Triterpene Derivatives as Potential Inhibitors of the RBD Spike Protein from SARS-CoV-2: An In Silico Approach
Abstract
1. Introduction
2. Results and Discussion
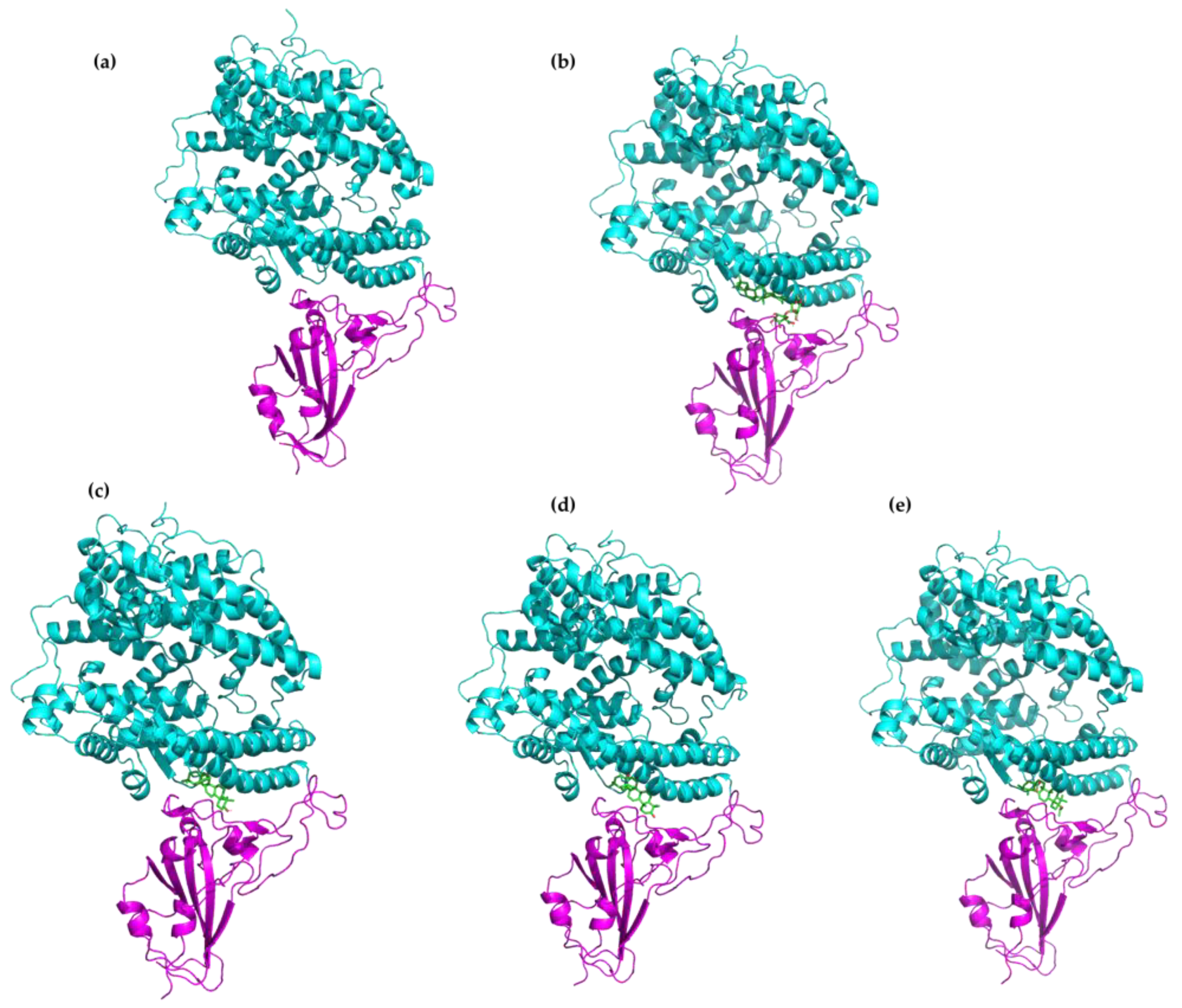
2.1. Molecular Docking Modeling
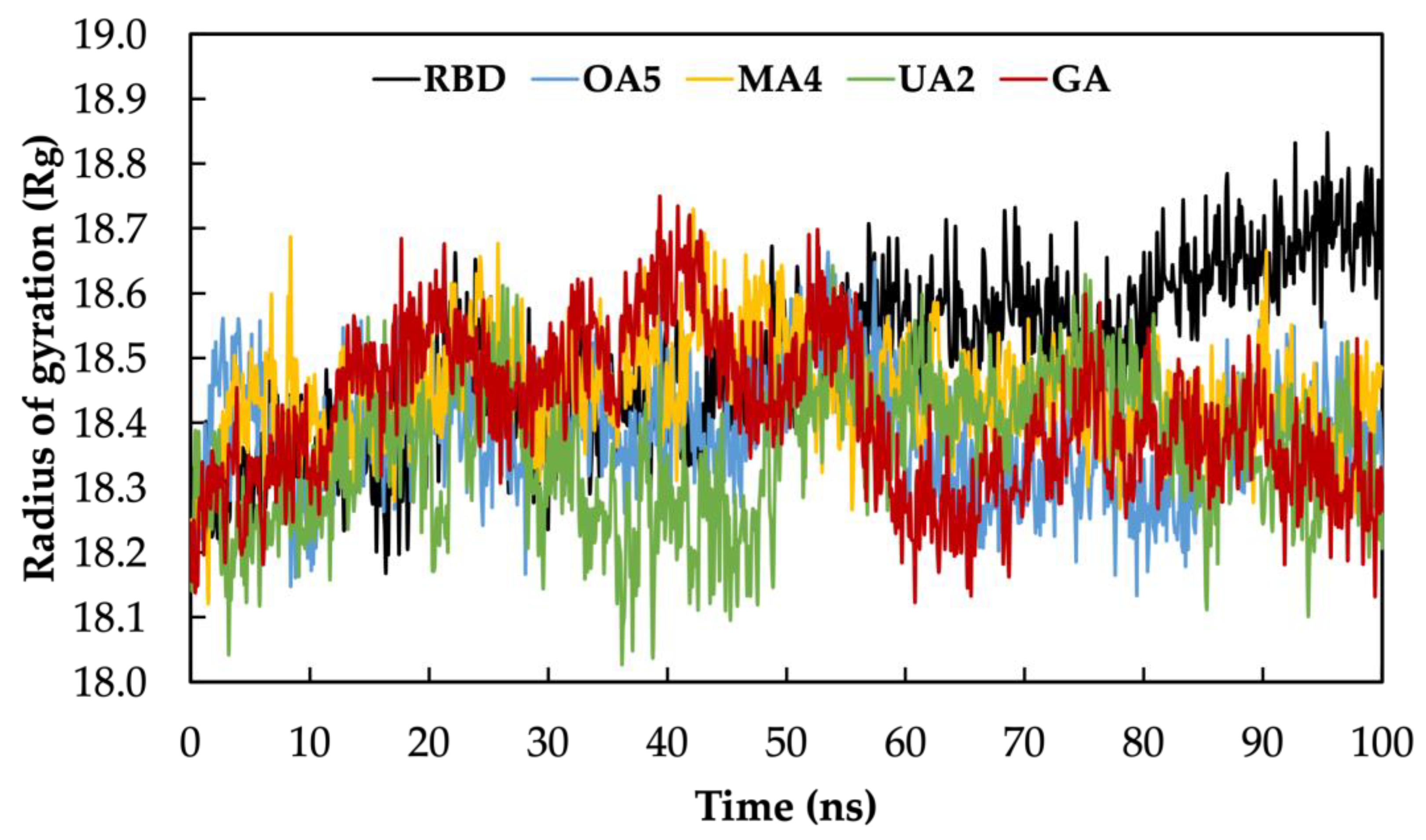
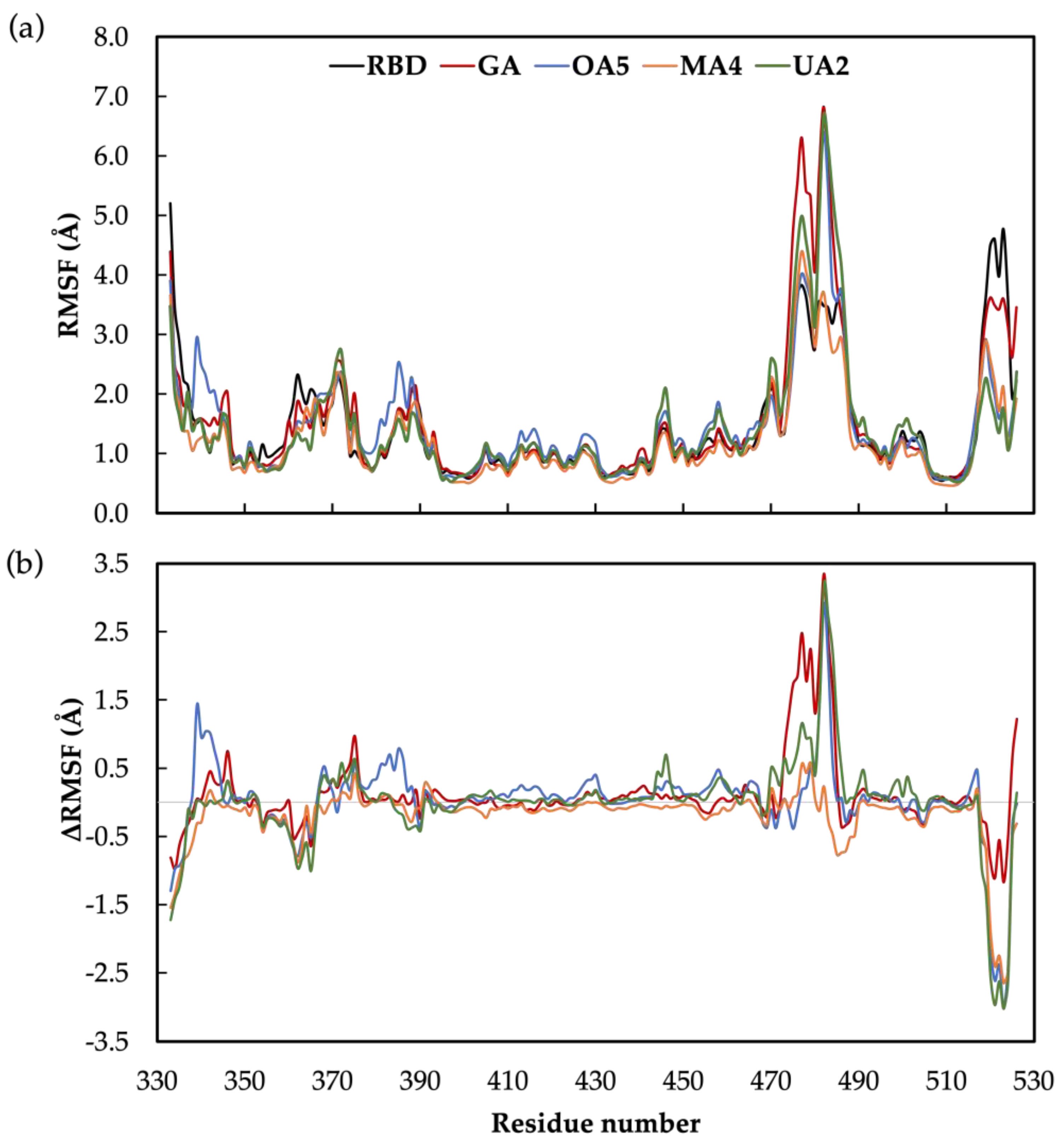
2.2. Molecular Dynamics Simulations
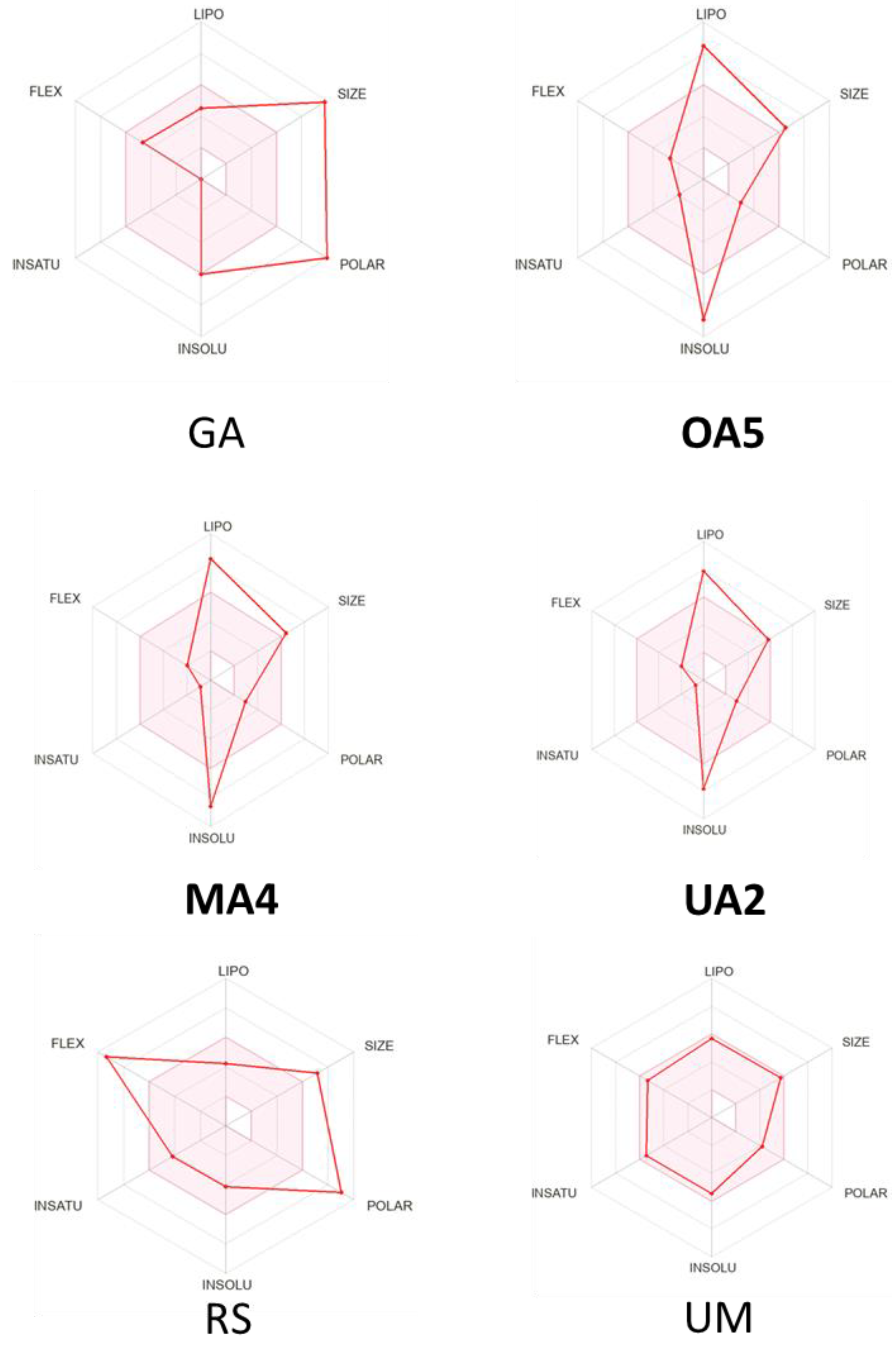
2.3. Physychochemical and Pharmacokinetic Properties
3. Materials and Methods
3.1. Triterpene Derivatives DFT Calculations
3.2. Protein Model Preparation
3.3. Molecular Docking Calculations
3.4. Molecular Dynamics Simulation
3.5. In Silico Prediction of Physicochemical and Pharmacokinetic Properties of Triterpene Acid Derivatives
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Robinson, P.C.; Liew, D.F.L.; Tanner, H.L.; Grainger, J.R.; Dwek, R.A.; Reisler, R.B.; Steinman, L.; Feldmann, M.; Ho, L.-P.; Hussell, T. COVID-19 Therapeutics: Challenges and Directions for the Future. Proc. Natl. Acad. Sci. USA 2022, 119, e2119893119. [Google Scholar] [CrossRef] [PubMed]
- Madison, A.A.; Shrout, M.R.; Renna, M.E.; Kiecolt-Glaser, J.K. Psychological and Behavioral Predictors of Vaccine Efficacy: Considerations for COVID-19. Perspect. Psychol. Sci. 2021, 16, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Chavarot, N.; Morel, A.; Leruez-Ville, M.; Vilain, E.; Divard, G.; Burger, C.; Serris, A.; Sberro-Soussan, R.; Martinez, F.; Amrouche, L. Weak Antibody Response to Three Doses of MRNA Vaccine in Kidney Transplant Recipients Treated with Belatacept. Am. J. Transplant. 2021, 21, 4043–4051. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Montez-Rath, M.E.; Han, J.; Garcia, P.; Cadden, L.; Hunsader, P.; Kerschmann, R.; Beyer, P.; Dittrich, M.; Block, G.A. Antibody Response to COVID-19 Vaccination in Patients Receiving Dialysis. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, S.D.; Goldwater, M.-S.; Jew, S.; Bujarski, S.; Regidor, B.; Daniely, D.; Chen, H.; Xu, N.; Li, M.; Green, T. Response to MRNA Vaccination for COVID-19 among Patients with Multiple Myeloma. Leukemia 2021, 35, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Lazniewski, M.; Dermawan, D.; Hidayat, S.; Muchtaridi, M.; Dawson, W.K.; Plewczynski, D. Drug Repurposing for Identification of Potential Spike Inhibitors for SARS-CoV-2 Using Molecular Docking and Molecular Dynamics Simulations. Methods 2022, 203, 498–510. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Zhang, J.; Shao, B.; Gong, H.; Wang, Y.; He, X.; Liu, S.; Liu, T. Exploring the Regulatory Function of the N-terminal Domain of SARS-CoV-2 Spike Protein through Molecular Dynamics Simulation. Adv. Theory Simul. 2021, 4, 2100152. [Google Scholar] [CrossRef]
- da Costa, C.H.S.; de Freitas, C.A.B.; Alves, C.N.; Lameira, J. Assessment of Mutations on RBD in the Spike Protein of SARS-CoV-2 Alpha, Delta and Omicron Variants. Sci. Rep. 2022, 12, 8540. [Google Scholar] [CrossRef]
- Awad, I.E.; Abu-Saleh, A.A.-A.A.; Sharma, S.; Yadav, A.; Poirier, R.A. High-Throughput Virtual Screening of Drug Databanks for Potential Inhibitors of SARS-CoV-2 Spike Glycoprotein. J. Biomol. Struct. Dyn. 2022, 40, 2099–2112. [Google Scholar] [CrossRef]
