An EPR Study on Highly Stable Nitroxyl-Nitroxyl Biradicals for Dynamic Nuclear Polarization Applications at High Magnetic Fields
Abstract
1. Introduction
2. Results and Discussion
2.1. EPR Measurements and Simulations
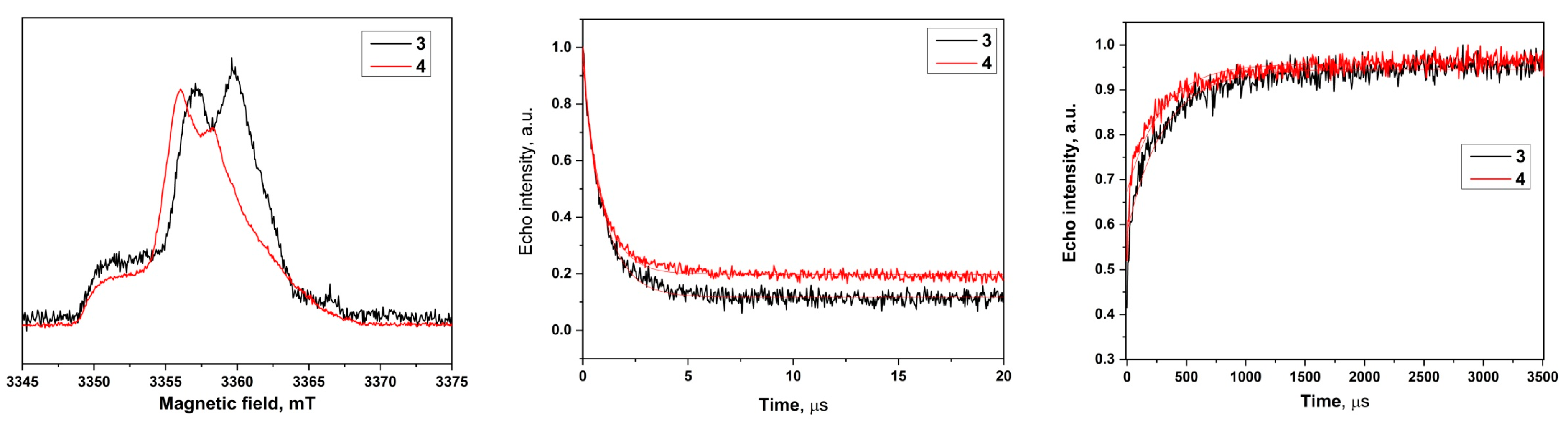
2.2. W-Band Echo-Detected Spectra
2.3. Relaxation Measurements
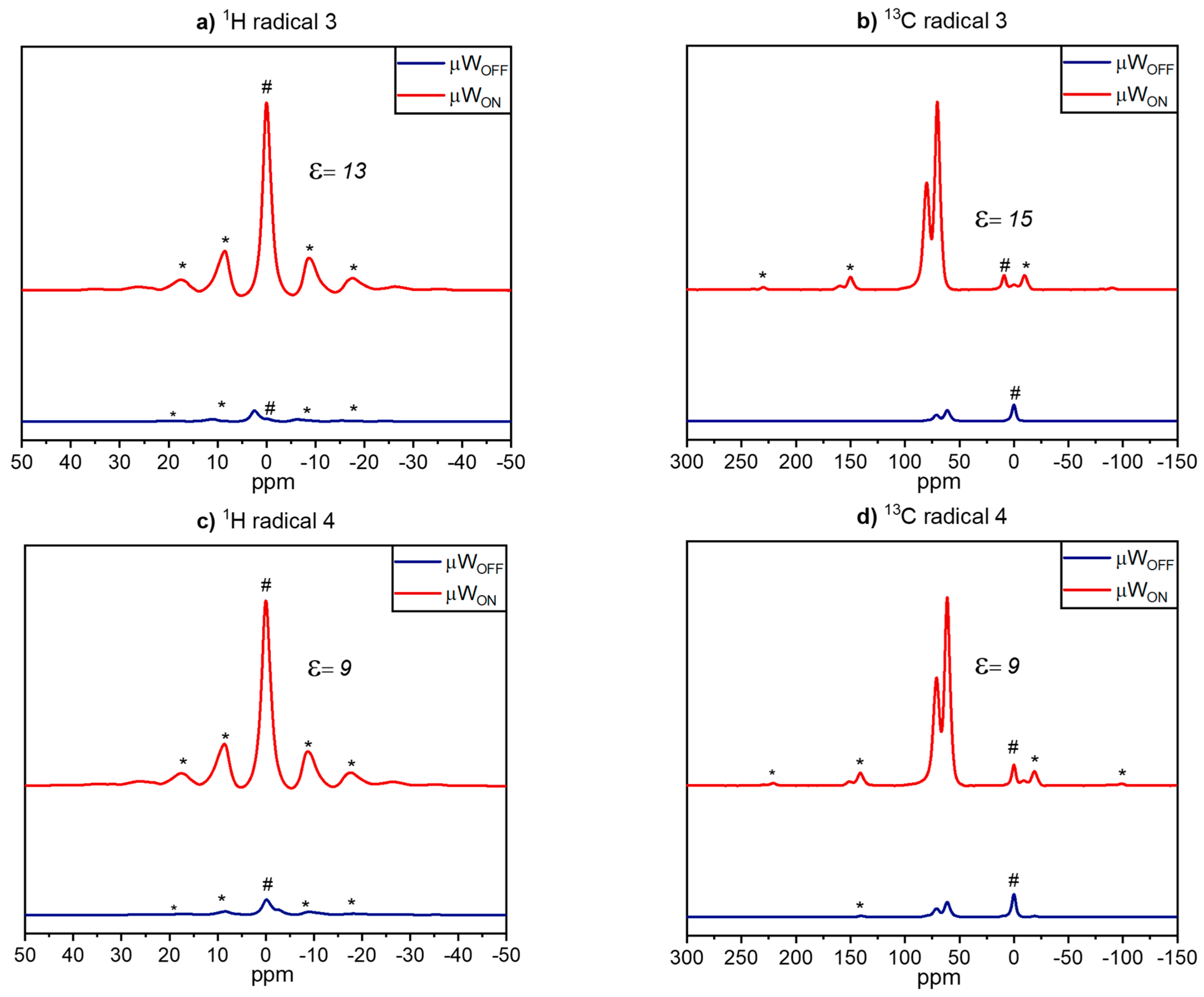
2.4. DNP Experiments
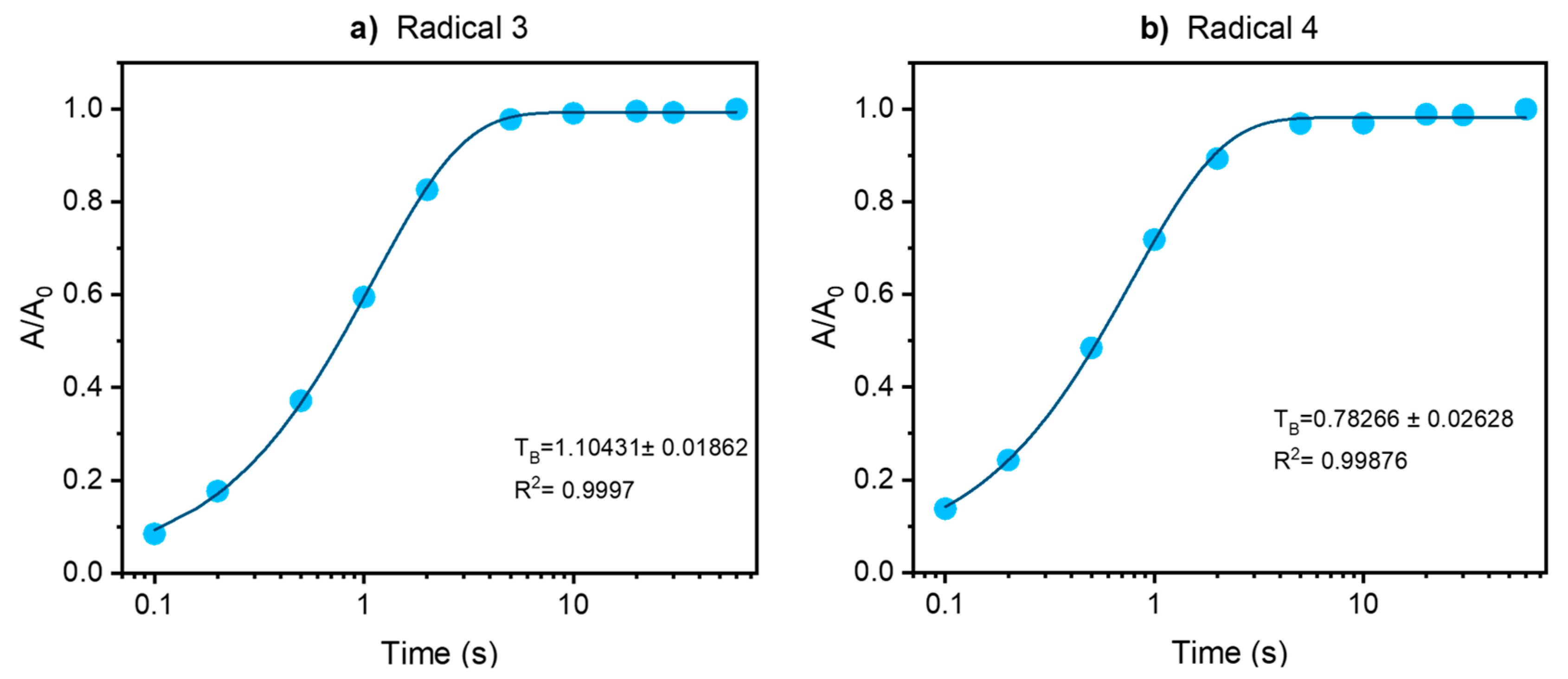
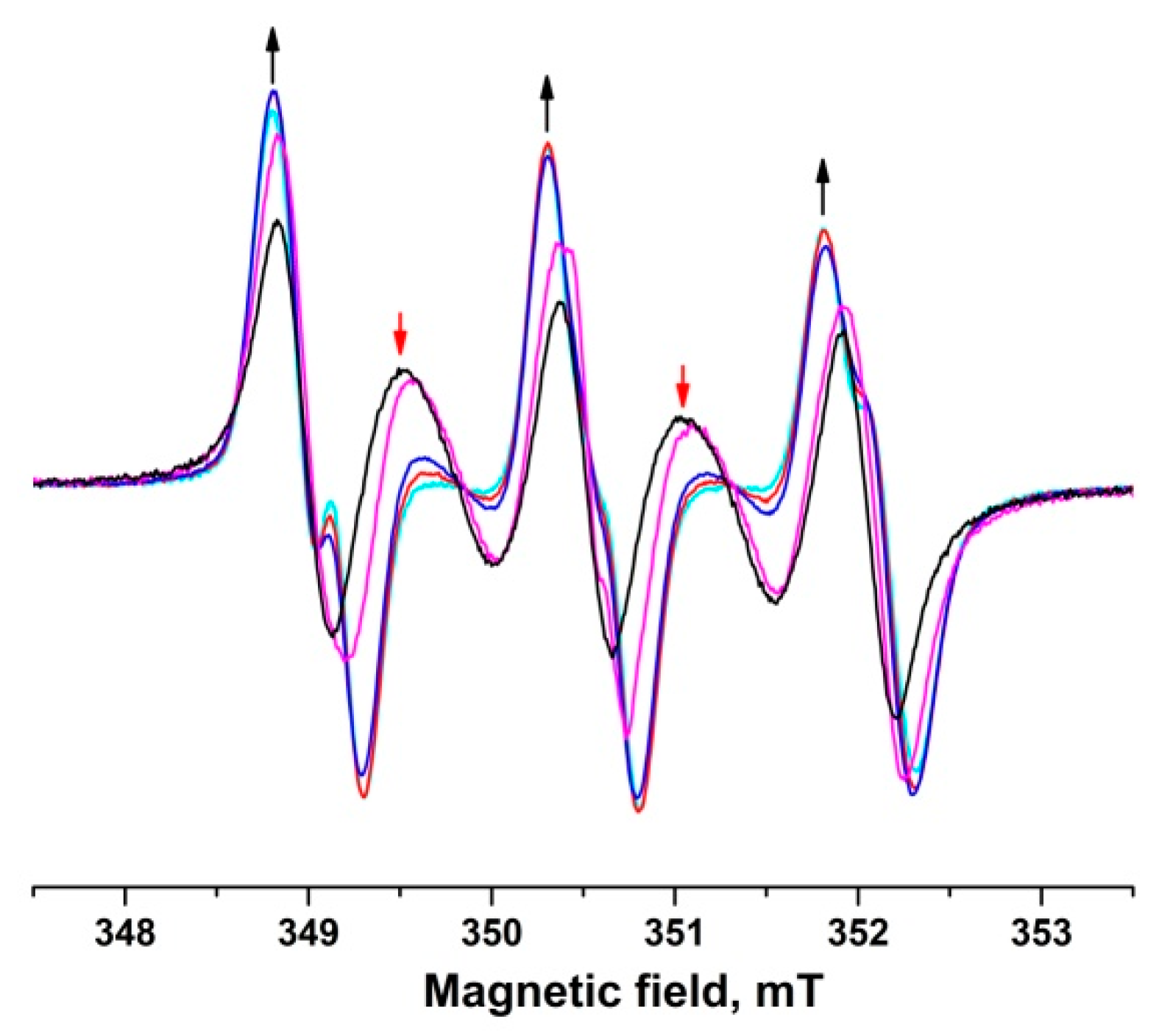
2.5. Resistance of the Biradicals to Reduction by Ascorbate
3. Materials and Methods
3.1. EPR Sample Preparation for W-Band EPR Experiments
3.2. X-Band EPR Experiments
3.3. Solid-State DNP Experiments
3.3.1. Sample Preparation
3.3.2. DNP Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lilly Thankamony, A.S.; Wittmann, J.J.; Kaushik, M.; Corzilius, B. Dynamic Nuclear Polarization for Sensitivity Enhancement in Modern Solid-State NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 102–103, 120–195. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, T.; Groszewicz, P.B.; Buntkowsky, G. Solid-State NMR of Nanocrystals. Annu. Rep. NMR Spectrosc. 2019, 97, 1–82. [Google Scholar] [CrossRef]
- Corzilius, B. High-Field Dynamic Nuclear Polarization. Annu. Rev. Phys. Chem. 2020, 71, 143–170. [Google Scholar] [CrossRef]
- Perras, F.A.; Wang, L.L.; Manzano, J.S.; Chaudhary, U.; Opembe, N.N.; Johnson, D.D.; Slowing, I.I.; Pruski, M. Optimal Sample Formulations for DNP SENS: The Importance of Radical-Surface Interactions. Curr. Opin. Colloid Interface Sci. 2018, 33, 9–18. [Google Scholar] [CrossRef]
- Ishiguro, K.; Ozaki, M.; Kamekura, Y.; Sekine, N.; Sawaki, Y. Preparation and Property of 2-(3′,5′-Di-Tert-Butylphenyl-4′-Oxyl)-4,4,5,5-Tetramethyl-4,5, -Dihydro-1H-Imidazole-3-Oxide-1-Oxyl. Mol. Cryst. Liq. Cryst. 1997, 306, 75–80. [Google Scholar] [CrossRef]
- Marx, L.; Rassat, A. Application of a Spin-Labeled Spin-Trap to the Detection of Nitric Oxide (NO). Angew. Chem. Int. Ed. 2000, 39, 4494–4496. [Google Scholar] [CrossRef]
