.G.; Schroeder, F.C.; Sternberg, P.W. Ascaroside Signaling is Widely Conserved Among Nematodes. Curr. Biol. 2012, 22, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Fox, B.W.; Palomino, D.C.F.; Panda, O.; Tenjo, F.J.; Koury, E.J.; Evans, K.S.; Stevens, L.; Rodrigues, P.R.; Kolodziej, A.R.; et al. Natural Genetic Variation in the Pheromone Production of C. elegans. bioRxiv 2022. [Google Scholar] [CrossRef]
- Jaffe, H.; Huettel, R.N.; Demilo, A.B.; Hayes, D.K.; Rebois, R.V. Isolation and Identification of a Compound from Soybean Cyst Nematode, Heterodera glycines, with Sex Pheromone Activity. J. Chem. Ecol. 1989, 15, 2031–2043. [Google Scholar] [CrossRef]
- Kaletta, T.; Hengartner, M.O. Finding Function in Novel Targets: C. elegans as a Model Organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar] [CrossRef]
- Cassada, R.C.; Russell, R.L. The Dauerlarva, A Post-Embryonic Developmental Variant of the Nematode Caenorhabditis elegans. Dev. Biol. 1975, 46, 326–342. [Google Scholar] [CrossRef]
- Klass, M.; Hirsh, D. Non-Ageing Developmental Variant of Caenorhabditis elegans. Nature 1976, 260, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, F.C. Modular Assembly of Primary Metabolic Building Blocks: A Chemical Language in C. elegans. Chem. Biol. 2015, 22, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.L.; Albert, P.S. Genetic and Environmental Regulation of Dauer Larva Development. In C. elegans II; Riddle, D.L., Blumenthal, T., Meyer, B.J., Priess, J.R., Eds.; Cold Spring Harbor Laboratory Press Copyright © 2023; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997. [Google Scholar]
- Kim, S.; Paik, Y.K. Developmental and Reproductive Consequences of Prolonged Non-aging Dauer in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2008, 368, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Golden, J.W.; Riddle, D.L. A Pheromone Influences Larval Development in the Nematode Caenorhabditis elegans. Science 1982, 218, 578–580. [Google Scholar] [CrossRef]
- Butcher, R.A. Small-Molecule Pheromones and Hormones Controlling Nematode Development. Nat. Chem. Biol. 2017, 13, 577–586. [Google Scholar] [CrossRef]
- Jeong, P.Y.; Jung, M.; Yim, Y.H.; Kim, H.; Park, M.; Hong, E.; Lee, W.; Kim, Y.H.; Kim, K.; Paik, Y.K. Chemical Structure and Biological Activity of the Caenorhabditis elegans Dauer-Inducing Pheromone. Nature 2005, 433, 541–545. [Google Scholar] [CrossRef]
- Butcher, R.A.; Fujita, M.; Schroeder, F.C.; Clardy, J. Small-Molecule Pheromones that Control Dauer Development in Caenorhabditis elegans. Nat. Chem. Biol. 2007, 3, 420–422. [Google Scholar] [CrossRef]
- Cohen, S.M.; Wrobel, C.J.J.; Prakash, S.J.; Schroeder, F.C.; Sternberg, P.W. Formation and Function of Dauer Ascarosides in the Nematodes Caenorhabditis briggsae and Caenorhabditis elegans. G3 Bethesda 2022, 12, jkac014. [Google Scholar] [CrossRef]
- Srinivasan, J.; Kaplan, F.; Ajredini, R.; Zachariah, C.; Alborn, H.T.; Teal, P.E.; Malik, R.U.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A Blend of Small Molecules Regulates Both Mating and Development in Caenorhabditis elegans. Nature 2008, 454, 1115–1118. [Google Scholar] [CrossRef]
