Radioactive Wastewater Treatment Technologies: A Review
Abstract
1. Introduction
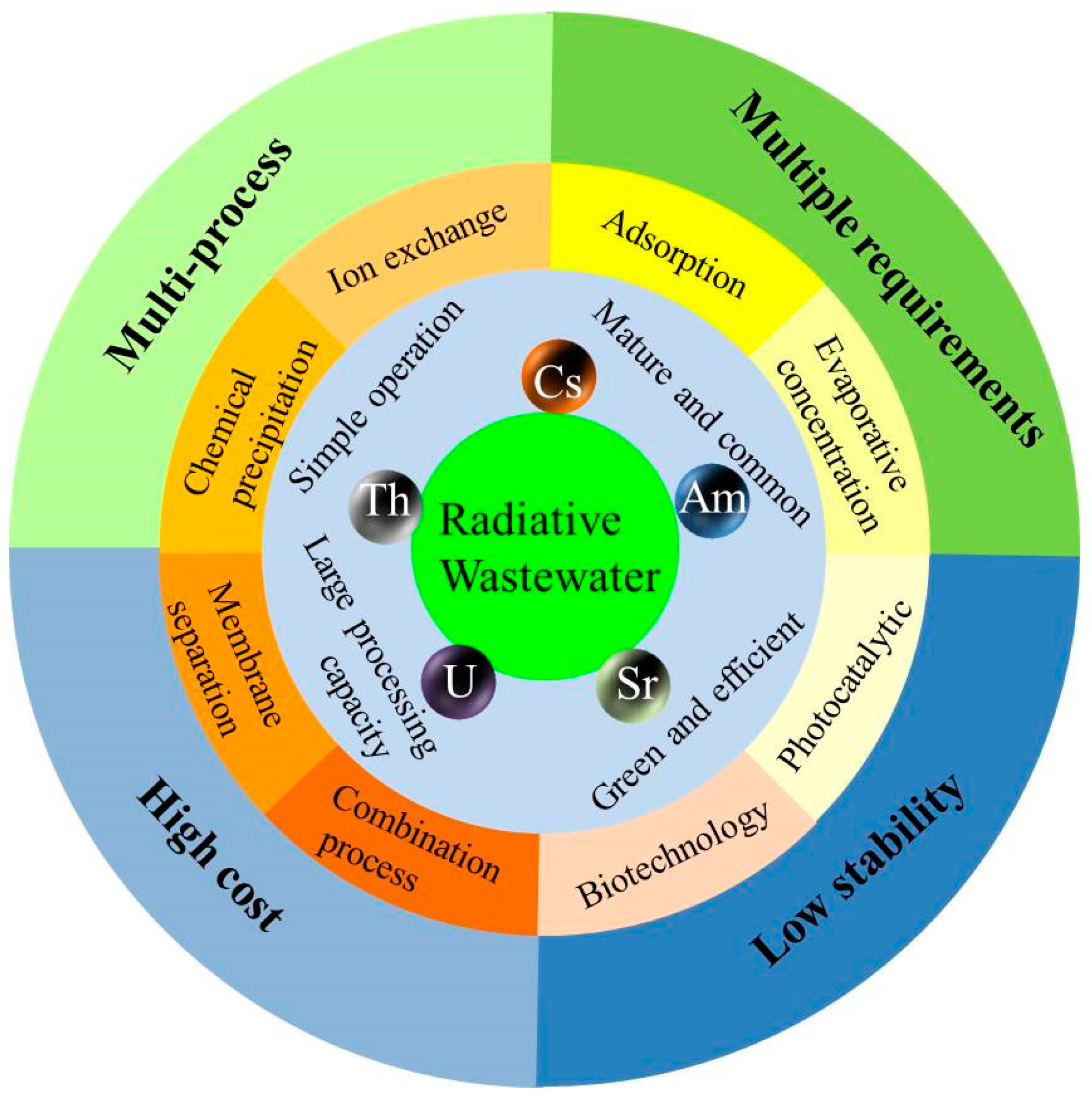
2. Properties and Challenges of Radioactive Wastewater
3. Treatment Technologies for Radioactive Wastewater
3.1. Ion Exchange
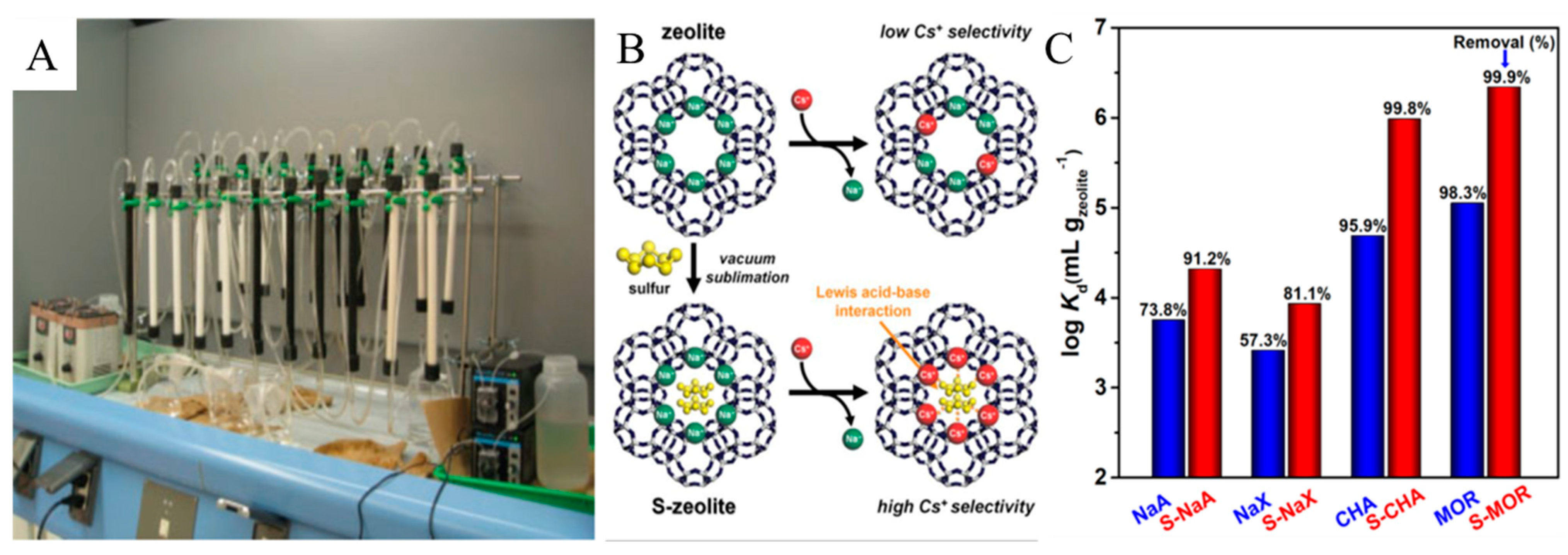
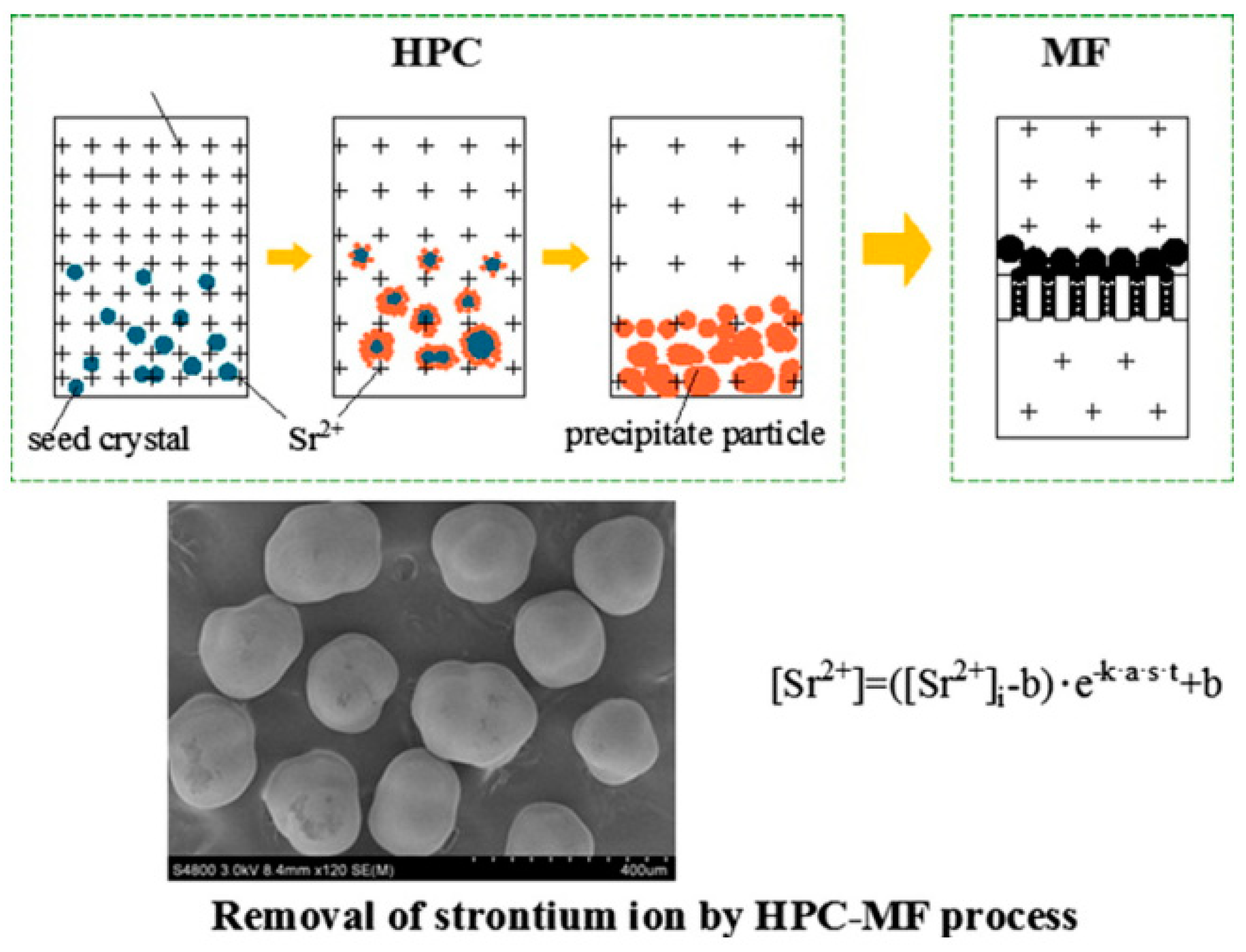
3.2. Chemical Precipitation
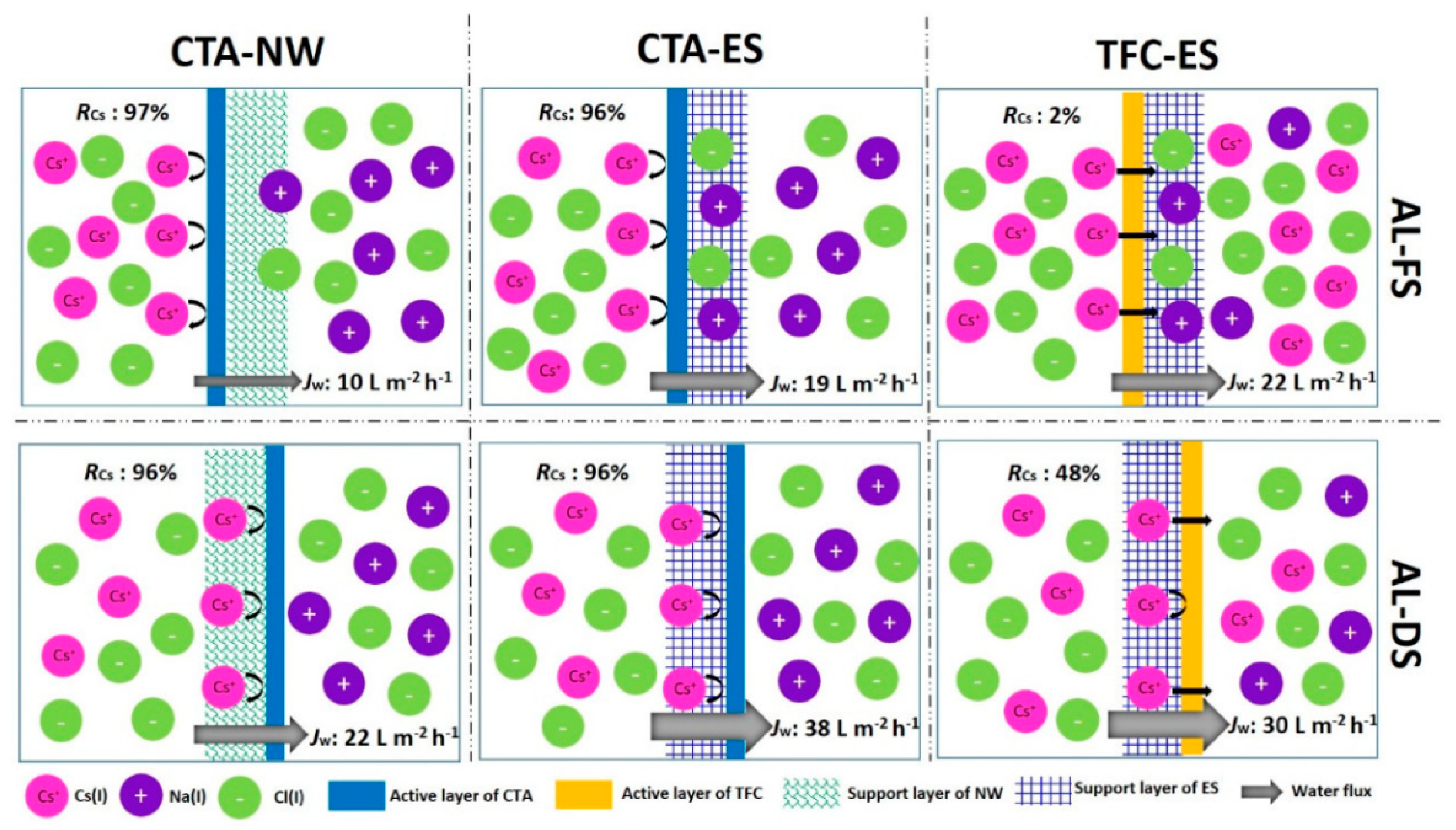
3.3. Membrane Separation
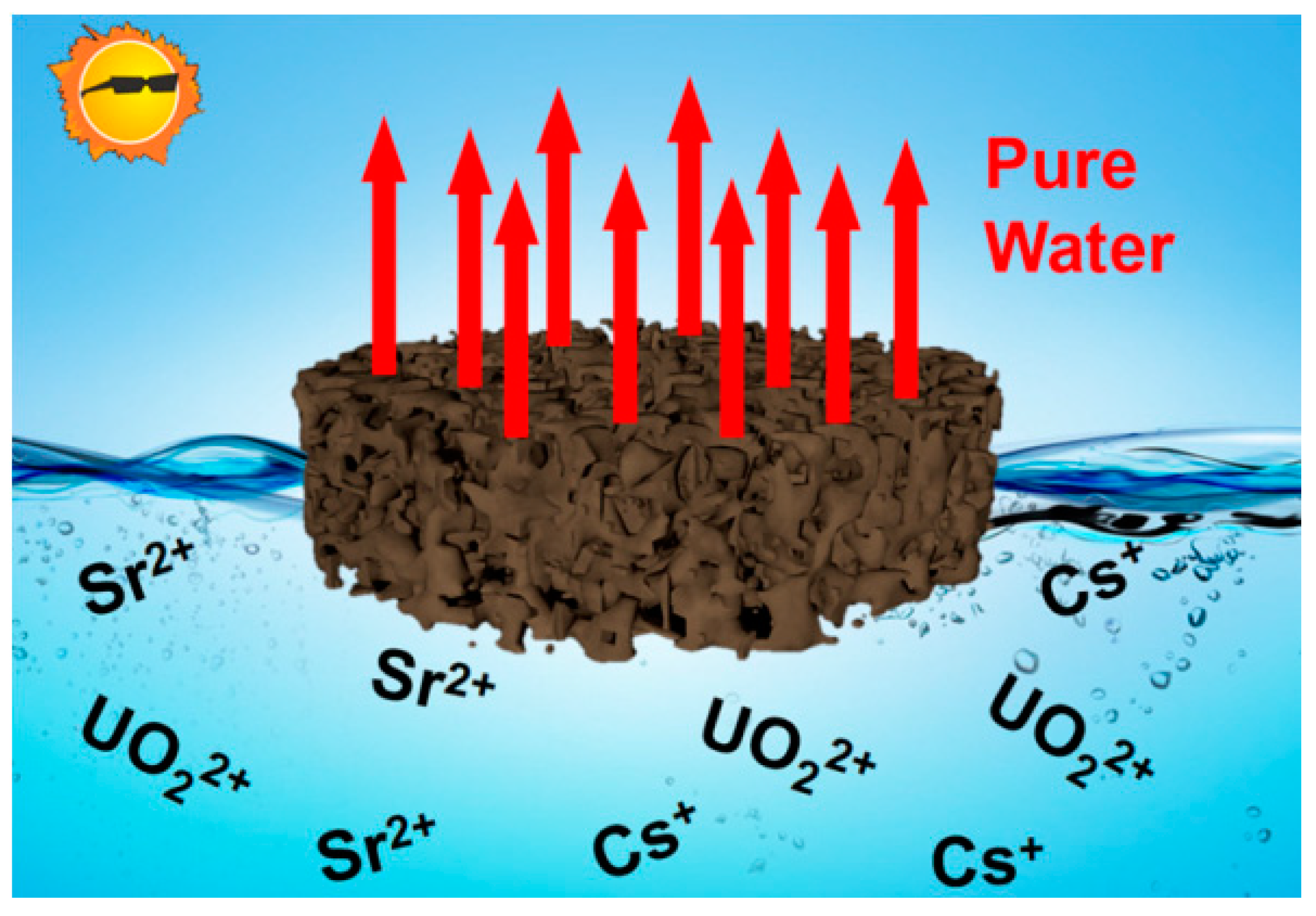
3.4. Evaporative Concentration
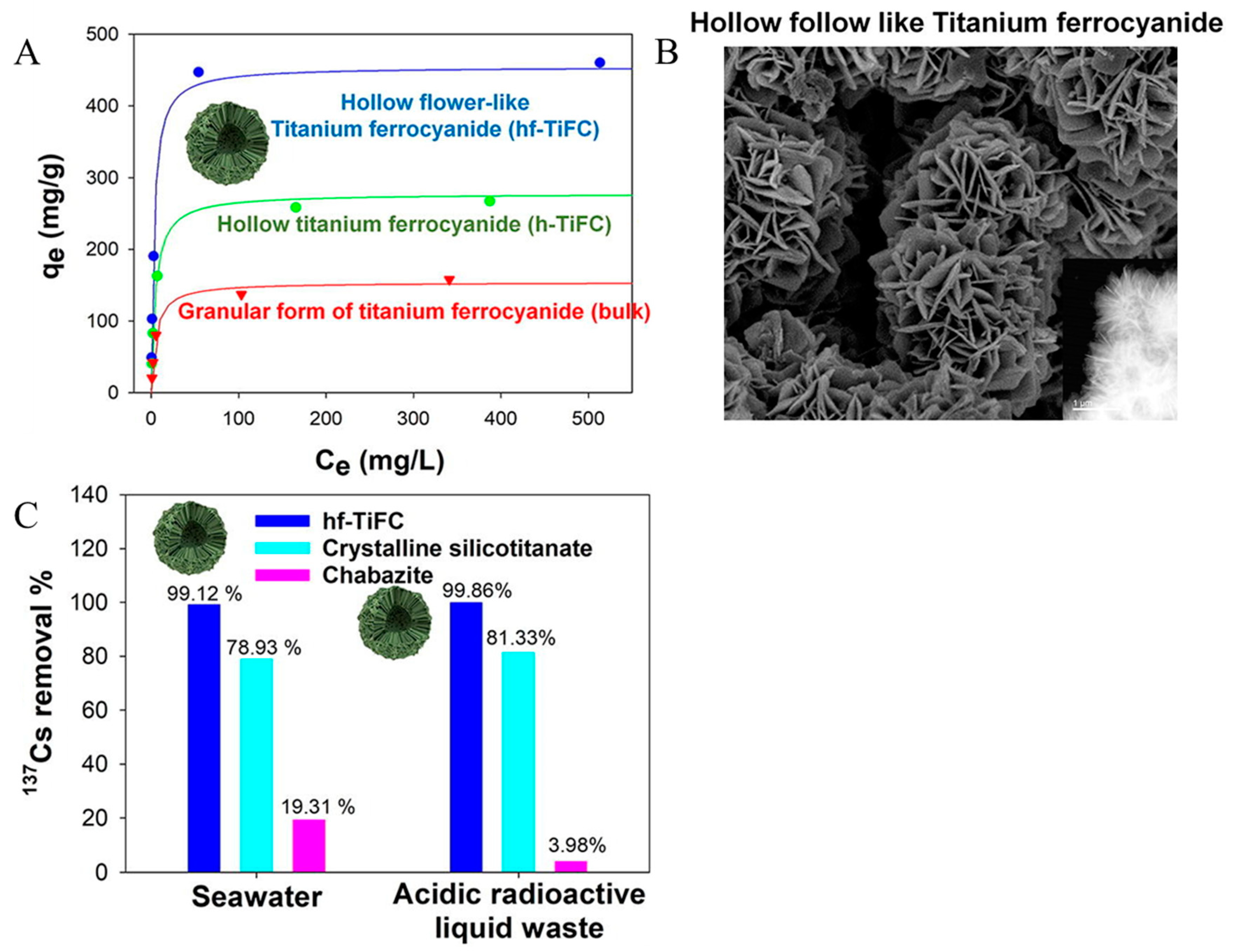
3.5. Adsorption
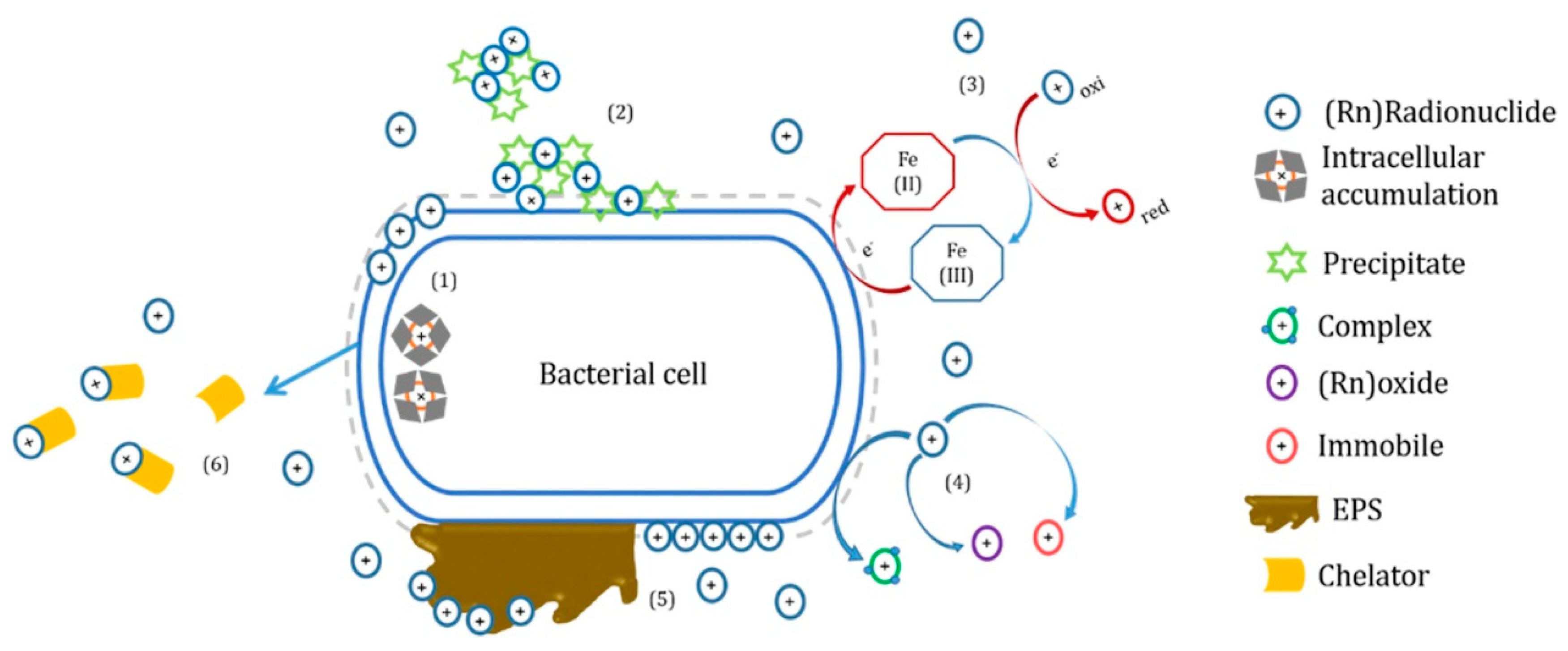
3.6. Biotechnology
3.7. Photocatalysis

4. Comparison of Different Radioactive Waste Treatment Technologies
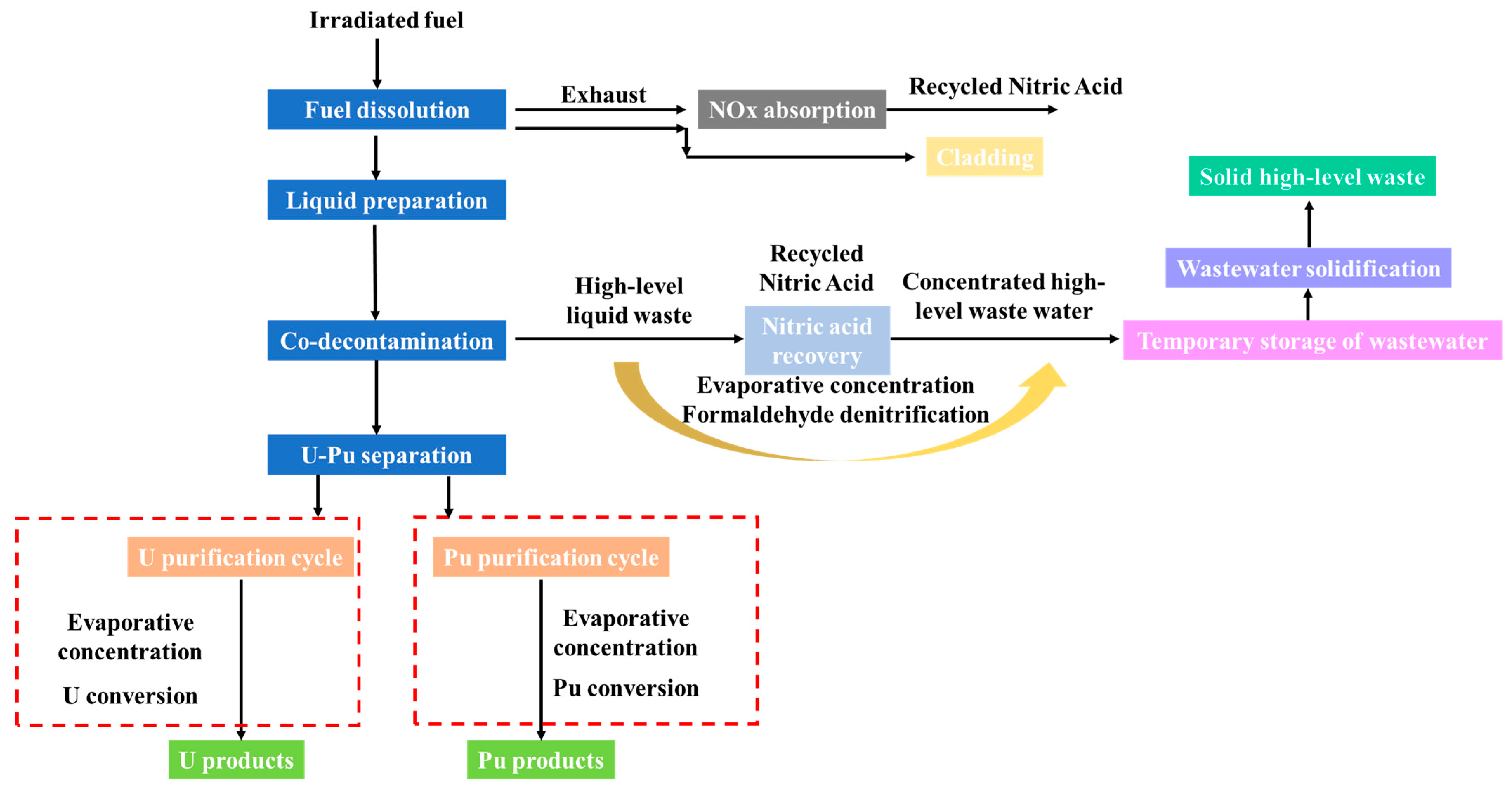
5. Integrated Treatment of Radioactive Wastewater
6. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menyah, K.; Wolde-Rufael, Y. CO2 emissions, nuclear energy, renewable energy and economic growth in the US. Energy Policy 2010, 38, 2911–2915. [Google Scholar] [CrossRef]
- Zohuri, B. Nuclear Energy Research and Development Roadmap. In Small Modular Reactors as Renewable Energy Sources; Springer: Berlin/Heidelberg, Germany, 2019; pp. 95–116. [Google Scholar]
- Shirizadeh, B.; Quirion, P. Low-carbon options for the French power sector: What role for renewables, nuclear energy and carbon capture and storage? Energy Econ. 2021, 95, 105004. [Google Scholar] [CrossRef]
- Ariew, S. Current Issues in Nuclear Energy: Radioactive Waste. Nuclear Sci. Eng. 2002. [Google Scholar]
- Johnson, J. Radioactive waste stranded as US shifts from nuclear energy. Chem. Eng. News 2018, 96, 28–29. [Google Scholar]
- Shannon, K.A.; Grimm, T.L.; Grimm, A.K.; Johnson, N.C.; Odeh, F.Y.; Starovoitova, V.N. Small Scale Recycling of Irradiated Nuclear Fuel for Isotope Production and Nuclear Energy R&D. Trans. Am. Nucl. Soc. 2018, 118, 131–132. [Google Scholar]
- International Atomic Energy Agency (IAEA). Chernobyl’s Legacy: Health, Environmental and Socio-Economic Impacts and Recommendations to the Governments of Belarus, the Russian Federation and Ukraine; International Atomic Energy Agency: Vienna, Austria, 2006; Volume 2, p. 55. [Google Scholar]
- Lu, W. Current Status and Analysis of World Spent Fuel Reprocessing; China Jiangxi Chemical Industry: Nanchang, China, 2018; Volume 140, pp. 16–18. [Google Scholar]
- Zhang, Q. Suggestions on Accelerating the Development of Spent Fuel Reprocessing in Nuclear Power Plants; China Energy: Beijing, China, 2019; Volume 41, pp. 44–47. [Google Scholar]
- Liao, Y.; Yun, H.; Wang, C. Research Status of Spent Fuel Reprocessing Technology; Sichuan Chemical Industry: Chengdu, China, 2012; pp. 12–15. [Google Scholar]
- Sood, D.D.; Patil, S.K. Chemistry of nuclear fuel reprocessing: Current status. J. Radioanal. Nucl. Chem. 1996, 203, 547–573. [Google Scholar] [CrossRef]
- Krishnaswami, S.; Graustein, W.C.; Turekian, K.K.; Dowd, J.F. Radium, Thorium and Radioactive Lead Isotopes in Groundwaters: Application to the in situ Determination of Adsorption-Desorption Rate Constants and Retardation Factors. Water Resour. Res. 1982, 18, 1663–1675. [Google Scholar] [CrossRef]
- Nishi, M.T. Activated carbon filter treatment of laundry waste water in nuclear power plants and filter recovery by heating in vacuum. Carbon 2000, 38, 709–714. [Google Scholar]
