Amine-Functionalized Natural Rubber/Mesostructured Silica Nanocomposites for Adsorptive Removal of Clofibric Acid in Aqueous Phase
Abstract
1. Introduction
2. Results and Discussion
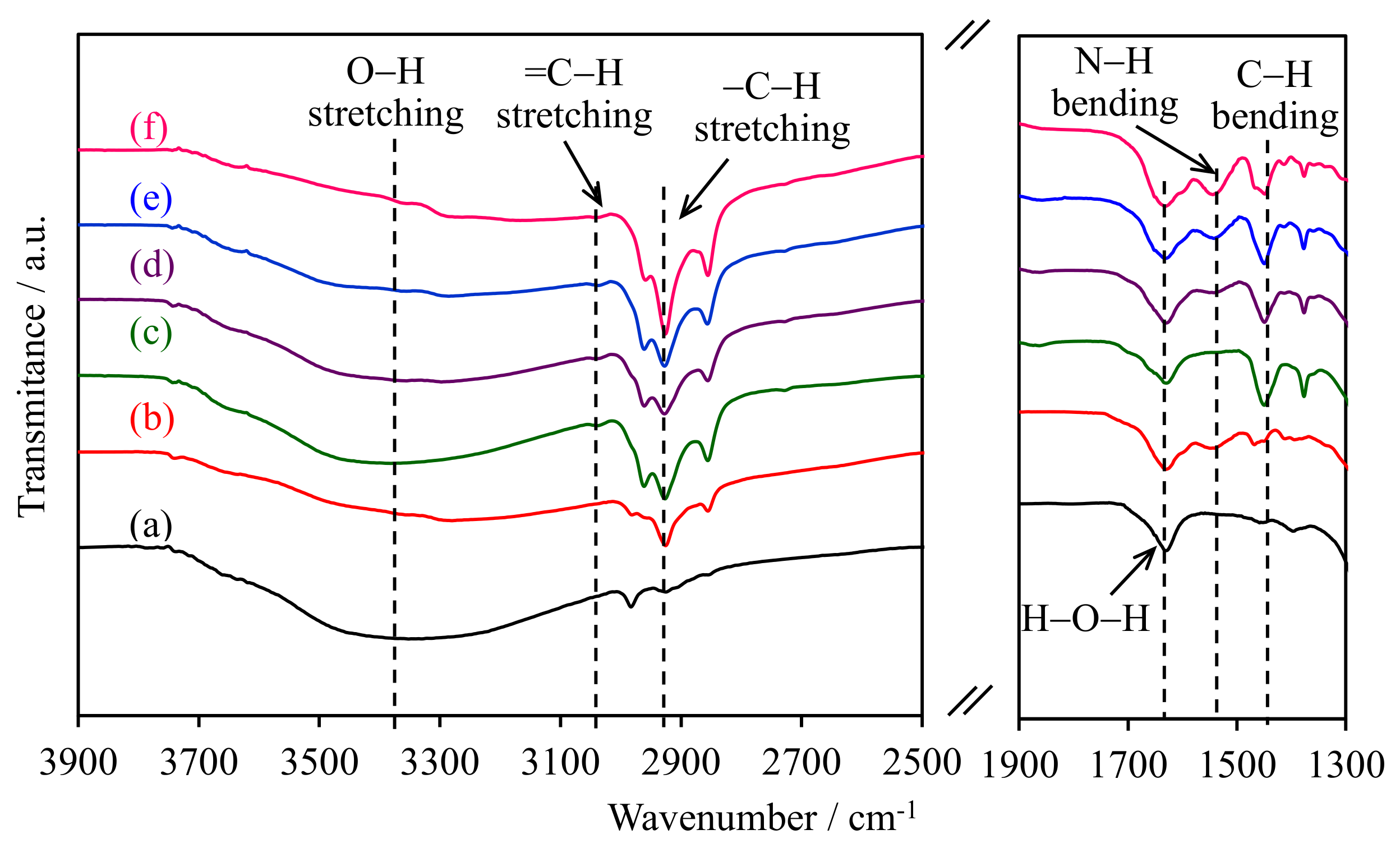
2.1. FTIR Spectroscopy
2.2. Solid State 29Si CP/MAS NMR Spectroscopy
2.3. Elemental Analysis
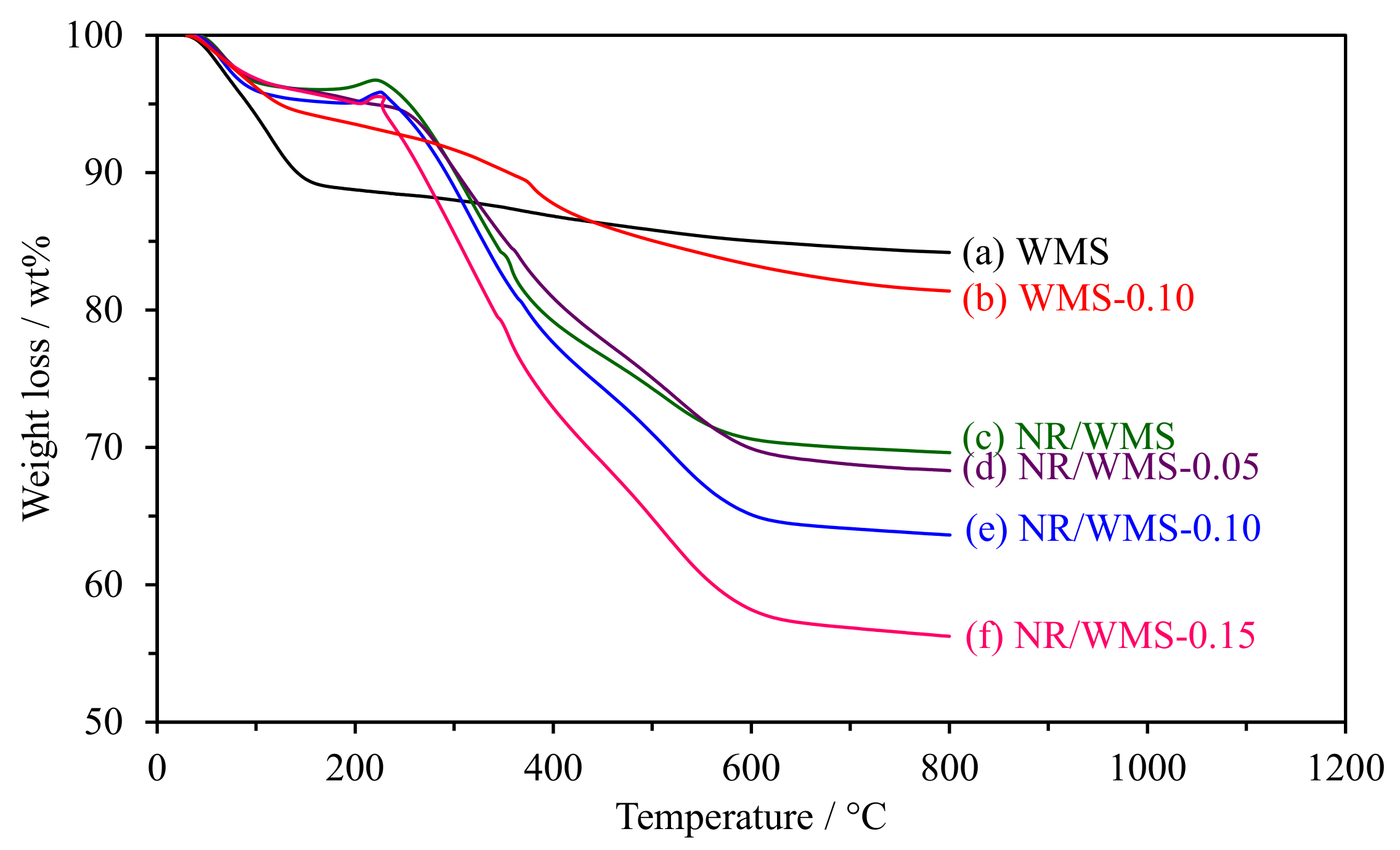
2.4. Thermogravimetric Analysis
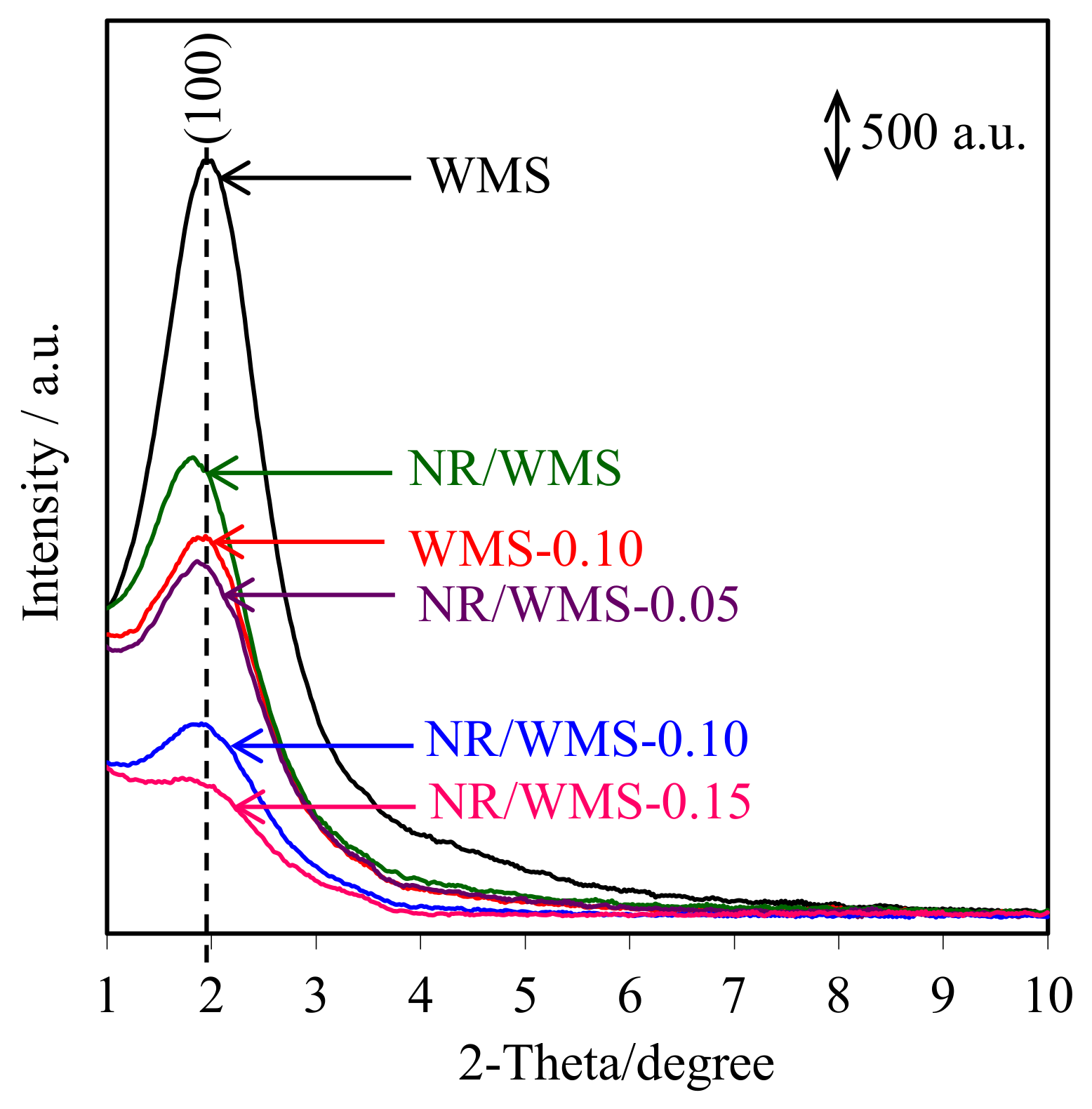
2.5. XRD Analysis
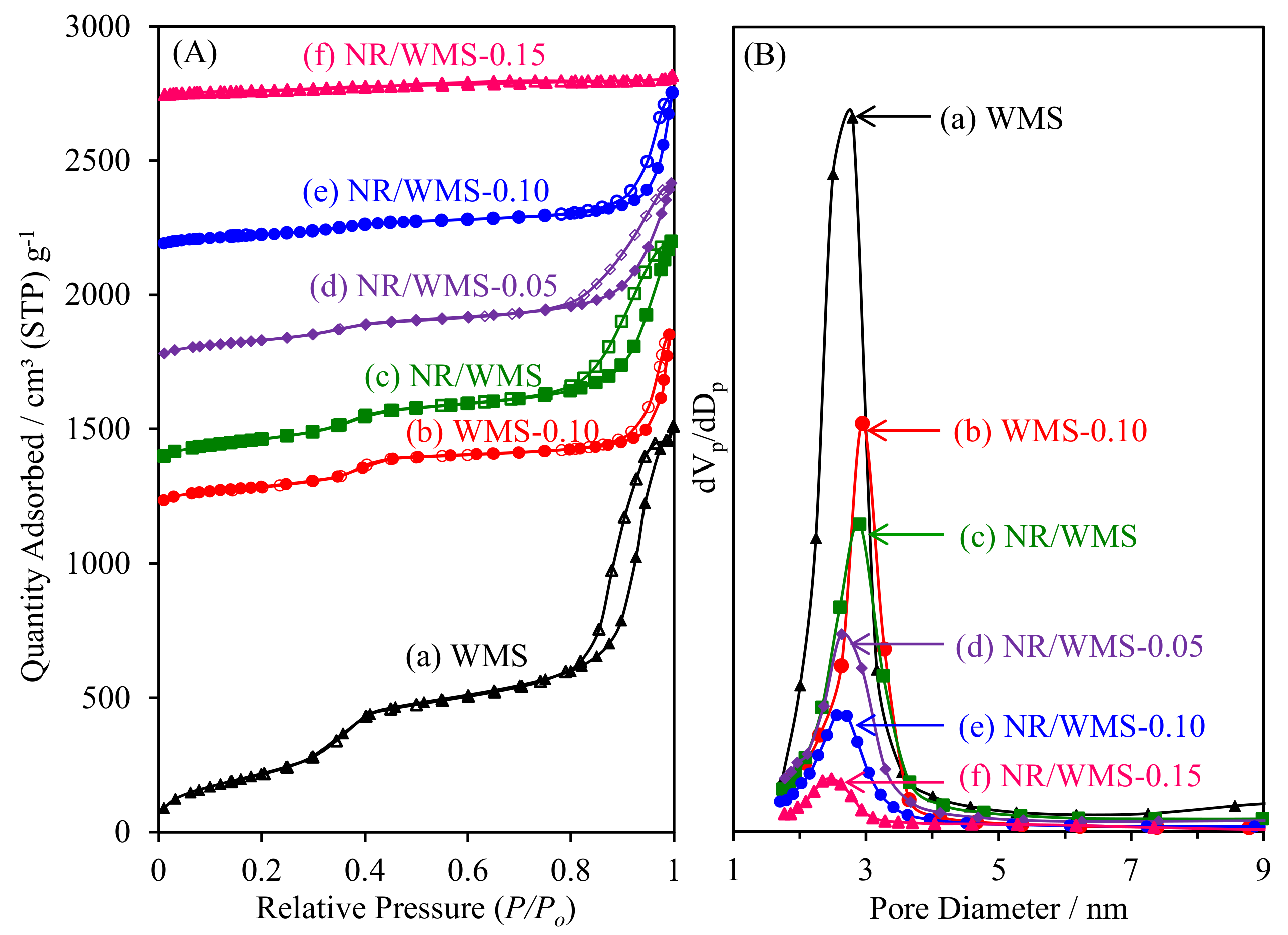
2.6. N2 Adsorption–Desorption Measurement
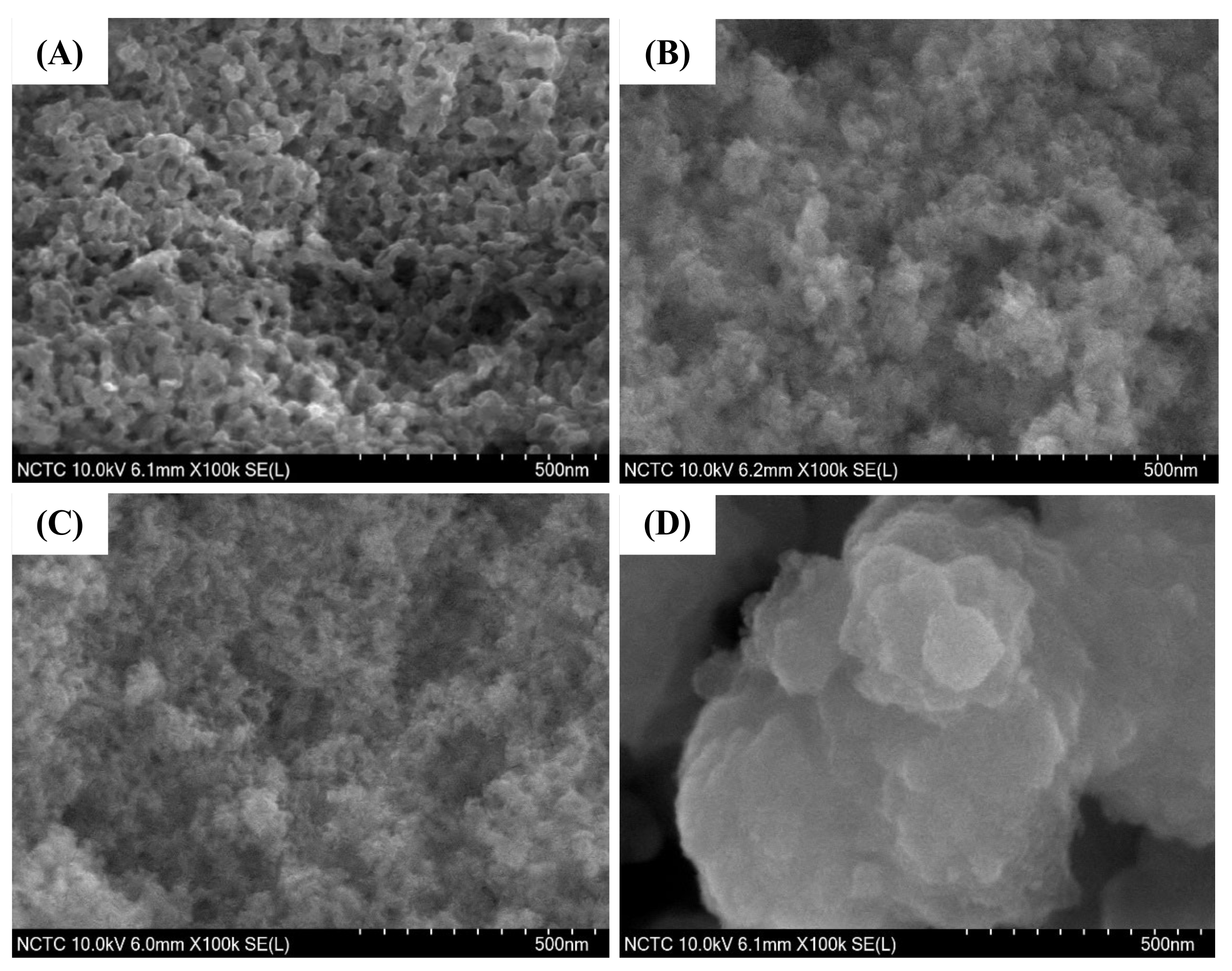
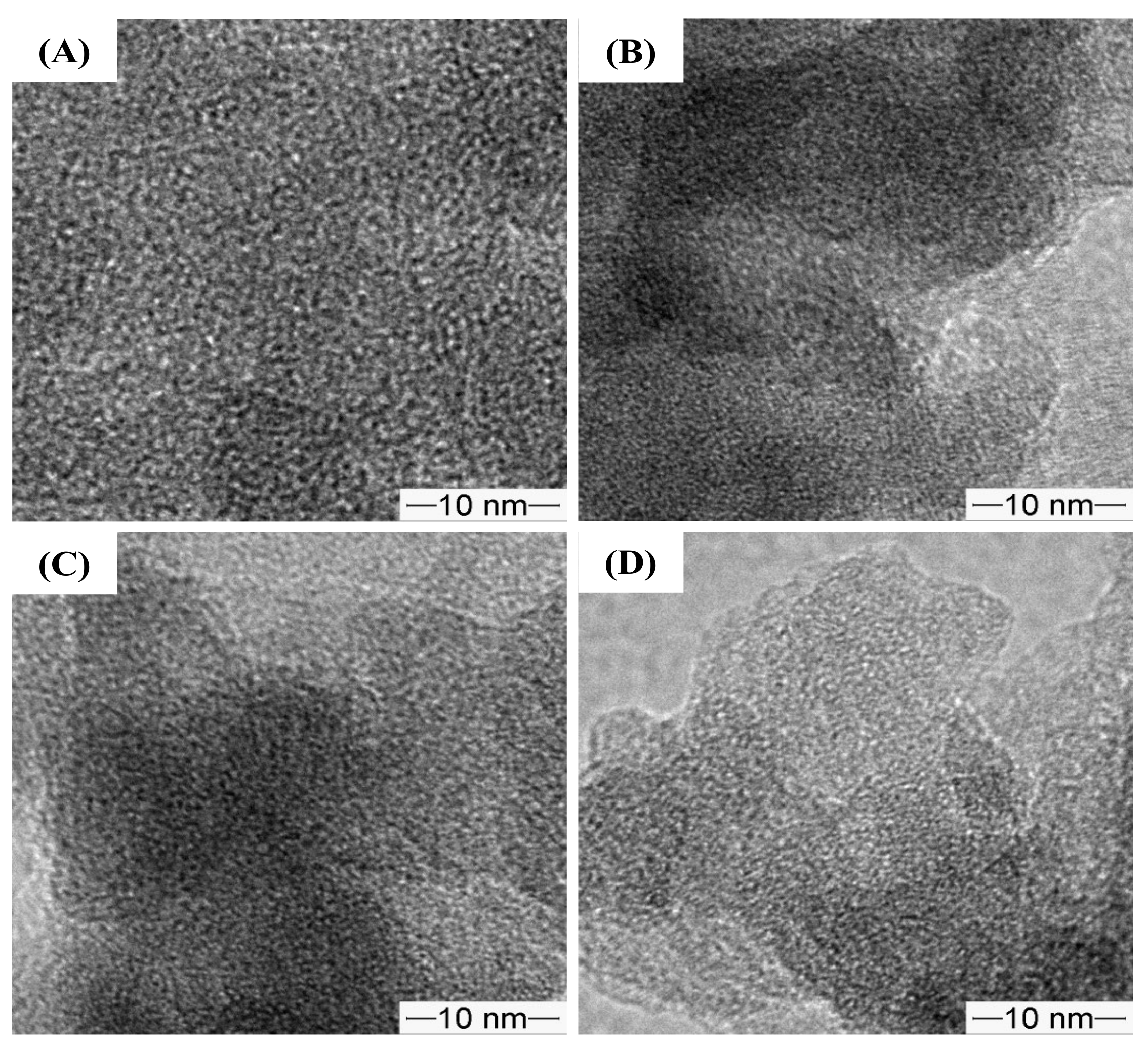
2.7. Electron Microscopy
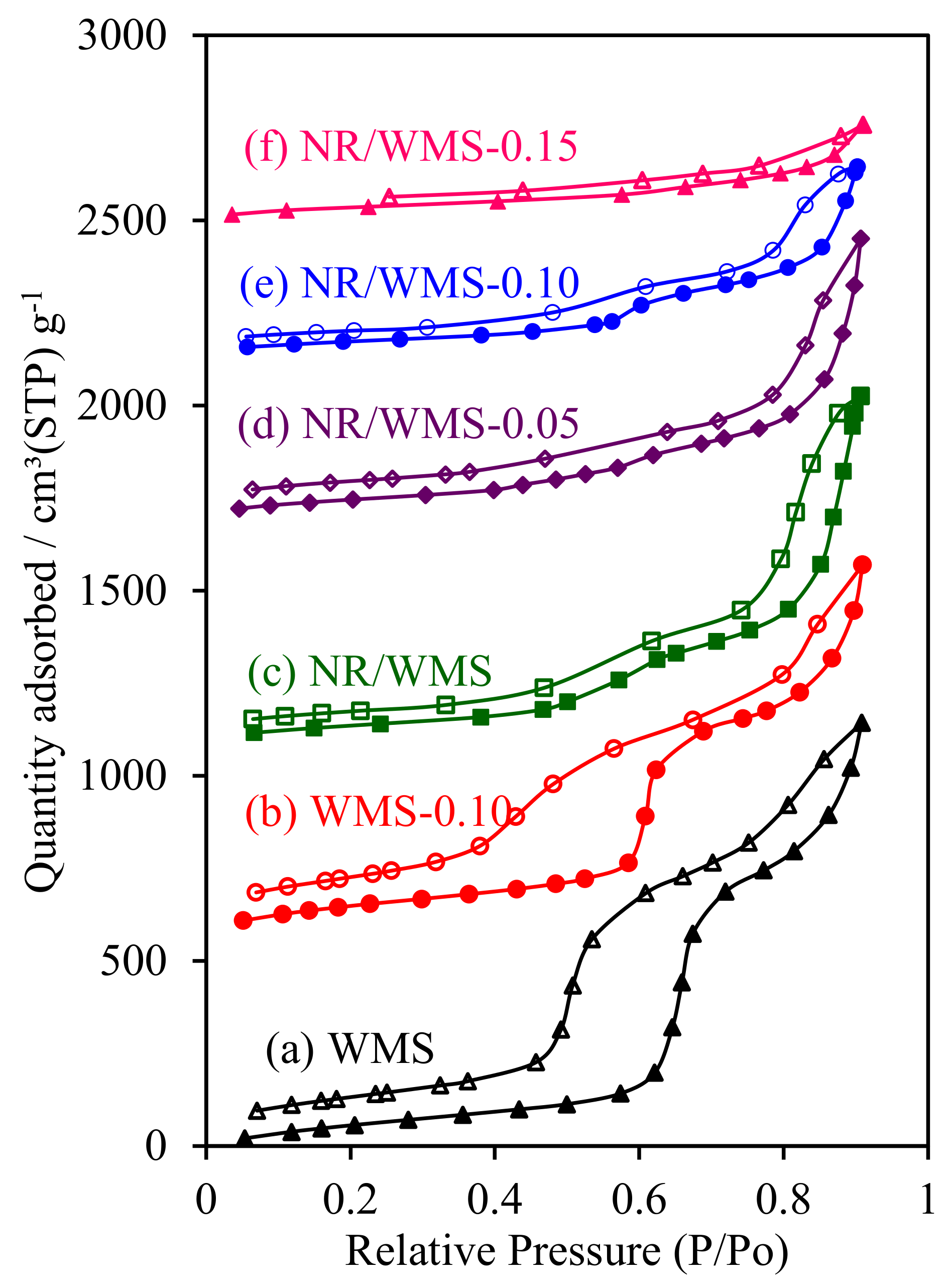
2.8. H2O Adsorption–Desorption Measurement
2.9. Adsorption Experiment
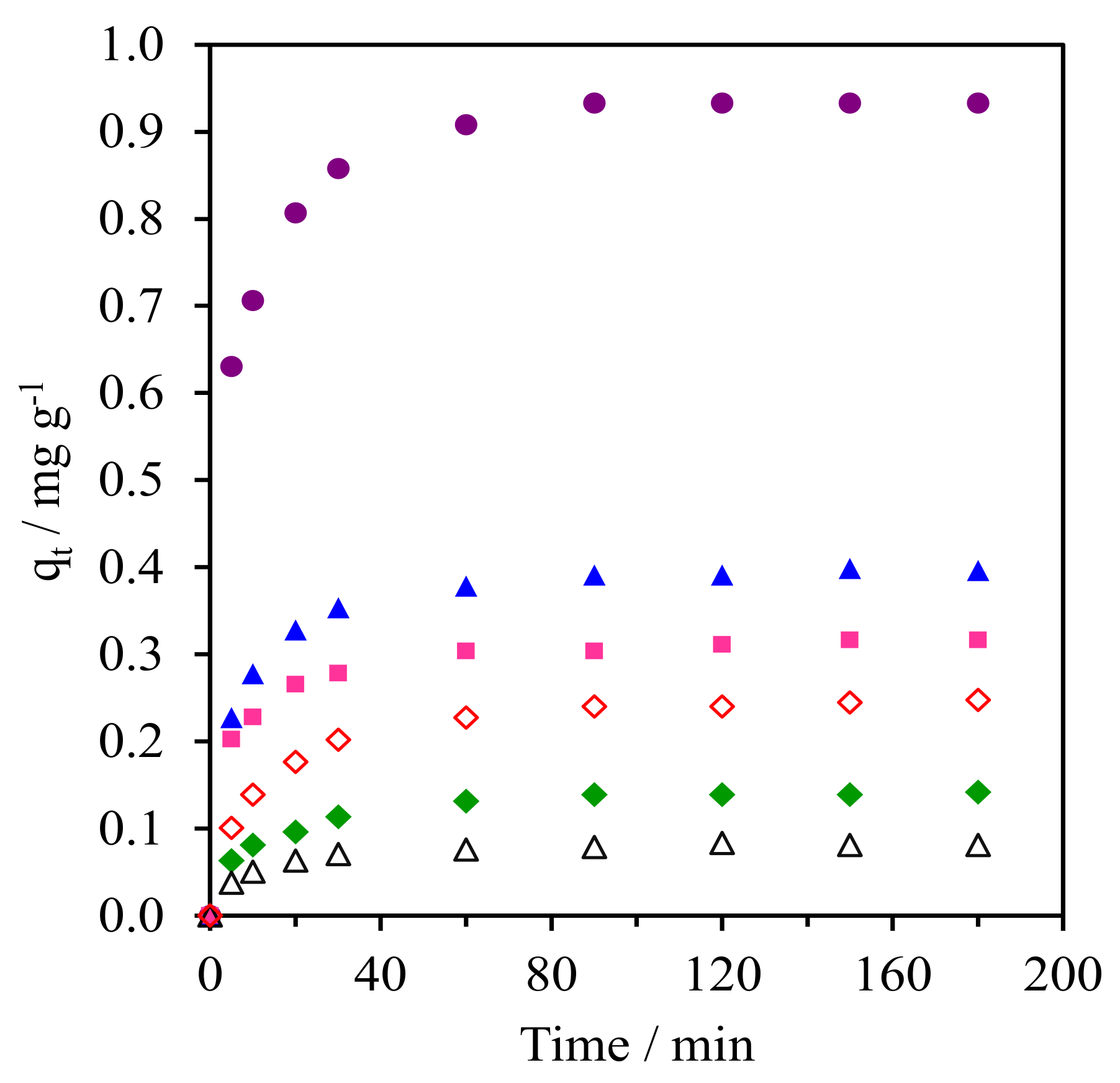
2.9.1. Adsorption Kinetics
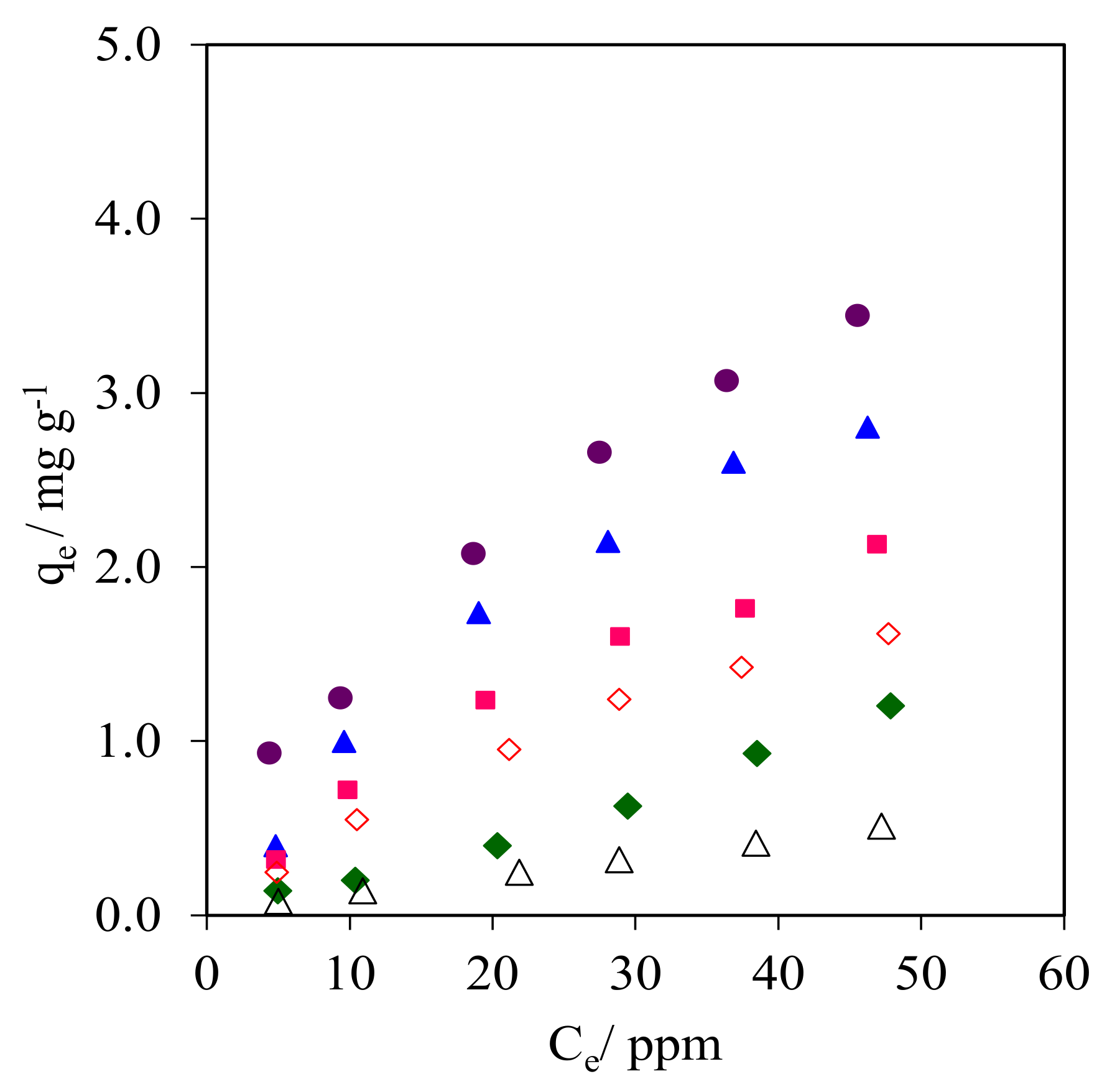
2.9.2. Adsorption Isotherms
2.9.3. Adsorption Thermodynamics
2.9.4. Reusability of NR/WMS-NH2
2.10. Comparison of Adsorbent Performance
3. Experimental Procedure
3.1. Material and Chemical Reagents
3.2. Synthesis of Pure Silica WMS and WMS-NH2 Materials
3.3. Synthesis of NR/WMS and NR/WMS-NH2 Nanocomposites
3.4. Characterization of Adsorbents
3.5. Adsorptive Removal of CFA
3.5.1. Adsorption Kinetics
3.5.2. Adsorption Isotherm
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- De Oliveira, M.; Frihling, B.E.F.; Velasques, J.; Filho, F.; Cavalheri, P.S.; Migliolo, L. Pharmaceuticals residues and xenobiotics contaminants: Occurrence, analytical techniques and sustainable alternatives for wastewater treatment. Sci. Total. Environ. 2020, 705, 135568. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Suriyanon, N.; Permrungruang, J.; Kaosaiphun, J.; Wongrueng, A.; Ngamcharussrivichai, C.; Punyapalakul, P. Selective adsorption mechanisms of antilipidemic and non-steroidal anti-inflammatory drug residues on functionalized silica-based porous materials in a mixed solute. Chemosphere 2015, 136, 222–231. [Google Scholar] [CrossRef]
