A Promising 1,3,5-Triazine-Based Anion Exchanger for Perrhenate Binding: Crystal Structures of Its Chloride, Nitrate and Perrhenate Salts
Abstract
1. Introduction
2. Results and Discussions
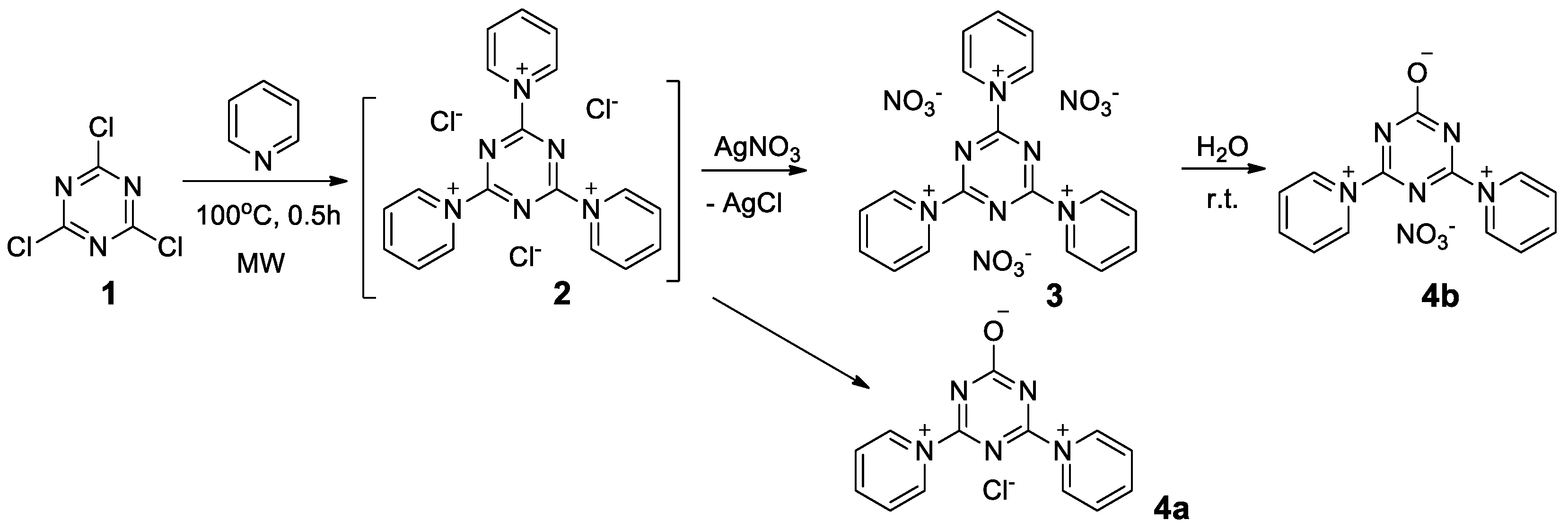
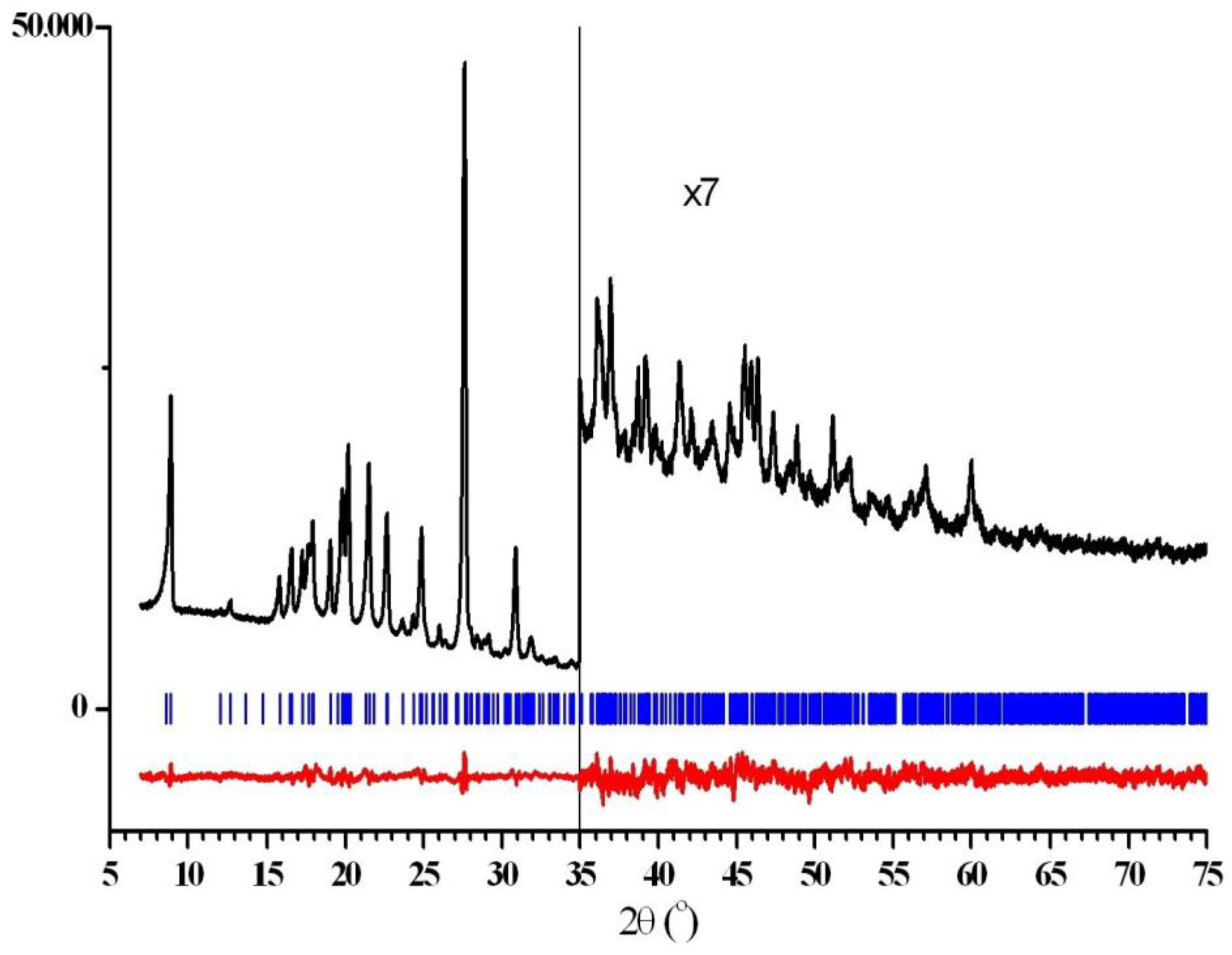
