Synthesis and Characterization of Novel Triphenylamine—Containing Electrochromic Polyimides with Benzimidazole Substituents
Abstract
1. Introduction
2. Results and Discussion
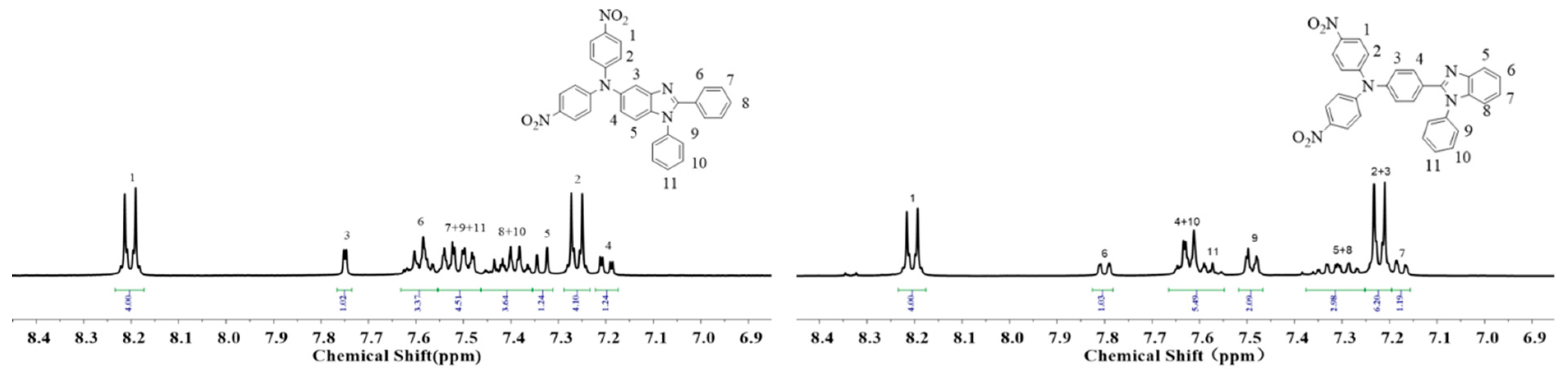
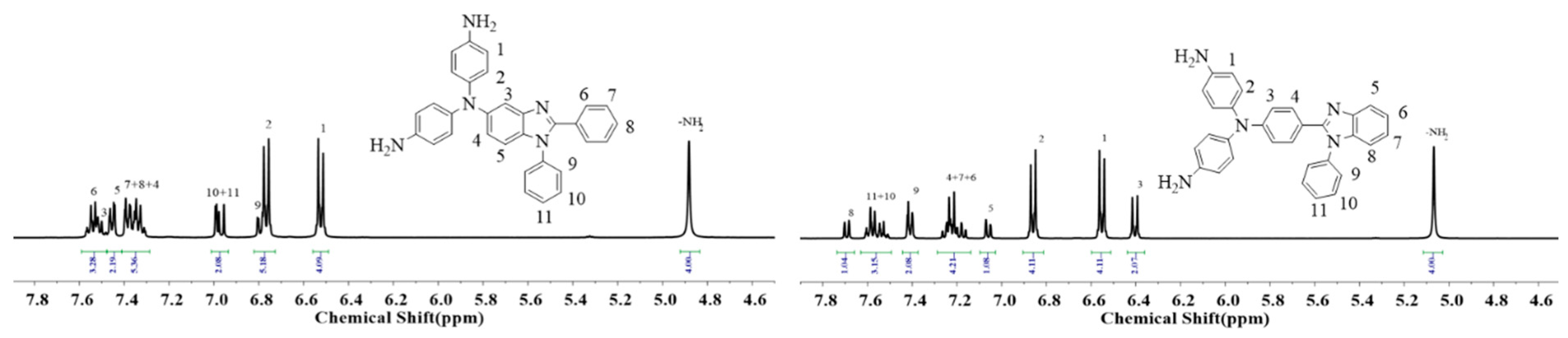
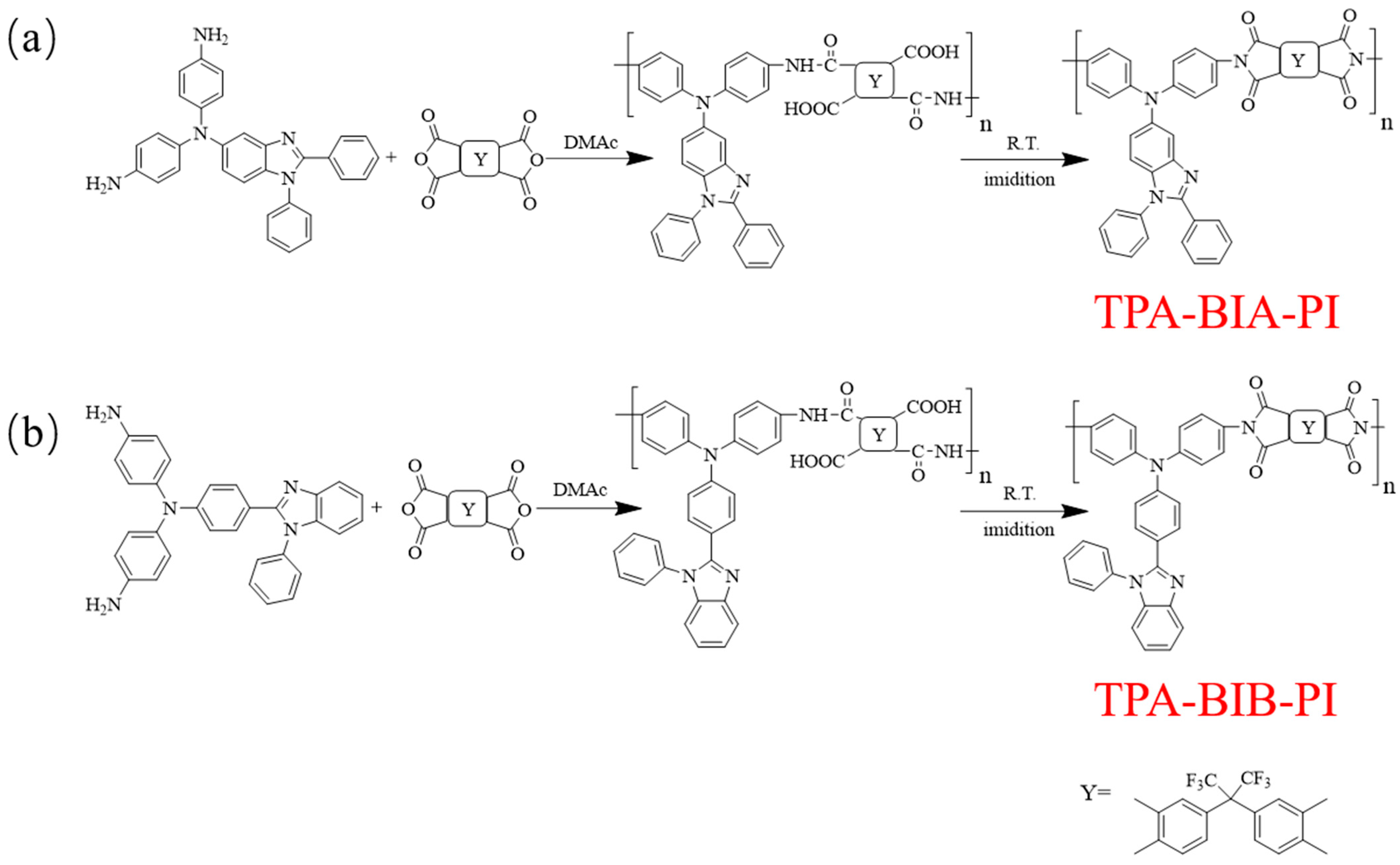
2.1. Monomer Synthesis
2.2. Polymer Synthesis
2.3. Basic Properties
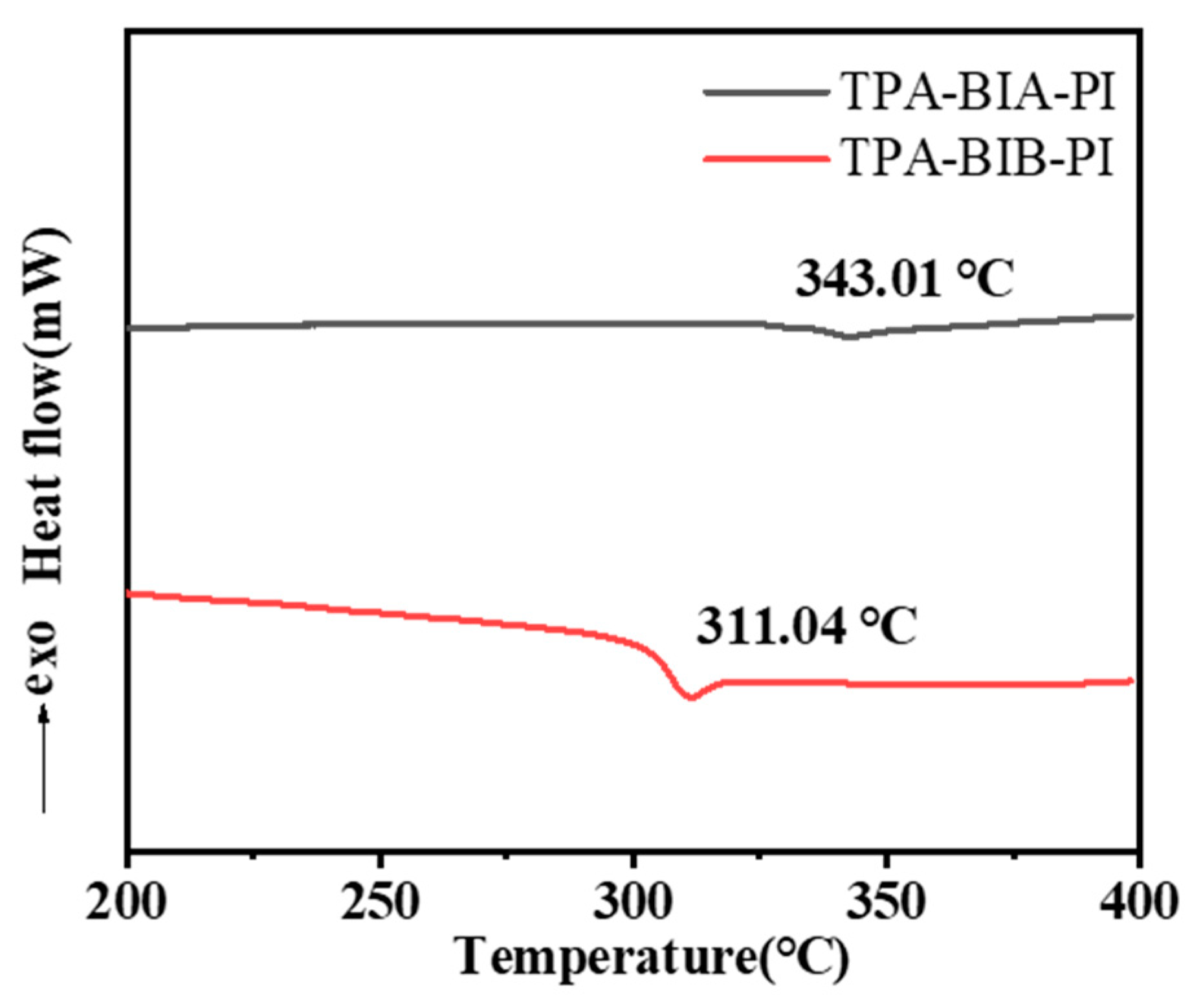
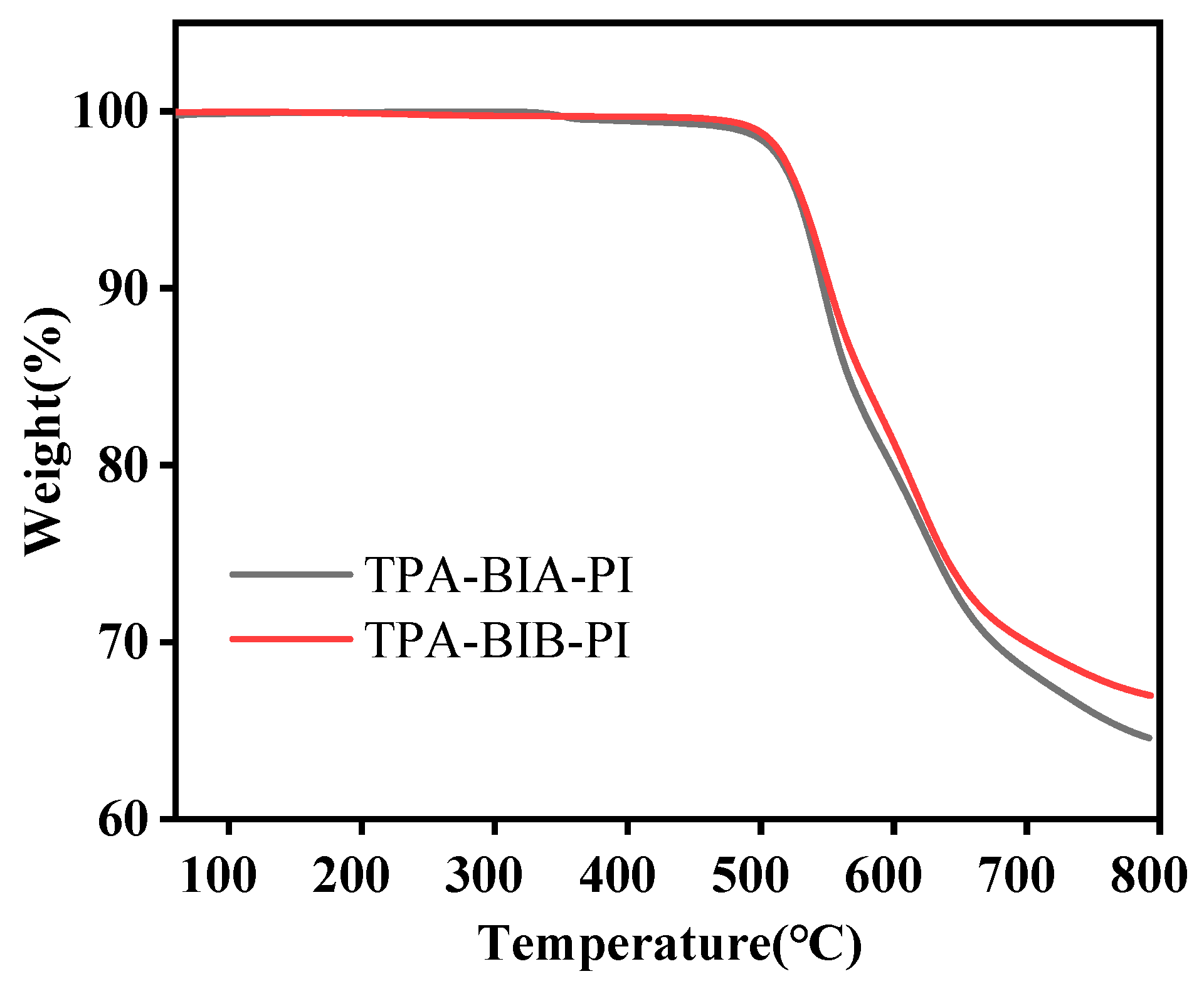
2.4. Thermal Properties
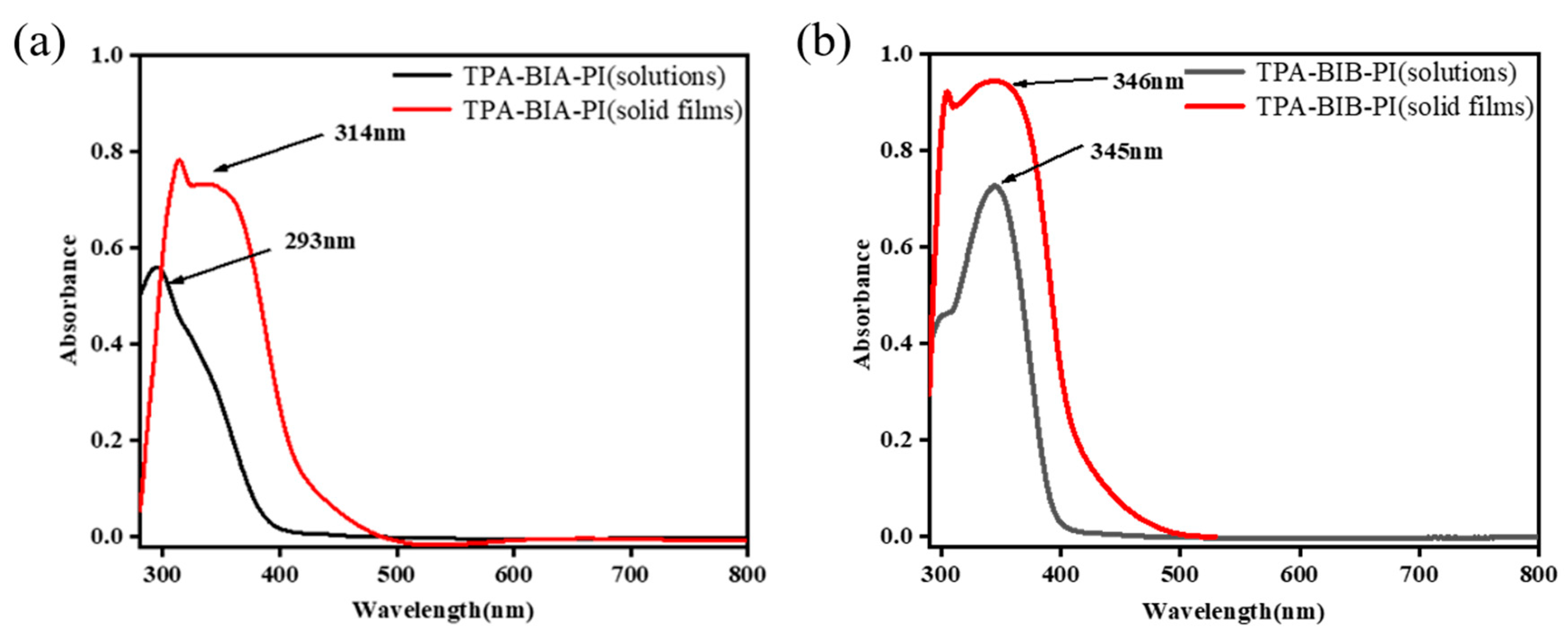
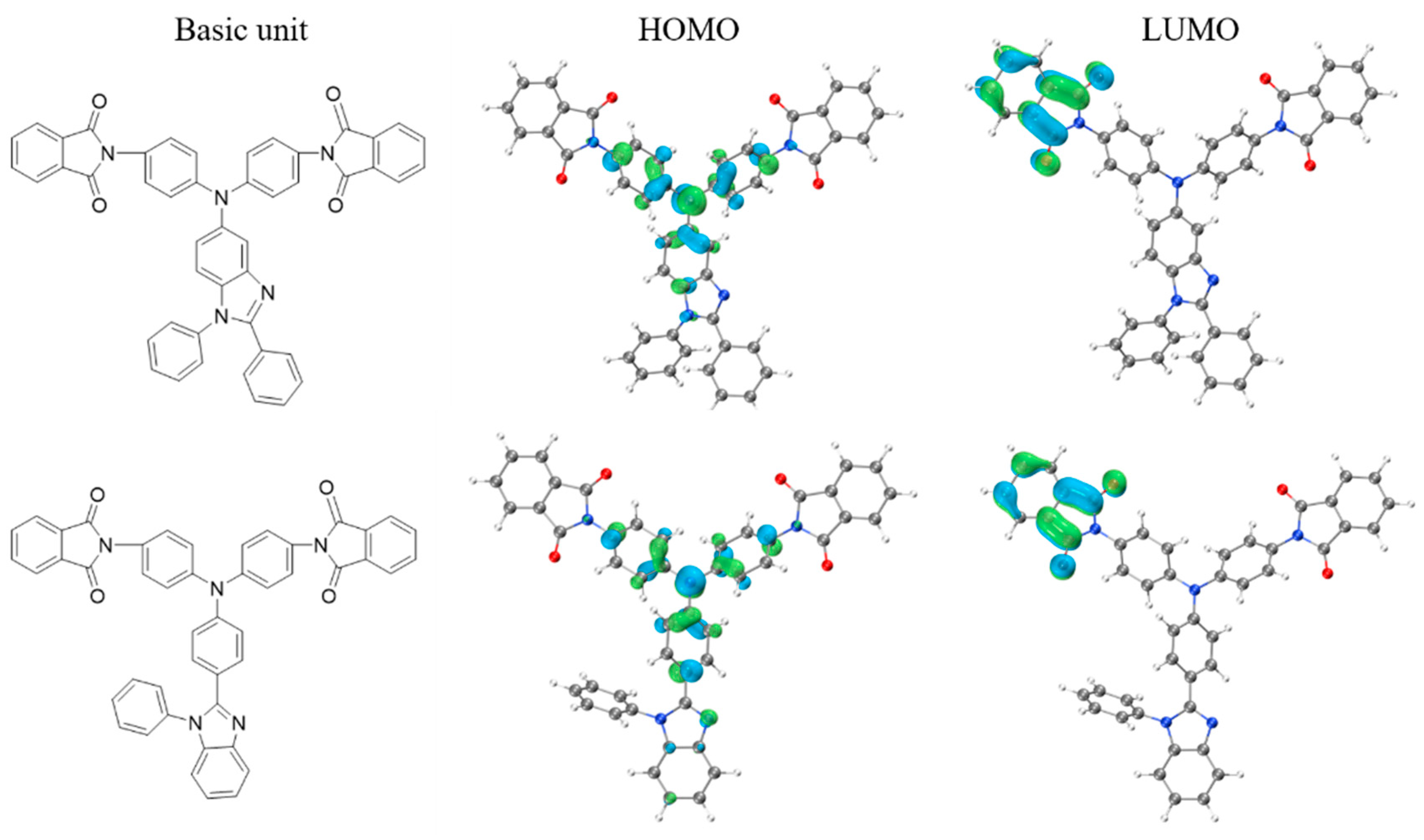
2.5. Optical Properties
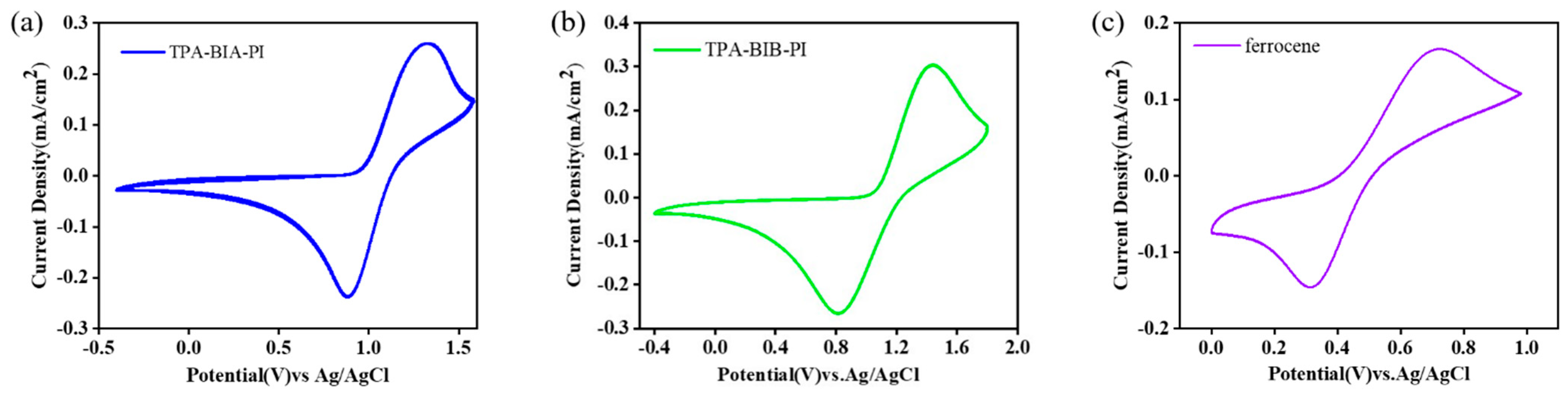
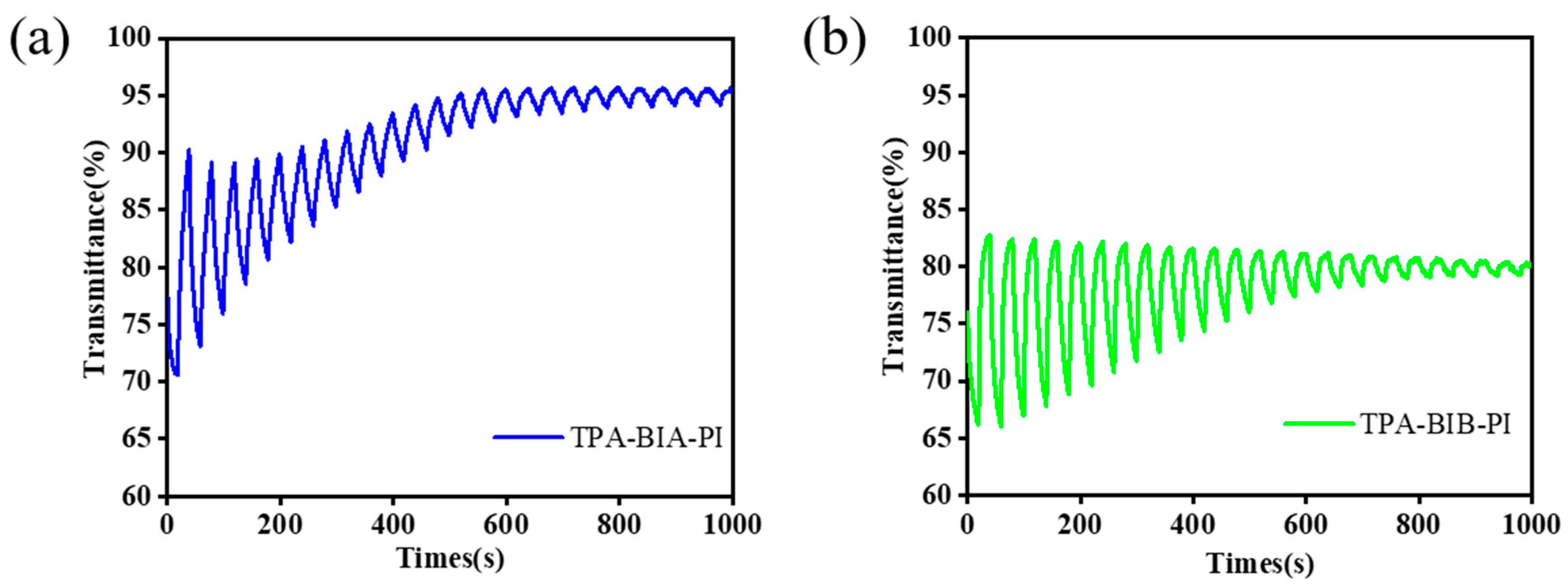
2.6. Electrochemical and Electrochromic Properties
3. Experimental
3.1. Materials
3.2. Synthesis of Compounds
3.2.1. Synthesis of 1,2-Diphenyl-N,N′-di-4-nitrophenyl-5-amine benzimidazole (TPA-BIA-NO2)
3.2.2. Synthesis of 1,2-Diphenyl-N,N′-di-4-aminophenyl-5-amino-benzimidazole (TPA-BIA-NH2)
3.2.3. Synthesis of 4-Nitro-4′-nitrophenyl-4″-1-phenyl-benzimidazolyl-phenyl-aniline (TPA-BIB-NO2)
3.2.4. Synthesis of 4-Amino-4′-aminophenyl-4″-1-phenyl-benzimidazolyl-phenyl-aniline (TPA-BIB-NH2)
3.3. Synthesis of Polyimides TPA-BIA-PI and TPA-BIB-PI
3.4. Preparation of Polyimide Films
3.5. Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Platt, J.R. Electrochromism, a Possible Change of Color Producible in Dyes by an Electric Field. J. Chem. Phys. 1961, 34, 862–863. [Google Scholar] [CrossRef]
- Deb, S.K. Optical and Photoelectric Properties and Colour Centres in Thin Films of Tungsten Oxide. Philos. Mag. 1973, 27, 801–822. [Google Scholar] [CrossRef]