- Butcher, R.A.; Ragains, J.R.; Kim, E.; Clardy, J. A Potent Dauer Pheromone Component in Caenorhabditis elegans that Acts Synergistically with Other Components. Proc. Natl. Acad. Sci. USA 2008, 105, 14288–14292. [Google Scholar] [CrossRef]
- Reilly, D.K.; McGlame, E.J.; Vandewyer, E.; Robidoux, A.N.; Muirhead, C.S.; Northcott, H.T.; Joyce, W.; Alkema, M.J.; Gegear, R.J.; Beets, I.; et al. Distinct Neuropeptide-Receptor Modules Regulate a Sex-Specific Behavioral Response to a Pheromone. Commun. Biol. 2021, 4, 1018. [Google Scholar] [CrossRef] [PubMed]
- Butcher, R.A.; Ragains, J.R.; Clardy, J. An Indole-Containing Dauer Pheromone Component with Unusual Dauer Inhibitory Activity at Higher Concentrations. Org. Lett. 2009, 11, 3100–3103. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, A.H.; Artyukhin, A.B.; Aprison, E.Z.; Rodrigues, P.R.; Pulido, D.C.; Burkhardt, R.N.; Panda, O.; Zhang, Y.K.; Gudibanda, P.; Ruvinsky, I.; et al. An Excreted Small Molecule Promotes C. elegans Reproductive Development and Aging. Nat. Chem. Biol. 2019, 15, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.L.; Sommer, R.J. Pristionchus pacificus: A Well-Rounded Nematode. BioEssays 2006, 28, 651–659. [Google Scholar] [CrossRef]
- Meyer, J.M.; Baskaran, P.; Quast, C.; Susoy, V.; Rödelsperger, C.; Glöckner, F.O.; Sommer, R.J. Succession and Dynamics of Pristionchus Nematodes and Their Microbiome During Decomposition of Oryctes Borbonicus on La Réunion Island. Environ. Microbiol. 2017, 19, 1476–1489. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R.J.; McGaughran, A. The Nematode Pristionchus pacificus as a Model System for Integrative Studies in Evolutionary Biology. Mol. Ecol. 2013, 22, 2380–2393. [Google Scholar] [CrossRef]
- Bento, G.; Ogawa, A.; Sommer, R.J. Co-Option of the Hormone-Signalling Module Dafachronic Acid-DAF-12 in Nematode Evolution. Nature 2010, 466, 494–497. [Google Scholar] [CrossRef]
- Bose, N.; Ogawa, A.; von Reuss, S.H.; Yim, J.J.; Ragsdale, E.J.; Sommer, R.J.; Schroeder, F.C. Complex Small-Molecule Architectures Regulate Phenotypic Plasticity in a Nematode. Angew. Chem. Int. Ed. Engl. 2012, 51, 12438–12443. [Google Scholar] [CrossRef]
- Falcke, J.M.; Bose, N.; Artyukhin, A.B.; Rödelsperger, C.; Markov, G.V.; Yim, J.J.; Grimm, D.; Claassen, M.H.; Panda, O.; Baccile, J.A.; et al. Linking Genomic and Metabolomic Natural Variation Uncovers Nematode Pheromone Biosynthesis. Cell Chem. Biol. 2018, 25, 787–796.e12. [Google Scholar] [CrossRef]
- Bose, N.; Meyer, J.M.; Yim, J.J.; Mayer, M.G.; Markov, G.V.; Ogawa, A.; Schroeder, F.C.; Sommer, R.J. Natural Variation in Dauer Pheromone Production and Sensing Supports Intraspecific Competition in Nematodes. Curr. Biol. 2014, 24, 1536–1541. [Google Scholar] [CrossRef]
