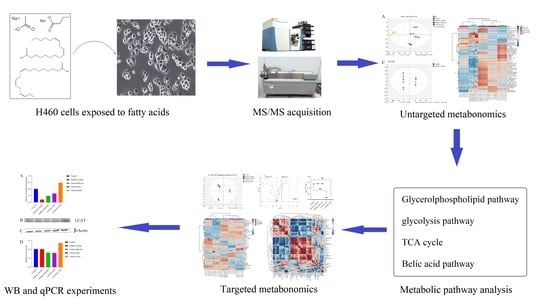
Effect of Short-Chain Fatty Acids and Polyunsaturated Fatty Acids on Metabolites in H460 Lung Cancer Cells
Abstract
1. Introduction
2. Results
2.1. CCK8 Experiment
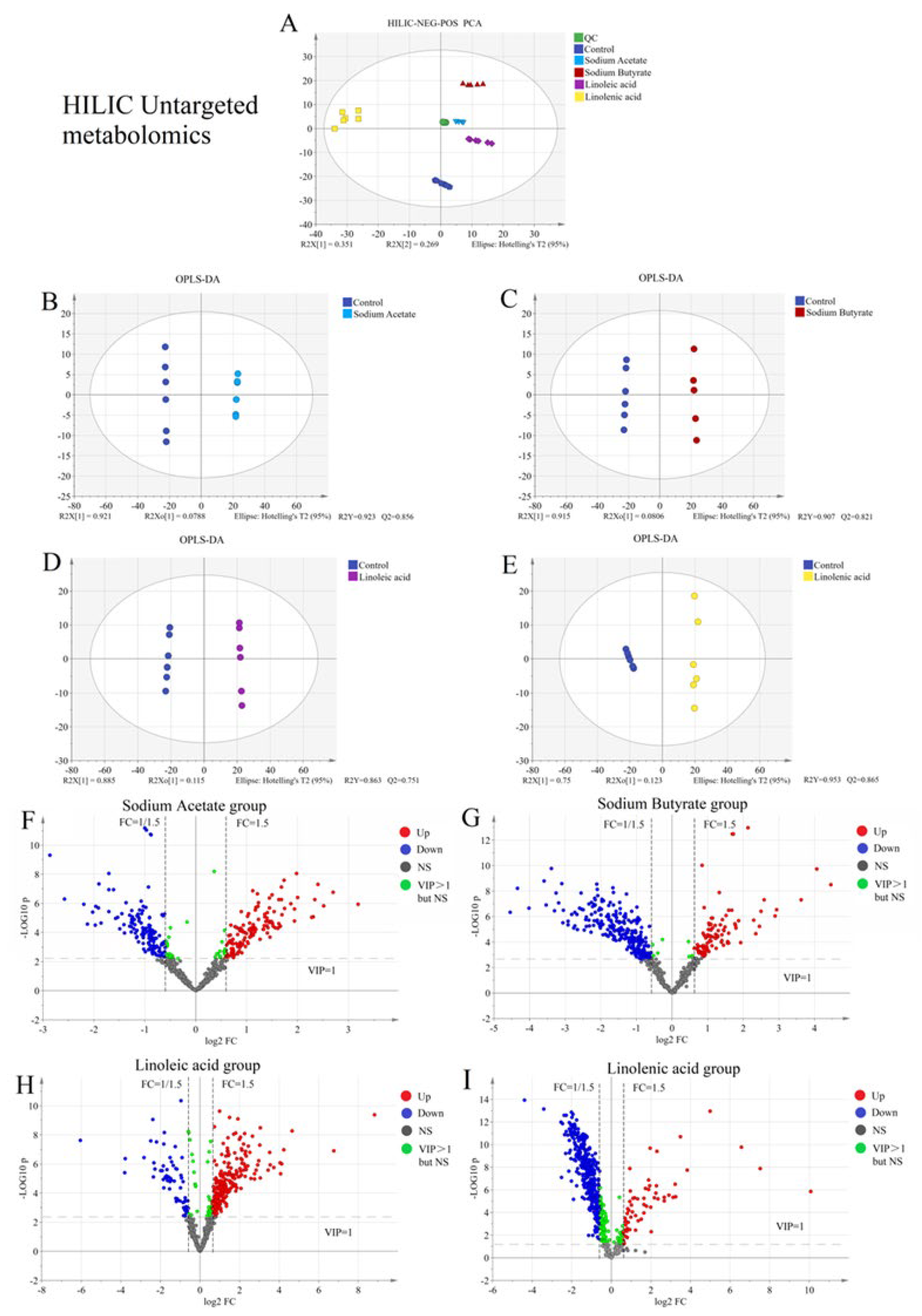
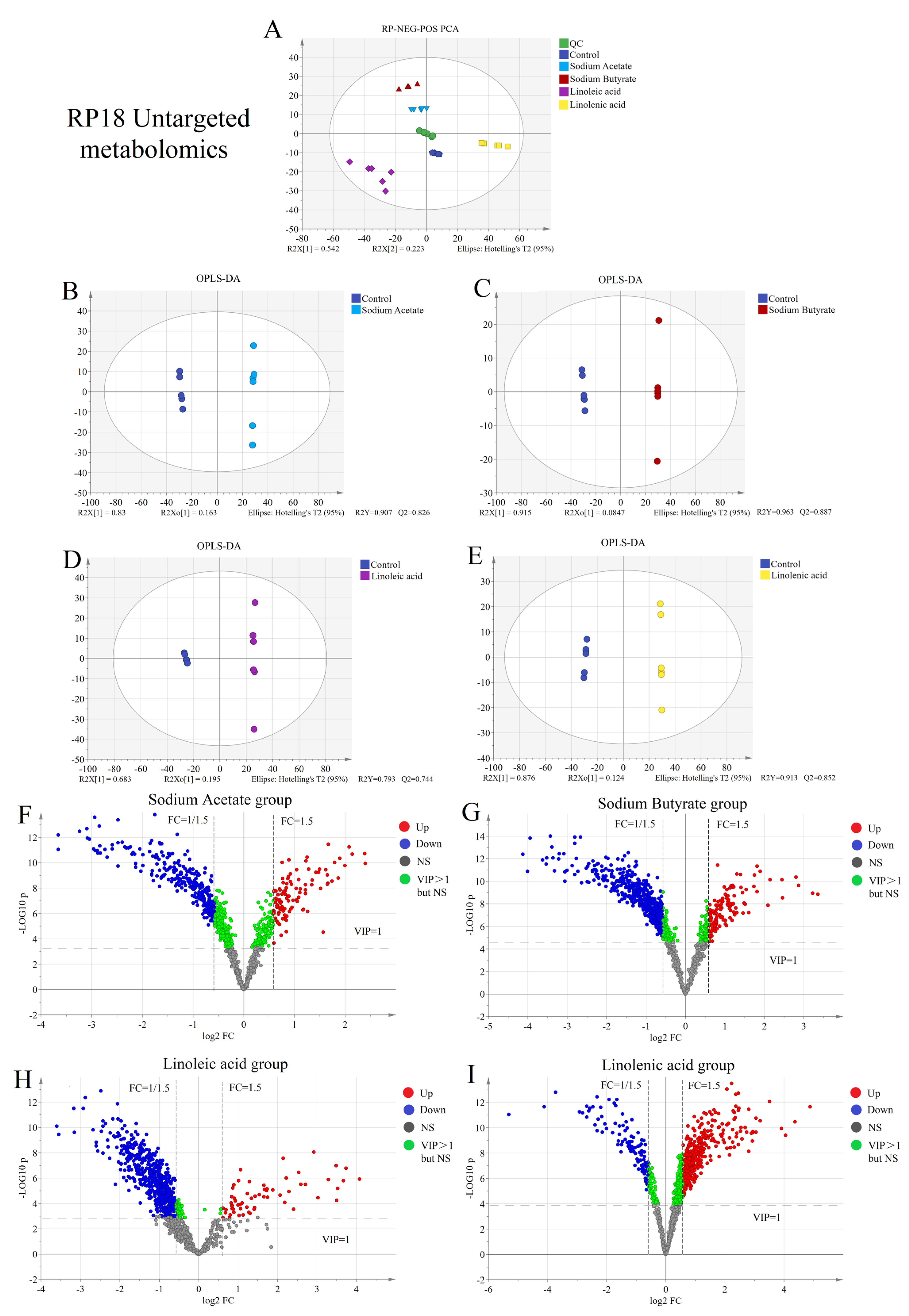
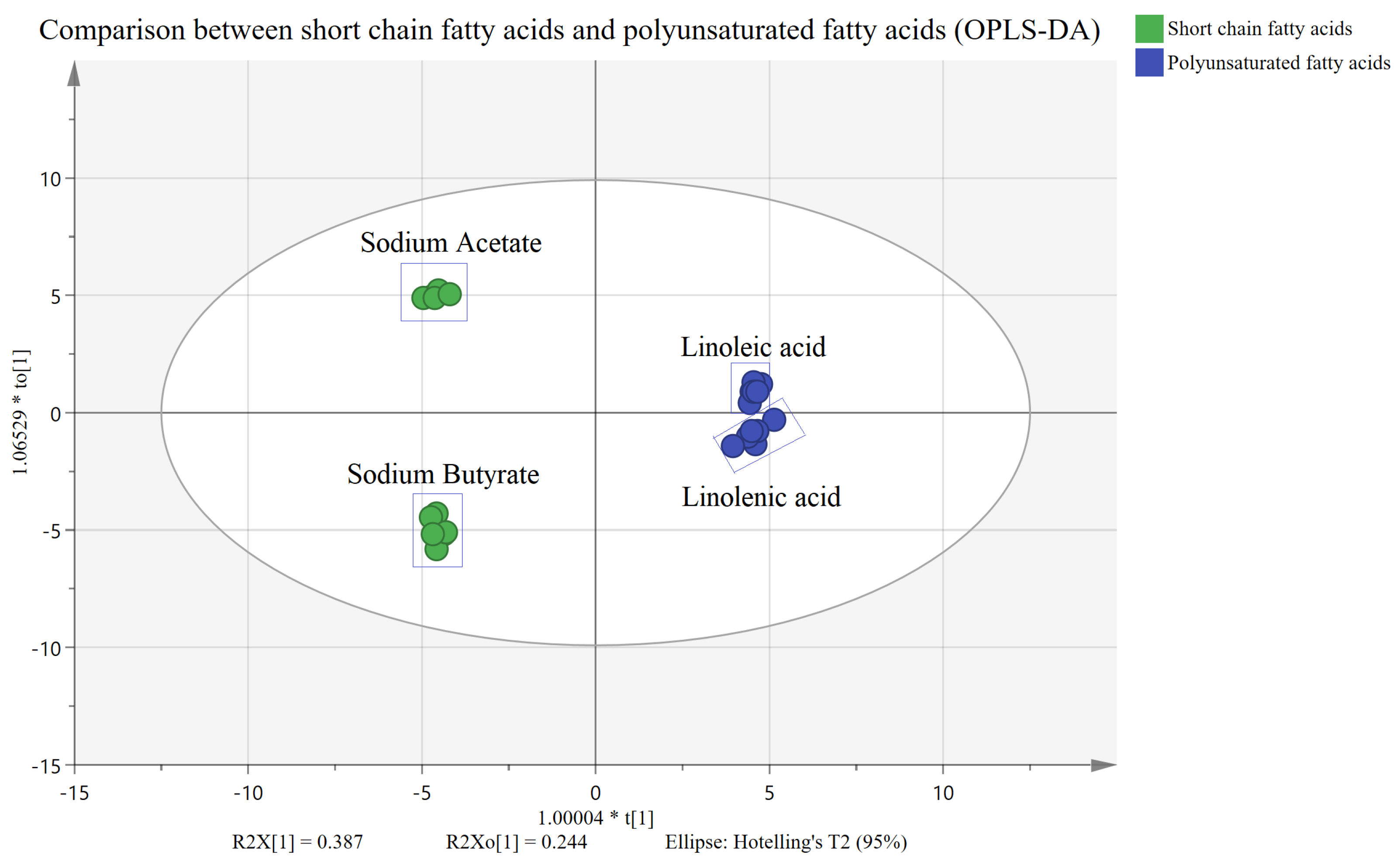
2.2. Untargeted Metabolism
2.3. Building and Optimizing the Analytical Method for Targeted Metabolome Detection
2.3.1. Pretreatment Optimization
2.3.2. LC-MS Method Optimization for Targeted Metabolism
2.3.3. Targeted Methodological Validation
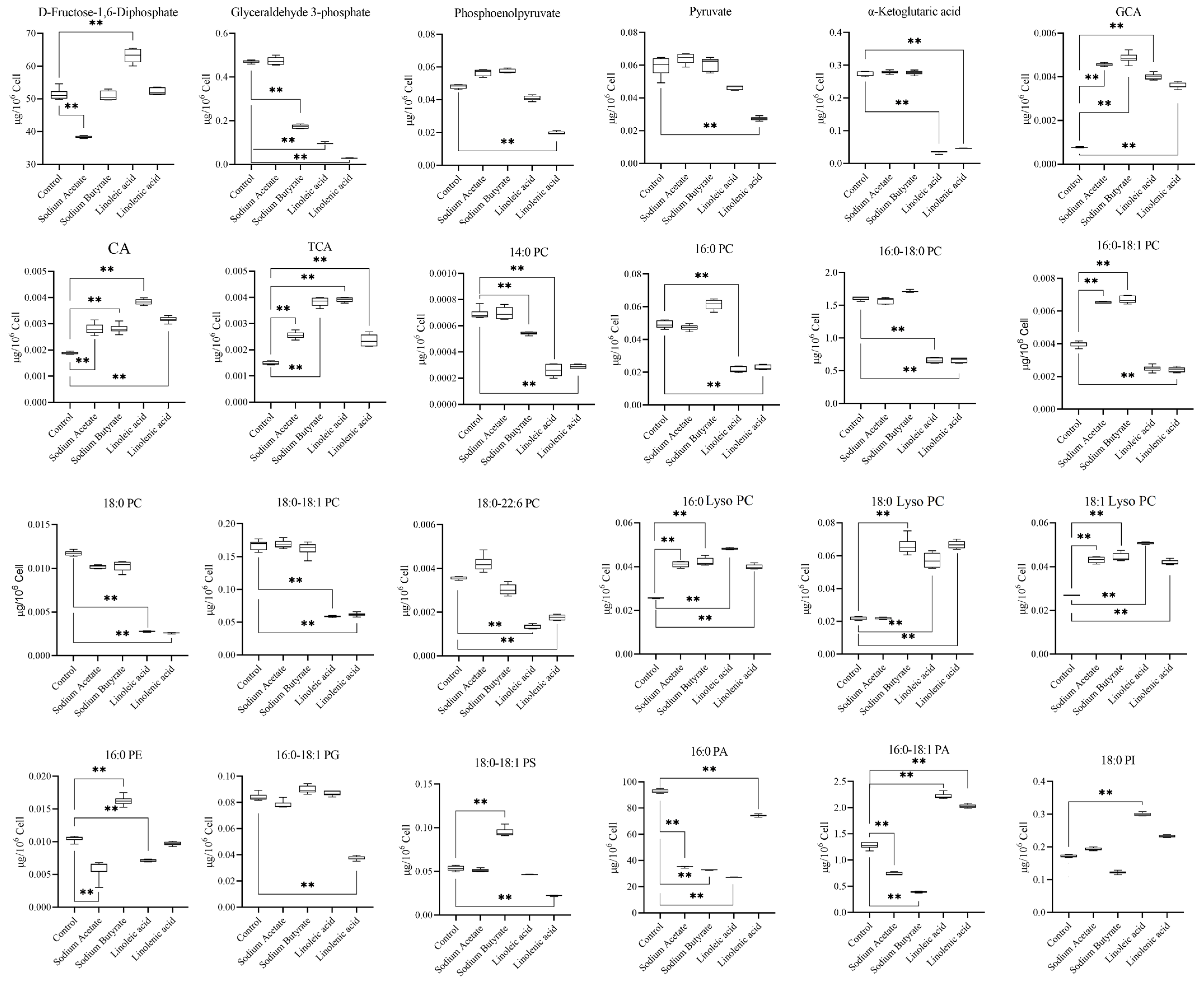
2.4. Targeted Metabolism
2.5. Effects of LCAT on Metabolome Changes and Biological Validation
2.5.1. mRNA Expression of LCAT
2.5.2. Protein Content of LCAT
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cultured Cells
4.3. CCK8 Experimental Procedure
4.4. LC-MS/MS Sample Preparation
4.5. Untargeted Metabonomics
4.6. Targeted Metabonomics
4.6.1. Calibrators and Quality Control Samples
4.6.2. Method Validation
4.6.3. LC-MS/MS Parameters for Targeted Metabonomics
4.7. PCR Experimental Procedure
4.8. WB Experimental Procedure
4.9. Statistical Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ahmed, N.; Kidane, B.; Wang, L.; Nugent, Z.; Moldovan, N.; McElrea, A.; Shariati-Ievari, S.; Qing, G.; Tan, L.; Buduhan, G.; et al. Metabolic Changes in Early-Stage Non-Small Cell Lung Cancer Patients after Surgical Resection. Cancers 2021, 13, 3012. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chiang, J.H.; Yu, C.S.; Hsia, T.C.; Wu, R.S.; Lien, J.C.; Lai, K.C.; Yu, F.S.; Chung, J.G. Antitumor effects of deguelin on H460 human lung cancer cells in vitro and in vivo: Roles of apoptotic cell death and H460 tumor xenografts model. Environ. Toxicol. 2017, 32, 84–98. [Google Scholar] [CrossRef]
- Correa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Guo, Y.; Xie, Y.; Yu, H.; Yao, W.; Cheng, Y.; Qian, H. Extraction, characterization of aloe polysaccharides and the in-depth analysis of its prebiotic effects on mice gut microbiota. Carbohydr. Polym. 2021, 261, 117874. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, S.; Huang, D.; Xiong, T.; Nie, S.; Xie, M. Polysaccharides from fermented Asparagus officinalis with Lactobacillus plantarum NCU116 alleviated liver injury via modulation of glutathione homeostasis, bile acid metabolism, and SCFA production. Food Funct. 2020, 11, 7681–7695. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Rauf, A.; Khalil, A.A.; Rahman, U.U.; Khalid, A.; Naz, S.; Shariati, M.A.; Rebezov, M.; Urtecho, E.Z.; de Albuquerque, R.D.D.G.; Anwar, S.; et al. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6034–6054. [Google Scholar] [CrossRef]
- Schwarz, A.; Philippsen, R.; Schwarz, T. Induction of Regulatory T Cells and Correction of Cytokine Disbalance by Short-Chain Fatty Acids: Implications for Psoriasis Therapy. J. Invest. Dermatol. 2021, 141, 95–104. [Google Scholar] [CrossRef]
- D’Archivio, M.; Scazzocchio, B.; Vari, R.; Santangelo, C.; Giovannini, C.; Masella, R. Recent Evidence on the Role of Dietary PUFAs in Cancer Development and Prevention. Curr. Med. Chem. 2018, 25, 1818–1836. [Google Scholar] [CrossRef]
- Luu, H.N.; Cai, H.; Murff, H.J.; Xiang, Y.B.; Cai, Q.; Li, H.; Gao, J.; Yang, G.; Lan, Q.; Gao, Y.T.; et al. A prospective study of dietary polyunsaturated fatty acids intake and lung cancer risk. Int. J. Cancer 2018, 143, 2225–2237. [Google Scholar] [CrossRef]
- Song, E.A.; Kim, H. Docosahexaenoic Acid Induces Oxidative DNA Damage and Apoptosis, and Enhances the Chemosensitivity of Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1257. [Google Scholar] [CrossRef]
