Abstract
The various chemicals in industrial wastewater can be beneficial for improving its circularity. If extraction methods are used to capture valuable components from the wastewater and then recirculate them throughout the process, the potential of the wastewater can be fully exploited. In this study, wastewater produced after the polypropylene deodorization process was evaluated. These waters remove the remains of the additives used to create the resin. With this recovery, contamination of the water bodies is avoided, and the polymer production process becomes more circular. The phenolic component was recovered by solid-phase extraction and HPLC, with a recovery rate of over 95%. FTIR and DSC were used to evaluate the purity of the extracted compound. After the phenolic compound was applied to the resin and its thermal stability was analyzed via TGA, the compound’s efficacy was finally determined. The results showed that the recovered additive improves the thermal qualities of the material.
1. Introduction
Pollution generated by petrochemical plants is of paramount concern today due to its adverse effects on the environment and human health [1,2,3,4]. The petrochemical industry is responsible for the emission of a wide variety of pollutants, including sulfur dioxide, polycyclic aromatic hydrocarbons (PAHs), and volatile organic compounds (VOCs), which contribute to climate change, to air pollution, and especially to the detriment of human health via the presence of carcinogens already determined, such as 1,3-butadiene and benzene, among others [1,2,3,5,6]. Water pollution is another problem commonly associated with petrochemical plants due to the accidental or intentional release of toxic chemicals into the water during production. Soil contamination can also be a problem in areas near petrochemical plants due to toxic residues and the possible infiltration of chemicals into the soil [7,8,9,10,11]. Pollution generated by petrochemical plants is a serious problem that requires effective measures to minimize its adverse effects on the environment and human health. Treating wastewater from petrochemical plants is of great importance due to the various chemical and toxic contaminants in this type of water [7,9,10,11,12]. Wastewater from petrochemical plants can contain hydrocarbons, heavy metals, acids, and other hazardous chemicals that can adversely affect the environment and human health if not treated properly [6,13,14,15,16,17]. Therefore, it is essential to implement effective treatments to remove these contaminants and ensure that wastewater meets the set quality standards before its discharge into the environment. COD (Chemical Oxygen Demand) and BOD (Biochemical Oxygen Demand) are critical parameters used to assess wastewater quality, especially in the context of petrochemical plants. Both parameters are important because they can provide valuable information on water’s organic load and biodegradation capacity, which can help determine the efficiency of treatment processes and assess the impact of wastewater on the environment [15,18,19,20].
Wastewater from petrochemical plants can contain various chemical and toxic contaminants, including volatile organic compounds (VOCs), polyhydroxyalkanoates (PHAs), phenols, and minerals. VOCs are a group of volatile organic compounds that can occur in wastewater from petrochemical plants due to the presence of refining and petrochemical processes. VOCs can adversely affect human health and the environment if not treated properly. On the other hand, PHAs are a group of biodegradable polymers produced from the biotransformation of organic matter in water and can be difficult to remove via conventional treatment processes [5,6,16,21,22,23,24]. Phenols can also be present in wastewater from petrochemical plants due to the presence of chemical processes and can have toxic effects on the environment and human health. Phenols are chemical compounds that are widely used in the petrochemical industry due to their unique properties and added value in producing various products [25,26,27,28]. Synthetic phenolic antioxidants (SPAs) are artificially manufactured chemical compounds with antioxidant properties similar to natural phenolic antioxidants (NPAs). SPAs can be used as additives in polymer manufacturing because they protect (inhibit) polymers from oxidative degradation [26,27,28,29,30,31]. SPAs can delay or prevent the oxidative degradation of polymers by neutralizing free radicals and other oxidizing agents [26,27,28,31]. SPAs are one of the families of emerging organic pollutants classified as anthropogenic, given their origin. Given the variety of uses and the breadth of their production, SPAs have been found in various environmental matrices such as marine sediments, river waters, and dust, among others. It has been shown that there is a migration of SPAs into the water from polymers. SPAs are also detected in urine and human (donated) sera at significant levels.
SPAs have been shown to generate toxic effects related to liver failure and damage to the endocrine system, and some could cause cancer. It is known that some of the SPAs, under certain conditions, can cause DNA strand breakage. SPAs are also of concern due to their bioaccumulation and high toxicity in aquatic environments [26,32,33,34,35,36,37,38,39,40,41]. 2,6-Di-tert-Butylphenol has been shown to pose a cancer risk [41]. Cyanox 1790 presents variants of 2,6-Di-tert-Butylphenol (the same variant three times) within its structure, as shown in Figure 1. Thus, given that this is a macromolecule, it could also represent a health risk with an increased multiplicity factor, so it is reasonable and indisputably necessary research that generates a knowledge base to establish the impact that this molecule can generate in the different aspects that concern us human beings (health, environment, economy), as is the present research. SPA removal from wastewater is essential due to its toxicity and resistance to biodegradation. Several techniques are available to remove SPAs from wastewater, including physical, chemical, and biological processes. Physical processes include adsorption and coagulation, which use materials such as activated carbon and polymers to remove phenols from water. Chemical processes include techniques such as chemical oxidation and neutralization, which rely on the use of chemical agents to oxidize or neutralize SPAs. Finally, biological processes include techniques such as biodegradation and biotransformation, which rely on the use of microorganisms to degrade SPAs into less toxic compounds [25,42,43]. Although several techniques are available to remove SPAs from wastewater, some may have deficiencies or limitations in their effectiveness [25,42,43].
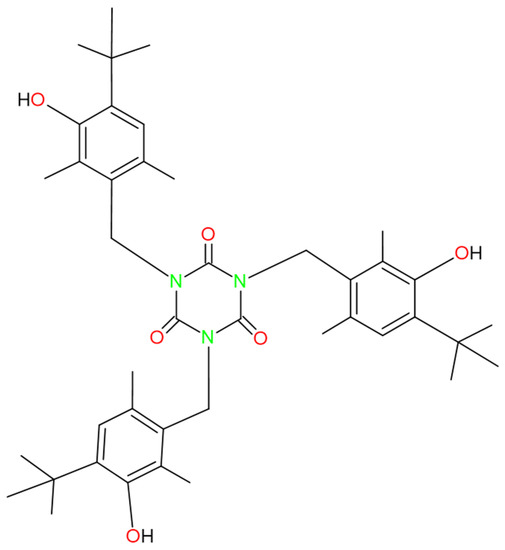
Figure 1.
Chemical structure of Cyanox 1790.
SPA degradation is essential for removing these toxic compounds from wastewater, but some degradation techniques may need to be improved or improved in their effectiveness [44]. Biodegradation is a technique commonly used to degrade SPAs into less toxic compounds but can be limited by factors such as the availability of microorganisms and the presence of other contaminants that can inhibit microbial activity [42]. SPA recovery is a critical practice to leverage resources and minimize the environmental impact of the petrochemical industry. The recovery of phenols can contribute to conserving natural resources and reducing dependence on fossil fuels. SPA recovery can also reduce waste generation and decrease the pollutant load in wastewater. SPA recovery can also positively impact the economy, as it can provide an additional source of raw materials and reduce production costs [45,46,47]. Prior to the analysis of AOs in environmental samples in recent years, procedures such as liquid phase microextraction (LPME), solid phase extraction (SPE), and solid phase microextraction (SPME) were used in sample treatments [48,49,50]. According to the most recent quantitative and qualitative investigations of these AOs, which were performed with HPLC, the LOD is less than 1 ug L−1, and the relative standard deviation (RSD) is less than 10% [51,52,53,54] in matrices of low chemical complexity. The situation that we are in for this project is quite different from the aforementioned situation. Wei and colleagues (2011) quantified AOs in simulant C (10% ethanol) and simulant D (oil) using a C18 sorbent preconditioned with 5 mL of ACN and 5 mL of distilled water and obtained an LOD and an LOQ between 0.09 and 1.72 ug mL−1 and between 0.20 and 5.64 ug mL−1, respectively. With an RSD between 2.8 and 9.8%, their recovery ranged from 67.5 to 108.6%.
