Confusoside from Anneslea fragrans Alleviates Acetaminophen-Induced Liver Injury in HepG2 via PI3K-CASP3 Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. HPLC Analysis of CF
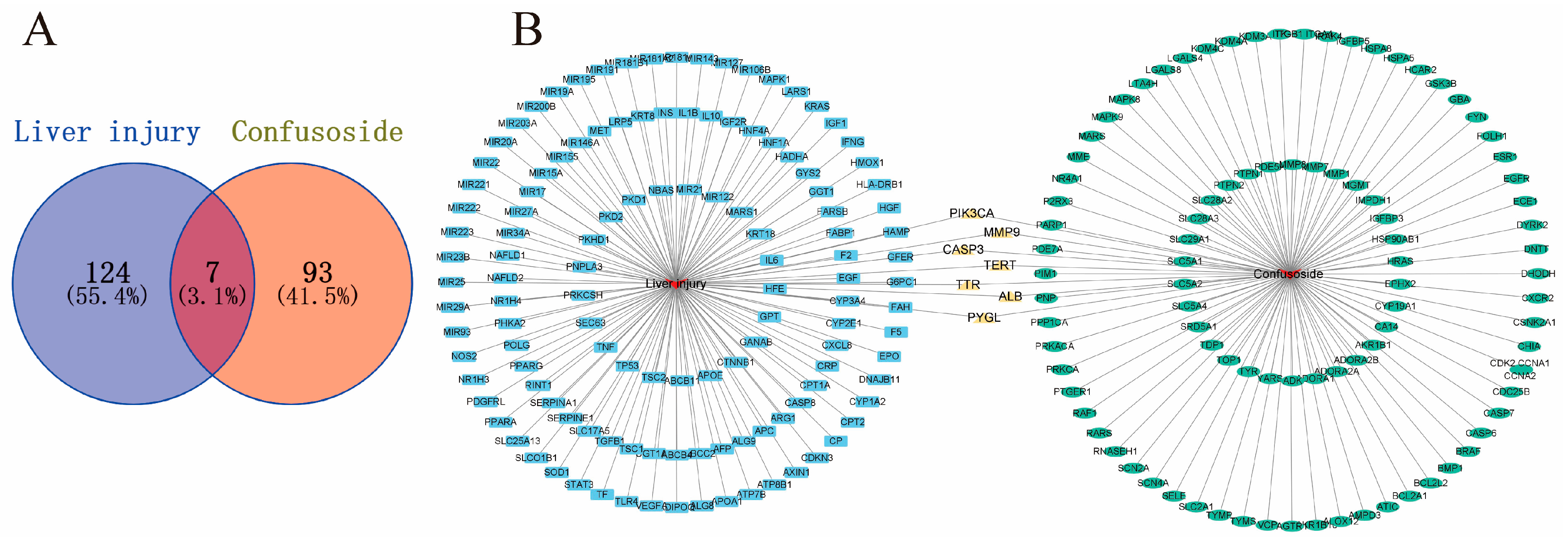
2.2. Potential Targets of CF Treatment of Liver Injury
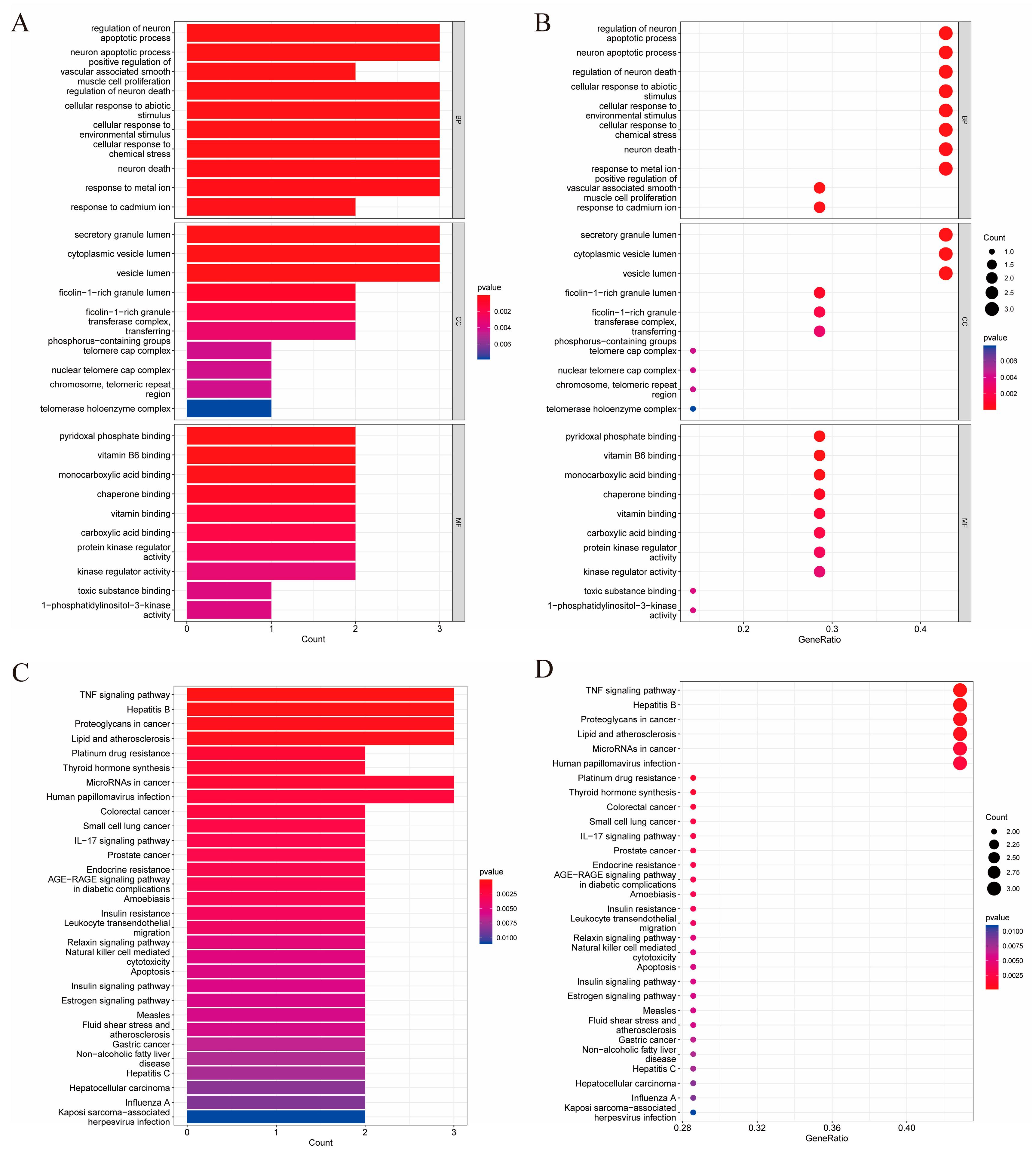
2.3. GO Biological Process and KEGG Pathway Enrichment Analysis
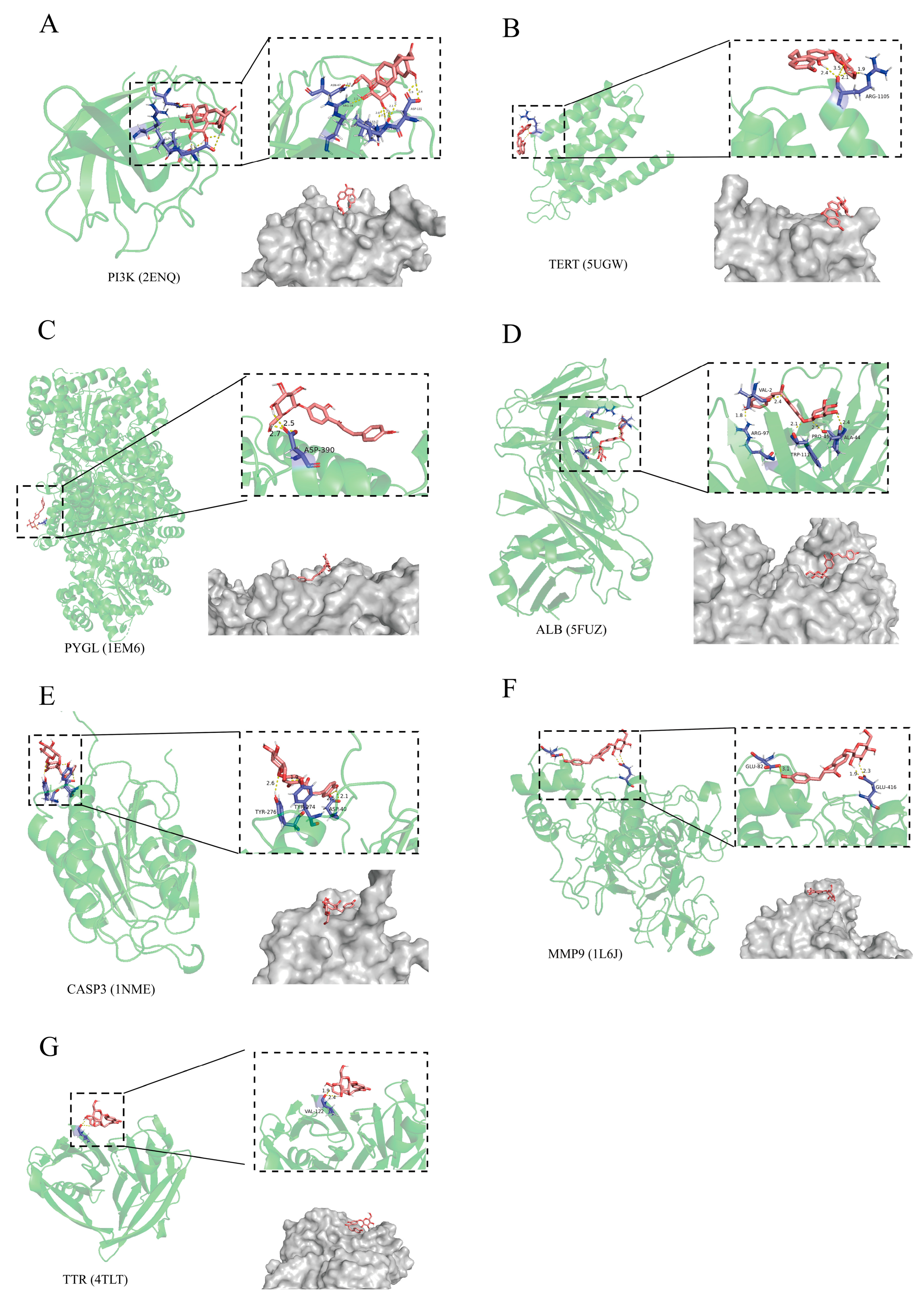
2.4. The Relationship of CF with Liver Injury Assessed by Molecular Docking
2.5. CF Reduced APAP-Induced Injury of Hepg2 Cells and Improved Their Viability
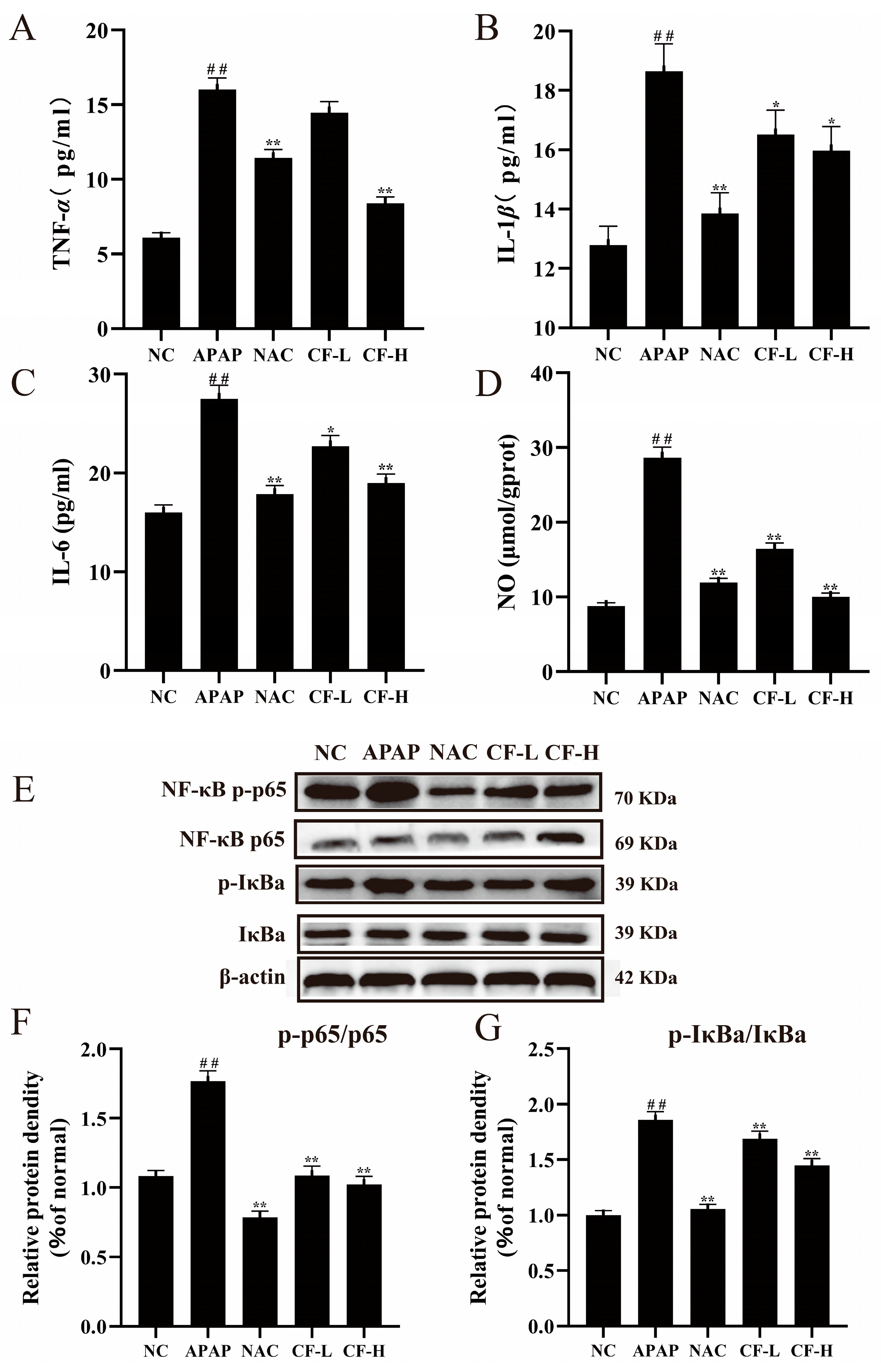
2.6. Inhibitory Effect of CF on APAP-Induced Inflammation in HepG2 Cells
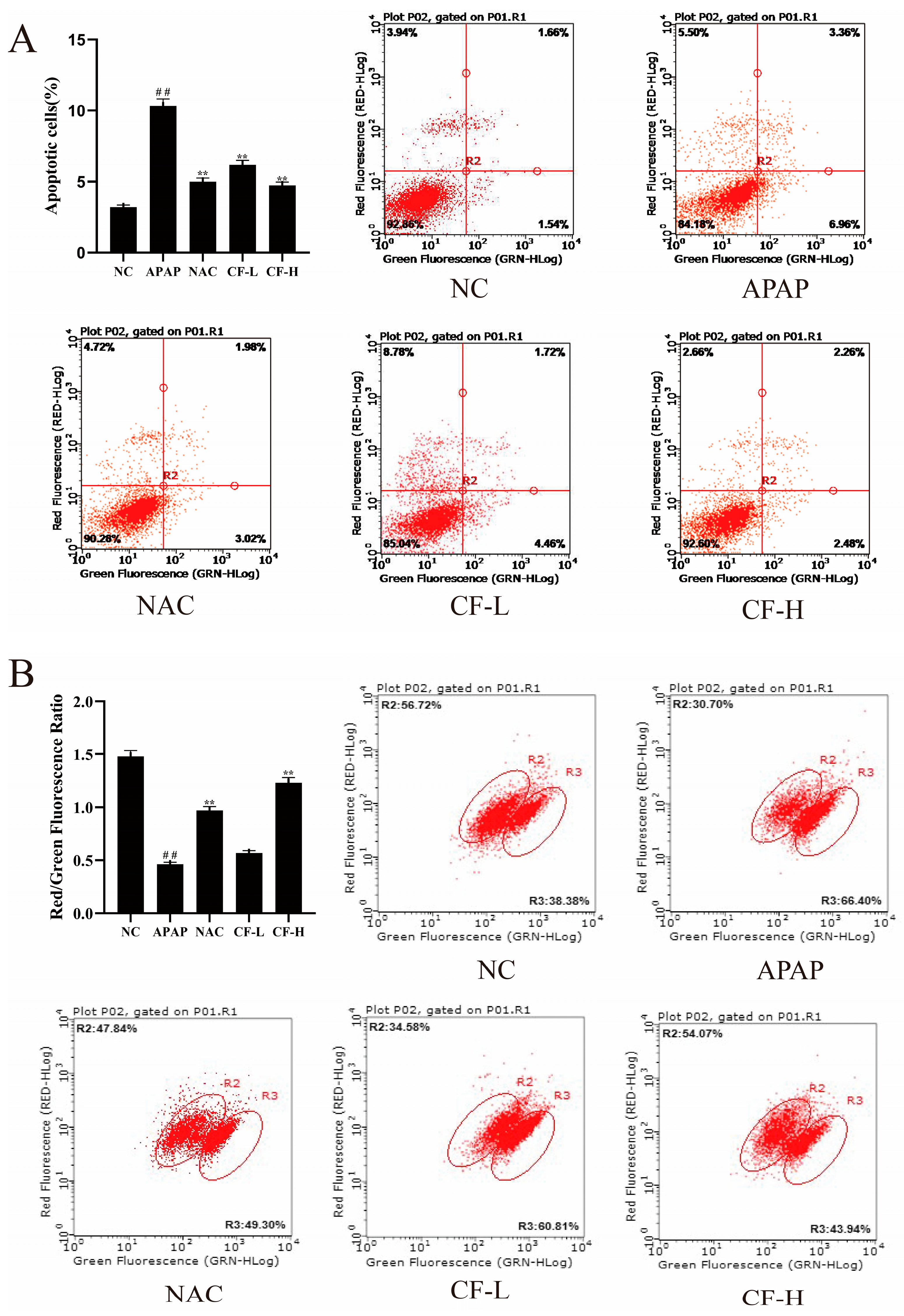
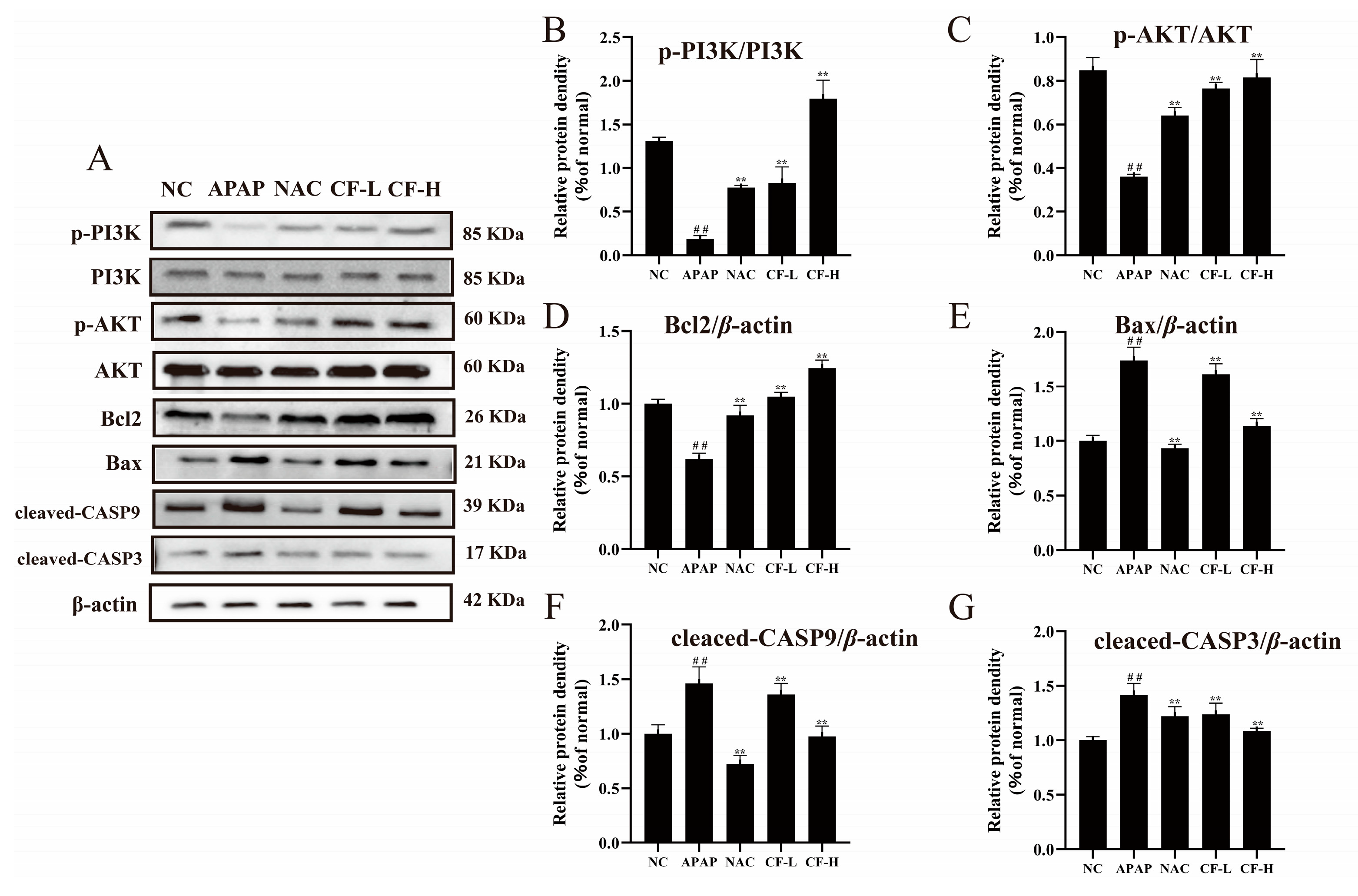
2.7. Inhibitory Effect of CF against APAP-Induced Apoptosis in HepG2 Cells
3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Preparation of CF from Leaves of A. fragrans
4.3. Network Pharmacology Analysis of the Effect of CF on Liver Injury
4.4. Molecular Docking Analysis of CF on Liver Injury
4.5. Cell Culture
4.6. Cell Culture
4.7. Analysis of Biochemical Indicators in APAP-Induced HepG2 Cells
4.8. Determination of Cell Apoptosis
4.9. Detection of Mitochondrial Membrane Potential (MMP)
4.10. Western Blotting Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers. 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, E.; Tawfik, A.; Abd-Elrahman, H. Egyptian perspectives on potential risk of paracetamol/acetaminophen-induced toxicities: Lessons learnt during COVID-19 pandemic. Toxicol. Rep. 2022, 9, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Bunchorntavakul, C.; Reddy, K.R. Acetaminophen (APAP or N-acetyl-p-aminophenol) and acute liver failure. Clin. Liver. Dis. 2018, 22, 325–346. [Google Scholar] [CrossRef]
