Effects of Antioxidant Combinations on the Renal Toxicity Induced Rats by Gold Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Changes in Body Weight and Kidney Function Markers
2.2. Changes in Renal Antioxidant and Inflammatory Markers
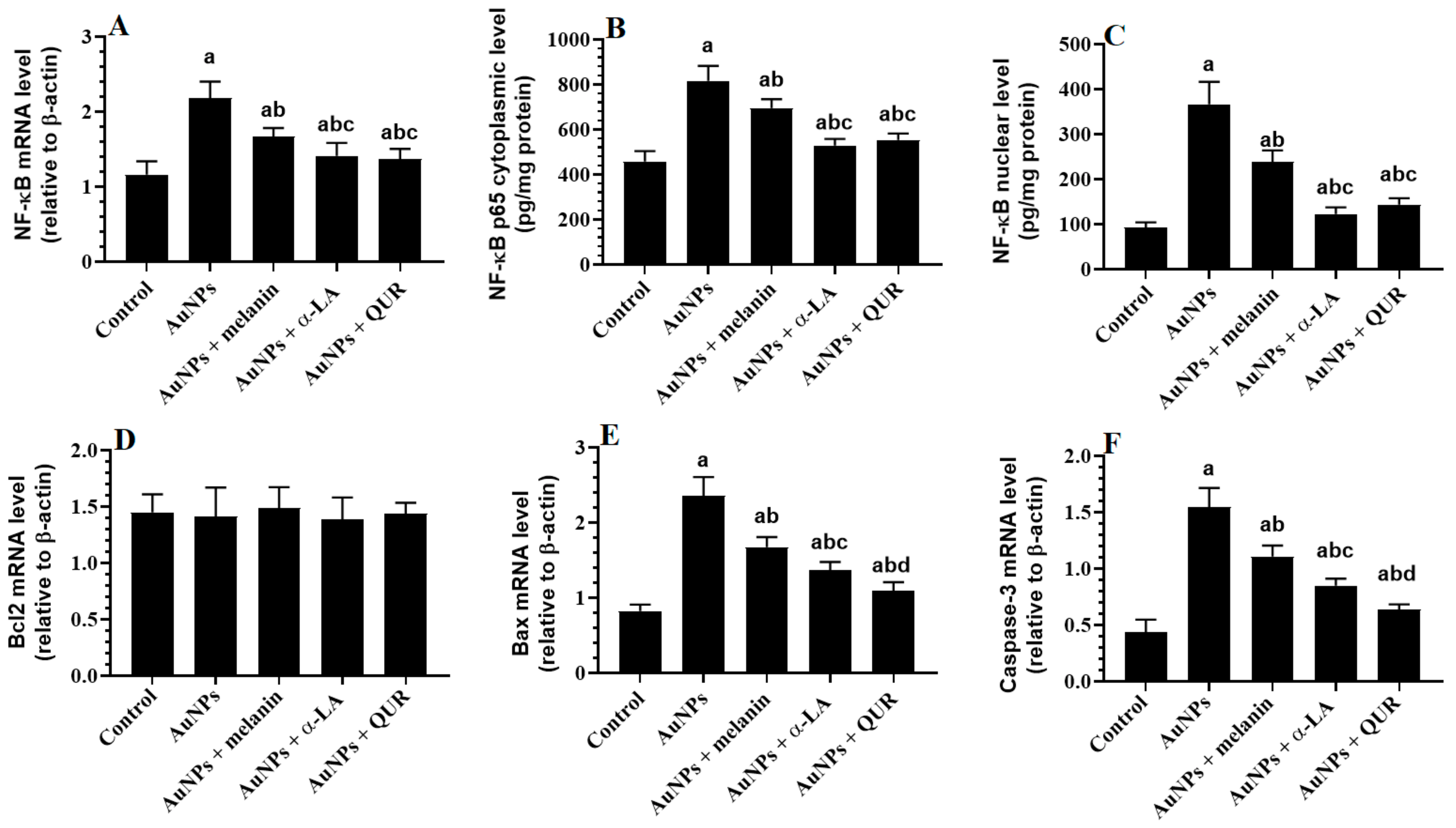
2.3. Changes in the Transcriptional Activity of NF-κB and Apoptotic/Anti-Apoptotic Markers
2.4. Changes in the Mitochondrial Coupling, Mitochondrial Membrane Potential (of mtPTP), and Expression of NOX4
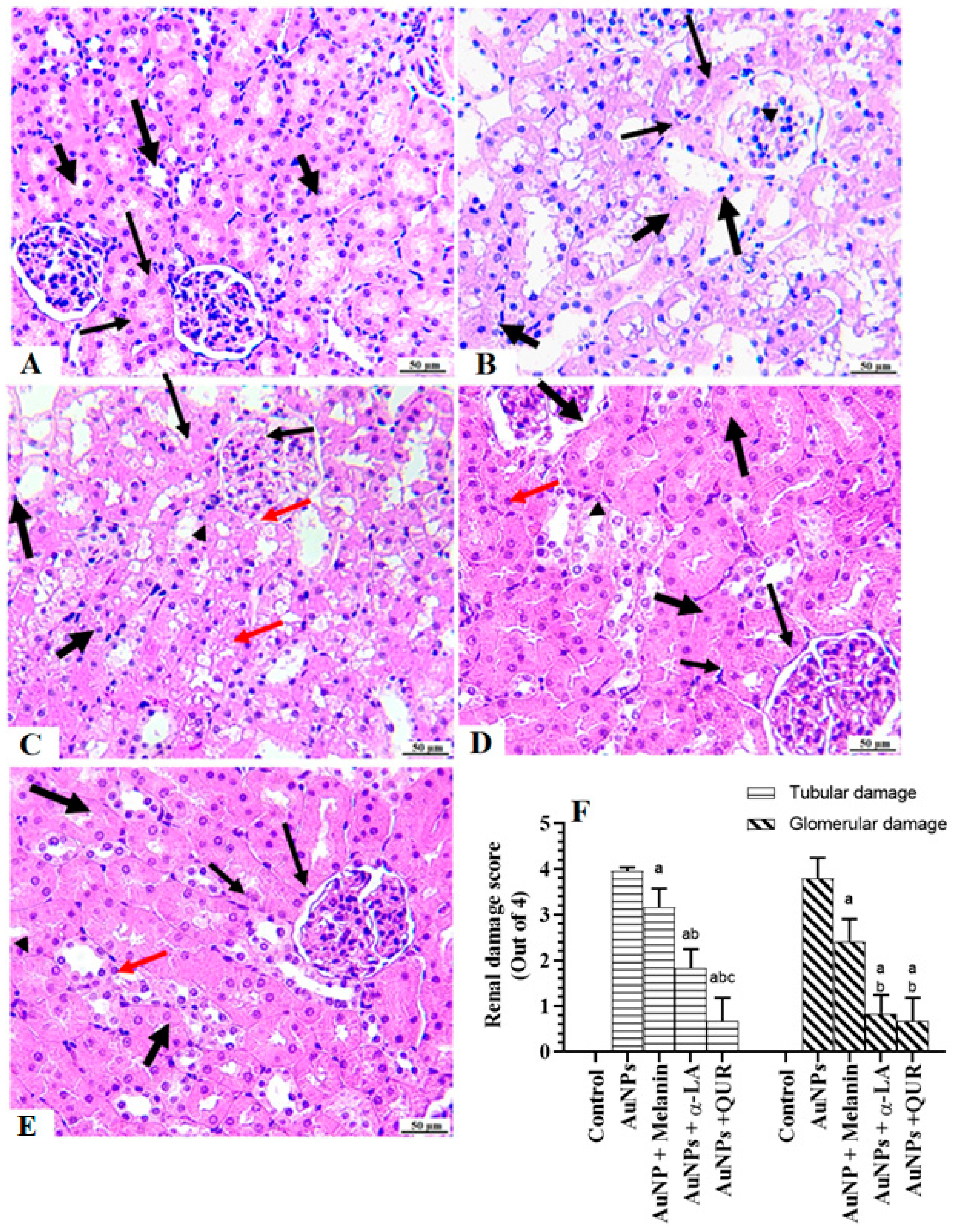
2.5. Histological Findings
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Urine, Blood, and Their Collection and Preparation
4.4. Biochemical Analysis of the Sera, Urine, and Homogenates
4.5. Analysis of mtPTP Potential and Mitochondrial Respiration
4.6. Real-Time PCR (qPCR)
4.7. Histopathological Evaluation
4.8. Statistical Analysis
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tejchman, K.; Kotfis, K.; Sieńko, J. Biomarkers and Mechanisms of Oxidative Stress-Last 20 Years of Research with an Emphasis on Kidney Damage and Renal Transplantation. Int. J. Mol. Sci. 2021, 22, 8010. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Su, H.; Zhang, C. Role of NADPH Oxidase in Metabolic Disease-Related Renal Injury: An Update. Oxid. Med. Cell Longev. 2016, 2016, 7813072. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Scorza, F.A. Renal manifestations of primary mitochondrial disorders. Biomed. Rep. 2017, 6, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Takemura, K.; Nishi, H.; Inagi, R. Mitochondrial Dysfunction in Kidney Disease and Uremic Sarcopenia. Front. Physiol. 2020, 11, 565023. [Google Scholar] [CrossRef] [PubMed]
- Holterman, C.E.; Read, N.C.; Kennedy, C.R. Nox and renal disease. Clin. Sci. 2015, 128, 465–481. [Google Scholar] [CrossRef]
- Ogura, S.; Shimosawa, T. Oxidative stress and organ damages. Curr. Hypertens. Rep. 2014, 16, 452. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Lei, X.G.; Zhu, J.H.; Cheng, W.H.; Bao, Y.; Ho, Y.S.; Reddi, A.R.; Holmgren, A.; Arnér, E.S. Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications. Physiol. Rev. 2016, 96, 307–364. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Mullins, L.J.; Conway, B.R.; Menzies, R.I.; Denby, L.; Mullins, J.J. Renal disease pathophysiology and treatment: Contributions from the rat. Dis. Model Mech. 2016, 9, 1419–1433. [Google Scholar] [CrossRef]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Tomino, Y. Pathogenesis and treatment of chronic kidney disease: A review of our recent basic and clinical data. Kidney Blood Press Res. 2014, 39, 450–489. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, P.; Khurana, S.; Ganguly, N.K.; Kukreti, R.; Saso, L.; Rana, D.S.; Taneja, V.; Bhargava, V. Implications of oxidative stress in chronic kidney disease: A review on current concepts and therapies. Kidney Res. Clin. Pract. 2021, 40, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Meng, X.F.; Zhang, C. Role of NADPH oxidase-mediated reactive oxygen species in podocyte injury. Biomed. Res. Int. 2013, 2013, 839761. [Google Scholar] [CrossRef]