- Singh, D.; Hareendran, K.; Sreenivas, T.; Kain, V.; Dey, G. Development of a phosphate precipitation method for the recovery of uranium from lean tenor alkaline leach liquor. Hydrometallurgy 2017, 171, 228–235. [Google Scholar] [CrossRef]
- Li, P.; Zhun, B.; Wang, X.; Liao, P.; Wang, G.; Wang, L.; Guo, Y.; Zhang, W. Highly Efficient Interception and Precipitation of Uranium(VI) from Aqueous Solution by Iron-Electrocoagulation Combined with Cooperative Chelation by Organic Ligands. Environ. Sci. Technol. 2017, 51, 14368–14378. [Google Scholar] [CrossRef]
- Fülöp, J. Process for Separating Heavy Metals from Waste Water by Sulfide Precipitation Using Calcium Polysulfide. Patent EP0349671 A1, 31 January 1996. [Google Scholar]
- Zhu, K.; Chen, C.; Wang, H.; Xie, Y.; Wakeel, M.; Wahid, A.; Zhang, X. Gamma-ferric oxide nanoparticles decoration onto porous layered double oxide belts for efficient removal of uranyl. J. Colloid Interface Sci. 2019, 535, 265–275. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Q.; Yu, S.; Chen, Z.; Wang, X. Retraction notice to “High sorption of U(VI) on graphene oxides studied by batch experimental and theoretical calculations” [Chem. Eng. J. 287 (2015) 448–455]. Chem. Eng. J. 2020, 390, 124272. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Pankov, A.; Lee, W.E. The ion exchange phase in corrosion of nuclear waste glasses. J. Nucl. Mater. 2006, 358, 57–68. [Google Scholar] [CrossRef]
- Tripatanasuwan, S.; Zhong, Z.; Reneker, D.H. Effect of evaporation and solidification of the charged jet in electrospinning of poly(ethylene oxide) aqueous solution. Polymer 2007, 48, 5742–5746. [Google Scholar] [CrossRef]
- Shen, J.; Schäfer, A. Removal of fluoride and uranium by nanofiltration and reverse osmosis: A review. Chemosphere 2014, 117, 679–691. [Google Scholar] [CrossRef]
- Stylo, M.; Alessi, D.S.; Shao, P.P.; Lezama-Pacheco, J.S.; Bargar, J.R.; Bernier-Latmani, R. Biogeochemical Controls on the Product of Microbial U(VI) Reduction. Environ. Sci. Technol. 2013, 47, 12351–12358. [Google Scholar] [CrossRef]
- Sylvester, P.; Milner TJensen, J. Radioactive liquid waste treatment at Fukushima Daiichi. J. Chem. Technol. Biotechnol. 2013, 88, 1592–1596. [Google Scholar] [CrossRef]
- Ayres John, A. Treatment of Radioactive Waste by Ion Exchange. Ind. Eng. Chem. 1963, 43, 1526–1531. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mohapatra, P.K.; Pathak, P.N.; Manchanda, V.K. Cation-exchangeseparation of uranium from thorium in nitric acid medium. J. Radioanal. Nucl. Chem. 2006, 268, 323–328. [Google Scholar] [CrossRef]
- Nur, T.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Removal of strontium from aqueous solutions and synthetic seawater using resorcinol formaldehyde polycondensate resin. Desalination 2017, 420, 283–291. [Google Scholar] [CrossRef]
- Figueiredo, B.R.; Cardoso, S.P.; Portugal, I.; Rocha, J.; Silva, C.M. Inorganic Ion Exchangers for Cesium Removal from Radioactive Wastewater. Sep. Purif. Rev. 2017, 47, 306–336. [Google Scholar] [CrossRef]
- Jia, Z.; Cheng, X.; Guo, Y.; Tu, L. In-situ preparation of iron(III) hexacyanoferrate nano-layer on polyacrylonitrile membranes for cesium adsorption from aqueous solutions. Chem. Eng. J. 2017, 325, 513–520. [Google Scholar] [CrossRef]