- Uddin, M.B.; Sajib, E.H.; Hoque, S.F.; Bappy, M.N.I.; Elahi, F.; Ghosh, A.; Muhit, S.; Hassan, M.M.; Hasan, M.; Chelliah, R. Genomic Diversity and Molecular Dynamics Interaction on Mutational Variances among RB Domains of SARS-CoV-2 Interplay Drug Inactivation. Infect. Genet. Evol. 2022, 97, 105128. [Google Scholar] [CrossRef]
- Brindani, N.; Munafò, F.; Menichetti, A.; Donati, E.; Nigro, M.; Ottonello, G.; Armirotti, A.; de Vivo, M. Design, Synthesis, Docking, and Biochemical Characterization of Non-Nucleoside SARS-CoV-2 RdRp Inhibitors. Bioorg. Med. Chem. 2023, 80, 117179. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bhardwaj, V.K.; Sharma, J.; Kumar, D.; Purohit, R. Identification of Potential Plant Bioactive as SARS-CoV-2 Spike Protein and Human ACE2 Fusion Inhibitors. Comput. Biol. Med. 2021, 136, 104631. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.P.; Schultz, K.J.; Wilson, J.W.; Kruel, A.; Varikoti, R.A.; Kombala, C.J.; Kneller, D.W.; Galanie, S.; Phillips, G.; Zhang, Q. AI-Accelerated Design of Targeted Covalent Inhibitors for SARS-CoV-2. J. Chem. Inf. Model. 2023. [Google Scholar] [CrossRef] [PubMed]
- Muratov, E.N.; Amaro, R.; Andrade, C.H.; Brown, N.; Ekins, S.; Fourches, D.; Isayev, O.; Kozakov, D.; Medina-Franco, J.L.; Merz, K.M. A Critical Overview of Computational Approaches Employed for COVID-19 Drug Discovery. Chem. Soc. Rev. 2021, 50, 9121–9151. [Google Scholar] [CrossRef]
- Ren, P.; Shang, W.; Yin, W.; Ge, H.; Wang, L.; Zhang, X.; Li, B.; Li, H.; Xu, Y.; Xu, E.H. A Multi-Targeting Drug Design Strategy for Identifying Potent Anti-SARS-CoV-2 Inhibitors. Acta Pharmacol. Sin. 2022, 43, 483–493. [Google Scholar] [CrossRef]
- Acharya, A.; Agarwal, R.; Baker, M.B.; Baudry, J.; Bhowmik, D.; Boehm, S.; Byler, K.G.; Chen, S.Y.; Coates, L.; Cooper, C.J. Supercomputer-Based Ensemble Docking Drug Discovery Pipeline with Application to COVID-19. J. Chem. Inf. Model. 2020, 60, 5832–5852. [Google Scholar] [CrossRef]
- Borgio, J.F.; Alsuwat, H.S.; al Otaibi, W.M.; Ibrahim, A.M.; Almandil, N.; al Asoom, L.I.; Salahuddin, M.; Kamaraj, B.; AbdulAzeez, S. State-of-the-Art Tools Unveil Potent Drug Targets amongst Clinically Approved Drugs to Inhibit Helicase in SARS-CoV-2. Arch. Med. Sci. 2020, 16, 508–518. [Google Scholar] [CrossRef]
- Singh, R.; Bhardwaj, V.K.; Purohit, R. Inhibition of Nonstructural Protein 15 of SARS-CoV-2 by Golden Spice: A Computational Insight. Cell Biochem. Funct. 2022, 40, 926–934. [Google Scholar] [CrossRef]
- Singh, R.; Bhardwaj, V.K.; Das, P.; Bhattacherjee, D.; Zyryanov, G.V.; Purohit, R. Benchmarking the Ability of Novel Compounds to Inhibit SARS-CoV-2 Main Protease Using Steered Molecular Dynamics Simulations. Comput. Biol. Med. 2022, 146, 105572. [Google Scholar] [CrossRef]
- Singh, R.; Bhardwaj, V.K.; Purohit, R. Potential of Turmeric-Derived Compounds against RNA-dependent RNA Polymerase of SARS-CoV-2: An in-Silico Approach. Comput. Biol. Med. 2021, 139, 104965. [Google Scholar] [CrossRef]
- Aljindan, R.Y.; Al-Subaie, A.M.; Al-Ohali, A.I.; Kamaraj, B. Investigation of Nonsynonymous Mutations in the Spike Protein of SARS-CoV-2 and Its Interaction with the ACE2 Receptor by Molecular Docking and MM/GBSA Approach. Comput. Biol. Med. 2021, 135, 104654. [Google Scholar] [CrossRef] [PubMed]
