Effect of Polyethylene Glycol Additive on the Structure and Performance of Fabric-Reinforced Thin Film Composite
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of TFC Membrane
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cheng, X.; Xu, Y.; Lei, Z.; Du, J. Investigation on operational parameters and membrane fouling performance in treating synthetic aquaculture wastewater via forward osmosis with sucrose as draw solution. Sci. Total. Environ. 2022, 847, 157573. [Google Scholar] [CrossRef] [PubMed]
- Giagnorio, M.; Morciano, M.; Zhang, W.; Hélix-Nielsen, C.; Fasano, M.; Tiraferri, A. Coupling of forward osmosis with desalination technologies: System-scale analysis at the water-energy nexus. Desalination 2022, 543, 116083. [Google Scholar] [CrossRef]
- Minier-Matar, J.; Al-Maas, M.; Hussain, A.; Nasser, M.S.; Adham, S. Pilot-scale evaluation of forward osmosis membranes for volume reduction of industrial wastewater. Desalination 2022, 531, 115689. [Google Scholar] [CrossRef]
- Mohammadifakhr, M.; de Grooth, J.; Trzaskus, K.; Roesink, H.D.W.; Kemperman, A.J.B. Single-step synthesis of a polyelectrolyte complex hollow-fiber membrane for forward osmosis. Sep. Purif. Technol. 2021, 264, 118430. [Google Scholar] [CrossRef]
- Pejman, M.; Dadashi Firouzjaei, M.; Aghapour Aktij, S.; Das, P.; Zolghadr, E.; Jafarian, H.; Arabi Shamsabadi, A.; Elliott, M.; Sadrzadeh, M.; Sangermano, M.; et al. In Situ Ag-MOF Growth on Pre-Grafted Zwitterions Imparts Outstanding Antifouling Properties to Forward Osmosis Membranes. ACS Appl. Mater. Interfaces 2020, 12, 36287–36300. [Google Scholar] [CrossRef]
- Sanahuja-Embuena, V.; Frauholz, J.; Oruc, T.; Trzaskus, K.; Hélix-Nielsen, C. Transport mechanisms behind enhanced solute rejection in forward osmosis compared to reverse osmosis mode. J. Membr. Sci. 2021, 636, 119561. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.H.