New Insight on Archaeological Metal Finds, Nails and Lead Sheathings of the Punic Ship from Battle of the Egadi Islands
Abstract
1. Introduction
2. The Punic Ship and the Metal Finds
3. Results and Discussion
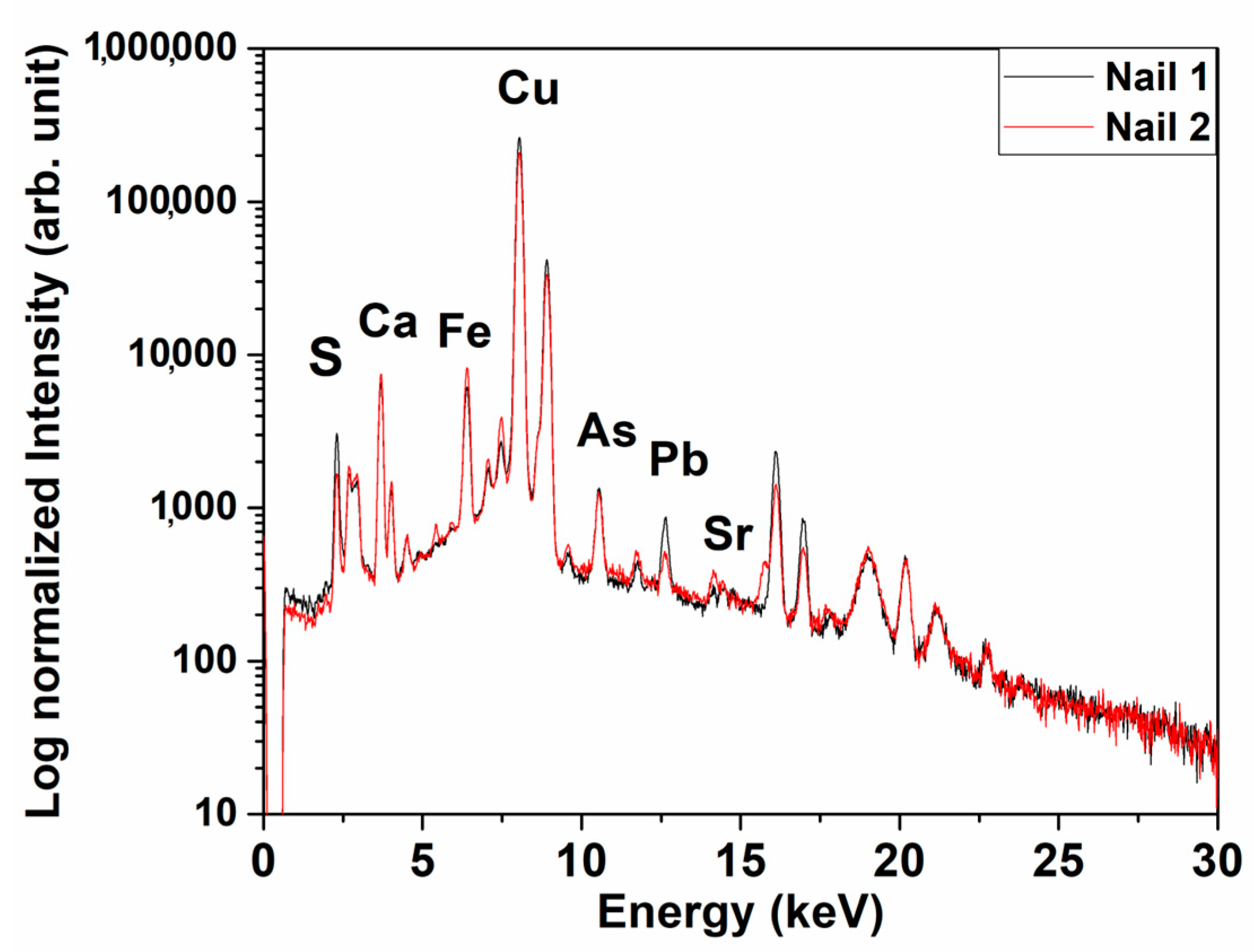
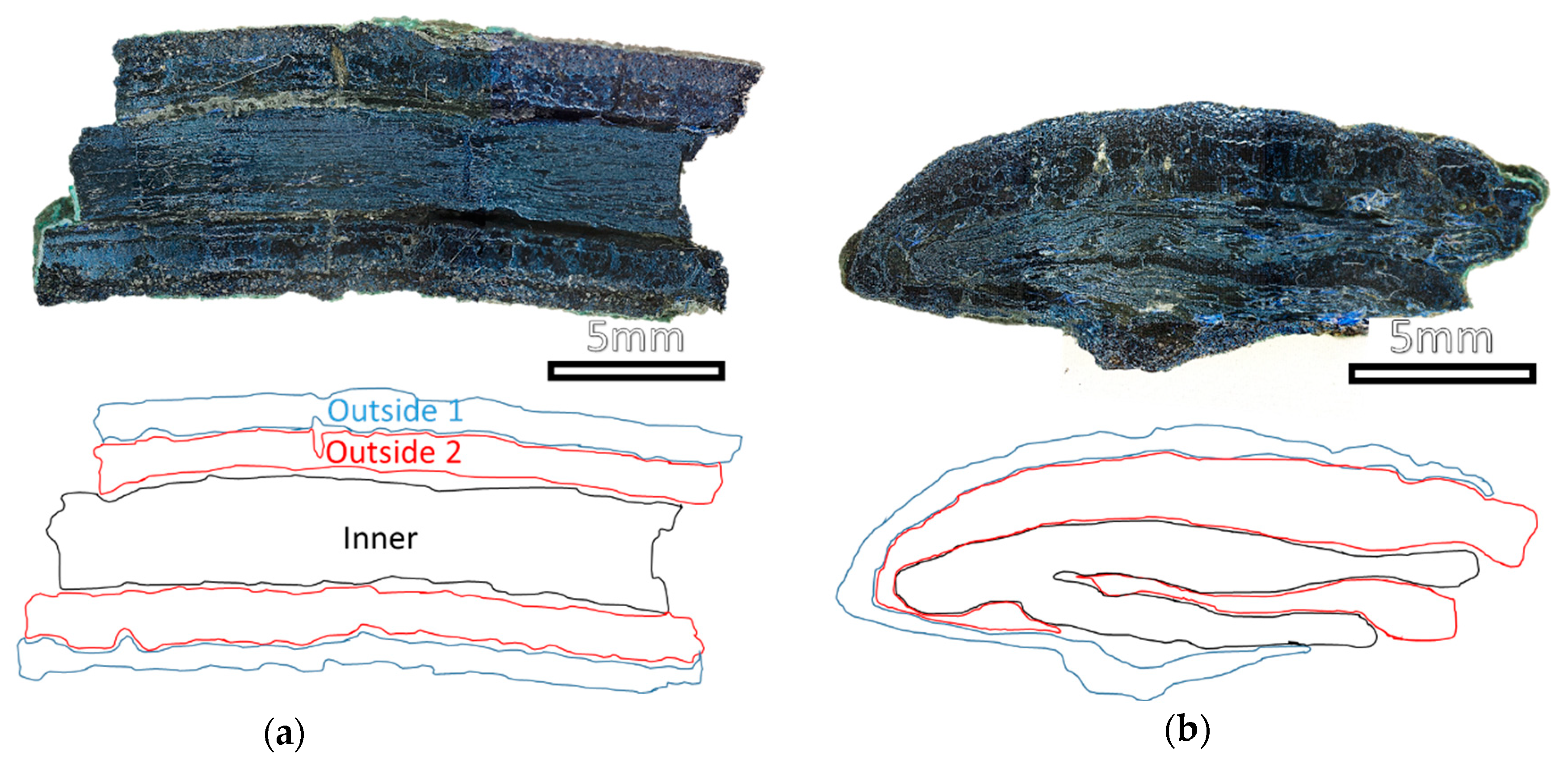
3.1. Nails
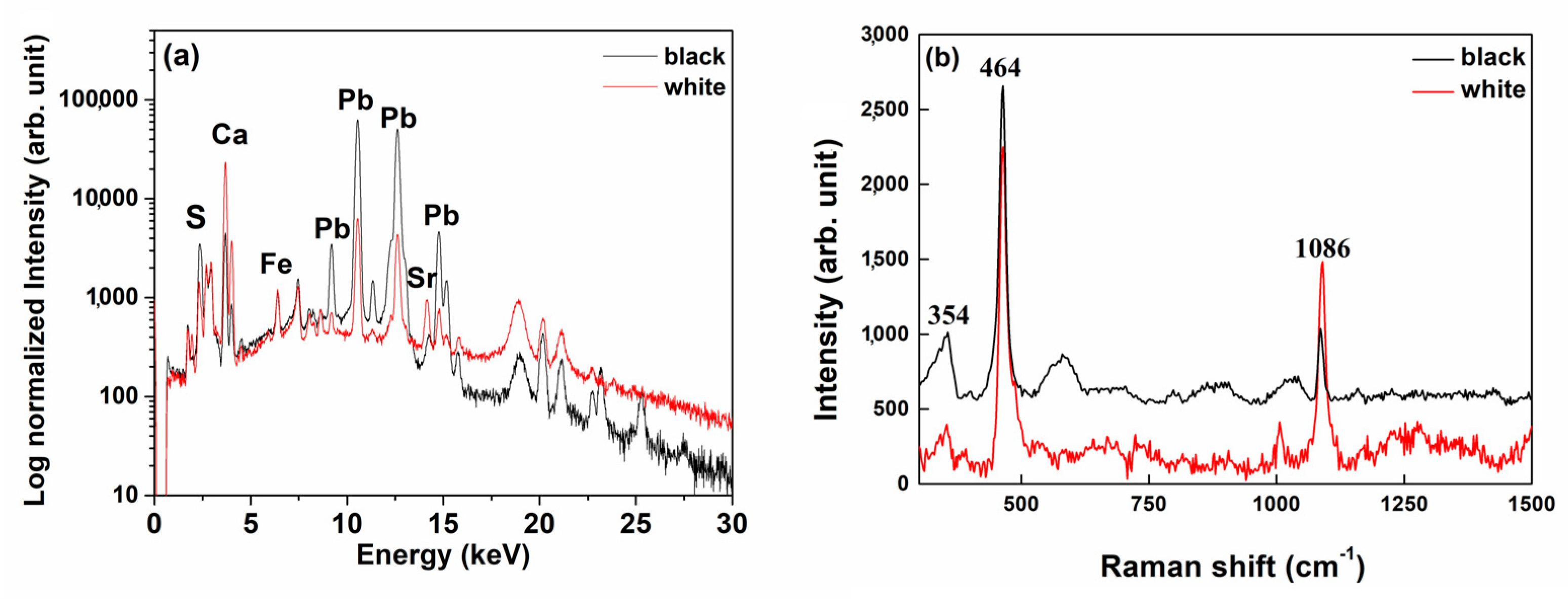
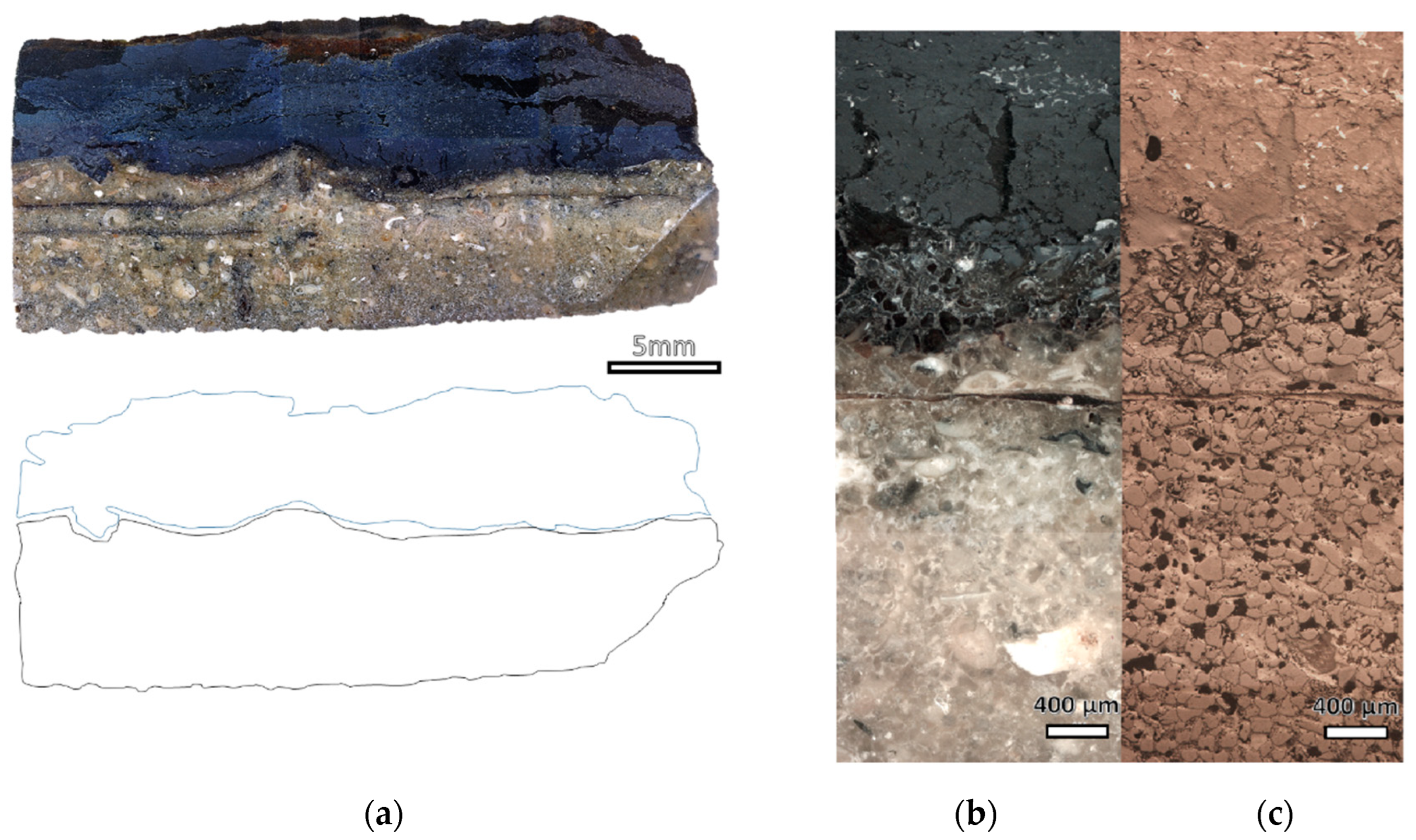
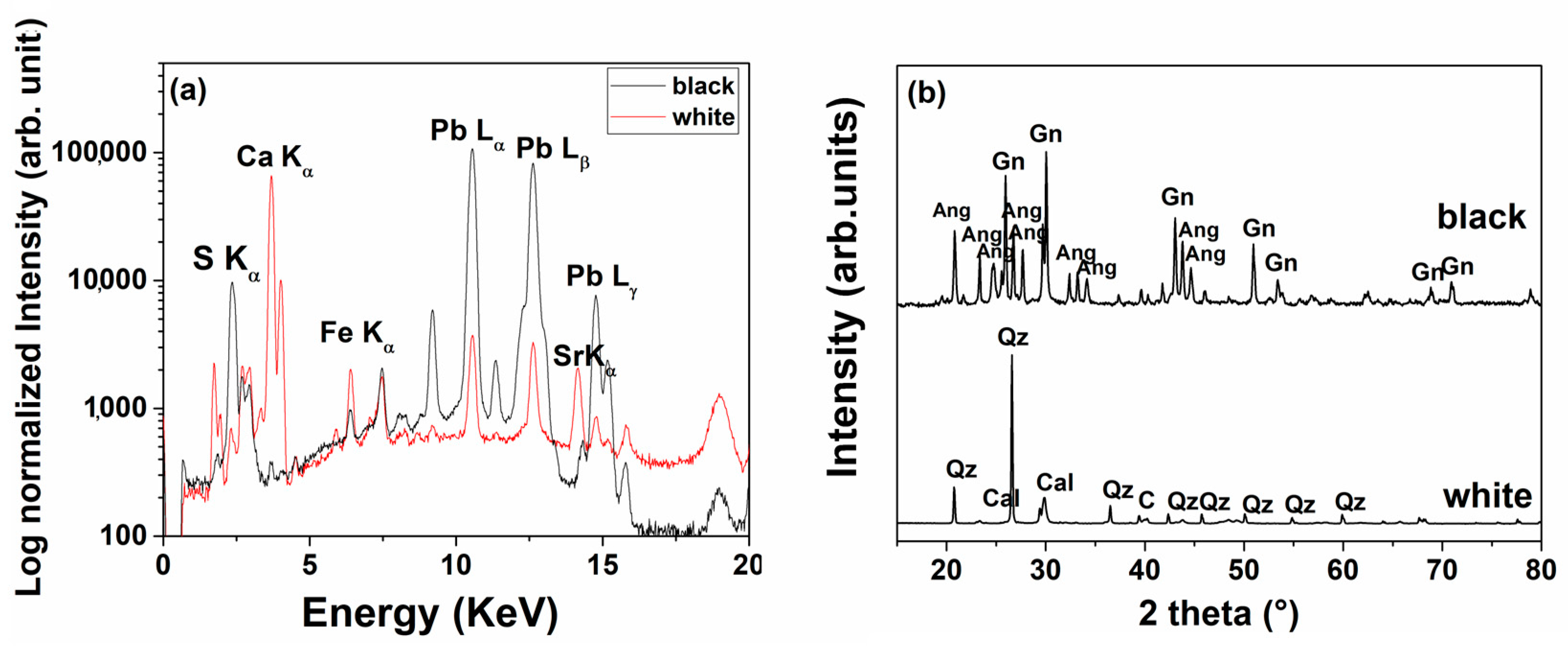
3.2. Sheathings
4. Conclusions
5. Methodology and Instrumentation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Huisman, D.; Manders, M.; Kretschmar, E.; Klaassen, R.; Lamersdorf, N. Burial conditions and wood degradation at archaeological sites in the Netherlands. Int. Biodeterior. Biodegrad. 2008, 61, 33–44. [Google Scholar] [CrossRef]