- Al-Karmalawy, A.A.; Dahab, M.A.; Metwaly, A.M.; Elhady, S.S.; Elkaeed, E.B.; Eissa, I.H.; Darwish, K.M. Molecular Docking and Dynamics Simulation Revealed the Potential Inhibitory Activity of ACEIs against SARS-CoV-2 Targeting the h ACE2 Receptor. Front. Chem. 2021, 9, 661230. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Liu, H.; Wu, C. Drug Repurposing against SARS-CoV-2 Receptor Binding Domain Using Ensemble-Based Virtual Screening and Molecular Dynamics Simulations. Comput. Biol. Med. 2021, 135, 104634. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Sarkar, A.; Maulik, U. Molecular Docking Study of Potential Phytochemicals and Their Effects on the Complex of SARS-CoV-2 Spike Protein and Human ACE2. Sci. Rep. 2020, 10, 17699. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.; Xu, W.; Liu, S. Structural and Functional Properties of SARS-CoV-2 Spike Protein: Potential Antivirus Drug Development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Yepes-Pérez, A.F.; Herrera-Calderon, O.; Quintero-Saumeth, J. Uncaria Tomentosa (Cat’s Claw): A Promising Herbal Medicine against SARS-CoV-2/ACE-2 Junction and SARS-CoV-2 Spike Protein Based on Molecular Modeling. J. Biomol. Struct. Dyn. 2022, 40, 2227–2243. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Liu, C.; Zhang, C.; Han, W.; Hong, X.; Wang, Y.; Hong, Q.; Wang, S.; Zhao, Q. Conformational Dynamics of SARS-CoV-2 Trimeric Spike Glycoprotein in Complex with Receptor ACE2 Revealed by Cryo-EM. Sci. Adv. 2021, 7, eabe5575. [Google Scholar] [CrossRef]
- Zhang, Y.; Kutateladze, T.G. Molecular Structure Analyses Suggest Strategies to Therapeutically Target SARS-CoV-2. Nat. Commun. 2020, 11, 2920. [Google Scholar] [CrossRef]
- Yang, J.; Petitjean, S.J.L.; Koehler, M.; Zhang, Q.; Dumitru, A.C.; Chen, W.; Derclaye, S.; Vincent, S.P.; Soumillion, P.; Alsteens, D. Molecular Interaction and Inhibition of SARS-CoV-2 Binding to the ACE2 Receptor. Nat. Commun. 2020, 11, 4541. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904. [Google Scholar] [CrossRef]
- Jawad, B.; Adhikari, P.; Podgornik, R.; Ching, W.-Y. Key Interacting Residues between RBD of SARS-CoV-2 and ACE2 Receptor: Combination of Molecular Dynamics Simulation and Density Functional Calculation. J. Chem. Inf. Model. 2021, 61, 4425–4441. [Google Scholar] [CrossRef]
- Maffucci, I.; Contini, A. In Silico Drug Repurposing for SARS-CoV-2 Main Proteinase and Spike Proteins. J. Proteome Res. 2020, 19, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhu, Z.; Shi, Y.; Wang, X.; Mu, K.; Yang, Y.; Zhang, X.; Xu, Z.; Zhu, W. Computational Insights into the Conformational Accessibility and Binding Strength of SARS-CoV-2 Spike Protein to Human Angiotensin-Converting Enzyme 2. J. Phys. Chem. Lett. 2020, 11, 10482–10488. [Google Scholar] [CrossRef]
- Alvarado, W.; Perez-Lemus, G.R.; Menéndez, C.A.; Byléhn, F.; de Pablo, J.J. Molecular Characterization of COVID-19 Therapeutics: Luteolin as an Allosteric Modulator of the Spike Protein of SARS-CoV-2. Mol. Syst. Des. Eng. 2022, 7, 58–66. [Google Scholar] [CrossRef]
- Deganutti, G.; Prischi, F.; Reynolds, C.A. Supervised Molecular Dynamics for Exploring the Druggability of the SARS-CoV-2 Spike Protein. J. Comput. Aided Mol. Des. 2021, 35, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.-T.; Cheng, T.-J.R.; Juang, Y.-P.; Ma, H.-H.; Wu, Y.-T.; Yang, W.-B.; Cheng, C.-W.; Chen, X.; Chou, T.-H.; Shie, J.-J. Identification of Existing Pharmaceuticals and Herbal Medicines as Inhibitors of SARS-CoV-2 Infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2021579118. [Google Scholar] [CrossRef] [PubMed]