- Lee, H.; Jose, P.A. Coordinated Contribution of NADPH Oxidase- and Mitochondria-Derived Reactive Oxygen Species in Metabolic Syndrome and Its Implication in Renal Dysfunction. Front. Pharmacol. 2021, 12, 670076. [Google Scholar] [CrossRef]
- Fortuño, A.; Beloqui, O.; San José, G.; Moreno, M.U.; Zalba, G.; Díez, J. Increased phagocytic nicotinamide adenine dinucleotide phosphate oxidase-dependent superoxide production in patients with early chronic kidney disease. Kidney Int. Suppl. 2005, 99, S71–S75. [Google Scholar] [CrossRef]
- DuPont, J.J.; Ramick, M.G.; Farquhar, W.B.; Townsend, R.R.; Edwards, D.G. NADPH oxidase-derived reactive oxygen species contribute to impaired cutaneous microvascular function in chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2014, 306, F1499–F1506. [Google Scholar] [CrossRef]
- Horie, M.; Tabei, Y. Role of oxidative stress in nanoparticles toxicity. Free. Radic. Res. 2021, 55, 331–342. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.; Qaid, H.A.; Al-Mohy, Y.; Al-Ayed, M.S. Effects of quercetin and arginine on the nephrotoxicity and lipid pe-roxidation induced by gold nanoparticles in vivo. Int. J. Nanomed. 2018, 13, 7765. [Google Scholar] [CrossRef]
- Sung, J.H.; Ji, J.H.; Park, J.D.; Song, M.Y.; Song, K.S.; Ryu, H.R.; Yoon, J.U.; Jeon, K.S.; Jeong, J.; Han, B.S.; et al. Subchronic inhalation toxicity of gold nanoparticles. Part Fibre. Toxicol. 2011, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.A.; Abdelmottaleb Moussa, S.A. The gold nanoparticle size and exposure duration effect on the liver and kidney function of rats: In vivo. Saudi J. Biol. Sci. 2013, 20, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Bartneck, M.; Ritz, T.; Keul, H.A.; Wambach, M.; Bornemann, J.; Gbureck, U.; Ehling, J.; Lammers, T.; Heymann, F.; Gassler, N.; et al. Peptide-functionalized gold nanorods increase liver injury in hepatitis. ACS Nano. 2012, 6, 8767–8777. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Abdelhalim, M.A.; Alhomida, A.S.; Al-Ayed, M.S. Effects of naked gold nanoparticles on proinflammatory cytokines mRNA expression in rat liver and kidney. Biomed. Res. Int. 2013, 2013, 590730. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.A.K.; Moussa, S.A.A.; Qaid, H.A.Y. The protective role of quercetin and arginine on gold nanoparticles induced hepatotoxicity in rats. Int. J. Nanomed. 2018, 1, 2821–2825. [Google Scholar] [CrossRef]
