Abstract
Supported Pt-based catalysts have been identified as highly selective catalysts for CO oxidation, but their potential for applications has been hampered by the high cost and scarcity of Pt metals as well as aggregation problems at relatively high temperatures. In this work, nanorod structured (TiO2−Pt)/CeO2 catalysts with the addition of 0.3 at% Pt and different atomic ratios of Ti were prepared through a combined dealloying and calcination method. XRD, XPS, SEM, TEM, and STEM measurements were used to confirm the phase composition, surface morphology, and structure of synthesized samples. After calcination treatment, Pt nanoparticles were semi-inlayed on the surface of the CeO2 nanorod, and TiO2 was highly dispersed into the catalyst system, resulting in the formation of (TiO2−Pt)/CeO2 with high specific surface area and large pore volume. The unique structure can provide more reaction path and active sites for catalytic CO oxidation, thus contributing to the generation of catalysts with high catalytic activity. The outstanding catalytic performance is ascribed to the stable structure and proper TiO2 doping as well as the combined effect of Pt, TiO2, and CeO2. The research results are of importance for further development of high catalytic performance nanoporous catalytic materials.
1. Introduction
Carbon monoxide is one of the most dangerous waste gases because of its harmful impact on the environment and high toxicity to animal and human lives. As catalytic CO oxidation is an efficient method to eliminate CO pollution under low temperature conditions, it has attracted widespread research interest in recent years [1,2]. Among them, the supported Pt-based catalysts have been widely investigated since Langmuir’s first discovery [3]. Pt-based catalysts are critical to industrial CO oxidation because of their superior catalytic activity and stable catalytic properties [4,5,6]. The catalytic mechanism of Pt catalysts has been widely investigated and the results show that the reaction generally follows Langmuir–Hinshelwood (L-H) models [7,8,9]. However, the relative high cost and scarcity of noble metals, as well as their aggregation tendency as temperature rises, have retarded their further development [10,11]. Both theoretical and experimental studies have demonstrated that combining transition metal oxides [12,13] or rare earth metal ions [14,15] with noble metals is an effective method to reduce cost while maintaining stable catalytic property, which has been widely used in fuel cell and energy conversion/storage equipment. TiO2, as a typical metal oxide, exhibits high oxygen storage capacity and redox properties as well as active catalytic performance by enhancing the migration rate of surface-active oxygen atoms and plays an important role in the catalysis field [16,17,18]. For example, Liou’s team [19] prepared Cu-doped TiO2 microsphere for catalytic CO oxidation. They think that the highly dispersed doping metals can increase the exposure of copper and TiO2 matrix, thus leading to the improvement of catalytic performance. However, the bulk metal oxides always show poor charge transfer ability and conductivity, which hinders their full play. Combining TiO2 with Pt is an effective strategy to avoid the aggregation of Pt and enhance the overall property of materials. Liu’s group [20] fabricated the Pt-Au/TiO2-CeO2 catalyst and found that the introduction of TiO2 into a system can improve CO oxidation by enhancing the charge transfer from Pt to Au sites. Nava’s team [21] investigated the loading amount of TiO2 on catalytic performance of Au/TiO2/SBA-15 systems and concluded that the catalyst reached the highest catalytic activity when 10 wt% TiO2 was added. Therefore, TiO2 is a good promoter in improving the catalytic performance of catalysts.
In practice, the metallic catalysts or metal–metal oxide composites are always supported on some nanostructured substrates to form heterogeneous catalysts [22]. This unique structure can allow good dispersion of noble metals and make full play use of the catalysts. It is well established that the noble catalysts supported on reducible metal oxides are more active than non-reducible oxides such as Al2O3 or SiO2 [23,24]. In comparison, as a unique rare metal oxide, CeO2 has been applied as a superior reducible supporting oxide due to its rich reservation and fast storage/release oxygen ability [25]. More importantly, the reversible Ce3+/Ce4+ redox reaction and easy generation of oxygen vacancies in CeO2 can contribute to the improvement in CO oxidation rate [26,27]. Previous studies also imply that the morphology and facets of CeO2-based nanocomposites can greatly influence the formation and migration of surface oxygen vacancies, and nanosized structured CeO2 materials, including nanospheres, nanorods, and nanocubes [28,29], have been synthesized. Among these structures, nanorod-shaped CeO2 has received a substantial amount of attention because of its potentially large surface area and abundance of oxygen vacancy defects. Li et al. [30] prepared Au cluster-CeO2 catalysts and concluded that the Au25 nanoclusters on CeO2 nanorods and nano polyhedra display higher activity than CeO2 nanocubes due to the difference in concentration of (O) species on ceria surface. Kwangjin An’s group [31] fabricated Pt/CeO2 with different morphologies and found that the Pt/CeO2 with cube morphology shows the best activity compared with other structured samples. It is therefore predicated that the catalytic activity of CeO2-based catalysts can be controlled by tuning their physicochemical properties. However, the conventional fabrication methods always require relatively high cost and complicated or time-consuming preparation processes, which limit their large-scale application.
The structure and activity of a catalyst is greatly related to the synthesis method. Compared with the traditional preparation method, dealloying is a simple and pollution-free method to fabricate three-dimensional nanoporous materials on a large-scale production basis [32]. The structure and pore size of samples can also be controlled by adjusting the dealloying temperature or composition of precursor alloys [33]. Metal oxides such as NiO [34] and CuO [35] or noble metals such as Ag [36], Au [37], and Pt [4] have been reported to be successfully supported on CeO2 and have displayed satisfying catalytic activity. Whereas the Pt/TiO2 composites supported onto CeO2 to improve catalytic activity has been rarely reported.