- Oleksiienko, O.; Wolkersdorfer, C.; Sillanpää, M. Titanosilicates in Cation Adsorption and Cation Exchange—A Review. Chem. Eng. J. 2017, 317, 570–585. [Google Scholar] [CrossRef]
- Cheng, Y.; Chuah, G.K. The synthesis and applications of α-zirconium phosphate. Chin. Chem. Lett. 2020, 31, 307–310. [Google Scholar] [CrossRef]
- He, W.; Ai, K.; Ren, X.; Wang, S.; Lu, L. Inorganic layered ion-exchangers for decontamination of toxic metal ions in aquatic systems. J. Mater. Chem. A 2017, 5, 19593–19606. [Google Scholar] [CrossRef]
- Han, E.; Young-Gu, K.; Yang, H.M.; In-Ho, Y.; Minkee, C. Synergy between Zeolite Framework and Encapsulated Sulfur for Enhanced Ion-Exchange Selectivity to Radioactive Cesium. Chem. Mater. 2018, 30, 5777–5785. [Google Scholar] [CrossRef]
- El-Naggar, M.; El-Kamash, A.; El-Dessouky, M.; Ghonaim, A. Two-step method for preparation of NaA-X zeolite blend from fly ash for removal of cesium ions. J. Hazard. Mater. 2008, 154, 963–972. [Google Scholar] [CrossRef]
- Galamboš, M.; Paučová, V.; Kufčáková, J.; Rosskopfová, O.; Rajec, P.; Adamcová, R. Cesium sorption on bentonites and montmorillonite K10. J. Radioanal. Nucl. Chem. 2010, 284, 55–64. [Google Scholar] [CrossRef]
- Banerjee, D.; Rao, M.A.; Khot, S.A.; Pawaskar, C.S.; Gangadharan, A.; Rao, S.N.; Jain, S.; Shah, J.G.; Banerjee, K. Removal of radiocesium from low level radioactive effluents by hexacyanoferrate loaded synthetic zeolite: Laboratory to pilot plant scale demonstration. Radiochim. Acta 2017, 105, 341–346. [Google Scholar] [CrossRef]
- Humelnicu, D.; Popovici, E.; Dvininov, E.; Mita, C. Study on the retention of uranyl ions on modified clays with titanium oxide. J. Radioanal. Nucl. Chem. 2009, 279, 131–136. [Google Scholar] [CrossRef]
- Yousefi, T.; Torab-Mostaedi, M.; Ali Moosavian, M.; Mobtaker, H.G. Potential application of a nanocomposite:HCNFe@polymer for effective removal of Cs (I) from nuclear waste. Prog. Nucl. Engergy 2015, 85, 631–639. [Google Scholar] [CrossRef]
- Nilchi, A.; Saberi, R.; Garmarodi, S.R.; Bagheri, A. Evaluation of PAN-based manganese dioxide composite for the sorptive removal of cesium-137 from aqueous solutions. Appl. Radiat. Isot. 2012, 70, 369–374. [Google Scholar] [CrossRef]
- Yang, H.-M.; Hwang, K.S.; Park, C.W.; Lee, K.-W. Sodium-copper hexacyanoferrate-functionalized magnetic nanoclusters for the highly efficient magnetic removal of radioactive caesium from seawater. Water Res. 2017, 125, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Genevois, N.; Villandier, N.; Chaleix, V.; Poli, E.; Jauberty, L.; Gloaguen, V. Removal of cesium ion from contaminated water: Improvement of Douglas fir bark biosorption by a combination of nickel hexacyanoferrate impregnation and TEMPO oxidation. Ecol. Eng. 2017, 100, 186–193. [Google Scholar] [CrossRef]
- Pangeni, B.; Paudyal, H.; Inoue, K.; Ohto, K.; Kawakita, H.; Alam, S. Preparation of natural cation exchanger from persimmon waste and its application for the removal of cesium from water. Chem. Eng. J. 2014, 242, 109–116. [Google Scholar] [CrossRef]