- Palabiyik, S.; Karakus, E.; Halici, Z.; Cadirci, E.; Ayaz, G.; Cinar, I. The protective effects of carvacrol and thymol against paracetamol–induced toxicity on human hepatocellular carcinoma cell lines (HepG2). Hum. Exp. Toxicol. 2016, 35, 1252–1263. [Google Scholar] [CrossRef]
- Yan, M.; Huo, Y.; Yin, S.; Hu, H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox. Biol. 2018, 17, 274–283. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, M.; Jin, Q.; Wu, Y.; Cui, Z.; Shang, Y.; Zhan, Z.; Lin, Y.; Jiao, J.; Piao, M.; et al. Modulation of HMGB1 release in APAP-induced liver injury: A possible strategy of chikusetsusaponin V targeting NETs formation. Front. Pharmacol. 2021, 12, 723881. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, Y.; Wang, J.; Li, P.; Ma, F. Structure-antioxidant capacity relationship of dihydrochalcone compounds in malus. Food Chem. 2019, 275, 354–360. [Google Scholar] [CrossRef]
- Shang, A.; Liu, H.-Y.; Luo, M.; Xia, Y.; Yang, X.; Li, H.-Y.; Wu, D.-T.; Sun, Q.-C.; Geng, F.; Gan, R.Y. Sweet tea (Lithocarpus polystachyus rehd.) as a new natural source of bioactive dihydrochalcones with multiple health benefits. Crit. Rev. Food Sci. 2022, 62, 917–934. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Turkiewicz, I.P.; Tkacz, K.; Hernandez, F. Comparison of bioactive compounds and health promoting properties of fruits and leaves of apple, pear and quince. Sci. Rep. 2021, 11, 20253. [Google Scholar] [CrossRef]