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- Rafiee, H.; Shakeri, A.; Mahdavi, H. Layer-by-layer assembly of alginate/Ca2+ as interlayer on microfiltration substrate: Fabrication of high selective thin-film composite forward osmosis membrane for efficient heavy metal ions removal. Chem. Eng. Res. Des. 2022, 188, 564–574. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Xiao, P.; Li, J.; Tian, E.; Zhao, Y.; Ren, Y. High water permeable free-standing cellulose triacetate/graphene oxide membrane with enhanced antibiofouling and mechanical properties for forward osmosis. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 327–335. [Google Scholar] [CrossRef]
- Tavakol, I.; Hadadpour, S.; Shabani, Z.; Ahmadzadeh Tofighy, M.; Mohammadi, T.; Sahebi, S. Synthesis of novel thin film composite (TFC) forward osmosis (FO) membranes incorporated with carboxylated carbon nanofibers (CNFs). J. Environ. Chem. Eng. 2020, 8, 104614. [Google Scholar] [CrossRef]
- Nguyen, T.P.N.; Jun, B.M.; Lee, J.H.; Kwon, Y.N. Comparison of integrally asymmetric and thin film composite structures for a desirable fashion of forward osmosis membranes. J. Membr. Sci. 2015, 495, 457–470. [Google Scholar] [CrossRef]
- Zainal Abidin, N.H.; Shafie, S.N.A.; Suhaimi, H.; Sambudi, N.S.; Sapiaa Md Nordin, N.A.H. Incorporation of carboxyl and amino functionalized carbon quantum dots in thin film membrane for nanofiltration. Polym. Test. 2021, 100, 107270. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Tian, E.; Li, J.; Ren, Y. Graphene Oxide-Based Polymeric Membranes for Water Treatment. Adv. Mater. Interfaces 2018, 5, 1701427. [Google Scholar] [CrossRef]
- Deshmukh, A.; Yip, N.Y.; Lin, S.H.; Elimelech, M. Desalination by forward osmosis: Identifying performance limiting parameters through module-scale modeling. J. Membr. Sci. 2015, 491, 159–167. [Google Scholar] [CrossRef]