Herein, the nanorod structured (TiO2−Pt)/CeO2 catalysts with the addition of Pt and varied amount of TiO2 were fabricated through a combined dealloying and calcination method. The highly dispersed Pt and TiO2 nanoparticles are loaded onto CeO2 and form a nanoscale interface, which can accelerate the movement rate of electrons at the interface. The good framework structure also makes CO access catalysts more efficiently and gives full play to the role of active phases. The (0.5TiO2−Pt)/CeO2 catalyst shows optimal catalytic property of 50% and 99% at reaction temperatures as low as 55 °C and 90 °C, respectively. This work provides a new idea for preparation of high catalytic performance transition metal/CeO2-based catalysts for large-scale production.
2. Results and Discussion
2.1. Characterization of Catalysts
Figure 1a displays the XRD patterns of melt-spun and dealloyed Al91.2Ce8Pt0.3Ti0.5 ribbons. As observed, the melt-spun Al91.2Ce8Pt0.3Ti0.5 ribbons consisted of α-Al, Al4Ce and Al92Ce8 phases; after the dealloying procedure, only a new phase of CeOx was detected while α-Al, Al4Ce, and Al92Ce8 phases disappeared, implying that most of the Al has been removed. The diffraction peaks representing Pt/Ti cannot be detected, which is ascribed to their low content and high dispersion into alloy ribbons. The XRD patterns of Al91.4Ce8Pt0.3Ti0.3, Al91.2Ce8Pt0.3Ti0.5, and Al91Ce8Pt0.3Ti0.7 melt-spun ribbons after dealloying and calcination treatments are displayed in Figure 1b. The diffraction at 28.5°, 32.9°, 47.4°, 56.2°, 69.2°, and 76.7° corresponded to the (111), (200), (220), (311), (400), and (331) planes of cubic CeO2 (PDF#89-8436), respectively; the weak diffraction peak at 41o representing Pt was also discovered while no peaks related to Ti was found. However, the content of Al, Ce, Pt, and Ti in the (0.5TiO2-Pd)/CeO2 catalyst obtained from Al91.2Ce8Pt0.3Ti0.5 melt-spun ribbon is 3.81 at%, 90.14 at%, 1.66 at%, and 4.4 at%, respectively, as shown in the EDS spectrum in Figure S1, demonstrating that Pt and Ti have been added into Al-Ce precursor alloys successfully.
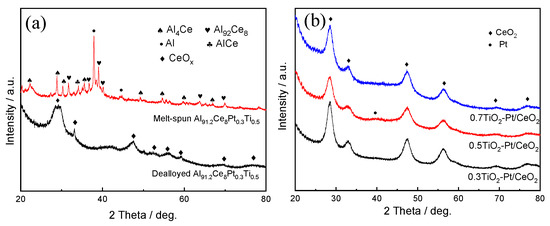
Figure 1.
The XRD patterns of (a) melt-spun and dealloyed Al91.2Ce8Pt0.3Ti0.5 ribbons; (b) (xTiO2−Pt0.3)/CeO2 (X = 0.3, 0.5, 0.7) calcined at 300 °C.
To further confirm the chemical state of Pt, Ti, and Ce, XPS characterization of (0.5TiO2−Pt)/CeO2 is conducted with results shown in Figure 2. The Ce 3d spectrum displayed in Figure 2a reveals that the sample exhibits both Ce4+ and Ce3+ ions. The five peaks at 881.9 eV, 888.3 eV, 897.7 eV, 900.4 eV, and 907.3 eV are ascribed to Ce4+, while the other two peaks at 885.1 eV and 903.7 eV corresponded to Ce3+. The existence of Ce3+ implies the generation of oxygen vacancies; Ce3+ can adsorb active oxygen at the catalytic interface, thus contributing to the formation of interfacial active center. The concentration of Ce3+ can be reflected from the integrated areas of the Ce3+ peak to the total (Ce3+ + Ce4+) peaks. As a result, the surface concentration of Ce3+ on the (0.5TiO2−Pt)/CeO2 catalyst is 21.58% according to the fitting calculation of the Ce 3d spectrum. For the Pt 4f spectrum in Figure 2b, the binding energies at 70.8 eV for Pt 4f7/2 and 73.9 eV for Pt 4f5/2 are assigned to metallic state platinum (Pt0), while the peaks at 71.9 eV and 76.4 eV corresponded to Pt2+ [38,39]. Likewise, the content of Pt0 accounts for 61.6% of the total (Pt0 + Pt2+). The Ti 2p spectrum in Figure 2c displays a Ti4+ binding energy, in which the two peaks at 463.6 eV and 457.8eV corresponded to Ti 2p1/2 and Ti 2p3/2, respectively [40]. Since Ti mainly existed in the form of Ti4+ in the product, it is deduced that TiO2 existed in the composite material. The O 1s spectrum in Figure 2d can be fitted to three peaks. The binding energies centered around ~529.3 eV, ~531 eV, and ~532.2 eV corresponded to lattice oxygen species (Olat), surface adsorbed oxygen (Osur), and weakly bonded specific oxygen species such as adsorbed O2, H2O, and CO2 (Obon), respectively. The active surface oxygen can be evaluated by Osur, and the ratio of active oxygen species for (0.5TiO2−Pt)/CeO2 is 20.8%.
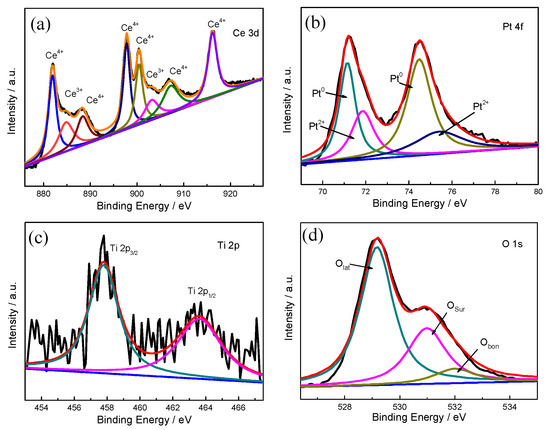
Figure 2.