- Ikeda-Ohno, A.; Harrison, J.J.; Thiruvoth, S.; Wilsher, K.; Wong, H.K.Y.; Johansen, M.P.; Waite, T.D.; Payne, T.E. Solution Speciation of Plutonium and Americium at an Australian Legacy Radioactive Waste Disposal Site. Environ. Sci. Technol. 2014, 48, 10045–10053. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, G.; Xue, W.; Ping, G. Research on a pellet co-precipitation micro-filtration process for the treatment of liquid waste containing strontium. J. Radioanal. Nucl. Chem. 2013, 298, 931–939. [Google Scholar] [CrossRef]
- Tenson, T.; Syojiro, K. Treatment of Radioactive Liquid Waste in High Salt Concentration by Chemical Precipitation (I) Removal of Radioactive Strontium by Co-precipitation with Barium Sulfate. Jpn. J. Health Phys. 1980, 15, 33–39. [Google Scholar]
- Bobrov, P.A.; Slyunchev, O.M.; Semenova, T.A. Radionuclide removal from radioactively contaminated drainage water and groundwater by precipitation and sorption methods. Radiochemistry 2015, 57, 537–541. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, P.; Jia, L.; Zhang, G. An investigation into the use of cuprous chloride for the removal of radioactive iodide from aqueous solutions. J. Hazard. Mater. 2016, 302, 82–89. [Google Scholar] [CrossRef]
- Rogers, H.; Bowers, J.; Gates-Anderson, D. An isotope dilution–precipitation process for removing radioactive cesium from wastewater. J. Hazard. Mater. 2012, 243, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, G.; Wang, Q.; Gu, P. Removal of strontium from liquid waste using a hydraulic pellet co-precipitation microfiltration (HPC-MF) process. Desalination 2014, 349, 31–38. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J. Study on Radioactive Wastewater Treatment by Precipitation and Membrane Separation. Appl. Mech. Mater. 2014, 490–491, 972–975. [Google Scholar] [CrossRef]
- Ambashta, R.D.; Sillanp, M.E.T. Membrane purification in radioactive waste management: A short review. J. Environ. Radioact. 2012, 105, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Matsuura, T.; Kassim, M.A.; Ismail, A.F. Radioactive decontamination of water by membrane processes—A review. Desalination 2013, 321, 77–92. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, T.; Zhang, D.; Liu, X.J.; Fu, Y.B. Treatment of plutonium-containing wastewater by a combined flocculation-microfiltration process. Nucl. Radiochem. 2007, 29, 113–117. [Google Scholar]
- Zhang, X.; Niu, L.; Li, F.; Zhao, X.; Hu, H. Enhanced rejection of cations by low-level cationic surfactant during ultrafiltration of low-level radioactive wastewater. Sep. Purif. Technol. 2017, 175, 314–320. [Google Scholar] [CrossRef]
- Chen, L.; Bian, X.; Lu, X. Removal of strontium from simulated low-level radioactive wastewater by nanofiltration. Water Sci. Technol. 2018, 78, 1733–1740. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, T.; Chen, X.; Qiu, M.; Fan, Y. Fabrication of TiO2-doped ZrO2 nanofiltration membranes by using a modified colloidal sol-gel process and its application in simulative radioactive effluent. J. Membr. Sci. 2016, 514, 476–486. [Google Scholar] [CrossRef]
- Gu, J.; Wang, S.; Wang, X. Research on reverse osmosis treatment of boron-containing radioactive waste liquid from nuclear power plants. China Nucl. Power 2015, 8, 219–224. [Google Scholar]