- Alexpandi, R.; de Mesquita, J.F.; Pandian, S.K.; Ravi, A.V. Quinolines-Based SARS-CoV-2 3CLpro and RdRp Inhibitors and Spike-RBD-ACE2 Inhibitor for Drug-Repurposing against COVID-19: An in Silico Analysis. Front. Microbiol. 2020, 11, 1796. [Google Scholar] [CrossRef]
- da Silva, J.K.R.; Figueiredo, P.L.B.; Byler, K.G.; Setzer, W.N. Essential Oils as Antiviral Agents, Potential of Essential Oils to Treat SARS-CoV-2 Infection: An in-Silico Investigation. Int. J. Mol. Sci. 2020, 21, 3426. [Google Scholar] [CrossRef]
- Khare, P.; Sahu, U.; Pandey, S.C.; Samant, M. Current Approaches for Target-Specific Drug Discovery Using Natural Compounds against SARS-CoV-2 Infection. Virus Res. 2020, 290, 198169. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Nagarajan, S.K.; Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational Evaluation of Major Components from Plant Essential Oils as Potent Inhibitors of SARS-CoV-2 Spike Protein. J. Mol. Struct. 2020, 1221, 128823. [Google Scholar] [CrossRef]
- Darshani, P.; sen Sarma, S.; Srivastava, A.K.; Baishya, R.; Kumar, D. Anti-Viral Triterpenes: A Review. Phytochem. Rev. 2022, 21, 1761–1842. [Google Scholar] [CrossRef] [PubMed]
- Hisham Shady, N.; Youssif, K.A.; Sayed, A.M.; Belbahri, L.; Oszako, T.; Hassan, H.M.; Abdelmohsen, U.R. Sterols and Triterpenes: Antiviral Potential Supported by in-Silico Analysis. Plants 2021, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, J.; Xiao, S.; Zhang, L.; Zhou, D. Triterpenoid-Mediated Inhibition of Virus–Host Interaction: Is Now the Time for Discovering Viral Entry/Release Inhibitors from Nature? J. Med. Chem. 2020, 63, 15371–15388. [Google Scholar] [CrossRef] [PubMed]
- Elshabrawy, H.A. SARS-CoV-2: An Update on Potential Antivirals in Light of SARS-CoV Antiviral Drug Discoveries. Vaccines 2020, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent Progress in the Antiviral Activity and Mechanism Study of Pentacyclic Triterpenoids and Their Derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an Active Component of Liquorice Roots, and Replication of SARS-Associated Coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Li, J.; Xu, D.; Wang, L.; Zhang, M.; Zhang, G.; Li, E.; He, S. Glycyrrhizic Acid Inhibits SARS-CoV-2 Infection by Blocking Spike Protein-Mediated Cell Attachment. Molecules 2021, 26, 6090. [Google Scholar] [CrossRef]
- Giofrè, S.V.; Napoli, E.; Iraci, N.; Speciale, A.; Cimino, F.; Muscarà, C.; Molonia, M.S.; Ruberto, G.; Saija, A. Interaction of Selected Terpenoids with Two SARS-CoV-2 Key Therapeutic Targets: An in Silico Study through Molecular Docking and Dynamics Simulations. Comput. Biol. Med. 2021, 134, 104538. [Google Scholar] [CrossRef]
- Yi, Y.; Li, J.; Lai, X.; Zhang, M.; Kuang, Y.; Bao, Y.-O.; Yu, R.; Hong, W.; Muturi, E.; Xue, H.; et al. Natural Triterpenoids from Licorice Potently Inhibit SARS-CoV-2 Infection. J. Adv. Res. 2022, 36, 201–210. [Google Scholar] [CrossRef]