- Werner, M.S.; Claaßen, M.H.; Renahan, T.; Dardiry, M.; Sommer, R.J. Adult Influence on Juvenile Phenotypes by Stage-Specific Pheromone Production. iScience 2018, 10, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Ciche, T.A.; Kim, K.S.; Kaufmann-Daszczuk, B.; Nguyen, K.C.; Hall, D.H. Cell Invasion and Matricide during Photorhabdus luminescens Transmission by Heterorhabditis bacteriophora Nematodes. Appl. Environ. Microbiol. 2008, 74, 2275–2287. [Google Scholar] [CrossRef] [PubMed]
- Ciche, T.A.; Ensign, J.C. For the Insect Pathogen Photorhabdus luminescens, Which End of a Nematode is Out? Appl. Environ. Microbiol. 2003, 69, 1890–1897. [Google Scholar] [CrossRef]
- Ciche, T. The Biology and Genome of Heterorhabditis bacteriophora. WormBook 2007, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Noguez, J.H.; Conner, E.S.; Zhou, Y.; Ciche, T.A.; Ragains, J.R.; Butcher, R.A. A Novel Ascaroside Controls the Parasitic Life Cycle of the Entomopathogenic Nematode Heterorhabditis bacteriophora. ACS Chem. Biol. 2012, 7, 961–966. [Google Scholar] [CrossRef]
- Gomez-Diaz, C.; Benton, R. The Joy of Sex Pheromones. EMBO Rep. 2013, 14, 874–883. [Google Scholar] [CrossRef]
- Wyatt, T.D. Pheromones and Signature Mixtures: Defining Species-Wide Signals and Variable Cues for Identity in Both Invertebrates and Vertebrates. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2010, 196, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Leighton, D.H.; Sternberg, P.W. Mating Pheromones of Nematoda: Olfactory Signaling with Physiological Consequences. Curr. Opin. Neurobiol. 2016, 38, 119–124. [Google Scholar] [CrossRef]
- Simon, J.M.; Sternberg, P.W. Evidence of a Mate-Finding Cue in the Hermaphrodite Nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 1598–1603. [Google Scholar] [CrossRef]
- Pungaliya, C.; Srinivasan, J.; Fox, B.W.; Malik, R.U.; Ludewig, A.H.; Sternberg, P.W.; Schroeder, F.C. A Shortcut to Identifying Small Molecule Signals that Regulate Behavior and Development in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 7708–7713. [Google Scholar] [CrossRef]
- Artyukhin, A.B.; Yim, J.J.; Srinivasan, J.; Izrayelit, Y.; Bose, N.; von Reuss, S.H.; Jo, Y.; Jordan, J.M.; Baugh, L.R.; Cheong, M.; et al. Succinylated Octopamine Ascarosides and a New Pathway of Biogenic Amine Metabolism in Caenorhabditis elegans. J. Biol. Chem. 2013, 288, 18778–18783. [Google Scholar] [CrossRef] [PubMed]
- Izrayelit, Y.; Srinivasan, J.; Campbell, S.L.; Jo, Y.; von Reuss, S.H.; Genoff, M.C.; Sternberg, P.W.; Schroeder, F.C. Targeted Metabolomics Reveals a Male Pheromone and Sex-Specific Ascaroside Biosynthesis in Caenorhabditis elegans. ACS Chem. Biol. 2012, 7, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Choe, A.; Chuman, T.; von Reuss, S.H.; Dossey, A.T.; Yim, J.J.; Ajredini, R.; Kolawa, A.A.; Kaplan, F.; Alborn, H.T.; Teal, P.E.; et al. Sex-Specific Mating Pheromones in the Nematode Panagrellus redivivus. Proc. Natl. Acad. Sci. USA 2012, 109, 20949–20954. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Runnels, A.M.; Murphy, C.T. Mating and Male Pheromone Kill Caenorhabditis Males Through Distinct Mechanisms. Elife 2017, 6, 23493. [Google Scholar] [CrossRef]