- Bach, U.; Corr, D.; Lupo, D.; Pichot, F.; Ryan, M. Nanomaterials-Based Electrochromics for Paper-Quality Displays. Adv. Mater. 2002, 14, 845. [Google Scholar] [CrossRef]
- Österholm, A.M.; Shen, D.E.; Gottfried, D.S.; Reynolds, J.R. Full Color Control and High-Resolution Patterning from Inkjet Printable Cyan/Magenta/Yellow Colored-to-Colorless Electrochromic Polymer Inks. Adv. Mater. Technol. 2016, 1, 1600063. [Google Scholar] [CrossRef]
- Sibilio, S.; Rosato, A.; Scorpio, M.; Iuliano, G.; Ciampi, G.; Vanoli, G.P.; de Rossi, F. A Review of Electrochromic Windows for Residential Applications. IJHT 2016, 34, S481–S488. [Google Scholar] [CrossRef]
- Beaupré, S.; Breton, A.-C.; Dumas, J.; Leclerc, M. Multicolored Electrochromic Cells Based On Poly(2,7-Carbazole) Derivatives For Adaptive Camouflage. Chem. Mater. 2009, 21, 1504–1513. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Properties, Requirements and Possibilities of Smart Windows for Dynamic Daylight and Solar Energy Control in Buildings: A State-of-the-Art Review. Sol. Energy Mater. Sol. Cells 2010, 94, 87–105. [Google Scholar] [CrossRef]
- Shin, H.; Kim, Y.; Bhuvana, T.; Lee, J.; Yang, X.; Park, C.; Kim, E. Color Combination of Conductive Polymers for Black Electrochromism. ACS Appl. Mater. Interfaces 2012, 4, 185–191. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromic Tungsten Oxide Films: Review of Progress 1993–1998. Sol. Energy Mater. Sol. Cells 2000, 60, 201–262. [Google Scholar] [CrossRef]
- Bange, K. Colouration of Tungsten Oxide ”lms: A Model for Optically Active Coatings. Sol. Energy Mater. 1999, 58, 1–131. [Google Scholar] [CrossRef]