XPS spectra of (a) Ce 3d, (b) Pt 4f, (c) Ti 2p, and (d) O 1s of the (0.5TiO2−Pt)/CeO2 catalyst.
Figure 3 presents the surface and cross-sectional morphologies of (TiO2−Pt)/CeO2 with different TiO2 content. As observed, all the three samples display a robust framework, which are composed of a nanoporous matrix with nanorods embedded in them. The nanorods pile up on each other to form rich pores among them. Notably, the slight increase in TiO2 content from 0.3 at% to 0.5 at% does not influence the overall morphologies of samples and only fine-tunes the arrangement of pores, as shown in Figure 3a,d,g. Moreover, the cross-sectional SEM image of (0.5TiO2−Pt)/CeO2 in Figure S2 further reflects the presence of rich pores and independent arrangement of nanorods. The unique and robust nanorod-embedded matrix structure is beneficial to stabilize the overall structure of samples during the catalytic process; the existence of lots of pores distributed among matrix and nanorods can also provide more channels for reacted gas to enter and exit; therefore, the catalytic CO oxidation performance is expected to be improved.
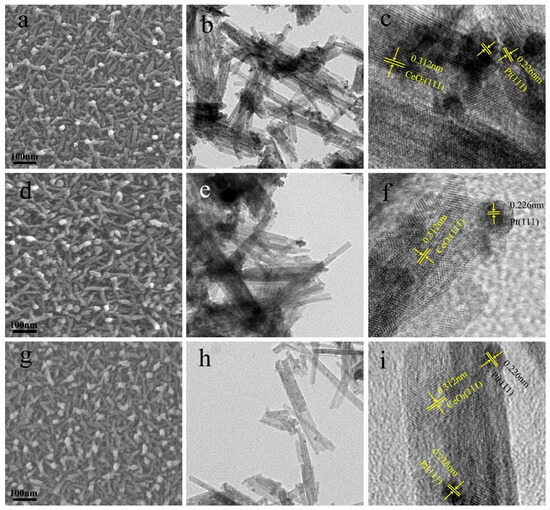
Figure 3.
The SEM images of (a) (0.3TiO2−Pt)/CeO2, (d) (0.5TiO2−Pt)/CeO2, and (g) (0.7TiO2−Pt)/CeO2; the TEM and HRTEM images of (b,c) (0.3TiO2−Pt)/CeO2, (e,f) (0.5TiO2−Pt)/CeO2, and (h,i) (0.7TiO2−Pt)/CeO2.
TEM and HRTEM characterization are performed to further understand the microstructure of (TiO2−Pt)/CeO2 catalysts. As shown in the TEM images of (0.3TiO2−Pt)/CeO2, (0.5TiO2−Pt)/CeO2, and (0.7TiO2−Pt)/CeO2 presented in Figure 3b,e,h, respectively, the samples are composed of a large number of uniform nanorods with an average diameter of 10 nm, which are interconnected and stacked on each other; some dark nanoparticles with diameter of 3–5 nm on average are uniformly embedded on the surface of nanorods. These are consistent with SEM results. The corresponding HRTEM images of (0.3TiO2−Pt)/CeO2, (0.5TiO2−Pt)/CeO2, and (0.7TiO2−Pt)/CeO2 are displayed in Figure 3c,f,i, respectively. The lattice fringe with a space of 0.32 nm corresponded to the (111) plane of CeO2, implying the cubic structured CeO2 nanorod in the (111) crystal plane. The dark nanoparticles with lattice space of 0.229 nm are assigned to the (111) plane of Pt, which further indicates that Pt has been added into Al-Ce alloy successfully. However, no results related to Ti are found in TEM characterization. This may be because the calcination temperature in the (TiO2−Pt)/CeO2 system is relatively low (300 °C); CeO2 can inhibit the crystallization of other oxides during the calcination process under such low temperatures [41]. Our previous work also found that CeO2 can inhibit the crystallization of NiO; as temperature rises, the structure of NiO in the system is transformed from the amorphous state into the crystallization state [34]. Therefore, the reason why the lattice fringe related to TiO2 is not detected in TEM characterization may be the amorphous state of TiO2 in the system, which is in line with XRD results.
The distribution of elements on the surface of the CeO2 nanorod is further investigated via STEM mapping, with results presented in Figure 4. Figure 4a displays the SEM image of (0.5TiO2−Pt)/CeO2. For (0.5TiO2−Pt)/CeO2 obtained from Al91.2Ce8Pt0.3Ti0.5 through the dealloying and calcination processes, Pt is semi-embedded onto the surface of the CeO2 nanorod, while Ti is uniformly distributed into the CeO2 nanorod, as reflected in Figure 4b–d. Combined with XPS and STEM results, it can be concluded that Ti mainly exists as the TiO2 phase in the composite system; thus, the obtained composite material is named as (TiO2−Pt)/CeO2.
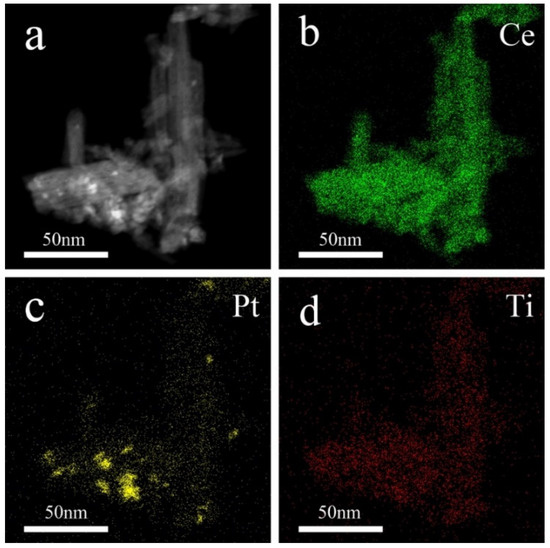
Figure 4.
