Flavonoids and Phenolic Acids Content in Cultivation and Wild Collection of European Cranberry Bush Viburnum opulus L.
Abstract
1. Introduction
2. Results
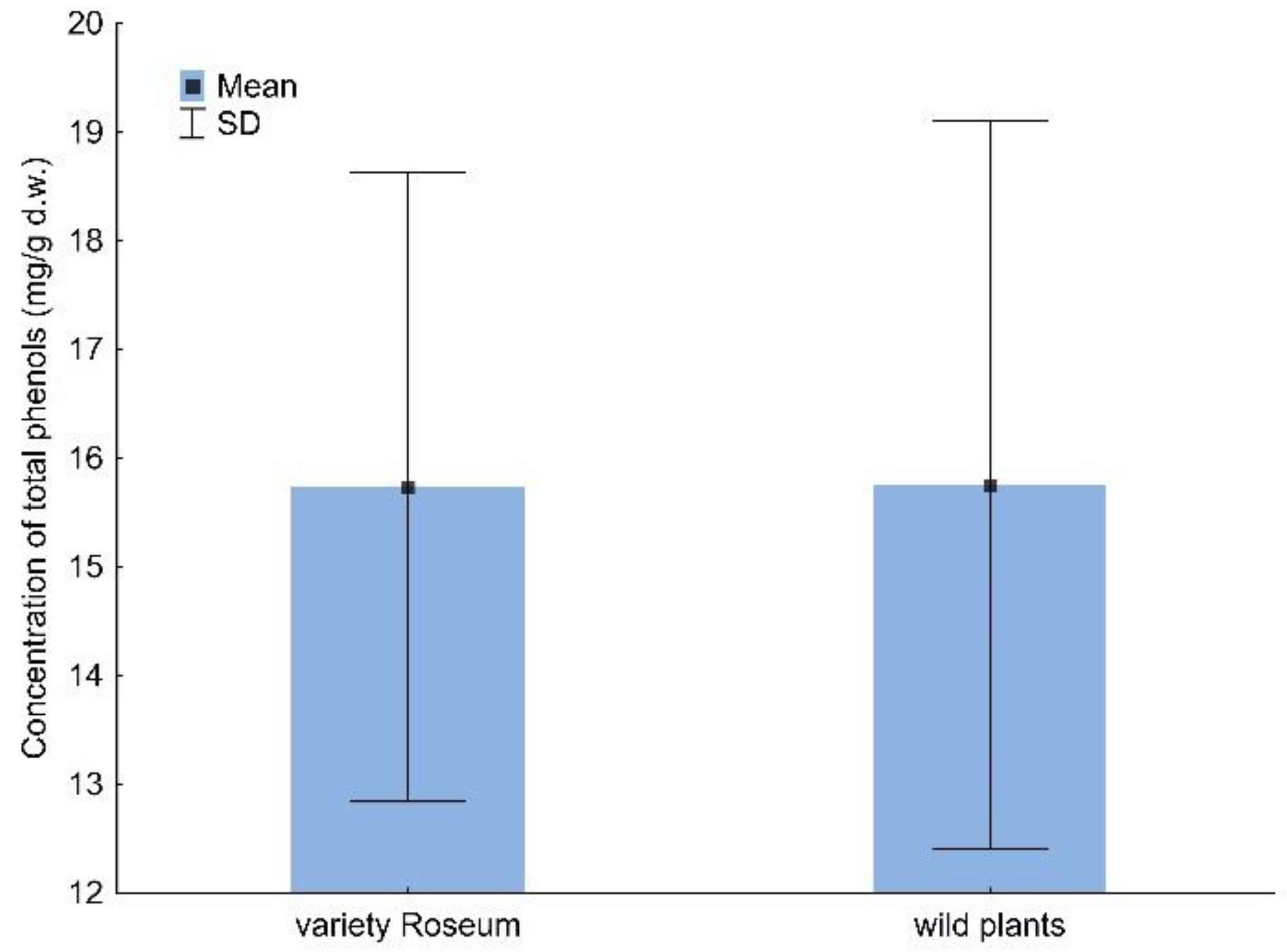
2.1. Total Phenols
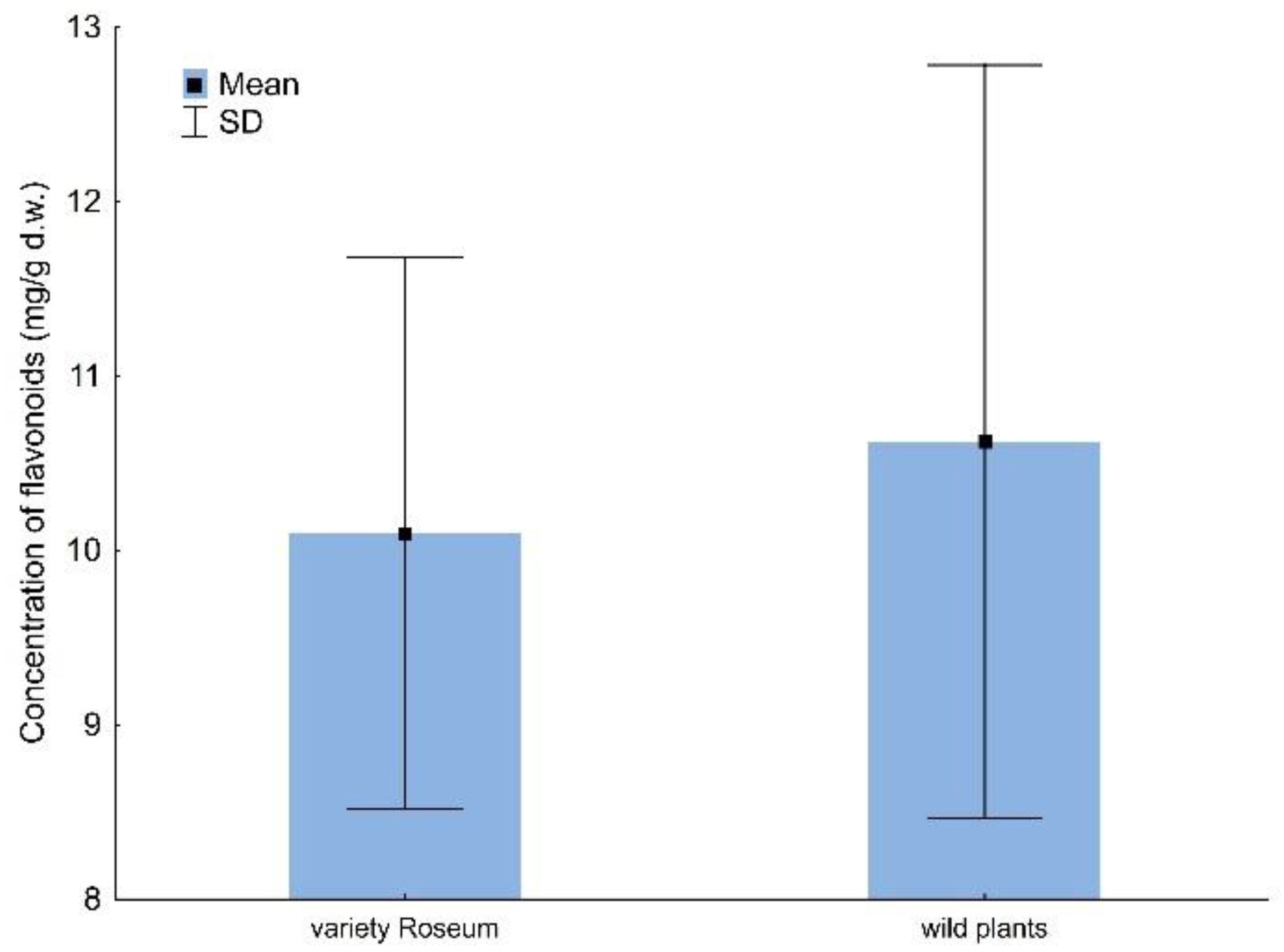
2.2. Total Flavonoids
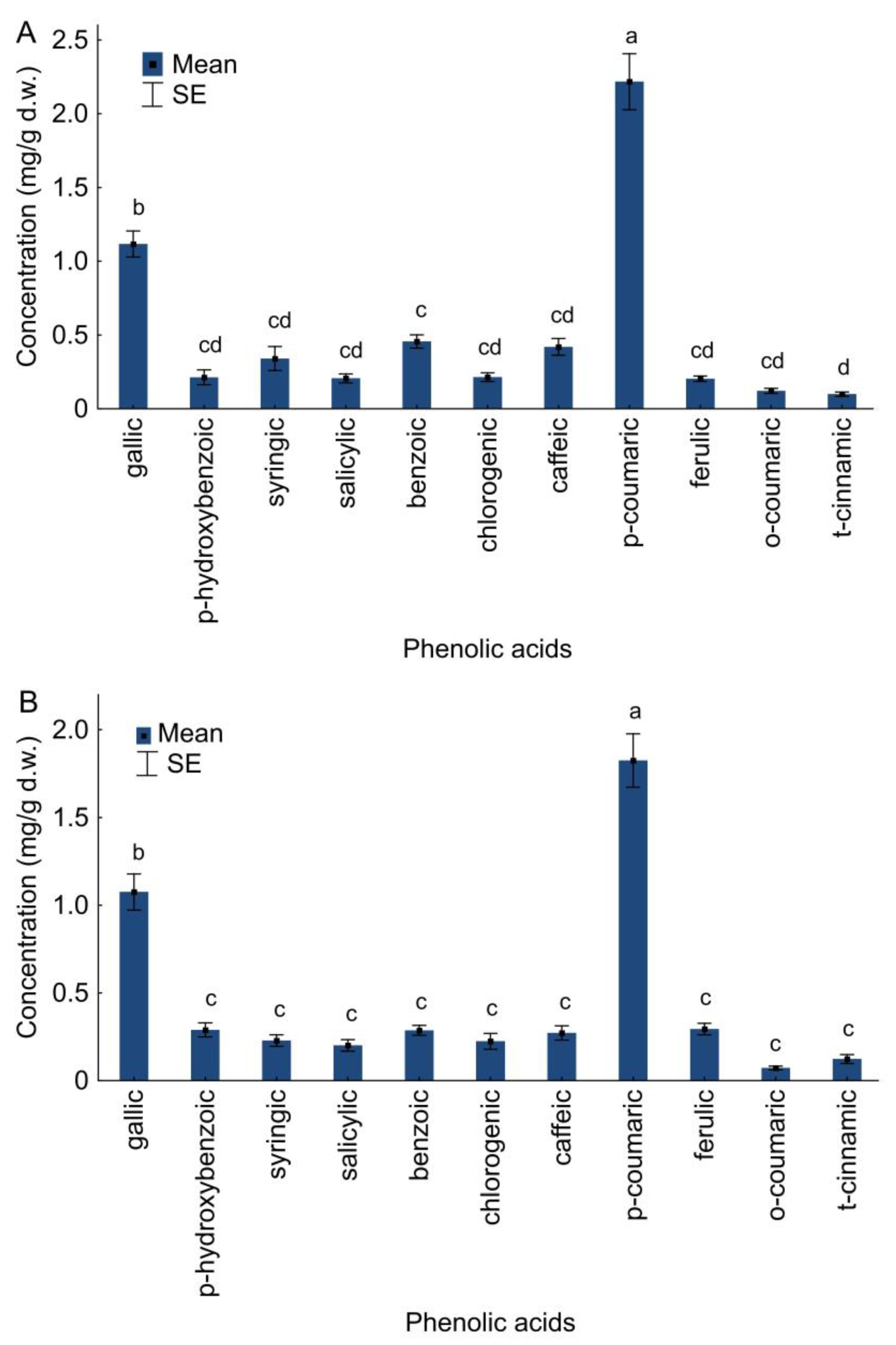
2.3. Phenolic Acids
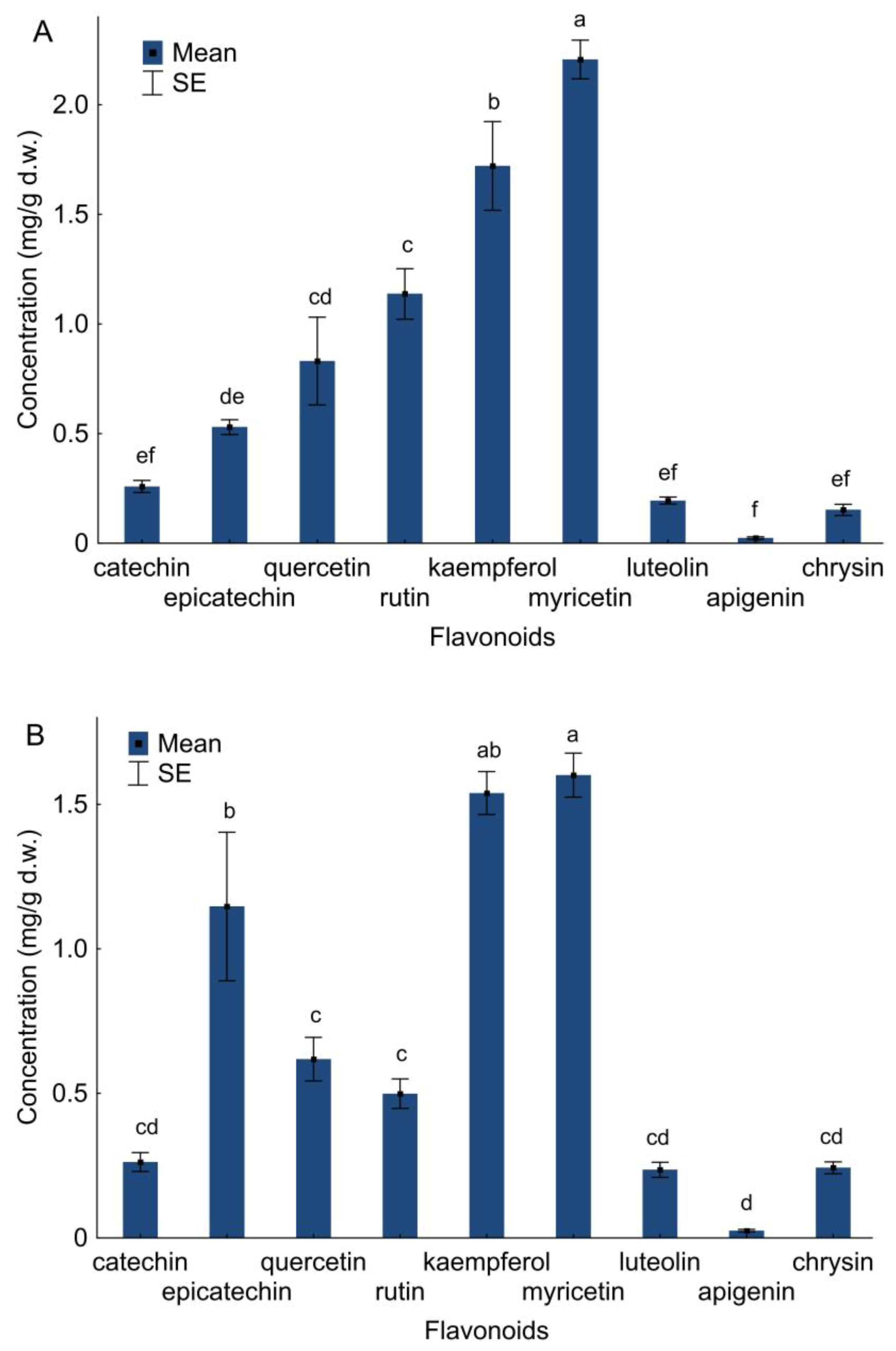
2.4. Flavonoids
3. Discussion
4. Materials and Methods
4.1. Plant Material and Study Area
4.2. Chemical Analysis
4.2.1. Determination of Individual Phenolic Acids
4.2.2. Determination of Individual Flavonoids
4.2.3. Total Phenols Determination
4.2.4. Flavonoids Determination
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Goławska, S.; Łukasik, I. Antifeedant activity of luteolin and genistein against the pea aphid, Acyrthosiphon pisum. J. Pest Sci. 2012, 85, 443–450. [Google Scholar] [CrossRef]
- Goławska, S.; Sprawka, I.; Łukasik, I.; Goławski, A. Are naringenin and quercetin useful chemicals in pest-management strategies? J. Pest Sci. 2014, 87, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jeond, S.; Lee, C. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Matsuda, H.; Pongpiriyadacha, Y.; Morikawa, T.; Ochi, M.; Yoshikawa, M. Gastroprotetive effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: Structural requirements and mode of action. Eur. J. Pharmacol. 2003, 471, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.A.C.; Leon, L.L. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz. 2001, 96, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.T.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef]
- Yu, Z.F.; Kong, L.D.; Chen, Y. Antidepressant activity of aqueous extracts of Curcuma longa in mice. J. Ethnopharmacol. 2002, 83, 161–165. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Liu, J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef]
- Kollmann, J.; Grubb, P.J. Viburnum lantana L. and Viburnum opulus L. (V. lobatum Lam., Opulus vulgaris Borkh.). J. Ecol. 2002, 90, 1044–1070. [Google Scholar] [CrossRef]
- Velioglu, Y.; Ekici, L.; Poyrazoglu, E. Phenolic composition of European cranberry bush (Viburnum opulus L.) berries and astringency removal of its commercial juice. Int. J. Food Sci. Technol. 2006, 41, 1011–1015. [Google Scholar] [CrossRef]
- Perova, I.; Zhogova, A.; Cherkashin, A.; Éller, K.; Ramenskaya, G.; Samylina, I. Biologically active substances from European guelder berry fruits. Pharm. Chem. J. 2014, 48, 332–339. [Google Scholar] [CrossRef]