- Song, X.X.; Liu, Z.Y.; Sun, D.R.D.L. Nano Gives the Answer: Breaking the Bottleneck of Internal Concentration Polarization with a Nanofiber Composite Forward Osmosis Membrane for a High Water Production Rate. Adv. Mater. 2011, 23, 3256–3260. [Google Scholar] [CrossRef]
- Liang, H.-Q.; Hung, W.-S.; Yu, H.-H.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y.; Xu, Z.-K. Forward osmosis membranes with unprecedented water flux. J. Membr. Sci. 2017, 529, 47–54. [Google Scholar] [CrossRef]
- Tiraferri, A.; Yip, N.Y.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. Relating performance of thin-film composite forward osmosis membranes to support layer formation and structure. J. Membr. Sci. 2011, 367, 340–352. [Google Scholar] [CrossRef]
- Ren, J.; O’Grady, B.; deJesus, G.; McCutcheon, J.R. Sulfonated polysulfone supported high performance thin film composite membranes for forward osmosis. Polymer 2016, 103, 486–497. [Google Scholar] [CrossRef]
- Park, M.J.; Phuntsho, S.; He, T.; Nisola, G.M.; Tijing, L.D.; Li, X.M.; Chen, G.; Chung, W.J.; Shon, H.K. Graphene oxide incorporated polysulfone substrate for the fabrication of flat-sheet thin-film composite forward osmosis membranes. J. Membr. Sci. 2015, 493, 496–507. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, X.; Zuo, J.; Wang, Y. Performance enhancement of TFC FO membranes with polyethyleneimine modification and post-treatment. J. Membr. Sci. 2017, 534, 46–58. [Google Scholar] [CrossRef]
- Hegab, H.M.; ElMekawy, A.; Barclay, T.G.; Michelmore, A.; Zou, L.; Saint, C.P.; Ginic-Markovic, M. Fine-Tuning the Surface of Forward Osmosis Membranes via Grafting Graphene Oxide: Performance Patterns and Biofouling Propensity. ACS Appl. Mater. Inter. 2015, 7, 18004–18016. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Xiong, S.; Wang, Y. Graphene oxide incorporated thin-film composite membranes for forward osmosis applications. Chem. Eng. Sci. 2016, 143, 194–205. [Google Scholar] [CrossRef]