- Randazzo, L.; Ricca, M.; Ruffolo, S.; Aquino, M.; Davidde Petriaggi, B.; Enei, F.; La Russa, M.F. An Integrated Analytical Approach to Define the Compositional and Textural Features of Mortars Used in the Underwater Archaeological Site of Castrum Novum (Santa Marinella, Rome, Italy). Minerals 2019, 9, 268. [Google Scholar] [CrossRef]
- Estalayo, E.; Aramendia, J.; Matés Luque, J.M.; Madariaga, J.M. Chemical study of degradation processes in ancient metallic materials rescued from underwater medium. Raman Art Archeol. 2019, 50, 289–298. [Google Scholar] [CrossRef]
- Ingo, G.M.; Riccucci, C.; Faraldi, F.; Casaletto, M.P.; Guida, G. Micro-chemical and micro-structural investigation of the corrosion products on “The dancing satyr” (Mazara del Vallo, Sicily, Italy). Appl. Phys. A 2010, 100, 785–792. [Google Scholar] [CrossRef]
- Buccolieri, G.; Buccolieri, A.; Donati, P.; Marabelli, M.; Castellano, A. Portable EDXRF investigation of the patinas on the Riace Bronzes. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Interact. Mater. At. 2015, 343, 101–109. [Google Scholar] [CrossRef]
- Caponetti, E.; Armetta, F.; Brusca, L.; Chillura Martino, D.; Saladino, M.L.; Ridolfi, S.; Chirco, G.; Berrettoni, M.; Conti, P.; Nicolò, B.; et al. First discovery of orichalcum ingots from the remains of a 6th century BC shipwreck near gela (Sicily) seabed. Mediterr. Archaeol. Archaeom. 2017, 17, 11–18. [Google Scholar]
- Scott, D.A. Bronze Disease: A review of some chemical problems and the role of relative humidity. J. Am. Inst. Conserv. 1990, 29, 193–206. [Google Scholar] [CrossRef]
