Emerging Immunotherapeutic and Diagnostic Modalities in Carcinoid Tumors
Abstract
1. Introduction
2. Carcinoid
3. Carcinoid Biomarkers
3.1. Carcinoid Biomarkers
3.2. Multianalyte Biomarkers
3.3. Carcinoid Immune Biomarkers
3.3.1. Gastrointestinal Carcinoids
3.3.2. Pulmonary Carcinoids
4. Non-Immune Therapy for Carcinoid
4.1. Advantages and Disadvantages of Surgical Intervention
4.2. Advantages and Disadvantages of Pharmacotherapy
| Intervention | Results |
|---|---|
| STZ with cyclophosphamide [55] | Carcinoids primary to small bowel: overall response rate (ORR) 37% Carcinoids of pulmonary or unknown region: ORR 17% |
| STZ with 5-flourouracil (5-FU) [56] | Metastatic carcinoid tumors: ORR 22% |
| STZ with doxorubicin [57] | Advanced carcinoid tumors: ORR 16% |
| Recombinant IFN-alpha-2a [58] | Metastatic carcinoid tumors: Progression-free survival median of 14.1 months |
| Capecitabine paired with temozolomide [49] | Pancreatic, lung, and small bowel-origin NETs: ORR 21% |
| Immunotherapy | Target | Carcinoid Typed |
|---|---|---|
| Combined Ipilimumab/Nivolumab | CTLA-4/PD-1 | Locations 32 patients: 18 with high-grade disease, 10 with intermediate-grade disease, and 4 with low-grade disease. Gastrointestinal (GI): 15, Lung: 6 NCT02834013 [59,60] |
| Pembrolizumab | PD-1 | 25 PD-1-positive advanced or metastatic carcinoid tumors. Lung: 9, GI: 7, Other: 9 NCT02054806 [61] |
| Pembrolizumab | PD-1 | GI tumors: 14, Pancreatic NETs: 8 NCT03043664 [62] |
| Peptide Receptor Radionuclide Therapy (PRRT) 177 Lu-Dotatate | SSTR (somatostatin receptor) | Midgut carcinoid tumors NCT01578239 [63,64] |
| Spartalizumab (PDR001) | PD-1 | Advanced NETs from pancreatic, GI, and thoracic origins including 116 pts with well-differentiated NETs NCT02955069 [65] |
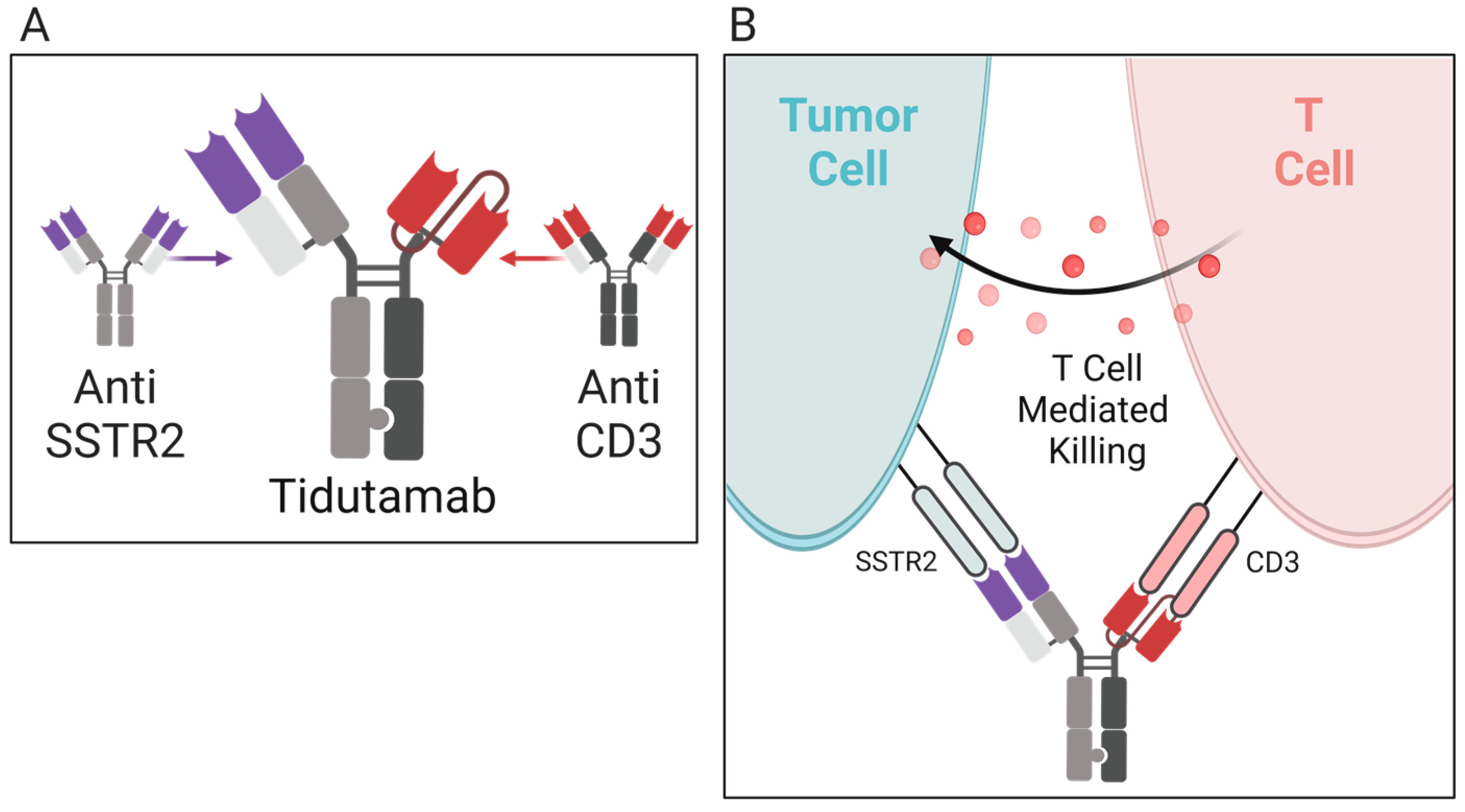
| Tidutamab (previously XmAb18087) | SSTR2 and CD3 | Advanced NETs including 41 participants comprised of the following: 46% pancreas, 22% intestine, 20% lung, and 12% GEP-NET/unknown NCT03411915 [66] |
| AdVince | Recombinant Adenovirus | Treat liver metastases from NETs including metastatic midgut carcinoids that express Chromogranin A NCT02749331 [67] |
5. Immunotherapy for Carcinoid
5.1. Overview of Immunotherapy and Carcinoid [27]
5.2. Active Immunotherapy for Carcinoid
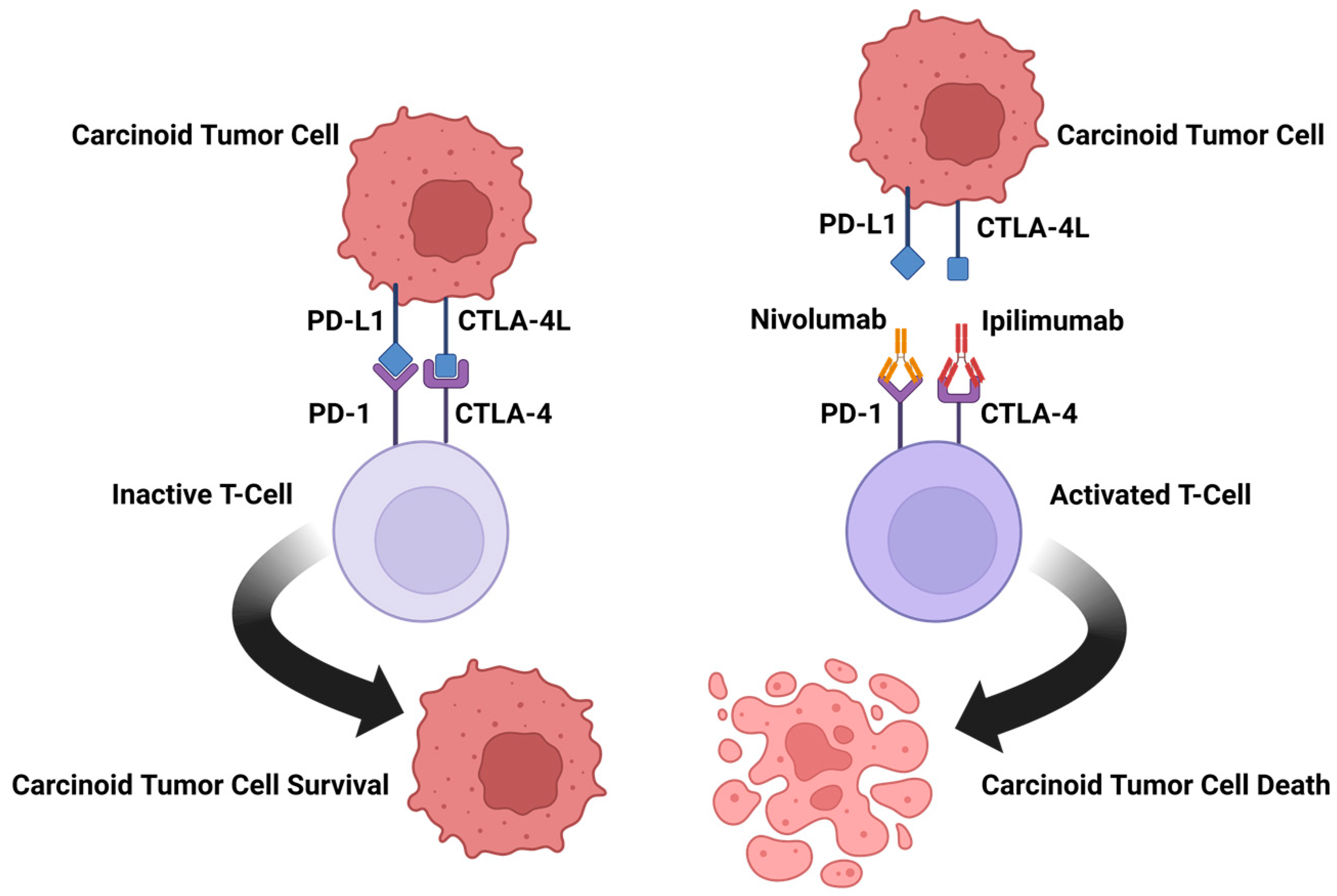
5.2.1. Ipilimumab/Nivolumab
5.2.2. Pembrolizumab
5.2.3. Spartalizumab
5.3. Passive Immunotherapy for Carcinoid
5.3.1. Tidutamab