- Kassab, A.A.; Moustafa, K.A.; Ragab, M.H.; Ragab, A.M. The Biological Effect of Different Doses of Gold Nanoparticles on the Liver of Female Rats: A Histological and Immunohistochemical Study. Egypt. J. Histol. 2021, 44, 489–502. [Google Scholar] [CrossRef]
- Chen, T.Y.; Chen, M.R.; Liu, S.W.; Lin, J.Y.; Yang, Y.T.; Huang, H.Y.; Chen, J.K.; Yang, C.S.; Lin, K.M. Assessment of Polyethylene Gly-col-Coated Gold Nanoparticle Toxicity and Inflammation In Vivo Using NF-κB Reporter Mice. Int. J. Mol. Sci. 2020, 21, 8158. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.; Jarrar, B.M. Gold nanoparticles induced cloudy swelling to hydropic degeneration, cytoplasmic hyaline vacuolation, polymorphism, binucleation, karyopyknosis, karyolysis, karyorrhexis and necrosis in the liver. Lipids Health Dis. 2011, 10, 166. [Google Scholar] [CrossRef]
- Tamadon, M.R.; Zahmatkesh, M.; Beladi Mousavi, S.S. Administration of antioxidants in chronic kidney disease. J. Nephro-Pharm. 2015, 4, 9–11. [Google Scholar]
- Rochette, L.; Ghibu, S.; Richard, C.; Zeller, M.; Cottin, Y.; Vergely, C. Direct and indirect antioxidant properties of α-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 2013, 57, 114–125. [Google Scholar] [CrossRef]
- Kim, E.; Panzella, L.; Napolitano, A.; Payne, G.F. Redox Activities of Melanins Investigated by Electrochemical Reverse Engi-neering: Implications for their Roles in Oxidative Stress. J. Invest. Dermatol. 2020, 140, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; McCullough, P.A. Lipoic Acid in the Prevention of Acute Kidney Injury. Nephron 2016, 134, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Vicente, L.; González-Calle, D.; Casanova, A.G.; Hernández-Sánchez, M.T.; Prieto, M.; Rama-Merchán, J.C.; Martín-Moreiras, J.; Martín-Herrero, F.; Sánchez, P.L.; López-Hernández, F.J.; et al. Quercetin, a Promising Clinical Candidate for The Prevention of Contrast-Induced Nephropathy. Int. J. Mol. Sci. 2019, 20, 4961. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Jiang, D.; Rosenkrans, Z.T.; Ehlerding, E.B.; Ni, D.; Qi, C.; Kutyreff, C.J.; Barnhart, T.E.; Engle, J.W.; Huang, P.; et al. A Mela-nin-Based Natural Antioxidant Defense Nanosystem for Theranostic Application in Acute Kidney Injury. Adv. Funct. Mater. 2019, 29, 1904833. [Google Scholar] [CrossRef]
- Hu, Q.; Qu, C.; Xiao, X.; Zhang, W.; Jiang, Y.; Wu, Z.; Song, D.; Peng, X.; Ma, X.; Zhao, Y. Flavonoids on diabetic nephropathy: Advances and therapeutic opportunities. Chin. Med. 2021, 16, 74. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.; Moussa, S.A.; Qaid, H.A.; Al-Ayed, M.S. Potential effects of different natural antioxidants on inflammatory damage and oxidative-mediated hepatotoxicity induced by gold nanoparticles. Int. J. Nanomed. 2018, 13, 7931. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K.; Qaid, H.A.; Al-Mohy, Y.H.; Ghannam, M.M. The Protective Roles of Vitamin E and α-Lipoic Acid Against Nephrotoxicity, Lipid Peroxidation, and Inflammatory Damage Induced by Gold Nanoparticles. Int. J. Nanomed. 2020, 15, 729–734. [Google Scholar] [CrossRef]