- Liu, J.; Mazzone, P.J.; Cata, J.P.; Kurz, A.; Bauer, M.; Mascha, E.J.; Sessler, D.I. Serum free fatty acid biomarkers of lung cancer. Chest 2014, 146, 670–679. [Google Scholar] [CrossRef]
- Montecillo-Aguado, M.; Tirado-Rodriguez, B.; Antonio-Andres, G.; Morales-Martinez, M.; Tong, Z.; Yang, J.; Hammock, B.D.; Hernandez-Pando, R.; Huerta-Yepez, S. Omega-6 Polyunsaturated Fatty Acids Enhance Tumor Aggressiveness in Experimental Lung Cancer Model: Important Role of Oxylipins. Int. J. Mol. Sci. 2022, 23, 6179. [Google Scholar] [CrossRef]
- Yan, L.; Cao, L.M.; Zhang, X.J.; Chu, B. Metabolomes of Lewis lung carcinoma metastases and normal lung tissue from mice fed different diets. J. Nutr. Biochem. 2022, 107, 109051. [Google Scholar] [CrossRef]
- Zhang, G.; Panigrahy, D.; Mahakian, L.M.; Yang, J.; Liu, J.Y.; Stephen Lee, K.S.; Wettersten, H.I.; Ulu, A.; Hu, X.; Tam, S.; et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 6530–6535. [Google Scholar] [CrossRef]
- Jia, M.; Peng, Z.; Yang, K.; Su, C.; Wang, Y.; Yan, C. A high-throughput targeted metabolomics method for the quantification of 104 non-polar metabolites in cholesterol, eicosanoid, and phospholipid metabolism: Application in the study of a CCl4-induced liver injury mouse model. Analyst 2020, 145, 3575–3591. [Google Scholar] [CrossRef]
- Lu, H.; Guo, Y.; Yang, P. Using amino acids for probing structural information of cytochrome c by electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 1612–1615. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, J.L.; Gao, H.; Fan, L.L.; Chen, H.H.; Nie, S.P. Polysaccharide from Plantago asiatica L. attenuates hyperglycemia, hyperlipidemia and affects colon microbiota in type 2 diabetic rats. Food Hydrocoll. 2019, 86, 34–42. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, J.; Gao, H.; Li, M.; Sun, Y.; Chen, H.; Zuo, S.; Fang, Q.; Huang, X.; Yin, J.; et al. Bioactive Dietary Fibers Selectively Promote Gut Microbiota to Exert Antidiabetic Effects. J. Agric. Food Chem. 2021, 69, 7000–7015. [Google Scholar] [CrossRef]
- Wu, Q.; Fan, L.; Tan, H.; Zhang, Y.; Fang, Q.; Yang, J.; Cui, S.W.; Nie, S. Impact of pectin with various esterification degrees on the profiles of gut microbiota and serum metabolites. Appl. Microbiol. Biotechnol. 2022, 106, 3707–3720. [Google Scholar] [CrossRef]
- Yan, Y.; Zeng, M.M.; Zheng, Z.P.; He, Z.Y.; Tao, G.J.; Zhang, S.; Gao, Y.H.; Chen, J. Simultaneous analysis of PhIP, 4′-OH-PhIP, and their precursors using UHPLC-MS/MS. J. Agric. Food Chem. 2014, 62, 11628–11636. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, S.; Tao, G.J.; You, F.H.; Chen, J.; Zeng, M.M. Acetonitrile extraction coupled with UHPLC-MS/MS for the accurate quantification of 17 heterocyclic aromatic amines in meat products. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1068–1069, 173–179. [Google Scholar] [CrossRef]
- Zeng, M.; Zhang, M.; Chen, J.; He, Z.; Qin, F.; Hu, C.; Xu, H.; Tao, G.; Zhang, S.; Chen, J. UPLC-MS/MS and multivariate analysis of inhibition of heterocyclic amine profiles by black pepper and piperine in roast beef patties. Chemom. Intell. Lab. Syst. 2017, 168, 96–106. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, Z.; Guo, R.; Wang, X.; Gong, W.; Duan, J.; He, Z.; Ding, R.; Zhang, X.; Nie, S.; et al. Metabolomics reveal dynamic changes in eicosanoid profile in patients with ST-elevation myocardial infarction after percutaneous coronary intervention. Clin. Exp. Pharmacol. Physiol. 2021, 48, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; Nie, S.; Xie, M.; Li, S. MALDI mass spectrometry in food carbohydrates analysis: A review of recent researches. Food Chem. 2023, 399, 133968. [Google Scholar] [CrossRef]
