Manipulation in Culture Conditions of Nanofrustulum shiloi for Enhanced Fucoxanthin Production and Isolation by Preparative Chromatography
Abstract
1. Introduction
2. Results
2.1. Enhanced Productivity of Fucoxanthin
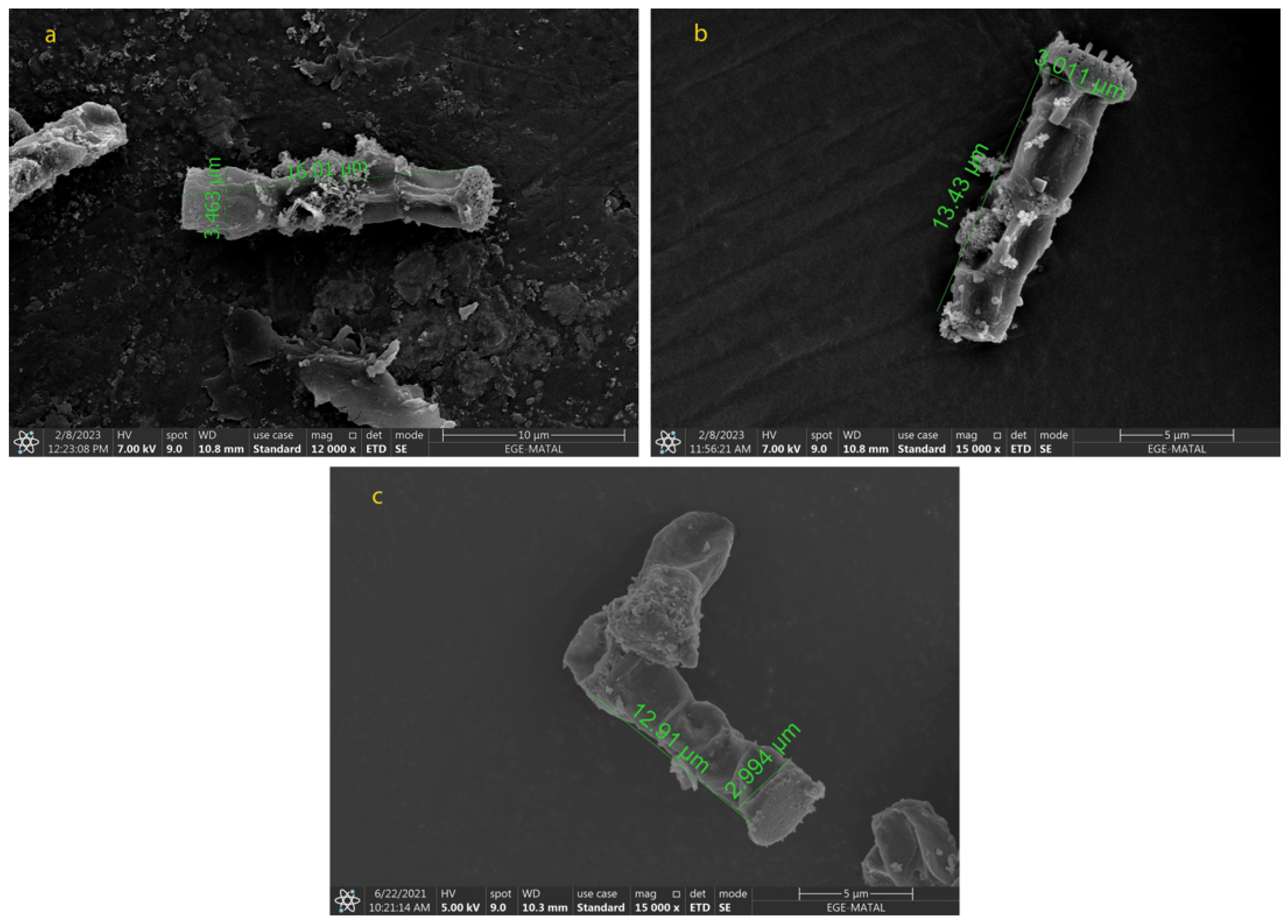
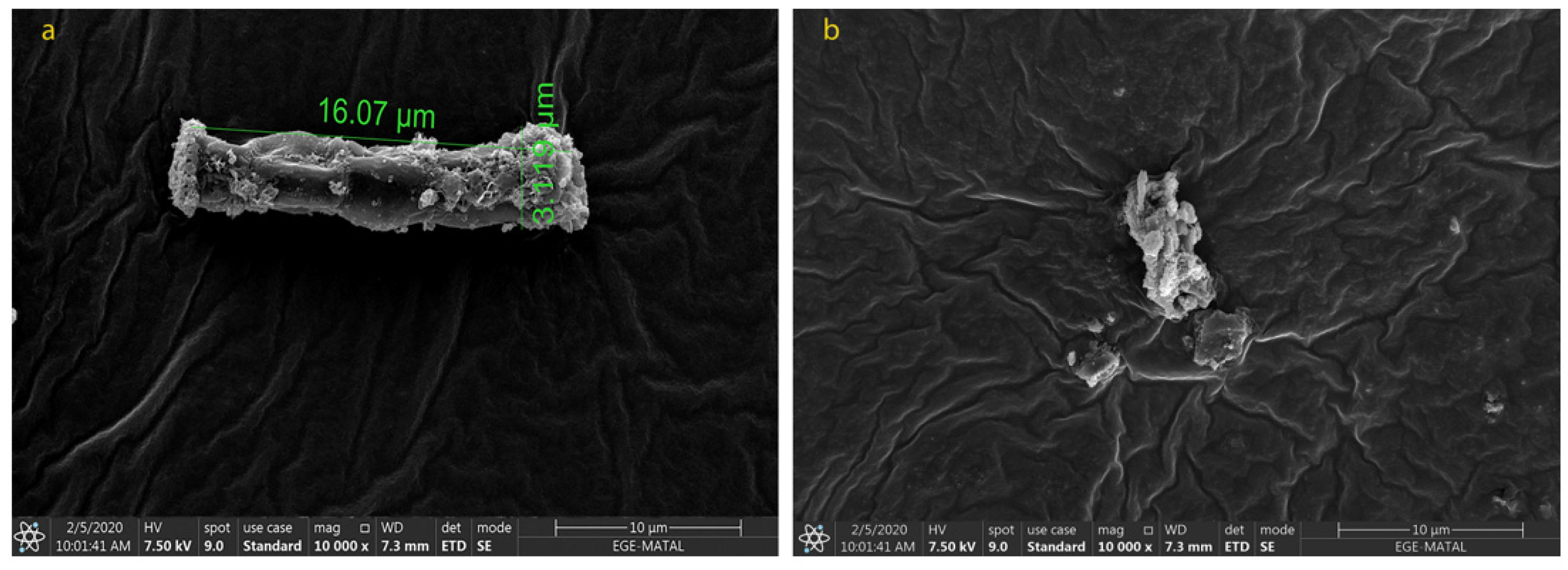
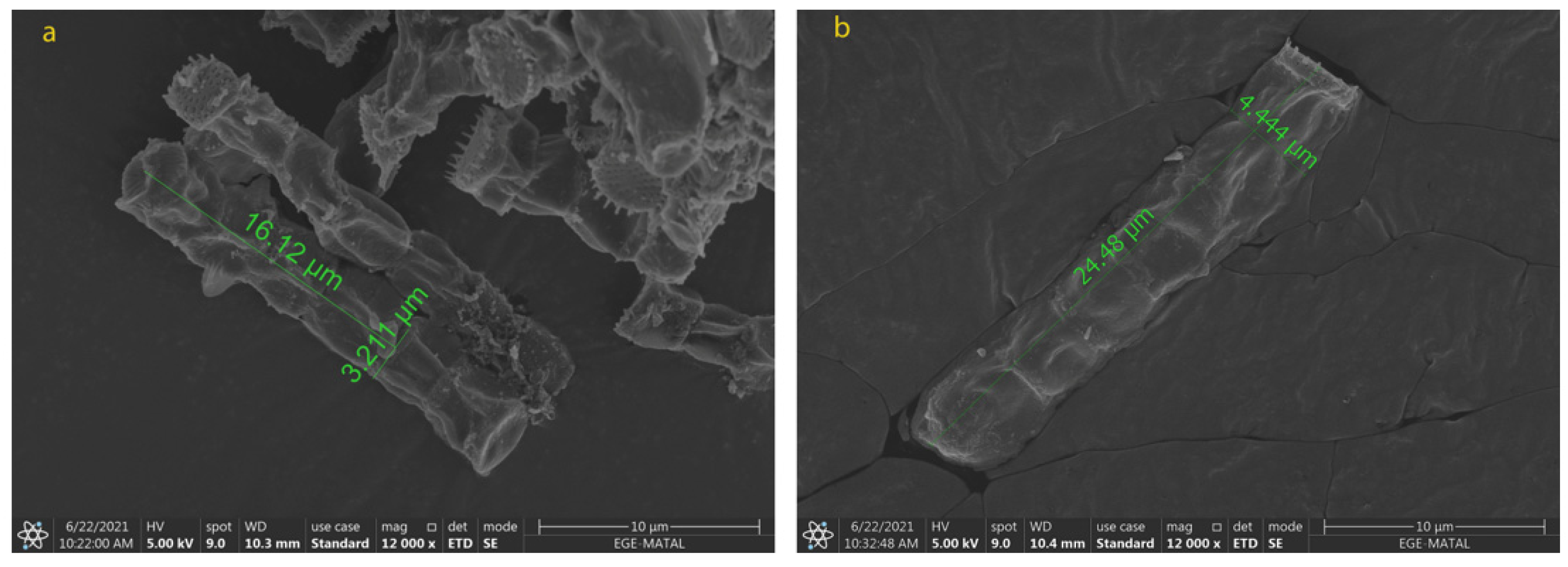
2.2. Morphological Changes in N. shiloi
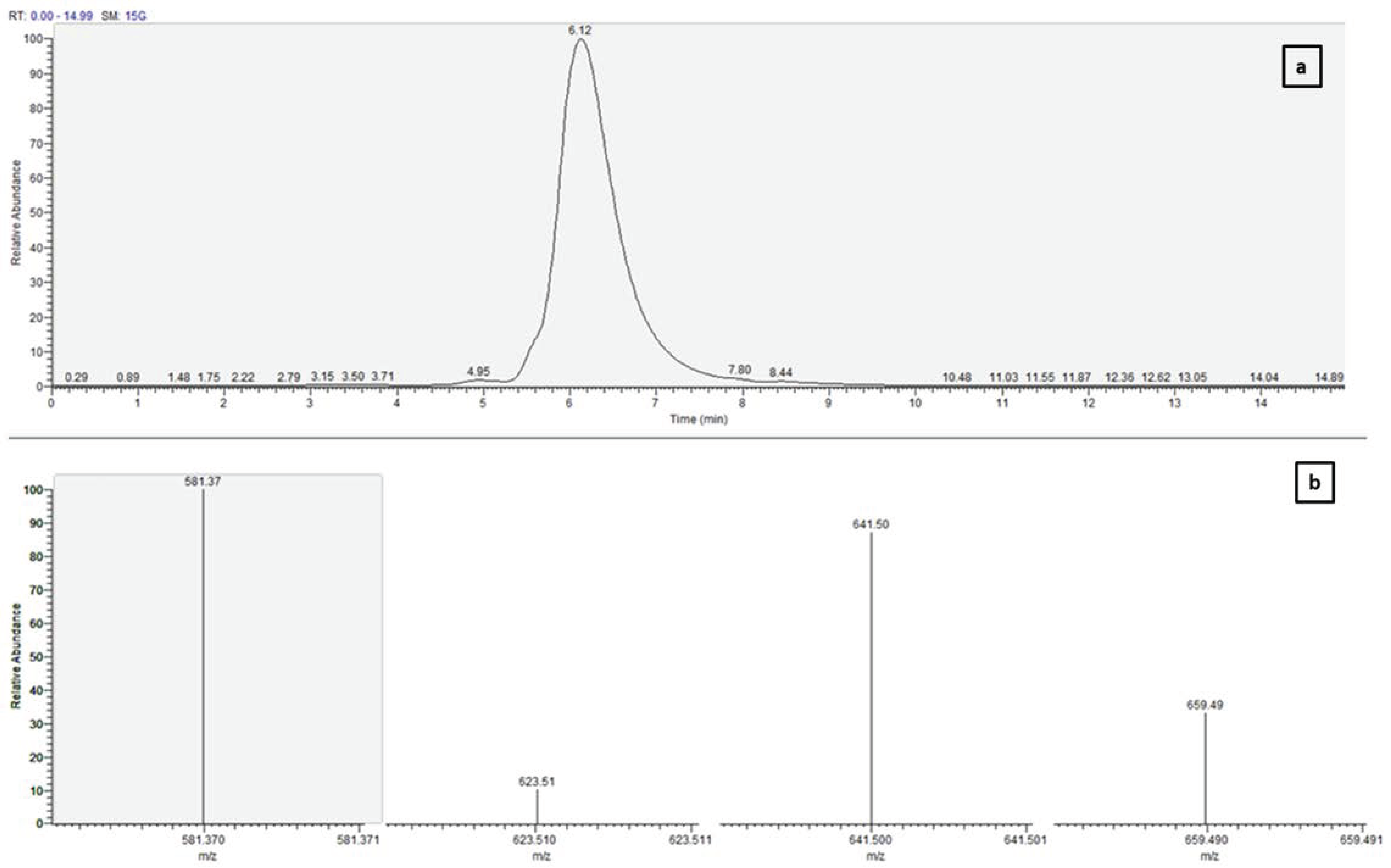
2.3. Fucoxanthin Analysis and Isolation by Preparative Chromatography
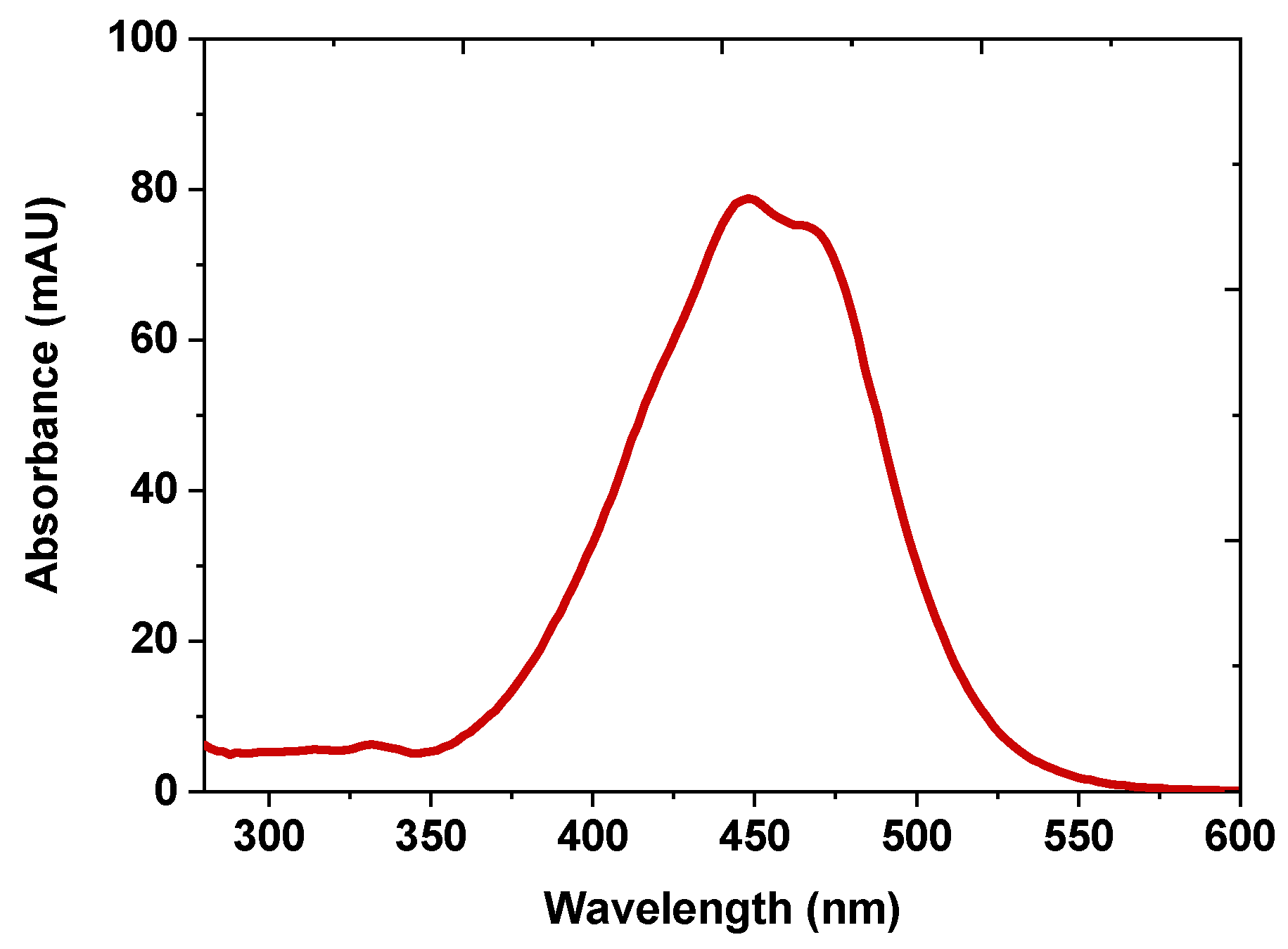
2.4. Confirmation of Purified Fucoxanthin with UV-vis Spectroscopy and Mass Spectrometry
3. Discussion
3.1. Variation of Fucoxanthin Content and Biomass Productivity under Different Light Intensities and Aeration Dates
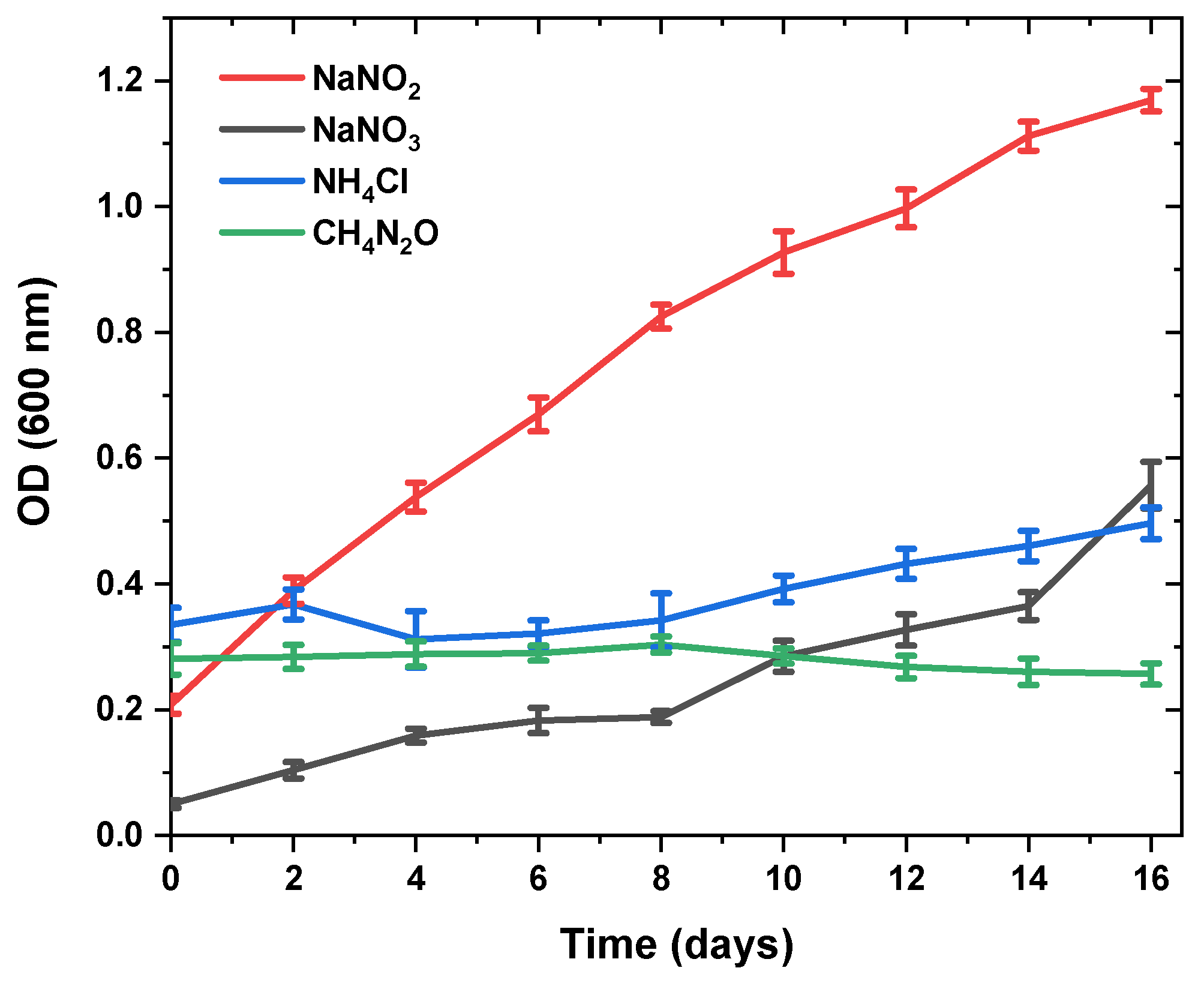
3.2. Changes in Fucoxanthin Content and Biomass Productivity Using Different Nitrogen Sources
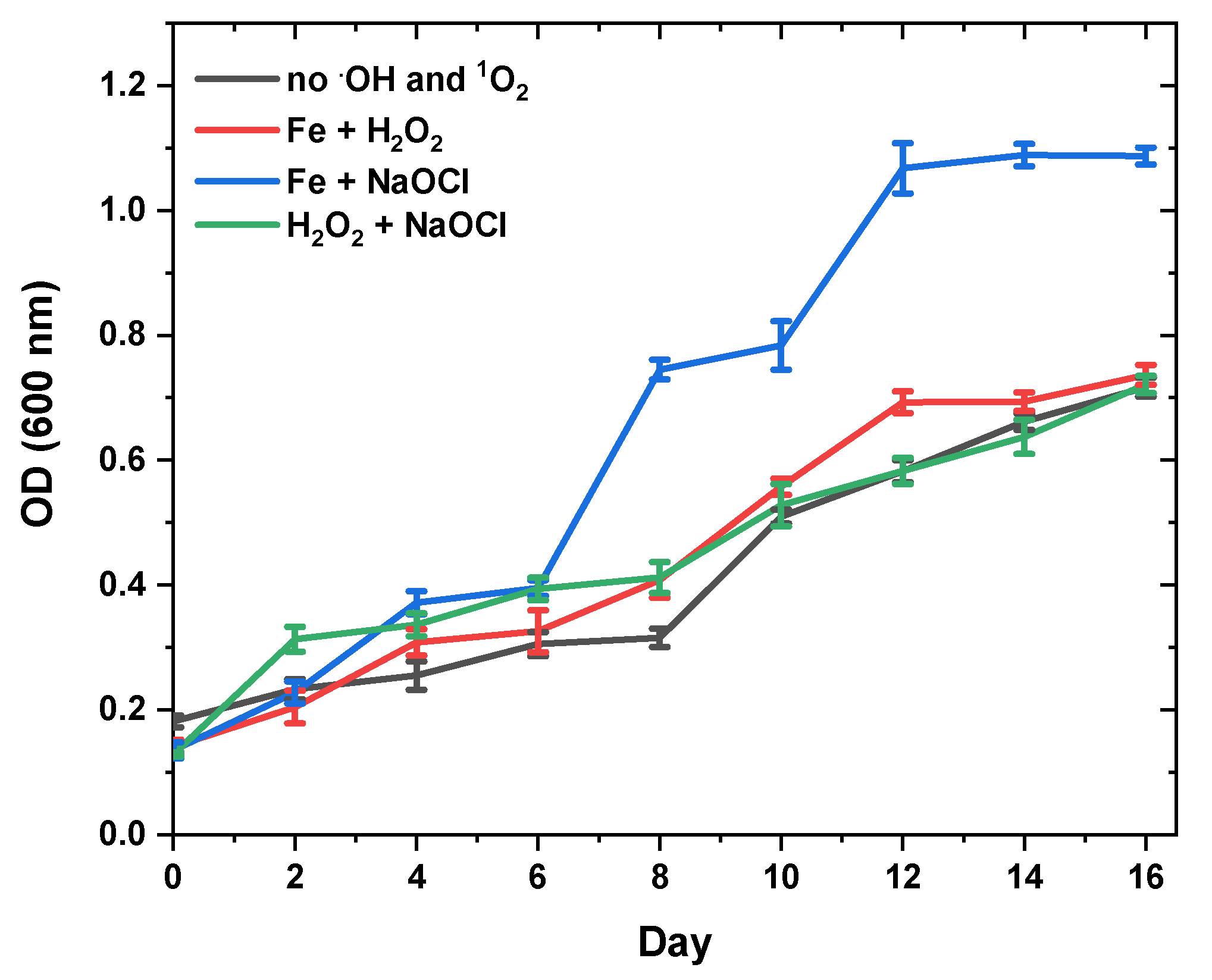
3.3. Effect of Oxidative Stress on Fucoxanthin Content and Biomass Productivity
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Molecular Identification of N. shiloi
4.3. Cultivation and Harvesting of N. shiloi
- R1:
- Fe2+ + H2O2 → Fe3+ + OH− + ∙OH
- R2:
- NaClO + H2O → HOCl + Na+ + OH− and HOCl + Fe2+ → Fe3+ + Cl− + ∙OH
- R3:
- H2O2 + NaClO → H2O + NaCl + 1O2
4.4. Preparation of Stock and Standard Fucoxanthin Solutions
4.5. Ultrasound-Assisted Extraction (UAE) of Fucoxanthin from N. shiloi
4.6. HPLC-DAD and LC-APCI-MS/MS Analyses of Fucoxanthin
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cohen, Z. Chemicals from Microalgae; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Saini, D.K.; Chakdar, H.; Pabbi, S.; Shukla, P. Enhancing production of microalgal biopigments through metabolic and genetic engineering. Crit. Rev. Food Sci. Nutr. 2019, 60, 391–405. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Raposo, M.F.; de Morais, R.M.; de Morais, A.M. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Garcia-Gonzalez, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Wijffels, R.H. Potential of sponges and microalgae for marine biotechnology. Trends Biotechnol. 2008, 26, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Jimenez, A.R.; Paredes-Lopez, O. Natural pigments: Carotenoids, anthocyanins, and betalains--characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Chatfield, D.C.; Mebel, A.M.; Alvarez-Calderon, F.; Fernandez, M.V. The conformation of end-groups is one determinant of carotenoid topology suitable for high fidelity molecular recognition: A study of β-and ε-end-groups. Arch. Biochem. Biophys. 2010, 493, 169–174. [Google Scholar] [CrossRef]