- Rana, S.V.S. A Comprehensive Assessment of Hepatotoxicity Induced by Engineered Nanoparticles-A Review. J. Toxicol. Risk Assess. 2020, 6, 035. [Google Scholar]
- Vargas, F.; Romecín, P.; García-Guillén, A.I.; Wangesteen, R.; Vargas-Tendero, P.; Paredes, M.D.; Atucha, N.M.; García-Estañ, J. Fla-vonoids in Kidney Health and Disease. Front. Physiol. 2018, 9, 394. [Google Scholar] [CrossRef]
- Yang, H.; Song, Y.; Liang, Y.N.; Li, R. Quercetin Treatment Improves Renal Function and Protects the Kidney in a Rat Model of Adenine-Induced Chronic Kidney Disease. Med. Sci. Monit. 2018, 24, 4760–4766. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Souza, M.T.S.; Duarte, A.B.S.; Sousa, D.P. Mechanistic Aspects and Therapeutic Potential of Quercetin against COVID-19-Associated Acute Kidney Injury. Molecules 2020, 25, 5772. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Ma, D.; Luo, Y.; Tang, S.; Li, Y.; Chen, G.; Wang, L.; Hou, Z.; Shen, C.; Lu, H.; et al. Quercetin alleviates chronic renal failure by targeting the PI3k/Akt pathway. Bioengineered 2021, 12, 6538–6558. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Dis-ease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Lossi, L. The concept of intrinsic versus extrinsic apoptosis. Biochem. J. 2022, 479, 357–384. [Google Scholar] [CrossRef] [PubMed]
- Kulsoom, B.; Shamsi, T.S.; Afsar, N.A.; Memon, Z.; Ahmed, N.; Hasnain, S.N. Bax, Bcl-2, and Bax/Bcl-2 as prognostic markers in acute myeloid leukemia: Are we ready for Bcl-2-directed therapy? Cancer Manag. Res. 2018, 10, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Kaltschmidt, C.; Hofmann, T.G.; Hehner, S.P.; Dröge, W.; Schmitz, M.L. The pro- or anti-apoptotic function of NF-kappaB is determined by the nature of the apoptotic stimulus. Eur. J. Biochem. 2000, 267, 3828–3835. [Google Scholar] [CrossRef]
- Khandelwal, N.; Simpson, J.; Taylor, G.; Rafique, S.; Whitehouse, A.; Hiscox, J.; Stark, L.A. Nucleolar NF-κB/RelA mediates apoptosis by causing cytoplasmic relocalization of nucleophosmin. Cell Death Differ. 2011, 18, 1889–1903. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- White, S.; Lin, L.; Hu, K. NF-κB and tPA Signaling in Kidney and Other Diseases. Cells 2020, 9, 1348. [Google Scholar] [CrossRef]
- Song, N.; Thaiss, F.; Guo, L. NFκB and Kidney Injury. Front. Immunol. 2019, 10, 815. [Google Scholar] [CrossRef]