- Aprison, E.Z.; Dzitoyeva, S.; Angeles-Albores, D.; Ruvinsky, I. A Male Pheromone that Improves the Quality of the Oogenic Germline. Proc. Natl. Acad. Sci. USA 2022, 119, e2015576119. [Google Scholar] [CrossRef]
- Aprison, E.Z.; Ruvinsky, I. Sex Pheromones of C. elegans Males Prime the Female Reproductive System and Ameliorate the Effects of Heat Stress. PLoS Genet. 2015, 11, e1005729. [Google Scholar] [CrossRef]
- Aprison, E.Z.; Ruvinsky, I. Sexually Antagonistic Male Signals Manipulate Germline and Soma of C. elegans Hermaphrodites. Curr. Biol. 2016, 26, 2827–2833. [Google Scholar] [CrossRef]
- Aprison, E.Z.; Ruvinsky, I. Counteracting Ascarosides Act through Distinct Neurons to Determine the Sexual Identity of C. elegans Pheromones. Curr. Biol. 2017, 27, 2589–2599.e3. [Google Scholar] [CrossRef]
- Dong, C.; Dolke, F.; von Reuss, S.H. Selective MS Screening Reveals a Sex Pheromone in Caenorhabditis briggsae and Species-Specificity in Indole Ascaroside Signalling. Org. Biomol. Chem. 2016, 14, 7217–7225. [Google Scholar] [CrossRef]
- Balakanich, S.; Samoiloff, M.R. Development of Nematode Behavior: Sex Attraction Among Different Strains of the Free-Living Panagrellus redivivus. Can. J. Zool. 1974, 52, 835–845. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Kache, V.; Pires-daSilva, A. Regulation of Sexual Plasticity in a Nematode that Produces Males, Females, and Hermaphrodites. Curr. Biol. 2011, 21, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.; Bose, N.; Tandonnet, S.; Adams, S.; Zuco, G.; Kache, V.; Parihar, M.; von Reuss, S.H.; Schroeder, F.C.; Pires-daSilva, A. Mating Dynamics in a Nematode with Three Sexes and Its Evolutionary Implications. Sci. Rep. 2015, 5, 17676. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.L.; Johnson, G.; Dimock, M.; Fahey, J.W.; Huettel, R.N. Field Efficacy of Verticillium lecanii, Sex Pheromone, and Pheromone Analogs as Potential Management Agents for Soybean Cyst Nematode. J. Nematol. 1997, 29, 282–288. [Google Scholar] [PubMed]
- Aumann, J.; Dietsche, E.; Rutencrantz, S.; Ladehoff, H. Physico-Chemical Properties of the Female Sex Pheromone of Heterodera schachtii (Nematoda: Heteroderidae). Int. J. Parasitol. 1998, 28, 1691–1694. [Google Scholar] [CrossRef]
- Shinya, R.; Chen, A.; Sternberg, P.W. Sex Attraction and Mating in Bursaphelenchus okinawaensis and B. xylophilus. J. Nematol. 2015, 47, 176–183. [Google Scholar] [PubMed]
- Cepulyte, R.; Bu da, V. Toward Chemical Ecology of Plant-Parasitic Nematodes: Kairomones, Pheromones, and Other Behaviorally Active Chemical Compounds. J. Agric. Food Chem. 2022, 70, 1367–1390. [Google Scholar] [CrossRef] [PubMed]
- Riga, E.; Holdsworth, D.R.; Perry, R.N.; Barrett, J.; Johnston, M.R. Electrophysiological Analysis of the Response of Males of the Potato Cyst Nematode, Globodera rostochiensis, to Fractions of Their Homospecific Sex Pheromone. Parasitology 1997, 115 Pt 3, 311–316. [Google Scholar] [CrossRef]
- Faghih, N.; Bhar, S.; Zhou, Y.; Dar, A.R.; Mai, K.; Bailey, L.S.; Basso, K.B.; Butcher, R.A. A Large Family of Enzymes Responsible for the Modular Architecture of Nematode Pheromones. J. Am. Chem. Soc. 2020, 142, 13645–13650. [Google Scholar] [CrossRef]