5.3.2. 177Lu-Dotatate
6. Conclusions
7. Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breasted, J.H. (Ed.) The Edwin Smith Surgical Papyrus: Published in Facsimile and Hieroglyphic Transliteration with Translation and Commentary in Two Volumes; University of Chicago Press: Chicago, IL, USA, 1930. [Google Scholar]
- Hajdu, S.I. A note from history: Landmarks in history of cancer, part 1. Cancer 2011, 117, 1097–1102. [Google Scholar] [CrossRef]
- Di Lonardo, A.; Nasi, S.; Pulciani, S. Cancer: We Should Not Forget The Past. J. Cancer 2015, 6, 29–39. [Google Scholar] [CrossRef]
- Haridy, Y.; Witzmann, F.; Asbach, P.; Schoch, R.R.; Fröbisch, N.; Rothschild, B.M. Triassic Cancer—Osteosarcoma in a 240-Million-Year-Old Stem-Turtle. JAMA Oncol. 2019, 5, 425–426. [Google Scholar] [CrossRef]
- Inthagard, J.; Edwards, J.; Roseweir, A.K. Immunotherapy: Enhancing the efficacy of this promising therapeutic in multiple cancers. Clin. Sci. 2019, 133, 181–193. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.M.; Phan, A.T.; Dagohoy, C.G.; Leary, C.C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Cingam, S.R.; Kashyap, S.; Karanchi, H. Carcinoid Tumors. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kuracinová, K.M.; Janega, P.; Janegová, A.; Čierna, Z. Histopathology of Neuroendocrine Neoplasms of the Gastrointestinal System. Klin. Onkol. 2018, 31, 167–177. [Google Scholar] [CrossRef]
- Manrow, R.E.; Beckwith, M.; Johnson, L.E. NCI’s Physician Data Query (PDQ®) Cancer Information Summaries: History, Editorial Processes, Influence, and Reach. J. Cancer Educ. 2013, 29, 198–205. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Oiseth, S.J.; Aziz, M.S. Cancer immunotherapy: A brief review of the history, possibilities, and challenges ahead. J. Cancer Metastasis Treat. 2017, 3, 250. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Coley, W.B. The treatment of malignant tumors by repeated inoculations of erysipelas: With a report of ten original cases. Am. J. Med. Sci. 1893, 105, 487. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.M.; Buqué, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.-P.; Agostinis, P.; et al. Classification of current anticancer immunotherapies. Oncotarget 2014, 5, 12472–12508. [Google Scholar] [CrossRef]
- Wu, X.; Gu, Z.; Chen, Y.; Chen, B.; Chen, W.; Weng, L.; Liu, X. Application of PD-1 Blockade in Cancer Immunotherapy. Comput. Struct. Biotechnol. J. 2019, 17, 661–674. [Google Scholar] [CrossRef]