- Yan, J.; Xiang, B.; Wang, D.; Tang, S.; Teng, M.; Yan, S.; Zhou, Z.; Zhu, W. Different Toxic Effects of Racemate, Enantiomers, and Metabolite of Malathion on HepG2 Cells Using High-Performance Liquid Chromatography-Quadrupole-Time-of-Flight-Based Metabolomics. J. Agric. Food Chem. 2019, 67, 1784–1794. [Google Scholar] [CrossRef]
- Lim, S.L.; Jia, Z.; Lu, Y.; Zhang, H.; Ng, C.T.; Bay, B.H.; Shen, H.M.; Ong, C.N. Metabolic signatures of four major histological types of lung cancer cells. Metabolomics 2018, 14, 118. [Google Scholar] [CrossRef]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Yin, J.; Nie, S. Multiomics Approach to Explore the Amelioration Mechanisms of Glucomannans on the Metabolic Disorder of Type 2 Diabetic Rats. J. Agric. Food Chem. 2021, 69, 2632–2645. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Sun, W.; Tseng, K.; Zhang, J.; Guo, Y. Efficient determination of enantiomeric ratios of alpha-hydroxy/amino acids from fermented milks via ion mobility-mass spectrometry. Food Chem. 2023, 400, 134092. [Google Scholar] [CrossRef]
- Bai, Z.; Huang, X.; Wu, G.; Ye, H.; Huang, W.; Nie, Q.; Chen, H.; Yin, J.; Chen, Y.; Nie, S. Polysaccharides from red kidney bean alleviating hyperglycemia and hyperlipidemia in type 2 diabetic rats via gut microbiota and lipid metabolic modulation. Food Chem. 2023, 404, 134598. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Li, R.; Yin, J.; Sun, H.; Zhu, C.; Kong, Q.; Mou, H.; Nie, S. Amelioration of hydrolyzed guar gum on high-fat diet-induced obesity: Integrated hepatic transcriptome and metabolome. Carbohydr. Polym. 2022, 297, 120051. [Google Scholar] [CrossRef]
- Mirzaei, R.; Afaghi, A.; Babakhani, S.; Sohrabi, M.R.; Hosseini-Fard, S.R.; Babolhavaeji, K.; Akbari, S.K.A.; Yousefimashouf, R.; Karampoor, S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021, 139, 18. [Google Scholar] [CrossRef]
- Gui, Q.; Li, H.; Wang, A.; Zhao, X.; Tan, Z.; Chen, L.; Xu, K.; Xiao, C. The association between gut butyrate-producing bacteria and non-small-cell lung cancer. J. Clin. Lab. Anal. 2020, 34, e23318. [Google Scholar] [CrossRef]
- Kim, K.; Kwon, O.; Ryu, T.Y.; Jung, C.R.; Kim, J.; Min, J.K.; Kim, D.S.; Son, M.Y.; Cho, H.S. Propionate of a microbiota metabolite induces cell apoptosis and cell cycle arrest in lung cancer. Mol. Med. Rep. 2019, 20, 1569–1574. [Google Scholar] [CrossRef]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.; Do, Q.N.; Doucette, S.; Burguete, D.; et al. Lactate Metabolism in Human Lung Tumors. Cell 2017, 171, 358–371. [Google Scholar] [CrossRef]
- Huang, J.; Mondul, A.M.; Weinstein, S.J.; Koutros, S.; Derkach, A.; Karoly, E.; Sampson, J.N.; Moore, S.C.; Berndt, S.I.; Albanes, D. Serum metabolomic profiling of prostate cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Br. J. Cancer 2016, 115, 1087–1095. [Google Scholar] [CrossRef]
- Liu, H.; Xu, F.; Gao, Y.; Pang, Y.; Xie, C.; Jiang, C. An Integrated LC-MS/MS Strategy for Quantifying the Oxidative-Redox Metabolome in Multiple Biological Samples. Anal. Chem. 2020, 92, 8810–8818. [Google Scholar] [CrossRef]
- Muthu, M.; Kumar, R.; Syed Khaja, A.S.; Gilthorpe, J.D.; Persson, J.L.; Nordstrom, A. GLUL Ablation Can Confer Drug Resistance to Cancer Cells via a Malate-Aspartate Shuttle-Mediated Mechanism. Cancers 2019, 11, 1945. [Google Scholar] [CrossRef]
- Nagarajan, S.R.; Butler, L.; Hoy, A. The diversity and breadth of cancer cell fatty acid metabolism. Cancer Metab. 2021, 9, 2. [Google Scholar] [CrossRef]
- Namikawa-Kanai, H.; Miyazaki, T.; Matsubara, T.; Shigefuku, S.; Ono, S.; Nakajima, E.; Morishita, Y.; Honda, A.; Furukawa, K.; Ikeda, N. Comparison of the amino acid profile between the nontumor and tumor regions in patients with lung cancer. Am. J. Cancer Res. 2020, 10, 2145–2159. [Google Scholar]