- Li, H.; Cheng, C.; Li, S.; Wu, Y.; Liu, Z.; Liu, M.; Chen, J.; Zhong, Q.; Zhang, X.; Liu, S. Discovery and Structural Optimization of 3-O-β-Chacotriosyl Oleanane-Type Triterpenoids as Potent Entry Inhibitors of SARS-CoV-2 Virus Infections. Eur. J. Med. Chem. 2021, 215, 113242. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Ávila, R.; Flores-Morales, V.; Paoli, P.; Camici, G.; Ramírez-Espinosa, J.J.; Cerón-Romero, L.; Navarrete-Vázquez, G.; Hidalgo-Figueroa, S.; Yolanda Rios, M.; Villalobos-Molina, R. Ursolic Acid Derivatives as Potential Antidiabetic Agents: In Vitro, in Vivo, and in Silico Studies. Drug Dev. Res. 2018, 79, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Cerón-Romero, L.; Paoli, P.; Camici, G.; Flores-Morales, V.; Rios, M.Y.; Ramírez-Espinosa, J.J.; Hidalgo-Figueroa, S.; Navarrete-Vázquez, G.; Estrada-Soto, S. In Vitro and in Silico PTP-1B Inhibition and in Vivo Antidiabetic Activity of Semisynthetic Moronic Acid Derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 2018–2022. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Espinosa, J.J.; Rios, M.Y.; Paoli, P.; Flores-Morales, V.; Camici, G.; de la Rosa-Lugo, V.; Hidalgo-Figueroa, S.; Navarrete-Vázquez, G.; Estrada-Soto, S. Synthesis of Oleanolic Acid Derivatives: In Vitro, in Vivo and in Silico Studies for PTP-1B Inhibition. Eur. J. Med. Chem. 2014, 87, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, W.P.; Brylinski, M. Calculating an Optimal Box Size for Ligand Docking and Virtual Screening against Experimental and Predicted Binding Pockets. J. Cheminform. 2015, 7, 18. [Google Scholar] [CrossRef]
- Huang, S.; Zou, X. An Iterative Knowledge-based Scoring Function for Protein–Protein Recognition. Proteins Struct. Funct. Bioinform. 2008, 72, 557–579. [Google Scholar] [CrossRef]
- Yan, Y.; Tao, H.; He, J.; Huang, S.-Y. The HDOCK Server for Integrated Protein–Protein Docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef]
- Weng, G.; Wang, E.; Wang, Z.; Liu, H.; Zhu, F.; Li, D.; Hou, T. HawkDock: A Web Server to Predict and Analyze the Protein–Protein Complex Based on Computational Docking and MM/GBSA. Nucleic Acids Res. 2019, 47, W322–W330. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Tian, S.; Xu, L.; Hou, T. Assessing the Performance of MM/PBSA and MM/GBSA Methods. 4. Accuracies of MM/PBSA and MM/GBSA Methodologies Evaluated by Various Simulation Protocols Using PDBbind Data Set. Phys. Chem. Chem. Phys. 2014, 16, 16719–16729. [Google Scholar] [CrossRef]
- Chen, F.; Liu, H.; Sun, H.; Pan, P.; Li, Y.; Li, D.; Hou, T. Assessing the Performance of the MM/PBSA and MM/GBSA Methods. 6. Capability to Predict Protein–Protein Binding Free Energies and Re-Rank Binding Poses Generated by Protein–Protein Docking. Phys. Chem. Chem. Phys. 2016, 18, 22129–22139. [Google Scholar] [CrossRef]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the Performance of the MM/PBSA and MM/GBSA Methods. 1. The Accuracy of Binding Free Energy Calculations Based on Molecular Dynamics Simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.K.; Wang, B.; Sam, A.; Hoop, C.L.; Case, D.A.; Baum, J. Molecular Dynamics Analysis of a Flexible Loop at the Binding Interface of the SARS-CoV-2 Spike Protein Receptor-Binding Domain. Proteins Struct. Funct. Bioinform. 2022, 90, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.S.; Pacheco, B.D.C.; Pinheiro, S.; Muri, E.M.F.; Dias, L.R.S.; Lima, C.H.S.; Garrett, R.; de Moraes, M.B.M.; de Souza, B.E.G.; Puzer, L. 3-Acyltetramic Acids as a Novel Class of Inhibitors for Human Kallikreins 5 and 7. Bioorg. Med. Chem. Lett. 2019, 29, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Baral, P.; Gerstman, B.S.; Chapagain, P.P. Structural and Dynamical Differences in the Spike Protein RBD in the SARS-CoV-2 Variants B. 1.1. 7 and B. 1.351. J. Phys. Chem. B 2021, 125, 7101–7107. [Google Scholar] [CrossRef] [PubMed]