- Roshchupkina, G.I.; Bobko, A.A.; Bratasz, A.; Reznikov, V.A.; Kuppusamy, P.; Khramtsov, V.V. In Vivo EPR Measurement of Glutathione in Tumor-Bearing Mice Using Improved Disulfide Biradical Probe. Free Radic. Biol. Med. 2008, 45, 312–320. [Google Scholar] [CrossRef]
- Song, C.; Hu, K.N.; Joo, C.G.; Swager, T.M.; Griffin, R.G. TOTAPOL: A Biradical Polarizing Agent for Dynamic Nuclear Polarization Experiments in Aqueous Media. J. Am. Chem. Soc. 2006, 128, 11385–11390. [Google Scholar] [CrossRef]
- Nagaraj, M.; Franks, T.W.; Saeidpour, S.; Schubeis, T. Surface Binding of TOTAPOL Assists Structural Investigations of Amyloid Fibrils by Dynamic Nuclear Polarization NMR Spectroscopy. ChemBioChem 2016, 17, 1308–1311. [Google Scholar] [CrossRef]
- Geiger, M.A.; Jagtap, A.P.; Kaushik, M.; Sun, H.; Stöppler, D.; Sigurdsson, S.T.; Corzilius, B.; Oschkinat, H. Efficiency of Water-Soluble Nitroxide Biradicals for Dynamic Nuclear Polarization in Rotating Solids at 9.4 T: BcTol-M and Cyolyl-TOTAPOL as New Polarizing Agents. Chem. A Eur. J. 2018, 24, 13485–13494. [Google Scholar] [CrossRef]
- Jagtap, A.P.; Geiger, M.A.; Stöppler, D.; Orwick-Rydmark, M.; Oschkinat, H.; Sigurdsson, S.T. BcTol: A Highly Water-Soluble Biradical for Efficient Dynamic Nuclear Polarization of Biomolecules. Chem. Commun. 2016, 52, 7020–7023. [Google Scholar] [CrossRef]
- Sauvée, C.; Casano, G.; Abel, S.; Rockenbauer, A.; Akhmetzyanov, D.; Karoui, H.; Siri, D.; Aussenac, F.; Maas, W.; Weber, R.T.; et al. Tailoring of Polarizing Agents in the BTurea Series for Cross-Effect Dynamic Nuclear Polarization in Aqueous Media. Chem. A Eur. J. 2016, 22, 5598–5606. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Dumarieh, R.; Xiao, Y.; Frederick, K.K. Stability of the Nitroxide Biradical AMUPol in Intact and Lysed Mammalian Cells. J. Magn. Reson. 2022, 336, 107150. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, V.K.; Keeler, E.G.; Bahri, S.; Ong, T.