- Yang, W.-M.; Liu, J.-K.; Qin, X.-D.; Wu, W.-L.; Chen, Z.-H. Activities of three dihydrochalcone glucosides from leaves of Lithocarpus pachyphyllus. Antioxidant 2004, 59, 481–484. [Google Scholar] [CrossRef]
- An, R.-B.; Park, E.-J.; Jeong, G.-S.; Sohn, D.-H.; Kim, Y.-C. Cytoprotective constituent of hoveniae lignum on both HepG2 cells and rat primary hepatocytes. Arch. Pharm. Res. 2007, 30, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Zhao, T.; Cao, J.; Liu, Y.; Wang, Y.; Wang, Y.; Cheng, G. E Se tea alleviates acetaminophen-induced liver injury by activating the Nrf2 signaling pathway. Food Funct. 2022, 13, 7240–7250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yauk, Y.-K.; Zhao, Q.; Hamiaux, C.; Xiao, Z.; Gunaseelan, K.; Zhang, L.; Tomes, S.; López-Girona, E.; Cooney, J.; et al. Biosynthesis of the dihydrochalcone sweetener trilobatin requires phloretin glycosyltransferase2. Plant Physiol. 2020, 184, 738–752. [Google Scholar] [CrossRef]
- Wang, X. Structure, mechanism and engineering of plant natural product glycosyltransferases. FEBS Lett. 2009, 583, 3303–3309. [Google Scholar] [CrossRef]
- Gutmann, A.; Bungaruang, L.; Weber, H.; Leypold, M.; Breinbauer, R.; Nidetzky, B. Towards the synthesis of glycosylated dihydrochalcone natural products using glycosyltransferase-catalysed cascade reactions. Green Chem. 2014, 16, 4417–4425. [Google Scholar] [CrossRef]
- Desire, O.; Rivière, C.; Razafindrazaka, R.; Goossens, L.; Moreau, S.; Guillon, J.; Uverg-Ratsimamanga, S.; Andriamadio, P.; Moore, N.; Randriantsoa, A.; et al. Antispasmodic and antioxidant activities of fractions and bioactive constituent davidigenin isolated from Mascarenhasia arborescens. J. Ethnopharmacol. 2010, 130, 320–328. [Google Scholar] [CrossRef]