- Bò, L.; Miotto, M.; di Rienzo, L.; Milanetti, E.; Ruocco, G. Exploring the Association between Sialic Acid and SARS-CoV-2 Spike Protein through a Molecular Dynamics-Based Approach. Front. Med. Technol. 2021, 2, 614652. [Google Scholar] [CrossRef]
- Moneriz, C.; Castro-Salguedo, C. Fármacos Prometedores y Potenciales Para El Tratamiento de COVID-19. Rev. Chil. Infectol. 2020, 37, 205–215. [Google Scholar] [CrossRef]
- Vankadari, N. Arbidol: A Potential Antiviral Drug for the Treatment of SARS-CoV-2 by Blocking Trimerization of the Spike Glycoprotein. Int. J. Antimicrob. Agents 2020, 56, 105998. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Kohn, W.; Becke, A.D.; Parr, R.G. Density Functional Theory of Electronic Structure. J. Phys. Chem. 1996, 100, 12974–12980. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.01 2016; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Søndergaard, C.R.; Olsson, M.H.M.; Rostkowski, M.; Jensen, J.H. Improved Treatment of Ligands and Coupling Effects in Empirical Calculation and Rationalization of p K a Values. J. Chem. Theory Comput. 2011, 7, 2284–2295. [Google Scholar] [CrossRef]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical p K a Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, Tampa, FL, USA, 11–17 November 2006; p. 84–es. [Google Scholar] [CrossRef]
- Banks, J.L.; Beard, H.S.; Cao, Y.; Cho, A.E.; Damm, W.; Farid, R.; Felts, A.K.; Halgren, T.A.; Mainz, D.T.; Maple, J.R. Integrated Modeling Program, Applied Chemical Theory (IMPACT). J. Comput. Chem. 2005, 26, 1752–1780. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.v.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings1PII of Original Article: S0169-409X(96)00423-1. The Article Was Originally Published in Advanced Drug Delivery Reviews 23 (1997) 3–25.1. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Egan, W.J.; Merz Kenneth, M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple Selection Criteria for Drug-like Chemical Matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Bakan, A.; Meireles, L.M.; Bahar, I. ProDy: Protein Dynamics Inferred from Theory and Experiments. Bioinformatics 2011, 27, 1575–1577. [Google Scholar] [CrossRef] [PubMed]
- Bakan, A.; Dutta, A.; Mao, W.; Liu, Y.; Chennubhotla, C.; Lezon, T.R.; Bahar, I. Evol and ProDy for Bridging Protein Sequence Evolution and Structural Dynamics. Bioinformatics 2014, 30, 2681–2683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Krieger, J.M.; Zhang, Y.; Kaya, C.; Kaynak, B.; Mikulska-Ruminska, K.; Doruker, P.; Li, H.; Bahar, I. ProDy 2.0: Increased Scale and Scope after 10 Years of Protein Dynamics Modelling with Python. Bioinformatics 2021, 37, 3657–3659. [Google Scholar] [CrossRef] [PubMed]
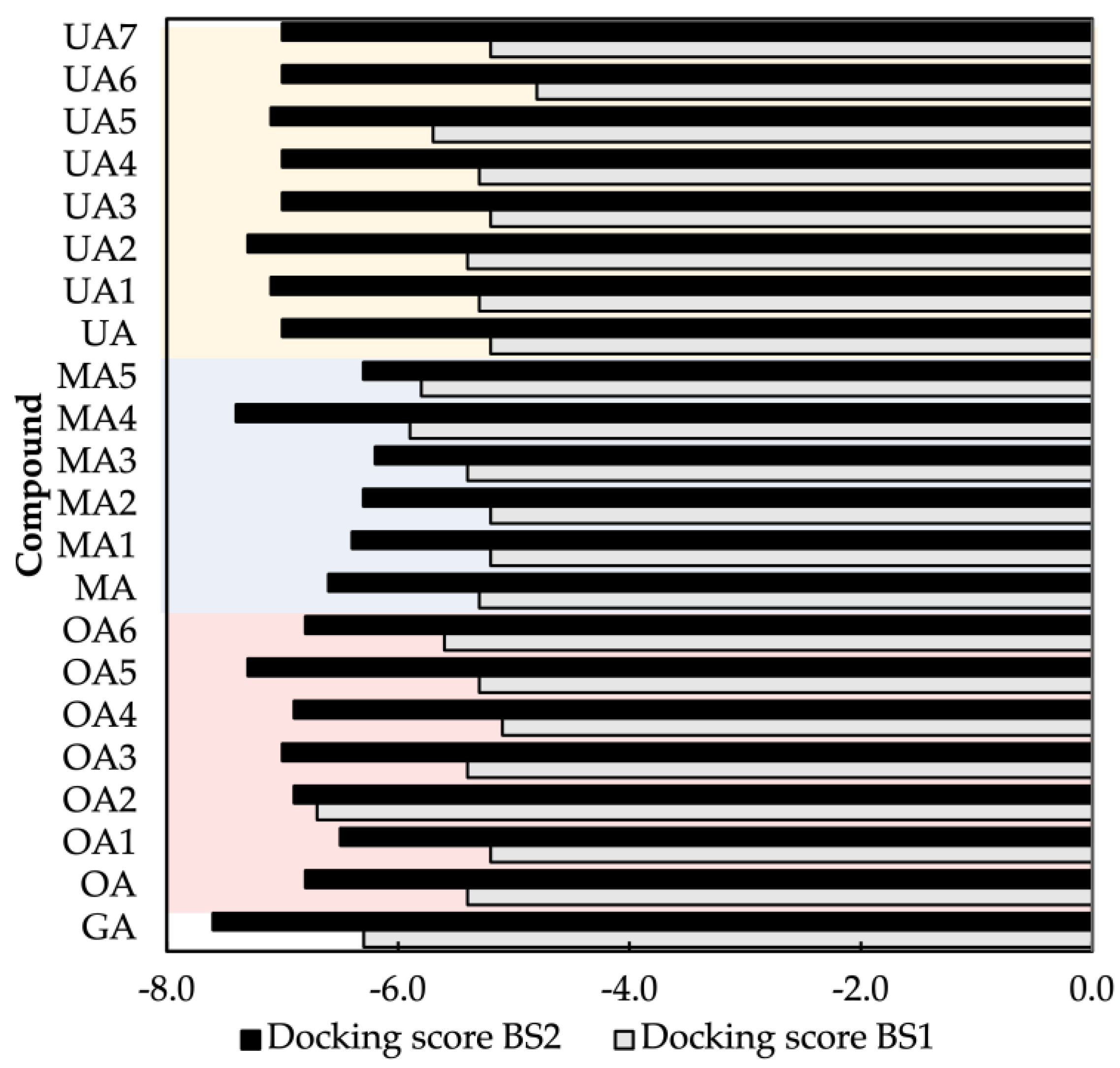
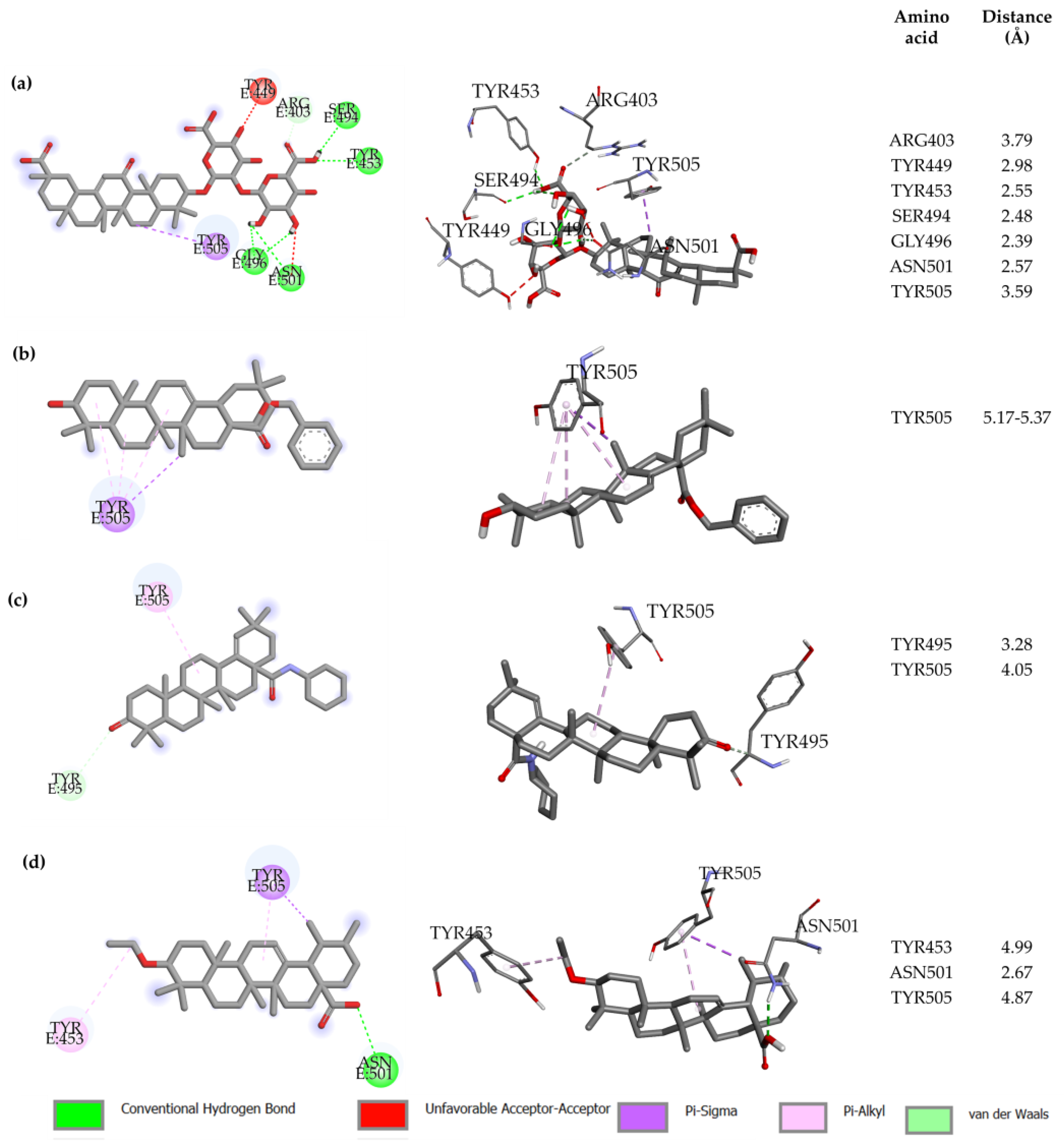
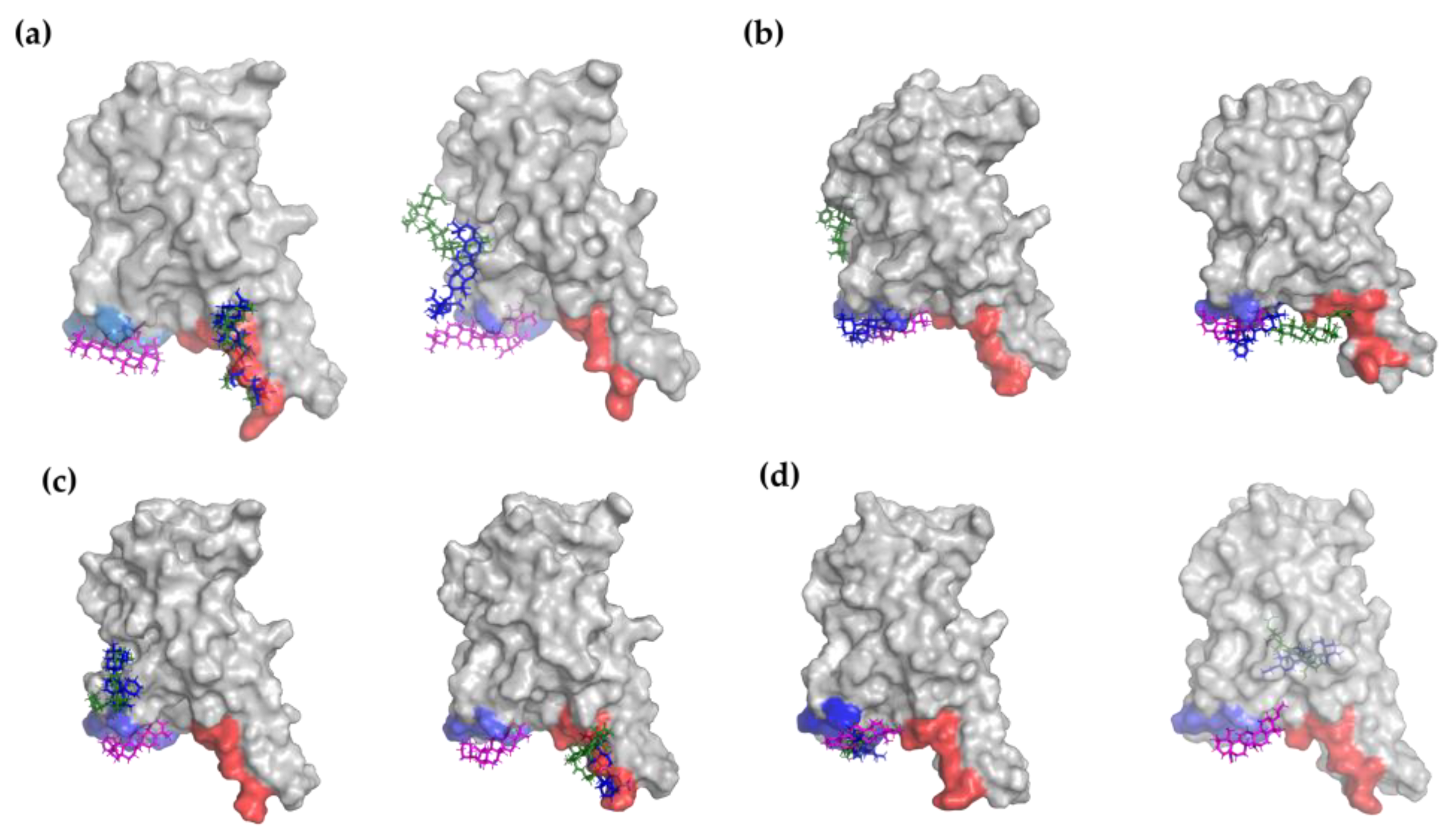
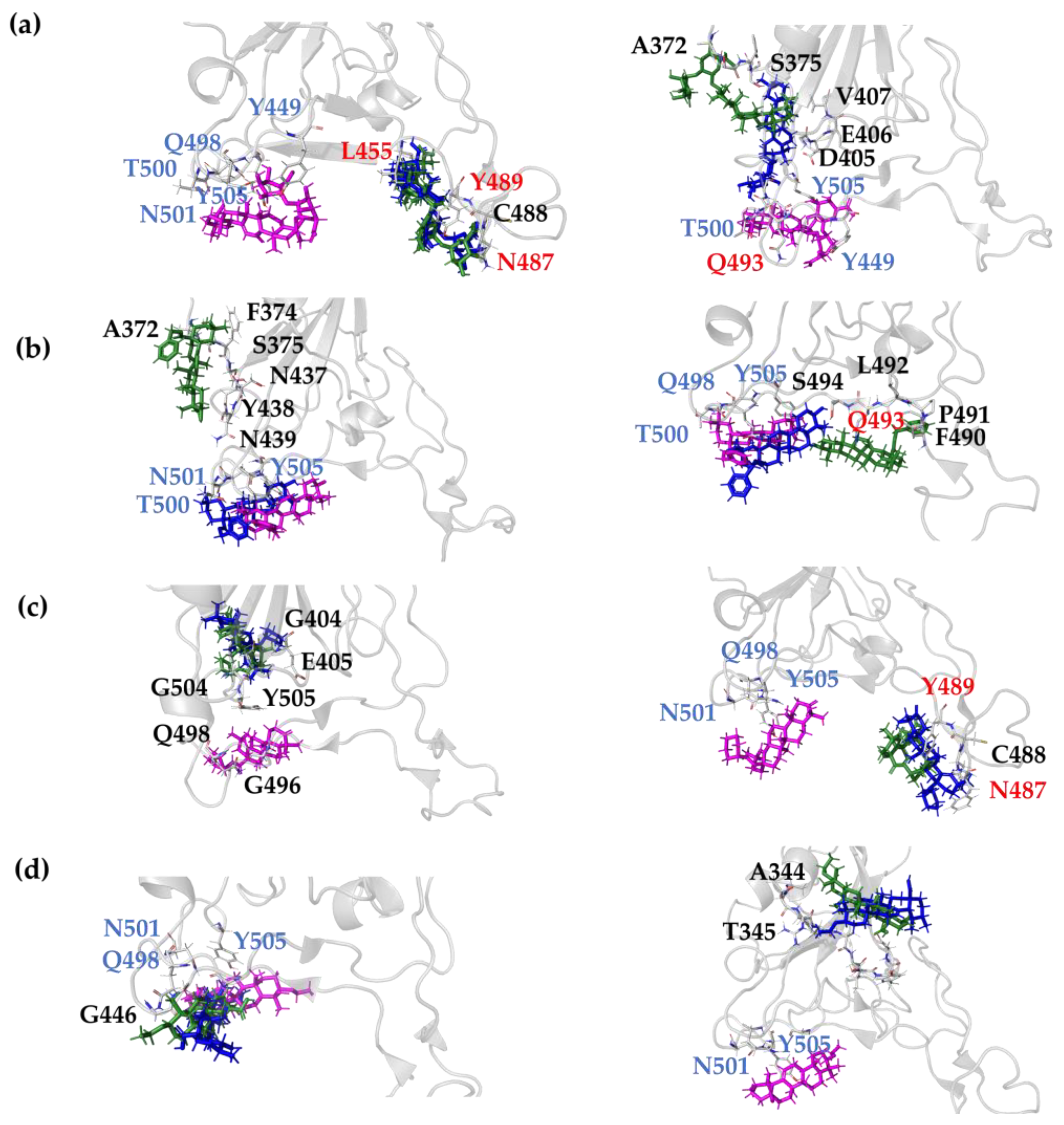


| Compound Key Name | Chemical Structure | BS1 Docking Score | BS2 Docking Score | Residues at a 5 Å Sphere of Interaction |
|---|---|---|---|---|
