Abstract
In the present study, biogenic selenium nanoparticles (SeNPs) have been prepared using Paenibacillus terreus and functionalized with nystatin (SeNP@PVP_Nystatin nanoconjugates) for inhibiting growth, morphogenesis, and a biofilm in Candida albicans. Ultraviolet–visible spectroscopy analysis has shown a characteristic absorption at 289, 303, and 318 nm, and X-ray diffraction analysis has shown characteristic peaks at different 2θ values for SeNPs. Electron microscopy analysis has shown that biogenic SeNPs are spherical in shape with a size in the range of 220–240 nm. Fourier transform infrared spectroscopy has confirmed the functionalization of nystatin on SeNPs (formation of SeNP@PVP_Nystatin nanoconjugates), and the zeta potential has confirmed the negative charge on the nanoconjugates. Biogenic SeNPs are inactive; however, nanoconjugates have shown antifungal activities on C. albicans (inhibited growth, morphogenesis, and a biofilm). The molecular mechanism for the action of nanoconjugates via a real-time polymerase chain reaction has shown that genes involved in the RAS/cAMP/PKA signaling pathway play an important role in antifungal activity. In cytotoxic studies, nanoconjugates have inhibited only 12% growth of the human embryonic kidney cell line 293 cells, indicating that the nanocomposites are not cytotoxic. Thus, the biogenic SeNPs produced by P. terreus can be used as innovative and effective drug carriers to increase the antifungal activity of nystatin.
1. Introduction
Candida albicans is an opportunistic pathogenic yeast commonly associated with superficial and systemic infections [1]. C. albicans has become difficult to kill due to its virulence factors and increasing antifungal resistance [2,3]. The virulence factors include yeast-to-hyphae transition and biofilm formation [2,4,5]. Although yeast and hyphal forms have a role in the pathogenicity of C. albicans, the hyphal form of C. albicans is the main invasive form [6]. The transition from yeast to hyphae is known as dimorphism [7]. Various factors induce dimorphism, which includes pH, starvation, serum, N-acetylglucosamine, temperature, and CO2 [8]. Dimorphism is the first committed step by which C. albicans invade the host [5]. The hyphal protrusions formed during dimorphism help C. albicans invade the host tissue. Generally, under the appropriate conditions, and after dimorphic, biofilm formation starts; the latter increases the survival rates and traits of multidrug resistance in C. albicans, which has become a challenge for therapeutic intervention [9]. A significant proportion of hospital morbidity has been caused by Candida invaginating the blood (candidemia) in severe infections. This is especially true with implanted medical devices prone to C. albicans biofilms. Removing these infected implant devices is often the only option to avoid deadly bloodstream and organ infections. However, this is more expensive and dangerous for the patient. Therefore, there is always a need for new and non-invasive therapeutic strategies. Furthermore, current antifungal drugs are ineffective against C. albicans biofilms, because antifungal molecules are absorbed through the extracellular polymers of the biofilm, which have upregulated efflux systems [4]. One of the promising antifungal molecules, nystatin, is used to prevent and treat C. albicans for recurrent topical or oral fungal infections [10,11,12]. However, a higher dose of nystatin can cause kidney problems, rashes, nausea, diarrhea, and vomiting. Nystatin also has the drawback of not working against resistant Candida strains, thus requiring alternative therapies [12]. The combination of nystatin with nanoparticles (NPs) will likely increase the bioavailability and efficacy of nystatin and also a reduction in its toxicity [13,14]. The NPs have a high surface area, facilitating enhanced drug loading, more efficient drug delivery, and reduced toxicity and dosage for anti-Candida activity [15,16,17,18]. The NPs can be used as smart drug delivery systems by encapsulating or attaching the antifungal agents with biocompatible NPs. When using NPs to deliver nystatin, it is important to ensure that the NPs are not cytotoxic. The majority of studies demonstrating the potential of NPs as nystatin carriers have used cytotoxic nanomaterials [19,20,21]. Furthermore, the colloidal stability of the NPs in a solution is another issue for the application of NPs as carriers of nystatin. SeNPs, for example, exhibit good antimicrobial activity and low cytotoxicity toward mammalian cells in addition to their antimicrobial effect. Several chemical methods for synthesizing SeNPs require reducing and stabilizing chemicals that can be toxic when used in biological systems and prevent their efficient utilization [22,23]. In order to improve biocompatibility and stability, SeNPs must be synthesized biologically. Over the last two decades, researchers have become increasingly interested in the green synthesis of NPs [24,25,26,27,28] including SeNPs [23]. Microorganism-mediated methods are regarded as superior to chemical methods when preparing low-cost and nonhazardous biological agents. Biogenic SeNPs are safe to use as drug carriers due to their high degradability, low toxicity, and gradual elimination from the body [29,30].