The selection of the instrumental technique to quantify these OA and its precision and analytical errors are of great importance for carrying out reliable measurements of these OA, given the protective function they have in the PP matrix to guarantee its thermal stability. Oxidative degradation is a process that occurs when polymers come into contact with oxygen and other oxidizing agents, such as light and heat, and degrade due to the formation of free radicals. Oxidative degradation can affect the service life of polymers and reduce their performance and properties [25,44]. Oxidative degradation can manifest itself in various ways, such as color change, strength deterioration, and the modification of mechanical and thermal properties. Oxidation is a process that can occur during the manufacture of polyolefins and can affect the quality and properties of polyolefins. Polymers can be sensitive to oxidation due to the presence of double bonds or carbonyl groups, which are vulnerable points to oxidation [29,30,55,56]. Due to its extremely low density, polypropylene (PP) is a polymer of petrochemical origin that is widely used in many sectors because it can be heated, cooled, and reheated without losing its composition. PP is characterized by its high mechanical strength, chemical resistance, thermal stability, and low production cost [26,27,57,58,59]. In addition, it is in high demand and is a product of great commercial interest, which drives production growth and can raise questions about potential environmental issues related to its manufacture and disposal. Phenolic antioxidants play an essential role in polypropylene’s increased resistance to oxidation [60]. Cyanox 1970, a phenolic antioxidant and non-bleaching stabilizer that works well on materials such as polyolefins and is recommended for polymer processing, is easily accessible [61,62,63,64]. Figure 1 shows the molecule in question from this research.
Unfortunately, many pollutants are produced in the PP production process with possible adverse effects on human health and the environment. These contaminants include synthetic phenols and volatile organic chemicals, which are added to PP as additives to enhance their qualities [27,55,65,66]. The existence of these materials in industrial waste supports the need to evaluate their processing efficiency and drives the creation of a more efficient and environmentally friendly PP production method. Deodorization is a step in manufacturing PP which consists of removing the substances that were not absorbed and that cause odor from the polymer. If these substances are recovered, they can be used in the PP extrusion process to give PP a new value, increase its manufacturing productivity, and reduce losses during its synthesis. This article proposes recovering the Cyanox 1970 additive and introducing it in the process to check if the recovered additive can improve the thermal qualities of PP.
In the present research, we intend to recover a high percentage (≥90%) of a high value additive (Cyanox 1790) for polymers and characterize its efficiency via competitive and reliable techniques to determine the feasibility of its reuse in the industry.
2. Analysis and Discussion
2.1. Identification, Quantification, Repeatability, Reproducibility, and Linearity Analysis of Multiple Cyanox 1790 Standards
This analysis is performed in a very detailed and rigorous way in some respects because, from the results obtained, it will be possible to determine if recovering this additive is workable.
2.1.1. Multistandard Repeatability Analysis of Cyanox 1790 in CH2Cl2 and Multistandard per SPE in CH2CN
For the repeatability (precision) of the process, five tests were carried out using the HPLC-MS technique, with the same standard, on the same day, with the same analyst, depending on the relative standard deviation (RSD) and seven concentrations between 0 (white) and 5000 ppm. Accuracy within the day was validated if the average global values were less than 20%. A divergence of less than 15% from the expected value is suggested as an acceptable condition [66,67,68,69]. Table 1 shows the values and precision parameters calculated from the data obtained. These data were analyzed in an ANOVA and using Tukey’s method; it was found that all means were grouped in the same literal (A), which indicates that there are no significant differences in the means of the data, which allows us to infer with 95% certainty that the standard analysis is repeatable. Figure 2a shows a box plot to graphically observe the distribution of the repeatability data of the standard and the location of its means.

Table 1.
Tabulation of data for repeatability and reproducibility of standards in HPLC-MS.
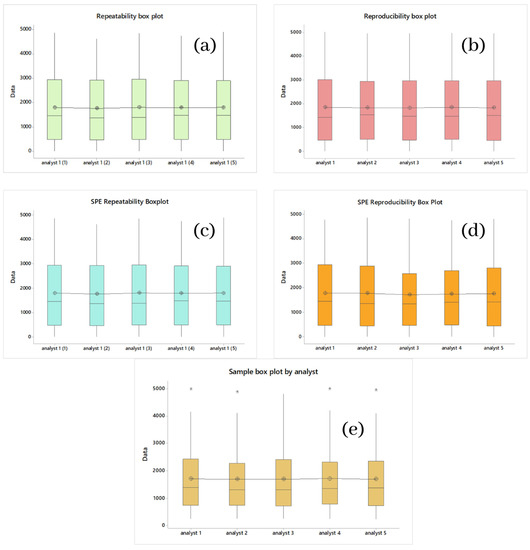
Figure 2.
(a) Box Chart Repeatability of Banners; (b) Box Graph Reproducibility of Banners; (c) Box Graph Repeatability of Recovery of Banners; (d) Box Graph Reproducibility of Recovery of Banners; (e) Graph of Box Reproducibility of Samples. *, p ≤ 0.05.
Table 2 shows the data obtained. We obtained RSDs much lower than 15%, where the highest was 3.6% (1500 ppm), errors less than 5.3%, and recoveries equal to or greater than 95% on all occasions, all of which were obtained by the same analyst on the same day. The highest yield obtained for these conditions was 98%. The ANOVA analysis performed for the SPE of Cyanox (standard), which is shown in Table 3, showed that the means did not have significant differences, and similarly to all the ANOVA performed so far, the grouping of the means occurred under the same bunk (A), which implies the significance of the difference between means. Figure 2c graphically complements the ANOVA set.

Table 2.
Tabulation of data for repeatability and reproducibility of standards in the SPE.

Table 3.
ANOVA analysis for standards and samples using the Tukey method with 95% confidence.
2.1.2. Multistandard Reproducibility Analysis of Cyanox 1790 in CH2Cl2 and Multistandard by SPE in CH2CN
For the reproducibility of the process, five tests were performed using the same analytical technique with the same standard on different and consecutive days, with different analysts. For reproducibility, the same validation criteria apply for repeatability. As for repeatability and reproducibility, an ANOVA analysis was also performed (Table 3), with the same degree of confidence and under the same method (Tukey) shown in Table 1, and the same result was obtained as repeatability, that is, the grouping of all means in the same literal (A), which indicates that for the means of reproducibility, there is no significant difference either, that is, the process is reproducible. As for repeatability and reproducibility, Figure 2b shows the box plot for these data, which corroborate the ANOVA analysis.
