Inhibition of TLR4 Alleviates Heat Stroke-Induced Cardiomyocyte Injury by Down-Regulating Inflammation and Ferroptosis
Abstract
1. Introduction
2. Results
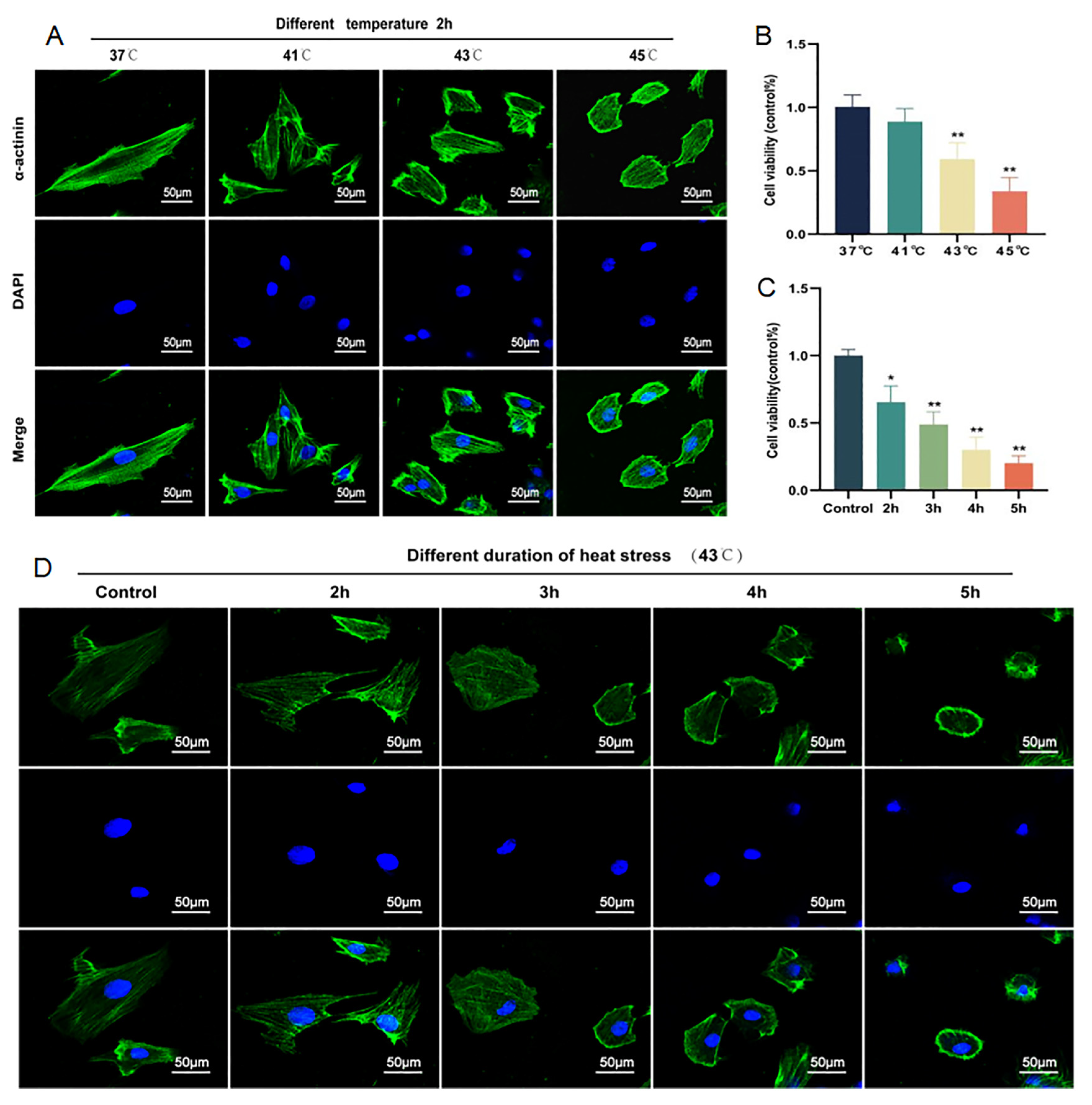
2.1. Cardiomyocyte Injury Can Be Induced by HS in a Time- and Temperature-Dependent Manner