The STEM image of (a) (0.5TiO2−Pt)/CeO2 and element mapping of (b) Ce, (c) Pt, and (d) Ti.
The specific surface area, pore size distribution, and pore volume of (TiO2−Pt)/CeO2 composite materials with varied TiO2 proportions are measured via the N2 adsorption-desorption test, with results displayed in Figure 5. The isotherms of three catalysts belong to type IV and possess H3 hysteresis loops at relative pressure of 0.7–1.0 P/P0 according to the IUPAC classification (Figure 5a), indicating the mesoporous structure of (TiO2−Pt)/CeO2 [42]. The BET surface area of (0.3TiO2−Pt)/CeO2, (0.5TiO2−Pt)/CeO2, and (0.7TiO2−Pt)/CeO2 is 101.88, 108.88, and 110.11 m2 g−1, respectively, while their corresponding pore size is centered at 14.36, 12.71, and 13.58 nm, and pore volume is 0.36, 0.37, and 0.35 cm3 g−1, respectively, as displayed in the BHJ pore size distribution curves in Figure 5b. Obviously, the three catalysts possess similar results in specific surface area and pore size distribution, which illustrates that the variation in the amount of Pt and TiO2 does not influence the physical structure of materials significantly, nor their mesoporous properties. In contrast, (0.5TiO2−Pt)/CeO2 has higher specific surface area, larger pore volume, and smaller porosity, which is beneficial for gas penetration during the catalytic process by providing more reaction paths and active sites for catalytic CO oxidation, and thus improving its catalytic performance.
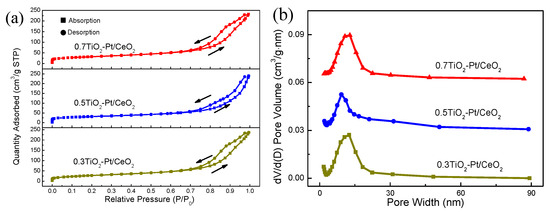
Figure 5.
(a) Nitrogen adsorption–desorption isotherms and (b) the BHJ pore size distribution of (TiO2−Pt)/CeO2.
Raman spectroscopy measurement is conducted to understand the structural phase changes of (TiO2−Pt)/CeO2 catalysts. In Figure 6, the weak peaks of Raman shift around 306 and 534 cm−1 indicate the existence of anatase TiO2; the appearance of new and broad peaks around 269 cm−1 is attributed to co-doping of Pt [43,44]. Moreover, compared with Raman peaks of pure CeO2 in Figure S3, the diffraction peak is shifted from 459 cm−1 to 439 cm−1, which is ascribed to the formation of more grain boundaries after the addition of TiO2 and Pt nanoparticles. It is expected that the Pt and TiO2 nanoparticles that are highly dispersed on CeO2 nanorods can cause a large number of defects including oxygen vacancies, grain boundaries, and dislocations, which are helpful for improvement in catalytic activity of catalysts.
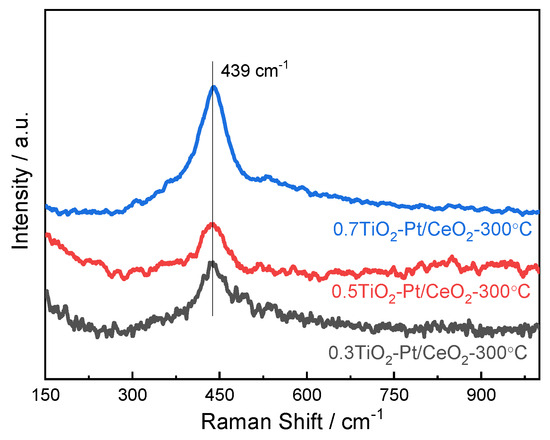
Figure 6.
Raman spectra of (TiO2−Pt)/CeO2 catalysts.
2.2. Catalytic Performance
Figure 7 presents the catalytic CO oxidation performance of (TiO2−Pt)/CeO2 catalysts. For Pt0.3/CeO2 without the addition of TiO2, the temperature for 50% CO conversion (T50) and 99% CO conversion (T99) is 91°C and 113 °C, respectively, which is much higher than that of the CeO2 matrix (T50 = 235 °C, T99 = 320 °C), as observed in Figure S4. The catalytic activity is greatly improved after the addition of TiO2. The T50 and T99 of (0.3TiO2−Pt)/CeO2 is 65 °C and 110 °C, respectively, when 0.3 at% Ti is added into alloy system. As Ti content increases to 0.5 at%, the catalytic activity reaches the optimum with a T50 and T99 decrease to 55 °C and 90 °C, respectively; on further increasing Ti content to 0.7 at%, catalytic performance decreases with T50 and T99 of 65 °C and 100 °C, respectively, as displayed in Figure 7a. The influence of calcination temperature on catalytic property of the (0.5TiO2−Pt)/CeO2 catalyst is shown in Figure 7b, in which the T99 of (0.5TiO2−Pt)/CeO2 without calcination treatment, calcined at 200 °C, 300 °C, 400 °C, and 500 °C is 120 °C, 110 °C, 90 °C, 100 °C, and 120 °C, respectively. The catalytic performance of (0.5TiO2−Pt)/CeO2 was stable after three repeated tests (Figure S5), implying good reusability of (0.5TiO2−Pt)/CeO2. The catalytic activity of (0.5TiO2−Pt)/CeO2 also surpasses the state-of-the-art TiO2/CeO2-based catalysts reported in the literature, as shown in Table 1 [22,45,46,47,48], indicating its superior catalytic property. It is clearly observed that the catalytic activity is improved as calcination temperature increases from room temperature to 300 °C, which is reduced as calcination temperature further increases. The (0.5TiO2−Pt)/CeO2 exhibits optimum catalytic performance after calcination at 300 °C. Furthermore, the addition of Ti into the Pt-CeO2 catalytic system can partly make up for the deficiency of the single precious metal Pt and realize the purpose of the experiment.