For the reproducibility of the SPE, the same conditions are used. For reproducibility under the above conditions, we obtained a maximum RSD value of 5.2% (3000 ppm), errors less than 6.9%, and recoveries equal to or greater than 93%. In the case of errors, despite being higher than in the case of repeatability of the SPE, they are still below 15%, so it meets the established criteria. The ANOVA analysis (Table 3) performed for SPE of Cyanox (standard) under reproducibility conditions showed that the means did not have significant differences for the reasons explained by the previous ANOVA. Figure 2d shows the box plot for the reproducibility of the SPE in order to complete the information provided by the ANOVA visually.
2.1.3. Repeatability Linearity and Reproducibility of Cyanox 1790 Multistandards
Figure 3a shows in the graph the theoretical concentrations established for SPE versus the concentrations obtained by the analyst. Each test has an independent linear regression that shows the same linear trend, in which a high degree of precision can be graphically demonstrated between the data. To obtain more specific information about (theory vs. reality), Figure 3b shows the theoretical values of the concentration of the SPE against the average of the data obtained per test, and here we find an (R2) of 0.99985, and a correlation coefficient of 0.99981, which allows us to establish first: that the model can satisfactorily predict the concentrations obtained experimentally, and second: that there is a relationship directly proportional between the theoretical and experimental values, representing the relevant fact that recovery values can be established with certainty, which is considered high, and this has significant economic and environmental implications.
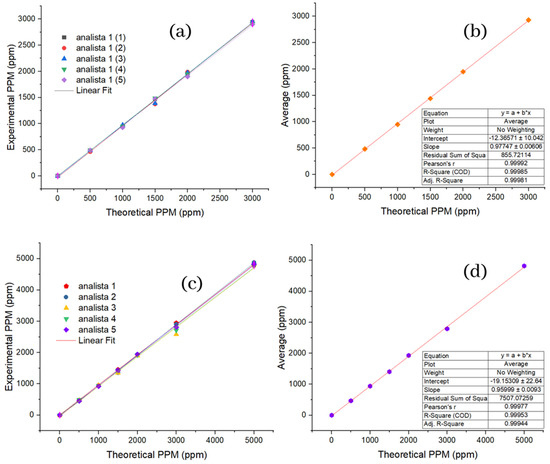
Figure 3.
Linearity of interday and intraday measurements. (a) Repeatability for standard; (b) mean repeatability for standard; (c) reproducibility for standard; (d) mean reproducibility for standard.
As for repeatability, for the reproducibility of the SPE, the figure shows in Figure 3c the theoretical concentrations versus the experimental concentrations with their respective linear settings; here, it should be noted that there is a lower precision in the points corresponding to the concentration of 3000 ppm concerning the same concentration for the repeatability of the PSE. The graph in Figure 3d shows the theoretical values of concentration against the reproducibility averages, for which an (R2) of 0.99953 and a correlation coefficient of 0.99977 is obtained so that exactly the same conclusions can be reached as for the repeatability of the PES. All of the above was performed with the sole and exclusive purpose of guaranteeing the effectiveness and efficiency of the methodology to measure the concentration of Cyanox 1790 in actual samples.
2.2. Identification, Quantification, Repeatability, Reproducibility, and Linearity Analysis of Cyanox 1790 in Industrial Wastewater Samples
2.2.1. Reproducibility Analysis Industrial Wastewater Samples
The actual samples were 40 in total, which were taken for 40 consecutive days and analyzed by five different analysts on the same day. For this sample, we obtained RSDs and the standard values below 15%, where the highest was 4.8%, and the recoveries were equal to or greater than 95.07%. The highest concentration value found in the samples occurred on day 26 and had a mean value of 4896 ppm, and the lowest value was found on day 38 with a mean value of 311.8 ppm, as shown in Table 4.

Table 4.
Data tabulation for sample reproducibility.
2.2.2. Linearity and Distribution of Industrial Wastewater Sample Data
The first thing that should be appropriately noted of the sample is that during the forty days, totally different concentrations were found from one day to another, evidenced in the randomness of the values obtained, as shown in Figure 4a, in which the days are plotted against the average concentration of the sample obtained for each day. This high variability of concentrations is verified by the (R2) values and the correlation coefficient for the samples, which were 1.22 × 10−4 and 1.10 × 10−2, respectively. In Figure 4b, the samples are plotted against the values obtained for each analyst in each of the samples, and it is possible to observe that for each sample, the values of the analysts are not too far from each other, so graphically, the precision that they retain is evident. The values furthest from each other concerning the other samples correspond to samples 20, 21, 22, and 23. However, they remain within the acceptable range. In Figure 4c, we can see the average values recorded by analysts against the net recovery, all in ppm and with the values ordered from highest to lower. Here, we can appreciate the linear correspondence of the average values and the net recovery, which highlights that the methodology used for the recovery of our additive has a high recovery performance because the linear regression conducted for this dataset shows an (R2) of 0.99955 and a correlation coefficient of 0.99977. If the values obtained for the standards are taken as a reference, it is obtained that the square of the sample had an error of 0.002%, and the correlation coefficient had an error of 0%, because the same value was obtained.
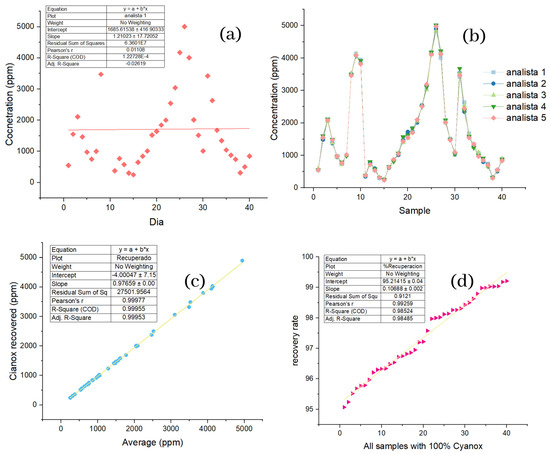
Figure 4.
(a) Distribution of concentration data per day for samples; (b) repeatability of the data by analysts per sample; (c) analyte recovery based on mean sample concentrations; (d) percentage recovery of analyte based on 100% analyte per sample.
If it is assumed that each sample contains 100% analyte, Figure 4d plots the samples against the percentage of recovery ordered from lowest to highest, such that a linear trend is seen. Once the difference in scales is more noticeable, separating the points concerning the linear regression—the trend and the minimum recovery value, which was 95.07%—is still essential. The ANOVA analysis (Table 3) performed for the concentration values of the samples obtained by the five analysts shows no significant differences between the means of the 40 samples under the same analysis conditions for the previous ANOVA. Figure 4e shows the box graph corresponding to the ANOVA analysis; here, you can see that there are outliers for four of the five analysts; apart from that, there is nothing.
2.3. Characterization of Recovered Dust and Comparison with a Pattern
2.3.1. Thermogravimetry Analysis (TGA) of Pure Standard and Recovered Dust
In the thermal degradation of pure Cyanox 1790, the percentage by weight remained relatively stable at 100% until it reached 344 °C; from this point, the weight loss of the sample begins. The most significant percentage weight loss of this substance occurs between 344 and 445 °C from this temperature point. The degradation continues tenuously until the maximum temperature compared to the previous range. It is evident that the recovered Cyanox 1970 had exactly the same behavior as pure Cyanox in thermal degradation, so the analysis is the same for the recovered Cyanox, including the degradation range. The degradation starts temperature and attenuated degradation from 445 °C. All of the above is shown graphically in the TGAs in Figure 5. To corroborate the high degree of similarity between pure and recovered Cyanox, the pure and recovered DTGAs, respectively, were plotted in Figure 5 immediately below the TGAs, and here, the same maximum value is evidenced, precisely at the same temperature (396.59 °C, 0.138), for both derivatives. It indicates the tipping point for pure and recovered Cyanox.