- Song, X.J.; Wang, L.; Mao, L.L.; Wang, Z.N. Nanocomposite Membrane with Different Carbon Nanotubes Location for Nanofiltration and Forward Osmosis Applications. ACS Sustain. Chem. Eng. 2016, 4, 2990–2997. [Google Scholar] [CrossRef]
- Xu, S.J.; Li, F.; Su, B.W.; Hu, M.Z.; Gao, X.L.; Gao, C.J. Novel graphene quantum dots (GQDs)-incorporated thin film composite (TFC) membranes for forward osmosis (FO) desalination. Desalination 2019, 451, 219–230. [Google Scholar] [CrossRef]
- Li, X.S.; Loh, C.H.; Wang, R.; Widjajanti, W.; Torres, J. Fabrication of a robust high-performance FO membrane by optimizing substrate structure and incorporating aquaporin into selective layer. J. Membr. Sci. 2017, 525, 257–268. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Ren, Y.; Pei, D. Mesh-Embedded Polysulfone/Sulfonated Polysulfone Supported Thin Film Composite Membranes for Forward Osmosis. ACS Appl. Mater. Interfaces 2018, 10, 2918–2928. [Google Scholar] [CrossRef]
- Yip, N.Y.; Tiraferri, A.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. High Performance Thin-Film Composite Forward Osmosis Membrane. Environ. Sci. Technol. 2010, 44, 3812–3818. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, C.Q.; Tang, C.Y.Y.; Wang, R.; Fane, A.G. Synthesis and characterization of flat-sheet thin film composite forward osmosis membranes. J. Membr. Sci. 2011, 372, 292–302. [Google Scholar] [CrossRef]
- Han, G.; Zhao, B.W.; Fu, F.J.; Chung, T.S.; Weber, M.; Staudt, C.; Maletzko, C. High performance thin-film composite membranes with mesh-reinforced hydrophilic sulfonated polyphenylenesulfone (sPPSU) substrates for osmotically driven processes. J. Membr. Sci. 2016, 502, 84–93. [Google Scholar] [CrossRef]
- Liu, Y.; Koops, G.H.; Strathmann, H. Characterization of morphology controlled polyethersulfone hollow fiber membranes by the addition of polyethylene glycol to the dope and bore liquid solution. J. Membr. Sci. 2003, 223, 187–199. [Google Scholar] [CrossRef]