- Mestre, A.S.; Nabiço, A.; Figueiredo, P.L.; Pinto, M.L.; Santos, M.S.C.S.; Fonseca, I.M. Enhanced clofibric acid removal by activated carbons: Water hardness as a key parameter. Chem. Eng. J. 2016, 286, 538–548. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, G.; Liu, K.; Deng, S.; Wang, B.; Huang, J.; Wang, Y. Integrated adsorption and visible-light photodegradation of aqueous clofibric acid and carbamazepine by a Fe-based metal-organic framework. Chem. Eng. J. 2017, 330, 157–165. [Google Scholar] [CrossRef]
- Valdes, M.E.; Huerta, B.; Wunderlin, D.A.; Bistoni, M.A.; Barcelo, D.; Rodriguez-Mozaz, S. Bioaccumulation and bioconcentration of carbamazepine and other pharmaceuticals in fish under field and controlled laboratory experiments. Evidences of carbamazepine metabolization by fish. Sci. Total. Environ. 2016, 557-558, 58–67. [Google Scholar] [CrossRef]
- Cunningham, V.L.; Perino, C.; D’Aco, V.J.; Hartmann, A.; Bechter, R. Human health risk assessment of carbamazepine in surface waters of North America and Europe. Regul. Toxicol. Pharmacol. 2010, 56, 343–351. [Google Scholar] [CrossRef]
- Qiang, L.; Cheng, J.; Yi, J.; Rotchell, J.M.; Zhu, X.; Zhou, J. Environmental concentration of carbamazepine accelerates fish embryonic development and disturbs larvae behavior. Ecotoxicology 2016, 25, 1426–1437. [Google Scholar] [CrossRef]
- Zhang, D.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Removal of pharmaceuticals and personal care products in aquatic plant-based systems: A review. Environ. Pollut. 2014, 184, 620–639. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total. Environ. 2017, 596#x2013;597, 303–320. [Google Scholar] [CrossRef]
- Tan, L.; Shuang, C.; Wang, Y.; Wang, J.; Su, Y.; Li, A. Effect of pore structure on the removal of clofibric acid by magnetic anion exchange resin. Chemosphere 2018, 191, 817–824. [Google Scholar] [CrossRef]
- Yousatit, S.; Pitayachinchot, H.; Wijitrat, A.; Chaowamalee, S.; Nuntang, S.; Soontaranon, S.; Rugmai, S.; Yokoi, T.; Ngamcharussrivichai, C. Natural rubber as a renewable carbon source for mesoporous carbon/silica nanocomposites. Sci. Rep. 2020, 10, 12977. [Google Scholar] [CrossRef]