- Smolle, E.; Leko, P.; Stacher-Priehse, E.; Brcic, L.; El-Heliebi, A.; Hofmann, L.; Quehenberger, F.; Hrzenjak, A.; Popper, H.H.; Olschewski, H.; et al. Distribution and prognostic significance of gluconeogenesis and glycolysis in lung cancer. Mol. Oncol. 2020, 14, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Swennen, E.L.; Ummels, V.; Buss, I.; Jaehde, U.; Bast, A.; Dagnelie, P.C. ATP sensitizes H460 lung carcinoma cells to cisplatin-induced apoptosis. Chem. Biol. Interact. 2010, 184, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cao, L.M.; Zhang, X.J.; Chu, B. Targeting ferroptosis as a vulnerability in pulmonary diseases. Cell Death Dis. 2022, 13, 649. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Gao, H.; Wu, Z.; Chen, J.; Zhang, J.; Huang, Q. Comprehensive metabolomics insights into benzo[a]pyrene-induced metabolic reprogramming related to H460 cell invasion and migration. Sci. Total Environ. 2021, 774, 145763. [Google Scholar] [CrossRef]
- Johnson, C.H.; Santidrian, A.F.; LeBoeuf, S.E.; Kurczy, M.E.; Rattray, N.J.W.; Rattray, Z.; Warth, B.; Ritland, M.; Hoang, L.T.; Loriot, C.; et al. Metabolomics guided pathway analysis reveals link between cancer metastasis, cholesterol sulfate, and phospholipids. Cancer Metab. 2017, 5, 9. [Google Scholar] [CrossRef]
- Louis, E.; Adriaensens, P.; Guedens, W.; Vanhove, K.; Vandeurzen, K.; Darquennes, K.; Vansteenkiste, J.; Dooms, C.; de Jonge, E.; Thomeer, M.; et al. Metabolic phenotyping of human blood plasma: A powerful tool to discriminate between cancer types? Ann. Oncol. 2016, 27, 178–184. [Google Scholar] [CrossRef]
- Marien, E.; Meister, M.; Muley, T.; Fieuws, S.; Bordel, S.; Derua, R.; Spraggins, J.; Van de Plas, R.; Dehairs, J.; Wouters, J.; et al. Non-small cell lung cancer is characterized by dramatic changes in phospholipid profiles. Int. J. Cancer 2015, 137, 1539–1548. [Google Scholar] [CrossRef]
- Ni, K.; Wang, D.; Xu, H.; Mei, F.; Wu, C.; Liu, Z.; Zhou, B. miR-21 promotes non-small cell lung cancer cells growth by regulating fatty acid metabolism. Cancer Cell. Int. 2019, 19, 219. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, H.; Li, S. The role of glycometabolic plasticity in cancer. Pathol. Res. Pract. 2021, 226, 153595. [Google Scholar] [CrossRef]
- Stoica, C.; Ferreira, A.K.; Hannan, K.; Bakovic, M. Bilayer Forming Phospholipids as Targets for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 5266. [Google Scholar] [CrossRef]
- Vriens, K.; Christen, S.; Parik, S.; Broekaert, D.; Yoshinaga, K.; Talebi, A.; Dehairs, J.; Escalona-Noguero, C.; Schmieder, R.; Cornfield, T.; et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature 2019, 566, 403–406. [Google Scholar] [CrossRef]
- Zaidi, N.; Lupien, L.; Kuemmerle, N.B.; Kinlaw, W.B.; Swinnen, J.V.; Smans, K. Lipogenesis and lipolysis, the pathways exploited by the cancer cells to acquire fatty acids. Prog. Lipid Res. 2013, 52, 585–589. [Google Scholar] [CrossRef]
- Zuijdgeest-van Leeuwen, S.D.; van der Heijden, M.S.; Rietveld, T.; van den Berg, J.W.; Tilanus, H.W.; Burgers, J.A.; Wilson, J.H.; Dagnelie, P.C. Fatty acid composition of plasma lipids in patients with pancreatic, lung and oesophageal cancer in comparison with healthy subjects. Clin. Nutr. 2002, 21, 225–230. [Google Scholar] [CrossRef]
- Sun, L.; Xu, H.; Ye, J.; Gaikwad, N.W. Comparative effect of black, green, oolong, and white tea intake on weight gain and bile acid metabolism. Nutrition 2019, 65, 208–215. [Google Scholar] [CrossRef]
- Tsai, C.L.; Changchien, C.Y.; Chen, Y.; Lai, C.R.; Chen, T.M.; Chang, H.H.; Tsai, W.C.; Tsai, Y.L.; Tsai, H.C.; Lin, H.-Y.; et al. Survival Benefit of Statin with Anti-Angiogenesis Efficacy in Lung Cancer-Associated Pleural Fluid through FXR Modulation. Cancers 2022, 14, 2765. [Google Scholar] [CrossRef]