- Yunos, M.Z.; Harun, Z.; Basri, H.; Ismail, A.F. Studies on fouling by natural organic matter (NOM) on polysulfone membranes: Effect of polyethylene glycol (PEG). Desalination 2014, 333, 36–44. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, F.; Wang, Z.; Wu, M.; Ma, J.; Gao, C. Preparation and characterization of PSf/clay nanocomposite membranes with PEG 400 as a pore forming additive. Desalination 2012, 286, 131–137. [Google Scholar] [CrossRef]
- Idris, A.; Mat Zain, N.; Noordin, M.Y. Synthesis, characterization and performance of asymmetric polyethersulfone (PES) ultrafiltration membranes with polyethylene glycol of different molecular weights as additives. Desalination 2007, 207, 324–339. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Karslyan, Y.A.; Ovcharova, A.A.; Volkov, V.V. Development of high flux ultrafiltration polyphenylsulfone membranes applying the systems with upper and lower critical solution temperatures: Effect of polyethylene glycol molecular weight and coagulation bath temperature. J. Membr. Sci. 2018, 565, 266–280. [Google Scholar] [CrossRef]
- Li, S.; Cui, Z.; Zhang, L.; He, B.; Li, J. The effect of sulfonated polysulfone on the compatibility and structure of polyethersulfone-based blend membranes. J. Membr. Sci. 2016, 513, 1–11. [Google Scholar] [CrossRef]
- Feng, Y.N.; Han, G.; Zhang, L.L.; Chen, S.B.; Chung, T.S.; Weber, M.; Staudt, C.; Maletzko, C. Rheology and phase inversion behavior of polyphenylenesulfone (PPSU) and sulfonated PPSU for membrane formation. Polymer 2016, 99, 72–82. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Aroujalian, A.; Raisi, A. Effect of lag time in interfacial polymerization on polyamide composite membrane with different hydrophilic sub layers. Desalination 2012, 284, 32–41. [Google Scholar] [CrossRef]
- Misdan, N.; Lau, W.J.; Ismail, A.F.; Matsuura, T.; Rana, D. Study on the thin film composite poly(piperazine-amide) nanofiltration membrane: Impacts of physicochemical properties of substrate on interfacial polymerization formation. Desalination 2014, 344, 198–205. [Google Scholar] [CrossRef]