- Dennis, J.M.; Witting, P.K. Protective Role for Antioxidants in Acute Kidney Disease. Nutrients 2017, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.L.; Leung, J.C.; Silverstein, D.M. Pro- and anti-inflammatory cytokines in chronic pediatric dialysis patients: Effect of aspirin. Clin. J. Am. Soc. Nephrol. 2006, 1, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. 2006, 48, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Bazeley, J.; Bieber, B.; Li, Y.; Morgenstern, H.; de Sequera, P.; Combe, C.; Yamamoto, H.; Gallagher, M.; Port, F.K.; Robinson, B.M. C-reactive protein and prediction of 1-year mortality in prevalent hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2452–2461. [Google Scholar] [CrossRef]
- De Oliveira Júnior, W.V.; Sabino Ade, P.; Figueiredo, R.C.; Rios, D.R. Inflammation and poor response to treatment with eryth-ropoietin in chronic kidney disease. J. Bras. Nefrol. 2015, 37, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef]
- Krata, N.; Zagożdżon, R.; Foroncewicz, B.; Mucha, K. Oxidative Stress in Kidney Diseases: The Cause or the Consequence? Arch. Immunol. Exp. 2018, 66, 211–220. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid. Med. Cell Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran Biomed. J. 2016, 20, 1–11. [Google Scholar]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Chornenka, N.; Raetska, Y.; Grebinyk, D.; Dranitsina, A.; Savchuk, O.; Beregova, T.; Ostapchenko, L. Protective antioxidant effect of melanin against chemical burn-induced esophageal injury. Biomed. Res. Ther. 2018, 5, 2712–2718. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Shi, F.; Yang, L.; Ye, M. Protection effect of intracellular melanin from Lachnum YM156 and Haikunshenxi capsule combination on adenine-induced chronic renal failure in mice. Medchemcomm 2017, 8, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Marsh, S.A.; Laursen, P.B.; Pat, B.K.; Gobe, G.C.; Coombes, J.S. Bcl-2 in endothelial cells is increased by vitamin E and alpha-lipoic acid supplementation but not exercise training. J. Mol. Cell Cardiol. 2005, 38, 445–451. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Yang, L.; Li, M.; Zhong, X. Effect of quercetin on the expression of Bcl-2/Bax apoptotic proteins in endometrial cells of lipopolysaccharide-induced-abortion. J. Tradit. Chin. Med. 2016, 36, 737–742. [Google Scholar]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef]
- Quan, J.H.; Gao, F.F.; Chu, J.Q.; Cha, G.H.; Yuk, J.M.; Wu, W.; Lee, Y.H. Silver nanoparticles induce apoptosis via NOX4-derived mi-tochondrial reactive oxygen species and endoplasmic reticulum stress in colorectal cancer cells. Nanomedicine 2021, 16, 1357–1375. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Y.; Shi, J.; Wang, C.; Yu, Z.; Zhang, H. NOX4- and Nrf2-mediated oxidative stress induced by silver nanoparticles in vascular endothelial cells. J. Appl. Toxicol. 2017, 37, 1428–1437. [Google Scholar] [CrossRef]