- Macosko, E.Z.; Pokala, N.; Feinberg, E.H.; Chalasani, S.H.; Butcher, R.A.; Clardy, J.; Bargmann, C.I. A Hub-and-Spoke Circuit Drives Pheromone Attraction and Social Behaviour in C. elegans. Nature 2009, 458, 1171–1175. [Google Scholar] [CrossRef]
- Hussey, R.; Stieglitz, J.; Mesgarzadeh, J.; Locke, T.T.; Zhang, Y.K.; Schroeder, F.C.; Srinivasan, S. Pheromone-Sensing Neurons Regulate Peripheral Lipid Metabolism in Caenorhabditis elegans. PLoS Genet. 2017, 13, e1006806. [Google Scholar] [CrossRef]
- Luo, J.; Portman, D.S. Sex-Specific, Pdfr-1-Dependent Modulation of Pheromone Avoidance by Food Abundance Enables Flexibility in C. elegans Foraging Behavior. Curr. Biol. 2021, 31, 4449–4461.e4. [Google Scholar] [CrossRef]
- Jang, H.; Kim, K.; Neal, S.J.; Macosko, E.; Kim, D.; Butcher, R.A.; Zeiger, D.M.; Bargmann, C.I.; Sengupta, P. Neuromodulatory State and Sex Specify Alternative Behaviors Through Antagonistic Synaptic Pathways in C. elegans. Neuron 2012, 75, 585–592. [Google Scholar] [CrossRef]
- Fagan, K.A.; Luo, J.; Lagoy, R.C.; Schroeder, F.C.; Albrecht, D.R.; Portman, D.S. A Single-Neuron Chemosensory Switch Determines the Valence of a Sexually Dimorphic Sensory Behavior. Curr. Biol. 2018, 28, 902–914.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Sanchez-Ayala, M.A.; Sternberg, P.W.; Srinivasan, J.; Schroeder, F.C. Improved Synthesis for Modular Ascarosides Uncovers Biological Activity. Org. Lett. 2017, 19, 2837–2840. [Google Scholar] [CrossRef]
- Ben Arous, J.; Laffont, S.; Chatenay, D. Molecular and Sensory Basis of a Food Related Two-State Behavior in C. elegans. PLoS ONE 2009, 4, e7584. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.S.; Brown, M.; Dobosiewicz, M.; Ishida, I.G.; Macosko, E.Z.; Zhang, X.; Butcher, R.A.; Cline, D.J.; McGrath, P.T.; Bargmann, C.I. Balancing Selection Shapes Density-Dependent Foraging Behaviour. Nature 2016, 539, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Dal Bello, M.; Perez-Escudero, A.; Schroeder, F.C.; Gore, J. Inversion of Pheromone Preference Optimizes Foraging in C. elegans. Elife 2021, 10, 58144. [Google Scholar] [CrossRef]
- Nuttley, W.M.; Atkinson-Leadbeater, K.P.; Van Der Kooy, D. Serotonin Mediates Food-Odor Associative Learning in the Nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 12449–12454. [Google Scholar] [CrossRef]
- Hong, M.; Ryu, L.; Ow, M.C.; Kim, J.; Je, A.R.; Chinta, S.; Huh, Y.H.; Lee, K.J.; Butcher, R.A.; Choi, H.; et al. Early Pheromone Experience Modifies a Synaptic Activity to Influence Adult Pheromone Responses of C. elegans. Curr. Biol. 2017, 27, 3168–3177.e3. [Google Scholar] [CrossRef]
- Manosalva, P.; Manohar, M.; von Reuss, S.H.; Chen, S.; Koch, A.; Kaplan, F.; Choe, A.; Micikas, R.J.; Wang, X.; Kogel, K.H.; et al. Conserved Nematode Signalling Molecules Elicit Plant Defenses and Pathogen Resistance. Nat. Commun. 2015, 6, 7795. [Google Scholar] [CrossRef]