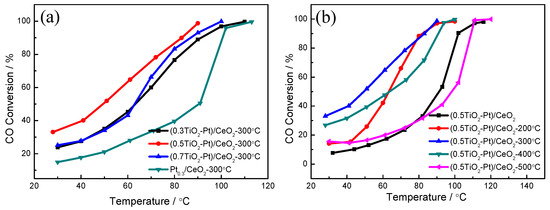
Figure 7.
(a) The catalytic performance of Pt0.3/CeO2, (0.3TiO2−Pt)/CeO2, (0.5TiO2−Pt)/CeO2, (0.7TiO2−Pt)/CeO2 catalysts; (b) the (0.5TiO2−Pt)/CeO2 catalyst obtained at different calcination temperatures.

Table 1.
Comparison on catalytic performance of (0.5TiO2−Pt)/CeO2 with previous reports.
The catalytic performance of (0.5TiO2−Pt)/CeO2 as a function of flow rate at 70 °C is detected, with corresponding catalytic activities shown in Figure 8a. As the total gas flow rate increases from 40 to 120 mL min−1, the CO conversion decreases from 97% to 58%. It can be also clearly detected that the reaction rate is positively related to flow rate. Figure 8b further explores the influence of O2 concentration in feed gas on catalytic performance of (0.5TiO2−Pt)/CeO2. The test temperature is kept at 90 °C with a flow rate of 100 mL min−1. The CO conversion rate can reach 99% as 10% O2 is initially infused into the system thanks to the sufficient O2 environment; CO conversion rate is reduced first and then kept stable at 10% when O2 supply is suddenly decreased to zero, which may be ascribed to the existence of surface lattice oxygen that can migrate to active sites and combine with adsorbed CO to form oxygen vacancies. However, CO conversion rate increases in poor oxygen conditions (0.3–5% O2) and then recovers to initial 99% value and stays unchanged when O2 is resupplied into feed gas, implying the superior catalytic CO oxidation property of (0.5TiO2−Pt)/CeO2.
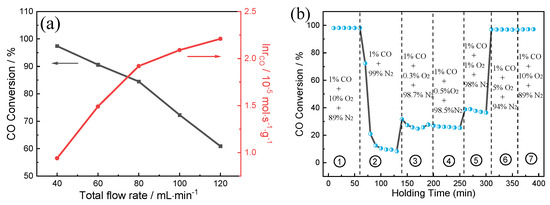
Figure 8.
(a) Catalytic activity of (0.5TiO2−Pt)/CeO2 under different space velocities at 70 °C. The measurement was performed using 100 mg of the catalyst with a mixed gas of 1% CO, 10% O2, and rest N2 at a flow rate ranging from 40 to 120 mL min−1. (b) Catalytic performance under varied oxygen concentrations.
The long-term stability of the (0.5TiO2−Pt)/CeO2 catalyst is also evaluated to investigate its practical application potential, as shown in Figure 9a. The (0.5TiO2−Pt)/CeO2 catalyst exhibits above 95% CO conversion under mixed atmosphere (1% CO, 10% O2, 89% N2) and is stable without deterioration after successive reaction of 55 h, indicating outstanding catalytic activity of the nanorod-shaped (0.5TiO2−Pt)/CeO2 catalyst. The outstanding catalytic performance of the (TiO2−Pt)/CeO2 catalyst can be attributed to the unique structure and phase composition. The existence of Ce3+ on catalytic interface can adsorb active oxygen, which is conducive to the formation of the interfacial active center; highly dispersed TiO2 can accelerate the migration rate of active oxygen species on the surface of CeO2 so that the oxygen atoms can react with activated CO to form CO2 [34], as reflected in the mechanism diagram in Figure 9b. The introduction of Pt nanoparticles and highly dispersed TiO2 can form a large number of nanoscale interfaces, which greatly promotes the movement of electrons at the interface. The electrons can not only activate the CO gas adsorbed by noble metals quickly but also accelerate the dissociation of generated CO2 on the catalyst surface, thus ultimately making the reaction rate increase. In addition, the robust framework structure provides a place for catalysts to contact harmful gases effectively; it also stimulates the effect of noble metals that are loaded on the CeO2 structure and inhibits the agglomeration or growth of loaded nanoparticles during heating or catalytic processes, guaranteeing the high catalytic stability of the catalysts.
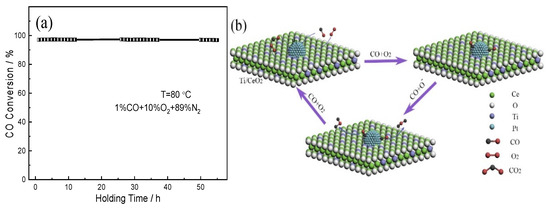
Figure 9.
(a) The long-term stability of the (0.5TiO2−Pt)/CeO2 catalyst; (b) a possible mechanism for reaction of CO on the (TiO2−Pt)/CeO2 catalyst.
3. Materials and Methods
3.1. Material Preparation
The Al92Ce8, Al91.7Ce8Pt0.3, Al91.4Ce8Pt0.3Ti0.3, Al91.2Ce8Pt0.3Ti0.5, and Al91Ce8Pt0.3Ti0.7 alloys were achieved from pure Al, Ce, Pt, and Pd through the arc-melting method under high-purity Ar atmosphere. After being remelted and solidified, the Al-Ce−Pt-Ti alloy ribbons with 4–6 mm width and 40–70 μm thickness were prepared. The quenched alloy ribbons were dealloyed in 20 wt% NaOH aqueous solution at room temperature for 2 h until no obvious bubbles were generated and most of Al were removed. After this, the samples were then further corroded at 80 °C for 10 h. Finally, after cleaning and drying, the dealloyed samples were calcined at 200–500 °C for 2 h under pure O2 environment.