- Rajesh, K.; Rohit, M.; Mohan, S.V. Microalgae-Based Carotenoids Production. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 139–147. [Google Scholar]
- Kumar, S.R.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 2013, 11, 5130–5147. [Google Scholar] [CrossRef]
- Beppu, F.; Niwano, Y.; Sato, E.; Kohno, M.; Tsukui, T.; Hosokawa, M.; Miyashita, K. In vitro and in vivo evaluation of mutagenicity of fucoxanthin (FX) and its metabolite fucoxanthinol (FXOH). J. Toxicol. Sci. 2009, 34, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, M.C.; Lopez, D.; Sáiz, M.P.; Poquet, M.; Perez, J.; Puig-Parellada, P.; Marmol, F.; Simonetti, P.; Gardana, C.; Lerat, Y.; et al. Toxicity and antioxidant activity in vitro and in vivo of two Fucus vesiculosus extracts. J. Agric. Food Chem. 2008, 56, 7773–7780. [Google Scholar] [CrossRef]
- Dominguez, H. Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Koduvayur Habeebullah, S.F.; Surendraraj, A.; Jacobsen, C. Isolation of Fucoxanthin from Brown Algae and Its Antioxidant Activity: In Vitro and 5% Fish Oil-In-Water Emulsion. J. Am. Oil Chem. Soc. 2018, 95, 835–843. [Google Scholar] [CrossRef]
- Sun, P.; Wong, C.C.; Li, Y.; He, Y.; Mao, X.; Wu, T.; Ren, Y.; Chen, F. A novel strategy for isolation and purification of fucoxanthinol and fucoxanthin from the diatom Nitzschia laevis. Food Chem. 2019, 277, 566–572. [Google Scholar] [CrossRef]
- Zailanie, K.; Purnomo, H. Fucoxanthin content of five species brown seaweed from Talango District, Madura Island. J. Agric. Sci. Technol. A 2011, 1, 1103–1105. [Google Scholar]
- Ishika, T.; Laird, D.W.; Bahri, P.A.; Moheimani, N.R. Co-cultivation and stepwise cultivation of Chaetoceros muelleri and Amphora sp. for fucoxanthin production under gradual salinity increase. J. Appl. Phycol. 2019, 31, 1535–1544. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Yang, J.; Jin, L.; Gao, Z.; Xue, L.; Hou, L.; Sui, L.; Liu, J.; Zou, X. Fucoxanthin inhibits tumour-related lymphangiogenesis and growth of breast cancer. J. Cell. Mol. Med. 2019, 23, 2219–2229. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chen, C.-Y.; Varjani, S.; Chang, J.-S. Producing fucoxanthin from algae–Recent advances in cultivation strategies and downstream processing. Bioresour. Technol. 2022, 344, 126170. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A. Enhancing production of fucoxanthin by the optimization of culture media of the microalga Tisochrysis lutea. Aquaculture 2021, 533, 736074. [Google Scholar] [CrossRef]
- Lu, X.; Sun, H.; Zhao, W.; Cheng, K.-W.; Chen, F.; Liu, B. A hetero-photoautotrophic two-stage cultivation process for production of fucoxanthin by the marine diatom Nitzschia laevis. Mar. Drugs 2018, 16, 219. [Google Scholar] [CrossRef]
- Mao, X.; Chen, S.H.Y.; Lu, X.; Yu, J.; Liu, B. High silicate concentration facilitates fucoxanthin and eicosapentaenoic acid (EPA) production under heterotrophic condition in the marine diatom Nitzschia laevis. Algal Res. 2020, 52, 102086. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Liu, J. Screening of Isochrysis strains for simultaneous production of docosahexaenoic acid and fucoxanthin. Algal Res. 2019, 41, 101545. [Google Scholar] [CrossRef]
- Mousavi Nadushan, R.; Hosseinzade, I. Optimization of production and antioxidant activity of fucoxanthin from marine haptophyte algae, Isochrysis galbana. Iran. J. Fish. Sci. 2020, 19, 2901–2908. [Google Scholar]
- Gao, F.; Teles, I.; Wijffels, R.H.; Barbosa, M.J. Process optimization of fucoxanthin production with Tisochrysis lutea. Bioresour. Technol. 2020, 315, 123894. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wu, T.; Fu, Y.; He, Y.; Mao, X.; Chen, F. Storage carbon metabolism of Isochrysis zhangjiangensis under different light intensities and its application for co-production of fucoxanthin and stearidonic acid. Bioresour. Technol. 2019, 282, 94–102. [Google Scholar] [CrossRef]
- Khaw, Y.S.; Yusoff, F.M.; Tan, H.T.; Noor Mazli, N.A.I.; Nazarudin, M.F.; Shaharuddin, N.A.; Omar, A.R.; Takahashi, K. Fucoxanthin Production of Microalgae under Different Culture Factors: A Systematic Review. Mar. Drugs 2022, 20, 592. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xing, R.; Liu, S.; Li, P. Effect of Fucoxanthin administration on thyroid gland injury induced by cadmium in mice. Biol. Trace Elem. Res. 2021, 199, 1877–1884. [Google Scholar] [CrossRef]