This study aims to synthesize biogenic SeNPs as biocompatible carriers for nystatin (SeNP@PVP_Nystatin nanoconjugates). Additionally, we have investigated the molecular mechanisms that regulate the antifungal activity of nanoconjugates by real-time polymerase chain reaction (RT-PCR). According to this study’s results, using biogenic SeNPs in conjunction with antimicrobial drugs is beneficial, as the quantities of both agents (biogenic SeNPs and drugs) can be significantly reduced, and the number of adverse side effects can also be reduced. The novelty of this study is the use of biogenic SeNPs as carriers for delivering nystatin, an antifungal drug, to inhibit the virulence factors of C. albicans.
2. Results and Discussion
2.1. Synthesis of Biogenic SeNPs and SeNP@PVP_Nystatin Nanoconjugates
The synthesis of the SeNPs was followed by observing the change in the color of the Tryptic Soy Broth (TSB) broth. We used lab isolates to screen the synthesis of biogenic SeNPs. Among the numerous isolates tested for tolerance toward sodium selenite at various concentrations, P. terreus (NCBI accession number KM203682) showed the highest tolerance, up to 5 mM (Figure S1). As seen in Figure S1, the growth kinetics of P. terreus during the synthesis of biogenic SeNPs was similar to the control (up to 10 mM sodium selenite) for 24 h; therefore, the P. terreus isolate was used to synthesize SeNPs.
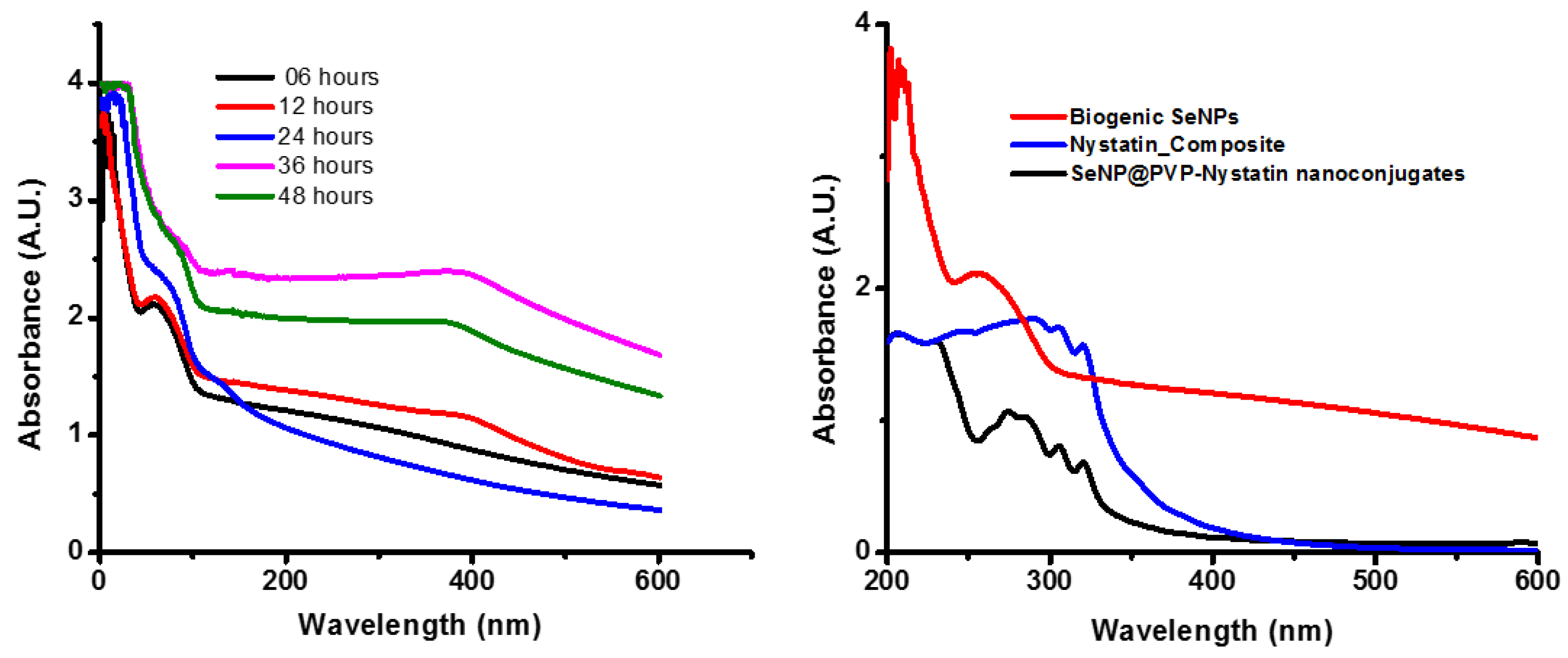
After P. terreus cells were added to the TSB medium supplemented with 1 mM sodium selenite, the color of the TSB medium changed from yellow to brick red within 6 h, and the intensity increased gradually over 48 h. The red coloration of the TSB was in agreement with previous reports [31,32] and indicated the formation of SeNPs [33,34]. The biogenic SeNPs were orange in color due to the surface plasmon resonance of SeNPs [35]. A UV-Vis spectroscopy observation during biogenic SeNP synthesis is illustrated in Figure 1. The SeNPs exhibited a characteristic peak at around 270 nm [35]. Using UV-Vis spectroscopy, we found that the synthesis of biogenic SeNPs started within 6 h and the synthesis of SeNPs increased with an increase in incubation time. As seen in Figure S1, the colony-forming units of P. terreus during the synthesis of biogenic SeNPs were nearly the same as the control, indicating that the biogenic SeNPs were produced during the logarithmic growth phase of P. terreus, which is in agreement with the available reports [36,37,38]. The size of the