-C.; Daviso, E.; Colvin, M.T.; Griffin, R.G. Biradical Polarizing Agents at High Fields. J. Phys. Chem. B 2022, 126, 7847–7856. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, M.K.; Michaelis, V.K.; Walish, J.J.; Griffin, R.G.; Swager, T.M. High Field Dynamic Nuclear Polarization NMR with Surfactant Sheltered Biradicals. J. Phys. Chem. B 2014, 118, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Zagdoun, A.; Casano, G.; Ouari, O.; Schwarzwälder, M.; Rossini, A.J.; Aussenac, F.; Yulikov, M.; Jeschke, G.; Copéret, C.; Lesage, A.; et al. Large Molecular Weight Nitroxide Biradicals Providing Efficient Dynamic Nuclear Polarization at Temperatures up to 200 K. J. Am. Chem. Soc. 2013, 135, 12790–12797. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.; Casano, G.; Menzildjian, G.; Kaushik, M.; Stevanato, G.; Yulikov, M.; Jabbour, R.; Wisser, D.; Renom-Carrasco, M.; Thieuleux, C.; et al. TinyPols: A Family of Water-Soluble Binitroxides Tailored for Dynamic Nuclear Polarization Enhanced NMR Spectroscopy at 18.8 and 21.1 T. Chem. Sci. 2020, 11, 2810–2818. [Google Scholar] [CrossRef]
- Harrabi, R.; Halbritter, T.; Aussenac, F.; Dakhlaoui, O.; van Tol, J.; Damodaran, K.K.; Lee, D.; Paul, S.; Hediger, S.; Mentink-Vigier, F.; et al. Highly Efficient Polarizing Agents for MAS-DNP of Proton-Dense Molecular Solids. Angew. Chem. Int. Ed. 2022, 61, e202114103. [Google Scholar] [CrossRef]
- Mathies, G.; Caporini, M.A.; Michaelis, V.K.; Liu, Y.; Hu, K.N.; Mance, D.; Zweier, J.L.; Rosay, M.; Baldus, M.; Griffin, R.G. Efficient Dynamic Nuclear Polarization at 800 MHz/527 GHz with Trityl-Nitroxide Biradicals. Angew. Chem. Int. Ed. 2015, 54, 11770–11774. [Google Scholar] [CrossRef]
- Zhai, W.; Lucini Paioni, A.; Cai, X.; Narasimhan, S.; Medeiros-Silva, J.; Zhang, W.; Rockenbauer, A.; Weingarth, M.; Song, Y.; Baldus, M.; et al. Postmodification via Thiol-Click Chemistry Yields Hydrophilic Trityl-Nitroxide Biradicals for Biomolecular High-Field Dynamic Nuclear Polarization. J. Phys. Chem. B 2020, 124, 9047–9060. [Google Scholar] [CrossRef]