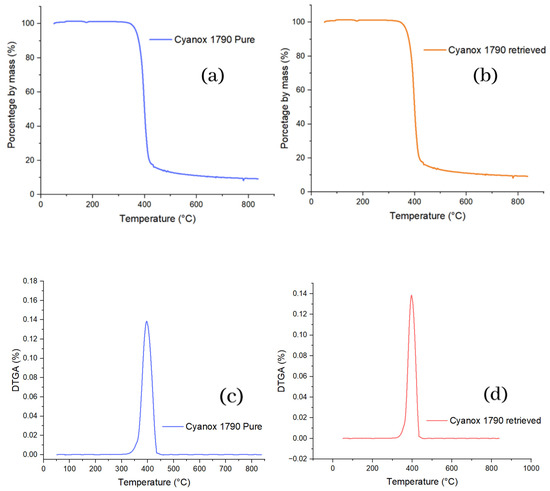
Figure 5.
(a) TGA graphics of pure and (b) recovered Cyanox 1790 and (c) DTGA Cyanox 1790 pure and (d) recovered.
This suggests that this additive is a fairly stable species in the surrounding environment (minimally reactive or reactive very specifically), because otherwise, changes would be expected to be at least perceptible in tests.
2.3.2. Pure Standard DSC Analysis and Recovered Dust
Differential scanning calorimetry, similarly, to TGA and more similarly to DTGA, specifically in the graph aspect, shows the same peaks for pure and recovered Cyanox. The calorimetric curve shows that for both samples of Cyanox between 155 and 176 °C, there is heat release (peak up) from the sample, which is related to the decomposition of the sample, that is, the breaking of the bonds of the molecule, which release energy in the form of heat. For both Cyanox samples, the maximum heat flux released was 8.69 J/s, as shown in Figure 6.
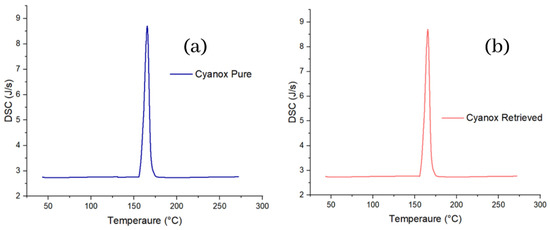
Figure 6.
DSC charts for pure (a) and recovered (b) Cyanox 1790.
2.3.3. Pure Standard FTIR Analysis and Recovered Dust
Before and after reintegration of Cyanox into the PP to distinguish the spectra of the recovered substance, and after its addition to the PP to study the compatibility of the components in the composite matrix, both the recovered Cyanox 1790 and the pure Cyanox 1790 were subjected to Fourier transform infrared spectroscopy (FTIR). The spectrum obtained from the recovered Cyanox 1790 (Figure 7) [70] is remarkably comparable to the spectrum of the pure additive obtained from the literature, according to the first results of the FTIR before it joined the PP. Both spectra show the most intense peaks and the fingerprint of the spectrum in a comparable way. Given the noise of the signals acquired for this test and the low concentrations of the analyte, which are represented by lower absorbance values in the range of 2250–1900 cm−1, slight discrepancies in the spectra can be observed [71].
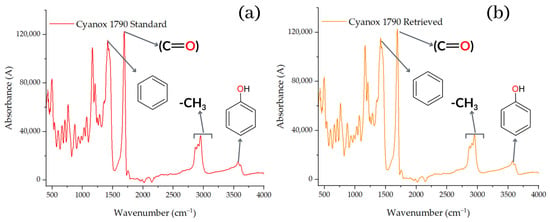
Figure 7.
FTIR charts for pure (a) and recovered (b) Cyanox 1790.
The resulting spectrum (Figure 7) shows a peak at approximately 1735 cm−1, which is indicative of the ester group (O=C) present in the structure of the Cyanox, and two bands between 1300 and 1050 cm−1, which correspond to the symmetrical and asymmetrical stretches of the Cyanox ester group. The intensity of one stretch is greater than that of the other. However, the signal at 3670 cm−1 shows that the group formed from phenols is present in Cyanox 1790. The usual band of the CH3 group is observed in the range of 2950 to 2970 cm−1. The chemical composition of Cyanox also reveals these categories. Another indicator of the presence of aromatic groups in the spectrum is a moderately strong absorption in the range of 1450–1500 cm−1, which is typical of the spectra of aromatic compounds. Because spectrophotometry is less desirable for this study because it is more desirable to measure the coupling and the difference between the peaks when the Cyanox enters a couple in the PP matrix, the analysis of the peak in the Cyanox spectrum allows us to confirm the presence of the recovered analyte, as well as its effectiveness. The molecular composition of Cyanox 1790 is shown in Figure 1 [71,72].
2.3.4. Use of Recovered Dust and Application to Polypropylene Resins to Evaluate Their Efficiency
The integration of recovered Cyanox 1790 and pure Cyanox 1790 into virgin PP resin was evaluated by FTIR (Figure 8a). Figure 8b,c shows that the distinctive peak of the carbonyl functional group at 1735 cm−1 is absent from the IR spectra of virgin PP. According to Figure 7a,b, this functional group represents Cyanox 1790 on a regular basis. In the IR spectrum of virgin PP + pure Cyanox 1790 and virgin PP + Cyanox 1790 recovered, this carbonyl peak can be clearly recognized. Figure 7 shows more clearly the characteristic bands of the functional groups typical of the molecular structure (Figure 1) of Cyanox. The distinct phenol peaks, aromatic rings, and methyl groups are masked by the PP saturations in the infrared spectrum [3,27,28,72,73,74,75,76].
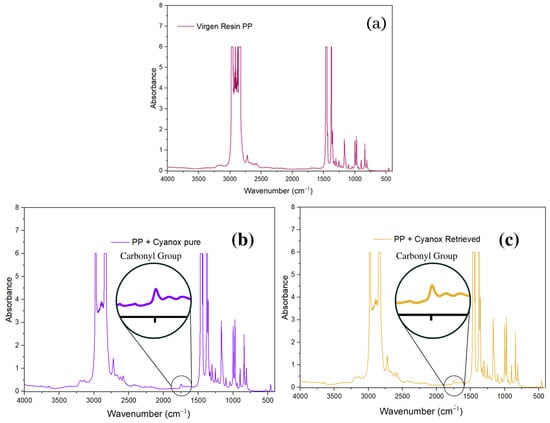
Figure 8.
(a) Virgin PP infrared spectrum, (b) pure PP + Cyanox 1790 infrared spectrum, (c) recovered PP + Cyanox 1790 infrared spectrum.
2.3.5. OIT PP, (PP + Cyanox Pure), and (PP + Cyanox Recovered)
To determine the oxidation time, changes in the slope of the curve generated by the DSC with respect to the expected time and heat flow are taken into account. As shown in Figure 9, an endothermic peak was initially observed, but over time and with changes in the atmosphere, the slope of the curves changed, revealing a new exothermic behavior that is consistent with oxidation. It can be observed that, for unstabilized PP (Figure 9a), a significantly lower value of the induced oxidation time (OIT) is provided than when PP has a certain amount of additive. The oxidation time refers to the moment in which the displacement of this slope occurs. The value of OIT for the non-stabilized PP is 0.7 min, taking as reference the change in slope at 17.3 min, and the OIT for the PP with the added additive is 8 min, demonstrating how the presence of the additive slows down the oxidation processes of PP and improves its thermal stability due to its coupling in the polymer matrix. That mechanism reduces thermo-oxidation due to the presence of OH in its rings, which helps eliminate free radicals (Figure 9b,c).