- Robbiola, L.; Blengino, J.-M.; Fiaud, C. Morphology and mechanisms of formation of natural patinas on archaeological Cu–Sn Alloys. Corros. Sci. 1998, 40, 2083–2111. [Google Scholar] [CrossRef]
- Piccardo, P.; Mille, B.; Robbiola, L. Tin and copper oxides in corroded archaeological bronzes. Corros. Met. Herit. Artefacts 2007, 239–262. [Google Scholar] [CrossRef]
- Ingo, G.M.; Riccucci, C.; Guida, G.; Pascucci, M.; Giuliani, C.; Messina, E.; Fierro, G.; Di Carlo, G. Micro-chemical investigation of corrosion products naturally grown on archaeological Cu-based artefacts retrieved from the Mediterranean Sea. Appl. Surf. Sci. 2019, 470, 695–706. [Google Scholar] [CrossRef]
- Elmouaden, K.; Jodeh, S.; Chaouay, A.; Oukhrib, R.; Salghi, R.; Bazzi, L.; Hilali, M. Sulfate-reducing bacteria impact on copper corrosion behavior in natural seawater environment. J. Surf. Eng. Mater. Adv. Technol. 2016, 6, 36–46. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Neff, D.; Kergourlay, F.; Foy, E.; Conforto, E.; Guilminot, E.; Reguer, S.; Refait, P.; Dillmann, P. Mechanisms of long-term anaerobic corrosion of iron archaeological artefacts in seawater. Corros. Sci. 2009, 51, 2932–2941. [Google Scholar] [CrossRef]
- Kergourlay, F.; Rémazeilles, C.; Neff, D.; Foy, E.; Conforto, E.; Guilminot, E.; Reguer, S.; Dillmann, P.; Nicot, F.; Mielcarek, F.; et al. Mechanisms of the dechlorination of iron archaeological artefacts extracted from seawater. Corros. Sci. 2011, 53, 2474–2483. [Google Scholar] [CrossRef]
- Hu, P.; Jia, M.; Li, M.; Sun, J.; Cui, Y.; Hu, D.; Hu, G. Corrosion Behavior of Ancient White Cast Iron Artifacts from Marine Excavations at Atmospheric Condition. Metals 2022, 12, 921. [Google Scholar] [CrossRef]
- Towarek, A.; Mistewicz, A.; Pilecka-Pietrusińska, E.; Zdunek, J.; Mizera, J. Corrosion degradation of archaeological lead: A review and case study. J. Archaeol. Sci. Rep. 2022, 45, 103611. [Google Scholar]
- Festa, G.; Saladino, M.L.; Mollica Nardo, V.; Armetta, F.; Renda, V.; Nasillo, G.; Pitonzo, R.; Spinella, A.; Borla, M.; Ferraris, E.; et al. Identifying the unknown content of an ancient Egyptian sealed alabaster vase from Kha and merit’s tomb using multiple techniques and multicomponent sample analysis in an interdisciplinary Applied Chemistry course. J. Chem. Educ. 2020, 98, 461–468. [Google Scholar] [CrossRef]
- Available online: http://www.parcolilibeo.it/ (accessed on 6 February 2023).
- Available online: https://honorfrostfoundation.org/the-punic-ship-of-marsala/ (accessed on 6 February 2023).