- Nuntang, S.; Yousatit, S.; Chaowamalee, S.; Yokoi, T.; Tatsumi, T.; Ngamcharussrivichai, C. Mesostructured natural rubber/in situ formed silica nanocomposites: A simple way to prepare mesoporous silica with hydrophobic properties. Microporous Mesoporous Mater. 2018, 259, 79–88. [Google Scholar] [CrossRef]
- Nuntang, S.; Yokoi, T.; Tatsumi, T.; Ngamcharussrivichai, C. Enhanced esterification of carboxylic acids with ethanol using propylsulfonic acid-functionalized natural rubber/hexagonal mesoporous silica nanocomposites. Catal. Commun. 2016, 80, 5–9. [Google Scholar] [CrossRef]
- Nuntang, S.; Poompradub, S.; Butnark, S.; Yokoi, T.; Tatsumi, T.; Ngamcharussrivichai, C. Organosulfonic acid-functionalized mesoporous composites based on natural rubber and hexagonal mesoporous silica. Mater. Chem. Phys. 2014, 147, 583–593. [Google Scholar] [CrossRef]
- Nuntang, S.; Poompradub, S.; Butnark, S.; Yokoi, T.; Tatsumi, T.; Ngamcharussrivichai, C. Novel mesoporous composites based on natural rubber and hexagonal mesoporous silica: Synthesis and characterization. Mater. Chem. Phys. 2014, 143, 1199–1208. [Google Scholar] [CrossRef]
- Yousatit, S.; Jittapasata, T.; Leelaphattharaphan, N.; Nuntang, S.; Ngamcharussrivichai, C. One-pot synthesis of wormhole-like mesostructured silica with a high amine loading for enhanced adsorption of clofibric acid. J. Porous Mater. 2018, 25, 1611–1623. [Google Scholar] [CrossRef]
- Suriyanon, N.; Punyapalakul, P.; Ngamcharussrivichai, C. Mechanistic study of diclofenac and carbamazepine adsorption on functionalized silica-based porous materials. Chem. Eng. J. 2013, 214, 208–218. [Google Scholar] [CrossRef]
- Zhao, X.S.; Lu, G.Q.; Whittaker, A.K.; Millar, G.J.; Zhu, H.Y. Comprehensive study of surface chemistry of MCM-41 using 29Si CP/MAS NMR, FTIR, Pyridine-TPD, and TGA. J. Phys. Chem. B 1997, 101, 6525–6531. [Google Scholar] [CrossRef]
- Rubio, F.; Rubio, J.; Oteo, J.L. A FT-IR study of the hydrolysis of tetraethylorthosilicate (TEOS). Spectrosc. Lett. 1998, 31, 199–219. [Google Scholar] [CrossRef]