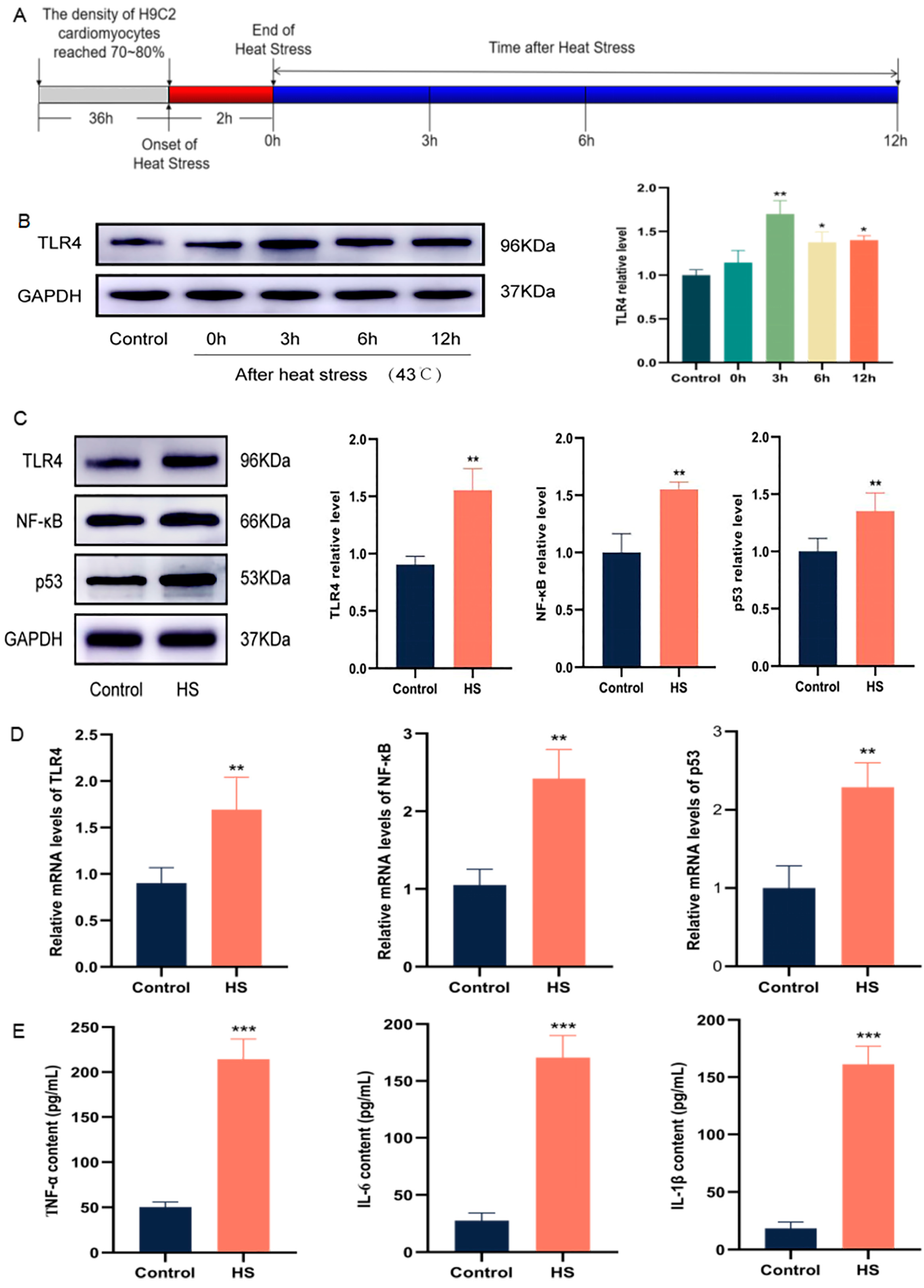
2.2. HS Activates a TLR4-Mediated Inflammatory Environment in Cardiomyocytes
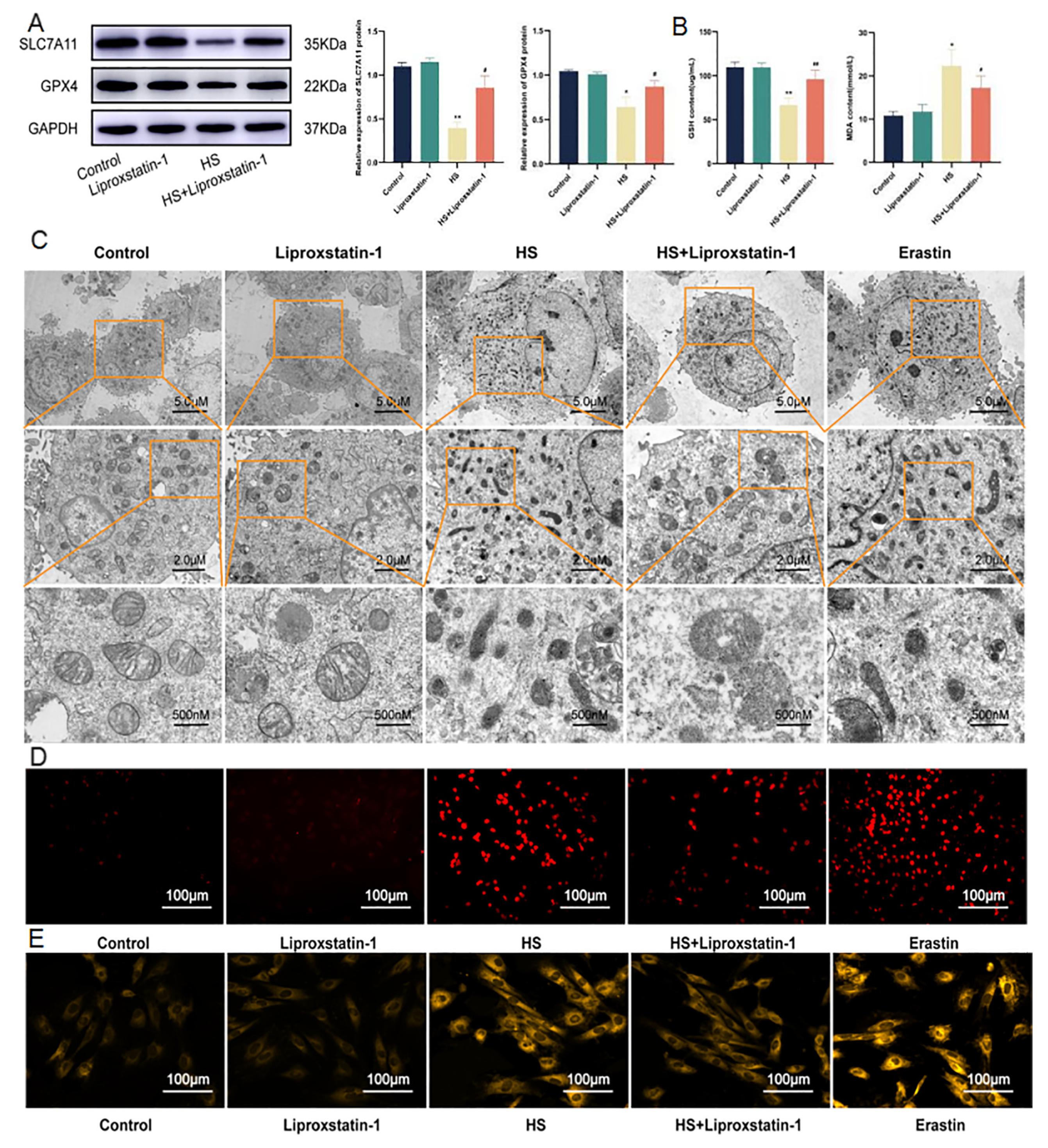
2.3. Ferroptosis of Cardiomyocytes Induced by HS Can Be Inhibited by Liproxstatin-1