- Klaysom, C.; Hermans, S.; Gahlaut, A.; Van Craenenbroeck, S.; Vankelecom, I.F.J. Polyamide/Polyacrylonitrile (PA/PAN) thin film composite osmosis membranes: Film optimization, characterization and performance evaluation. J. Membr. Sci. 2013, 445, 25–33. [Google Scholar] [CrossRef]
- Feng, Y.; Han, G.; Chung, T.-S.; Weber, M.; Widjojo, N.; Maletzko, C. Effects of polyethylene glycol on membrane formation and properties of hydrophilic sulfonated polyphenylenesulfone (sPPSU) membranes. J. Membr. Sci. 2017, 531, 27–35. [Google Scholar] [CrossRef]
- Tiraferri, A.; Yip, N.Y.; Straub, A.P.; Castrillon, S.R.V.; Elimelech, M. A method for the simultaneous determination of transport and structural parameters of forward osmosis membranes. J. Membr. Sci. 2013, 444, 523–538. [Google Scholar] [CrossRef]
- Stillman, D.; Krupp, L.; La, Y.H. Mesh-reinforced thin film composite membranes for forward osmosis applications: The structure-performance relationship. J. Membr. Sci. 2014, 468, 308–316. [Google Scholar] [CrossRef]
- Qiu, C.; Setiawan, L.; Wang, R.; Tang, C.Y.; Fane, A.G. High performance flat sheet forward osmosis membrane with an NF-like selective layer on a woven fabric embedded substrate. Desalination 2012, 287, 266–270. [Google Scholar] [CrossRef]
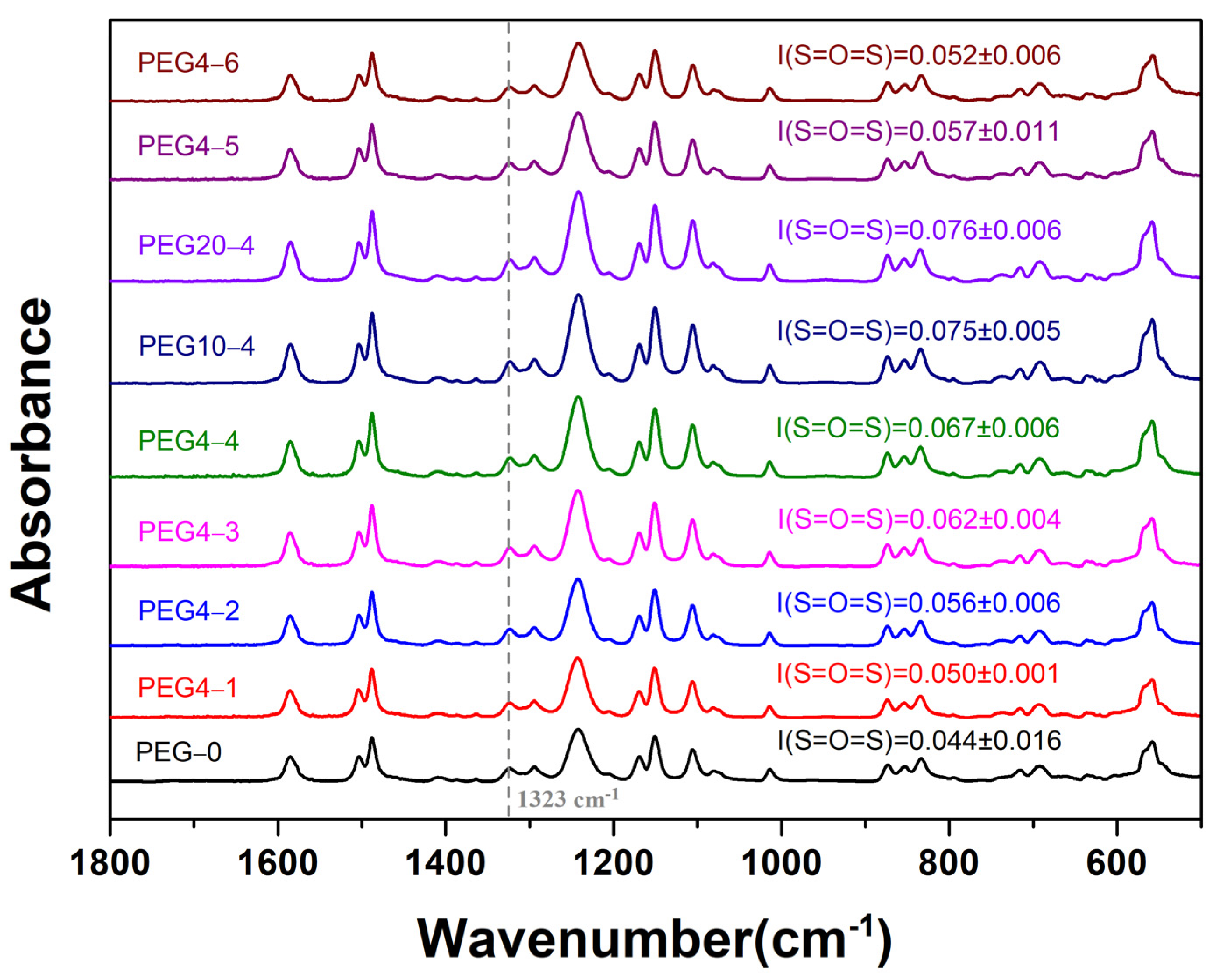


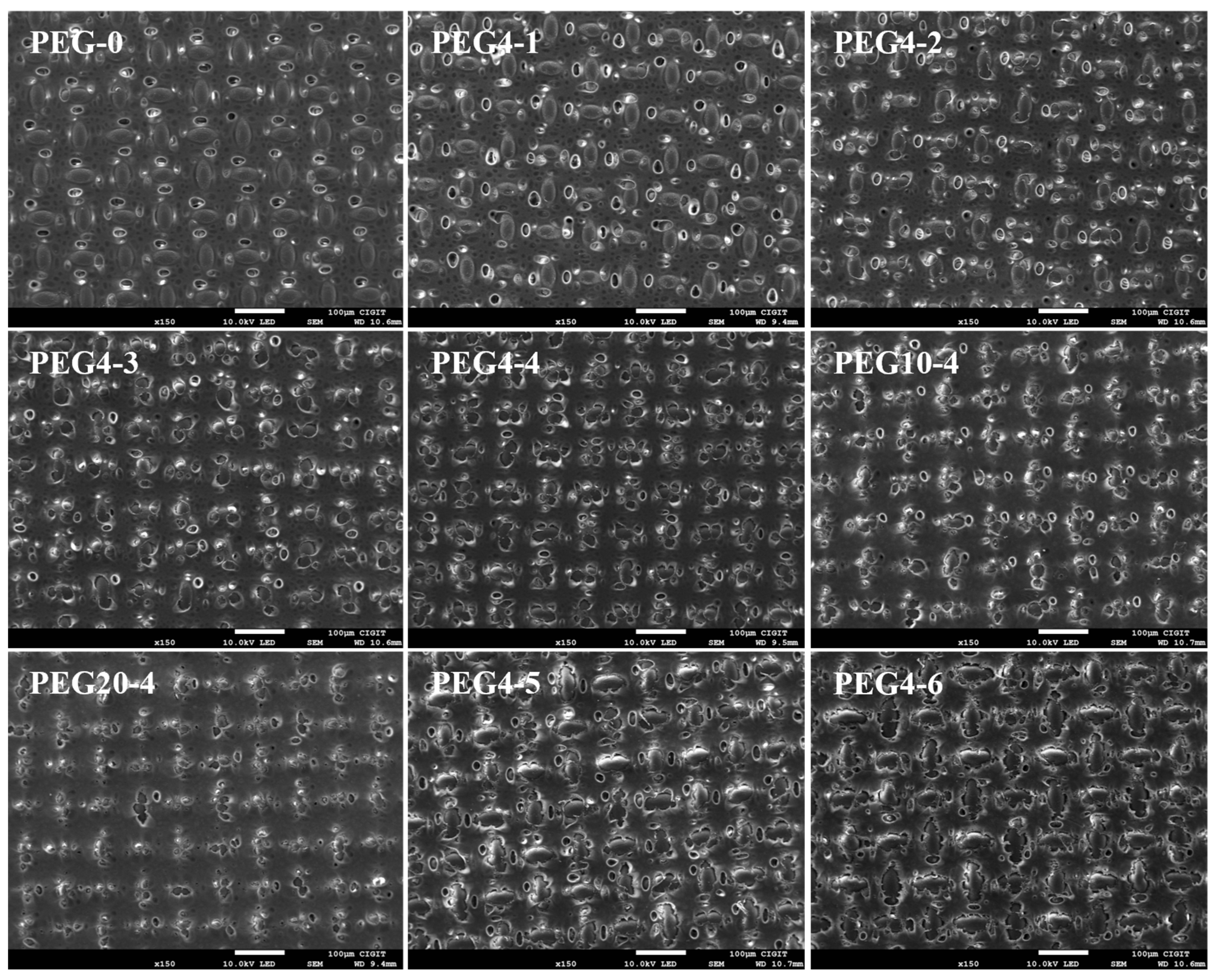


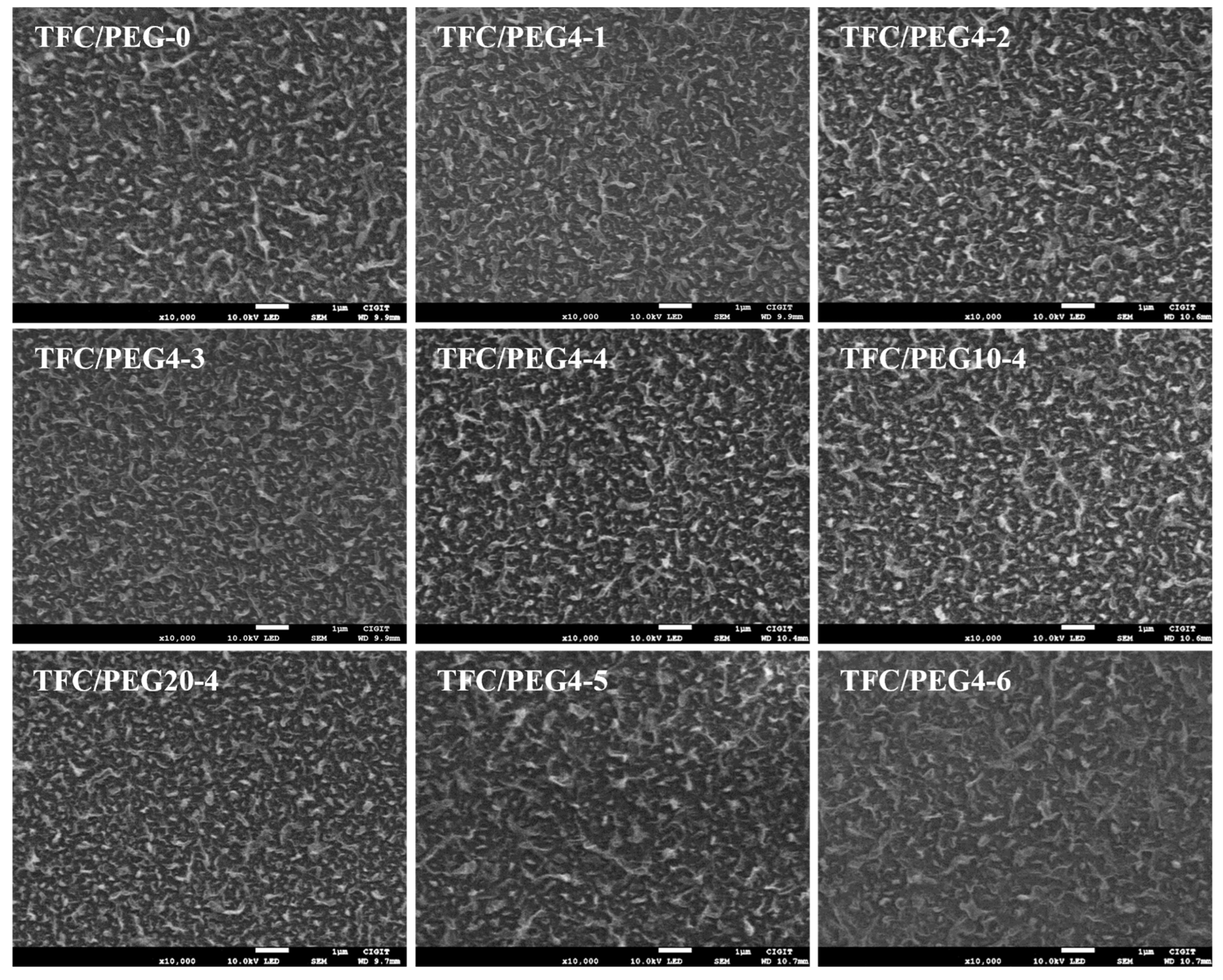

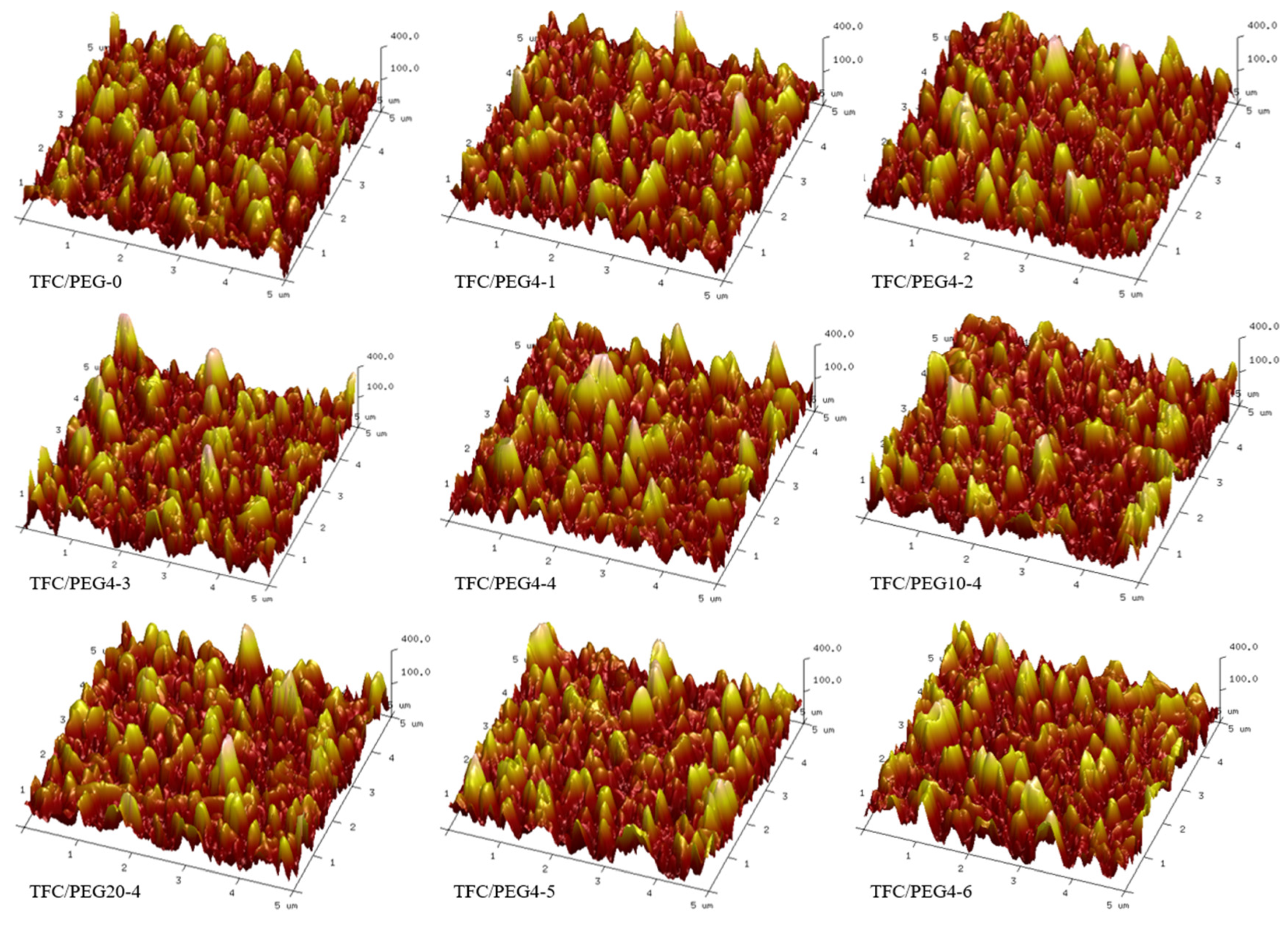
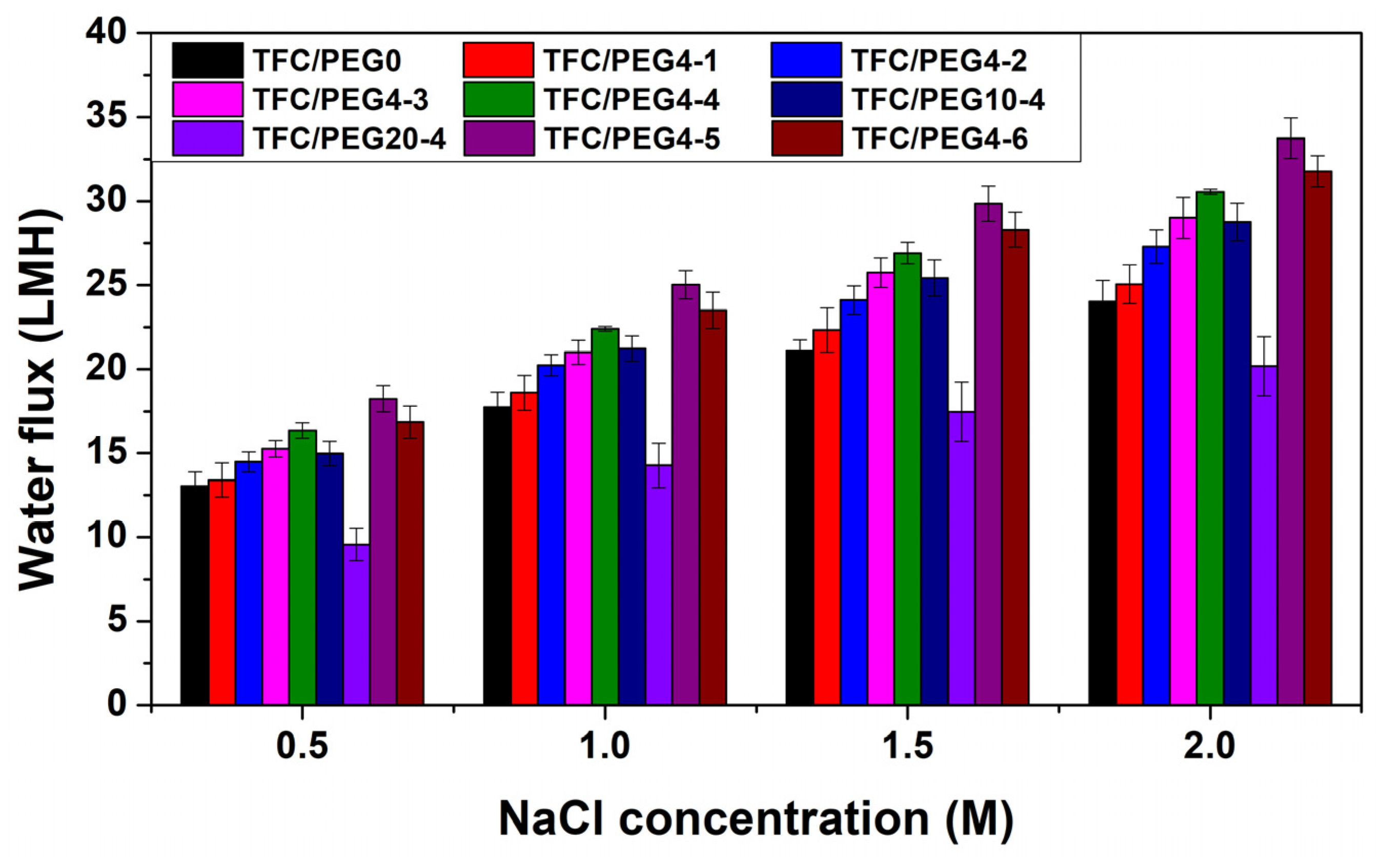

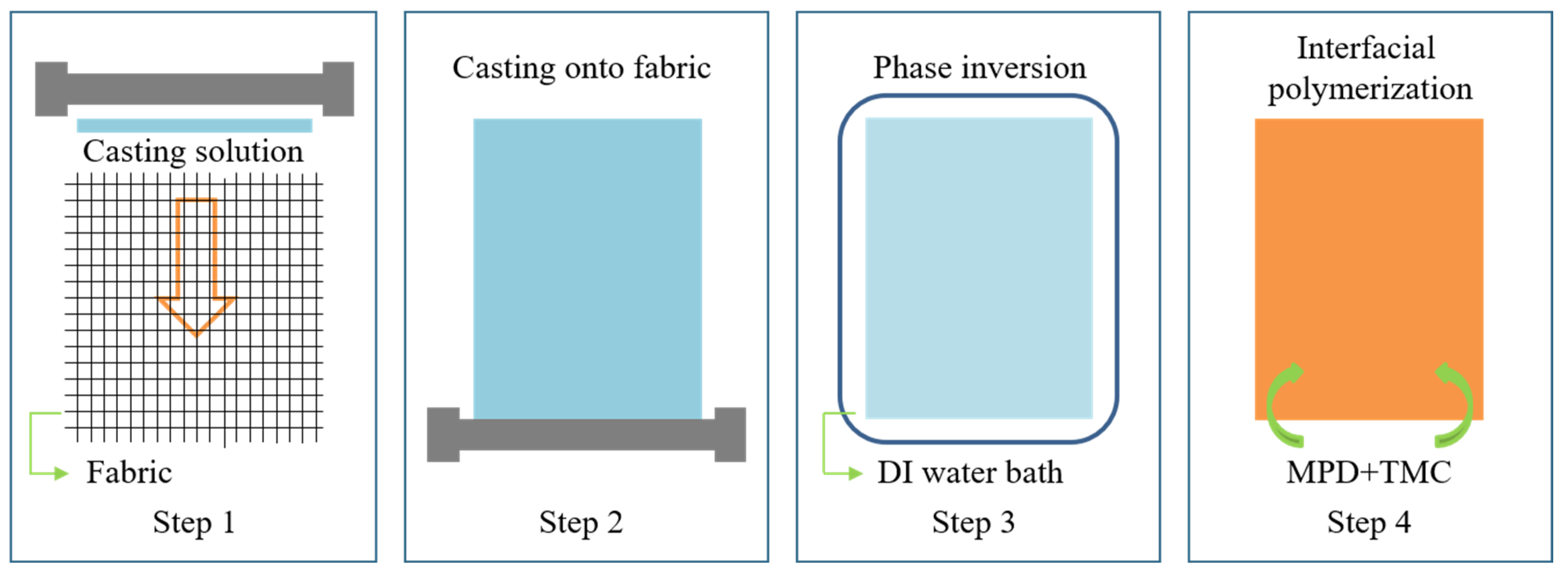
| Substrate Code | Composition (wt.%) | ||
|---|---|---|---|
| PSU | PEG | NMP | |
| PEG-0 | 15 | 0 | 85 |
| PEG4-1, PEG10-1, PEG20-1 | 15 | 5 | 80 |
| PEG4-2, PEG10-2, PEG20-2 | 15 | 10 | 75 |
| PEG4-3, PEG10-3, PEG20-3 | 15 | 15 | 70 |
| PEG4-4, PEG10-4, PEG20-4 | 15 | 20 | 65 |
| PEG4-5 | 12 | 20 | 68 |