3.2. Characterization
X-ray diffraction patterns were collected on Bruker D8 Advance to analysis phase composition. Field emission scanning electron microscopy (FESEM, JEOL, JSM-7000F) and high-resolution transmission electron microscopy (HRTEM, JEOL, JEM-2100) were employed to characterize surface morphologies and microstructures. A scanning transmission electron microscope (STEM, FEI-200) equipped with an Oxford Instruments EDS spectrometer was utilized to conduct EDS analysis and mapping. X-ray photoelectron spectroscopy (XPS) was performed on ESCALAB Xi+ to confirm element composition and valence state. Nitrogen sorption was tested on Micromeritics ASAP 2020 at 77 K, and the Barrett–Joyner–Halenda algorithm was adopted to evaluate pore size and pore volume. Raman spectra were collected on an HR 800 fully automatic laser Raman spectrometer.
3.3. Catalytic Evaluations
The catalytic activity was detected in a tubular reactor at atmospheric pressure. A 100 mg sample was added to the reactor and fixed with quartz wool. The mixed reaction gas consisting of 1% CO, 10% O2, and 89% N2 (volume fraction) was entered into the test system at a flow rate of 100 mL min−1 (space velocity 60,000 h−1). The inflowed and outflowed gases were collected using an Anglit 7890B gas chromatograph equipped with a hydrogen flame detector (FID). The CO conversion was determined by:
where Cin and Cout stand for the concentration of the CO inlet and outlet of the reactor, respectively.
4. Conclusions
In conclusion, the nanorod structured (TiO2−Pt)/CeO2 catalysts are fabricated via the combined dealloying and calcination method. SEM, TEM, and STEM measurements imply that the Pt nanoparticles were semi-inlayed on the surface of the CeO2 nanorod, while TiO2 were highly dispersed into the catalyst system. By rationally adjusting the proportion of TiO2 in the system, the obtained (0.5TiO2−Pt)/CeO2 displays unique nanorod structure and large pore volume, which contributes to exceptional catalytic activity with T50 and T99 temperature as low as 55 °C and 90 °C, respectively. It is considered that the stable structure, proper TiO2 doping, and jointed effect of Pt and TiO2 as well as rich nanopores contribute to the enhanced catalytic performance of (TiO2−Pt)/CeO2 catalysts. This work provides a new idea and facile strategy for the fabrication of noble metal/metal oxide composites with high catalytic performance.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules28041867/s1, Figure S1: The EDS spectrum of Al91.2Ce8Pt0.3Ti0.5 melt-spun ribbons after dealloying and calcination treatment. Figure S2: The cross-sectional SEM image of (0.5TiO2−Pt)/CeO2. Figure S3: The Raman spectrum of pure CeO2. Figure S4: The catalytic performance of CeO2 matrix. Figure S5: The reusability test of (0.5TiO2−Pt)/CeO2.
Author Contributions
H.W. conceived the idea and directed the experiments. R.Y. fabricated all of the samples. R.Z., H.M. and J.G. conducted the characterizations. M.L. and Y.Z. analyzed the data. H.W. drafted the manuscript, and Z.M. revised it. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 52173263), the Regional Innovation Capability Guidance Program of Shaanxi (No. 2022QFY03-02), the Natural Science Foundation of Anhui Province, China (No. 2108085J11), Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2022JQ-139), the Scientific Research Program Funded by Shaanxi Provincial Education Department (22JK0590, 22JP100), the Fundamental Research Funds for the Central Universities, Northwestern Polytechnical University (No. D5000210825), the Scientific research fund for high-level talents of Xijing University (No. XJ21B17), and the Youth Innovation Team of Shaanxi Universities.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data are available from the corresponding author upon reasonable request.
Acknowledgments
All authors are grateful for the financial support of this work provided by the Natural Science Foundation. The authors also thank his outstanding contributions in language polishing.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Wang, L.N.; Li, X.N.; He, S.G. Recent research progress in the study of catalytic CO oxidation by gas phase atomic clusters. Sci. China Mater. 2019, 63, 892–902. [Google Scholar] [CrossRef]
- Soliman, N.K. Factors affecting CO oxidation reaction over nanosized materials: A review. J. Mater. Res. Technol. 2019, 8, 2395–2407. [Google Scholar] [CrossRef]
- Langmuir, I. The mechanism of the catalytic action of platinum in the reactions 2CO + O2 = 2CO2 and 2H2+ O2 = 2H2O. Trans. Faraday Soc. 1922, 17, 621–654. [Google Scholar] [CrossRef]
- Camposeco, R.; Torres, A.E.; Zanella, R. Influence of the Preparation Method of Au, Pd, Pt, and Rh/TiO2 Nanostructures and Their Catalytic Activity on the CO Oxidation at Low Temperature. Top. Catal. 2022, 65, 798–816. [Google Scholar] [CrossRef]