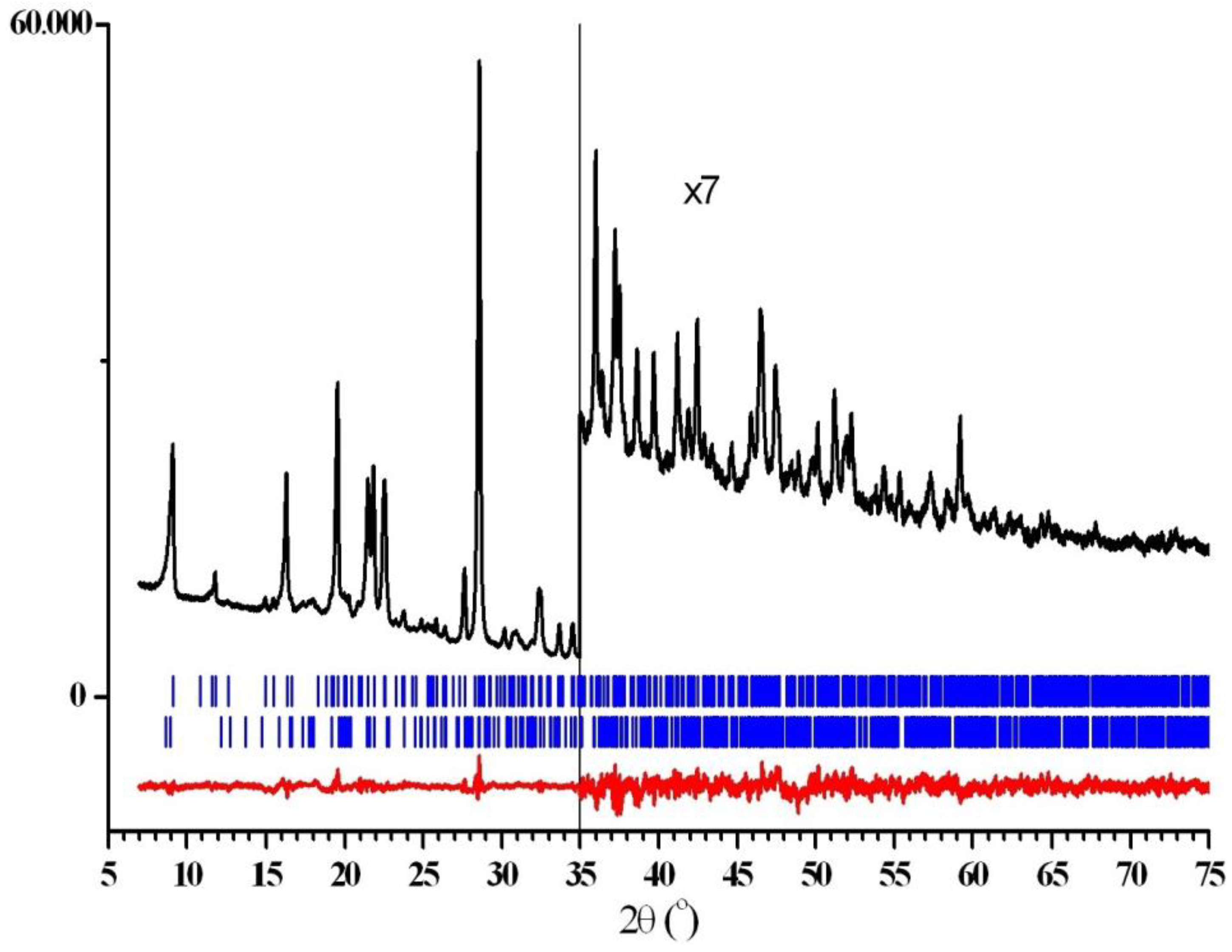
2.1. Synthesis and X-ray Phase Analysis
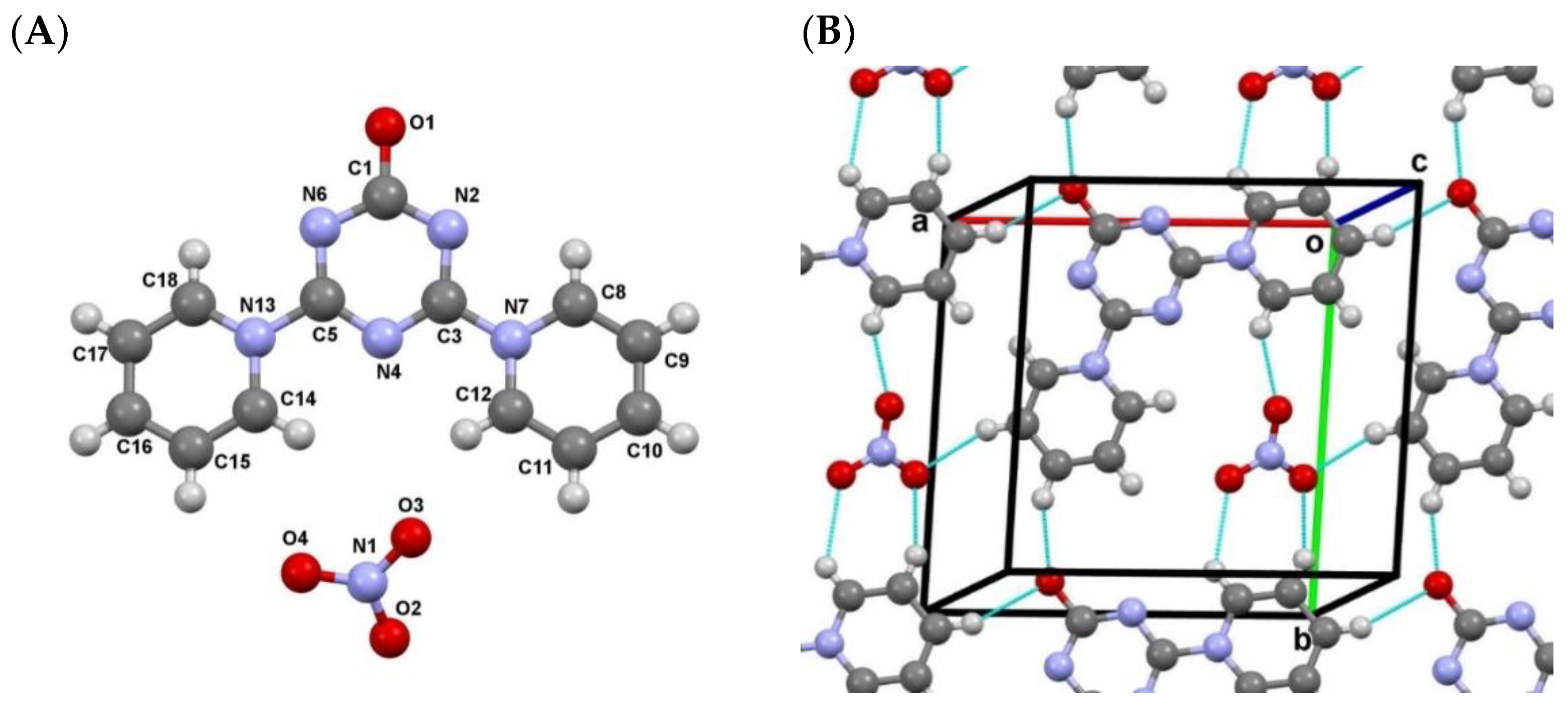
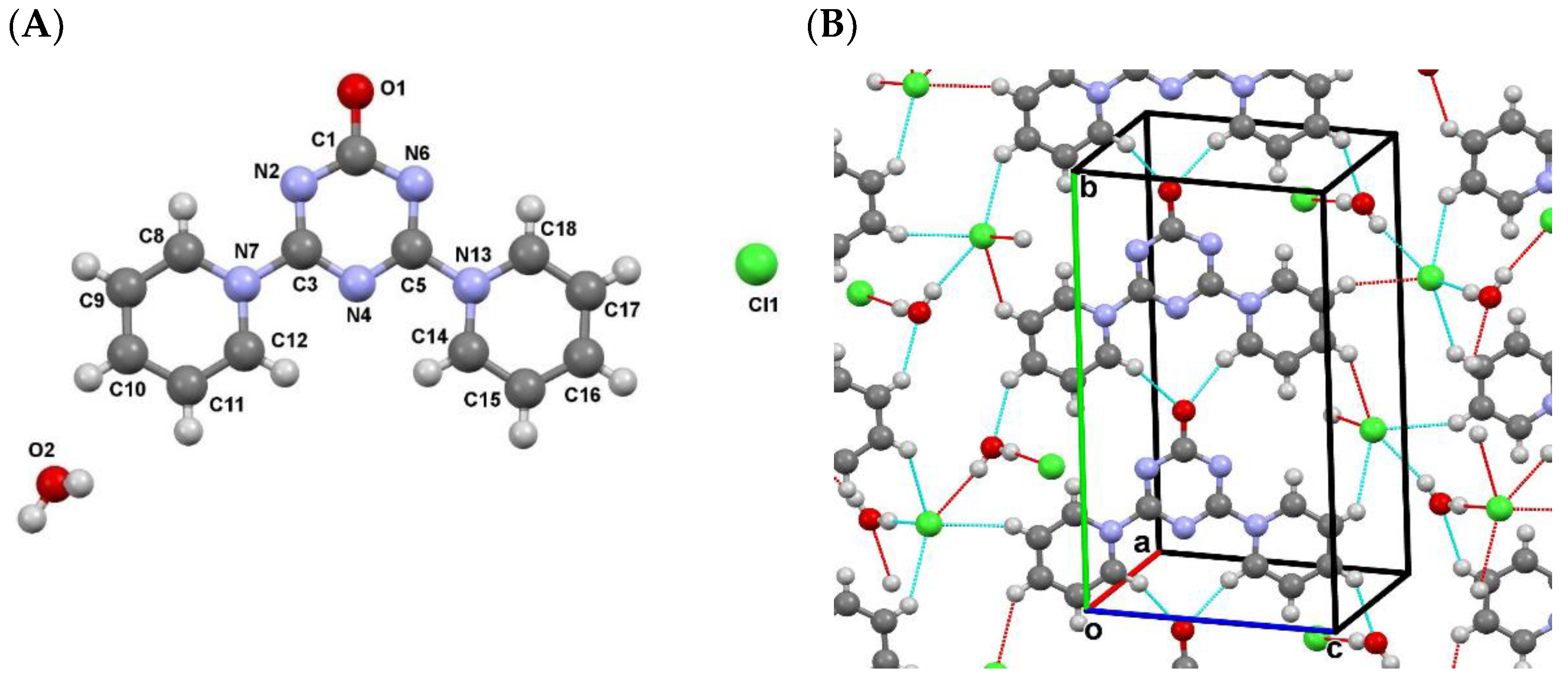
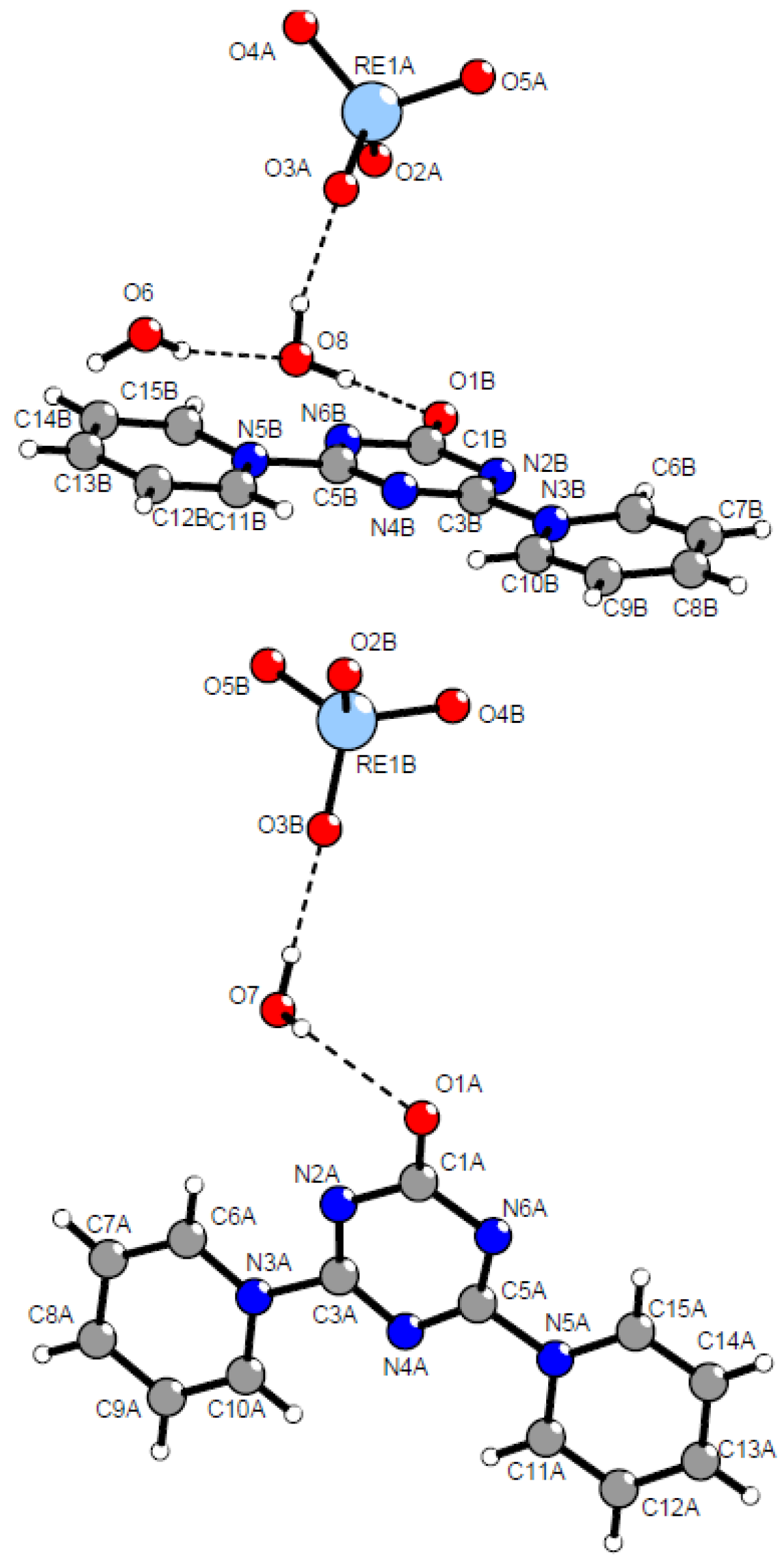
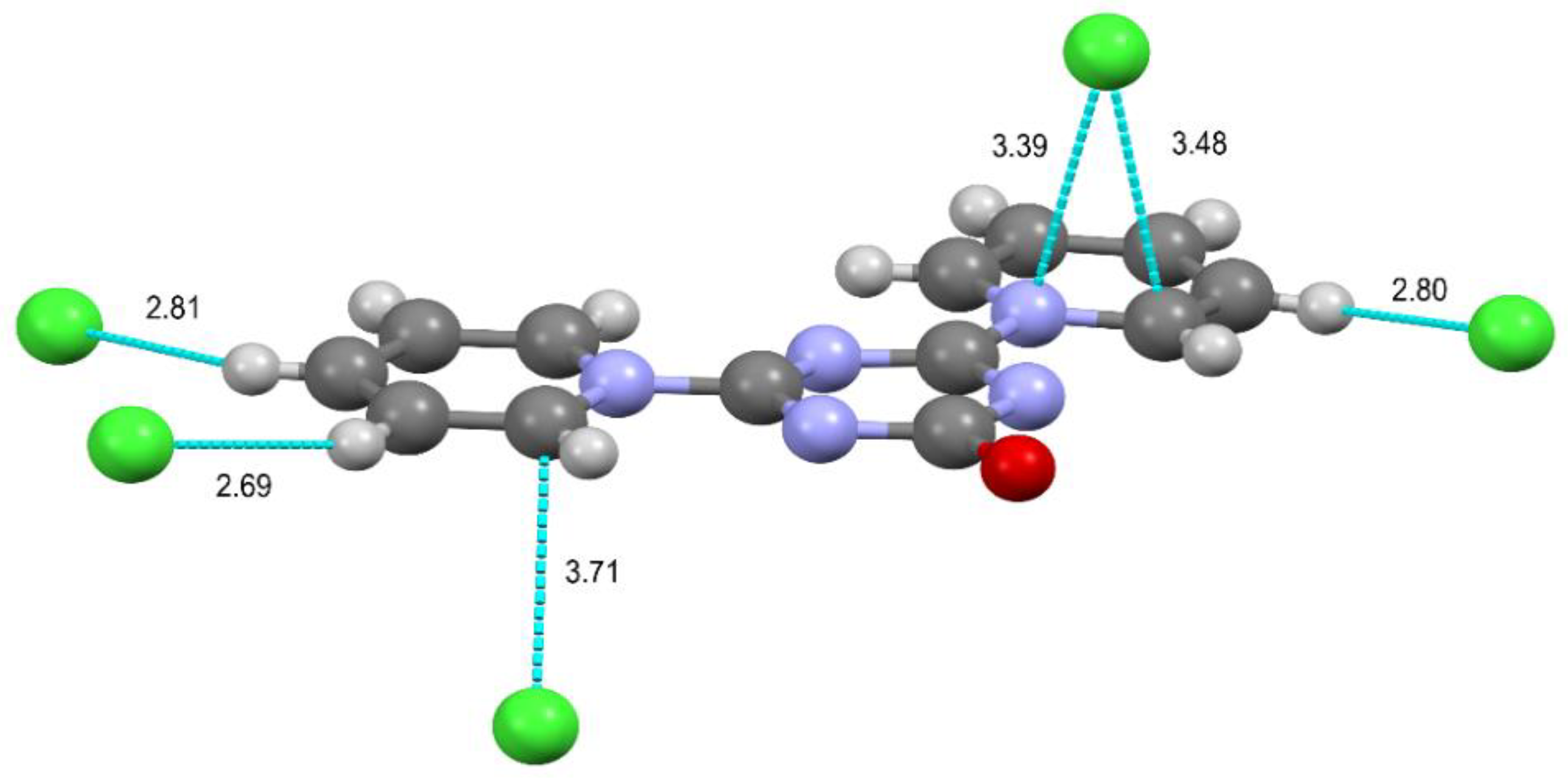