| GA | 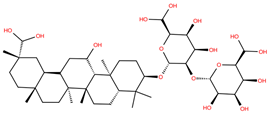 | −6.3 | −7.6 | BS1: K417, Y453, L455, F456, E484, G485, F486, N487, C488, Y489, F490, L492, Q493 BS2: R403, Y449, Y453, S494, Y495, G496, F497, Q498, T500, N501, G502, Y505 |
| OA | 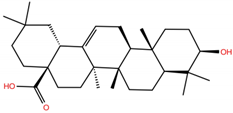 | −5.4 | −6.8 | BS1: K17, L455, F456, E484, G485, F486, N487, Y489 BS2: R403, Y453, Q493, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| OA1 | 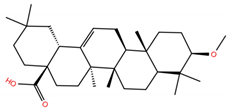 | −5.2 | −6.5 | BS1: K417, L455, F456, E484, G485, F486, N487 Y489 BS2: R403, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| OA2 | 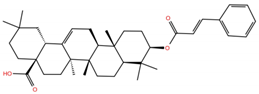 | −6.7 | −6.9 | BS1: L455, F456, Y473, A475, E484, N487, Y489, F490, Q493 BS2: R403, E406, K417, Y453, S494, Y495, G496, F497, Q498, T500, N501, Y505 |
| OA3 | 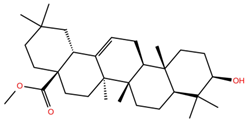 | −5.4 | −7.0 | BS1: K417, L455, F456, E484, G485, F486, N487, C488, Y489, F490 BS2: R403, Y453, S494, Y495, G496, Q498, T500, R501, G502, Y505 |
| OA4 |  | −5.1 | −6.9 | BS1: K417, L455, F456, E484, G485, F486, N487, C488, Y489, F490 BS2: R403, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| OA5 | 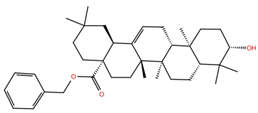 | −5.3 | −7.3 | BS1: K417, L455, F456, E484, N487, Y489, F490, L492, Q493 BS2: G446, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| OA6 | 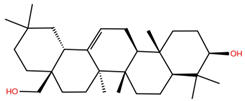 | −5.6 | −6.8 | BS1: K417, L455, F456, E484, G485, F486, N487, C488, Y489 BS2: R403, Y453, Y495, G496, Q498, T500, N501, G502, Y505 |
| MA | 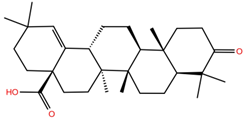 | −5.3 | −6.6 | BS1: K417, L455, F456, E484, G485, C488, Y489, F490, Q493 BS2: R403, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| MA1 | 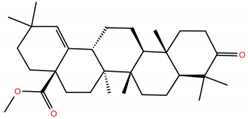 | −5.2 | −6.4 | BS1: K417, L455, F456, E484, G485, C488, Y489, F490, Q493 BS2: R403, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| MA2 | 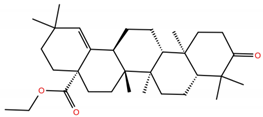 | −5.2 | −6.3 | BS1: K417, L455, F456, Y473, A475, E484, N487, Y489 BS2: R403, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| MA3 | 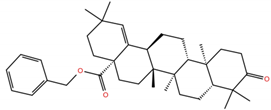 | −5.4 | −6.2 | BS1: L455, F456, E484, G485, F486, C488, Y489, Q493 BS2: R403, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| MA4 |  | −5.9 | −7.4 | BS1: K417, L455, F456, A475, E484, N487, Y489, Q493 BS2: R403, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| MA5 |  | −5.8 | −6.3 | BS1: K417, L455, F456, E484, Y489, F490, Q493 BS2: R403, Y449, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| UA | 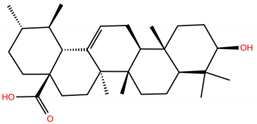 | −5.2 | −7.0 | BS1: K417, L455, F456, E484, G485, C488, Y489, F490, Q493 BS2: R403, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| UA1 |  | −5.3 | −7.1 | BS1: K417, L455, F456, E484, G485, C488, Y489, F490, Q493 BS2: R403, E406, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| UA2 | 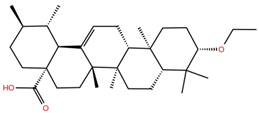 | −5.4 | −7.3 | BS1: K417, Y453, L455, F456, E484, G485, F486, C488, Y489, F490, Q493 BS2: R403, E406, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| UA3 |  | −5.2 | −7.0 | BS1: K417, L455, F456, E484, G485, F486, C488, Y489, F490, Q493 BS2: R403, E406, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| UA4 | 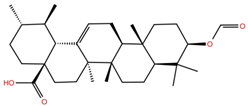 | −5.3 | −7.0 | BS1: K417, Y453, L455, F456, E484, G485, F486, C488, Y489, F490, Q493 BS2: R403, E406, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| UA5 | 