- Beaujuge, P.; Reynolds, J. Color Control in Π-Conjugated Organic Polymers for Use in Electrochromic Devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef]
- Huang, C.-L.; Kung, Y.-R.; Shao, Y.-J.; Liou, G.-S. Synthesis and Characteristics of Novel TPA-Containing Electrochromic Poly(Ether Sulfone)s with Dimethylamino Substituents. Electrochim. Acta 2021, 368, 137552. [Google Scholar] [CrossRef]
- van Mullekom, H.A.M.; Vekemans, J.A.J.M.; Havinga, E.E.; Meijer, E.W. Developments in the Chemistry and Band Gap Engineering of Donor–Acceptor Substituted Conjugated Polymers. Mater. Sci. Eng. R Rep. 2001, 32, 1–40. [Google Scholar] [CrossRef]
- Beaujuge, P.; Amb, M.; Reynolds, J. Spectral Engineering in π-Conjugated Polymers with Intramolecular Donor−Acceptor Interactions. Acc. Chem. Res. 2010, 43, 1396–1407. [Google Scholar] [CrossRef]
- Lv, X.; Li, W.; Ouyang, M.; Zhang, Y.; Wright, D.S.; Zhang, C. Polymeric Electrochromic Materials with Donor–Acceptor Structures. J. Mater. Chem. C 2017, 5, 12–28. [Google Scholar] [CrossRef]
- Qian, G.; Chen, H.; Song, G.; Yao, J.; Hu, M.; Chen, C. Synthesis of Polyimides with Lower H 2 O-absorption and Higher Thermal Properties by Incorporation of Intramolecular H-bonding. J. Polym. Sci. 2020, 58, 969–976. [Google Scholar] [CrossRef]
- Qian, G.; Chen, H.; Song, G.; Dai, F.; Chen, C.; Yao, J. Superheat-Resistant Polyimides with Ultra-Low Coefficients of Thermal Expansion. Polymer 2020, 196, 122482. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Sadrzadeh, M.; Matsuura, T. Thermally Stable Polymers for Advanced High-Performance Gas Separation Membranes. Prog. Energy Combust. Sci. 2018, 66, 1–41. [Google Scholar] [CrossRef]