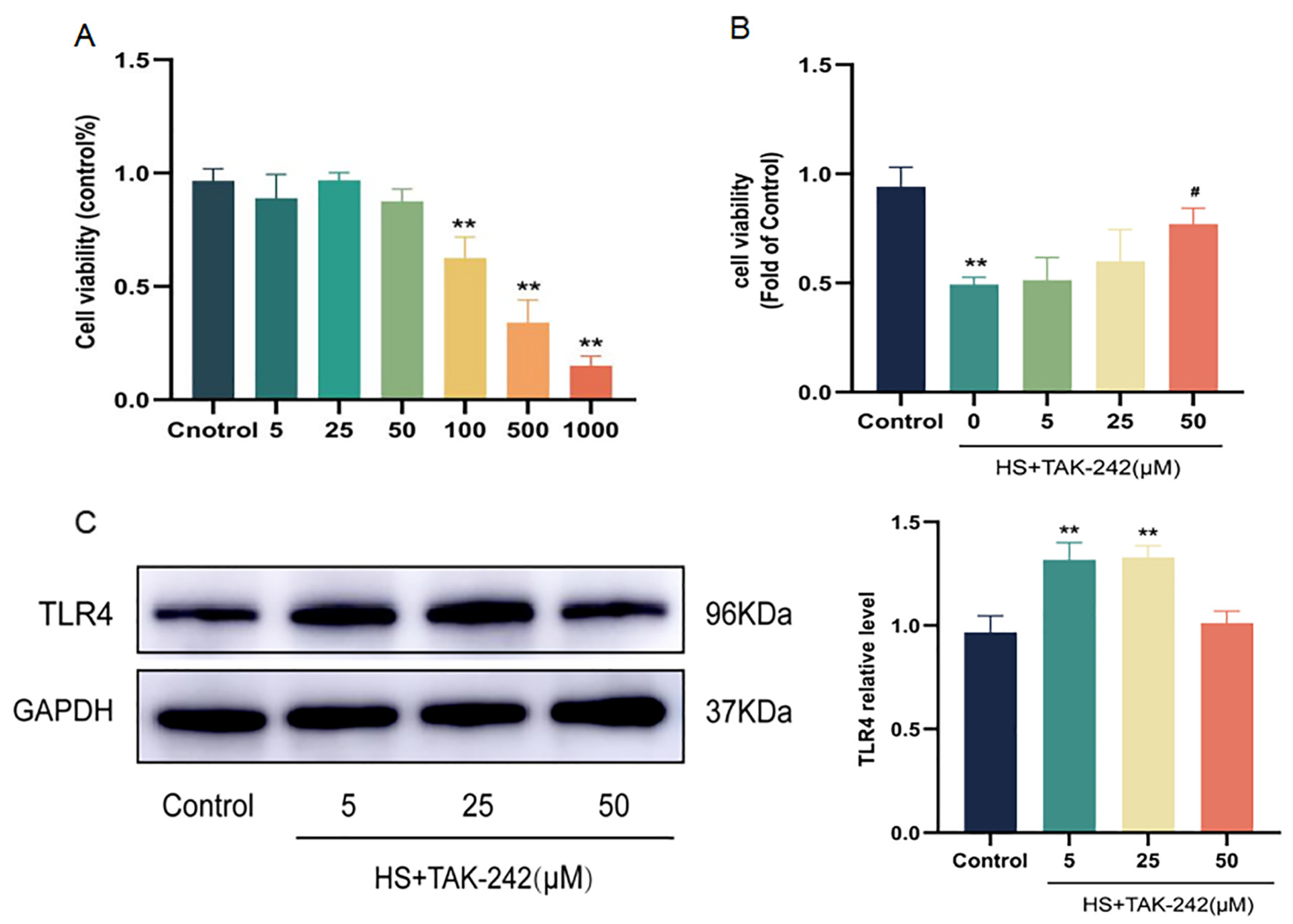
2.4. Inhibition of TLR4 Expression Can Improve Cardiomyocyte Injury Induced by HS
2.5. Inhibition of TLR4 Down-Regulates the Inflammatory Environment of Cardiomyocytes Induced by HS