- Zarekarizi, A.; Hoffmann, L.; Burritt, D. Approaches for the sustainable production of fucoxanthin, a xanthophyll with potential health benefits. J. Appl. Phycol. 2019, 31, 281–299. [Google Scholar] [CrossRef]
- Zheng, J.; Tian, X.; Zhang, W.; Zheng, P.; Huang, F.; Ding, G.; Yang, Z. Protective effects of fucoxanthin against alcoholic liver injury by activation of Nrf2-mediated antioxidant defense and inhibition of TLR4-mediated inflammation. Mar. Drugs 2019, 17, 552. [Google Scholar] [CrossRef] [PubMed]
- Telussa, I.; Nurachman, Z. Dynamics of β-carotene and fucoxanthin of tropical marine Navicula sp. as a response to light stress conditions. Algal Res. 2019, 41, 101530. [Google Scholar] [CrossRef]
- Csavina, J.L.; Stuart, B.J.; Riefler, R.G.; Vis, M.L. Growth optimization of algae for biodiesel production. J Appl Microbiol 2011, 111, 312–318. [Google Scholar] [CrossRef]
- Erdoğan, A.; Karataş, A.B.; Demirel, Z.; Dalay, M.C. Purification of fucoxanthin from the diatom Amphora capitellata by preparative chromatography after its enhanced productivity via oxidative stress. J. Appl. Phycol. 2022, 34, 301–309. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H.; Mercadante, A.; Egeland, E. Carotenoids: Handbook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Wang, W.; Yu, L.-J.; Xu, C.; Tomizaki, T.; Zhao, S.; Umena, Y.; Chen, X.; Qin, X.; Xin, Y.; Suga, M. Structural basis for blue-green light harvesting and energy dissipation in diatoms. Science 2019, 363, eaav0365. [Google Scholar] [CrossRef]
- Gardian, Z.; Litvín, R.; Bína, D.; Vácha, F. Supramolecular organization of fucoxanthin–chlorophyll proteins in centric and pennate diatoms. Photosynth. Res. 2014, 121, 79–86. [Google Scholar] [CrossRef]
- Truong, T.Q.; Park, Y.J.; Koo, S.Y.; Choi, J.-H.; Enkhbayar, A.; Song, D.-G.; Kim, S.M. Interdependence of fucoxanthin biosynthesis and fucoxanthin-chlorophyll a/c binding proteins in Phaeodactylum tricornutum under different light intensities. J. Appl. Phycol. 2023, 35, 25–42. [Google Scholar] [CrossRef]
- Nagao, R.; Yokono, M.; Ueno, Y.; Suzuki, T.; Kumazawa, M.; Kato, K.-H.; Tsuboshita, N.; Dohmae, N.; Ifuku, K.; Shen, J.-R. Enhancement of excitation-energy quenching in fucoxanthin chlorophyll a/c-binding proteins isolated from a diatom Phaeodactylum tricornutum upon excess-light illumination. Biochim. Biophys. Acta (BBA)-Bioenerg. 2021, 1862, 148350. [Google Scholar] [CrossRef] [PubMed]