- Deng, X.; Wang, Y.; Tian, L.; Yang, M.; He, S.; Liu, Y.; Khan, A.; Li, Y.; Cao, J.; Cheng, G. Anneslea fragrans Wall. ameliorates ulcerative colitis via inhibiting NF-κB and MAPK activation and mediating intestinal barrier integrity. J. Ethnopharmacol. 2021, 278, 114304. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, Y.; Zhou, W.; He, S.; Yang, M.; Xue, Q.; Wang, Y.; Zhao, T.; Cao, J.; Khan, A.; et al. Phenolic composition, antioxidant and cytoprotective effects of aqueous-methanol extract from Anneslea fragrans leaves as affected by drying methods. Int. J. Food Sci. Technol. 2021, 56, 4807–4819. [Google Scholar] [CrossRef]
- He, S.; Cui, X.; Khan, A.; Liu, Y.; Wang, Y.; Cui, Q.; Zhao, T.; Cao, J.; Cheng, G. Activity guided isolation of phenolic compositions from Anneslea fragrans Wall. and their cytoprotective effect against hydrogen peroxide induced oxidative stress in HepG2 Cells. Molecules 2021, 26, 3690. [Google Scholar] [CrossRef]
- Gong, L.; Zhou, H.; Wang, C.; He, L.; Guo, C.; Peng, C.; Li, Y. Hepatoprotective effect of forsythiaside against acetaminophen-induced liver injury in zebrafish: Coupling network pharmacology with biochemical pharmacology. J. Ethnopharmacol. 2021, 271, 113890. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.; Blake, J.; Botstein, D.; Butler, H.; Cherry, J.; Davis, A.; Dolinski, K.; Dwight, S.; Eppig, J.; et al. Gene ontology: Tool for the unification of biology. The gene ontology consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ge, J.; Li, M.; Deng, S.; Li, J.; Ma, Y.; Zhang, J.; Zheng, Y.; Ma, L. Network pharmacology, molecular docking technology integrated with pharmacodynamic study to reveal the potential targets of Schisandrol A in drug-induced liver injury by acetaminophen. Bioorg. Chem. 2022, 118, 105476. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Yi, R.; Mu, J.; Zhao, X.; Yang, Z. Hepatoprotective effects of lactobacillus on carbon tetrachloride-induced acute liver injury in mice. Int. J. Mol. Sci. 2018, 19, 2212. [Google Scholar] [CrossRef] [PubMed]
- Abdullah-Al-Shoeb, M.; Sasaki, K.; Kikutani, S.; Namba, N.; Ueno, K.; Kondo, Y.; Maeda, H.; Maruyama, T.; Irie, T.; Ishitsuka, Y. The late-stage protective effect of mito-tempo against acetaminophen-induced hepatotoxicity in mouse and three-dimensional cell culture models. Antioxidants-Basel 2020, 9, 965. [Google Scholar] [CrossRef]
- Ramirez, T.; Strigun, A.; Verlohner, A.; Huener, H.; Peter, E.; Herold, M.; Bordag, N.; Mellert, W.; Walk, T.; Spitzer, M.; et al. Prediction of liver toxicity and mode of action using metabolomics in vitro in HepG2 cells. Arch. Toxicol. 2018, 92, 893–906. [Google Scholar] [CrossRef]
- Yuan, L.; Qiu, Z.; Yang, Y.; Liu, C.; Zhang, R. Preparation, structural characterization and antioxidant activity of water-soluble polysaccharides and purified fractions from blackened jujube by an activity-oriented approach. Food Chem. 2022, 385, 132637. [Google Scholar] [CrossRef]
- Rada, P.; Pardo, V.; Mobasher, M.A.; García-Martínez, I.; Ruiz, L.; González-Rodríguez, Á.; Sanchez-Ramos, C.; Muntané, J.; Alemany, S.; James, L.; et al. SIRT1 controls acetaminophen hepatotoxicity by modulating inflammation and oxidative stress. Antioxid. Redox. Sign. 2017, 28, 1187–1208. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhang, L.; Niu, G.; Zhang, X.; Yang, L.; Ji, W.; Ren, L. Coinfection of porcine circovirus 2 and pseudorabies virus enhances immunosuppression and inflammation through NF-κB, JAK/STAT, MAPK, and NLRP3 pathways. Int. J. Mol. Sci. 2022, 23, 4469. [Google Scholar] [CrossRef]