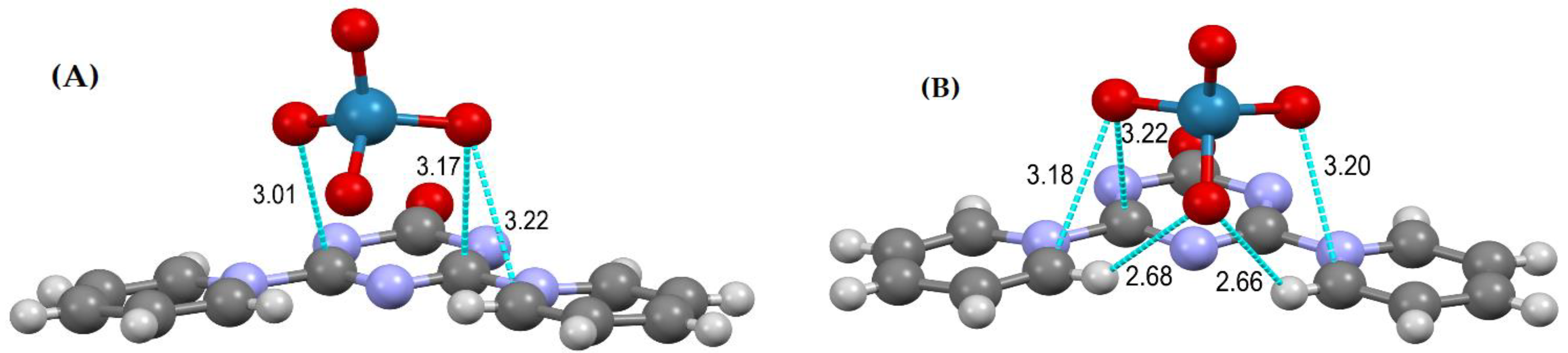
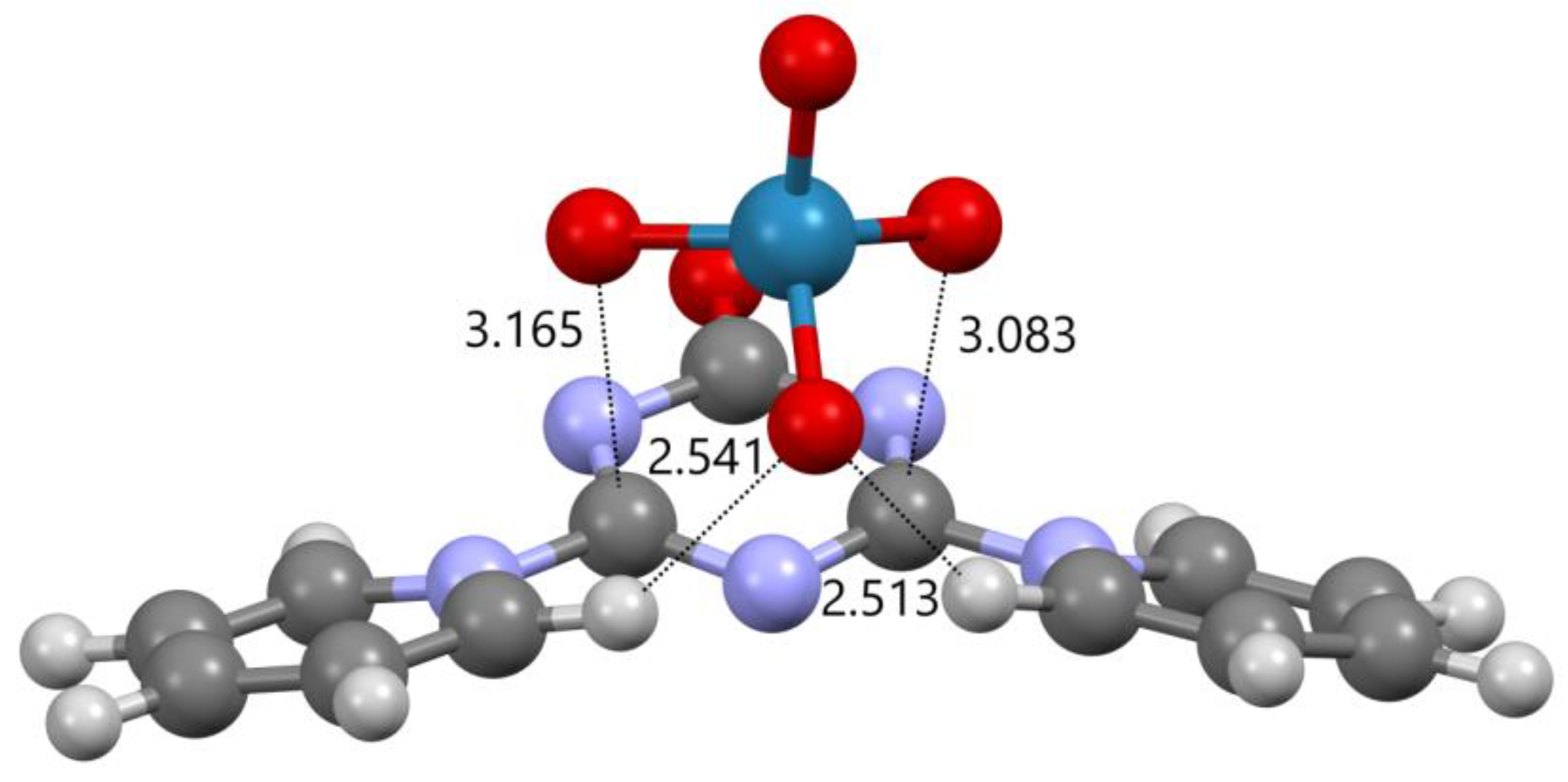
2.2. Mutual Arrangement of Cations and Anions in the Structures 4a, 4b and 4c
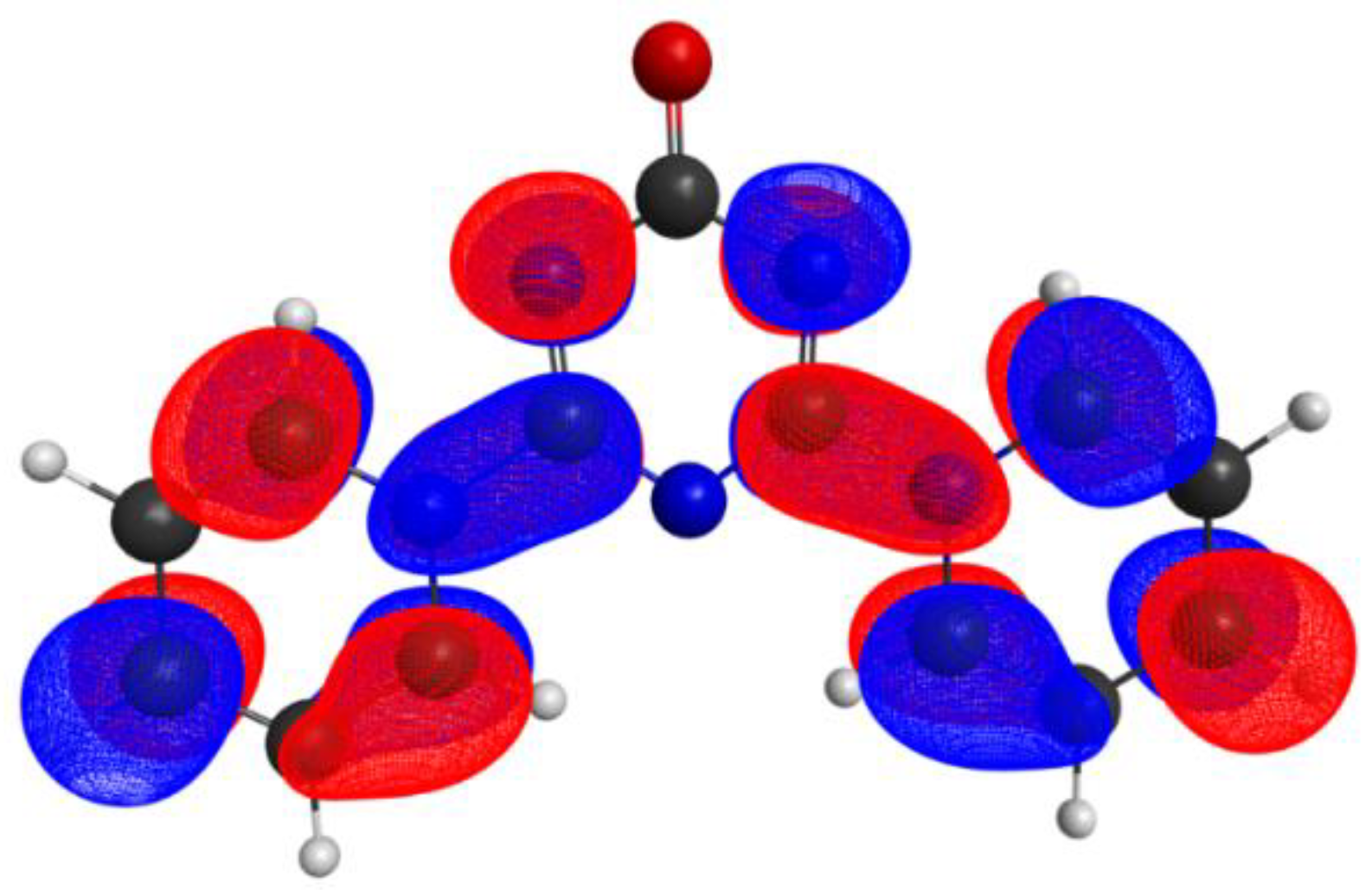
2.3. DFT Calculations
3. Materials and Methods
3.1. Materials
3.2. Synthesis Conditions
3.3. X-ray Analysis
3.3.1. Powder Diffraction
3.3.2. Single-Crystal Diffraction
3.3.3. IR, NMR and Mass Spectra
3.4. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Popova, N.N.; Tananaev, I.G.; Rovnyi, S.I.; Myasoedov, B.F. Technetium: Behaviour during reprocessing of spent nuclear fuel and in environmental objects. Russ. Chem. Rev. 2003, 72, 101–121. [Google Scholar] [CrossRef]
- Meena, A.H.; Arai, Y. Environmental geochemistry of technetium. Environ. Chem. Lett. 2017, 15, 241–263. [Google Scholar] [CrossRef]
- Croff, A.G. Identification and Summary Characterization of Materials Potentially Requiring Vitrifiction: Background Information; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1996.