- Li, Q.; Fang, X.; Wang, Z.; Gao, L.; Ding, M. Polyimides from Isomeric Oxydiphthalic Anhydrides. J. Polym. Sci. A Polym. Chem. 2003, 41, 3249–3260. [Google Scholar] [CrossRef]
- Espeso, J.; Ferrero, E.; de la Campa, J.; Lozano, A.; de Abajo, J. Synthesis and Characterization of New Soluble Aromatic Polyamides Derived from 1,4-Bis(4-carboxyphenoxy)-2, 5-di-tert-butylbenzene. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 475–485. [Google Scholar] [CrossRef]
- Myung, B.Y.; Ahn, C.J.; Yoon, T.H. Synthesis and Characterization of Polyimides from Novel 1-(3′,5′-Bis(Trifluoromethyl)Benzene) Pyromelliticdianhydride (6FPPMDA). Polymer 2004, 45, 3185–3193. [Google Scholar] [CrossRef]
- Sahadeva Reddy, D.; Chou, C.-H.; Shu, C.-F.; Lee, G.-H. Synthesis and Characterization of Soluble Poly(Ether Imide)s Based on 2,2′-Bis(4-Aminophenoxy)-9,9′-Spirobifluorene. Polymer 2003, 44, 557–563. [Google Scholar] [CrossRef]
- Liou, G.-S.; Hsiao, S.-H.; Ishida, M.; Kakimoto, M.; Imai, Y. Synthesis and Characterization of Novel Soluble Triphenylamine-Containing Aromatic Polyamides Based OnN,N′-Bis(4-Aminophenyl)-N,N′-Diphenyl-1,4-Phenylenediamine. J. Polym. Sci. A Polym. Chem. 2002, 40, 2810–2818. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Yang, C.-P.; Chen, C.-W.; Liou, G.-S. Synthesis and Properties of Novel Poly(Amide-Imide)s Derived from 2,4-Diaminotriphenylamine and Imide Ring-Preformed Dicarboxylic Acids. J. Polym. Res. 2005, 12, 289–294. [Google Scholar] [CrossRef]
- Liaw, D.J.; Hsu, P.N.; Chen, W.H.; Lin, S.-L. High Glass Transitions of New Polyamides, Polyimides, and Poly(Amide−imide)s Containing a Triphenylamine Group: Synthesis and Characterization. Macromolecules 2002, 35, 4669–4676. [Google Scholar] [CrossRef]