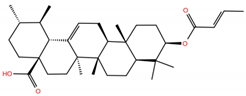 | −5.7 | −7.1 | BS1: K417, Y421, L455, F456, R457, Y473, A475, E484, Y489, Q493 BS2: R403, E406, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| UA6 | 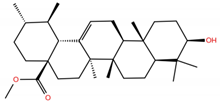 | −4.8 | −7.0 | BS1: L455, F456, E484, G485, C488, Y489, Q493 BS2: R403, E406, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| UA7 |  | −5.2 | −7.0 | BS1: K417, L455, F456, E484, C488, Y489, F490, Q493 BS2: R403, E406, Y453, S494, Y495, G496, Q498, T500, N501, G502, Y505 |
| Compound | M. Wt g/mol | TPSA Å2 | Log P o/w | LogS (ESOL) | HBA | HBD | Rotatable Bonds | Druglikeness * | Bioavailability Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WLOGP | MLOGP | Lipinski | Ghose | Veber | Egan | Mugue | ||||||||
| OA5 | 546.02 | 46.53 | 8.74 | 6.83 | −8.94 | 3 | 1 | 4 | 2 | 4 | 0 | 1 | 1 | 0.17 |
| MA4 | 535.84 | 46.17 | 7.52 | 5.90 | −8.62 | 2 | 1 | 3 | 2 | 4 | 0 | 1 | 1 | 0.17 |
| UA2 | 484.75 | 46.53 | 8.13 | 6.20 | −7.64 | 3 | 1 | 3 | 1 | 4 | 0 | 1 | 1 | 0.85 |
| GA | 833.01 | 279.68 | −0.20 | −0.67 | −6.05 | 16 | 12 | 7 | 3 | 3 | 1 | 1 | 4 | 0.17 |
| RS | 602.58 | 213.36 | 2.21 | 0.18 | −4.12 | 12 | 4 | 14 | 2 | 3 | 2 | 1 | 3 | 0.17 |
| UM | 477.41 | 80.00 | 4.87 | 3.59 | −5.45 | 4 | 1 | 8 | 0 | 0 | 0 | 0 | 0 | 0.55 |
| Compound | Pharmacokinetics | Medicinal Chemistry | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GI Absorption | BBB Permeant | P-gp Substrate | CY1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor | PAINS | Brenk | Leadlikeness | |
| OA5 | Low | No | No | No | No | No | No | No | 0 | 1 | 2 |
| MA4 | Low | No | No | No | No | No | No | No | 0 | 1 | 2 |
| UA2 | Low | No | No | No | No | No | No | No | 0 | 1 | 2 |
| GA | Low | No | Yes | No | No | No | No | No | 0 | 2 | 1 |
| RS | Low | No | Yes | No | No | No | No | Yes | 0 | 1 | 2 |
| UM | High | No | No | No | Yes | Yes | Yes | Yes | 1 | 0 | 3 |
| Compound | Antiviral (Influenza) | 3CLpro (Human Coronavirus) Inhibitor | ||
|---|---|---|---|---|
| Pa | Pi | Pa | Pi | |
| OA5 | 0.764 | 0.004 | 0.361 | 0.005 |
| MA4 | 0.746 | 0.004 | NR | NR |
| UA2 | 0.737 | 0.004 | 0.278 | 0.041 |
| GA | 0.833 | 0.002 | NR | NR |
| RS | 0.216 | 0.174 | NR | NR |
| UM | 0.740 | 0.004 | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avelar, M.; Pedraza-González, L.; Sinicropi, A.; Flores-Morales, V. Triterpene Derivatives as Potential Inhibitors of the RBD Spike Protein from SARS-CoV-2: An In Silico Approach. Molecules 2023, 28, 2333. https://doi.org/10.3390/molecules28052333
Avelar M, Pedraza-González L, Sinicropi A, Flores-Morales V. Triterpene Derivatives as Potential Inhibitors of the RBD Spike Protein from SARS-CoV-2: An In Silico Approach. Molecules. 2023; 28(5):2333. https://doi.org/10.3390/molecules28052333
Chicago/Turabian StyleAvelar, Mayra, Laura Pedraza-González, Adalgisa Sinicropi, and Virginia Flores-Morales. 2023. "Triterpene Derivatives as Potential Inhibitors of the RBD Spike Protein from SARS-CoV-2: An In Silico Approach" Molecules 28, no. 5: 2333. https://doi.org/10.3390/molecules28052333
APA StyleAvelar, M., Pedraza-González, L., Sinicropi, A., & Flores-Morales, V. (2023). Triterpene Derivatives as Potential Inhibitors of the RBD Spike Protein from SARS-CoV-2: An In Silico Approach. Molecules, 28(5), 2333. https://doi.org/10.3390/molecules28052333