| PEG4-6 | 9 | 20 | 71 |
| Substrate | Porosity (%) | dp (nm) | Contact Angle (°) | Thickness (μm) | ||
|---|---|---|---|---|---|---|
| Porous Layer | Substrate | Dry Membrane | Wet Membrane | |||
| PEG-0 | 80.1 ± 1.0 | 55.0 ± 1.1 | 19.0 ± 0.7 | 94.9 ± 0.7 | 77.0 ± 3.8 | 57.8 ± 1.4 |
| PEG4-1 | 82.9 ± 2.6 | 55.7 ± 1.5 | 15.0 ± 1.2 | 94.0 ± 0.6 | 72.7 ± 2.0 | 59.3 ± 0.9 |
| PEG4-2 | 85.3 ± 1.0 | 56.8 ± 3.1 | 14.0 ± 0.8 | 94.2 ± 1.2 | 74.1 ± 0.6 | 62.0 ± 2.2 |
| PEG4-3 | 85.5 ± 1.3 | 57.6 ± 0.7 | 11.8 ± 0.6 | 93.8 ± 0.8 | 73.0 ± 1.4 | 61.4 ± 0.3 |
| PEG4-4 | 84.6 ± 1.0 | 56.6 ± 1.4 | 10.8 ± 0.2 | 93.3 ± 1.0 | 73.8 ± 2.1 | 61.9 ± 1.9 |
| PEG10-4 | 84.7 ± 1.4 | 56.8 ± 0.8 | 9.9 ± 0.5 | 95.2 ± 1.6 | 73.1 ± 3.1 | 62.7 ± 2.2 |
| PEG20-4 | 84.6 ± 1.9 | 58.4 ± 2.6 | 7.8 ± 0.4 | 95.8 ± 1.0 | 74.1 ± 2.1 | 67.0 ± 2.5 |
| PEG4-5 | 85.7 ± 2.0 | 57.8 ± 3.2 | 17.2 ± 0.9 | 95.3 ± 0.5 | 75.6 ± 2.9 | 62.5 ± 2.4 |
| PEG4-6 | 89.5 ± 1.5 | 61.5 ± 0.5 | 23.3 ± 1.1 | 95.9 ± 0.8 | 76.1 ± 2.2 | 61.4 ± 3.4 |
| Membrane | Atomic Composition (%) | O I/O II Ratio | O/N Ratio | ||||
|---|---|---|---|---|---|---|---|
| C 1s | N 1s | O 1s | O I | O II | |||
| TFC/PEG-0 | 74.34 | 11.78 | 13.89 | 84.79 | 15.21 | 5.57 | 1.18 |
| TFC/PEG4-1 | 74.35 | 11.51 | 14.13 | 87.37 | 12.63 | 6.92 | 1.23 |
| TFC/PEG4-2 | 73.95 | 11.31 | 14.74 | 83.91 | 16.09 | 5.22 | 1.30 |
| TFC/PEG4-3 | 74.56 | 10.90 | 14.54 | 83.68 | 16.32 | 5.13 | 1.33 |
| TFC/PEG4-4 | 74.65 | 10.64 | 14.71 | 81.43 | 18.57 | 4.39 | 1.38 |
| TFC/PEG10-4 | 74.96 | 9.78 | 15.26 | 72.93 | 27.07 | 2.69 | 1.56 |
| TFC/PEG20-4 | 74.07 | 9.59 | 16.34 | 62.45 | 37.55 | 1.66 | 1.70 |
| TFC/PEG4-5 | 73.97 | 12.22 | 13.81 | 85.80 | 14.20 | 6.04 | 1.13 |
| TFC/PEG4-6 | 74.91 | 11.21 | 13.88 | 85.69 | 14.31 | 5.99 | 1.24 |
| Membrane | Rq (nm) | Ra (nm) |
|---|---|---|
| TFC/PEG-0 | 93.6 ± 5.9 | 75.2 ± 5.0 |
| TFC/PEG4-1 | 95.0 ± 7.9 | 75.9 ± 5.4 |
| TFC/PEG4-2 | 96.8 ± 2.3 | 77.4 ± 1.6 |