- Wang, X.; Lin, K.S.; Chan, J.C.; Cheng, S. Direct synthesis and catalytic applications of ordered large pore aminopropyl-functionalized SBA-15 mesoporous materials. J. Phys. Chem. B 2005, 109, 1763–1769. [Google Scholar] [CrossRef]
- Zakeri Siavashani, A.; Haghbin Nazarpak, M.; Fayyazbakhsh, F.; Toliyat, T.; McInnes, S.J.P.; Solati-Hashjin, M. Effect of amino-functionalization on insulin delivery and cell viability for two types of silica mesoporous structures. J. Mater. Sci. 2016, 51, 10897–10909. [Google Scholar] [CrossRef]
- Maria Chong, A.S.; Zhao, X.S. Functionalization of SBA-15 with APTES and characterization of functionalized materials. J. Phys. Chem. B 2003, 107, 12650–12657. [Google Scholar] [CrossRef]
- Hakiki, A.; Boukoussa, B.; Habib Zahmani, H.; Hamacha, R.; Hadj Abdelkader, N.e.H.; Bekkar, F.; Bettahar, F.; Nunes-Beltrao, A.P.; Hacini, S.; Bengueddach, A.; et al. Synthesis and characterization of mesoporous silica SBA-15 functionalized by mono-, di-, and tri-amine and its catalytic behavior towards Michael addition. Mater. Chem. Phys. 2018, 212, 415–425. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Ghezelbash, M.; Shabanian, M.; Aryanasab, F.; Saeb, M.R. Efficient removal of cationic dyes from colored wastewaters by dithiocarbamate-functionalized graphene oxide nanosheets: From synthesis to detailed kinetics studies. J. Taiwan Inst. Chem. Eng. 2017, 81, 239–246. [Google Scholar] [CrossRef]
- Nakanishi, K.; Tomita, M.; Kato, K. Synthesis of amino-functionalized mesoporous silica sheets and their application for metal ion capture. J. Asian Ceram. Soc. 2018, 3, 70–76. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Asghari, M.; Mahmoodi, N.M. Chitosan-wrapped multiwalled carbon nanotube as filler within PEBA thin film nanocomposite (TFN) membrane to improve dye removal. Carbohydr. Polym. 2020, 237, 116128. [Google Scholar] [CrossRef]
- Mello, M.R.; Phanon, D.; Silveira, G.Q.; Llewellyn, P.L.; Ronconi, C.M. Amine-modified MCM-41 mesoporous silica for carbon dioxide capture. Microporous Mesoporous Mater. 2011, 143, 174–179. [Google Scholar] [CrossRef]