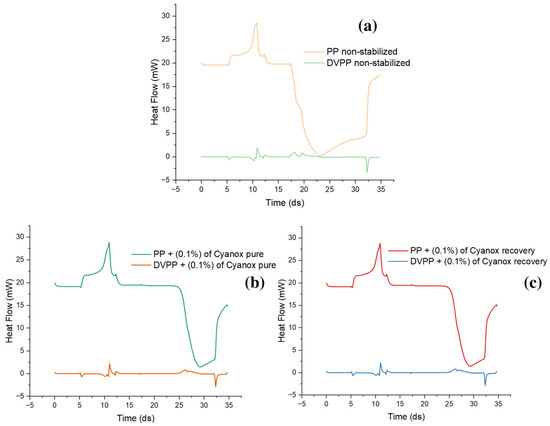
Figure 9.
(a) Unstabilized PP oxidation induction time; (b) PP oxidation induction time with pure Cyanox 1790; (c) PP oxidation induction time with recovered Cyanox 1790.
3. Materials and Methods
3.1. Sampling
The samples were collected in a polypropylene manufacturing plant (Figure 10). Figure 10 shows three stages of the production process. Stage 1 corresponds to the receipt, purification, and storage of propylene. Tank 1 stores the untreated propylene. Column 2 is used for the elimination of traces of contaminants, and tank 3 stores the purified propylene. In stage 2, polymerization occurs, and propylene is transformed into polypropylene. This process happens at point 4, which corresponds to the polymerization reactor. Stage 3 corresponds to activation and pelletizing. Point 5 is a silo in which the virgin PP resin is received; at point 6, the virgin resin is mixed with the additives; at point 7, extrusion and pelletizing are carried out. Sampling is carried out at point 7. At this point, a flow of water comes out of the extruder. Water is used in the polymer extrusion process to lower the temperature and create a more uniform grain, as it is carried out at a high temperature [62].
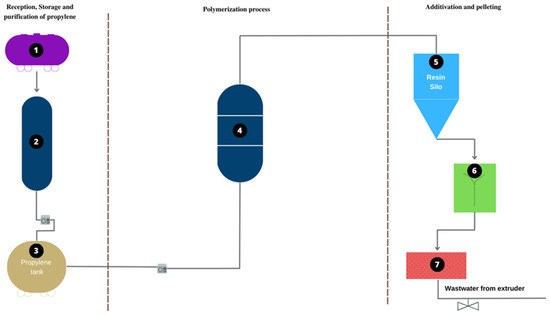
Figure 10.
Flowchart of the plant from which the samples were taken.
Due to the large processing volume and fast processing speed, samples were obtained every 12 min, with a throughput of 5 samples. Approximately 30 tons of PP can be processed in 50 min. Given that the desorption process lasts four hours, samples of the desorption unit were obtained in duplicate every hour. This method investigates both the way phenols are present in the condensates of these two units and the impact of sampling time on phenol migration in condensates.
3.2. Extraction Solid Phases (SPE)
The sample was filtered via a polytetrafluoroethylene filter prior to the extraction (PTFE). Strata X-33 tubes (500 mg, 6 mL) were filled with 15 mL of the sample for the SPE at a flow rate of 1 mL/min. In total, 5 mL of methanol and 5 mL of distilled water were used to condition the filtrate earlier. MeOH: H67O 80:20 was used to wash the filtrate. The retentate was stripped with nitrogen at 5 psi after being eluted with 10 mL of acetonitrile (ACN). The extracted solution was dissolved in 1 mL of ACN [7] and then subjected to HPLC analysis. Until a concentrate volume of 500 mL was produced, the preconcentration procedure was repeated. By using a stream of N2, this 500 mL sample was preconcentrated and dried. The resulting solid was examined by FTIR, DSC, TGA, and HPLC-MS. For subsequent mixing of the recovered solid (Cyanox 1790) with virgin PP resin, it is kept in a humidity- and temperature-controlled cabinet.
3.3. HPLC-DAD/MS/MS (High-Performance Liquid Chromatography with Diode Array Detector and Mass Spectrometry)
For this analysis, an Agilent 1100 HPCL and a Micromass Quattro II triple quadrupole mass spectrometer were used. It is possible to acquire spectra using MS and MS/MS. As part of the system, a degasser (G1322A), a quaternary pump (G1311A), an automatic sampling system (G1313A), a column carrier (G1316A), a DAD detector (G1315B) with a chemical station, a Lichrosorb RP-18 column (4.6x200mmx5microns), 5 and 10 syringes, and a precision balance were used. A separate double pump system and self-sampler were also used for automatic injection into the MS with the Cyanox 1790 solution at ACN, which was used to establish chromatographic conditions. With the mixture of ACN and H2O solvents, which were mixed in various proportions, the following separation was carried out: 84 and 16 percent (1 min, 15 mL/min); 92 and 8 percent (2 min, 2 mL/min); 96 and 4 percent (3.5 min, 3.5 mL/min); and 100 and 0 percent (8 min, 3.5 mL/min). The temperature, irrigation volume, and wavelength of the column were adjusted to 50 °C. For the identification of the additive, the mass data of MS fragment and MS/MS ions are used [26,62,63].
3.4. The Recovered Additive Is Added to the PP Matrix
3.4.1. Preparation of PP Sampling
The PP resin in question was produced from virgin resin devoid of additives. The recovered PP and Cyanox 1790 mixture samples were premixed by adding 0.1 wt% recovered Cyanox 1790 additive to PP powder using a standard Prodex Henschel 115JSS mixer at 800 rpm for 7 min at room temperature. The resin was then combined with the recovered Cyanox 1790. The samples were combined using a Welex-200 24.1 extruder, and melted extrusion was carried out at operating temperatures of 190, 195, 200, 210, and 220 °C on the extruder tracks. The mixtures underwent an additional transformation into films (300 mm diameter films with a thickness of about 100 mm) by compression molding in a hot press, the CARVER 3895. After the PP solid was granulated, 1000 mg/kg of recovered granules containing Cyanox 1790 were produced. Cyanox 1790 was diluted in ACN to solve with a concentration of 500 mg/L to manufacture the standard, and 2.5 mL of this solution was dissolved to a volume of 50 mL to obtain 25 mg/L.
3.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
A Nicolet 6700 infrared spectrometer was used for the FTIR study, with readings between 4000 and 600 cm−1 and a resolution of 2 cm−1 (reflection). The sample was pre-heated to 400 °C in preparation for this study to cause thermal deterioration and allow examination of changes in the polymer matrix [63,64].
3.4.3. Differential Scanning Calorimeter (DSC)
The oxidation induction time (OIT) was calculated using DSC Q2000 V24.11 Build 124 equipment for calorimetric analysis. A 6.1 mg sample was used to obtain the results under atmospheric conditions of nitrogen and oxygen. The environment of the experiment was changed to examine how oxidation affects the volatility of the material. On the other hand, nitrogen provides a controlled and inert environment that allows us to examine how decomposition affects the sample. This procedure was carried out in various conditions, such as isothermal at 60 °C for 5 min, an atmosphere of 50 mL/min of nitrogen, and a temperature increase of 60 °C at 200 °C for 20 min. The ambient conditions of the analysis were then adjusted, and the sample was subjected for 30 min to an airflow of 50 mL/min under oxidation conditions at a temperature of 200 °C. A displacement of the slope of exothermic heat with oxidation flow was observed. The value of OIT corresponding to the time when the slope changes can be calculated using this transformation [63].