- Sarıözkan, S.; Türk, G.; Eken, A.; Bayram, L.; Baldemir, A.; Doğan, G. Gilaburu (Viburnum opulus L.) fruit extract alleviates testis and sperm damages induced by taxane-based chemotherapeutics. Biomed. Pharmacother. 2017, 95, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Česonienė, L.; Daubaras, R.; Venclovienė, J.; Viškelis, P. Biochemical and agrobiological diversity of Viburnum opulus genotypes. Cent. Eur. J. Biol. 2010, 5, 864–871. [Google Scholar]
- Rychlińska, I. Sterols and triterpenes in Viburnum opulus L. leaves. Herba Pol. 2008, 54, 59–65. [Google Scholar]
- Rop, O.; Reznicek, V.; Valsikova, M.; Jurikova, T.; Mlcek, J.; Kramarova, D. Antioxidant properties of European cranberry bush fruit (Viburnum opulus var. edule). Molecules 2010, 15, 4467–4477. [Google Scholar] [CrossRef]
- Andreeva, T.I.; Komarova, E.N.; Yusubov, M.S.; Korotkova, E.I. Antioxidant activity of cranberry tree (Viburnum opulus L.) bark extract. Pharm. Chem. J. 2004, 38, 548–550. [Google Scholar] [CrossRef]
- Altun, M.; Çitoğlu, G.; Yilmaz, B.; Çoban, T. Antioxidant properties of Viburnum opulus and Viburnum lantana growing in Turkey. Int. J. Food Sci. Nutr. 2008, 59, 175–180. [Google Scholar] [CrossRef]
- Česonienė, L.; Daubaras, R.; Viškelis, P. Evaluation of productivity and biochemical components in fruit of different Viburnum accessions. Biologija 2008, 54, 93–96. [Google Scholar] [CrossRef]
- Yilmaz, N.; Yayli, N.; Misir, G.; Çoskunçelebi, K.; Karaoglu, S.; Yayli, N. Chemical composition and antimicrobial activities of the essential oils of Viburnum opulus, Viburnum lantana and Viburnum orientala. Asian J. Chem. 2008, 5, 3324–3330. [Google Scholar]
- Li, W.; Hydamaka, A.W.; Lowry, L.; Beta, T. Comparison of antioxidant capacity and phenolic compounds of berries, chokecherry and sea buckthorn. Cent. Eur. J. Biol. 2009, 4, 499–506. [Google Scholar]
- Erdogan-Orhan, I.; Altun, M.; Sever-Yilmaz, B.; Saltan, G. Antiacetylcholinesterase and antioxidant assets of the major components (salicin, amentoflavone, and chlorogenic acid) and the extracts of Viburnum opulus and Viburnum lantana and their total phenol and flavonoid contents. J. Med. Food 2011, 14, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Kraujalytė, V.; Venskutonis, P.; Pukalskas, A.; Česonienė, L.; Daubaras, R. Antioxidant properties and polyphenolic compositions of fruits from different European cranberry bush (Viburnum opulus L.) genotypes. Food Chem. 2013, 141, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Zayachkivska, O.; Gzhegotsky, M.; Terletska, O.; Lutsyk, D.; Yaschenko, A.; Dzhura, O. Influence of Viburnum opulus proanthocyanidins on stress-induced gastrointestinal mucosal damage. J. Physiol. Pharmacol. 2006, 57, 155–167. [Google Scholar]