- Wisser, D.; Karthikeyan, G.; Lund, A.; Casano, G.; Karoui, H.; Yulikov, M.; Menzildjian, G.; Pinon, A.C.; Purea, A.; Engelke, F.; et al. BDPA-Nitroxide Biradicals Tailored for Efficient Dynamic Nuclear Polarization Enhanced Solid-State NMR at Magnetic Fields up to 21.1 T. J. Am. Chem. Soc. 2018, 140, 13340–13349. [Google Scholar] [CrossRef]
- Dane, E.L.; Maly, T.; Debelouchina, G.T.; Griffin, R.G.; Swager, T.M. Synthesis of a BDPA-TEMPO Biradical. Org. Lett. 2009, 11, 1871–1874. [Google Scholar] [CrossRef]
- Mandal, S.; Sigurdsson, S.T. On the Limited Stability of BDPA Radicals. Chem. A Eur. J. 2020, 26, 7486–7491. [Google Scholar] [CrossRef]
- Mandal, S.; Sigurdsson, S.T. Water-Soluble BDPA Radicals with Improved Persistence. Chem. Commun. 2020, 56, 13121–13124. [Google Scholar] [CrossRef]
- Liu, Y.; Villamena, F.A.; Rockenbauer, A.; Zweier, J.L. Trityl-Nitroxide Biradicals as Unique Molecular Probes for the Simultaneous Measurement of Redox Status and Oxygenation. Chem. Commun. 2010, 46, 628–630. [Google Scholar] [CrossRef]
- Liu, Y.; Villamena, F.A.; Song, Y.; Sun, J.; Rockenbauer, A.; Zweier, J.L. Synthesis of 14N- and 15N-Labeled Trityl-Nitroxide Biradicals with Strong Spin-Spin Interaction and Improved Sensitivity to Redox Status and Oxygen. J. Org. Chem. 2010, 75, 7796–7802. [Google Scholar] [CrossRef]
- Bothe, S.; Nowag, J.; Klimavičius, V.; Hoffmann, M.; Troitskaya, T.I.; Amosov, E.V.; Tormyshev, V.M.; Kirilyuk, I.; Taratayko, A.; Kuzhelev, A.; et al. Novel Biradicals for Direct Excitation Highfield Dynamic Nuclear Polarization. J. Phys. Chem. C 2018, 122, 11422–11432. [Google Scholar] [CrossRef]
- Cai, X.; Lucini Paioni, A.; Adler, A.; Yao, R.; Zhang, W.; Beriashvili, D.; Safeer, A.; Gurinov, A.; Rockenbauer, A.; Song, Y.; et al. Highly Efficient Trityl-Nitroxide Biradicals for Biomolecular High-Field Dynamic Nuclear Polarization. Chem. A Eur. J. 2021, 27, 12758–12762. [Google Scholar] [CrossRef]