- Giovagnetti, V.; Ruban, A.V. Detachment of the fucoxanthin chlorophyll a/c binding protein (FCP) antenna is not involved in the acclimative regulation of photoprotection in the pennate diatom Phaeodactylum tricornutum. Biochim. Biophys. Acta (BBA)-Bioenerg. 2017, 1858, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Domingues, N.; Matos, A.R.; Marques da Silva, J.; Cartaxana, P. Response of the diatom Phaeodactylum tricornutum to photooxidative stress resulting from high light exposure. PLoS ONE 2012, 7, e38162. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- Van De Poll, W.H.; Van Leeuwe, M.A.; Roggeveld, J.; Buma, A.G. Nutrient Limitation And High Irradiance Acclimation Reduce Par And Uv-Induced Viability Loss In The Antarctic Diatom Chaetoceros Brevis (Bacillariophyceae) 1. J. Phycol. 2005, 41, 840–850. [Google Scholar] [CrossRef]
- Willemoës, M.; Monas, E. Relationship between growth irradiance and the xanthophyll cycle pool in the diatom Nitzschia palea. Physiol. Plant. 1991, 83, 449–456. [Google Scholar] [CrossRef]
- Kolber, Z.; Zehr, J.; Falkowski, P. Effects of Growth Irradiance and Nitrogen Limitation on Photosynthetic Energy Conversion in Photosystem II. Plant Physiol. 1988, 88, 923–929. [Google Scholar] [CrossRef]
- Coesel, S.; Obornik, M.; Varela, J.; Falciatore, A.; Bowler, C. Evolutionary origins and functions of the carotenoid biosynthetic pathway in marine diatoms. PLoS ONE 2008, 3, e2896. [Google Scholar] [CrossRef]
- Guo, B.B.; Liu, B.; Yang, B.; Sun, P.P.; Lu, X.; Liu, J.; Chen, F. Screening of Diatom Strains and Characterization of Cyclotella cryptica as A Potential Fucoxanthin Producer. Mar. Drugs 2016, 14, 125. [Google Scholar] [CrossRef]
- Pahl, S.L.; Lewis, D.M.; Chen, F.; King, K.D. Heterotrophic growth and nutritional aspects of the diatom Cyclotella cryptica (Bacillariophyceae): Effect of some environmental factors. J. Biosci. Bioeng. 2010, 109, 235–239. [Google Scholar] [CrossRef]
- Hao, T.B.; Yang, Y.F.; Balamurugan, S.; Li, D.W.; Yang, W.D.; Li, H.Y. Enrichment of f/2 medium hyperaccumulates biomass and bioactive compounds in the diatom Phaeodactylum tricornutum. Algal Res.-Biomass Biofuels Bioprod. 2020, 47, 101872. [Google Scholar] [CrossRef]