- Feister, H.A.; Auerbach, B.J.; Cole, L.A.; Krause, B.R.; Karathanasis, S.K. Identification of an IL-6 response element in the human LCAT promoter. J. Lipid Res. 2002, 43, 960–970. [Google Scholar] [CrossRef]
- Flores, R.; Jin, X.; Chang, J.; Zhang, C.; Cogan, D.G.; Schaefer, E.J.; Kruth, H.S. LCAT, ApoD, and ApoA1 Expression and Review of Cholesterol Deposition in the Cornea. Biomolecules 2019, 9, 785. [Google Scholar] [CrossRef]
- Liu, M.; Subbaiah, P. Hydrolysis and transesterification of platelet-activating factor by lecithin-cholesterol acyltransferase. Proc. Natl. Acad. Sci. USA 1994, 91, 6035–6039. [Google Scholar] [CrossRef]
- Manzini, S.; Pinna, C.; Busnelli, M.; Cinquanta, P.; Rigamonti, E.; Ganzetti, G.S.; Dellera, F.; Sala, A.; Calabresi, L.; Franceschini, G.; et al. Beta2-adrenergic activity modulates vascular tone regulation in lecithin:cholesterol acyltransferase knockout mice. Vascul. Pharmacol. 2015, 74, 114–121. [Google Scholar] [CrossRef]
- Solajic-Bozicevic, N.; Stavljenic-Rukavina, A.; Sesto, M. Lecithin-cholesterol acyltransferase activity in patients with coronary artery disease examined by coronary angiography. Clin. Investig. 1994, 72, 951–956. [Google Scholar] [CrossRef]
- Glukhova, A.; Hinkovska-Galcheva, V.; Kelly, R.; Abe, A.; Shayman, J.A.; Tesmer, J.J. Structure and function of lysosomal phospholipase A2 and lecithin:cholesterol acyltransferase. Nat. Commun. 2015, 6, 6250. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Montoya, M.; Pinzon-Cortes, J.A.; Silva-Bermudez, L.S.; Ruiz-Manco, D.; Perez-Matos, M.C.; Jimenez-Mora, M.A.; Mendivil, C.O. ApoE and apoC-III-defined HDL subtypes: A descriptive study of their lecithin cholesterol acyl transferase and cholesteryl ester transfer protein content and activity. Lipids Health Dis. 2020, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Forte, T.M.; Subbanagounder, G.; Berliner, J.A.; Blanche, P.J.; Clermont, A.O.; Jia, Z.; Oda, M.N.; Krauss, R.M.; Bielicki, J.K. Altered activities of anti-atherogenic enzymes LCAT, paraoxonase, and platelet-activating factor acetylhydrolase in atherosclerosis-susceptible mice. J. Lipid Res. 2002, 43, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Holleboom, A.G.; Daniil, G.; Fu, X.; Zhang, R.; Hovingh, G.K.; Schimmel, A.W.; Kastelein, J.J.; Stroes, E.S.; Witztum, J.L.; Hutten, B.A.; et al. Lipid oxidation in carriers of lecithin:cholesterol acyltransferase gene mutations. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 3066–3075. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Clair, R.; Subbaiah, P. Impaired function of lecithin:cholesterol acyltransferase in atherosclerosis-susceptible White Carneau pigeons: Possible effects on metabolism of oxidized phospholipids. J. Lipid Res. 1998, 39, 245–254. [Google Scholar] [CrossRef]
- Miller, K.R.; Parks, J.S. Influence of vesicle surface composition on the interfacial binding of lecithin:cholesterol acyltransferase and apolipoprotein A-I. J. Lipid Res. 1997, 38, 1094–1102. [Google Scholar] [CrossRef]
- Moore, C.E.; Alfin-Slater, R.B.; Aftergood, L. Effect of trans fatty acids on serum lecithin: Cholesterol acyltransferase in rats. J. Nutr. 1980, 110, 2284–2290. [Google Scholar] [CrossRef]
- Parks, J.S.; Huggins, K.W.; Gebre, A.K.; Burleson, E.R. Phosphatidylcholine fluidity and structure affect lecithin:cholesterol acyltransferase activity. J. Lipid Res. 2000, 41, 546–553. [Google Scholar] [CrossRef]
- Parks, J.S.; Thuren, T.Y.; Schmitt, J.D. Inhibition of lecithin:cholesterol acyltransferase activity by synthetic phosphatidylcholine species containing eicosapentaenoic acid or docosahexaenoic acid in the sn-2 position. J. Lipid Res. 1992, 33, 879–887. [Google Scholar] [CrossRef]