- Linden, A.H.; Lange, S.; Franks, W.T.; Akbey, Ü.; Specker, E.; van Rossum, B.-J.; Oschkinat, H. Neurotoxin II Bound to Acetylcholine Receptors in Native Membranes Studied by Dynamic Nuclear Polarization NMR. J. Am. Chem. Soc. 2011, 133, 19266–19269. [Google Scholar] [CrossRef]
- Mccoy, K.M.; Rogawski, R.; Stovicek, O.; Mcdermott, A.E. Stability of Nitroxide Biradical TOTAPOL in Biological Samples. J. Magn. Reson. 2019, 303, 115–120. [Google Scholar] [CrossRef]
- Kirilyuk, I.A.; Bobko, A.A.; Semenov, S.V.; Komarov, D.A.; Irtegova, I.G.; Grigorev, I.A.; Bagryanskaya, E. Effect of Sterical Shielding on the Redox Properties of Imidazoline and Imidazolidine Nitroxides. J. Org. Chem. 2015, 80, 9118–9125. [Google Scholar] [CrossRef]
- Mentink-Vigier, F.; Marin-Montesinos, I.; Jagtap, A.P.; Halbritter, T.; Van Tol, J.; Hediger, S.; Lee, D.; Sigurdsson, S.T.; De Paëpe, G. Computationally Assisted Design of Polarizing Agents for Dynamic Nuclear Polarization Enhanced NMR: The AsymPol Family. J. Am. Chem. Soc. 2018, 140, 11013–11019. [Google Scholar] [CrossRef]
- Equbal, A.; Tagami, K.; Han, S. Balancing Dipolar and Exchange Coupling in Biradicals to Maximize Cross Effect Dynamic Nuclear Polarization. Phys. Chem. Chem. Phys. 2020, 22, 13569–13579. [Google Scholar] [CrossRef]
- Kubicki, D.J.; Casano, G.; Schwarzwälder, M.; Abel, S.; Sauvée, C.; Ganesan, K.; Yulikov, M.; Rossini, A.J.; Jeschke, G.; Copéret, C.; et al. Rational Design of Dinitroxide Biradicals for Efficient Cross-Effect Dynamic Nuclear Polarization. Chem. Sci. 2016, 7, 550–558. [Google Scholar] [CrossRef]
- Zagdoun, A.; Casano, G.; Ouari, O.; Lapadula, G.; Rossini, A.J.; Lelli, M.; Baffert, M.; Gajan, D.; Veyre, L.; Maas, W.E.; et al. A Slowly Relaxing Rigid Biradical for Efficient Dynamic Nuclear Polarization Surface-Enhanced NMR Spectroscopy: Expeditious Characterization of Functional Group Manipulation in Hybrid Materials. J. Am. Chem. Soc. 2012, 134, 2284–2291. [Google Scholar] [CrossRef]