- Gokulakrishnan, N.; Karbowiak, T.; Bellat, J.P.; Vonna, L.; Saada, M.-A.; Paillaud, J.L.; Soulard, M.; Patarin, J.; Parmentier, J. Improved hydrophobicity of inorganic–organic hybrid mesoporous silica with cage-like pores. Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 34–43. [Google Scholar] [CrossRef]
- Harlick, P.J.E.; Sayari, A. Applications of pore-expanded mesoporous silicas. 3. triamine silane grafting for enhanced CO2 adsorption. Ind. Eng. Chem. Res. 2006, 45, 3248–3255. [Google Scholar] [CrossRef]
- Cullis, C.F.; Laver, H.S. The thermal degradation and oxidation of polybutadiene. Eur. Polym. J. 1978, 14, 571–573. [Google Scholar] [CrossRef]
- McNeill, I.C.; Stevenson, W.T.K. The structure and stability of oxidised polybutadiene. Polym. Degrad. Stab. 1985, 11, 123–143. [Google Scholar] [CrossRef]
- Tanev, P.T.; Pinnavaia, T.J. Mesoporous silica molecular sieves prepared by ionic and neutral surfactant templating: A comparison of physical properties. Chem. Mater. 1996, 8, 2068–2079. [Google Scholar] [CrossRef]
- Musso, G.E.; Bottinelli, E.; Celi, L.; Magnacca, G.; Berlier, G. Influence of surface functionalization on the hydrophilic character of mesoporous silica nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 13882–13894. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U.; Mezei, R.; Fratzl, P.; Riegel, B.; Kiefer, W.; Kohler, D.; Mader, W. Formation and Structure of Gel Networks from Si(OEt)4/(MeO)3Si(CH2)3NR‘2Mixtures (NR‘2= NH2or NHCH2CH2NH2). Chem. Mater. 1999, 11, 451–457. [Google Scholar] [CrossRef]
- Gao, C.; Qiu, H.; Zeng, W.; Sakamoto, Y.; Terasaki, O.; Sakamoto, K.; Chen, Q.; Che, S. Formation mechanism of anionic surfactant-templated mesoporous silica. Chem. Mater. 2006, 18, 3904–3914. [Google Scholar] [CrossRef]
- Nuntang, S.; Yousatit, S.; Yokoi, T.; Ngamcharussrivichai, C. Tunable mesoporosity and hydrophobicity of natural rubber/hexagonal mesoporous silica nanocomposites. Microporous Mesoporous Mater. 2019, 275, 235–243. [Google Scholar] [CrossRef]
- Bui, T.X.; Kang, S.Y.; Lee, S.H.; Choi, H. Organically functionalized mesoporous SBA-15 as sorbents for removal of selected pharmaceuticals from water. J. Hazard. Mater. 2011, 193, 156–163. [Google Scholar] [CrossRef]