biogenic SeNPs did not increase with an increase in the incubation period (Figure S1); in other words, there was no linear relationship between the incubation time and the NPs’ size. The relative, unaltered absorption spectra of the formed biogenic SeNPs, between 6 and 48 h, also indicated the stability in the sizes of the produced SeNPs [32]. However, biogenic SeNPs synthesized by using Pantoea agglomerans showed a linear relationship between incubation time and NPs size; as the incubation time increased from 15 h to 20 h, the average size of the biogenic SeNPs increased from 90–110 nm to 100–120 nm [39]. As the microbial cells reduced the selenium ions to elemental Se0, we excepted a reduction in the concentration of the selenium ions in the broth after separating the synthesized biogenic SeNPs and the cells from the TSB broth (Figure S1). In Figure S1, the concentration of selenium ions decreased linearly for 24 h, indicating that biogenic SeNPs were synthesized linearly during mediated synthesis. The microbial-mediated synthesis of SeNPs was well-documented [40,41,42,43,44,45]. The microbial-mediated synthesis did not require any specialized apparatus or condition. Whole cells or cell extracts of Bacillus sp. B2 [43], Penicillium chrysogenum [44], P. expansum [45], and Bacillus subtilis BSN313 [34] were used as reducing agents for the synthesis of SeNPs, with an incubation time of up to five days. However, SeNP biosynthesis using P. terreus has not been reported yet. Interestingly, unlike five days of incubation time for the synthesis of SeNPs in the aforementioned reports, in the present study, the synthesis of biogenic SeNPs starts within 6 h. Thus, the synthesis of biogenic SeNPs is an efficient and feasible method for the large-scale synthesis of SeNPs. The synthesis of biogenic SeNPs can be summarized as follows [39]
SeO32− + 4e− + 6H+ ⇆ Se0 + 3H2O
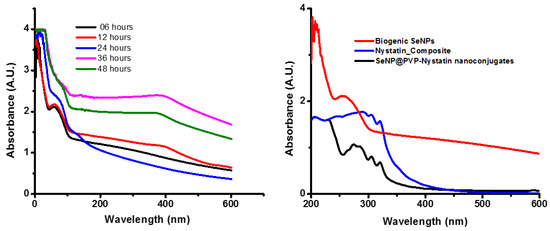
Figure 1.
Ultraviolet–visible (UV-Vis) spectra of biogenic SeNPs, Nystatin_Composite, and SeNP@PVP_Nystatin nanocomposites. The absorption peak at 270 nm for the synthesis of biogenic SeNPs at different time interval periods shows that the synthesis of biogenic SeNPs increases with an increase in the incubation period (left); absorption peaks of biogenic SeNPs upon functionalization with nystatin (SeNP@PVP_Nystatin nanoconjugates) show additional peaks at 289, 303, and 318, which are characteristic peaks for nystatin. The Nystatin_Composite also shows nystatin characteristic peaks.