- Weber, M.M.; Fottner, C. Immune Checkpoint Inhibitors in the Treatment of Patients with Neuroendocrine Neoplasia. Oncol. Res. Treat. 2018, 41, 306–312. [Google Scholar] [CrossRef]
- Linsley, P.S.; Wallace, P.M.; Johnson, J.; Gibson, M.G.; Greene, J.L.; Ledbetter, J.A.; Singh, C.; Tepper, M.A. Immunosuppression in Vivo by a Soluble Form of the CTLA-4 T Cell Activation Molecule. Science 1992, 257, 792–795. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef]
- Thakur, A.; Huang, M.; Lum, L.G. Bispecific antibody based therapeutics: Strengths and challenges. Blood Rev. 2018, 32, 339–347. [Google Scholar] [CrossRef]
- Shim, H. Bispecific Antibodies and Antibody–Drug Conjugates for Cancer Therapy: Technological Considerations. Biomolecules 2020, 10, 360. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; De Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef]
- Ahmed, M. Gastrointestinal neuroendocrine tumors in 2020. World J. Gastrointest. Oncol. 2020, 12, 791–807. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.M.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Gade, A.K.; Olariu, E.; Douthit, N.T. Carcinoid Syndrome: A Review. Cureus 2020, 12, e7186. [Google Scholar] [CrossRef]
- Vinik, A.; Hughes, M.S.; Feliberti, E.; Perry, R.R.; Casellini, C.; Sinesi, M.; Vingan, H.; Johnson, L. Carcinoid Tumors. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Strosberg, J.R. Clinical Characteristics of Well-Differentiated Neuroendocrine (Carcinoid) Tumors Arising in the Gastrointestinal and Genitourinary Tracts; Post, T.W., Ed.; UpToDate: Waltham, MA, USA, 2021. [Google Scholar]
- Al-Toubah, T.; Cives, M.; Strosberg, J. Novel immunotherapy strategies for treatment of neuroendocrine neoplasms. Transl. Gastroenterol. Hepatol. 2020, 5, 54. [Google Scholar] [CrossRef]
- Krishnan, M.; Tuma, F. Intestinal Carcinoid Cancer. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rossi, R.E.; Elvevi, A.; Citterio, D.; Coppa, J.; Invernizzi, P.; Mazzaferro, V.; Massironi, S. Gastrinoma and Zollinger Ellison syndrome: A roadmap for the management between new and old therapies. World J. Gastroenterol. 2021, 27, 5890–5907. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Spilker, B.A.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Capdevila, J.; Meeker, A.; García-Carbonero, R.; Pietras, K.; Astudillo, A.; Casanovas, O.; Scarpa, A. Molecular biology of neuroendocrine tumors: From pathways to biomarkers and targets. Cancer Metastasis Rev. 2013, 33, 345–351. [Google Scholar] [CrossRef]