3.4.4. Analysis by Thermogravimetry (TGA)
This analysis was performed using a Perkin Elmer TGA7 thermobalance at temperatures between 30 and 700 °C with a nitrogen flux of 50 mL/min. The temperature in the TGA at which 5% of the mass is lost was used to calculate the initial degradation temperature, and the DTG curve was used to determine the maximum degradation temperature [48,64,65].
4. Conclusions
There is evidence that industrial wastewater can be a source of numerous substances that can harm the environment and people’s health, but there is also evidence that it can be a source of substances that, once recovered, are very helpful in the industrial sector. Recovering these components, therefore, reduces the environmental effect and improves the circular economy. This study demonstrates how recovering phenolic compounds (Cyanox 1790), which are used as additives in polypropylene manufacturing, can yield high purity recoveries of over 98%. The thermal and thermo-oxidative stability of PP was significantly increased by the recovery and integration of Cyanox 1790 in the PP matrix. Without thermal stabilizers, PP would disintegrate throughout the extrusion process, preventing it from having any significant ultimate applications. Therefore, the recovery of this Cyanox 1790 at these high purity levels exemplifies a critical technology to be applied in the industrial sector and encourages sustainable raw material sources.
Author Contributions
Conceptualization, R.O.-T. and J.H.-F.; methodology, J.L.-M.; validation, J.H.-F., J.L.-M. and R.O.-T.; formal analysis, R.O.-T.; investigation, R.O.-T. and J.H.-F.; writing—original draft preparation, J.H.-F.; writing—review and editing, R.O.-T. and J.L.-M.; supervision, J.H.-F.; project administration, J.L.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Ministry of Science, Innovation, and Universities (MICIU) project number MAT2017-84909-C2-2-R Nanolignin.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Compound samples are available, please contact the lead author..
References
- Marquès, M.; Domingo, J.L.; Nadal, M.; Schuhmacher, M. Health risks for the population living near petrochemical industrial complexes. 2. Adverse health outcomes other than cancer. Sci. Total. Environ. 2020, 730, 139122. [Google Scholar] [CrossRef]
- Nyashina, G.; Kuznetsov, G.; Strizhak, P. Effects of plant additives on the concentration of sulfur and nitrogen oxides in the combustion products of coal-water slurries containing petrochemicals. Environ. Pollut. 2019, 258, 113682. [Google Scholar] [CrossRef]
- Fernández, J.H.; Rincón, D.; López-Martínez, J. Development and validation of a prototype for the on-line simultaneous analysis of quality caprolactam synthesized on an industrial scale. Methodsx 2022, 10, 101952. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Guerra, Y.; Espinosa, E. Development and Application of a Principal Component Analysis Model to Quantify the Green Ethylene Content in Virgin Impact Copolymer Resins During Their Synthesis on an Industrial Scale. J. Polym. Environ. 2022, 30, 4800–4808. [Google Scholar] [CrossRef]
- Kayyal-Tarabeia, I.; Blank, M.; Zick, A.; Agay-Shay, K. Residence near industrial complex and cancer incidence: A registry-based cohort of 1,022,637 participants with a follow-up of 21 years, Israel. Environ. Res. 2023, 216, 114471. [Google Scholar] [CrossRef]
- Lin, X.-Y.; Liu, Y.-X.; Zhang, Y.-J.; Shen, H.-M.; Guo, Y. Polycyclic aromatic hydrocarbon exposure and DNA oxidative damage of workers in workshops of a petrochemical group. Chemosphere 2022, 303, 135076. [Google Scholar] [CrossRef]
- Radelyuk, I.; Tussupova, K.; Klemeš, J.J.; Persson, K.M. Oil refinery and water pollution in the context of sustainable development: Developing and developed countries. J. Clean. Prod. 2021, 302, 126987. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Guerra, Y.; Puello-Polo, E.; Marquez, E. Effects of Different Concentrations of Arsine on the Synthesis and Final Properties of Polypropylene. Polymers 2022, 14, 3123. [Google Scholar] [CrossRef]
- Fernández, J.H.; Cano, H.; Guerra, Y.; Polo, E.P.; Ríos-Rojas, J.F.; Vivas-Reyes, R.; Oviedo, J. Identification and Quantification of Microplastics in Effluents of Wastewater Treatment Plant by Differential Scanning Calorimetry (DSC). Sustainability 2022, 14, 4920. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Cano-Cuadro, H.; Puello-Polo, E. Emission of Bisphenol A and Four New Analogs from Industrial Wastewater Treatment Plants in the Production Processes of Polypropylene and Polyethylene Terephthalate in South America. Sustainability 2022, 14, 10919. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Cano, H.; Rodríguez-Couto, S. Quantification and Removal of Volatile Sulfur Compounds (VSCs) in Atmospheric Emissions in Large (Petro) Chemical Complexes in Different Countries of America and Europe. Sustainability 2022, 14, 11402. [Google Scholar] [CrossRef]
- Barrington, D.; Prior, A.; Ho, G. The role of water auditing in achieving water conservation in the process industry. J. Clean. Prod. 2013, 52, 356–361. [Google Scholar] [CrossRef]
- Guo, L.; Hu, Y.; Lei, Y.; Wu, H.; Yang, G.; Wang, Y.; Wei, G. Vitrification of petrochemical sludge for rapid, facile, and sustainable fixation of heavy metals. J. Environ. Chem. Eng. 2022, 10, 108812. [Google Scholar] [CrossRef]
- Wang, S.; Kalkhajeh, Y.K.; Qin, Z.; Jiao, W. Spatial distribution and assessment of the human health risks of heavy metals in a retired petrochemical industrial area, south China. Environ. Res. 2020, 188, 109661. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, Z.; Cao, H.; Xu, M.; Xu, L. Concentrations, speciation, and ecological risk of heavy metals in the sediment of the Songhua River in an urban area with petrochemical industries. Chemosphere 2018, 219, 538–545. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Castro-Suarez, J.R.; Toloza, C.A.T. Iron Oxide Powder as Responsible for the Generation of Industrial Polypropylene Waste and as a Co-Catalyst for the Pyrolysis of Non-Additive Resins. Int. J. Mol. Sci. 2022, 23, 11708. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Vivas-Reyes, R.; Toloza, C.A.T. Experimental Study of the Impact of Trace Amounts of Acetylene and Methylacetylene on the Synthesis, Mechanical and Thermal Properties of Polypropylene. Int. J. Mol. Sci. 2022, 23, 12148. [Google Scholar] [CrossRef]
- Mesa, S.L.; Orjuela, J.; Ortega, A.; Sandoval, J.A. Revisión del panorama actual del manejo de agua de producción en la industria petrolera colombiana Review of the current state of wastewater management in the Colombian oil industry. Gestión Ambiente 2018, 21, 87–98. [Google Scholar] [CrossRef]
- Kanu, I.; Achi, O. Industrial Effluents and Their Impact on Water Quality of Receiving Rivers in Nigeria Medical Microbiology View Project Fermented Food Development View Project. 2011. Available online: https://www.researchgate.net/publication/287104597 (accessed on 15 December 2022).