- Subbaiah, P.V.; Subramanian, V.S.; Liu, M. Trans unsaturated fatty acids inhibit lecithin: Cholesterol acyltransferase and alter its positional specificity. J. Lipid Res. 1998, 39, 1438–1447. [Google Scholar] [CrossRef]
- Russell, M.R.; Graham, C.; D’Amato, A.; Gentry-Maharaj, A.; Ryan, A.; Kalsi, J.K.; Ainley, C.; Whetton, A.D.; Menon, U.; Jacobs, I.; et al. A combined biomarker panel shows improved sensitivity for the early detection of ovarian cancer allowing the identification of the most aggressive type II tumours. Br. J. Cancer 2017, 117, 666–674. [Google Scholar] [CrossRef]
- Park, H.M.; Kim, H.S.; Kim, D.W.; Yoon, J.H.; Kim, B.G.; Cho, J.Y. Common plasma protein marker LCAT in aggressive human breast cancer and canine mammary tumor. BMB Rep. 2020, 53, 664–669. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Gojkovic, T.; Vladimirov, S.; Miljkovic, M.; Stefanovic, A.; Vekic, J.; Zeljkovic, D.; Trifunovic, B.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; et al. Changes in lecithin: Cholesterol acyltransferase, cholesteryl ester transfer protein and paraoxonase-1 activities in patients with colorectal cancer. Clin. Biochem. 2019, 63, 32–38. [Google Scholar] [CrossRef]
- He, D.; Liao, S.; Cai, L.; Huang, W.; Xie, X.; You, M. Integrated analysis of methylation-driven genes and pretreatment prognostic factors in patients with hepatocellular carcinoma. BMC Cancer 2021, 21, 599. [Google Scholar] [CrossRef]






| Compound | Linear Range/μg·mL−1 | LOD/μg·mL−1 | LOQ/μg·mL−1 | LQC/0.03X | MQC/0.3X | HQC/1X | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RE% | CV% | CV% | RE% | CV% | CV% | RE% | CV% | CV% | ||||
| Intra (n = 5) | Inter (n = 5) | Intra (n = 5) | Inter (n = 5) | Intra (n = 5) | Inter (n = 5) | |||||||
| Pyruvate | 0.033–2.333 | 0.011 | 0.037 | 6.46 | 12.46 | 9.59 | 12.00 | 9.52 | 12.41 | 6.42 | 2.24 | 3.82 |
| Lactic acid | 0.023–2.333 | 0.008 | 0.027 | 6.91 | 9.82 | 9.38 | 8.94 | 8.36 | 6.70 | 8.80 | 3.95 | 4.45 |
| Fumarate | 0.033–2.333 | 0.011 | 0.037 | 11.22 | 6.55 | 13.95 | 13.78 | 8.17 | 7.31 | 14.28 | 6.59 | 1.73 |
| Succinate | 0.033–2.333 | 0.011 | 0.037 | 14.76 | 7.64 | 8.53 | 11.95 | 7.37 | 7.74 | 8.31 | 2.32 | 7.94 |
| Malic acid | 0.023–2.333 | 0.008 | 0.027 | 14.06 | 7.79 | 13.19 | 6.71 | 13.43 | 13.58 | 9.60 | 2.31 | 3.08 |
| α-Ketoglutaric acid | 0.233–10 | 0.766 | 2.553 | 5.91 | 6.00 | 11.83 | 5.54 | 8.73 | 10.65 | 14.92 | 9.71 | 8.87 |
| Phosphoenolpyruvate | 0.033–2.333 | 0.011 | 0.037 | 12.21 | 9.79 | 7.72 | 10.75 | 7.02 | 9.76 | 9.63 | 0.64 | 6.71 |
| Glyceraldehyde 3-phosphate | 0.033–2.333 | 0.011 | 0.037 | 7.72 | 10.26 | 13.42 | 12.60 | 13.40 | 14.86 | 13.57 | 2.88 | 1.53 |
| Cis-Aconitate | 0.003–2.333 | 0.001 | 0.003 | 10.79 | 14.12 | 6.53 | 10.52 | 10.75 | 11.96 | 6.44 | 2.51 | 1.55 |
| Glucose | 0.033–2.333 | 0.011 | 0.037 | 12.98 | 8.92 | 6.75 | 10.44 | 6.25 | 10.88 | 13.61 | 8.07 | 0.41 |
| 2-Phosphoglyceric acid | 0.233–10 | 0.766 | 2.553 | 11.96 | 10.18 | 7.51 | 11.66 | 14.33 | 14.79 | 14.00 | 4.78 | 8.91 |
| 3-Phosphoglyceric acid | 0.033–2.333 | 0.011 | 0.037 | 10.67 | 10.50 | 13.10 | 12.60 | 12.15 | 9.95 | 14.23 | 2.72 | 0.63 |
| Citric acid | 0.033–2.333 | 0.011 | 0.037 | 9.20 | 13.83 | 5.36 | 8.01 | 6.23 | 8.57 | 10.36 | 7.97 | 0.20 |
| Isocitric acid | 0.033–2.333 | 0.011 | 0.037 | 11.03 | 7.48 | 14.66 | 8.28 | 6.57 | 8.85 | 13.81 | 8.85 | 0.94 |
| Glucose 6-Phosphate | 3.333–233.333 | 1.111 | 3.703 | 12.41 | 5.36 | 8.14 | 5.90 | 10.87 | 13.68 | 9.89 | 5.94 | 3.90 |
| D-Fructose-1,6-Diphosphate | 3.333–233.333 | 1.111 | 3.703 | 14.18 | 5.45 | 6.36 | 11.59 | 13.39 | 6.30 | 12.45 | 7.83 | 2.48 |
| AMP | 0.033–2.333 | 0.011 | 0.037 | 7.78 | 8.58 | 12.01 | 13.97 | 13.69 | 13.60 | 6.05 | 5.77 | 0.96 |
| ADP | 0.233–23.333 | 0.766 | 2.553 | 13.92 | 10.69 | 5.54 | 9.74 | 7.41 | 6.70 | 11.02 | 7.82 | 8.63 |