- Česonienė, L.; Daubaras, R.; Viškelis, P.; Šarkinas, A. Determination of the total phenolic and anthocyanin contents and antimicrobial activity of Viburnum opulus fruit juice. Plant Foods Hum. Nutr. 2012, 67, 256–261. [Google Scholar] [CrossRef]
- Erdem, G.; Kesik, V.; Honca, T.; Özcan, A.; Uğuz, S.; Akgϋl, E.Ö.; Aykutlug, Ö.; Alp, B.F.; Korkmazer, N.; Saldir, M.; et al. Antinephrolithiatic activity of Persea americana (avocado) and Viburnum opulus (guelder rose) against ethylene glycol-induced nephrolithiasis in rats. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 110–119. [Google Scholar] [CrossRef]
- Adebayo, A.H.; Alade, A.; Yakubu, O.F. Gas chromatography-mass spectrometry analysis of Viburnum opulus (L.) extract and its toxicity studies in rats. Asian J. Pharm. Clin. Res. 2017, 10, 383–388. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Ivancic, A.; Todorovic, B.; Veberic, R.; Stampar, F. Fruit phenolic composition of different elderberry species and hybrids. J. Food Sci. 2015, 80, C2180–C2190. [Google Scholar] [CrossRef]
- Saridas, M.A.; Kafkas, N.E.; Zarifikhosroshahi, M.; Bozhaydar, O.; Kargi, S.P. Quality traits of green plums (Prunus cerasifera Ehrh.) at different maturity stages. Turk. J. Agric. For. 2016, 40, 655–663. [Google Scholar] [CrossRef]
- Yazici, K.; Sahin, A. Characterization of pomegranate (Punica granatum L.) hybrids and their potential use in further breeding. Turk. J. Agric. For. 2016, 40, 813–824. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Krska, B.; Kiprovski, B.; Veberic, R. Bioactive components and antioxidant capacity of fruits from nine sorbus genotypes. J. Food Sci. 2017, 82, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Raghubanshi, A.S.; Varshney, C.K. Integrated biodiversity research in India. Curr. Sci. 1994, 66, 109–112. [Google Scholar]
- Dansi, A.; Adjatin, A.; Adoukonou-Sagbadja, H.; Falade, V.; Ydomonhan, H.; Odou, D.; Dossou, B. Traditional leafy vegetables and their use in the Benin Republic. Genet. Resour. Crop Evol. 2008, 55, 1239–1256. [Google Scholar] [CrossRef]
- Mahapatra, A.K.; Albers, H.J.; Robinson, E.J.Z. The impact of NTFP sales on rural households’ cash income in India’s dry deciduous forest. Environ. Manage. 2005, 35, 258–265. [Google Scholar] [CrossRef]
- Bharucha, Z.; Pretty, J. The roles and values of wild foods in agricultural systems. Philos. Trans. R. Lond. B Biol. Sci. 2010, 365, 2913–2926. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. J. Agric. Food Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef] [PubMed]