2.6. Inhibition of TLR4 Alleviates Ferroptosis of Cardiomyocytes Induced by HS
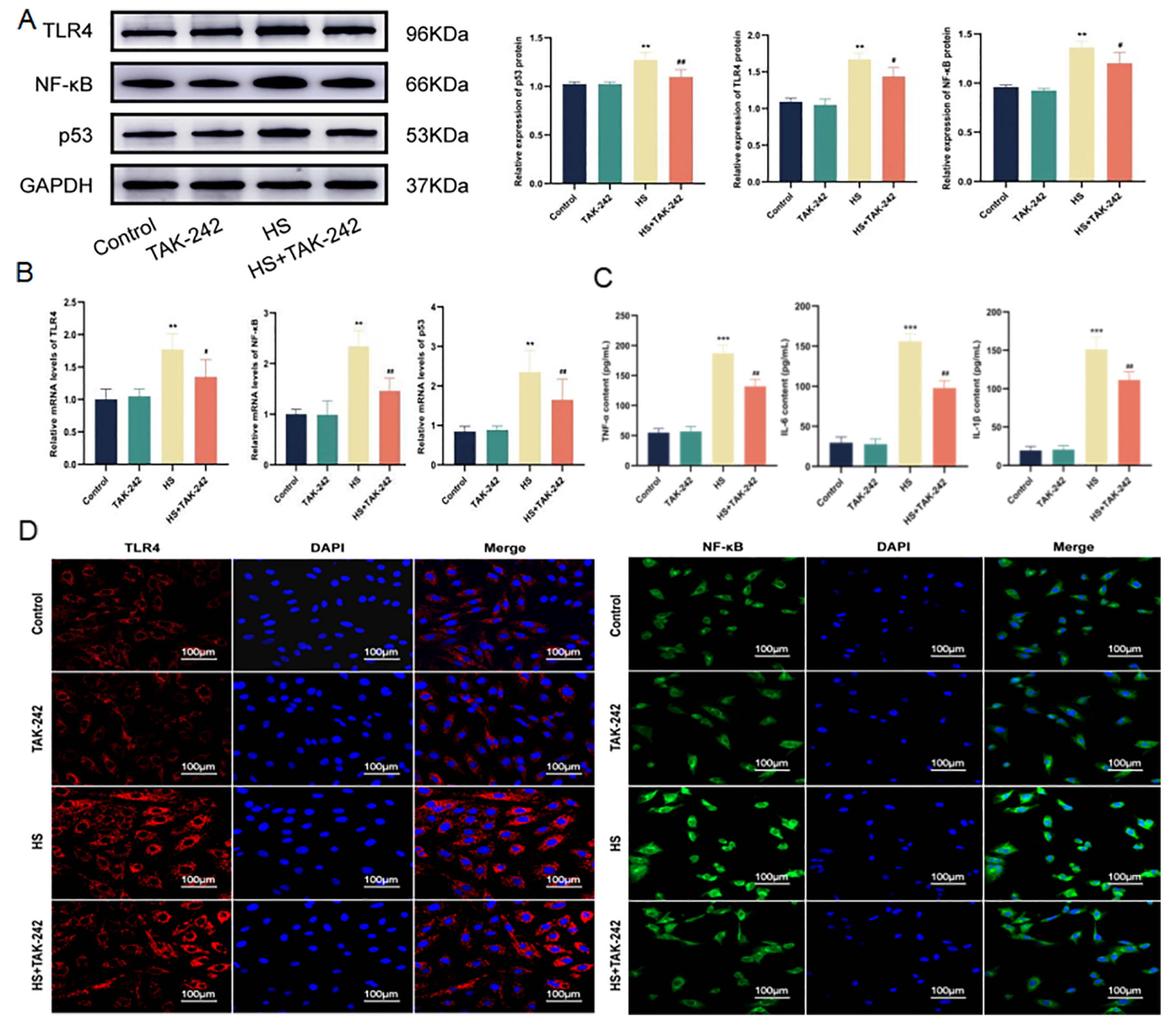
2.7. Inhibition of TLR4/NF-κB Signaling Pathway Alleviates the Inflammatory Environment and Ferroptosis of Cardiomyocytes Induced by HS
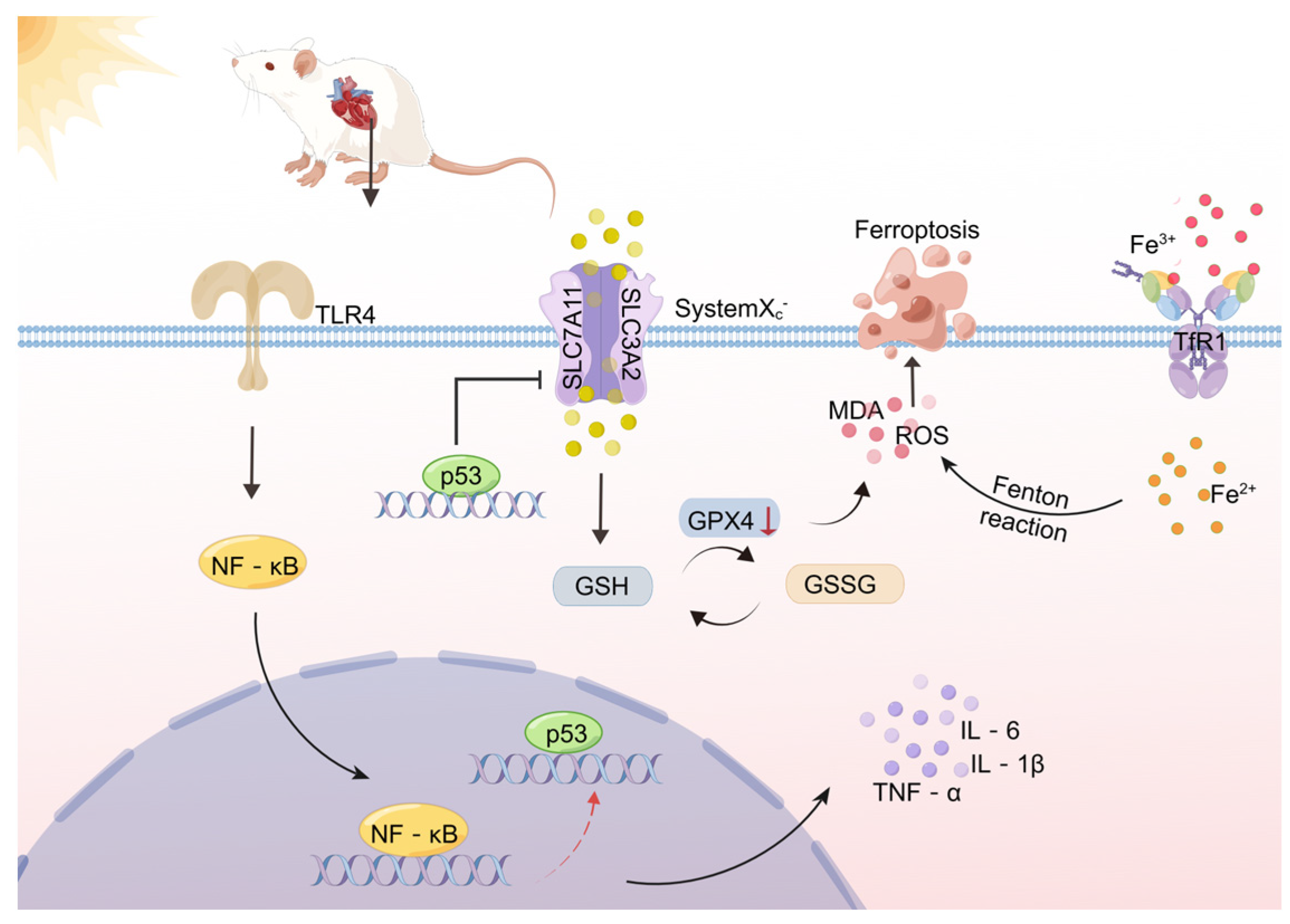
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Intervention
4.2. Cell Viability Assays
4.3. Western Blotting
4.4. RNA Extraction and Analysis
- TLR4 forward:5′-CCAGGTGTGAAATTGAGACAATTG-3′,
- TLR4 reverse: 3′-AAGCTGTCCAATATGGAAACCC-5′.