- Modlin, I.M.; Bodei, L.; Kidd, M. Neuroendocrine tumor biomarkers: From monoanalytes to transcripts and algorithms. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 59–77. [Google Scholar] [CrossRef]
- Malczewska, A.; Kos-Kudła, B.; Kidd, M.; Drozdov, I.; Bodei, L.; Matar, S.; Oberg, K.; Modlin, I.M. The clinical applications of a multigene liquid biopsy (NETest) in neuroendocrine tumors. Adv. Med Sci. 2019, 65, 18–29. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Malczewska, A.; Drozdov, I.; Bodei, L.; Matar, S.; Chung, K.-M. The NETest. Endocrinol. Metab. Clin. N. Am. 2018, 47, 485–504. [Google Scholar] [CrossRef]
- Liu, E.; Paulson, S.; Gulati, A.; Freudman, J.; Grosh, W.; Kafer, S.; Wickremesinghe, P.C.; Salem, R.R.; Bodei, L. Assessment of NETest Clinical Utility in a U.S. Registry-Based Study. Oncologist 2018, 24, 783–790. [Google Scholar] [CrossRef]
- Oberg, K.; Modlin, I.M.; De Herder, W.; Pavel, M.; Klimstra, D.; Frilling, A.; Metz, D.C.; Heaney, A.; Kwekkeboom, D.J.; Strosberg, J.R.; et al. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015, 16, e435–e446. [Google Scholar] [CrossRef]
- Onaitis, M.W.; Kirshbom, P.M.; Hayward, T.Z.; Quayle, F.J.; Feldman, J.M.; Seigler, H.F.; Tyler, D.S. Gastrointestinal Carcinoids: Characterization by Site of Origin and Hormone Production. Ann. Surg. 2000, 232, 549–556. [Google Scholar] [CrossRef]
- Ito, T.; Igarashi, H.; Jensen, R.T. Serum Pancreastatin. Pancreas 2012, 41, 505–507. [Google Scholar] [CrossRef]
- Cui, T.; Hurtig, M.; Elgue, G.; Li, S.-C.; Veronesi, G.; Essaghir, A.; Demoulin, J.-B.; Pelosi, G.; Alimohammadi, M.; Öberg, K.; et al. Paraneoplastic Antigen Ma2 Autoantibodies as Specific Blood Biomarkers for Detection of Early Recurrence of Small Intestine Neuroendocrine Tumors. PLoS ONE 2010, 5, e16010. [Google Scholar] [CrossRef]
- Kidd, M.; Drozdov, I.; Modlin, I. Blood and tissue neuroendocrine tumor gene cluster analysis correlate, define hallmarks and predict disease status. Endocr.-Relat. Cancer 2015, 22, 561–575. [Google Scholar] [CrossRef]
- Vikman, S.; Sommaggio, R.; De La Torre, M.; Öberg, K.; Essand, M.; Giandomenico, V.; Loskog, A.; Tötterman, T.H. Midgut carcinoid patients display increased numbers of regulatory T cells in peripheral blood with infiltration into tumor tissue. Acta Oncol. 2009, 48, 391–400. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J.; Al Diffalha, S.; Coppola, D. Analysis of the immune landscape of small bowel neuroendocrine tumors. Endocr.-Relat. Cancer 2019, 26, 119–130. [Google Scholar] [CrossRef]