- Kanamoto, A.; Kato, Y.; Yoshida, E.; Hasunuma, T.; Kondo, A. Development of a Method for Fucoxanthin Production Using the Haptophyte Marine Microalga Pavlova sp. OPMS 30543. Mar. Biotechnol. 2021, 23, 331–341. [Google Scholar] [CrossRef]
- Tokushima, H.; Inoue-Kashino, N.; Nakazato, Y.; Masuda, A.; Ifuku, K.; Kashino, Y. Advantageous characteristics of the diatom Chaetoceros gracilis as a sustainable biofuel producer. Biotechnol. Biofuels 2016, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.; Guerrero, M.G.; Losada, M. Short-Term Ammonium Inhibition of Nitrate Utilization by Anacystis-Nidulans and Other Cyanobacteria. Arch. Microbiol. 1980, 128, 137–144. [Google Scholar] [CrossRef]
- Hildebrand, M. Cloning and functional characterization of ammonium transporters from the marine diatom Cylindrotheca fusiformis (Bacillariophyceae) 1. J. Phycol. 2005, 41, 105–113. [Google Scholar] [CrossRef]
- Maestrini, S.Y.; Robert, J.M.; Leftley, J.W.; Collos, Y. Ammonium Thresholds for Simultaneous Uptake of Ammonium and Nitrate by Oyster-Pond Algae. J. Exp. Mar. Biol. Ecol. 1986, 102, 75–98. [Google Scholar] [CrossRef]
- Syrett, P.J. Nitrogen-Metabolism of Microalgae. Can. Bull. Fish. Aquat. Sci. 1981, 210, 182–210. [Google Scholar]
- Nur, M.M.A.; Muizelaar, W.; Boelen, P.; Buma, A.G.J. Environmental and nutrient conditions influence fucoxanthin productivity of the marine diatom Phaeodactylum tricornutum grown on palm oil mill effluent. J. Appl. Phycol. 2019, 31, 111–122. [Google Scholar] [CrossRef]
- Bekheet, I.t.; Syrett, P. Urea-degrading enzymes in algae. Br. Phycol. J. 1977, 12, 137–143. [Google Scholar] [CrossRef]
- Solomon, C.M.; Glibert, P.M. Urease activity in five phytoplankton species. Aquat. Microb. Ecol. 2008, 52, 149–157. [Google Scholar] [CrossRef]
- Collos, Y.; Harrison, P.J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo Paredes, P.; Cid, Á.; Abalde, J.; Herrero, C. Culture of the marine diatom Phaeodactylum tricornutum with different nitrogen sources: Growth, nutrient conversion and biochemical composition. Cah. Biol. Mar. 1995, 36, 165–173. [Google Scholar]
- Goiris, K.; Van Colen, W.; Wilches, I.; Leon-Tamariz, F.; De Cooman, L.; Muylaert, K. Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res.-Biomass Biofuels Bioprod. 2015, 7, 51–57. [Google Scholar] [CrossRef]