- NF-κB forward:5′-CAGATACCACTAAGACGCACCC-3′,
- NF-κB reverse: 3′-CTCCAGGTCTCGCTTCTTCACA-5′.
- p53 forward: 5′-GGAGGATTCACAGTCGGATATG-3′,
- p53 reverse: 3′-TGAGAAGGGACGGAAGATGAC-5′.
- SLC7A11 forward: 5′-TATGCTGAATTGGGTACGAGC-3′,
- SLC7A11 reverse: 3′-TATTACCAGCAGTTCCACCCA-5′.
- GPX4 forward: 5′-AGGCAGGAGCCAGGAAGTAATC-3′.
- GPX4 reverse: 3′-ACCACGCAGCCGTTCTTATC-5′.
- GAPDH forward: 5′-CTGGAGAAACCTGCCAAGTATG-3′,
- GAPDH reverse: 3′-GGTGGAAGAATGGGAGTTGCT-5′.
4.5. Immunofluorescence
4.6. Cytokine Measurements
4.7. Determination of ROS Levels
4.8. Transmission Electron Microscopy (TEM)
4.9. Measurement of Malonaldehyde (MDA) and Glutathione (GSH) Levels
4.10. Iron Content Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ouyang, L.; Ji, J.; Liu, Z. Research progress of heatstroke warning system. Zhonghua wei zhong bing ji jiu yi xue 2022, 34, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.F.; Ji, J.J.; Zheng, D.; Su, L.; Peng, T. Calpain-2 protects against heat stress-induced cardiomyocyte apoptosis and heart dysfunction by blocking p38 mitogen-activated protein kinase activation. J. Cell. Physiol. 2019, 234, 10761–10770. [Google Scholar] [CrossRef]
- Argaud, L.; Ferry, T.; Le, Q.H.; Marfisi, A.; Ciorba, D.; Achache, P.; Ducluzeau, R.; Robert, D. Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch. Intern. Med. 2007, 167, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, H.; Zhu, G.; Chen, X.; Tang, Z. Sodium tanshinone IIA sulfonate improves inflammation, aortic endothelial cell apoptosis, disseminated intravascular coagulation and multiple organ damage in a rat heat stroke model. Mol. Med. Rep. 2017, 16, 87–94. [Google Scholar] [CrossRef]
- Lin, X.; Lin, C.H.; Zhao, T.; Zuo, D.; Ye, Z.; Liu, L.; Lin, M.T. Quercetin protects against heat stroke-induced myocardial injury in male rats: Antioxidative and antiinflammatory mechanisms. Chem.-Biol. Interact. 2017, 265, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Jiang, X.; Gu, W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020, 30, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Zhang, S.; Zhou, X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics 2021, 11, 3052–3059. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, N.; Fan, S.; Zheng, X.; Yang, Y.; Zhu, Y.; Lu, Y.; Chen, Q.; Zhou, H.; Zheng, J. The citrus flavonoid naringenin confers protection in a murine endotoxaemia model through AMPK-ATF3-dependent negative regulation of the TLR4 signalling pathway. Sci. Rep. 2016, 6, 39735. [Google Scholar] [CrossRef]
- Zhou, Y.; Little, P.J.; Downey, L.; Afroz, R.; Wu, Y.; Ta, H.T.; Xu, S.; Kamato, D. The Role of Toll-like Receptors in Atherothrombotic Cardiovascular Disease. ACS Pharmacol. Transl. Sci. 2020, 3, 457–471. [Google Scholar] [CrossRef]
- Leon, L.R.; Helwig, B.G. Heat stroke: Role of the systemic inflammatory response. J. Appl. Physiol. 2010, 109, 1980–1988. [Google Scholar] [CrossRef]
- Ye, J.X.; Lin, H.; Mu, J.S.; Cui, X.P.; Yu, Z.L. Role of TLR4/NF-κB signaling pathway in brain injury of exertional heat stroke in rats. Med. J. Chin. People’s Lib. 2019, 40, 1170–1173+1178. [Google Scholar]
- Chen, D.D.; Xu, T.; Xue, S.J.; Zhu, L.Q.; Li, G.H. Role of Toll-like receptors in cardiac function injury and cardiac ferroptosis in heat stroke rats. Med. J. Chin. People’s Lib. 2022, 1–11. [Google Scholar]