- Royal, J.; Tusa, S.; Goldman, A.L.; Murray, W.M.; Prag, J.R.W. The Site of the Battle of the Egadi Islands at the end of the First Punic War: Fieldwork, Analyses and Perspectives 2005–2015; Bibliotheca Archaeologica: Rome, Italy, 2020; ISBN 9788891318350. [Google Scholar]
- Frost, H. The discovery of a Punic ship. Int. J. Naut. Archaeol. 1972, 1, 113–117. [Google Scholar] [CrossRef]
- Frost, H. The Punic Ship Museum, Marsala. Mar. Mirror 1981, 67, 65–75. [Google Scholar] [CrossRef]
- Basch, L. When is a ram not a ram? Mar. Mirror 1983, 69, 129–142. [Google Scholar] [CrossRef]
- Frost, H. Lilybaeum (Marsala). The Punic ship: Final excavation report. In Supplement to Notizie degli Scavi di Antichità Serie Ottava Vol. XXX; Accademia Nazionale dei Lincei: Roma, Italy, 1976. [Google Scholar]
- Alagna, P. The construction of the treatment tanks used in the conservation of the wood of the marsala punic ship. Stud. Conserv. 1977, 22, 158–160. [Google Scholar]
- La Pica, A.; Rodonò, G.; Volpes, R. A study on outdoor and indoor climate to satisfy particular requisites in museums: The case of the punic ship in the Marsala’s Archaeological Museum. Energy Environ. Proc. Int. Conf. Energy Environ. 2003, 2, 1522–1530. [Google Scholar]
- Albertin, F.; Baumer, L.E.; Bettuzzi, M.; Brancaccio, R.; Caruso, E.; Casali, F.; Cifarelli, L.; Festa, G.; Griffo, M.G.; Mistretta, A.; et al. X-ray computed tomography to study archaeological clay and wood artefacts at lilybaeum. Eur. Phys. J. Plus 2021, 136, 513. [Google Scholar] [CrossRef]
- Available online: https://cultureincrisis.org/projects/3d-documentation-of-the-marsala-punic-ship-digital-conservation-and-archiving (accessed on 14 November 2022).
- Crisci, G.M.; La Russa, M.F.; Macchione, M.; Malagodi, M.; Palermo, A.M.; Ruffolo, S.A. Study of archaeological underwater finds: Deterioration and conservation. Appl. Phys. A 2010, 100, 855–863. [Google Scholar] [CrossRef]
- Angelini, E.; Grassini, S.; Tusa, S. Underwater Corrosion of Metallic Heritage Artefacts. Corros. Conserv. Cult. Herit. Met. Artefacts 2013, 236–259. [Google Scholar] [CrossRef]
- Bethencourt, M.; Fernández-Montblanc, T.; Izquierdo, A.; González-Duarte, M.M.; Muñoz-Mas, C. Study of the influence of physical, chemical and biological conditions that influence the deterioration and protection of Underwater Cultural Heritage. Sci. Total Environ. 2018, 613–614, 98–114. [Google Scholar] [CrossRef]
- Khoo, H.W.; Mok, K.F.; Tang, S.M.; Yap, C.T. Strontium/calcium ratio analysis of molluscan shells in Singapore waters using the X-ray fluorescence technique. Environ. Monit. Assess. 1985, 5, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ingo, G.M.; de Caro, T.; Riccucci, C.; Angelini, E.; Grassini, S.; Balbi, S.; Bernardini, P.; Salvi, D.; Bousselmi, L.; Çilingiroglu, A.; et al. Large scale investigation of chemical composition, structure and corrosion mechanism of bronze archeological artefacts from Mediterranean Basin. Appl. Phys. A 2006, 83, 513–520. [Google Scholar] [CrossRef]
- Craddock, P.T. The composition of the copper alloys used by the Greek, Etruscan and Roman civilizations. J. Archaeol. Sci. 1976, 3, 93–113. [Google Scholar] [CrossRef]
- Giumlia-Mair, A.R. The composition of copper-based small finds from a West Phoenician settlement site and from Nimrud compared with that of contemporary Mediterranean small finds. Archaeometry 1992, 34, 107–119. [Google Scholar] [CrossRef]