- Cirulis, J.T.; Scott, J.A.; Ross, G.M. Management of oxidative stress by microalgae. Can. J. Physiol. Pharmacol. 2013, 91, 15–21. [Google Scholar] [CrossRef]
- Erdogan, A.; Demirel, Z.; Dalay, M.C.; Eroglu, A.E. Fucoxanthin Content of Cylindrotheca Closterium and Its Oxidative Stress Mediated Enhancement. Turk. J. Fish. Aquat. Sci. 2016, 16, 491–498. [Google Scholar] [CrossRef]
- Noctor, G.; Lelarge-Trouverie, C.; Mhamdi, A. The metabolomics of oxidative stress. Phytochemistry 2015, 112, 33–53. [Google Scholar] [CrossRef]
- Martinez, A.; Barbosa, A. Antiradical power of carotenoids and vitamin E: Testing the hydrogen atom transfer mechanism. J. Phys. Chem. B 2008, 112, 16945–16951. [Google Scholar] [CrossRef]
- Martίnez, A.; Vargas, R.; Galano, A. What is important to prevent oxidative stress? A theoretical study on electron-transfer reactions between carotenoids and free radicals. J. Phys. Chem. B 2009, 113, 12113–12120. [Google Scholar] [CrossRef] [PubMed]
- Demirel, Z.; Imamoglu, E.; Dalay, M.C. Growth kinetics of Nanofrustulum shiloi under different mixing conditions in flat-plate photobioreactor. Braz. Arch. Biol. Technol. 2020, 63, e20190201. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994; Volume 10, p. 293. [Google Scholar]
- Almeida, A.C.; Gomes, T.; Langford, K.; Thomas, K.V.; Tollefsen, K.E. Oxidative stress in the algae Chlamydomonas reinhardtii exposed to biocides. Aquat. Toxicol. 2017, 189, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Strati, I.F.; Oreopoulou, V. Effect of extraction parameters on the carotenoid recovery from tomato waste. Int. J. Food Sci. Technol. 2011, 46, 23–29. [Google Scholar] [CrossRef]


| * Aeration Rate and Light Intensity | Fucoxanthin (mg/gDW) | Specific Growth Rate (day−1) | Dry Weight (g/L) |
|---|---|---|---|
| N1-50 | 33.52 ± 0.67 b | 0.175 ± 0.03 a,b | 0.44 ± 0.02 e |
| N1-150 | 10.86 ± 0.21 d,e | 0.203 ± 0.02 a,b | 0.24 ± 0.08 f |
| N1-300 | 21.85 ± 0.43 e,f | 0.269 ± 0.02 a,b | 1.36 ± 0.08 a |
| N3-50 | 38.06 ± 0.76 b | 0.160 ± 0.03 b | 0.52 ± 0.03 e |
| N3-150 | 19.75 ± 0.39 f | 0.224 ± 0.03 a,b | 0.85 ± 0.07 d |
| N3-300 | 26.79 ± 0.53 c,d | 0.188 ± 0.02 a,b | 1.05 ± 0.06 b,c |
| N5-50 | 51.05 ± 1.02 a | 0.166 ± 0.01 b | 0.43 ± 0.09 e |
| N5-150 | 23.47 ± 0.46 d,e | 0.330 ± 0.04 a | 0.95 ± 0.03 c,d |
| N5-300 | 28.04 ± 0.56 c | 0.221 ± 0.02 b | 1.22 ± 0.08 a,b |