- Stankovic, B.; Aamodt, H.; Bjørhovde, H.A.K.; Müller, E.; Hammarström, C.; Brustugun, O.T.; Helland, D.; Øynebråten, I.; Corthay, A. The immune microenvironment in typical carcinoid lung tumour, a brief report of four cases. Scand. J. Immunol. 2020, 92, e12893. [Google Scholar] [CrossRef]
- Vesterinen, T.; Kuopio, T.; Ahtiainen, M.; Knuuttila, A.; Mustonen, H.K.; Salmenkivi, K.; Arola, J.; Haglund, C. PD-1 and PD-L1 expression in pulmonary carcinoid tumors and their association to tumor spread. Endocr. Connect. 2019, 8, 1168–1175. [Google Scholar] [CrossRef]
- Reuling, E.; Dickhoff, C.; Plaisier, P.; Bonjer, H.; Daniels, J. Endobronchial and surgical treatment of pulmonary carcinoid tumors: A systematic literature review. Lung Cancer 2019, 134, 85–95. [Google Scholar] [CrossRef]
- Filosso, P.L.; Ruffini, E.; Di Gangi, S.; Guerrera, F.; Bora, G.; Ciccone, G.; Galassi, C.; Solidoro, P.; Lyberis, P.; Oliaro, A.; et al. Prognostic factors in neuroendocrine tumours of the lung: A single-centre experience †. Eur. J. Cardio-Thorac. Surg. 2013, 45, 521–526. [Google Scholar] [CrossRef]
- Thomas, K.; Voros, B.A.; Meadows-Taylor, M.; Smeltzer, M.P.; Griffin, R.; Boudreaux, J.P.; Thiagarajan, R.; Woltering, E.A.; Ramirez, R.A. Outcomes of Capecitabine and Temozolomide (CAPTEM) in Advanced Neuroendocrine Neoplasms (NENs). Cancers 2020, 12, 206. [Google Scholar] [CrossRef]
- Pusceddu, S.; Verzoni, E.; Prinzi, N.; Mennitto, A.; Femia, D.; Grassi, P.; Concas, L.; Vernieri, C.; Russo, G.L.; Procopio, G. Everolimus treatment for neuroendocrine tumors: Latest results and clinical potential. Ther. Adv. Med. Oncol. 2017, 9, 183–188. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2015, 387, 968–977. [Google Scholar] [CrossRef]
- Larouche, V.; Akirov, A.; Alshehri, S.; Ezzat, S. Management of Small Bowel Neuroendocrine Tumors. Cancers 2019, 11, 1395. [Google Scholar] [CrossRef]
- Comaru-Schally, A.M.; Schally, A.V. A clinical overview of carcinoid tumors: Perspectives for improvement in treatment using peptide analogs (review). Int. J. Oncol. 2005, 26, 301–309. [Google Scholar] [CrossRef]
- Das, S.; Al-Toubah, T.; Strosberg, J. Chemotherapy in Neuroendocrine Tumors. Cancers 2021, 13, 4872. [Google Scholar] [CrossRef]