- Canan, I.; Gundogdu, M.; Seday, U.; Oluk, C.A.; Karasahın, Z.; Eroglu, E.C.; Yazıcı, E.; Unlu, M. Determination of antioxidant, total phenolic, total carotenoid, lycopene, ascorbic acid, and sugar contents of Citrus species and mandarin hybrids. Turk. J. Agric. For. 2016, 40, 894–899. [Google Scholar] [CrossRef]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D.; Mikulic-Petkovsek, M. Changes in berry quality of northern highbush blueberry (Vaccinium corymbosum L.) during the harvest season. Turk. J. Agric. For. 2016, 40, 855–867. [Google Scholar] [CrossRef]
- Çam, M.; Hisil, Y.; Kuscu, A. Organic acid, phenolic content, and antioxidant capacity of fruit flesh and seed of Viburnum opulus. Chem. Nat. Compd. 2007, 43, 460–461. [Google Scholar] [CrossRef]
- Özrenk, K.; Gündoğdu, M.; Keskin, N.; Kaya, T. Some physical and chemical characteristics of gilaburu (Viburnum opulus L.) fruits in Erzincan region. Iğdır Univ. J. Inst. Sci. Technol. 2011, 1, 9–14. [Google Scholar]
- Kalyoncu, I.H.; Ersoy, N.; Elidemir, A.Y.; Korali, M.E. Some physico-chemical characteristics and mineral contents of gilaburu (Viburnum opulus L.) fruits in Turkey. Int. J. Agric. Biosyst. Eng. 2013, 7, 424–426. [Google Scholar]
- Ersoy, N.; Ercisli, S.; Gundogdu, M. Evaluation of European cranberry bush (Viburnum opulus L.) genotypes for agro-morphological, biochemical and bioactive characteristics in Turkey. Folia Hortic. 2017, 29, 181–188. [Google Scholar] [CrossRef]
- Polka, D.; Podsędek, A.; Koziołkiewicz, M. Comparison of Chemical Composition and Antioxidant Capacity of Fruit, Flower and Bark of Viburnum opulus. Plant Foods Hum. Nutr. 2019, 74, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Ho, C.; Zhang, J.; Wan, X.; Zhang, K.; Lim, J. Antioxidants: Differing meanings in food science and health science. J. Agric. Food Chem. 2018, 66, 3063–3068. [Google Scholar] [CrossRef] [PubMed]
- Polka, D.; Podsędek, A. Phenolics composition and antioxidant capacity of guelder rose fruit, flower and bark extracts. Food Sci. Biotechnol. 2019, 83, 37–46. [Google Scholar]
- Zakłos-Szyda, M.; Pietrzyk, N.; Szustak, M.; Podsędek, A. Viburnum opulus L. juice phenolics inhibit mouse 3T3-L1 cells adipogenesis and pancreatic lipase activity. Nutrients 2020, 12, 2003. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Haynes, P.A.; Atwell, B.J. Physiological and molecular changes in Oryza meridionalis Ng., a heat-tolerant species of wild rice. J. Exp. Bot. 2010, 61, 191–202. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; Hao, T.; Jin, H.; Zhang, H.; He, L.; Zhou, Q.; Huang, D.; Hui, D.; Yu, J. Effects of heat shock on photosynthetic properties, antioxidant enzyme activity, and downy mildew of cucumber (Cucumis sativus L.). PLoS ONE 2016, 11, e0152429. [Google Scholar] [CrossRef]
- Dahal, K.; Li, X.Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving potato stress tolerance and tuber yield under a climate change scenario—A current overview. Front. Plant Sci. 2019, 10, 563. [Google Scholar] [CrossRef]