- Yen, H.-J.; Lin, H.-Y.; Liou, G.-S. Novel Starburst Triarylamine-Containing Electroactive Aramids with Highly Stable Electrochromism in Near-Infrared and Visible Light Regions. Chem. Mater. 2011, 23, 1874–1882. [Google Scholar] [CrossRef]
- Liou, G.-S.; Chang, C.-W. Highly Stable Anodic Electrochromic Aromatic Polyamides Containing N,N,N′,N′-Tetraphenyl-p-Phenylenediamine Moieties: Synthesis, Electrochemical, and Electrochromic Properties. Macromolecules 2008, 41, 1667–1674. [Google Scholar] [CrossRef]
- Chang, C.-W.; Chung, C.-H.; Liou, G.-S. Novel Anodic Polyelectrochromic Aromatic Polyamides Containing Pendent Dimethyltriphenylamine Moieties. Macromolecules 2008, 41, 8441–8451. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Recent Advances in Triphenylamine-Based Electrochromic Derivatives and Polymers. Polym. Chem. 2018, 9, 3001–3018. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Design and Preparation of Triphenylamine-Based Polymeric Materials towards Emergent Optoelectronic Applications. Prog. Polym. Sci. 2019, 89, 250–287. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chou, Y.-T. Synthesis and Electrochromic Properties of Aromatic Polyimides Bearing Pendent Triphenylamine Units. Polymer 2014, 55, 2411–2421. [Google Scholar] [CrossRef]
- Liou, G.-S.; Hsiao, S.-H.; Su, T.-H. Novel Thermally Stable Poly(Amine Hydrazide)s and Poly(Amine-1,3,4-Oxadiazole)s for Luminescent and Electrochromic Materials. J. Polym. Sci. A Polym. Chem. 2005, 43, 3245–3256. [Google Scholar] [CrossRef]
