Berberine-Based Carbon Quantum Dots Improve Intestinal Barrier Injury and Alleviate Oxidative Stress in C57BL/6 Mice with 5-Fluorouracil-Induced Intestinal Mucositis by Enhancing Gut-Derived Short-Chain Fatty Acids Contents
Abstract
1. Introduction
2. Results
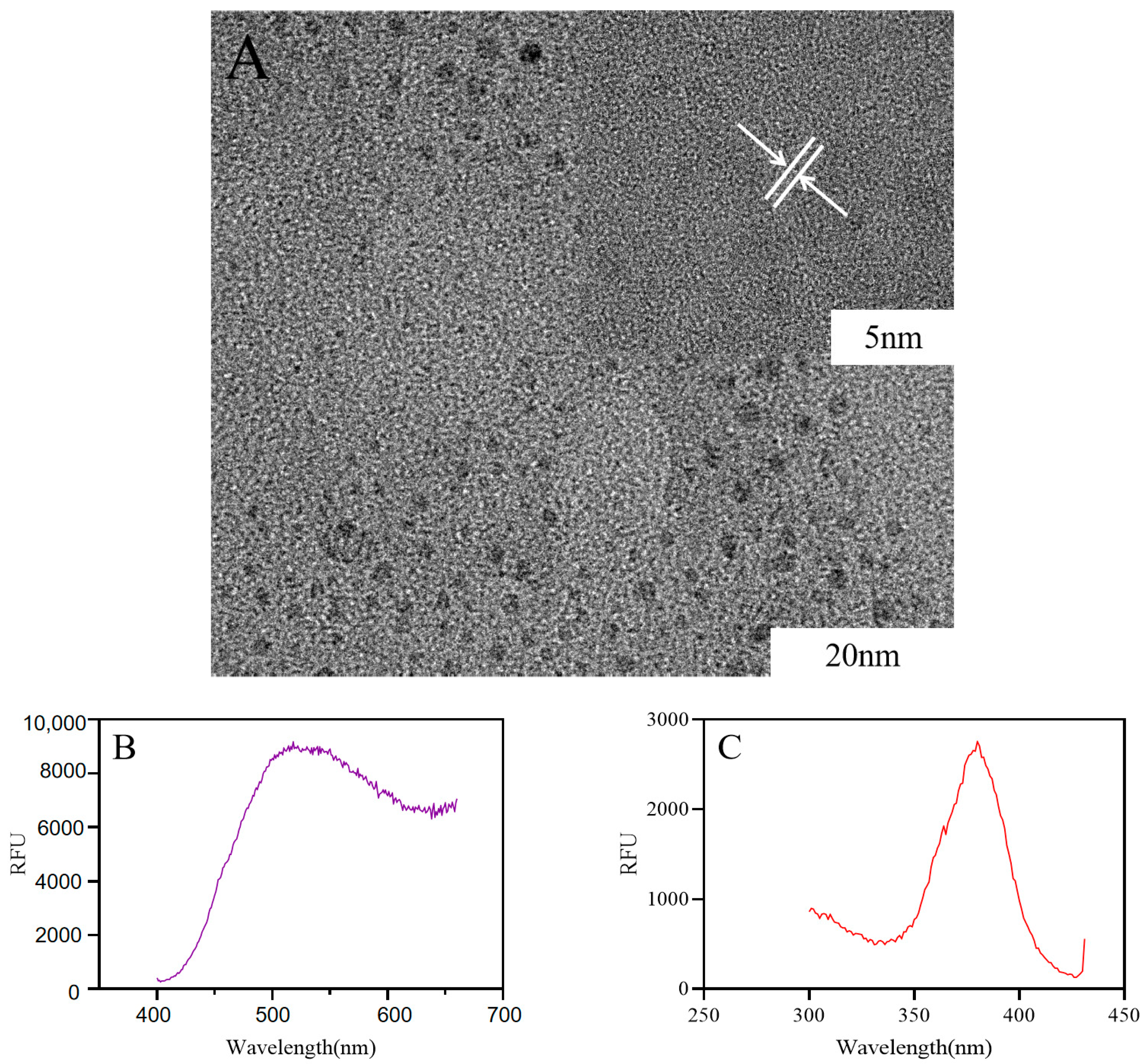
2.1. Preparation and Characterization of Ber-CDs
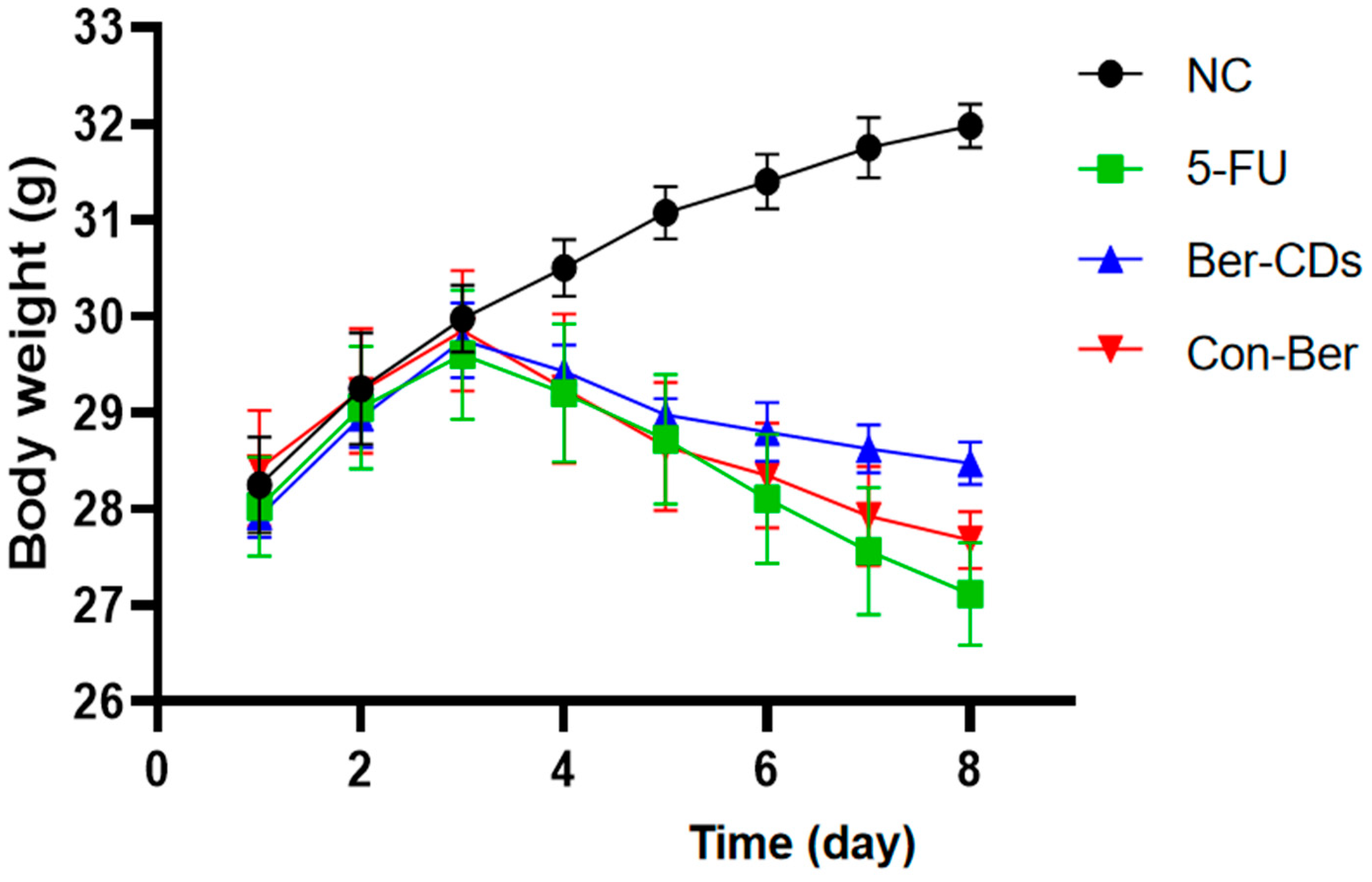
2.2. The Body Weight Changes of Experimental Mice
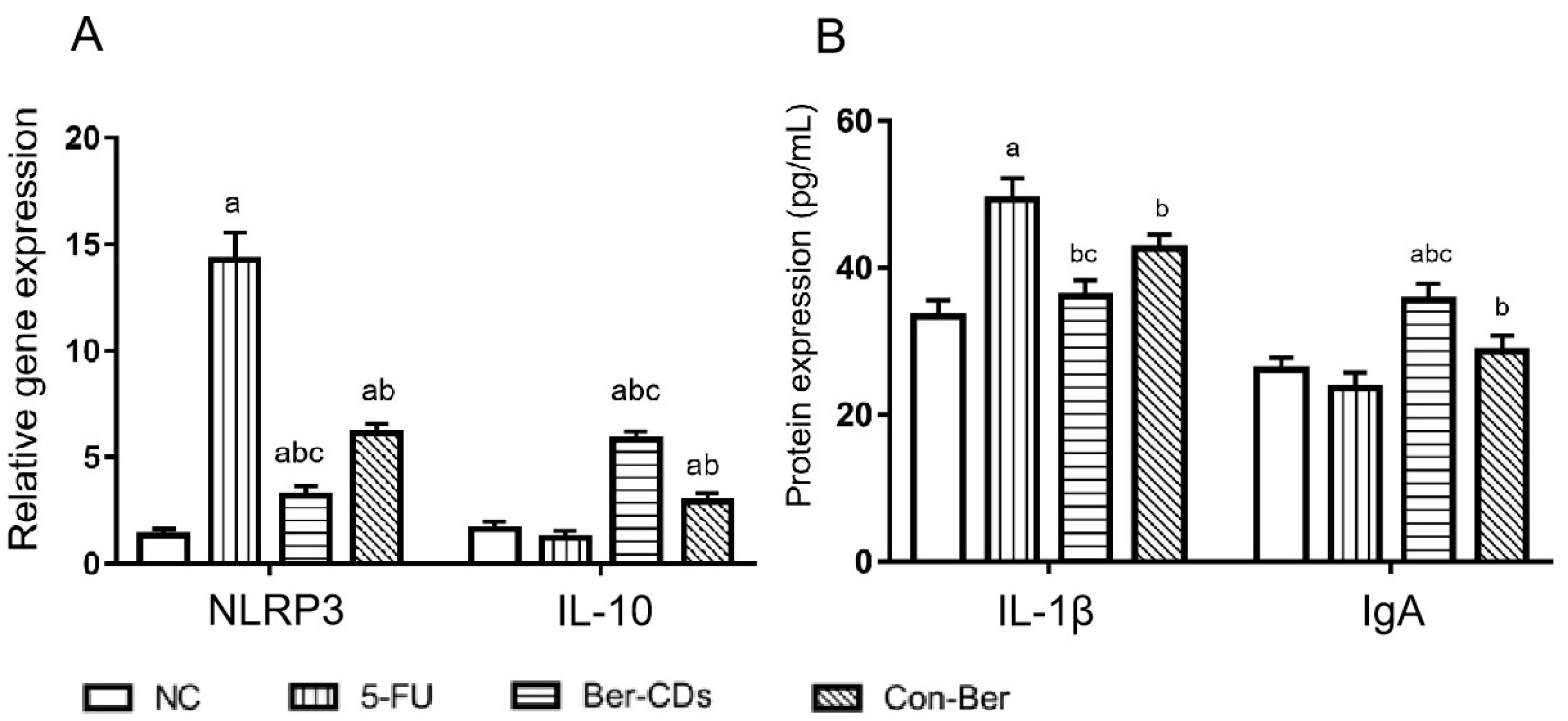
2.3. Expressions Levels of Inflammatory Factors in the Spleen and Serum of Mice
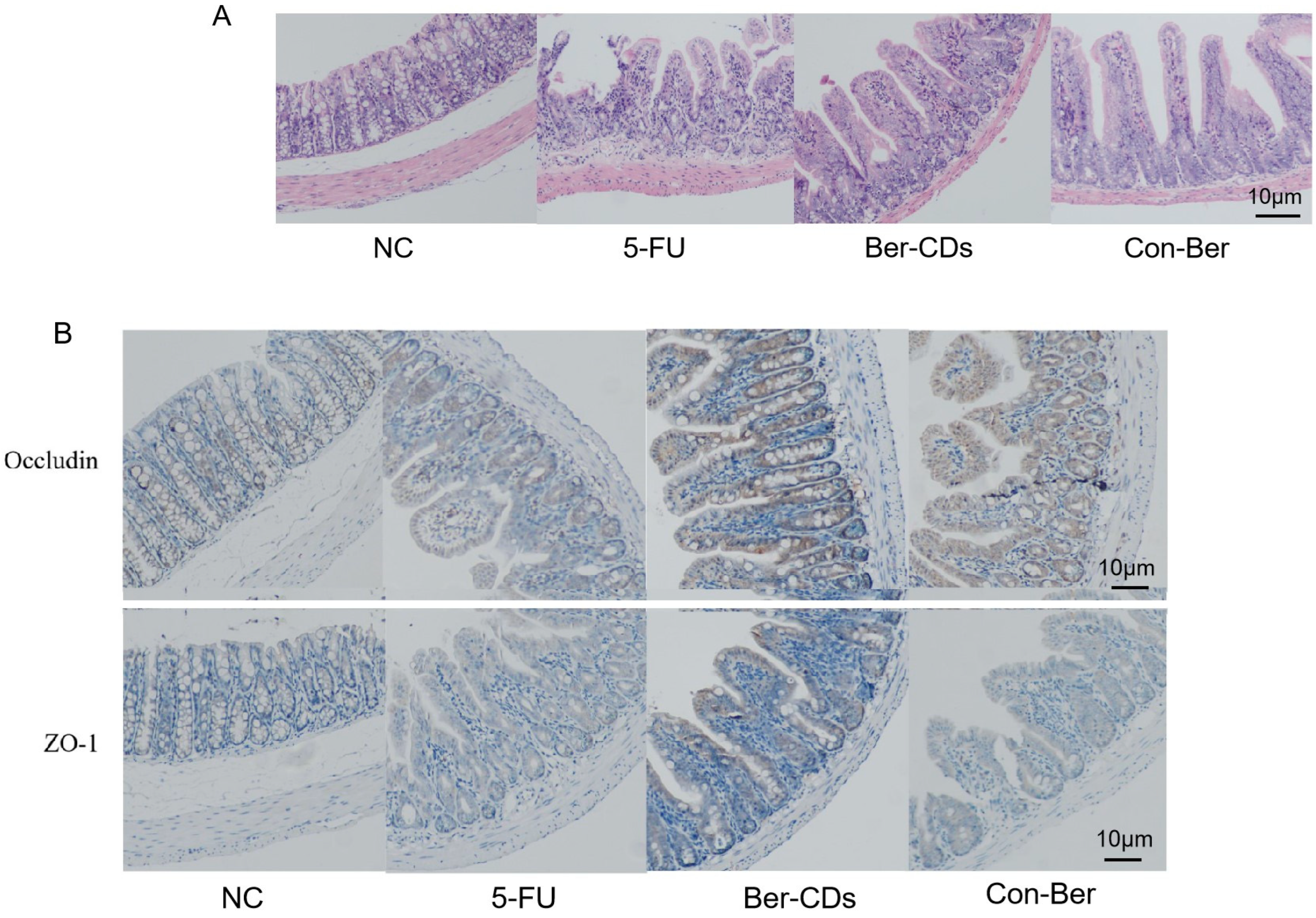
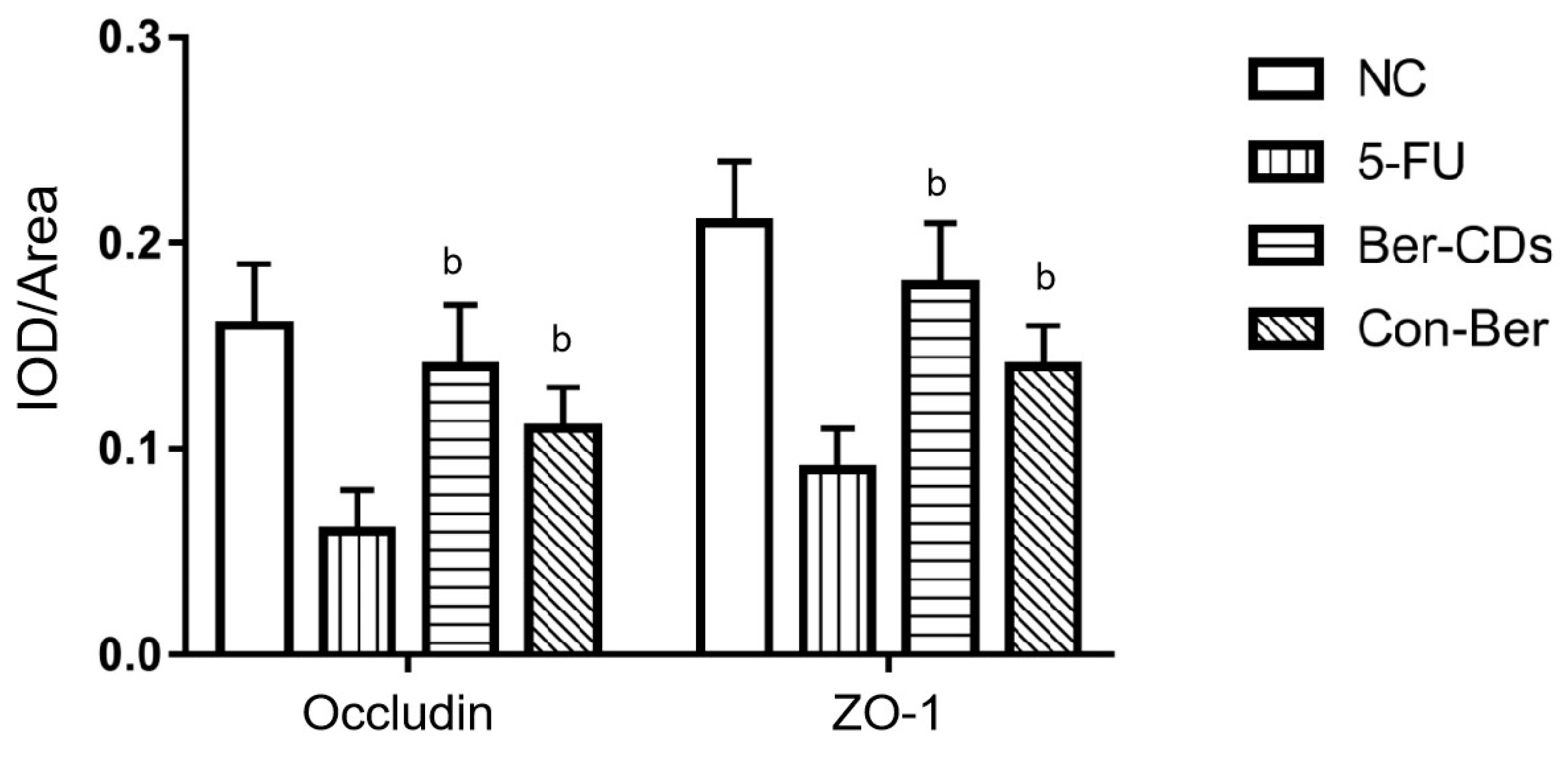
2.4. Morphology of the Intestinal Mucosa and Expression of Tight Junction Proteins
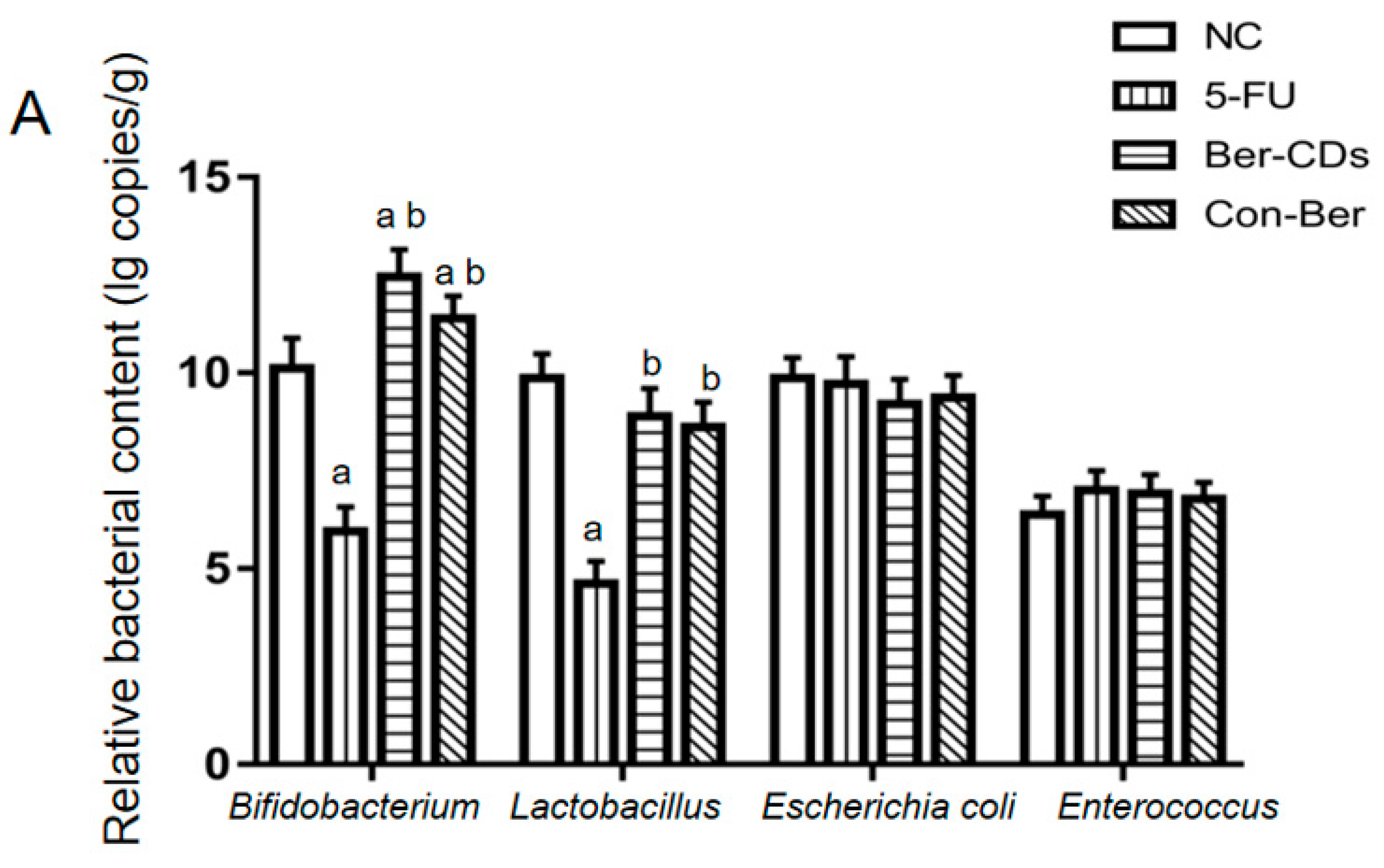
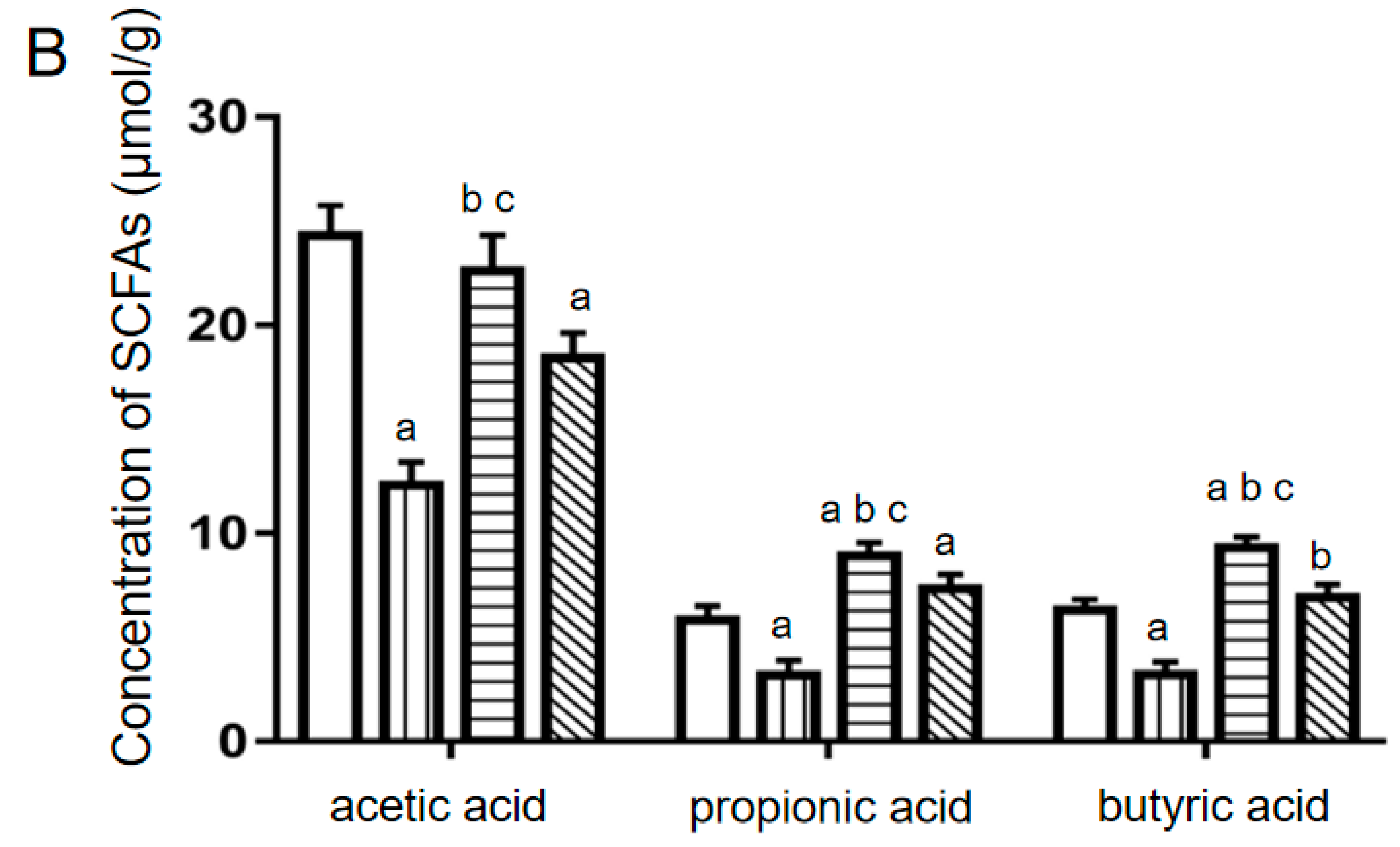
2.5. Relative Abundances of Intestinal Bacteria and Levels of SCFAs in Mouse Feces
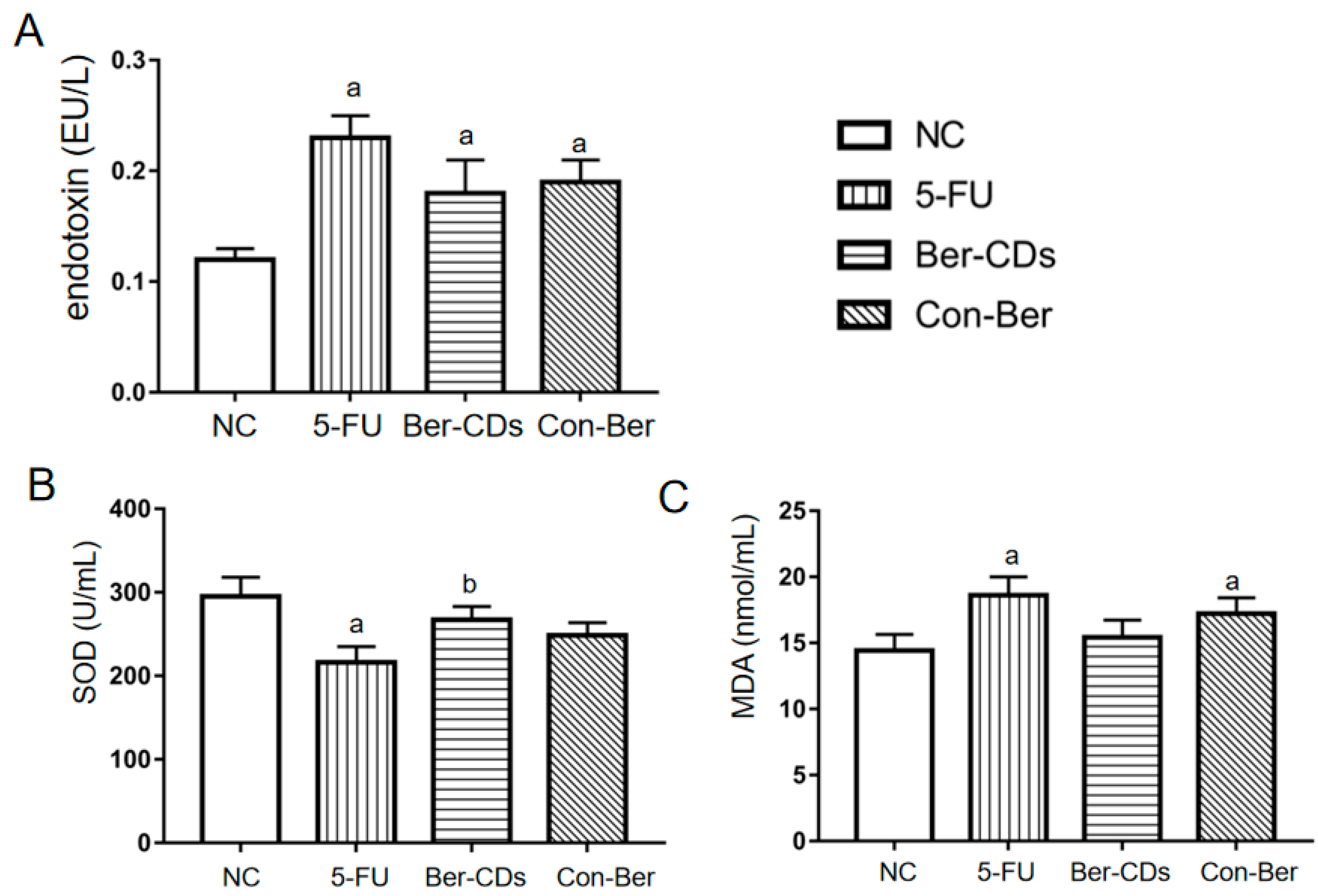
2.6. Levels of Plasma Endotoxin, Superoxide Dismutase and Malondialdehyde Concentrations in Mice
3. Discussion
4. Materials and Methods
4.1. Ber-CD Synthesis and Identification
4.2. Animal Experiment
4.3. qRT-PCR Assay
4.4. ELISA Assay
4.5. HE and Immunohistochemical Staining
4.6. Measurement of Relative Abundances of Intestinal Bacteria and Levels of SCFAs in Mouse Feces
4.7. Detection of Serum Endotoxin, SOD and MDA