- Ong, S.L.; Vohra, H.; Zhang, Y.; Sutton, M.; Whitworth, J.A. The effect of alpha-lipoic acid on mitochondrial superoxide and glu-cocorticoid-induced hypertension. Oxidative Med. Cell. Longev. 2013, 2013, 517045. [Google Scholar] [CrossRef]
- Sethumadhavan, S.; Chinnakannu, P. L-carnitine and alpha-lipoic acid improve age-associated decline in mitochondrial res-piratory chain activity of rat heart muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 650–659. [Google Scholar] [CrossRef]
- Huang, Y.P.; Jin, H.Y.; Yu, H.P. Inhibitory effects of alpha-lipoic acid on oxidative stress in the rostral ventrolateral medulla in rats with salt-induced hypertension. Int. J. Mol. Med. 2017, 39, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Elhadidy, M.G.; Elmasry, A.; Elsayed, H.R.H.; El-Nablaway, M.; Hamed, S.; Elalfy, M.M.; Rabei, M.R. Modulation of COX-2 and NADPH oxidase-4 by alpha-lipoic acid ameliorates busulfan-induced pulmonary injury in rats. Heliyon 2021, 7, e08171. [Google Scholar] [CrossRef] [PubMed]
- Kocak, A.; Ural, C.; Harmanci, D.; Oktan, M.A.; Afagh, A.; Sarioglu, S.; Yilmaz, O.; Birlik, M.; Akdogan, G.G.; Cavdar, Z. Protective effects of alpha-lipoic acid on bleomycin-induced skin fibrosis through the repression of NADPH Oxidase 4 and TGF-β1/Smad3 signaling pathways. Hum. Exp. Toxicol. 2022, 41, 9603271211065975. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, Z.; Chang, X.; Cong, G.; Hao, L. Quercetin attenuates vascular calcification by inhibiting oxidative stress and mito-chondrial fission. Vasc. Pharmacol. 2017, 88, 21–29. [Google Scholar] [CrossRef]
- Kicinska, A.; Jarmuszkiewicz, W. Flavonoids and Mitochondria: Activation of Cytoprotective Pathways? Molecules 2020, 25, 3060. [Google Scholar] [CrossRef]
- Waseem, M.; Kaushik, P.; Dutta, S.; Chakraborty, R.; Hassan, M.I.; Parvez, S. Modulatory Role of Quercetin in Mitochondrial Dysfunction in Titanium Dioxide Nanoparticle-Induced Hepatotoxicity. ACS Omega 2022, 7, 3192–3202. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, L.; Guo, X.; Zhang, S.; Wang, J.; Zhou, F.; Liu, L.; Tang, Y.; Yao, P. Quercetin attenuates high fat diet-induced athero-sclerosis in apolipoprotein E knockout mice: A critical role of NADPH oxidase. Food Chem. Toxicol. 2017, 105, 22–33. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway. Chem. Biol. Interact. 2018, 284, 32–40. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zhang, Z.Y.; Wang, R.X. Protective Mechanisms of Quercetin Against Myocardial Ischemia Reperfusion Injury. Front. Physiol. 2020, 11, 956. [Google Scholar] [CrossRef]