- Yuan Chiu, K.; Xiang Su, T.; Hong Li, J.; Lin, T.-H.; Liou, G.-S.; Cheng, S.-H. Novel Trends of Electrochemical Oxidation of Amino-Substituted Triphenylamine Derivatives. J. Electroanal. Chem. 2005, 575, 95–101. [Google Scholar] [CrossRef]
- Yen, H.-J.; Tsai, C.-L.; Chen, S.-H.; Liou, G.-S. Electrochromism and Nonvolatile Memory Device Derived from Triphenylamine-Based Polyimides with Pendant Viologen Units. Macromol. Rapid Commun. 2017, 38, 1600715. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-T.; Yen, H.-J.; Liou, G.-S. Substituent Effect on Electrochemical and Electrochromic Behaviors of Ambipolar Aromatic Polyimides Based on Aniline Derivatives. Macromolecules 2011, 44, 9595–9610. [Google Scholar] [CrossRef]
- Chang, C.-W.; Liou, G.-S.; Hsiao, S.-H. Highly Stable Anodic Green Electrochromic Aromatic Polyamides: Synthesis and Electrochromic Properties. J. Mater. Chem. 2007, 17, 1007–1015. [Google Scholar] [CrossRef]
- Han, Y.; Xing, Z.; Ma, P.; Li, S.; Wang, C.; Jiang, Z.; Chen, Z. Design Rules for Improving the Cycling Stability of High-Performance Donor–Acceptor-Type Electrochromic Polymers. ACS Appl. Mater. Interfaces 2020, 12, 7529–7538. [Google Scholar] [CrossRef]
- Bodedla, G.B.; Thomas, K.R.J.; Li, C.-T.; Ho, K.-C. Functional Tuning of Phenothiazine-Based Dyes by a Benzimidazole Auxiliary Chromophore: An Account of Optical and Photovoltaic Studies. RSC Adv. 2014, 4, 53588–53601. [Google Scholar] [CrossRef]
- Pina, J.; Seixas de Melo, J.S.; Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M. Triphenylamine–Benzimidazole Derivatives: Synthesis, Excited-State Characterization, and DFT Studies. J. Org. Chem. 2013, 78, 11389–11395. [Google Scholar] [CrossRef]