- Ericksen, P.J.; Ingram, J.S.I.; Liverman, D.M. Food security and global environmental change. Environ. Sci. Policy 2009, 12, 373–377. [Google Scholar] [CrossRef]
- Konarska, A.; Domaciuk, M. Diferences in the fruit structure and the location and content of bioactive substances in Viburnum opulus and Viburnum lantana fruits. Protoplasma 2018, 255, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Zarifikhosroshahi, M.; Tugba, Z.; Kafkas, E.; Okatan, V. Variation in volatile and fatty acid contents among Viburnum opulus L. fruits growing different locations. Sci. Hortic. 2020, 264, 109160. [Google Scholar] [CrossRef]
- Turek, S.; Cisowski, W. Free and chemically bonded phenolic acids in barks of Viburnum opulus L. and Sambucus nigra L. Acta Pol. Pharm. Drug Res. 2007, 64, 377–383. [Google Scholar]
- Erylimaz, M.; Ozbiligin, S.; Ergene, B.; Yilmaz, S.; Altun, M.L.; Saltan, G. Antimicrobial activity of Turkish Viburnum species. Bangladesh J. Bot. 2013, 42, 355–360. [Google Scholar] [CrossRef]
- Akbulut, M.; Calsir, S.; Marakoglu, T.; Coklar, H. Chemical and technological properties of European cranberrybush (Viburnum opulus L.) fruits. Asian J. Chem. 2008, 20, 1875–1885. [Google Scholar]
- Altun, M.L.; Yilmaz, B.S. HPLC method for the analysis of salicin and chlorogenic acid from Viburnum opulus and V. lantana. Chem. Nat. Compd. 2007, 43, 203–207. [Google Scholar]
- Danielewski, M.; Matuszewska, A.; Nowak, B.; Kucharska, A.Z.; Sozanski, T. The effects of natural iridoids and anthocyanins on selected parameters of liver and cardiovascular system functions. Oxid. Med. Cell. Longev. 2020, 1–12, e2735790. [Google Scholar] [CrossRef]
- Dienaite, L.; Pukalskiene, M.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Valorization of European cranberry bush (Viburnum opulus L.) berry pomace extracts isolated with pressurized ethanol and water by assessing their phytochemical composition, antioxidant, and antiproliferative activities. Foods 2020, 9, 1413. [Google Scholar] [CrossRef]
- Atoui, A.K.; Manouri, A.; Basou, G.; Kefalas, P. Tea and herbal infusions: Their antioxidant activity and phenolic profile. Food Chem. 2005, 89, 27–36. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Lefay, S.; Gil-Isquierdo, A. Bioavailability of phenolic acids. Phytochem. Res. 2008, 7, 301–311. [Google Scholar] [CrossRef]
- Vaquero, M.J.R.; Alberto, M.R.; de Nadra, M.C.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Hendra, R.; Ahmad, S.; Sukari, A.; Shukor, M.Y.; Oskoueian, E. Flavonoid Analyses and Antimicrobial Activity of Various Parts of Phaleria macrocarpa (Scheff.) Boerl Fruit. Int. J. Mol. Sci. 2011, 12, 3422–3431. [Google Scholar] [CrossRef] [PubMed]