- Jia, F.; Li, J.; Wang, J.; Sun, Y. Removal of strontium ions from simulated radioactive wastewater by vacuum membrane distillation. Ann. Nucl. Energy 2017, 103, 363–368. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Wang, J. Removal of Cs(I) from simulated radioactive wastewater by three forward osmosis membranes. Chem. Eng. J. 2018, 344, 353–362. [Google Scholar] [CrossRef]
- Zakrzewska-Trznadel, G. Advances in membrane technologies for the treatment of liquid radioactive waste. Desalination 2013, 321, 119–130. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Li, Z. Selection of operating conditions for the evaporation system of radioactive waste liquid. Nucl. Sci. Eng. 2017, 37, 1–4. [Google Scholar]
- Yang, Q.; Hou, L.; Wang, Y. Research progress in low- and medium-level radioactive wastewater treatment technology. Environ. Sci. Manag. 2007, 32, 103–106. [Google Scholar]
- McCullough, G.E. Concentration of Radioactive Liquid Waste by Evaporation. Ind. Eng. Chem. 1950, 43, 1505–1509. [Google Scholar] [CrossRef]
- Adamson, D.; Howe, A.; McCabe, D. Preparation and Evaporation of Hanford Waste Treatment Plant Direct Feed Low Activity Waste Effluent Management Facility Simulant; Savannah River Site: Aiken, SC, USA, 2017. [Google Scholar]
- Hu, Y.; Lu, J. Design and Problem Discussion of High-Level Radioactive Waste Liquid Kettle Evaporator in Post-Processing Plant. Ind. Technol. Forum 2018, 17, 53–55. [Google Scholar]
- Xu, Y.; Li, D.; Ren, L.; Hua, W. Application Research of MVR Evaporation in Nuclear Power Plant Accident Waste Liquid Treatment. Guangzhou Chem. Ind. 2016, 44, 171–172. [Google Scholar]
- Xia, Z.; Gan, S.; Yu, L. Discussion on the application of MVR technology in the evaporation and concentration process of radioactive waste liquid in nuclear power plants. Brick World 2019, 6, 126. [Google Scholar]
- Wei, F.; Fang, X. Application test of vacuum evaporation and concentration device in nuclear radiation wastewater treatment. Ind. Water Treat. 2009, 9, 62–65. [Google Scholar]
- Yu, K.; Shao, P.; Meng, P.; Chen, T.; Lei, J.; Yu, X.; He, R.; Yang, F.; Zhu, W.; Duan, T. Superhydrophilic and highly elastic monolithic sponge for efficient solar-driven radioactive wastewater treatment under one sun. J. Hazard. Mater. 2020, 392, 122350. [Google Scholar] [CrossRef]
- Peng, X. Research on the treatment of radioactive waste liquid by infrared heating evaporation method. Nucl. Power Eng. 1997, 18, 560–562. [Google Scholar]
- Xu, L.; Yao, C. Research on Concentration of Radioactive Waste Liquid in Infrared Heating Evaporator. Nucl. Power Eng. 1992, 13, 86–89. [Google Scholar]
- Wang, P.; Zhan, J.; Li, Y. Improving the scaling problem of Qinshan 320MW waste liquid evaporator. Sci. Technol. Vis. 2018, 27, 38–40. [Google Scholar]
- Gu, Y. Research progress in radioactive wastewater treatment methods. Sci. Technol. Vis. 2018, 2, 11–13. [Google Scholar]
- Osmanlioglu, A.E. Treatment of radioactive liquid waste by sorption on natural zeolite in Turkey. J. Hazard. Mater. 2006, 137, 332–335. [Google Scholar] [CrossRef]
- Hwang, K.S.; Park, C.W.; Lee, K.-W.; Park, S.-J.; Yang, H.-M. Highly efficient removal of radioactive cesium by sodium-copper hexacyanoferrate-modified magnetic nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 516, 375–382. [Google Scholar] [CrossRef]