| ATP | 0.233–23.333 | 0.766 | 2.553 | 14.79 | 13.98 | 11.47 | 5.89 | 8.70 | 6.63 | 9.78 | 0.40 | 5.44 |
| NAD | 0.01–2.333 | 0.003 | 0.010 | 8.94 | 14.50 | 12.18 | 10.11 | 8.16 | 11.40 | 6.01 | 3.90 | 7.53 |
| NADH | 0.233–23.333 | 0.766 | 2.553 | 13.19 | 8.10 | 14.24 | 11.14 | 13.03 | 9.51 | 7.12 | 5.81 | 7.10 |
| FAD | 0.01–2.333 | 0.003 | 0.010 | 7.02 | 11.39 | 6.82 | 10.75 | 14.54 | 5.75 | 11.37 | 3.57 | 5.28 |
| Acetyl CoA | 1–33.333 | 0.333 | 1.110 | 10.91 | 5.47 | 13.19 | 12.80 | 7.78 | 14.88 | 14.30 | 4.76 | 2.22 |
| TCA | 0.005–0.35 | 0.005 | 0.017 | 2.40 | 3.29 | 2.84 | 3.18 | 1.37 | 0.67 | 3.69 | 2.58 | 2.43 |
| GCA | 0.005–0.35 | 0.005 | 0.017 | 4.04 | 3.26 | 4.33 | 4.77 | 3.69 | 1.67 | 2.34 | 3.79 | 1.13 |
| CA | 0.005–0.35 | 0.005 | 0.017 | 5.46 | 3.37 | 0.64 | 5.20 | 4.53 | 2.91 | 3.08 | 3.55 | 1.38 |
| TCDCA | 0.005–0.35 | 0.005 | 0.017 | 3.02 | 2.66 | 4.54 | 4.64 | 2.28 | 1.45 | 3.78 | 5.07 | 5.33 |
| GCDCA | 0.005–0.35 | 0.005 | 0.017 | 1.79 | 4.75 | 2.60 | 1.61 | 1.63 | 0.56 | 0.86 | 0.64 | 1.04 |
| TDCA | 0.005–0.35 | 0.005 | 0.017 | 1.15 | 3.43 | 2.79 | 1.11 | 1.07 | 3.60 | 4.68 | 1.01 | 2.81 |
| CDCA | 0.005–0.35 | 0.005 | 0.017 | 3.85 | 1.06 | 2.54 | 3.39 | 4.94 | 3.77 | 2.50 | 3.58 | 1.50 |
| GDCA | 0.005–0.35 | 0.005 | 0.017 | 1.99 | 1.99 | 1.27 | 5.33 | 5.40 | 0.99 | 5.26 | 4.20 | 3.35 |
| DCA | 0.005–0.35 | 0.005 | 0.017 | 4.77 | 2.47 | 1.30 | 2.05 | 0.59 | 1.73 | 3.73 | 3.07 | 1.47 |
| TLCA | 0.005–0.35 | 0.005 | 0.017 | 4.69 | 2.03 | 4.88 | 3.69 | 1.84 | 0.95 | 5.17 | 0.83 | 5.49 |
| GLCA | 0.005–0.35 | 0.005 | 0.017 | 5.36 | 5.38 | 2.94 | 3.77 | 3.85 | 3.30 | 3.02 | 4.27 | 5.47 |
| LCA | 0.005–0.35 | 0.005 | 0.017 | 3.75 | 0.51 | 1.12 | 3.00 | 4.22 | 2.50 | 5.48 | 2.52 | 5.06 |
| TUDCA | 0.005–0.35 | 0.005 | 0.017 | 1.49 | 4.08 | 3.85 | 0.73 | 4.01 | 4.04 | 4.26 | 4.06 | 4.85 |
| GUDCA | 0.005–0.35 | 0.005 | 0.017 | 0.99 | 3.65 | 4.45 | 0.83 | 3.61 | 1.53 | 5.18 | 1.29 | 1.37 |
| UDCA | 0.005–0.35 | 0.005 | 0.017 | 1.64 | 2.68 | 4.11 | 1.06 | 1.15 | 1.06 | 5.05 | 3.43 | 3.77 |
| 14:0 PC | 0.03–0.7 | 0.03 | 0.100 | 4.79 | 3.27 | 0.51 | 1.00 | 1.10 | 1.40 | 4.87 | 2.01 | 3.44 |
| 16:0 PC | 0.01–0.7 | 0.01 | 0.033 | 0.79 | 1.05 | 0.59 | 0.97 | 5.04 | 5.18 | 2.67 | 5.33 | 1.05 |
| 16:0–18:0 PC | 0.01–0.7 | 0.01 | 0.033 | 2.04 | 3.43 | 2.67 | 4.19 | 2.79 | 4.10 | 3.63 | 1.74 | 1.16 |
| 16:0–18:1 PC | 0.01–0.7 | 0.01 | 0.033 | 2.80 | 5.34 | 0.69 | 0.86 | 2.64 | 0.65 | 4.80 | 1.95 | 5.03 |
| 16:0–22:4 PC | 0.01–0.7 | 0.01 | 0.033 | 3.05 | 2.03 | 0.83 | 0.65 | 4.62 | 3.32 | 4.73 | 2.65 | 5.46 |
| 18:0 PC | 0.01–0.7 | 0.01 | 0.033 | 3.16 | 4.16 | 1.67 | 2.44 | 1.19 | 5.49 | 1.53 | 2.97 | 2.68 |
| 18:0–18:1 PC | 0.01–0.7 | 0.01 | 0.033 | 1.28 | 0.73 | 2.46 | 1.55 | 1.66 | 4.53 | 0.62 | 4.82 | 0.64 |
| 18:0–22:6 PC | 0.01–0.7 | 0.01 | 0.033 | 0.90 | 4.53 | 0.62 | 1.93 | 4.28 | 3.07 | 5.26 | 4.39 | 2.50 |
| 20:0 PC | 0.01–0.7 | 0.01 | 0.033 | 3.34 | 2.35 | 3.73 | 4.81 | 2.34 | 2.81 | 3.79 | 1.75 | 2.95 |
| 16:0 Lyso PC | 0.01–0.7 | 0.01 | 0.033 | 1.47 | 2.12 | 0.98 | 1.03 | 2.77 | 4.30 | 0.70 | 1.97 | 2.85 |
| 18:1 Lyso PC | 0.01–0.7 | 0.01 | 0.033 | 5.44 | 2.38 | 1.03 | 5.00 | 3.82 | 1.93 | 1.65 | 4.12 | 2.59 |
| 18:0 Lyso PC | 0.03–0.7 | 0.03 | 0.100 | 1.70 | 4.89 | 5.33 | 5.11 | 1.42 | 2.17 | 0.76 | 3.82 | 4.23 |
| 20:0 Lyso PC | 0.01–0.7 | 0.01 | 0.033 | 2.85 | 3.71 | 2.24 | 2.12 | 4.26 | 3.73 | 2.73 | 1.89 | 1.53 |
| 16:0 PA | 0.006–0.42 | 0.006 | 0.020 | 2.62 | 3.07 | 2.40 | 1.61 | 3.92 | 4.05 | 1.49 | 2.56 | 3.14 |
| 16:0–18:1 PA | 0.006–0.42 | 0.006 | 0.020 | 1.83 | 4.01 | 3.78 | 2.28 | 4.24 | 3.62 | 1.31 | 2.04 | 2.30 |
| 16:0 Lyso PA | 0.006–0.42 | 0.006 | 0.020 | 1.34 | 2.99 | 4.01 | 1.54 | 3.29 | 3.24 | 4.72 | 4.65 | 1.82 |