- Cai, W.; Cai, J.; Niu, H.; Xiao, T.; Bai, X.; Wang, C.; Zhang, Y.; Wang, W. Synthesis and Electrochromic Properties of Polyimides with Pendent Benzimidazole and Triphenylamine Units. Chin. J. Polym. Sci. 2016, 34, 1091–1102. [Google Scholar] [CrossRef]
- Yan, X.; Dai, F.; Ke, Z.; Yan, K.; Chen, C.; Qian, G.; Li, H. Synthesis of Colorless Polyimides with High Tg from Asymmetric Twisted Benzimidazole Diamines. Eur. Polym. J. 2022, 164, 110975. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16, Revision a. 03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Sun, N.; Zhou, Z.; Chao, D.; Chu, X.; Du, Y.; Zhao, X.; Wang, D.; Chen, C. Novel Aromatic Polyamides Containing 2-Diphenylamino-(9,9-Dimethylamine) Units as Multicolored Electrochromic and High-Contrast Electrofluorescent Materials. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 213–222. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, H.; Chen, C.; Yu, Y. Preparation of Porous Polyamide Films with Enhanced Electrochromic Performance by Electrostatic Spray Deposition. J. Electroanal. Chem. 2021, 887, 115038. [Google Scholar] [CrossRef]
- Sun, N.; Meng, S.; Feng, F.; Zhou, Z.; Han, T.; Wang, D.; Zhao, X.; Chen, C. Electrochromic and Electrofluorochromic Polyimides with Fluorene-Based Triphenylamine. High. Perform. Polym. 2017, 29, 1130–1138. [Google Scholar] [CrossRef]




| Solvent a | GPC c (×104 g/mol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Polymer | NMP | DMAc | DMF | DMSO | THF | CHCl3 | ηinhb | Mw | Mn | PDI |
| TPA-BIA-PI | ++ | ++ | ++ | ++ | ++ | ++ | 0.7 | 15.3 | 12.7 | 1.2 |
| TPA-BIB-PI | ++ | ++ | ++ | +− | ++ | ++ | 0.5 | 15.0 | 5.8 | 2.6 |