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sougiannis, A.T.; Vanderveen, B.N.; Davis, J.M.; Fan, D.; Murphy, E.A. Understanding chemotherapy-induced intestinal mucositis and strategies to improve gut resilience. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zeng, H.; Li, X.; Liu, J.; Li, Z.; Xu, R.; Ma, Y.; Liu, C.; Xue, B. Activation of g protein coupled estrogen receptor prevents chemotherapy-induced intestinal mucositis by inhibiting the dna damage in crypt cell in an extracellular signal-regulated kinase 1- and 2- dependent manner. Cell Death Dis. 2021, 12, 1034. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, D.I.; Mendoza, T.R.; Fuller, C.D.; Hutcheson, K.A.; Wang, X.S.; Hanna, E.Y.; Lu, C.; Garden, A.S.; Morrison, W.H.; Cleeland, C.S.; et al. Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: A prospective analysis using the university of Texas MD Anderson Cancer Center symptom inventory-head and neck module. Cancer-Am. Cancer Soc. 2014, 120, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Elamin, E.E.; Masclee, A.A.; Dekker, J.; Pieters, H.J.; Jonkers, D.M. Short-chain fatty acids activate amp-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in caco-2 cell monolayers. J. Nutr. 2013, 143, 1872–1881. [Google Scholar]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic. Biol. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef]