| TFC/PEG4-3 | 98.1 ± 9.8 | 78.5 ± 8.3 |
| TFC/PEG4-4 | 96.2 ± 5.8 | 76.6 ± 4.5 |
| TFC/PEG10-4 | 95.1 ± 9.9 | 75.8 ± 7.3 |
| TFC/PEG20-4 | 94.2 ± 7.8 | 74.5 ± 6.1 |
| TFC/PEG4-5 | 98.0 ± 10.3 | 78.4 ± 8.5 |
| TFC/PEG4-6 | 93.2 ± 5.4 | 73.3 ± 4.2 |
| Membrane | A (L m−2 h−1 bar−1) | B (L m−2 h−1) | A/B (bar−1) | S (×10−6 m) |
|---|---|---|---|---|
| TFC/PEG-0 | 1.26 ± 0.29 | 0.14 ± 0.02 | 8.98 ± 1.65 | 363.7 ± 36.9 |
| TFC/PEG4-1 | 1.13 ± 0.15 | 0.15 ± 0.02 | 8.09 ± 1.65 | 313.0 ± 9.0 |
| TFC/PEG4-2 | 1.24 ± 0.15 | 0.17 ± 0.05 | 7.75 ± 1.82 | 300.0 ± 24.3 |
| TFC/PEG4-3 | 1.28 ± 0.15 | 0.15 ± 0.04 | 8.97 ± 2.49 | 276.0 ± 13.8 |
| TFC/PEG4-4 | 1.37 ± 0.01 | 0.17 ± 0.02 | 8.28 ± 0.80 | 265.5 ± 7.8 |
| TFC/PEG10-4 | 1.16 ± 0.07 | 0.12 ± 0.01 | 9.43 ± 1.43 | 253.0 ± 9.0 |
| TFC/PEG20-4 | 0.61 ± 0.07 | 0.09 ± 0.02 | 6.98 ± 2.18 | 286.7 ± 23.1 |
| TFC/PEG4-5 | 1.65 ± 0.09 | 0.17 ± 0.03 | 9.92 ± 1.35 | 248.8 ± 18.7 |
| TFC/PEG4-6 | 1.41 ± 0.23 | 0.22 ± 0.07 | 6.68 ± 1.31 | 242.5 ± 23.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, Y.; Wen, X. Effect of Polyethylene Glycol Additive on the Structure and Performance of Fabric-Reinforced Thin Film Composite. Molecules 2023, 28, 2318. https://doi.org/10.3390/molecules28052318
Wang X, Zhao Y, Wen X. Effect of Polyethylene Glycol Additive on the Structure and Performance of Fabric-Reinforced Thin Film Composite. Molecules. 2023; 28(5):2318. https://doi.org/10.3390/molecules28052318
Chicago/Turabian StyleWang, Xiao, Yuntao Zhao, and Xueyou Wen. 2023. "Effect of Polyethylene Glycol Additive on the Structure and Performance of Fabric-Reinforced Thin Film Composite" Molecules 28, no. 5: 2318. https://doi.org/10.3390/molecules28052318
APA StyleWang, X., Zhao, Y., & Wen, X. (2023). Effect of Polyethylene Glycol Additive on the Structure and Performance of Fabric-Reinforced Thin Film Composite. Molecules, 28(5), 2318. https://doi.org/10.3390/molecules28052318