- Yagmurca, M.; Yasar, Z.; Bas, O. Effects of quercetin on kidney injury induced by doxorubicin. Bratisl Lek Listy. 2015, 116, 486–489. [Google Scholar] [CrossRef]
- El-Sayed, E.M.; Mansour, A.M.; El-Sawy, W.S. Alpha lipoic acid prevents doxorubicin-induced nephrotoxicity by mitigation of oxidative stress, inflammation, and apoptosis in rats. J. Biochem. Mol. Toxicol. 2017, 31, e21940. [Google Scholar] [CrossRef] [PubMed]
- Zonooz, S.R.; Hasani, M.; Morvaridzadeh, M.; Pizarro, A.B.; Heydari, H.; Yosaee, S.; Rezamand, G.; Heshmati, J. Effect of alpha-lipoic acid on oxidative stress parameters: A systematic review and meta-analysis. J. Funct. Foods 2021, 87, 104774. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Q.; Li, J.; Hou, G.; Chen, T.; Ye, M. Nephroprotective effects of Lachnum melanin against acute kidney injury induced by cisplatin in mice. Process Biochem. 2019, 83, 198–205. [Google Scholar] [CrossRef]
- Bazzano, T.; Restel, T.I.; Porfirio, L.C.; Souza, A.S.; Silva, I.S. Renal biomarkers of male and female Wistar rats (Rattus norvegicus) undergoing renal ischemia and reperfusion. Acta Cir. Bras. 2015, 30, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, W.H.; Alshammari, G.M.; Alshuniaber, M.A.; Husain, M.; Alawwad, S.A.; Al-Ayesh, S.T.; Yahya, M.A.; Aldawood, A.S. The protective effect of isoliquiritigenin against doxorubicin-induced nephropathy in rats entails activation of Nrf2 signaling as one key mechanism. J. King Saud. Univ. Sci. 2022, 34, 102165. [Google Scholar] [CrossRef]
- Eid, R.A. Acylated ghrelin protection inhibits apoptosis in the remote myocardium post-myocardial infarction by inhibiting calcineurin and activating ARC. Arch. Physiol. Biochem. 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Li, C.; Jung, J.Y.; Shin, S.J.; Choi, B.S.; Lim, S.W.; Sun, B.K.; Kim, Y.S.; Kim, J.; Chang, Y.S.; et al. Preconditioning with erythropoietin protects against subsequent ischemia-reperfusion injury in rat kidney. FASEB J. 2003, 17, 1754–1755. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Control | AuNPs | AuNPs + Melanin | AuNPs + α-LA | AuNPs + QUR |
|---|---|---|---|---|---|
| Final body weight (g) | 331 ± 15.6 | 328 ± 17.1 | 318 ± 21 | 329 ± 16 | 322 ± 18 |
| Serum | |||||
| Albumin (g/dL) | 6.3 ± 0.74 | 3.4 ± 0.48 a | 4.12 ± 0.37 ab | 5.1 ± 0.61 abc | 5.9 ± 0.53 bcd |
| Urea (mg/dl) | 5.44 ± 0.87 | 35.6 ± 3.2 a | 19.5 ± 2.1 ab | 12.8 ± 1.9 abc | 7.8 ± 1.1 abcd |
| Creatinine (mg/dl) | 0.58 ± 0.08 | 2.56 ± 0.23 a | 1.64 ± 0.17 ab | 1.1 ± 0.15 abc | 0.74 ± 0.09 abcd |
| Urine | |||||
| Volume (mL/24 h) | 10.4 ± 2.1 | 22.3 ± 2.1 a | 17.5 ± 1.3 ab | 15.1 ± 1.2 abc | 12.2 ± 1.5 abcd |
| Urine flow (µL/min) | 6.8 ± 0.92 | 15.5 ± 1.2 a | 11.7 ± 0.89 ab | 9.7 ± 1.1 c | 8.3 ± 0.76 abcd |