- Banerjee, D.; Kim, D.; Schweiger, M.J.; Kruger, A.A.; Thallapally, P.K. Removal of TcO4− ions from solution: Materials and future outlook. Chem. Soc. Rev. 2016, 45, 2724–2739. [Google Scholar] [CrossRef]
- Pearce, C.I.; Moore, R.C.; Morad, J.W.; Asmussen, R.M.; Chatterjee, S.; Lawter, A.R.; Levitskaia, T.G.; Neeway, J.J.; Qafoku, N.P.; Rigali, M.J.; et al. Technetium immobilization by materials through sorption and redox-driven processes: A literature review. Sci. Total Environ. 2020, 716, 132849. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Macreadie, L.K.; Gale, P.A. Anion binding in metal-organic framework. Coord. Chem. Rev. 2021, 432, 213708. [Google Scholar] [CrossRef]
- Jin, K.; Lee, B.; Park, J. Metal-organic frameworks as a versatile platform for radionuclide management. Coord. Chem. Rev. 2021, 427, 213473. [Google Scholar] [CrossRef]
- Oseghe, E.O.; Idris, A.O.; Feleni, U.; Mamba, B.B.; Msagati, T.A.M. A review on water treatment technologies for the management of oxoanions: Prospects and challenges. Environ. Sci. Pollut. Res. 2021, 28, 61979–61997. [Google Scholar] [CrossRef]
- Gendy, E.A.; Oyekunle, D.T.; Ali, J.; Ifthikar, J.; Ramadan, A.M.M.; Chen, Z. High-performance removal of radionuclides by porous organic frameworks from the aquatic environment: A review. J. Environ. Radioactivity 2021, 238–239. [Google Scholar] [CrossRef]
- Patra, K.; Ansari, S.A.; Mohapatra, P.K. Metal-organic frameworks as superior porous adsorbents for radionuclide sequestration: Current status and perspectives. J. Chromatogr. A 2021, 1655, 462491. [Google Scholar] [CrossRef]
- Okubo, T.; Aoki, F. Solubilities of tetraphenylarsonium perrhenate and tetraphenylphosphonium perrhenate in water. Nippon Kagaku Zasshi 1966, 87, 1103–1104. [Google Scholar] [CrossRef]
- Refosco, F.; Cavoli-Belluco, P.; Pasquetto, A.; Roncari, E.; Mazzi, U. Recovery of technetium99 from laboratory wastes. Inorg. Chim. Acta 1982, 64, L205. [Google Scholar] [CrossRef]
- Chadwick, T.C. 1,2,4,6-Tetraphenylpyridinium acetate as a new anion precipitant. Anal. Chem. 1976, 48, 1201–1202. [Google Scholar] [CrossRef]
- Mausolf, E.; Droessler, J.; Poineau, F.; Hartmann, T.; Czerwinski, K. Tetraphenylpyridinium pertechnetate: A promising salt for the immobilization of technetium. Radiochim. Acta 2012, 100, 325–328. [Google Scholar] [CrossRef]
- Xie, R.; Shen, N.; Chen, X.; Li, J.; Wang, Y.; Zhang, C.; Xiao, C.; Chai, Z.; Wang, S. 99TcO4−Separation through Selective Crystallization Assisted by Polydentate Benzene-Aminoguanidinium Ligands. Inorg. Chem. 2021, 60, 6463–6471. [Google Scholar] [CrossRef] [PubMed]
- Artemjev, A.A.; Novikov, A.P.; Burkin, G.M.; Sapronov, A.A.; Kubasov, A.S.; Nenajdenko, V.G.; Khrustalev, V.N.; Borisov, A.V.; Kirichuk, A.A.; Kritchenkov, A.S.; et al. Towards Anion Recognition and Precipitation with Water-Soluble 1,2,4-Selenodiazolium Salts: Combined Structural and Theoretical Study. Int. J. Mol. Sci. 2022, 23, 6372. [Google Scholar] [CrossRef]
- Ghosh, P.; Ghosh, R.; Ghosh, T.K. Selective and efficient removal of perrhenate by an imidazolium based hexapodal receptor in water medium. Dalton Trans. 2020, 49, 3093–3097. [Google Scholar] [CrossRef]
- Sharma, A.; Sheyi, R.; De Torre, B.G.; El-Faham, A.; Albericio, F. s-Triazine: A Privileged Structure for Drug Discovery and Bioconjugation. Molecules 2021, 26, 864. [Google Scholar] [CrossRef] [PubMed]
- Bernat, Z.; Szymanowska, A.; Kciuk, M.; Kotwica-Mojzych, K.; Mojzych, M. Review of the Synthesis and Anticancer Properties of Pyrazolo[4,3-e] [1,2,4] triazine Derivatives. Molecules 2020, 25, 3948. [Google Scholar] [CrossRef]
- Sreeperumbuduru, R.S.; Abid, Z.M.; Claunch, K.M.; Chen, H.H.; McGillivray, S.M.; Simanek, E.E. Synthesis and antimicrobial activity of triazine dendrimers with DABCO groups. RSC Adv. 2016, 6, 8806–8810. [Google Scholar] [CrossRef]
- Bhat, H.R.; Singh, U.P.; Gahtori, P.; Ghosh, S.K.; Gogoi, K.; Prakash, A.; Singh, R.K. 4-Aminoquinoline-1,3,5-triazine: Design, synthesis, in vitroantimalarial activity and docking studies. New J. Chem. 2013, 37, 2654–2662. [Google Scholar] [CrossRef]
- Bhat, H.R.; Masih, A.; Shakya, A.; Ghosh, S.K.; Singh, U.P. Design, synthesis, anticancer, antibacterial, and antifungal evaluation of 4-aminoquinoline-1,3,5-triazine derivatives. J. Heterocycl. Chem. 2020, 57, 390–399. [Google Scholar] [CrossRef]
- Mogilski, S.; Kubacka, M.; Łażewska, D.; Więcek, M.; Głuch-Lutwin, M.; Tyszka-Czochara, M.; Bukowska-Strakova, K.; Filipek, B.; Kieć-Kononowicz, K. Aryl-1,3,5-triazine ligands of histamine H4 receptor attenuate inflammatory and nociceptive response to carrageen, zymosan and lipopolysaccharide. Inflamm Res. 2017, 66, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.; Narayanan, K.; Hari Prasad, K.H.; Karthikeyan, D.; Chandrasekaran, L.; Atchudan, R.; Chidambaranathan, V. Synthesis, characterization, and antiproliferative and apoptosis inducing effects of novel s-triazine derivatives. New J. Chem. 2018, 42, 1698–1714. [Google Scholar] [CrossRef]