- Hytti, M.; Korhonen, E.; Hyttinen, J.; Roehrich, H.; Kaarniranta, K.; Ferrington, D.A.; Kauppinen, A. Antimycin a-induced mitochondrial damage causes human rpe cell death despite activation of autophagy. Oxid. Med. Cell. Longev. 2019, 2019, 1583656. [Google Scholar] [CrossRef]
- Wan, Y.; Fu, Y.; Wang, F.; Sinclair, A.J.; Li, D. Protective effects of a lipid extract from hard-shelled mussel (mytilus coruscus) on intestinal integrity after lipopolysaccharide challenge in mice. Nutrients 2018, 10, 860. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhang, H. NLRP3 inflammasome and inflammatory bowel disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The anti-cancer mechanisms of berberine: A review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef]
- Mohammadian, H.S.; Momtazi-Borojeni, A.A. Berberine as a promising natural compound for the treatment of periodontal disease: A focus on anti-inflammatory properties. J. Cell. Mol. Med. 2021, 25, 11333–11337. [Google Scholar] [CrossRef]
- Cohen, E.N.; Kondiah, P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Carbon dots as nanotherapeutics for biomedical application. Curr. Pharm. Des. 2020, 26, 2207–2221. [Google Scholar] [CrossRef]
- Song, D.; Guo, H.; Huang, K.; Zhang, H.; Chen, J.; Wang, L.; Lian, C.; Wang, Y. Carboxylated carbon quantum dot-induced binary metal–organic framework nanosheet synthesis to boost the electrocatalytic performance. Mater. Today 2022, 54, 42–51. [Google Scholar] [CrossRef]
- Zhang, B.; An, G.; Chen, J.; Guo, H.; Wang, L. Surface state engineering of carbon dot/carbon nanotube heterojunctions for boosting oxygen reduction performance. J. Colloid Interface Sci. 2023, 637, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, H.; Fang, Y.; Wang, L.; Li, P. Rational design of nitrogen-doped carbon dots for inhibiting β-amyloid aggregation. Molecules 2023, 28, 1451. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.K.; Zhang, L.L.; Yang, Z.Y.; Guo, X.H.; Wu, Y.; Zhang, W.; Luo, J.K.; Tang, T.; Wang, Y. Herbal medicine derived carbon dots: Synthesis and applications in therapeutics, bioimaging and sensing. J. Nanobiotechnol. 2021, 19, 320. [Google Scholar] [CrossRef]
- Mirhadi, E.; Rezaee, M.; Malaekeh-Nikouei, B. Nano strategies for berberine delivery, a natural alkaloid of berberis. Biomed. Pharmacother. 2018, 104, 465–473. [Google Scholar] [CrossRef]
- Wareing, T.C.; Gentile, P.; Phan, A.N. Biomass-based carbon dots: Current development and future perspectives. ACS Nano 2021, 15, 15471–15501. [Google Scholar] [CrossRef]
- Hou, Q.; He, W.J.; Wu, Y.S.; Hao, H.J.; Xie, X.Y.; Fu, X.B. Berberine: A traditional natural product with novel biological activities. Altern. Ther. Health Med. 2020, 26, 20–27. [Google Scholar]