- Zhu, B.S.; Xing, C.G.; Lin, F.; Fan, X.Q.; Zhao, K.; Qin, Z.H. Blocking NF-κB nuclear translocation leads to p53-related autophagy activation and cell apoptosis. World J. Gastroenterol. 2011, 17, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Zhang, Y.; Hong, T.; Zhang, X.; Liu, X.; Mao, C.; Yan, Y.; Koppula, P.; Cheng, W.; Sood, A.K.; et al. Ferroptosis as a mechanism to mediate p53 function in tumor radiosensitivity. Oncogene 2021, 40, 3533–3547. [Google Scholar] [CrossRef]
- Li, Q.; Sun, R.; Liu, S.; Lyu, H.; Wang, H.; Hu, Q.; Wang, N.; Yan, J.; Wang, J.; Li, X. Effect of heat acclimatization training on inflammatory reaction and multiple organ dysfunction syndrome in patients with exertional heat stroke. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2018, 30, 599–602. [Google Scholar] [CrossRef]
- Lou, Y.; Lin, H.; Wang, H.; Chen, W.; Wu, Y.; Li, H. Research progress in the heatstroke-induced myocardial injury. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019, 31, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhao, T.; Lin, C.H.; Zuo, D.; Ye, Z.; Lin, S.; Wen, S.; Liu, L.; Lin, M.T.; Chang, C.P.; et al. Melatonin provides protection against heat stroke-induced myocardial injury in male rats. J. Pharm. Pharmacol. 2018, 70, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.D.; Surani, S.; Horseman, M.A. Endotoxin, Toll-like Receptor-4, and Atherosclerotic Heart Disease. Curr. Cardiol. Rev. 2017, 13, 86–93. [Google Scholar] [CrossRef]
- Wu, M. Clinical characteristics and molecular mechanisms of acute kidney injury induced by rhabdomyolysis after heatstroke. J. South. Med. Univ. 2021. [Google Scholar] [CrossRef]
- Koppula, P.; Zhang, Y.; Zhuang, L.; Gan, B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018, 38, 12. [Google Scholar] [CrossRef]
- Shin, C.S.; Mishra, P.; Watrous, J.D.; Carelli, V.; D’Aurelio, M.; Jain, M.; Chan, D.C. The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility. Nat. Commun. 2017, 8, 15074. [Google Scholar] [CrossRef]
- Xu, C.; Sun, S.; Johnson, T.; Qi, R.; Zhang, S.; Zhang, J.; Yang, K. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 2021, 35, 109235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qiao, W.; Zuo, L. A1 and A2b adenosine receptors regulate GPX4 against ferroptosis of cardiomyocytes in myocardial infarction rat model and in vitro. Tissue Cell 2022, 77, 101828. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; He, L.L.; Zhang, G.R.; Zuo, Q.J.; Wang, Z.L.; Zhai, J.L.; Zhang, T.T.; Wang, Y.; Ma, H.J.; Guo, Y.F. Canagliflozin mitigates ferroptosis and ameliorates heart failure in rats with preserved ejection fraction. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 945–962. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, G.; Gauthier, J.M.; Lokshina, I.; Higashikubo, R.; Evans, S.; Liu, X.; Hassan, A.; Tanaka, S.; Cicka, M.; et al. Astragaloside IV attenuates injury caused by myocardial ischemia/reperfusion in rats via regulation of toll-like receptor 4/nuclear factor-κB signaling pathway. Phytother. Res. PTR 2019, 29, 599–606. [Google Scholar] [CrossRef]
- Ma, X.H.; Liu, J.H.; Liu, C.Y.; Sun, W.Y.; Duan, W.J.; Wang, G.; Kurihara, H.; He, R.R.; Li, Y.F.; Chen, Y.; et al. ALOX15-launched PUFA-phospholipids peroxidation increases the susceptibility of ferroptosis in ischemia-induced myocardial damage. Signal Transduct. Target. Ther. 2022, 7, 288. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yan, D.; Sun, Q.; Tao, J.; Xu, L.; Sun, H.; Zhao, H. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J. Cell. Biochem. 2020, 121, 2994–3004. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, S.; Zhao, C.; Liu, B. Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem. Biophys. Res. Commun. 2019, 516, 37–43. [Google Scholar] [CrossRef]