- Attallah, M.; Hassan, H.; Youssef, M. Synthesis and sorption potential study of Al2O3ZrO2CeO2 composite material for removal of some radionuclides from radioactive waste effluent. Appl. Radiat. Isot. 2019, 147, 40–47. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Huang, H.; Shi, R. Advances in radioactive wastewater treatment technology. Appl. Chem. 2018, 1, 193–197. [Google Scholar]
- Yang, H.-M.; Park, C.W.; Kim, I.; Yoon, I.-H. Hollow flower-like titanium ferrocyanide structure for the highly efficient removal of radioactive cesium from water. Chem. Eng. J. 2019, 392, 123713. [Google Scholar] [CrossRef]
- Eka, B.; Ao, B.; Bfs, C.; Ost, D. A radioactively durable melamine-styrene based polymer: Highly efficient removal of 90 Sr. Appl. Radiat. Isot. 2019, 149, 96–103. [Google Scholar]
- Shukla, A.; Parmar, P.; Saraf, M. Radiation, radionuclides and bacteria: An in-perspective review. J. Environ. Radioact. 2017, 180, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Vanhoudt, N.; Vandenhove, H.; Leys, N.; Janssen, P. Potential of higher plants, algae, and cyanobacteria for remediation of radioactively contaminated waters. Chemosphere 2018, 207, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Miao, J. Overview of radioactive wastewater treatment technology. Sci. Technol. Inf. 2011, 23, 60. [Google Scholar]
- Ferreira, R.V.D.P.; Sakata, S.; Dutra, F.; Di Vitta, P.B.; Taddei, M.H.T.; Bellini, M.H.; Marumo, J.T. Treatment of radioactive liquid organic waste using bacteria community. J. Radioanal. Nucl. Chem. 2011, 292, 811–817. [Google Scholar] [CrossRef]
- Gorbunova, O.; Safonov, A.; Tregubova, V.; German, K. Cementation of biodegraded radioactive oils and organic waste. J. Radioanal. Nucl. Chem. 2015, 304, 371–375. [Google Scholar] [CrossRef]
- Liu, X.; Hu, W.; Huang, X.; Deng, H. Highly effective biosorption of Sr(II) from low level radioactive wastewater. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2015, 71, 1727. [Google Scholar] [CrossRef]
- Tsezos, M.; Volesky, B. Biosorption of uranium and thorium. Biotechnol. Bioeng. 2010, 23, 583–604. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Zabihi, M.; Tahmasbi, M.; Bastami, T.R. Effect of adsorbents and chemical treatments on the removal of strontium from aqueous solutions. J. Hazard. Mater. 2010, 182, 552–556. [Google Scholar] [CrossRef]
- Hidouri, S. Possible domestication of uranium oxides using biological assistance reduction. Saudi J. Biol. Sci. 2017, 24, 1–10. [Google Scholar] [CrossRef]
- Salomone, V.N.; Meichtry, J.M.; Schinelli, G.; Leyva, A.G.; Litter, M.I. Photochemical reduction of U(VI) in aqueous solution in the presence of 2-propanol. J. Photochem. Photobiol. A Chem. 2014, 277, 19–26. [Google Scholar] [CrossRef]
- Wang, G.; Zhen, J.; Zhou, L.; Wu, F.; Deng, N. Adsorption and photocatalytic reduction of U(VI) in aqueous TiO2 suspensions enhanced with sodium formate. J. Radioanal. Nucl. Chem. 2015, 304, 579–585. [Google Scholar] [CrossRef]
- Feng, J.; Yang, Z.; He, S.; Niu, X.; Zhang, T.; Ding, A.; Liang, H.; Feng, X. Photocatalytic reduction of Uranium(VI) under visible light with Sn-doped In2S3 microspheres. Chemosphere 2018, 212, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Chen, R.; Wu, X.; Fan, M.; Liu, Y.; Le, Z.; Jiang, S.; Song, S. Boron doped g-C3N4 with enhanced photocatalytic UO22+ reduction performance —ScienceDirect. Appl. Surf. Sci. 2016, 360, 1016–1022. [Google Scholar] [CrossRef]