- Zhang, X.; Han, Y.; Huang, W.; Jin, M.; Gao, Z. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm. Sin. B 2021, 11, 1789–1812. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Sun, W.; Ding, L.; Yan, M.; Sun, C.; Zhang, M.; Li, S.; Qian, X.; Ma, J.; Wu, L. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022, 23, 19. [Google Scholar]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 inflammasome and inflammatory diseases. Oxid. Med. Cell. Longev. 2020, 2020, 4063562. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van, M.B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Wu, J.; Gan, Y.; Luo, H.; Xu, N.; Chen, L.; Li, M.; Guan, F.; Su, Z.; Lin, Z.; Xie, J.; et al. Beta-patchoulene ameliorates water transport and the mucus barrier in 5-fluorouracil-induced intestinal mucositis rats via the cAMP/PKA/CREB signaling pathway. Front. Pharmacol. 2021, 12, 689491. [Google Scholar] [CrossRef]
- Song, M.K.; Park, M.Y.; Sung, M.K. 5-fluorouracil-induced changes of intestinal integrity biomarkers in BALB/c mice. J. Cancer Prev. 2013, 18, 322–329. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, F.; Li, R.; Liu, Y.; Wang, X.; Zhang, X.; Xu, C.; Li, Y.; Guo, Y.; Yao, Q. Berberine regulates fecal metabolites to ameliorate 5-fluorouracil induced intestinal mucositis through modulating gut microbiota. Biomed. Pharmacother. 2020, 124, 109829. [Google Scholar] [CrossRef]