- Lin, J.; Wang, X.; Zhang, T. Recent progress in CO oxidation over Pt-group-metal catalysts at low temperatures. Chin. J. Catal. 2016, 37, 1805–1813. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Zhu, T.; Tian, M. Catalytic oxidation of CO on noble metal-based catalysts. Environ. Sci. Pollut. Res. Int. 2021, 28, 24847–24871. [Google Scholar] [CrossRef]
- Therrien, A.J.; Hensley, A.J.R.; Marcinkowski, M.D.; Zhang, R.; Lucci, F.R.; Coughlin, B.; Schilling, A.C.; McEwen, J.S.; Sykes, E.C.H. An atomic-scale view of single-site Pt catalysis for low-temperature CO oxidation. Nat. Catal. 2018, 1, 192–198. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Wang, X.; Zhang, Z.; Zhao, J.; Yan, J.; Du, Y.; Zhang, H.; Ma, D. Complete CO Oxidation by O2 and H2O over Pt-CeO2-δ/MgO Following Langmuir-Hinshelwood and Mars-van Krevelen Mechanisms, Respectively. ACS Catal. 2021, 11, 11820–11830. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Y.; Li, L.; Liu, J.; Pan, X.; Liu, W.; Wei, F.; Cui, Y.; Qiao, B.; Sun, X.; et al. Identification of Active Sites on High-Performance Pt/Al2O3 Catalyst for Cryogenic CO Oxidation. ACS Catal. 2020, 10, 8815–8824. [Google Scholar] [CrossRef]
- Song, J.; Yang, Y.; Liu, S.; Li, L.; Yu, N.; Fan, Y.; Chen, Z.; Kuai, L.; Geng, B. Dispersion and support dictated properties and activities of Pt/metal oxide catalysts in heterogeneous CO oxidation. Nano Res. 2021, 14, 4841–4847. [Google Scholar] [CrossRef]
- Song, H.C.; Han, G.; Reddy, K.P.; Choi, M.; Ryoo, R.; Park, J.Y. Synergistic interactions between water and the metal/oxide interface in CO oxidation on Pt/CeO2 model catalysts. Catal. Today 2022, in press. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C.; Mohan, D.; Prasad, R. Advances in transition metal oxide catalysts for carbon monoxide oxidation: A review. Adv. Compos. Hybrid Mater. 2019, 2, 626–656. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, H.; Wang, C.; Long, R.; Xiong, Y. Engineering the surface charge states of nanostructures for enhanced catalytic performance. Mater. Chem. Front. 2017, 1, 1951–1964. [Google Scholar] [CrossRef]
- Siakavelas, G.I.; Charisiou, N.D.; AlKhoori, A.; AlKhoori, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Highly selective and stable Ni/La-M (M = Sm, Pr, and Mg)-CeO2 catalysts for CO2 methanation. J. CO2 Util. 2021, 51, 101618. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Xu, X.; Xi, R.; Liu, Y.; Fang, X.; Wang, X. Tailoring La2Ce2O7 catalysts for low temperature oxidative coupling of methane by optimizing the preparation methods. Catal. Today 2020, 355, 518–528. [Google Scholar] [CrossRef]
- Shutilov, A.A.; Zenkovets, G.A.; Kryukova, G.N.; Gavrilov, V.Y.; Paukshtis, E.A.; Boronin, A.I.; Koshcheev, S.V.; Tsybulya, S.V. Effect of the microstructure of Pt/CeO2-TiO2 catalysts on their catalytic properties in CO oxidation. Kinet. Catal. 2011, 49, 271–278. [Google Scholar] [CrossRef]
- Cai, J.; Yu, Z.; Fan, X.; Li, J. Effect of TiO2 Calcination Pretreatment on the Performance of Pt/TiO2 Catalyst for CO Oxidation. Molecules 2022, 27, 3875. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Kim, A.; Kim, Y.K. Observation of temperature-dependent kinetics for catalytic CO oxidation over TiO2-supported Pt catalysts. Chem. Phys. Lett. 2017, 685, 282–287. [Google Scholar] [CrossRef]
- Yang, W.T.; Lin, C.J.; Montini, T.; Fornasiero, P.; Ya, S.; Liou, S.Y.H. High-performance and long-term stability of mesoporous Cu-doped TiO(2) microsphere for catalytic CO oxidation. J. Hazard Mater. 2021, 403, 123630. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Sun, Y.; Zhu, T.; Liu, Z. Pt-Au/MOx-CeO(2) (M = Mn, Fe, Ti) Catalysts for the Co-Oxidation of CO and H(2) at Room Temperature. Molecules 2017, 22, 351. [Google Scholar] [CrossRef] [PubMed]
- Peza-Ledesma, C.L.; Escamilla-Perea, L.; Nava, R.; Pawelec, B.; Fierro, J.L.G. Supported gold catalysts in SBA-15 modified with TiO2 for oxidation of carbon monoxide. Appl. Catal. A Gen. 2010, 375, 37–48. [Google Scholar] [CrossRef]
- Deng, Y.; Tian, P.; Liu, S.; He, H.; Wang, Y.; Ouyang, L.; Yuan, S. Enhanced catalytic performance of atomically dispersed Pd on Pr-doped CeO2 nanorod in CO oxidation. J Hazard Mater 2022, 426, 127793. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wan, J.; Lin, J.; Zhou, R. Comparative study of three-way catalytic performance over Pd/CeO2-ZrO2-Al2O3 and Pd/La-Al2O3 catalysts: New insights into microstructure and thermal stability. Mol. Catal. 2022, 526, 112361. [Google Scholar] [CrossRef]
- Wang, C.; Ren, D.; Du, J.; Qin, Q.; Zhang, A.; Chen, L.; Cui, H.; Chen, J.; Zhao, Y. In Situ Investigations on the Facile Synthesis and Catalytic Performance of CeO2−Pt/Al2O3 Catalyst. Catalysts 2020, 10, 143. [Google Scholar] [CrossRef]
- Konsolakis, M.; Lykaki, M. Facet-Dependent Reactivity of Ceria Nanoparticles Exemplified by CeO2-Based Transition Metal Catalysts: A Critical Review. Catalysts 2021, 11, 452. [Google Scholar] [CrossRef]