- Sharma, A.; Chaudhry, S.A. Adsorption of pharmaceutical pollutants using lignocellulosic materials. In Green Materials for Wastewater Treatment; Naushad, M., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 277–289. [Google Scholar]
- Barczak, M. Amine-modified mesoporous silicas: Morphology-controlled synthesis toward efficient removal of pharmaceuticals. Microporous Mesoporous Mater. 2019, 278, 354–365. [Google Scholar] [CrossRef]
- Lotfi, R.; Hayati, B.; Rahimi, S.; Shekarchi, A.A.; Mahmoodi, N.M.; Bagheri, A. Synthesis and characterization of PAMAM/SiO2 nanohybrid as a new promising adsorbent for pharmaceuticals. Microchem. J. 2019, 146, 1150–1159. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef]
- Saxena, M.; Kushwaha, J.P.; Kulshreshtha, S.; Kaur, G.; Singh, N. Doxycycline adsorptive interaction with mesoporous MCM-41: Kinetic and isotherm modelling with thermodynamics. Chem. Afr. 2022, 5, 1055–1068. [Google Scholar] [CrossRef]
- Bouyahmed, F.; Cai, M.; Reinert, L.; Duclaux, L.; Dey, R.; Youcef, H.; Lahcini, M.; Muller, F.; Delpeux-Ouldriane, S. A wide adsorption range hybrid material based on chitosan, activated carbon and montmorillonite for water treatment. C 2018, 4, 35. [Google Scholar] [CrossRef]
- Cabrera-Lafaurie, W.A.; Roman, F.R.; Hernandez-Maldonado, A.J. Transition metal modified and partially calcined inorganic-organic pillared clays for the adsorption of salicylic acid, clofibric acid, carbamazepine, and caffeine from water. J. Colloid Interface Sci. 2012, 386, 381–391. [Google Scholar] [CrossRef]
- Bui, T.X.; Choi, H. Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J. Hazard. Mater. 2009, 168, 602–608. [Google Scholar] [CrossRef]
- Bo, L.; Gao, N.; Liu, J.; Gao, B. The competitive adsorption of pharmaceuticals on granular activated carbon in secondary effluent. Desalination Water Treat. 2015, 57, 17023–17029. [Google Scholar] [CrossRef]
- Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Alahabadi, A.; Hosseini-Bandegharaei, A.; Moussavi, G.; Amin, B.; Rastegar, A.; Karimi-Sani, H.; Fattahi, M.; Miri, M. Comparing adsorption properties of NH 4 Cl-modified activated carbon towards chlortetracycline antibiotic with those of commercial activated carbon. J. Mol. Liq. 2017, 232, 367–381. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
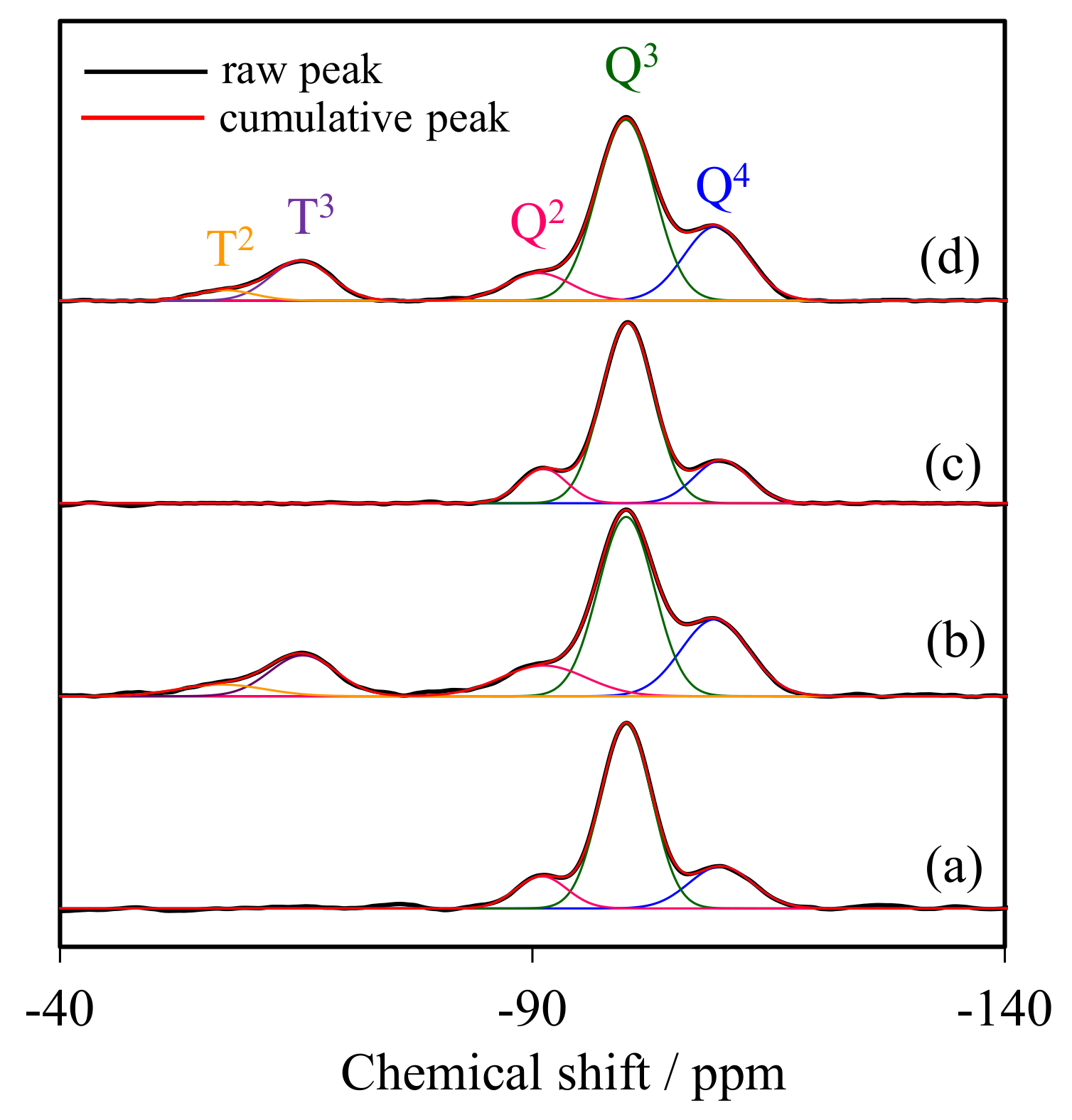

| Sample | Si Species Distribution (%) | ΣTm/Σ(Tm + Qn) (%) | ||||
|---|---|---|---|---|---|---|
| T2 | T3 | Q2 | Q3 | Q4 | ||
| WMS | – | – | 11.89 | 68.79 | 19.32 | – |
| WMS-0.10 | 4.78 | 12.75 | 8.54 | 49.38 | 24.55 | 17.53 |
| NR/WMS | – | – | 17.56 | 70.43 | 12.01 | – |
| NR/WMS-0.10 | 3.12 | 11.97 | 8.93 | 52.31 | 23.67 | 15.09 |
| Sample a | Amine Concentration (mmolN g−1) | SBET c (m2 g−1) | Sext d (m2 g−1) | Dp e (nm) | Vt f (cm3 g−1) | Vp g (cm3 g−1) | d100 h (nm) | a0 i (nm) | Wt j (nm) | Vm k (cm3 g−1) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Theoretical | Experimental b | ||||||||||
| WMS | 0.00 | 0.00 | 986 | 469 | 2.80 | 2.41 | 0.52 | 4.51 | 5.21 | 2.41 | 70.4 |
| WMS-0.10 | 1.54 | 1.47 | 420 | 102 | 2.94 | 1.05 | 0.29 | 4.70 | 5.43 | 2.49 | 58.1 |
| NR/WMS | 0.00 | 0.00 | 492 | 241 | 2.90 | 1.34 | 0.23 | 4.91 | 5.67 | 2.77 | 43.7 |
| NR/WMS-0.05 | 0.81 | 0.43 | 378 | 184 | 2.63 | 1.06 | 0.15 | 4.80 | 5.55 | 2.92 | 42.1 |
| NR/WMS-0.10 | 1.54 | 1.23 | 238 | 110 | 2.55 | 0.92 | 0.10 | 4.75 | 5.49 | 2.94 | 36.2 |
| NR/WMS-0.15 | 2.20 | 1.84 | 115 | 21 | 2.48 | 0.14 | 0.08 | 4.73 | 5.46 | 2.98 | 33.2 |
| Adsorbent | qe,exp (mg g−1) | Pseudo-First Order | Pseudo-Second Order | Ritchie-Second Order | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe,cal (mg g−1) | R2 | k2 (g mg−1 min−1) | qe,cal (mg g−1) | R2 | kr (L min−1) | qe,cal (mg g−1) | R2 | ||
| WMS | 0.08 | 0.07 | 0.07 | 0.981 | 1.84 | 0.08 | 1.000 | 0.17 | 0.08 | 0.997 |
| WMS-0.10 | 0.25 | 0.06 | 0.20 | 0.974 | 0.46 | 0.26 | 0.999 | 0.14 | 0.23 | 0.996 |
| NR/WMS | 0.14 | 0.05 | 0.11 | 0.977 | 0.79 | 0.15 | 0.995 | 0.18 | 0.13 | 0.968 |
| NR/WMS-0.05 | 0.93 | 0.07 | 0.30 | 0.986 | 0.33 | 0.95 | 1.000 | 0.42 | 0.92 | 0.959 |
| NR/WMS-0.10 | 0.40 | 0.05 | 0.18 | 0.981 | 0.56 | 0.41 | 1.000 | 0.27 | 0.39 | 0.991 |