| 16:0 PS | 0.006–0.42 | 0.006 | 0.020 | 2.56 | 4.85 | 1.55 | 3.62 | 3.29 | 1.22 | 1.79 | 4.66 | 1.53 |
| 18:0–18:1 PS | 0.006–0.42 | 0.006 | 0.020 | 3.97 | 3.05 | 2.16 | 3.09 | 4.92 | 1.31 | 2.17 | 3.29 | 1.53 |
| 18:0 Lyso PS | 0.006–0.42 | 0.006 | 0.020 | 1.44 | 3.76 | 4.75 | 3.23 | 1.17 | 4.45 | 4.05 | 2.29 | 3.25 |
| 16:0 PI | 0.02–0.14 | 0.02 | 0.067 | 1.02 | 4.20 | 4.59 | 4.72 | 4.32 | 1.44 | 2.54 | 3.54 | 1.93 |
| 18:0 PI | 0.02–0.14 | 0.02 | 0.067 | 3.52 | 4.26 | 3.32 | 4.37 | 1.65 | 4.23 | 1.92 | 3.65 | 3.74 |
| 16:0–18:1 PI | 0.014–0.14 | 0.014 | 0.047 | 1.52 | 4.66 | 1.69 | 3.99 | 1.73 | 4.10 | 4.72 | 3.02 | 3.25 |
| 18:0–20:4 PI | 0.006–0.14 | 0.006 | 0.020 | 1.57 | 1.31 | 4.55 | 1.37 | 4.53 | 1.24 | 3.70 | 2.81 | 1.20 |
| 18:0 Lyso PI | 0.006–0.14 | 0.006 | 0.020 | 3.11 | 2.49 | 2.05 | 3.23 | 2.53 | 2.85 | 2.21 | 2.11 | 2.21 |
| 14:0 PG | 0.025–0.35 | 0.025 | 0.083 | 1.57 | 2.22 | 3.29 | 3.80 | 2.46 | 4.67 | 2.07 | 4.84 | 4.34 |
| 16:0–18:1 PG | 0.005–0.35 | 0.005 | 0.017 | 3.50 | 2.59 | 1.85 | 3.52 | 2.64 | 3.78 | 3.75 | 3.79 | 2.15 |
| 18:0 PG | 0.005–0.35 | 0.005 | 0.017 | 2.55 | 1.10 | 4.61 | 4.81 | 2.24 | 1.27 | 2.43 | 1.29 | 4.88 |
| 14:0 PE | 0.005–0.35 | 0.005 | 0.017 | 1.85 | 1.02 | 1.19 | 2.53 | 1.52 | 2.77 | 1.31 | 4.38 | 4.68 |
| 16:0 PE | 0.005–0.35 | 0.005 | 0.017 | 4.69 | 2.95 | 4.99 | 1.13 | 4.04 | 3.96 | 3.76 | 2.68 | 4.27 |
| 18:0 PE | 0.005–0.35 | 0.005 | 0.017 | 3.51 | 2.85 | 1.03 | 2.24 | 3.22 | 1.29 | 1.15 | 4.49 | 4.64 |
| 16:0 Lyso PE | 0.005–0.35 | 0.005 | 0.017 | 3.52 | 2.86 | 3.25 | 1.13 | 2.19 | 1.53 | 2.34 | 3.61 | 2.22 |
| 18:0 Lyso PE | 0.005–0.35 | 0.005 | 0.017 | 3.60 | 1.14 | 3.51 | 1.63 | 3.74 | 4.70 | 4.13 | 4.71 | 2.20 |
| IS | MQC (n = 6) | |
|---|---|---|
| Recovery Rate (% ± SD) | Matrix Effect (% ± SD) | |
| 13C-GCA | 95.2 ± 2.9 | 101.2 ± 2.1 |
| 17:0 Lyso PC | 85.2 ± 4.5 | 93.4 ± 5.8 |
| 17:0 Lyso PA | 81.9 ± 3.6 | 89.4 ± 6.9 |
| 17:1 Lyso PS | 84.4 ± 8.6 | 95.8 ± 8.1 |
| 17:1 Lyso PI | 88.4 ± 1.9 | 102.7 ± 2.2 |
| 15:0 PG | 85.6 ± 3.3 | 87.6 ± 2.1 |
| 17:1 Lyso PE | 94.6 ± 4.5 | 93.5 ± 3.4 |
| 13C-AMP | 86.6 ± 2.9 | 94.8 ± 2.8 |
| d4-Succinic acid | 81.9 ± 5.2 | 89.6 ± 4.3 |
| IS | Cell (n = 6) | |
|---|---|---|
| Short-Term Stability (% ± SD) | Long-Term Stability (% ± SD) | |
| 13C-GCA | 98.2 ± 2.2 | 99.1 ± 3.8 |
| 17:0 Lyso PC | 88.9 ± 10.9 | 100.5 ± 9.8 |
| 17:0 Lyso PA | 86.2 ± 8.2 | 100.4 ± 7.1 |
| 17:1 Lyso PS | 90.8 ± 3.2 | 91.1 ± 7.9 |
| 17:1 Lyso PI | 88.9 ± 3.2 | 96.4 ± 1.9 |
| 15:0 PG | 83.6 ± 9.8 | 91.6 ± 1.7 |
| 17:1 Lyso PE | 95.7 ± 0.9 | 91.1 ± 0.3 |
| 13C-AMP | 88.7 ± 5.6 | 92.7 ± 2.1 |
| d4-Succinic acid | 91.8 ± 1.4 | 85.9 ± 0.8 |
| Short-term stability (% ± SD): 4 °C 24 h | ||
| Long-term stability (% ± SD): −20 °C 2 months | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Yang, K.; Huang, J.; Fu, W.; Yan, C.; Wang, Y. Effect of Short-Chain Fatty Acids and Polyunsaturated Fatty Acids on Metabolites in H460 Lung Cancer Cells. Molecules 2023, 28, 2357. https://doi.org/10.3390/molecules28052357
Zhou T, Yang K, Huang J, Fu W, Yan C, Wang Y. Effect of Short-Chain Fatty Acids and Polyunsaturated Fatty Acids on Metabolites in H460 Lung Cancer Cells. Molecules. 2023; 28(5):2357. https://doi.org/10.3390/molecules28052357
Chicago/Turabian StyleZhou, Tianxiao, Kaige Yang, Jin Huang, Wenchang Fu, Chao Yan, and Yan Wang. 2023. "Effect of Short-Chain Fatty Acids and Polyunsaturated Fatty Acids on Metabolites in H460 Lung Cancer Cells" Molecules 28, no. 5: 2357. https://doi.org/10.3390/molecules28052357
APA StyleZhou, T., Yang, K., Huang, J., Fu, W., Yan, C., & Wang, Y. (2023). Effect of Short-Chain Fatty Acids and Polyunsaturated Fatty Acids on Metabolites in H460 Lung Cancer Cells. Molecules, 28(5), 2357. https://doi.org/10.3390/molecules28052357