- Cam, T.S.; Omarov, S.O.; Chebanenko, M.I.; Izotova, S.G.; Popkov, V.I. Recent progress in the synthesis of CeO2-based nanocatalysts towards efficient oxidation of CO. J. Sci. Adv. Mater. Devices 2022, 7, 100399. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. 1996, 4, 439–520. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, X.; Sun, X.; Peng, Q.; Li, Y. Enhanced catalytic activity of ceria nanorods from well-defined reactive crystal planes. J. Catal. 2005, 229, 206–212. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, D.; Shi, L.; Fang, J. Template-Free Synthesis, Controlled Conversion, and CO Oxidation Properties of CeO2 Nanorods, Nanotubes, Nanowires, and Nanocubes. Eur. J. Inorg. Chem. 2008, 15, 2429–2436. [Google Scholar]
- Li, Z.; Zhang, X.; Shi, Q.; Gong, X.; Xu, H.; Li, G. Morphology effect of ceria supports on gold nanocluster catalyzed CO oxidation. Nanoscale Adv 2021, 3, 7002–7006. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Ha, H.; Kim, J.; Nam, E.; Yoo, M.; Jeong, B.; Kim, H.Y.; An, K. Influence of the Pt size and CeO2 morphology at the Pt-CeO2 interface in CO oxidation. J. Mater. Chem. A 2021, 9, 26381–26390. [Google Scholar] [CrossRef]
- McCue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and Dealloyed Materials. Annu. Rev. Mater. Res. 2016, 46, 263–286. [Google Scholar] [CrossRef]
- Erlebacher, J. An Atomistic Description of Dealloying. J. Electrochem. Soc. 2004, 151, C614. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Shi, W.; Wei, C.; Song, X.; Yang, S.; Sun, Z. Baize-like CeO2 and NiO/CeO2 nanorod catalysts prepared by dealloying for CO oxidation. Nanotechnology 2017, 28, 045602. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, X.; Zhang, X.J.; Zhang, W.S.; Wu, Z.F.; Wang, H.Y.; Han, D.X.; Niu, L. Enhanced photocatalytic CO2 reduction by constructing an In2O3-CuO heterojunction with CuO as a cocatalyst. Catal. Sci. Technol. 2021, 11, 2713–2717. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Feng, W.; Fang, X.; Liu, J. Nanoporous CeO2-Ag catalysts prepared by etching the CeO2/CuO/Ag2O mixed oxides for CO oxidation. Corros. Sci. 2018, 134, 140–148. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Perdjon, M.; Oh, R.; Zhao, W.; Huang, X.; Liu, S. Investigations of supported Au-Pd nanoparticles on synthesized CeO2 with different morphologies and application in solvent-free benzyl alcohol oxidation. Appl. Surf. Sci. 2020, 505, 144473. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, Y.; Tian, C.; Wang, L.; Zhou, W.; Dong, Y.; Han, Q.; Liu, Y.; Yuan, F.; Fu, H. Synergistic effect of Mo2N and Pt for promoted selective hydrogenation of cinnamaldehyde over Pt–Mo2N/SBA-15. Catal. Sci. Technol. 2016, 6, 2403–2412. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Zhang, J.; Pan, S.; Bian, C.; Wang, L.; Chen, F.; Meng, X.; Zheng, X.; Gao, X.; et al. Superior Performance in Catalytic Combustion of Toluene over KZSM-5 Zeolite Supported Platinum Catalyst. Catal. Lett. 2014, 144, 1851–1859. [Google Scholar] [CrossRef]
- Xiao, Z.; Xia, F.; Xu, L.; Wang, X.; Meng, J.; Wang, H.; Zhang, X.; Geng, L.; Wu, J.; Mai, L. Suppressing the Jahn–Teller Effect in Mn-Based Layered Oxide Cathode toward Long-Life Potassium-Ion Batteries. Adv. Funct. Mater. 2021, 32, 2108244. [Google Scholar] [CrossRef]
- Zhang, C.; He, H.; Tanaka, K.-i. Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature. Appl. Catal. B: Environ. 2006, 65, 37–43. [Google Scholar] [CrossRef]
- Sun, B.; Xu, D.; Wang, Z.; Zhan, Y.; Zhang, K. Interfacial structure design for triboelectric nanogenerators. Battery Energy 2022, 1, 20220001. [Google Scholar] [CrossRef]
- Wei, S.; Fu, X.P.; Wang, W.W.; Jin, Z.; Song, Q.S.; Jia, C.J. Au/TiO2 Catalysts for CO Oxidation: Effect of Gold State to Reactivity. J. Phys. Chem. C 2018, 122, 4928–4936. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Christodoulakis, A.; Kondarides, D.I.; Boghosian, S. Particle size effects on the reducibility of titanium dioxide and its relation to the water–gas shift activity of Pt/TiO2 catalysts. J. Catal. 2006, 240, 114–125. [Google Scholar] [CrossRef]
- Wang, Y.; Pei, C.; Zhao, Z.J.; Gong, J. Kinetically rate-determining step modulation by metal—Support interactions for CO oxidation on Pt/CeO2. Sci. China Chem. 2022, 65, 2038–2044. [Google Scholar] [CrossRef]
- Zong, C.; Wang, C.; Hu, L.; Zhang, R.; Jiang, P.; Chen, J.; Wei, L.; Chen, Q. The Enhancement of the Catalytic Oxidation of CO on Ir/CeO(2) Nanojunctions. Inorg. Chem. 2019, 58, 14238–14243. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, C.; Wang, H.; Yan, H.; Lu, J. Revisiting the Au Particle Size Effect on TiO2-Coated Au/TiO2 Catalysts in CO Oxidation Reaction. J. Phys. Chem. C 2016, 120, 9174–9183. [Google Scholar] [CrossRef]
- Zhen, J.; Wang, X.; Liu, D.; Wang, Z.; Li, J.; Wang, F.; Wang, Y.; Zhang, H. Mass production of Co3O4@CeO2 core@shell nanowires for catalytic CO oxidation. Nano Res. 2015, 8, 1944–1955. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).