- Stevanato, G.; Casano, G.; Kubicki, D.; Rao, Y.; Esteban Hofer, L.; Menzildjian, G.; Karoui, H.; Siri, D.; Cordova, M.; Yulikov, M.; et al. Open and Closed Radicals: Local Geometry Around Unpaired Electrons Governs MAS DNP Performance. J. Am. Chem. Soc. 2020, 142, 16587–16599. [Google Scholar] [CrossRef]
- Fehling, P.; Buckenmaier, K.; Dobrynin, S.A.; Morozov, D.A.; Polienko, Y.F.; Khoroshunova, Y.V.; Borozdina, Y.; Mayer, P.; Engelmann, J.; Scheffler, K.; et al. The Effects of Nitroxide Structure upon 1H Overhauser Dynamic Nuclear Polarization Efficacy at Ultralow-Field. J. Chem. Phys. 2021, 155, 144203. [Google Scholar] [CrossRef]
- Asanbaeva, N.B.; Gurskaya, L.Y.; Polienko, Y.F.; Rybalova, T.V.; Kazantsev, M.S.; Dmitriev, A.A.; Gritsan, N.P.; Haro-Mares, N.; Gutmann, T.; Buntkowsky, G.; et al. Effects of Spiro-Cyclohexane Substitution of Nitroxyl Biradicals on Dynamic Nuclear Polarization. Molecules 2022, 27, 3252. [Google Scholar] [CrossRef]
- White, J.L.; Haw, J.F. Nuclear Overhauser Effect in Solids. J. Am. Chem. Soc. 1990, 112, 5896–5898. [Google Scholar] [CrossRef]
- Hoffmann, M.M.; Bothe, S.; Gutmann, T.; Buntkowsky, G. Unusual Local Molecular Motions in the Solid State Detected by Dynamic Nuclear Polarization Enhanced NMR Spectroscopy. J. Phys. Chem. C 2017, 121, 22948–22957. [Google Scholar] [CrossRef]
- Kuzhelev, A.A.; Strizhakov, R.K.; Krumkacheva, O.A.; Polienko, Y.F.; Morozov, D.A.; Yu, G.; Pyshnyi, D.V.; Kirilyuk, I.A.; Fedin, M.V.; Bagryanskaya, E.G. Room-Temperature Electron Spin Relaxation of Nitroxides Immobilized in Trehalose: Effect of Substituents Adjacent to NO-Group. J. Magn. Reson. 2016, 266, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dobrynin, S.A.; Glazachev, Y.I.; Gatilov, Y.V.; Chernyak, E.I.; Salnikov, G.E.; Kirilyuk, I.A. Synthesis of 3,4-Bis(Hydroxymethyl)-2,2,5,5-Tetraethylpyrrolidin-1-Oxyl via 1,3-Dipolar Cycloaddition of Azomethine Ylide to Activated Alkene. J. Org. Chem. 2018, 83, 5392–5397. [Google Scholar] [CrossRef] [PubMed]