- Teffo, L.; Aderogba, M.; Eloff, J. Antibacterial and antioxidant activities of four kaempferol methyl ethers isolated from Dodonaea viscosa Jacq. var. angustifolia leaf extracts. S. Afr. J. Bot. 2010, 76, 25–29. [Google Scholar] [CrossRef]
- Demetzos, C.; Angelopoulou, D.; Kolocouris, A.; Daliani, I.; Mavromoustakos, T. Structure elucidation, conformational analysis and thermal effects on membrane bilayers of an antimicrobial myricetin ether derivative. J. Heterocycl. Chem. 2001, 38, 703–710. [Google Scholar] [CrossRef]
- Li, M.; Xu, Z. Quercetin in a lotus leaves extract may be responsible for antibacterial activity. Arch. Pharm. Res. 2008, 31, 640–644. [Google Scholar] [CrossRef]
- Mandalari, G.; Bennett, R.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.; Gasson, M.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef]
- Gouvea, D.R.; Gobbo-Neto, L.; Sakamoto, H.T.; Lopes, N.P.; Callegari Lopes, J.L. Seasonal variation of the major secondary metabolites present in the extract of Eremanthus mattogrossensis less (Asteraceae: Vernonieae) leaves. Quim. Nova 2012, 35, 2139–2145. [Google Scholar] [CrossRef]
- Dębski, H.; Wiczkowski, W.; Horbowicz, M. Effect of Elicitation with Iron Chelate and sodium metasilicate on phenolic compounds in legume sprouts. Molecules 2021, 26, 1345. [Google Scholar] [CrossRef]
- Holopainen, J.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef]
- Wen, P.F.; Chen, J.Y.; Wan, S.B.; Kong, W.F.; Zhang, P.; Wang, W.; Zhan, J.C.; Pan, Q.H.; Huang, W.D. Salicylic acid activates phenylalanine ammonia-lyase in grape berry in response to high temperature stress. Plant Growth Regul. 2008, 55, 1–10. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Zivcak, M.; Bruckova, K.; Brestic, M.; Hemmerich, I.; Rauh, C.; Simko, I. Shift in accumulation of flavonoids and phenolic acids in lettuce attributable to changes in ultraviolet radiation and temperature. Sci. Hortic. 2018, 239, 193–204. [Google Scholar] [CrossRef]
- Lancaster, J.E.; Reay, P.F.; Norris, J.; Butler, R.C. Induction of flavonoids and phenolic acids in apple by UV-B and temperature. J. Hortic. Sci. Biotechnol. 2000, 75, 142–148. [Google Scholar] [CrossRef]
- Barański, M.; Lacko-Bartošová, M.; Rembiałkowska, E.; Lacko-Bartošová, L. The Effect of Species and Cultivation Year on Phenolic Acids Content in Ancient Wheat. Agronomy 2020, 10, 673. [Google Scholar] [CrossRef]