- Moertel, C.G.; Hanley, J.A. Combination chemotherapy trials in metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer Clin. Trials 1979, 2, 327–334. [Google Scholar]
- Engstrom, P.F.; Lavin, P.T.; Moertel, C.G.; Folsch, E.; Douglass, H.O. Streptozocin plus fluorouracil versus doxorubicin therapy for metastatic carcinoid tumor. J. Clin. Oncol. 1984, 2, 1255–1259. [Google Scholar] [CrossRef]
- Sun, W.; Lipsitz, S.; Catalano, P.; Mailliard, J.A.; Haller, D.G. Phase II/III Study of Doxorubicin With Fluorouracil Compared With Streptozocin With Fluorouracil or Dacarbazine in the Treatment of Advanced Carcinoid Tumors: Eastern Cooperative Oncology Group Study E1281. J. Clin. Oncol. 2005, 23, 4897–4904. [Google Scholar] [CrossRef]
- Dahan, L.; Bonnetain, F.; Rougier, P.; Raoul, J.-L.; Gamelin, E.; Etienne, P.-L.; Cadiot, G.; Mitry, E.; Smith, D.; Cvitkovic, F.; et al. Phase III trial of chemotherapy using 5-fluorouracil and streptozotocin compared with interferon α for advanced carcinoid tumors: FNCLCC–FFCD 9710. Endocr.-Relat. Cancer 2009, 16, 1351–1361. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Al Baghdadi, T.; et al. A Phase II Basket Trial of Dual Anti–CTLA-4 and Anti–PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef]
- Patel, S.P.; Mayerson, E.; Chae, Y.K.; Strosberg, J.; Wang, J.; Konda, B.; Hayward, J.; McLeod, C.M.; Chen, H.X.; Sharon, E.; et al. A phase II basket trial of Dual Anti–CTLA–4 and Anti–PD–1 Blockade in Rare Tumors (DART) SWOG S1609: High-grade neuroendocrine neoplasm cohort. Cancer 2021, 127, 3194–3201. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Bergsland, E.; O’Neil, B.H.; Santoro, A.; Schellens, J.H.M.; Cohen, R.B.; Doi, T.; Ott, P.A.; Pishvaian, M.J.; Puzanov, I.; et al. Pembrolizumab for the treatment of programmed death–ligand 1–positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020, 126, 3021–3030. [Google Scholar] [CrossRef]
- Morse, M.; Halperin, D.M.; Uronis, H.E.; Hsu, D.S.; Hurwitz, H.; Bolch, E.; Warren, D.; Haley, S.; John, L.; Moyer, A.; et al. Phase Ib/II study of pembrolizumab with lanreotide depot for advanced, progressive gastroenteropancreatic neuroendocrine tumors (PLANET). J. Clin. Oncol. 2021, 39, 369. [Google Scholar] [CrossRef]
- Strosberg, J.R.; E Caplin, M.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; E Pavel, M.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Mandriani, B.; Pelle’, E.; Mannavola, F.; Ingravallo, G.; Cazzato, G.; Ramello, M.; Porta, C.; Strosberg, J.; Abate-Daga, D.; Cives, M. 1101MO Development of CAR T-cells for future treatment of NETs. Ann. Oncol. 2021, 32, S911. [Google Scholar] [CrossRef]