- Ghosh, P.; Samanta, A.N.; Ray, S. COD reduction of petrochemical industry wastewater using Fenton’s oxidation. Can. J. Chem. Eng. 2010, 88, 1021–1026. [Google Scholar] [CrossRef]
- Lee, W.-J.; Liow, M.-C.; Tsai, P.-J.; Hsieh, L.-T. Emission of polycyclic aromatic hydrocarbons from medical waste incinerators. Atmos. Environ. 2002, 36, 781–790. [Google Scholar] [CrossRef]
- Bonachela, S.; López, J.C.; Granados, M.R.; Magán, J.J.; Hernández, J.; Baille, A. Effects of gravel mulch on surface energy balance and soil thermal regime in an unheated plastic greenhouse. Biosyst. Eng. 2020, 192, 1–13. [Google Scholar] [CrossRef]
- Joaquin, H.-F.; Juan, L. Quantification of poisons for Ziegler Natta catalysts and effects on the production of polypropylene by gas chromatographic with simultaneous detection: Pulsed discharge helium ionization, mass spectrometry and flame ionization. J. Chromatogr. A 2019, 1614, 460736. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Puello-Polo, E.; Trilleras, J. Characterization of Microplastics in Total Atmospheric Deposition Sampling from Areas Surrounding Industrial Complexes in Northwestern Colombia. Sustainability 2022, 14, 13613. [Google Scholar] [CrossRef]
- Farzadkia, M.; Shahamat, Y.D.; Nasseri, S.; Mahvi, A.H.; Gholami, M.; Shahryari, A. Catalytic Ozonation of Phenolic Wastewater: Identification and Toxicity of Intermediates. J. Eng. 2014, 2014, 520929. [Google Scholar] [CrossRef]
- Hernández-Fernandez, J.; Rodríguez, E. Determination of phenolic antioxidants additives in industrial wastewater from polypropylene production using solid phase extraction with high-performance liquid chromatography. J. Chromatogr. A 2019, 1607, 460442. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Lopez-Martinez, J.; Barceló, D. Quantification and elimination of substituted synthetic phenols and volatile organic compounds in the wastewater treatment plant during the production of industrial scale polypropylene. Chemosphere 2020, 263, 128027. [Google Scholar] [CrossRef]
- Hernández-Fernández, J. Quantification of oxygenates, sulphides, thiols and permanent gases in propylene. A multiple linear regression model to predict the loss of efficiency in polypropylene production on an industrial scale. J. Chromatogr. A 2020, 1628, 461478. [Google Scholar] [CrossRef]
- Wiles, D.M.; Scott, G. Polyolefins with controlled environmental degradability. Polym. Degrad. Stab. 2006, 91, 1581–1592. [Google Scholar] [CrossRef]
- Al-Malaika, S. Perspectives in Stabilisation of Polyolefins. In Long Term Properties of Polyolefins; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2004; pp. 121–150. [Google Scholar] [CrossRef]
- Pospíšil, J. Mechanistic Action of Phenolic Antioxidants in Polymers—A Review. Polym. Degrad. Stab. 1988, 20, 181–202. [Google Scholar] [CrossRef]
- Dương, T.-B.; Dwivedi, R.; Bain, L.J. 2,4-di-tert-butylphenol exposure impairs osteogenic differentiation. Toxicol. Appl. Pharmacol. 2023, 461, 116386. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Synthetic Phenolic Antioxidants: A Review of Environmental Occurrence, Fate, Human Exposure, and Toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Synthetic phenolic antioxidants and transformation products in dust from different indoor environments in Toronto, Canada. Sci. Total. Environ. 2019, 672, 23–29. [Google Scholar] [CrossRef]
- Makahleh, A.; Saad, B.; Bari, M. Synthetic phenolics as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 51–78. [Google Scholar] [CrossRef]
- Gonçalves-Filho, D.; De Souza, D. Detection of Synthetic Antioxidants: What Factors Affect the Efficiency in the Chromatographic Analysis and in the Electrochemical Analysis? Molecules 2022, 27, 7137. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Q.; Zhang, Q.; Zuo, C.; Shi, H. An Overview of Chemical Additives on (Micro)Plastic Fibers: Occurrence, Release, and Health Risks. Rev. Environ. Contam. Toxicol. 2022, 22, 260. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: A review. Environ. Res. 2021, 201, 111531. [Google Scholar] [CrossRef]
- Wang, W.; Asimakopoulos, A.G.; Abualnaja, K.O.; Covaci, A.; Gevao, B.; Johnson-Restrepo, B.; Kumosani, T.A.; Malarvannan, G.; Minh, T.B.; Moon, H.-B.; et al. Synthetic Phenolic Antioxidants and Their Metabolites in Indoor Dust from Homes and Microenvironments. Environ. Sci. Technol. 2015, 50, 428–434. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.-R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.-A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Cui, S.; Yu, Y.; Zhan, T.; Gao, Y.; Zhang, J.; Zhang, L.; Ge, Z.; Liu, W.; Zhang, C.; Zhuang, S. Carcinogenic Risk of 2,6-Di-tert-Butylphenol and Its Quinone Metabolite 2,6-DTBQ Through Their Interruption of RARβ: In Vivo, In Vitro, and In Silico Investigations. Environ. Sci. Technol. 2021, 56, 480–490. [Google Scholar] [CrossRef]
- Aggelis, G.; Iconomou, D.; Christou, M.; Bokas, D.; Kotzailias, S.; Christou, G.; Tsagou, V.; Papanikolaou, S. Phenolic removal in a model olive oil mill wastewater using Pleurotus ostreatus in bioreactor cultures and biological evaluation of the process. Water Res. 2003, 37, 3897–3904. [Google Scholar] [CrossRef]
- Lee, Y.G.; Hwang, S.H.; Kim, S.D. Predicting the Toxicity of Substituted Phenols to Aquatic Species and Its Changes in the Stream and Effluent Waters. Arch. Environ. Contam. Toxicol. 2006, 50, 213–219. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Yavuz, Y.; Koparal, A.S.; Öğütveren, Ü.B. Treatment of petroleum refinery wastewater by electrochemical methods. Desalination 2010, 258, 201–205. [Google Scholar] [CrossRef]
- Universidad Iberoamericana. Estudios con Reconocimiento de Validez Oficial por Decreto Presidencial Del 3 de Abril de 1981 DORIAN PRATO GARCÍA; Universidad Iberoamericana: Mexico City, Mexico, 2007. [Google Scholar]
- Serna, I.; Torres, J. Recuperación de fenol de aguas residuales industriales por tratamiento con NaCl. Dyna 2003, 70, 25–34. [Google Scholar]
- Ma, J.; Xiao, R.; Li, J.; Yu, J.; Zhang, Y.; Chen, L. Determination of 16 polycyclic aromatic hydrocarbons in environmental water samples by solid-phase extraction using multi-walled carbon nanotubes as adsorbent coupled with gas chromatography–mass spectrometry. J. Chromatogr. A 2010, 1217, 5462–5469. [Google Scholar] [CrossRef]
- Galán-Cano, F.; Bernabé-Zafón, V.; Lucena, R.; Cárdenas, S.; Herrero-Martínez, J.M.; Ramis-Ramos, G.; Valcárcel, M. Sensitive determination of polycyclic aromatic hydrocarbons in water samples using monolithic capillary solid-phase extraction and on-line thermal desorption prior to gas chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 1802–1807. [Google Scholar] [CrossRef]