- Huang, R.; Ai, G.; Zhong, L.; Mai, L.; Chen, J.N.; Liu, Y.; Li, Y.; Huang, X.; Su, Z.; Zhan, J.Y. Protective effects of oxyberberine in 5-fluorouracil-induced intestinal mucositis in the mice model. Evid. Based Complement. Alternat. Med. 2022, 2022, 1238358. [Google Scholar] [CrossRef]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.P.; Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef] [PubMed]
- de Barros, P.; Rabelo, A.M.; de Vasconcelos, G.S.; Mendes, M.S.; Dos, R.D.; Lacerda, L.P.; de Sales, E.S.E.; Dos, S.M.F.; Da, G.M.; Cassali, G.D.; et al. Conjugated linoleic acid prevents damage caused by intestinal mucositis induced by 5-fluorouracil in an experimental model. Biomed. Pharmacother. 2018, 103, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Sun, W.; Ding, L.; Yan, M.; Sun, C.; Qiu, C.; Wang, D.; Wu, L. Short-chain fatty acids weaken ox-ldl-induced cell inflammatory injury by inhibiting the NLRP3/Caspase-1 pathway and affecting cellular metabolism in THP-1 cells. Molecules 2022, 27, 8801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xi, Y.; Yang, C.; Gong, W.; Wang, C.; Wu, L.; Wang, D. Short-chain fatty acids attenuate 5-fluorouracil-induced THP-1 cell inflammation through inhibiting NF-kappaB/NLRP3 signaling via glycerolphospholipid and sphingolipid metabolism. Molecules 2023, 28, 494. [Google Scholar] [CrossRef]