- Yao, J.; Strosberg, J.; Fazio, N.; Pavel, M.; Ruszniewski, P.; Bergsland, E.; Li, D.; Tafuto, S.; Raj, N.; Campana, D.; et al. Activity & safety of spartalizumab (PDR001) in patients (pts) with advanced neuroendocrine tumors (NET) of pancreatic (Pan), gastrointestinal (GI), or thoracic (T) origin, & gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progressed on prior treatment (Tx). Ann. Oncol. 2018, 29, viii467–viii468. [Google Scholar] [CrossRef]
- El-Rayes, B.F.; Hendifar, A.E.; Pant, S.; Wilky, B.A.; Reilley, M.J.; Benson, A.B.; Chow, W.A.; Konda, B.; Starr, J.; Ahn, D.H.; et al. Safety, Pharmacodynamic, and Antitumor Activity of Tidutamab, an SSTR2 x CD3 Bispecific Antibody, in Subjects with Advanced Neuroendocrine Tumors. In Proceedings of the NANETS Multidisciplinary NET Symposium, Virtual, 3–6 November 2021. [Google Scholar]
- Strosberg, J.R.; Mizuno, N.; Doi, T.; Grande, E.; Delord, J.-P.; Shapira-Frommer, R.; Bergsland, E.K.; Shah, M.H.; Fakih, M.; Takahashi, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin. Cancer Res. 2020, 26, 2124–2130. [Google Scholar] [CrossRef]
- Liu, M.; Guo, F. Recent updates on cancer immunotherapy. Precis. Clin. Med. 2018, 1, 65–74. [Google Scholar] [CrossRef]
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Kwok, G.; Yau, T.C.C.; Chiu, J.W.; Tse, E.; Kwong, Y.-L. Pembrolizumab (Keytruda). Hum. Vaccines Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef]
- Yao, J.C.; Strosberg, J.; Fazio, N.; Pavel, M.E.; Bergsland, E.; Ruszniewski, P.; Halperin, D.M.; Li, D.; Tafuto, S.; Raj, N.; et al. Spartalizumab in metastatic, well/poorly differentiated neuroendocrine neoplasms. Endocr. Relat. Cancer 2021, 28, 161–172. [Google Scholar] [CrossRef]
- Kuhns, M.S.; Davis, M.M.; Garcia, K.C. Deconstructing the Form and Function of the TCR/CD3 Complex. Immunity 2006, 24, 133–139. [Google Scholar] [CrossRef]

| Biomarker | Carcinoid Location | Assess/Correlations | Sensitivity and Specificity |
|---|---|---|---|
| Chromogranin A(CgA) | All locations | Confirm diagnosis, assess treatment progress, tumor burden, correlated to tumor load, background levels variable in different populations [32,33,34,35] | 43–100% sensitivity 10–96% specificity [36] |
| Serotonin (5-HT) | Foregut, Midgut | Blood serum analysis, carcinoid syndrome [32,37] | 35% sensitivity up to ≈100% specificity [36] |