- Fan, W.; Liu, X.; Zhang, J.; Qin, L.; Du, J.; Li, X.; Qian, S.; Chen, H.; Qian, P. TRIM67 suppresses TNFα-triggered NF-κB activation by competitively binding β-TrCP to IkBa. Front. Immunol. 2022, 13, 793147. [Google Scholar] [CrossRef]
- Gardner, C.; Laskin, J.; Dambach, D.; Chiu, H.; Durham, S.; Zhou, P.-H.; Bruno, M.; Gerecke, D.; Gordon, M.; Laskin, D. Exaggerated hepatotoxicity of acetaminophen in mice lacking tumor necrosis factor receptor-1: Potential role of inflammatory mediators. Toxicol. Appl. pharm. 2003, 192, 119–130. [Google Scholar] [CrossRef]
- Zhang, S.-S.; Liu, M.; Liu, D.-N.; Shang, Y.-F.; Du, G.-H.; Wang, Y.-H. Network pharmacology analysis and experimental validation of kaempferol in the treatment of ischemic stroke by inhibiting apoptosis and regulating neuroinflammation involving neutrophils. Int. J. Mol. Sci. 2022, 23, 12694. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, F.; Wang, Y.; Khan, A.; Liu, Y.; Yang, M.; Zhao, T.; Cao, J.; Cheng, G. Anti-leukemic effect and molecular mechanism of 11-methoxytabersonine from Melodinus cochinchinensis via network pharmacology, ROS-mediated mitochondrial dysfunction and PI3K/Akt signaling pathway. Bioorg. Chem. 2022, 120, 105607. [Google Scholar] [CrossRef]
- Leung, K.H.; Luo, S.; Kwarteng, R.; Chen, S.; Yap, M.; Huang, C.; Yip, S. The myopia susceptibility locus vasoactive intestinal peptide receptor 2 (VIPR2) contains variants with opposite effects. Sci. Rep. 2019, 9, 18165. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2020, 49, D480–D489. [Google Scholar] [CrossRef]