- Paletta, J.T.; Pink, M.; Foley, B.; Rajca, S.; Rajca, A. Synthesis and Reduction Kinetics of Sterically Shielded Pyrrolidine Nitroxides. Org. Lett. 2012, 14, 5322–5325. [Google Scholar] [CrossRef]
- Ovcherenko, S.S.; Chinak, O.A.; Chechushkov, A.V.; Dobrynin, S.A.; Kirilyuk, I.A.; Krumkacheva, O.A.; Richter, V.A.; Bagryanskaya, E.G. Uptake of Cell-Penetrating Peptide RL2 by Human Lung Cancer Cells: Monitoring by Electron Paramagnetic Resonance and Confocal Laser Scanning Microscopy. Molecules 2021, 26, 5442. [Google Scholar] [CrossRef] [PubMed]
- Bobko, A.A.; Kirilyuk, I.A.; Grigor’ev, I.A.; Zweier, J.L.; Khramtsov, V.V. Reversible Reduction of Nitroxides to Hydroxylamines: Roles for Ascorbate and Glutathione. Free Radic. Biol. Med. 2007, 42, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.; Schweiger, A. EasySpin, a Comprehensive Software Package for Spectral Simulation and Analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Cory, D.G.; Ritchey, W.M. Suppression of Signals from the Probe in Bloch Decay Spectra. J. Magn. Reson. 1988, 80, 128–132. [Google Scholar] [CrossRef]
- Comellas, G.; Lopez, J.J.; Nieuwkoop, A.J.; Lemkau, L.R.; Rienstra, C.M. Straightforward, Effective Calibration of SPINAL-64 Decoupling Results in the Enhancement of Sensitivity and Resolution of Biomolecular Solid-State NMR. J. Magn. Reson. 2011, 209, 131–135. [Google Scholar] [CrossRef]
- Hoffmann, M.M.; Bothe, S.; Brodrecht, M.; Klimavicius, V.; Haro-Mares, N.B.; Gutmann, T.; Buntkowsky, G. Direct and Indirect Dynamic Nuclear Polarization Transfer Observed in Mesoporous Materials Impregnated with Nonionic Surfactant Solutions of Polar Polarizing Agents. J. Phys. Chem. C. 2020, 124, 5145–5156. [Google Scholar] [CrossRef]
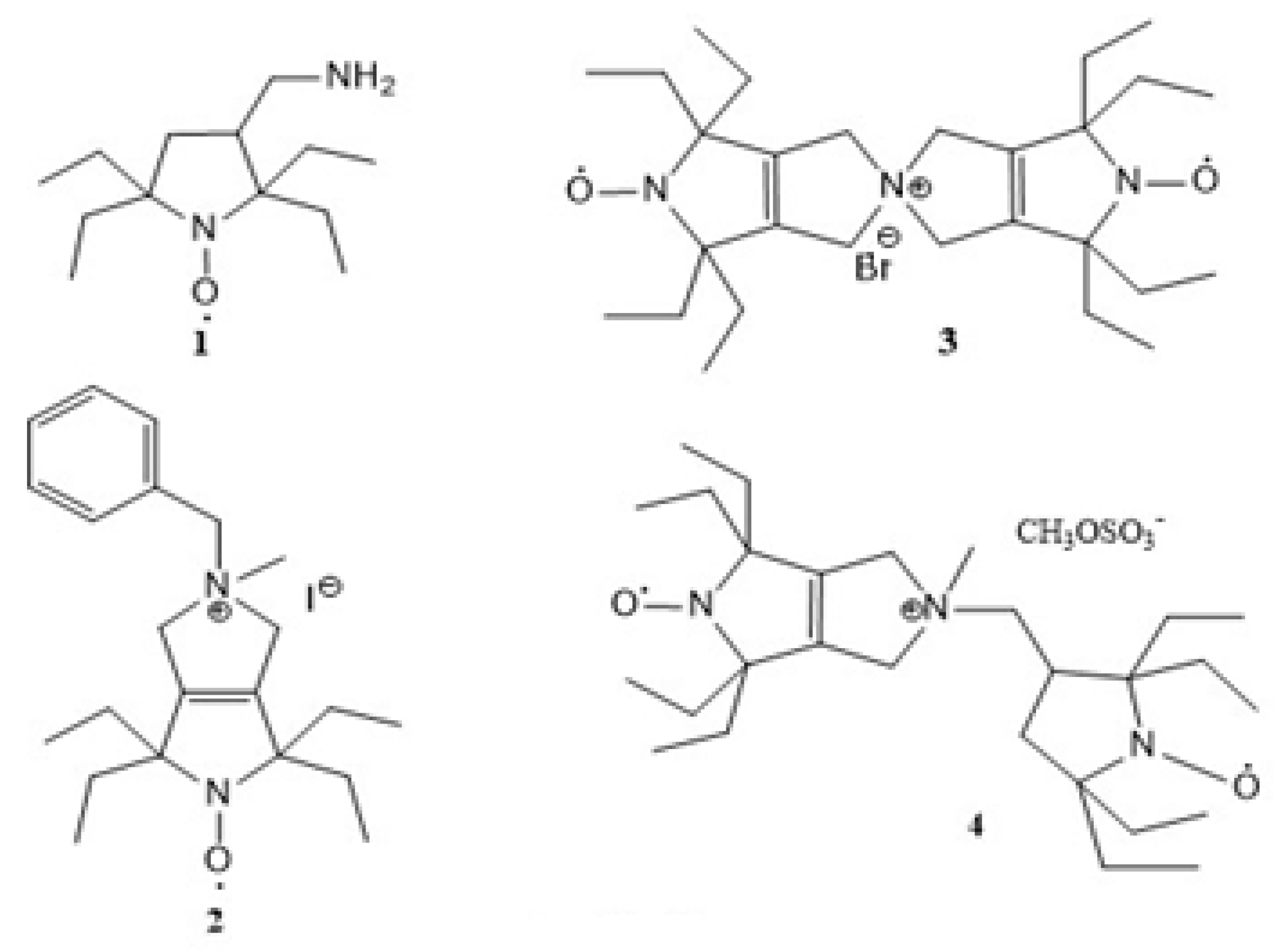
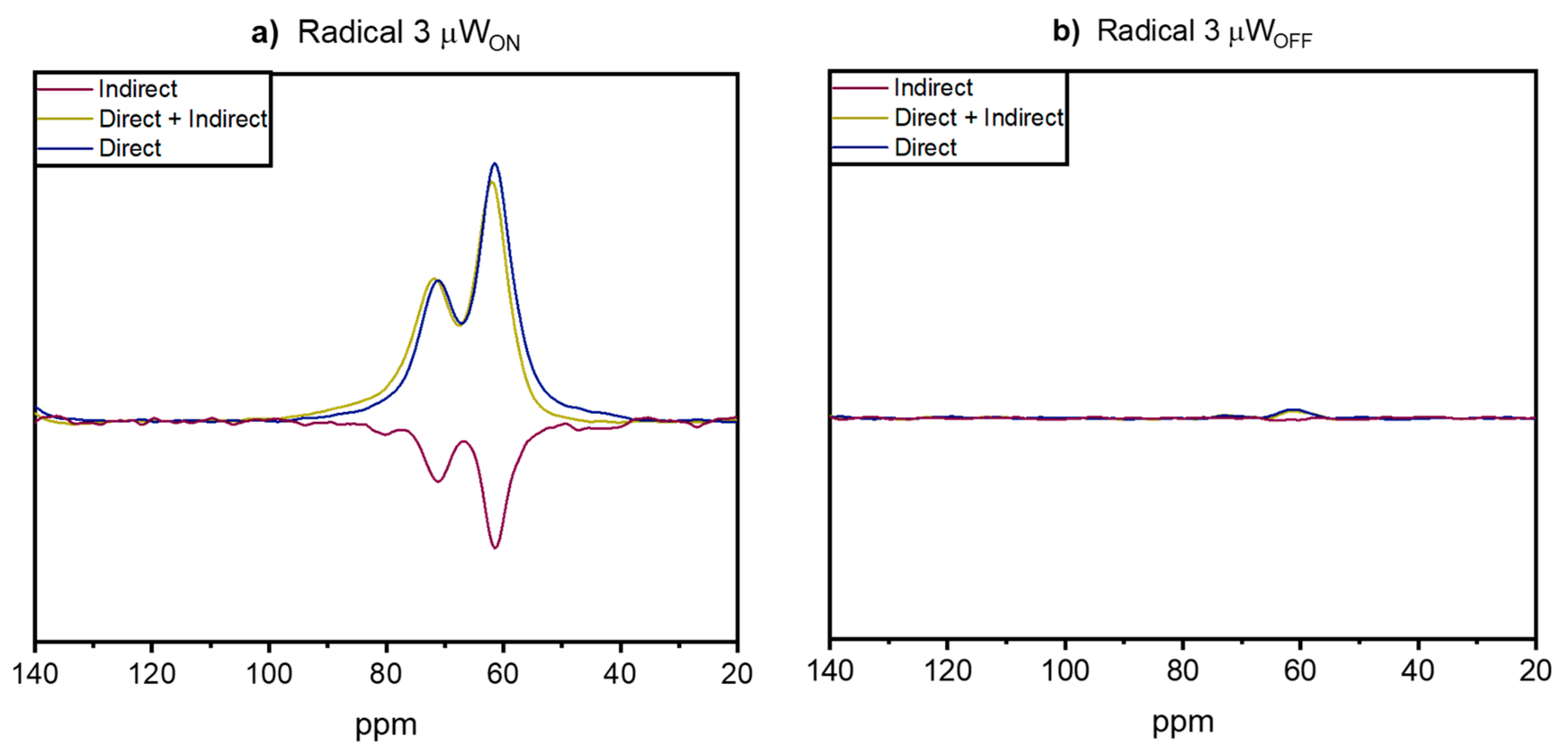
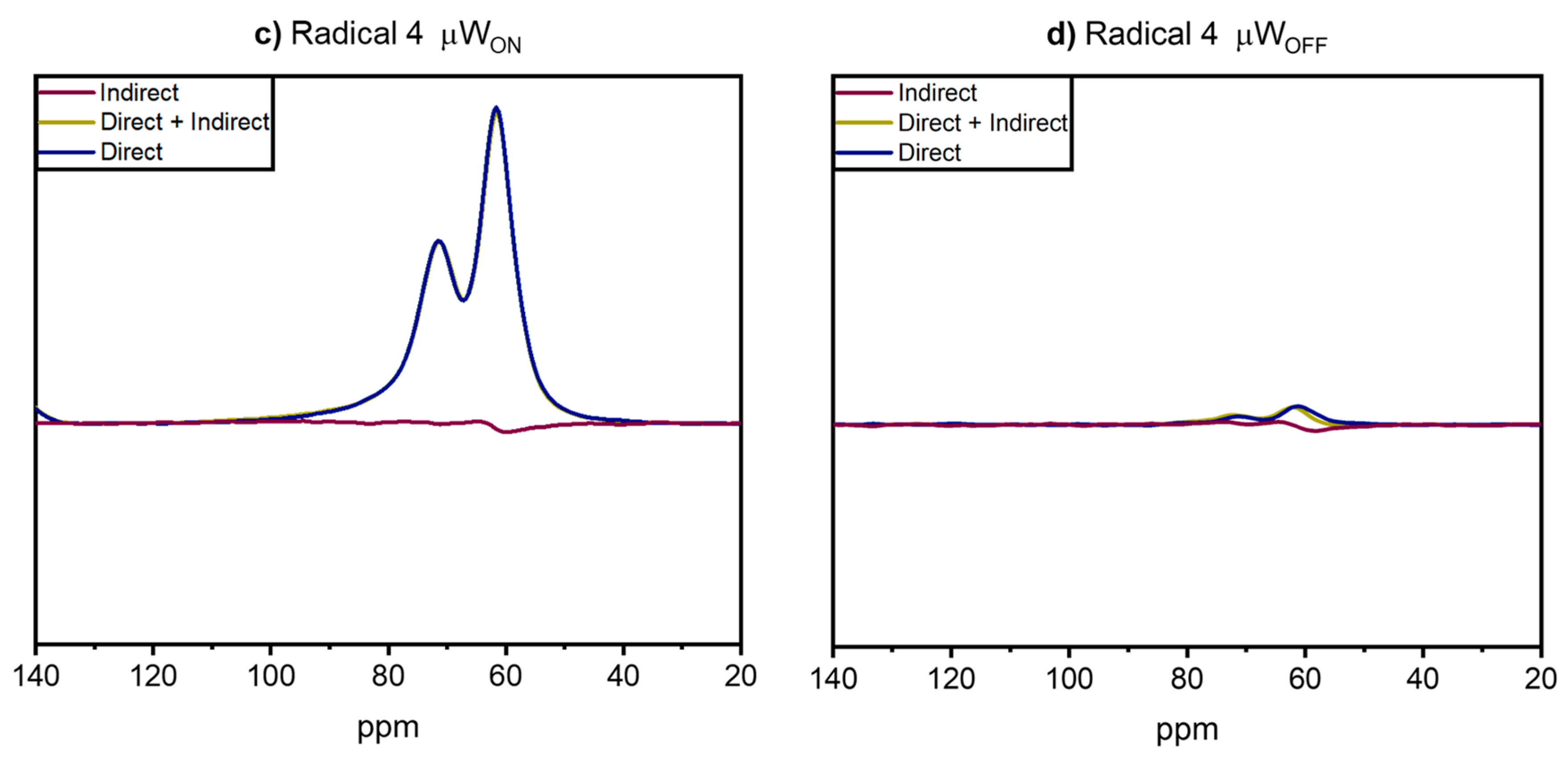

| Radical | EPR Spectrum | Parameters of Simulations |