- Kaplan, F.; Perret-Gentil, A.; Giurintano, J.; Stevens, G.; Erdogan, H.; Schiller, K.C.; Mirti, A.; Sampson, E.; Torres, C.; Sun, J.; et al. Conspecific and Heterospecific Pheromones Stimulate Dispersal of Entomopathogenic Nematodes During Quiescence. Sci. Rep. 2020, 10, 5738. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Alborn, H.T.; von Reuss, S.H.; Ajredini, R.; Ali, J.G.; Akyazi, F.; Stelinski, L.L.; Edison, A.S.; Schroeder, F.C.; Teal, P.E. Interspecific Nematode Signals Regulate Dispersal Behavior. PLoS ONE 2012, 7, e38735. [Google Scholar] [CrossRef]
- Hartley, C.J.; Lillis, P.E.; Owens, R.A.; Griffin, C.T. Infective Juveniles of Entomopathogenic Nematodes (Steinernema and Heterorhabditis) Secrete Ascarosides and Respond to Interspecific Dispersal Signals. J. Invertebr. Pathol. 2019, 168, 107257. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, L.; Huang, Z.; Gu, X.; Cui, Y.; Li, J.; Li, Y.; Xu, C.; Han, R. Influence of the Ascarosides on the Recovery, Yield and Dispersal of Entomopathogenic Nematodes. J. Invertebr. Pathol. 2022, 188, 107717. [Google Scholar] [CrossRef]
- Kong, X.; Huang, Z.; Gu, X.; Cui, Y.; Li, J.; Han, R.; Jin, Y.; Cao, L. Dimethyl Sulfoxide and Ascarosides Improve the Growth and Yields of Entomopathogenic Nematodes in Liquid Cultures. J. Invertebr. Pathol. 2022, 193, 107800. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Wei, Y.; Zhou, J.; Zhang, W.; Qin, P.; Chinta, S.; Kong, X.; Liu, Y.; Yu, H.; et al. Ascarosides Coordinate the Dispersal of a Plant-Parasitic Nematode with the Metamorphosis of Its Vector Beetle. Nat. Commun. 2016, 7, 12341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wickham, J.D.; Zhao, L.; Sun, J. Major Ascaroside Pheromone Component asc-C5 Influences Reproductive Plasticity Among Isolates of the Invasive Species Pinewood Nematode. Integr. Zool. 2021, 16, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wickham, J.D.; Ren, W.; Zhao, L.; Sun, J. Species Displacement Facilitated by Ascarosides Between Two Sympatric Sibling Species: A Native and Invasive Nematode. J. Pest Sci. 2020, 93, 1059–1071. [Google Scholar] [CrossRef]
- Yamada, K.; Hirotsu, T.; Matsuki, M.; Butcher, R.A.; Tomioka, M.; Ishihara, T.; Clardy, J.; Kunitomo, H.; Iino, Y. Olfactory Plasticity is Regulated by Pheromonal Signaling in Caenorhabditis elegans. Science 2010, 329, 1647–1650. [Google Scholar] [CrossRef]
- Zhou, Y.; Loeza-Cabrera, M.; Liu, Z.; Aleman-Meza, B.; Nguyen, J.K.; Jung, S.K.; Choi, Y.; Shou, Q.; Butcher, R.A.; Zhong, W. Potential Nematode Alarm Pheromone Induces Acute Avoidance in Caenorhabditis elegans. Genetics 2017, 206, 1469–1478. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.K.; Manohar, M.; Artyukhin, A.B.; Kumari, A.; Tenjo-Castano, F.J.; Nguyen, H.; Routray, P.; Choe, A.; Klessig, D.F.; et al. Nematode Signaling Molecules are Extensively Metabolized by Animals, Plants, and Microorganisms. ACS Chem. Biol. 2021, 16, 1050–1058. [Google Scholar] [CrossRef]
- Ning, S.; Zhang, L.; Ma, J.; Chen, L.; Zeng, G.; Yang, C.; Zhou, Y.; Guo, X.; Deng, X. Modular and Scalable Synthesis of Nematode Pheromone Ascarosides: Implications in Eliciting Plant Defense Response. Org. Biomol. Chem. 2020, 18, 4956–4961. [Google Scholar] [CrossRef]