- Uleberg, E.; Rohloff, J.; Jaakola, L.; Trôst, K.; Junttila, O.; Häggman, H.; Martinussen, I. Effects of Temperature and Photoperiod on Yield and Chemical Composition of Northern and Southern Clones of Bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 2012, 60, 10406–10414. [Google Scholar] [CrossRef]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Effect of thermal treatment and storage on the stability of organic acids and the functional value of grapefruit juice. Food Chem. 2010, 118, 291–299. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Sytykiewicz, H.; Durak, R.; Borowiak-Sobkowiak, B.; Chrzanowski, G. Role of phenolic compounds during antioxidative responses of winter triticale to aphid and beetle attack. Plant Physiol. Biochem. 2017, 118, 529–540. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kuban, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables—Evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Czapski, J.; Szwejda, J. Thermal processing effects on antioxidant constituents and properties of tomatoes. Veg. Crop. Res. Bull. 2006, 65, 49–62. [Google Scholar]
| Parameter | F21,146 | p |
|---|---|---|
| Total phenols | ||
| Temperature | 2.21 | 0.140 |
| Place | 6.04 | 0.003 |
| Survey number | 45.67 | <0.001 |
| Interaction | 7.96 | <0.001 |
| Flavonoids | ||
| Temperature | 0.20 | 0.657 |
| Place | 0.00 | 0.999 |
| Survey number | 4.75 | <0.001 |
| Interaction | 9.66 | <0.001 |
| Parameter | F4,19 | p |
|---|---|---|
| Hydroxybenzoic acids | ||
| gallic | ||
| Temperature | 2.44 | 0.134 |
| Place | 0.36 | 0.558 |
| Survey number | 0.22 | 0.647 |
| Interaction | 2.38 | 0.139 |
| p-hydroxybenzoic | ||
| Temperature | 1.20 | 0.288 |
| Place | 5.91 | 0.025 |
| Survey number | 1.72 | 0.205 |
| Interaction | 1.22 | 0.283 |
| syringic | ||
| Temperature | 2.75 | 0.114 |
| Place | 1.82 | 0.193 |
| Survey number | 2.32 | 0.144 |
| Interaction | 2.95 | 0.102 |
| salicylic | ||
| Temperature | 1.64 | 0.215 |
| Place | 0.02 | 0.899 |
| Survey number | 1.89 | 0.184 |
| Interaction | 11.15 | 0.003 |
| benzoic | ||
| Temperature | 0.65 | 0.431 |
| Place | 9.69 | 0.006 |
| Survey number | 0.29 | 0.598 |
| Interaction | 0.80 | 0.382 |
| Parameter | F4,19 | p |
|---|---|---|
| Hydroxycinnamic acids | ||
| chlorogenic | ||
| Temperature | 5.65 | 0.028 |
| Place | 0.09 | 0.764 |
| Survey number | 0.79 | 0.387 |
| Interaction | 20.65 | <0.001 |
| caffeic | ||
| Temperature | 0.43 | 0.521 |
| Place | 8.42 | 0.009 |
| Survey number | 0.64 | 0.433 |
| Interaction | 0.23 | 0.634 |
| p-coumaric | ||
| Temperature | 0.01 | 0.941 |
| Place | 5.29 | 0.033 |
| Survey number | 2.18 | 0.156 |
| Interaction | 0.00 | 0.967 |
| ferulic | ||
| Temperature | 5.37 | 0.032 |
| Place | 11.74 | 0.003 |
| Survey number | 1.53 | 0.232 |
| Interaction | 11.48 | 0.003 |
| o-coumaric | ||
| Temperature | 2.67 | 0.118 |
| Place | 6.08 | 0.023 |
| Survey number | 2.48 | 0.132 |