|---|---|---|
| 1 |  | AN iso = 43.0 ± 0.2 MHz AH iso = 7.2 ± 0.2 MHz |
| 2 |  | AN iso = 43.7 ± 0.2 MHz |
| 3 |  | AN iso = 43.7 ± 0.2 MHz J = 206 ± 2 MHz |
| 4 | 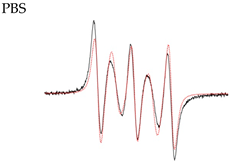 | AN1 iso = 44.0 ± 0.2 MHz AN2 iso = 43.2 ± 0.2 MHz J = 0 σ(J) = 120 MHz J distribution |
| Biradical | T2, μs | T1, ms |
|---|---|---|
| 3 | 1.01 ± 0.01 | 0.31 ± 0.01 |
| 4 | 0.92 ± 0.01 | 0.29 ± 0.01 |
| Radical | kobs × 103, min−1 | k × 103, M−1s−1 |
|---|---|---|
| 1 | 1.4 ± 0.1 | 0.23 ± 0.02 |
| 2 | 11.6 ± 0.3 | 1.93 ± 0.05 |
| 3 | 9.7 ± 0.2 | 1.62 ± 0.03 |
| 4 | 6.2 ± 0.2 | 1.03 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asanbaeva, N.B.; Dobrynin, S.A.; Morozov, D.A.; Haro-Mares, N.; Gutmann, T.; Buntkowsky, G.; Bagryanskaya, E.G. An EPR Study on Highly Stable Nitroxyl-Nitroxyl Biradicals for Dynamic Nuclear Polarization Applications at High Magnetic Fields. Molecules 2023, 28, 1926. https://doi.org/10.3390/molecules28041926
Asanbaeva NB, Dobrynin SA, Morozov DA, Haro-Mares N, Gutmann T, Buntkowsky G, Bagryanskaya EG. An EPR Study on Highly Stable Nitroxyl-Nitroxyl Biradicals for Dynamic Nuclear Polarization Applications at High Magnetic Fields. Molecules. 2023; 28(4):1926. https://doi.org/10.3390/molecules28041926
Chicago/Turabian StyleAsanbaeva, Nargiz B., Sergey A. Dobrynin, Denis A. Morozov, Nadia Haro-Mares, Torsten Gutmann, Gerd Buntkowsky, and Elena G. Bagryanskaya. 2023. "An EPR Study on Highly Stable Nitroxyl-Nitroxyl Biradicals for Dynamic Nuclear Polarization Applications at High Magnetic Fields" Molecules 28, no. 4: 1926. https://doi.org/10.3390/molecules28041926
APA StyleAsanbaeva, N. B., Dobrynin, S. A., Morozov, D. A., Haro-Mares, N., Gutmann, T., Buntkowsky, G., & Bagryanskaya, E. G. (2023). An EPR Study on Highly Stable Nitroxyl-Nitroxyl Biradicals for Dynamic Nuclear Polarization Applications at High Magnetic Fields. Molecules, 28(4), 1926. https://doi.org/10.3390/molecules28041926