- Gregory, D. Experiments into the deterioration characteristics of materials on the Duart Point wreck site: An interim report. IJNA 1995, 24, 61–65. [Google Scholar] [CrossRef]
- Griesser, M.; Kockelmann, W.; Hradil, K.; Traum, R. New insights into the manufacturing technique and corrosion of high leaded antique bronze coins. Microchem. J. 2016, 126, 181–193. [Google Scholar] [CrossRef]
- Di Turo, F.; Montoya, N.; Piquero-Cilla, J.; De Vito, C.; Coletti, F.; Favero, G.; Doménech-Carbó, A. Archaeometric analysis of Roman bronze coins from the Magna Mater Temple using solid-state voltammetry and Electrochemical Impedance Spectroscopy. Anal. Chim. Acta 2017, 955, 36–47. [Google Scholar] [CrossRef]
- North, N.; MacLeod, I. Corrosion of metals. Conserv. Mar. Archaeol. Objects 1986, 68–98. [Google Scholar] [CrossRef]
- Festa, G.; Caroppi, P.A.; Filabozzi, A.; Andreani, C.; Arancio, M.L.; Triolo, R.; Celso, F.L.; Benfante, V.; Imberti, S. Composition and corrosion phases of Etruscan bronzes from villanovan age. Meas. Sci. Technol. 2008, 19, 034004. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Langlet-Marzloff, V.; Creus, J.; Lotte, G.; Deshayes, C.; Baleux, F.; Robbiola, L. Remarkable corrosion resumption of archaeological bronzes, induced by the oxidation of ternary Cu-sn-s phases in atmosphere, after long-term burial with sulfides. Corros. Sci. 2020, 175, 108865. [Google Scholar] [CrossRef]
- Almkvist, G.; Persson, I. Distribution of iron and sulfur and their speciation in relation to degradation processes in wood from the Swedish warship Vasa. New J. Chem. 2011, 35, 1491–1502. [Google Scholar] [CrossRef]
- Dowsett, M.G.; Sabbe, P.-J.; Alves Anjos, J.; Schofield, E.J.; Walker, D.; Thomas, P.; York, S.; Brown, S.; Wermeille, D.; Adriaens, M. Synchrotron X-ray diffraction investigation of the surface condition of artefacts from king Henry VIII's warship the mary rose. J. Synchrotron Radiat. 2020, 27, 653–663. [Google Scholar] [CrossRef]
- Armetta, F.; Saladino, M.L.; Scherillo, A.; Caponetti, E. Microstructure and phase composition of Bronze Montefortino helmets discovered Mediterranean seabed to explain an unusual corrosion. Sci. Rep. 2021, 11, 23022. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armetta, F.; Ponterio, R.C.; Pibiri, I.; Saladino, M.L. New Insight on Archaeological Metal Finds, Nails and Lead Sheathings of the Punic Ship from Battle of the Egadi Islands. Molecules 2023, 28, 1968. https://doi.org/10.3390/molecules28041968
Armetta F, Ponterio RC, Pibiri I, Saladino ML. New Insight on Archaeological Metal Finds, Nails and Lead Sheathings of the Punic Ship from Battle of the Egadi Islands. Molecules. 2023; 28(4):1968. https://doi.org/10.3390/molecules28041968
Chicago/Turabian StyleArmetta, Francesco, Rosina Celeste Ponterio, Ivana Pibiri, and Maria Luisa Saladino. 2023. "New Insight on Archaeological Metal Finds, Nails and Lead Sheathings of the Punic Ship from Battle of the Egadi Islands" Molecules 28, no. 4: 1968. https://doi.org/10.3390/molecules28041968
APA StyleArmetta, F., Ponterio, R. C., Pibiri, I., & Saladino, M. L. (2023). New Insight on Archaeological Metal Finds, Nails and Lead Sheathings of the Punic Ship from Battle of the Egadi Islands. Molecules, 28(4), 1968. https://doi.org/10.3390/molecules28041968