| Interaction | 0.04 | 0.843 |
| t-cinnamic | ||
| Temperature | 0.85 | 0.368 |
| Place | 0.73 | 0.404 |
| Survey number | 1.78 | 0.198 |
| Interaction | 1.38 | 0.255 |
| Parameter | F4,19 | p |
|---|---|---|
| Flavanols | ||
| (+)-catechin | ||
| Temperature | 3.87 | 0.064 |
| Place | 0.00 | 0.946 |
| Survey number | 7.70 | 0.012 |
| Interaction | 6.33 | 0.021 |
| (−)-epicatechin | ||
| Temperature | 3.21 | 0.089 |
| Place | 231.69 | <0.001 |
| Survey number | 59.41 | <0.001 |
| Interaction | 490.67 | <0.001 |
| Parameter | F4,19 | p |
|---|---|---|
| Flavonols | ||
| quercetin | ||
| Temperature | 0.83 | 0.372 |
| Place | 17.51 | <0.001 |
| Survey number | 11.51 | 0.003 |
| Interaction | 300.37 | <0.001 |
| rutin | ||
| Temperature | 1.03 | 0.322 |
| Place | 276.26 | <0.001 |
| Survey number | 0.83 | 0.374 |
| Interaction | 178.18 | <0.001 |
| kaempferol | ||
| Temperature | 0.00 | 0.973 |
| Place | 2.94 | 0.103 |
| Survey number | 1.44 | 0.245 |
| Interaction | 57.35 | <0.001 |
| myricetin | ||
| Temperature | 1.04 | 0.321 |
| Place | 44.97 | <0.001 |
| Survey number | 0.51 | 0.482 |
| Interaction | 16.24 | 0.001 |
| Parameter | F4,19 | p |
|---|---|---|
| Flavones | ||
| luteolin | ||
| Temperature | 2.96 | 0.101 |
| Place | 1.85 | 0.189 |
| Survey number | 4.04 | 0.059 |
| Interaction | 0.00 | 0.960 |
| apigenin | ||
| Temperature | 4.53 | 0.047 |
| Place | 0.04 | 0.843 |
| Survey number | 4.36 | 0.051 |
| Interaction | 1.29 | 0.269 |
| chrysin | ||
| Temperature | 12.61 | 0.002 |
| Place | 19.79 | 0.000 |
| Survey number | 16.27 | 0.001 |
| Interaction | 22.86 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goławska, S.; Łukasik, I.; Chojnacki, A.A.; Chrzanowski, G. Flavonoids and Phenolic Acids Content in Cultivation and Wild Collection of European Cranberry Bush Viburnum opulus L. Molecules 2023, 28, 2285. https://doi.org/10.3390/molecules28052285
Goławska S, Łukasik I, Chojnacki AA, Chrzanowski G. Flavonoids and Phenolic Acids Content in Cultivation and Wild Collection of European Cranberry Bush Viburnum opulus L. Molecules. 2023; 28(5):2285. https://doi.org/10.3390/molecules28052285
Chicago/Turabian StyleGoławska, Sylwia, Iwona Łukasik, Adrian Arkadiusz Chojnacki, and Grzegorz Chrzanowski. 2023. "Flavonoids and Phenolic Acids Content in Cultivation and Wild Collection of European Cranberry Bush Viburnum opulus L." Molecules 28, no. 5: 2285. https://doi.org/10.3390/molecules28052285
APA StyleGoławska, S., Łukasik, I., Chojnacki, A. A., & Chrzanowski, G. (2023). Flavonoids and Phenolic Acids Content in Cultivation and Wild Collection of European Cranberry Bush Viburnum opulus L. Molecules, 28(5), 2285. https://doi.org/10.3390/molecules28052285