| Nitrogen Sources | Fucoxanthin (mg/gDW) | Specific Growth Rate (day−1) | Dry Weight (g/L) |
|---|---|---|---|
| NaNO3 | 51.05 ± 1.58 a | 0.166 ± 0.04 a | 0.43 ± 0.09 b |
| NaNO2 | 19.18± 0.39 b | 0.162 ± 0.03 a | 0.69 ± 0.07 a |
| NH4 Cl | 2.56 ± 0.04 c | 0.040 ± 0.00 b | 0.64 ± 0.08 a |
| CH4N2O | - | - | - |
| During Growth Phase | pH of Medium with | |||
|---|---|---|---|---|
| NaNO3 | NaNO2 | NH4Cl | CH4N2O | |
| Day 0 (culture medium without cells) | 8.50 ± 0.23 a | 8.69 ± 0.19 a | 7.87 ± 0.21 a | 8.67 ± 0.30 a |
| Day 16 (culture medium before harvesting) | 9.02 ± 0.25 a | 8.95 ± 0.21 a | 4.2 ± 0.14 b | 3.7 ± 0.13 b |
| Oxidative Stress Sources in Seawaterbg-11 | Fucoxanthin (mg/gDW) | Specific Growth Rate (day−1) | Dry Weight (g/L) |
|---|---|---|---|
| no.OH and 1O2 | 50.17 ± 1.02 d | 0.166 ± 0.03 a | 0.66 ± 0.01 a |
| 0.1 mM H2O2 + 0.1 mM Fe2+ | 58.20 ± 1.16 c | 0.142 ± 0.01 c | 0.15 ± 0.03 d |
| 0.1 mM NaClO + 0.1 mM Fe2+ | 65.14 ± 1.95 b | 0.152 ± 0.00 b | 0.28 ± 0.01 c |
| 0.1 mM H2O2 + 0.1 mM NaOCl | 97.45 ± 2.64 a | 0.163 ± 0.04 a | 0.48 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdoğan, A.; Karataş, A.B.; Demir, D.; Demirel, Z.; Aktürk, M.; Çopur, Ö.; Conk-Dalay, M. Manipulation in Culture Conditions of Nanofrustulum shiloi for Enhanced Fucoxanthin Production and Isolation by Preparative Chromatography. Molecules 2023, 28, 1988. https://doi.org/10.3390/molecules28041988
Erdoğan A, Karataş AB, Demir D, Demirel Z, Aktürk M, Çopur Ö, Conk-Dalay M. Manipulation in Culture Conditions of Nanofrustulum shiloi for Enhanced Fucoxanthin Production and Isolation by Preparative Chromatography. Molecules. 2023; 28(4):1988. https://doi.org/10.3390/molecules28041988
Chicago/Turabian StyleErdoğan, Ayşegül, Ayça Büşra Karataş, Dilan Demir, Zeliha Demirel, Merve Aktürk, Öykü Çopur, and Meltem Conk-Dalay. 2023. "Manipulation in Culture Conditions of Nanofrustulum shiloi for Enhanced Fucoxanthin Production and Isolation by Preparative Chromatography" Molecules 28, no. 4: 1988. https://doi.org/10.3390/molecules28041988
APA StyleErdoğan, A., Karataş, A. B., Demir, D., Demirel, Z., Aktürk, M., Çopur, Ö., & Conk-Dalay, M. (2023). Manipulation in Culture Conditions of Nanofrustulum shiloi for Enhanced Fucoxanthin Production and Isolation by Preparative Chromatography. Molecules, 28(4), 1988. https://doi.org/10.3390/molecules28041988