- Hu, N.; Zhang, Y. TLR4 knockout attenuated high fat diet-induced cardiac dysfunction via NF-κB/JNK-dependent activation of autophagy. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Marchand, M.; Gin, K. The Cardiovascular System in Heat Stroke. CJC Open 2021, 4, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Cheng, P.; Feng, Z.; Zhang, X. Transmembrane p24 trafficking protein 2 regulates inflammation through the TLR4/NF-κB signaling pathway in lung adenocarcinoma. World J. Surg. Oncol. 2022, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Mao, W.; Jin, L.; Fang, M.; Liu, X.; Lang, J.; Jin, L.; Cao, B.; Shou, Q.; Fu, H. Crude Radix Aconiti Lateralis Preparata (Fuzi) with Glycyrrhiza Reduces Inflammation and Ventricular Remodeling in Mice through the TLR4/NF-κB Pathway. Mediat. Inflamm. 2020, 2020, 5270508. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Y.; Xue, Y.; Han, X.; Zhang, X.; Ma, Z.; Sun, S.; Chu, X.; Cheng, J.; Guan, S.; et al. Crocin attenuates isoprenaline-induced myocardial fibrosis by targeting TLR4/NF-κB signaling: Connecting oxidative stress, inflammation, and apoptosis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 13–23. [Google Scholar] [CrossRef]
- Li, M.; Tan, H.; Gao, T.; Han, L.; Teng, X.; Wang, F.; Zhang, X. Gypensapogenin I Ameliorates Isoproterenol (ISO)-Induced Myocardial Damage through Regulating the TLR4/NF-κB/NLRP3 Pathway. Molecules 2022, 27, 5298. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Liao, X.Q.; Fan, X.Y.; Wang, Z.Y.; Hu, S.Y.; Hu, Z.X. Protective effect of Shenfu Injection on rats with chronic heart failure based on HMGB1/TLR4/NF-κB signaling pathway. Zhongguo Zhong Yao Za Zhi 2022, 47, 5556–5563. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ding, Z.X.; Cui, Y.; Li, Y.P.; Rao, Y.H. Effects of pulmonary infection on peripheral blood monocyte Toll-like receptor4 and nuclear factor-κB signaling pathway in elderly patients with chronic heart failure. Chin. J. Nosocomiology 2021, 31, 2452–2456. [Google Scholar] [CrossRef]
- Su, Q.; Li, L.; Sun, Y.; Yang, H.; Ye, Z.; Zhao, J. Effects of the TLR4/Myd88/NF-κB Signaling Pathway on NLRP3 Inflammasome in Coronary Microembolization-Induced Myocardial Injury. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 47, 1497–1508. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Geng, Y.; Deng, Z.; Li, P.; Xue, S.; Xu, T.; Li, G. Inhibition of TLR4 Alleviates Heat Stroke-Induced Cardiomyocyte Injury by Down-Regulating Inflammation and Ferroptosis. Molecules 2023, 28, 2297. https://doi.org/10.3390/molecules28052297
Chen D, Geng Y, Deng Z, Li P, Xue S, Xu T, Li G. Inhibition of TLR4 Alleviates Heat Stroke-Induced Cardiomyocyte Injury by Down-Regulating Inflammation and Ferroptosis. Molecules. 2023; 28(5):2297. https://doi.org/10.3390/molecules28052297
Chicago/Turabian StyleChen, Dandan, Yao Geng, Ziwei Deng, Peiling Li, Shujing Xue, Tao Xu, and Guanghua Li. 2023. "Inhibition of TLR4 Alleviates Heat Stroke-Induced Cardiomyocyte Injury by Down-Regulating Inflammation and Ferroptosis" Molecules 28, no. 5: 2297. https://doi.org/10.3390/molecules28052297
APA StyleChen, D., Geng, Y., Deng, Z., Li, P., Xue, S., Xu, T., & Li, G. (2023). Inhibition of TLR4 Alleviates Heat Stroke-Induced Cardiomyocyte Injury by Down-Regulating Inflammation and Ferroptosis. Molecules, 28(5), 2297. https://doi.org/10.3390/molecules28052297