- Guo, Y.; Li, L.; Li, Y.; Li, Z.; Wang, X.; Wang, G. Adsorption and photocatalytic reduction activity of uranium(VI) on zinc oxide/rectorite composite enhanced with methanol as sacrificial organics. J. Radioanal. Nucl. Chem. 2016, 310, 883–890. [Google Scholar] [CrossRef]
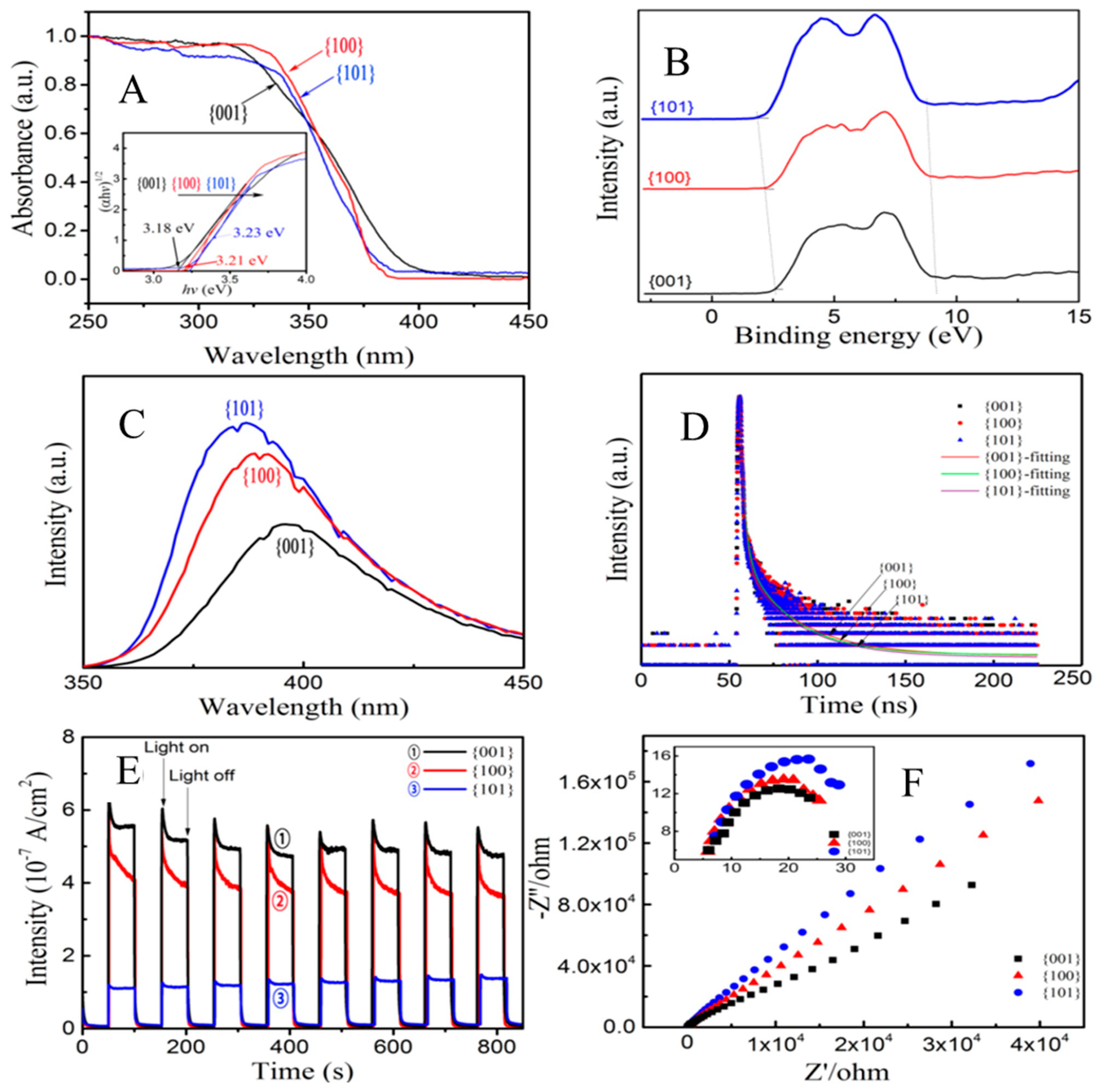
- Chen, K.; Chen, C.; Ren, X.; Alsaedi, A.; Hayat, T. Interaction mechanism between different facet TiO2 and U (VI): Experimental and density-functional theory investigation. Chem. Eng. J. 2019, 359, 944–954. [Google Scholar] [CrossRef]
- Ping, L.; Wang, J.; Wang, Y.; Liang, J.; Fan, Q. An overview and recent progress in the heterogeneous photocatalytic reduction of U(VI). J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100320. [Google Scholar]
- Chen, J.; Ollis, D.F.; Rulkens, W.H.; Bruning, H. Photocatalyzed deposition and concentration of soluble uranium(VI) from TiO2 suspensions. Colloids Surfaces A Physicochem. Eng. Asp. 1999, 151, 339–349. [Google Scholar] [CrossRef]
- Salomone, V.N.; Meichtry, J.M.; Litter, M.I. Heterogeneous photocatalytic removal of U(VI) in the presence of formic acid: U(III) formation. Chem. Eng. J. 2015, 270, 28–35. [Google Scholar] [CrossRef]
- Li, Z.J.; Huang, Z.W.; Guo, W.L.; Wang, L.; Zheng, L.R.; Chai, Z.F.; Shi, W.Q. Enhanced Photocatalytic Removal of Uranium(VI) from Aqueous Solution by Magnetic TiO2/Fe3O4 and Its Graphene Composite. Environ. Sci. Technol. 2017, 51, 5666. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, Y.; Wang, X.; Li, P.; Kong, L.; Wang, G.; Li, X.; Li, Y. Enhanced photocatalytic reduction activity of uranium(VI) from aqueous solution using the Fe2O3–graphene oxide nanocomposite. Dalton Trans. 2017, 46, 14762–14770. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, P.; Jiang, S.; Wu, X.; Song, S.; Zhu, M.; Lou, Z.; Li, Z.; Liu, F.; Liu, Y.; et al. Photocatalytic reduction elimination of UO22+ pollutant under visible light with metal-free sulfur doped g-C3N4 photocatalyst. Appl. Catal. B: Environ. 2016, 200, 378–385. [Google Scholar] [CrossRef]
- Jiang, X.; Xing, Q.-J.; Luo, X.-B.; Li, F. Simultaneous photoreduction of Uranium(VI) and photooxidation of Arsenic (III) in aqueous solution over g-C3N4/TiO2 heterostructured catalysts under simulated sunlight irradiation. Appl. Catal. B Environ. 2018, 228, 29–38. [Google Scholar] [CrossRef]
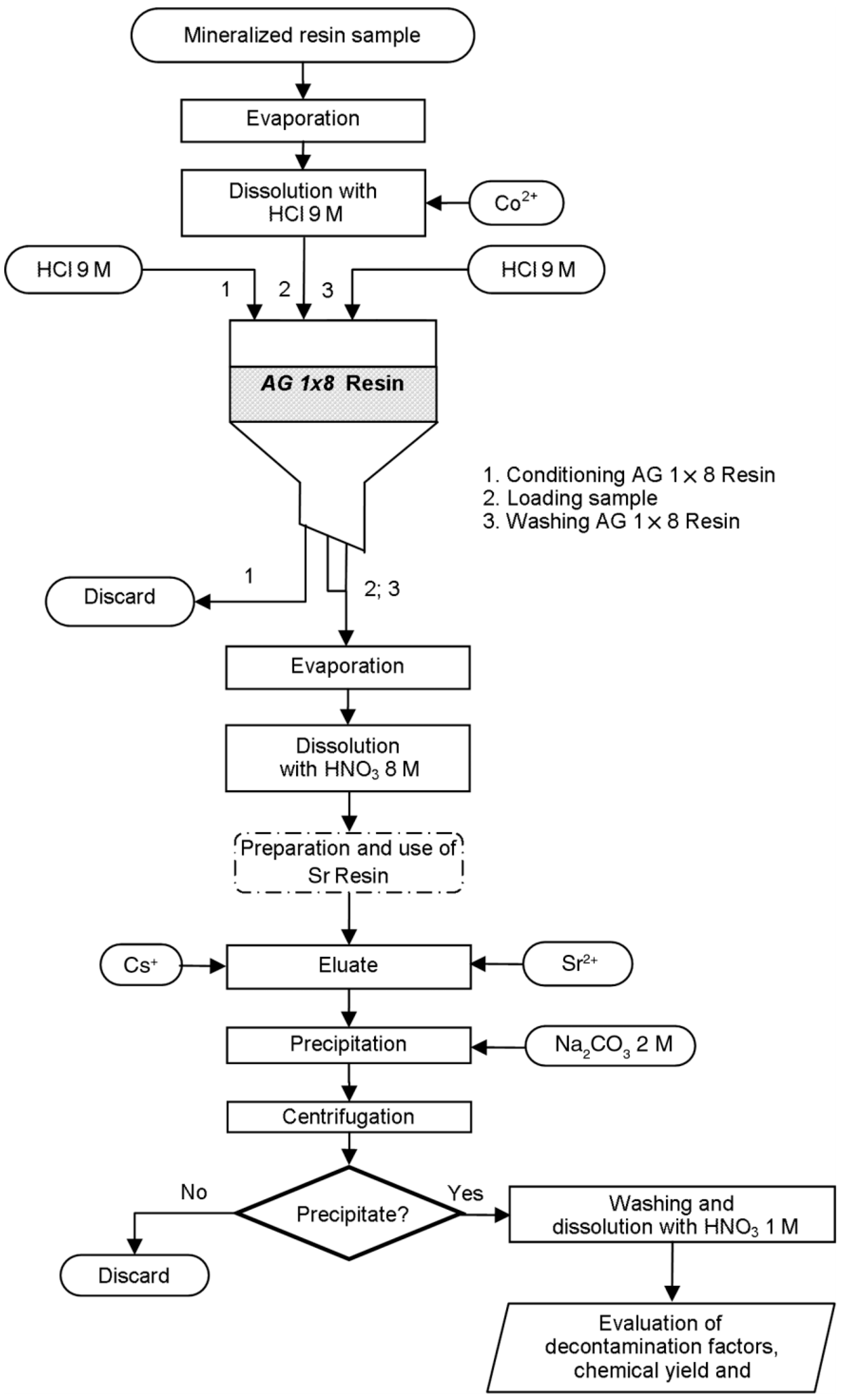
- Dianu, A.M.; Dobrin, R.I. Separation and quantification of 90Sr from ion-exchange resin radioactive waste: Methods and techniques of analysis. Radiochim. Acta 2020, 108, 627–640. [Google Scholar] [CrossRef]
- Xu, M. “Red Oil” Explosion Safety Analysis of Spent Fuel Reprocessing Plant. Nucl. Saf. 2011, 1, 22–27. [Google Scholar]
| Sources | Typical Radioisotope | Characteristics |
|---|---|---|
| Nuclear Research Center/Radioisotope Laboratory | According to the target’s yield and purity, there are many varieties, short-term active nuclides, and long-term radionuclide mixure. | a. After the ion-exchange resin is regenerated, the batches whose pH value is close to neutral are generally more uniform; b. Small size, high specific activity, high chemical concentration; |