| Polymer | Tg a | Td5% b | Rw800(%) c |
|---|---|---|---|
| TPA-BIA-PI | 343 | 529 | 64 |
| TPA-BIB-PI | 311 | 531 | 67 |
| Solution(nm) a | Film(nm) | Oxidation | Potional(V) | Eg c | HOMO d | LUMO e | ||
|---|---|---|---|---|---|---|---|---|
| Polymer | Abs. Max | Abs. onset | Abs. Max | Eonset | E1/2 b | |||
| TPA-BIA-PI | 293 | 465 | 318 | 0.96 | 1.11 | 2.67 | −4.21 | −1.54 |
| TPA-BIB-PI | 345 | 471 | 344 | 1.05 | 1.13 | 2.63 | −4.19 | −1.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, K.; Chen, H.; Zhu, C.; Ke, Z.; Li, D.; Wang, M.; Dai, F.; Yu, Y. Synthesis and Characterization of Novel Triphenylamine—Containing Electrochromic Polyimides with Benzimidazole Substituents. Molecules 2023, 28, 2029. https://doi.org/10.3390/molecules28052029
Yan K, Chen H, Zhu C, Ke Z, Li D, Wang M, Dai F, Yu Y. Synthesis and Characterization of Novel Triphenylamine—Containing Electrochromic Polyimides with Benzimidazole Substituents. Molecules. 2023; 28(5):2029. https://doi.org/10.3390/molecules28052029
Chicago/Turabian StyleYan, Kuangguo, Haiquan Chen, Chenjie Zhu, Zhao Ke, Dongwu Li, Mengxia Wang, Fengna Dai, and Youhai Yu. 2023. "Synthesis and Characterization of Novel Triphenylamine—Containing Electrochromic Polyimides with Benzimidazole Substituents" Molecules 28, no. 5: 2029. https://doi.org/10.3390/molecules28052029
APA StyleYan, K., Chen, H., Zhu, C., Ke, Z., Li, D., Wang, M., Dai, F., & Yu, Y. (2023). Synthesis and Characterization of Novel Triphenylamine—Containing Electrochromic Polyimides with Benzimidazole Substituents. Molecules, 28(5), 2029. https://doi.org/10.3390/molecules28052029