| 5-HIAA | Midgut | Urinary or serum analysis, carcinoid syndrome, used for screening and diagnosis [32,36] | 35% sensitivity Up to ≈100% specificity [36] |
| Pancreastatin | Pancreas, Midgut | Tumor activity [32,36,38] | 64% sensitivity 58–100% specificity [36] |
| Neurokinin A (NKA), Substance P | Midgut | Prognostic value, correlated with poor outcome [36] | 88% sensitivity No data for specificity [36] |
| Neuron-specific enolase (NSE) | All locations | Elevated levels suggest poor differentiation [32,36] | 33% sensitivity Up to 100% specificity [36] |
| Progastrin- releasing peptide (proGRP) | Lung | Expression associated with survival, >90 ng/L negatively correlated with outcome [36] | 99% sensitivity 43% specificity [36] |
| Pancreatic Polypeptide (PP) | Pancreas, Midgut, Colon | No known clinical utility [36] | 50–80% sensitivity No data for specificity [36] |
| N-terminal pro-brain natriuretic peptide (NT-proBNP) | Midgut | Prognostic value, correlates with survival in carcinoid heart disease [36] | 87% sensitivity 80% specificity [36] |
| Connective Tissue Growth Factor (CTGF) | Midgut | Elevations predict reduced right ventricular function in carcinoid heart disease [36] | 88% sensitivity 69% specificity [36] |
| Paraneoplastic Ma antigen 2 (PNMA2) | Small intestines, Lung | Assess recurrence risk [39] | 46–50% sensitivity SI-NETs 35% sensitivity lung 98% overall specificity [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vellani, S.D.; Nigro, A.; Varatharajan, S.; Dworkin, L.D.; Creeden, J.F. Emerging Immunotherapeutic and Diagnostic Modalities in Carcinoid Tumors. Molecules 2023, 28, 2047. https://doi.org/10.3390/molecules28052047
Vellani SD, Nigro A, Varatharajan S, Dworkin LD, Creeden JF. Emerging Immunotherapeutic and Diagnostic Modalities in Carcinoid Tumors. Molecules. 2023; 28(5):2047. https://doi.org/10.3390/molecules28052047
Chicago/Turabian StyleVellani, Shahnaz D., Anthony Nigro, Shangari Varatharajan, Lance D. Dworkin, and Justin Fortune Creeden. 2023. "Emerging Immunotherapeutic and Diagnostic Modalities in Carcinoid Tumors" Molecules 28, no. 5: 2047. https://doi.org/10.3390/molecules28052047
APA StyleVellani, S. D., Nigro, A., Varatharajan, S., Dworkin, L. D., & Creeden, J. F. (2023). Emerging Immunotherapeutic and Diagnostic Modalities in Carcinoid Tumors. Molecules, 28(5), 2047. https://doi.org/10.3390/molecules28052047