| Nuclear Power Plant | 3H, 14C, U (233U, 234U, 235U, 238U) and Th (228Th, 232Th), etc. | a. The volume may be large, and the chemical composition is uncertain; b. Very high specific activity and chemical concentration; |
| Scientific research | Variable, short-lived, and long-lived radioisotopes | Extremely variable inactivity, volume, chemical concentration, etc.; |
| Radiolabels and radiopharmaceuticals/medical diagnosis and treatment | 14C, 3H, 32P, 35S, 125I, 99Tcm, 131I, 85Sr | a. Predictable small volume of chemical composition; b. Mainly comes from the patient’s large amount of urine, and a small amount comes from the preparation and processing process; |
| Rare earth metal mine beneficiation wastewater | It varies greatly depending on the type of ore | a. Large size and uncertain chemical composition; b. Often mixed with other toxic heavy metals; |
| Industrial and pilot plants | Depends on the application, for example in the instrument industry (226Ra, 147Pm) | The volume may be large, and the chemical composition is uncertain. |
| Processing Technology | Advantages | Disadvantages |
|---|---|---|
| Ion exchange | High selectivity and simple operation | Affected by salinity, regeneration is difficult, secondary waste is generated |
| Chemical precipitation | Suitable for processing large volumes of high-concentration waste liquid, simple, convenient, and low-cost | Difficulty in solid–liquid separation, poor treatment of anionic radionuclides |
| Evaporative concentration | High decontamination coefficient, large concentration ratio, mature method, strong versatility, and great flexibility | High energy consumption, low heat utilization rate, equipment corrosion, and scaling |
| Membrane separation | Large processing capacity, flexibility, easy to be combined with multiple methods | High cost, easy to pollute the membrane, poor radiation stability |
| Biotechnology | Environmentally friendly, no secondary pollution | Microbes have poor tolerance to radiation |
| Adsorption | Simple operation | High requirements for adsorbent |
| Photocatalytic | Low cost, high safety, high efficiency, no secondary pollution | Affected by the environment, the charge separation efficiency is low, and the utilization rate of sunlight is low |
| Combination process | A high degree of purification | High process design requirements and high operating costs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Shen, M.; Tong, Y.; Wang, X. Radioactive Wastewater Treatment Technologies: A Review. Molecules 2023, 28, 1935. https://doi.org/10.3390/molecules28041935
Ma H, Shen M, Tong Y, Wang X. Radioactive Wastewater Treatment Technologies: A Review. Molecules. 2023; 28(4):1935. https://doi.org/10.3390/molecules28041935
Chicago/Turabian StyleMa, Hailing, Minghai Shen, Yao Tong, and Xiao Wang. 2023. "Radioactive Wastewater Treatment Technologies: A Review" Molecules 28, no. 4: 1935. https://doi.org/10.3390/molecules28041935
APA StyleMa, H., Shen, M., Tong, Y., & Wang, X. (2023). Radioactive Wastewater Treatment Technologies: A Review. Molecules, 28(4), 1935. https://doi.org/10.3390/molecules28041935