| NR/WMS-0.15 | 0.32 | 0.05 | 0.13 | 0.987 | 0.82 | 0.32 | 0.999 | 0.38 | 0.30 | 0.953 |
| Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| kL (L mg−1) | qm (mg g−1) | R2 | n | kF | R2 | |
| WMS | 0.008 | 1.81 | 0.996 | 1.13 | 0.017 | 0.999 |
| WMS-0.10 | 0.016 | 3.73 | 0.993 | 1.38 | 0.103 | 0.976 |
| NR/WMS | 0.006 | 3.13 | 0.969 | 0.84 | 0.012 | 0.999 |
| NR/WMS-0.05 | 0.026 | 6.29 | 0.999 | 1.55 | 0.305 | 0.993 |
| NR/WMS-0.10 | 0.022 | 5.66 | 0.998 | 1.50 | 0.230 | 0.993 |
| NR/WMS-0.15 | 0.020 | 4.30 | 0.997 | 1.44 | 0.149 | 0.991 |
| Temperature (K) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (kJ mol−1 K−1) |
|---|---|---|---|
| 298 | −13.18 | 8.08 | 71.36 |
| 308 | −13.90 | ||
| 318 | −14.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousatit, S.; Rungruangwattanachot, W.; Yuwawanitchakorn, N.; Nuntang, S.; Punyapalakul, P.; Ngamcharussrivichai, C. Amine-Functionalized Natural Rubber/Mesostructured Silica Nanocomposites for Adsorptive Removal of Clofibric Acid in Aqueous Phase. Molecules 2023, 28, 2330. https://doi.org/10.3390/molecules28052330
Yousatit S, Rungruangwattanachot W, Yuwawanitchakorn N, Nuntang S, Punyapalakul P, Ngamcharussrivichai C. Amine-Functionalized Natural Rubber/Mesostructured Silica Nanocomposites for Adsorptive Removal of Clofibric Acid in Aqueous Phase. Molecules. 2023; 28(5):2330. https://doi.org/10.3390/molecules28052330
Chicago/Turabian StyleYousatit, Satit, Witsarut Rungruangwattanachot, Natthakit Yuwawanitchakorn, Sakdinun Nuntang, Patiparn Punyapalakul, and Chawalit Ngamcharussrivichai. 2023. "Amine-Functionalized Natural Rubber/Mesostructured Silica Nanocomposites for Adsorptive Removal of Clofibric Acid in Aqueous Phase" Molecules 28, no. 5: 2330. https://doi.org/10.3390/molecules28052330
APA StyleYousatit, S., Rungruangwattanachot, W., Yuwawanitchakorn, N., Nuntang, S., Punyapalakul, P., & Ngamcharussrivichai, C. (2023). Amine-Functionalized Natural Rubber/Mesostructured Silica Nanocomposites for Adsorptive Removal of Clofibric Acid in Aqueous Phase. Molecules, 28(5), 2330. https://doi.org/10.3390/molecules28052330