- Gosetti, F.; Chiuminatto, U.; Mazzucco, E.; Robotti, E.; Calabrese, G.; Gennaro, M.C.; Marengo, E. Simultaneous determination of thirteen polycyclic aromatic hydrocarbons and twelve aldehydes in cooked food by an automated on-line solid phase extraction ultra high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 6308–6318. [Google Scholar] [CrossRef]
- Gao, Y.; Gu, Y.; Wei, Y. Determination of Polymer Additives–Antioxidants and Ultraviolet (UV) Absorbers by High-Performance Liquid Chromatography Coupled with UV Photodiode Array Detection in Food Simulants. J. Agric. Food Chem. 2011, 59, 12982–12989. [Google Scholar] [CrossRef]
- Arias, M.; Penichet, I.; Ysambertt, F.; Bauza, R.; Zougagh, M.; Ríos, Á. Fast supercritical fluid extraction of low- and high-density polyethylene additives: Comparison with conventional reflux and automatic Soxhlet extraction. J. Supercrit. Fluids 2009, 50, 22–28. [Google Scholar] [CrossRef]
- Burman, L.; Albertsson, A.-C.; Höglund, A. Solid-phase microextraction for qualitative and quantitative determination of migrated degradation products of antioxidants in an organic aqueous solution. J. Chromatogr. A 2005, 1080, 107–116. [Google Scholar] [CrossRef]
- Dopico, S.; Vilariño, J.M.L.; Rodríguez, M.V.G. Determination of antioxidants by solid-phase extraction method in aqueous food simulants. Talanta 2005, 66, 1103–1107. [Google Scholar] [CrossRef]
- Joaquin, H.-F.; Juan, L.-M. Autocatalytic influence of different levels of arsine on the thermal stability and pyrolysis of polypropylene. J. Anal. Appl. Pyrolysis 2021, 161, 105385. [Google Scholar] [CrossRef]
- Pavón, C.; Aldas, M.; Hernández-Fernández, J.; López-Martínez, J. Comparative characterization of gum rosins for their use as sustainable additives in polymeric matrices. J. Appl. Polym. Sci. 2022, 139, 51734. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Rayón, E.; López, J.; Arrieta, M.P. Enhancing the Thermal Stability of Polypropylene by Blending with Low Amounts of Natural Antioxidants. Macromol. Mater. Eng. 2019, 304, 1900379. [Google Scholar] [CrossRef]
- Thörnblom, K.; Palmlöf, M.; Hjertberg, T. The extractability of phenolic antioxidants into water and organic solvents from polyethylene pipe materials—Part I. Polym. Degrad. Stab. 2011, 96, 1751–1760. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Environmental impacts of polypropylene (PP) production and prospects of its recycling in the GCC region. Mater. Today Proc. 2021, 56, 2245–2251. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; López-Martínez, J. Experimental Study of the Auto-Catalytic Effect of Triethylaluminum and TiCl4 Residuals at the Onset of Non-Additive Polypropylene Degradation and Their Impact on Thermo-Oxidative Degradation and Pyrolysis. J. Anal. Appl. Pyrolysis. 2021, 155, 105052. [Google Scholar] [CrossRef]
- Yu, W.; Reitberger, T.; Hjertberg, T.; Oderkerk, J.; Costa, F.; Englund, V.; Gedde, U. Chlorine dioxide resistance of different phenolic antioxidants in polyethylene. Polym. Degrad. Stab. 2014, 111, 1–6. [Google Scholar] [CrossRef]
- Reingruber, E.; Himmelsbach, M.; Sauer, C.; Buchberger, W. Identification of degradation products of antioxidants in polyolefins by liquid chromatography combined with atmospheric pressure photoionisation mass spectrometry. Polym. Degrad. Stab. 2010, 95, 740–745. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Chung, D. Antioxidant-Based Phase-Change Thermal Interface Materials with High Thermal Stability. J. Electron. Mater. 2008, 37, 448–461. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Chung, D.D.L. Effects of antioxidants and the solid component on the thermal stability of polyol-ester-based thermal pastes. J. Mater. Sci. 2007, 42, 2358–2375. [Google Scholar] [CrossRef]
- Gómez-Contreras, P.; Figueroa-Lopez, K.J.; Hernández-Fernández, J.; Rodríguez, M.C.; Ortega-Toro, R. Effect of Different Essential Oils on the Properties of Edible Coatings Based on Yam (Dioscorea rotundata L.) Starch and Its Application in Strawberry (Fragaria vesca L.) Preservation. Appl. Sci. 2021, 11, 11057. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; López-Martínez, J.; Hernández-Fernández, J.; Arrieta, M. Films Based on Thermoplastic Starch Blended with Pine Resin Derivatives for Food Packaging. Foods 2021, 10, 1171. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Puello-Polo, E.; Castro-Suarez, J.R. Characterization of the Morphological and Chemical Profile of Different Families of Microplastics in Samples of Breathable Air. Molecules 2023, 28, 1042. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.H.; Guerra, Y.; Cano, H. Detection of Bisphenol A and Four Analogues in Atmospheric Emissions in Petrochemical Complexes Producing Polypropylene in South America. Molecules 2022, 27, 4832. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, H.E.; Yagoubi, N.; Ferrier, D. Extraction of Polypropylene Additives and Their Analysis by HPLC. Chromatographia 1998, 48, 491–496. [Google Scholar] [CrossRef]
- Jordan, S.L.; Taylor, L.T. HPLC Separation with Solvent Elimination FTIR Detection of Polymer Additives. J. Chromatogr. Sci. 1997, 35, 7–13. [Google Scholar] [CrossRef]
- Francenia Santos Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Shalaby, E., Ed.; Intechopen: London, UK, 2019; pp. 1–28. [Google Scholar]
- Chacon, H.; Cano, H.; Fernández, J.H.; Guerra, Y.; Puello-Polo, E.; Ríos-Rojas, J.F.; Ruiz, Y. Effect of Addition of Polyurea as an Aggregate in Mortars: Analysis of Microstructure and Strength. Polymers 2022, 14, 1753. [Google Scholar] [CrossRef]
- Alladio, E.; Amante, E.; Bozzolino, C.; Seganti, F.; Salomone, A.; Vincenti, M.; Desharnais, B. Effective validation of chromatographic analytical methods: The illustrative case of androgenic steroids. Talanta 2020, 215, 120867. [Google Scholar] [CrossRef]
- Coutinho, F.M.B.; da Costa, M.; Simão, R.; Nicolini, L.F. Estudo da interação entre as fases da mistura poliestireno/elastômeros butadiênicos: Aspectos morfológicos e térmicos. In Proceedings of the 10° Congresso Brasileiro de Polímeros, Foz do Iguaçu, Brazil, 13–17 October 2009. [Google Scholar]
- Spectrabase. Cyanox 1790—FTIR—Spectrum—SpectraBase, 22 August 2022. CYANOX 1790 ANTIOXIDANT. Available online: https://spectrabase.com/spectrum/I3g5xqi3f4q (accessed on 18 January 2023).
- McMurry, J.; Mondragón, C.H.; Pozo, V.G. Química Orgánica, 8th ed.; Cengage Learning: Mexico City, Mexico, 2008. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).