- Geng, H.; Chen, X.; Wang, C. Systematic elucidation of the pharmacological mechanisms of rhynchophylline for treating epilepsy via network pharmacology. BMC Complement Med. 2021, 21, 9. [Google Scholar] [CrossRef]
- Shi, M.-J.; Yan, X.-L.; Dong, B.-S.; Yang, W.-N.; Su, S.-B.; Zhang, H. A network pharmacology approach to investigating the mechanism of Tanshinone IIA for the treatment of liver fibrosis. J. Ethnopharmacol. 2020, 253, 112689. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. Cluster Profiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M. KEGG mapping tools for uncovering hidden features in biological data. Protein. Sci. 2022, 31, 47–53. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Luo, Z.; Xia, L.; Tang, Y.; Huang, L.; Liu, D.; Huai, W.; Zhang, C.; Wang, Y.; Xie, Y.; Yin, Q.; et al. Action mechanism underlying improvement effect of Fuzi Lizhong decoction on nonalcoholic fatty liver disease: A study based on network pharmacology and molecular docking. Evid-Based Compl. Alt. 2022, 2022, 1670014. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Patel, G.; Xue, Q.; Njateng, G.S.; Cai, S.; Cheng, G.; Kai, G. In vitro and in vivo anti-inflammatory effects of different extracts from Epigynum auritum through down-regulation of NF-κB and MAPK signaling pathways. J. Ethnopharmacol. 2020, 261, 113105. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Ko, S.Y.; Jun, M.; Jeong, W.S. Quercitrin from Toona sinensis (Juss.) M.Roem. attenuates acetaminophen-induced acute liver toxicity in HepG2 cells and mice through induction of antioxidant machinery and inhibition of inflammation. Nutrients 2016, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, L.; Wang, Y.; Zhao, T.; Khan, A.; Wang, Y.; Cao, J.; Cheng, G. Protective effect of Que Zui tea hot-water and aqueous ethanol extract against acetaminophen-induced liver injury in mice via inhibition of oxidative stress, inflammation, and apoptosis. Food Funct. 2021, 12, 2468–2480. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.-H.; Li, J.; Zhang, X.-Y.; Shi, S.; Wang, L.; Yuan, M.-L.; Liu, Y.-P.; Wang, Y.-D. Confusoside from Anneslea fragrans Alleviates Acetaminophen-Induced Liver Injury in HepG2 via PI3K-CASP3 Signaling Pathway. Molecules 2023, 28, 1932. https://doi.org/10.3390/molecules28041932
Zhao J-H, Li J, Zhang X-Y, Shi S, Wang L, Yuan M-L, Liu Y-P, Wang Y-D. Confusoside from Anneslea fragrans Alleviates Acetaminophen-Induced Liver Injury in HepG2 via PI3K-CASP3 Signaling Pathway. Molecules. 2023; 28(4):1932. https://doi.org/10.3390/molecules28041932
Chicago/Turabian StyleZhao, Jing-Hao, Jing Li, Xiao-Yu Zhang, Shang Shi, Lin Wang, Ming-Long Yuan, Ya-Ping Liu, and Yu-Dan Wang. 2023. "Confusoside from Anneslea fragrans Alleviates Acetaminophen-Induced Liver Injury in HepG2 via PI3K-CASP3 Signaling Pathway" Molecules 28, no. 4: 1932. https://doi.org/10.3390/molecules28041932
APA StyleZhao, J.-H., Li, J., Zhang, X.-Y., Shi, S., Wang, L., Yuan, M.-L., Liu, Y.-P., & Wang, Y.-D. (2023). Confusoside from Anneslea fragrans Alleviates Acetaminophen-Induced Liver Injury in HepG2 via PI3K-CASP3 Signaling Pathway. Molecules, 28(4), 1932. https://doi.org/10.3390/molecules28041932