- Rauf, A.; Khalil, A.A.; Rahman, U.U.; Khalid, A.; Naz, S.; Shariati, M.A.; Rebezov, M.; Urtecho, E.Z.; de Albuquerque, R.; Anwar, S.; et al. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6034–6054. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut microbiota and short chain fatty acids: Implications in glucose homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Hernandez, M.; Canfora, E.E.; Jocken, J.; Blaak, E.E. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Jiang, Q.; Yin, Y. Butyrate in energy metabolism: There is still more to learn. Trends Endocrinol. Metab. 2021, 32, 159–169. [Google Scholar] [CrossRef]
- Getachew, B.; Csoka, A.B.; Bhatti, A.; Copeland, R.L.; Tizabi, Y. Butyrate protects against salsolinol-induced toxicity in SH-SY5Y cells: Implication for Parkinson’s disease. Neurotox. Res. 2020, 38, 596–602. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef] [PubMed]
- Bach, K.K.; Laerke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund, N.D.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Liu, C.; Chen, Y.W.; Fan, J.G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Xi, Y.; Yan, M.; Sun, C.; Tan, J.; He, J.; Li, H.; Wang, D. Berberine-Based Carbon Quantum Dots Improve Intestinal Barrier Injury and Alleviate Oxidative Stress in C57BL/6 Mice with 5-Fluorouracil-Induced Intestinal Mucositis by Enhancing Gut-Derived Short-Chain Fatty Acids Contents. Molecules 2023, 28, 2148. https://doi.org/10.3390/molecules28052148
Wu L, Xi Y, Yan M, Sun C, Tan J, He J, Li H, Wang D. Berberine-Based Carbon Quantum Dots Improve Intestinal Barrier Injury and Alleviate Oxidative Stress in C57BL/6 Mice with 5-Fluorouracil-Induced Intestinal Mucositis by Enhancing Gut-Derived Short-Chain Fatty Acids Contents. Molecules. 2023; 28(5):2148. https://doi.org/10.3390/molecules28052148
Chicago/Turabian StyleWu, Liang, Yue Xi, Man Yan, Chang Sun, Jiajun Tan, Jiayuan He, Haitao Li, and Dongxu Wang. 2023. "Berberine-Based Carbon Quantum Dots Improve Intestinal Barrier Injury and Alleviate Oxidative Stress in C57BL/6 Mice with 5-Fluorouracil-Induced Intestinal Mucositis by Enhancing Gut-Derived Short-Chain Fatty Acids Contents" Molecules 28, no. 5: 2148. https://doi.org/10.3390/molecules28052148
APA StyleWu, L., Xi, Y., Yan, M., Sun, C., Tan, J., He, J., Li, H., & Wang, D. (2023). Berberine-Based Carbon Quantum Dots Improve Intestinal Barrier Injury and Alleviate Oxidative Stress in C57BL/6 Mice with 5-Fluorouracil-Induced Intestinal Mucositis by Enhancing Gut-Derived Short-Chain Fatty Acids Contents. Molecules, 28(5), 2148. https://doi.org/10.3390/molecules28052148