- Kothayer, H.; Elshanawani, A.A.; Abu Kull, M.E.; El-Sabbagh, O.I.; Shekhar, M.P.V.; Brancale, A.; Jones, A.T.; Westwell, A.D. Design, synthesis and in vitro anticancer evaluation of 4,6-diamino-1,3,5-triazine-2-carbohydrazides and -carboxamides. Bioorg. Med. Chem. Lett. 2013, 23, 6886–6889. [Google Scholar] [CrossRef] [PubMed]
- Zvarych, V.; Stasevych, M.; Novikov, V.; Rusanov, E.; Vovk, M.; Szweda, P.; Grecka, K.; Milewski, S. Anthra[1,2-d][1,2,3]triazine-4,7,12(3H)-triones as a New Class of Antistaphylococcal Agents: Synthesis and Biological Evaluation. Molecules 2019, 24, 4581. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, V.N.; Kudryavtsev, I.K.; Dunaev, S.F.; Paseshnichenko, K.A.; Aslanov, L.A. Triazine 2D Nanosheets as a New Class of Nanomaterials: Crystallinity, Properties and Applications. Colloids Interfaces 2022, 6, 20. [Google Scholar] [CrossRef]
- Saure, S. The reaction of cyanuric chloride with pyridine. (The alkaline hydrolysis of cyanuric chloride). ChemischeBerichte 1950, 83, 335–340. [Google Scholar] [CrossRef]
- Kangani, C.O.; Day, B.W. Mild, Efficient Friedel-Crafts Acylations from Carboxylic Acids Using Cyanuric Chloride and AlCl3. Org. Lett. 2008, 10, 2645–2648. [Google Scholar] [CrossRef]
- Murakami, M.; Hajima, M.; Takami, F.; Yoshioka, M. 2,4,6-Tripyridinio-1,3,5-twine trichloride. A new and mild esterification agent for preparation of penicillin esters. Heterocycles 1990, 31, 2055–2064. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Dallas, P.; Sanakis, Y.; Stamopoulos, D.; Trapalis, C.; Niarchos, D. Synthesis and characterization of a π -conjugate, covalent layered network derived from condensation polymerization of the 4,4’-bipyridine-cyanuric chloride couple. Eur. Polym. J. 2006, 42, 2940–2948. [Google Scholar] [CrossRef]
- Dickson, J.O.; Harsh, J.B.; Lukens, W.W.; Pierce, E.M. Perrhenate incorporation into binary mixed sodalites: The role of anion size and implications for technetium-99 sequestration. Chem. Geol. 2015, 395, 138–143. [Google Scholar] [CrossRef]
- Zuckier, L.S.; Dohan, O.; Li, Y.; Chee, J.C.; Carrasco, N.; Dadachova, E. Kinetics of perrhenate uptake and comparative biodistribution of perrhenate, pertechnetate, and iodide by NaI symporter-expressing tissues in vivo. J. Nucl. Med. 2004, 45, 500–507. [Google Scholar] [PubMed]
- Kang, M.J.; Rhee, S.W.; Moon, H.; Neck, V.; Fanghaenel, T. Sorption of MO4-(M = Tc, Re) on Mg/Al layered double hydroxide by anion exchange. Radiochim. Acta 1996, 75, 169–174. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Zhukov, S.G.; Chernyshev, V.V.; Babaev, E.V.; Sonneveld, E.J.; Schenk, H. Application of simulated annealing approach for structure solution of molecular crystals from X-ray laboratory powder data. Z. Kristallogr. 2001, 216, 5–9. [Google Scholar] [CrossRef]
- Zlokazov, V.B.; Chernyshev, V.V. MRIA—A program for a full profile analysis of powder multiphase neutron-diffraction time-of-flight (direct and Fourier) spectra. J. Appl. Crystallogr. 1992, 25, 447–451. [Google Scholar] [CrossRef]
- Isbjakowa, A.S.; Chernyshev, V.V.; Tafeenko, V.A.; Aslanov, L.A. Crystal structures of rare earth cyamelurates obtained under kinetic and thermodynamic controls. Struct. Chem. 2022, 33, 607–615. [Google Scholar] [CrossRef]
- Isbjakowa, A.S.; Chernyshev, V.V.; Tafeenko, V.A.; Aslanov, L.A. Metal cyamelurates: Structural diversity caused by kinetic and thermodynamic controls. Struct. Chem. 2021, 32, 1745–1754. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Brandenburg, K. DIAMOND, Release 2.1d; Crystal Impact GbR: Bonn, Germany, 2000. [Google Scholar]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharov, V.N.; Lemport, P.S.; Chernyshev, V.V.; Tafeenko, V.A.; Yatsenko, A.V.; Ustynyuk, Y.A.; Dunaev, S.F.; Nenajdenko, V.G.; Aslanov, L.A. A Promising 1,3,5-Triazine-Based Anion Exchanger for Perrhenate Binding: Crystal Structures of Its Chloride, Nitrate and Perrhenate Salts. Molecules 2023, 28, 1941. https://doi.org/10.3390/molecules28041941
Zakharov VN, Lemport PS, Chernyshev VV, Tafeenko VA, Yatsenko AV, Ustynyuk YA, Dunaev SF, Nenajdenko VG, Aslanov LA. A Promising 1,3,5-Triazine-Based Anion Exchanger for Perrhenate Binding: Crystal Structures of Its Chloride, Nitrate and Perrhenate Salts. Molecules. 2023; 28(4):1941. https://doi.org/10.3390/molecules28041941
Chicago/Turabian StyleZakharov, Valery N., Pavel S. Lemport, Vladimir V. Chernyshev, Victor A. Tafeenko, Alexandr V. Yatsenko, Yuri A. Ustynyuk, Sergey F. Dunaev, Valentine G. Nenajdenko, and Leonid A. Aslanov. 2023. "A Promising 1,3,5-Triazine-Based Anion Exchanger for Perrhenate Binding: Crystal Structures of Its Chloride, Nitrate and Perrhenate Salts" Molecules 28, no. 4: 1941. https://doi.org/10.3390/molecules28041941
APA StyleZakharov, V. N., Lemport, P. S., Chernyshev, V. V., Tafeenko, V. A., Yatsenko, A. V., Ustynyuk, Y. A., Dunaev, S. F., Nenajdenko, V. G., & Aslanov, L. A. (2023). A Promising 1,3,5-Triazine-Based Anion Exchanger for Perrhenate Binding: Crystal Structures of Its Chloride, Nitrate and Perrhenate Salts. Molecules, 28(4), 1941. https://doi.org/10.3390/molecules28041941