| Albumin (Alb) (µg/dL) | 10.4 ± 1.1 | 45.6 ± 3.7 a | 29.5 ± 3.1 ab | 18.5 ± 1.9 abc | 14.2 ± 1.8 abcd |
| Creatinine (mg/dl) | 74.4 ± 4.8 | 33.4 ± 3.3 a | 48.9 ± 3.4 ab | 55.1 ± 6.1 abc | 63.7 ± 5.3 abcd |
| Urinary Alb/Cr ratio (µg/mg) | 0.14 ± 0.02 | 1.4 ± 0.15 a | 0.6 ± 0.04 ab | 0.33 ± 0.03 abc | 0.22 ± 0.02 abcd |
| CrCl (mL/min) | 0.91 ± 0.18 | 0.22 ± 0.04 a | 0.38 ± 0.02 ab | 0.52 ± 0.06 abc | 0.74 ± 0.03 abcd |
| Parameter | Control | AuNPs | AuNPs + Melanin | AuNPs + α-LA | AuNPs + QUR |
|---|---|---|---|---|---|
| MDA (µM/mg tissue) | 0.51 ± 0.07 | 1.95 ± 0.11 a | 1.31 ± 0.02 ab | 1.04 ± 0.09 abc | 0.78 ± 0.05 abcd |
| GSH (µg/mg tissue) | 65.3 ± 3.9 | 26.3 ± 2.7 a | 41.6 ± 3.1 ab | 47.6 ± 4.9 abc | 56.2 ± 5.2 abcd |
| SOD (U/mg tissue) | 15.4 ± 2.1 | 5.1 ± 0.78 a | 9.4 ± 1.1 ab | 10.6 ± 1.1 abc | 12.5 ± 1.7 abcd |
| CAT (U/mg tissue) | 11.5 ± 1.6 | 3.7 ± 0.85 a | 7.6 ± 0.59 ab | 7.9 ± 0.52 ab | 9.3 ± 1.2 abcd |
| TNF-α (pg/mg tissue) | 4.5 ± 0.73 | 25.5 ± 3.8 a | 16.7 ± 1.9 ab | 12.1 ± 1.4 abc | 8.1 ± 0.94 abcd |
| IL-6 (pg/mg tissue) | 2.1 ± 0.48 | 13.8 ± 1.4 | 8.5 ± 1.2 ab | 5.4 ± 0.67 abc | 3.2 ± 0.43 abcd |
| Gene | Primers (5′→3′) | Accession # | BP |
|---|---|---|---|
| NOX4 | F: AGGTGTCTGCATGGTGGTG R: GAGGGTGAGTGTCTAAATTGGT | NM_053524.1 | 182 |
| NF-κB | F: GAGATTGTGCCAAGAGTGAC R: CTTGTCTTCCATGGTGGATG | XM_342346.4 | 134 |
| Bcl2 | F: AACATCGCTCTGTGGATGAC R: GAGCAGCGTCTTCAGAGACA | U34964.1 | 150 |
| Bax | F: AGGATCGAGCAGAGAGGATGG R: GACACTCGCTCAGCTTCTTGG | NM_017059 | 93 |
| Caspase-3 | F: AATTCAAGGGACGGGTCATG R: GCTTGTGCGCGTACAGTTTC | U49930 | 67 |
| β-Actin | F: GACCTCTATGCCAACACAGT R: CACCAATCCACACAGAGTAC | NM_031144 | 154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, G.M.; Al-Ayed, M.S.; Abdelhalim, M.A.; Al-Harbi, L.N.; Yahya, M.A. Effects of Antioxidant Combinations on the Renal Toxicity Induced Rats by Gold Nanoparticles. Molecules 2023, 28, 1879. https://doi.org/10.3390/molecules28041879
Alshammari GM, Al-Ayed MS, Abdelhalim MA, Al-Harbi LN, Yahya MA. Effects of Antioxidant Combinations on the Renal Toxicity Induced Rats by Gold Nanoparticles. Molecules. 2023; 28(4):1879. https://doi.org/10.3390/molecules28041879
Chicago/Turabian StyleAlshammari, Ghedeir M., Mohammed S. Al-Ayed, Mohamed Anwar Abdelhalim, Laila Naif Al-Harbi, and Mohammed Abdo Yahya. 2023. "Effects of Antioxidant Combinations on the Renal Toxicity Induced Rats by Gold Nanoparticles" Molecules 28, no. 4: 1879. https://doi.org/10.3390/molecules28041879
APA StyleAlshammari, G. M., Al-Ayed, M. S., Abdelhalim, M. A., Al-Harbi, L. N., & Yahya, M. A. (2023). Effects of Antioxidant Combinations on the Renal Toxicity Induced Rats by Gold Nanoparticles. Molecules, 28(4), 1879. https://doi.org/10.3390/molecules28041879