- Zhao, L.; Ahmad, F.; Lu, M.; Zhang, W.; Wickham, J.D.; Sun, J. Ascarosides Promote the Prevalence of Ophiostomatoid Fungi and an Invasive Pathogenic Nematode, Bursaphelenchus xylophilus. J. Chem. Ecol. 2018, 44, 701–710. [Google Scholar] [CrossRef]
- Yang, C.T.; Vidal-Diez de Ulzurrun, G.; Goncalves, A.P.; Lin, H.C.; Chang, C.W.; Huang, T.Y.; Chen, S.A.; Lai, C.K.; Tsai, I.J.; Schroeder, F.C.; et al. Natural Diversity in the Predatory Behavior Facilitates the Establishment of a Robust Model Strain for Nematode-Trapping Fungi. Proc. Natl. Acad. Sci. USA 2020, 117, 6762–6770. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Mahanti, P.; Schroeder, F.C.; Sternberg, P.W. Nematode-Trapping Fungi Eavesdrop on Nematode Pheromones. Curr. Biol. 2013, 23, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Tian, M.; Huang, J.; Zheng, X.; Guo, Y.; Li, G.; Wang, X. Amphiphysin AoRvs167-Mediated Membrane Curvature Facilitates Trap Formation, Endocytosis, and Stress Resistance in Arthrobotrys oligospora. Pathogens 2022, 11, 997. [Google Scholar] [CrossRef]
- Kaplan, F.; Srinivasan, J.; Mahanti, P.; Ajredini, R.; Durak, O.; Nimalendran, R.; Sternberg, P.W.; Teal, P.E.; Schroeder, F.C.; Edison, A.S.; et al. Ascaroside Expression in Caenorhabditis elegans is Strongly Dependent on Diet and Developmental Stage. PLoS ONE 2011, 6, e17804. [Google Scholar] [CrossRef]
- Joo, H.J.; Kim, K.Y.; Yim, Y.H.; Jin, Y.X.; Kim, H.; Kim, M.Y.; Paik, Y.K. Contribution of the Peroxisomal Acox Gene to the Dynamic Balance of Daumone Production in Caenorhabditis elegans. J. Biol. Chem. 2010, 285, 29319–29325. [Google Scholar] [CrossRef] [PubMed]
- Butcher, R.A. Decoding Chemical Communication in Nematodes. Nat. Prod. Rep. 2017, 34, 472–477. [Google Scholar] [CrossRef]
- Hollister, K.A.; Conner, E.S.; Zhang, X.; Spell, M.; Bernard, G.M.; Patel, P.; de Carvalho, A.C.; Butcher, R.A.; Ragains, J.R. Ascaroside. Activity in Caenorhabditis elegans is Highly Dependent on Chemical Structure. Bioorg. Med. Chem. 2013, 21, 5754–5769. [Google Scholar] [CrossRef] [PubMed]
- Artyukhin, A.B.; Zhang, Y.K.; Akagi, A.E.; Panda, O.; Sternberg, P.W.; Schroeder, F.C. Metabolomic “Dark Matter” Dependent on Peroxisomal β-Oxidation in Caenorhabditis elegans. J. Am. Chem. Soc. 2018, 140, 2841–2852. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Wang, J.; Zheng, X.; Wang, X. Nematode Pheromones: Structures and Functions. Molecules 2023, 28, 2409. https://doi.org/10.3390/molecules28052409
Yang B, Wang J, Zheng X, Wang X. Nematode Pheromones: Structures and Functions. Molecules. 2023; 28(5):2409. https://doi.org/10.3390/molecules28052409
Chicago/Turabian StyleYang, Biyuan, Jie Wang, Xi Zheng, and Xin Wang. 2023. "Nematode Pheromones: Structures and Functions" Molecules 28, no. 5: 2409. https://doi.org/10.3390/molecules28052409
APA StyleYang, B., Wang, J., Zheng, X., & Wang, X. (2023). Nematode Pheromones: Structures and Functions. Molecules, 28(5), 2409. https://doi.org/10.3390/molecules28052409