Paenibacillus bacteria are rod-shaped Gram-positive or Gram-variable, endospore-forming, aerobic, or facultatively anaerobic bacteria. In facultative anaerobes, either the Se oxyanions act as the electron acceptors and form extracellular granules composed of stable, uniform nanospheres (with diameters around 200 nm) of SeNPs having trigonal structures, or the selenite is reduced by a Painter-type reaction in which Se-digluthathione intermediates are formed followed by SeNPs (because selenite is highly reactive with thiol groups) [44]. Following the synthesis of biogenic SeNPs, it is functionalized with nystatin via polyvinylpyrrolidone (PVP) to form SeNP@PVP_Nystatin nanoconjugates. PVP is a biocompatible polymer and is used as a binder to help nystatin to coat the surface of the biogenic SeNPs. Nystatin is a proven antifungal molecule of broad specificity and therefore has been used in the present study. The synthesis of nanoconjugates is shown in Figure 1. As can be seen in Figure 1, the characteristic peak for the biogenic SeNPs shifted from 270 nm to 245 nm in a Nystatin_Composite, due to the presence of polymeric layers, polyvinylpyrrolidone (PVP), which collect the n-electrons of PVP on the surface of biogenic SeNPs [46]. In nanocomposites, three additional peaks (289, 303, and 318 wavelengths) have been observed, which correspond to multiple nystatin functionalization on biogenic SeNPs [47].
2.2. Characterization of Biogenic SeNPs and SeNP@PVP_Nystatin Nanoconjugates
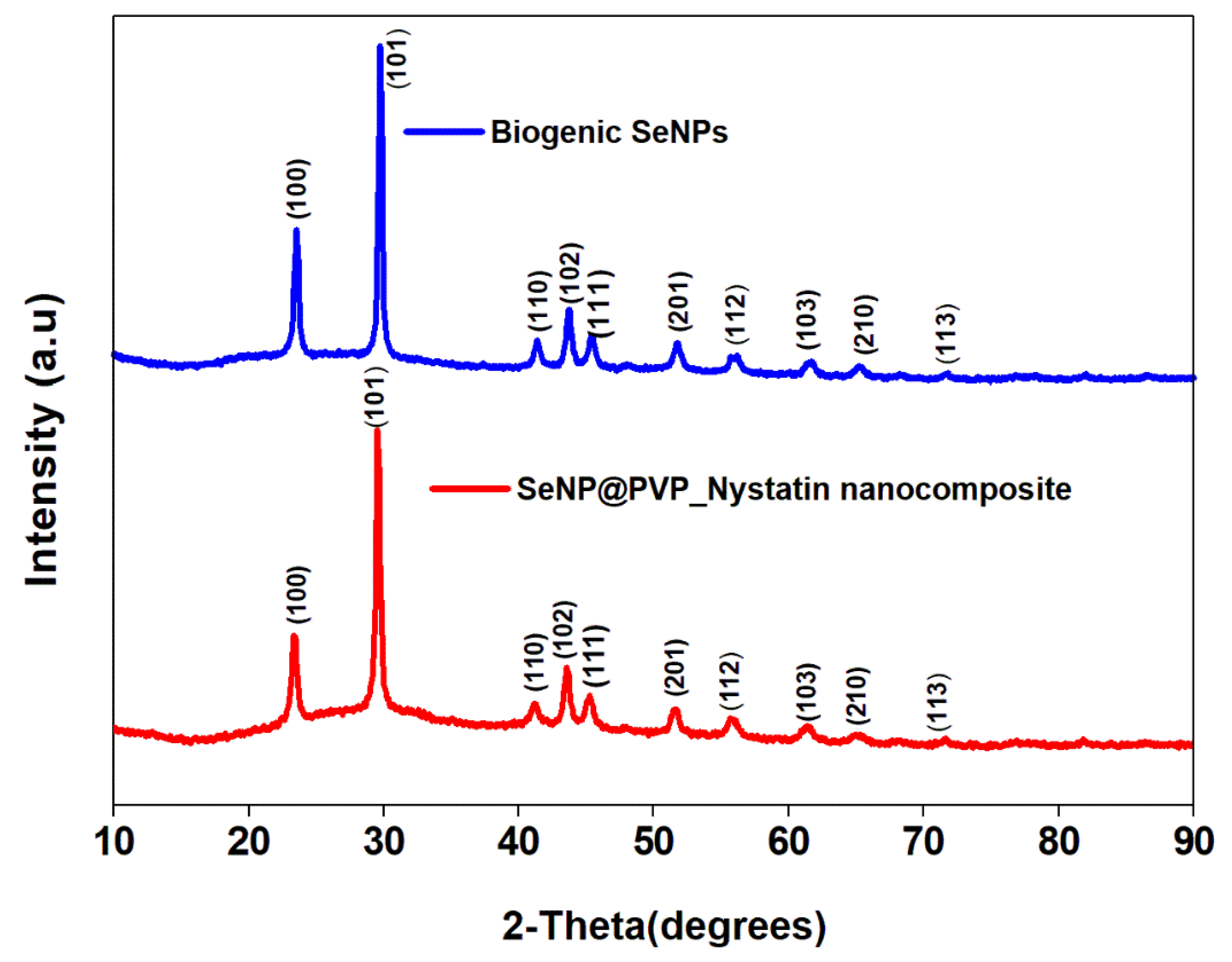
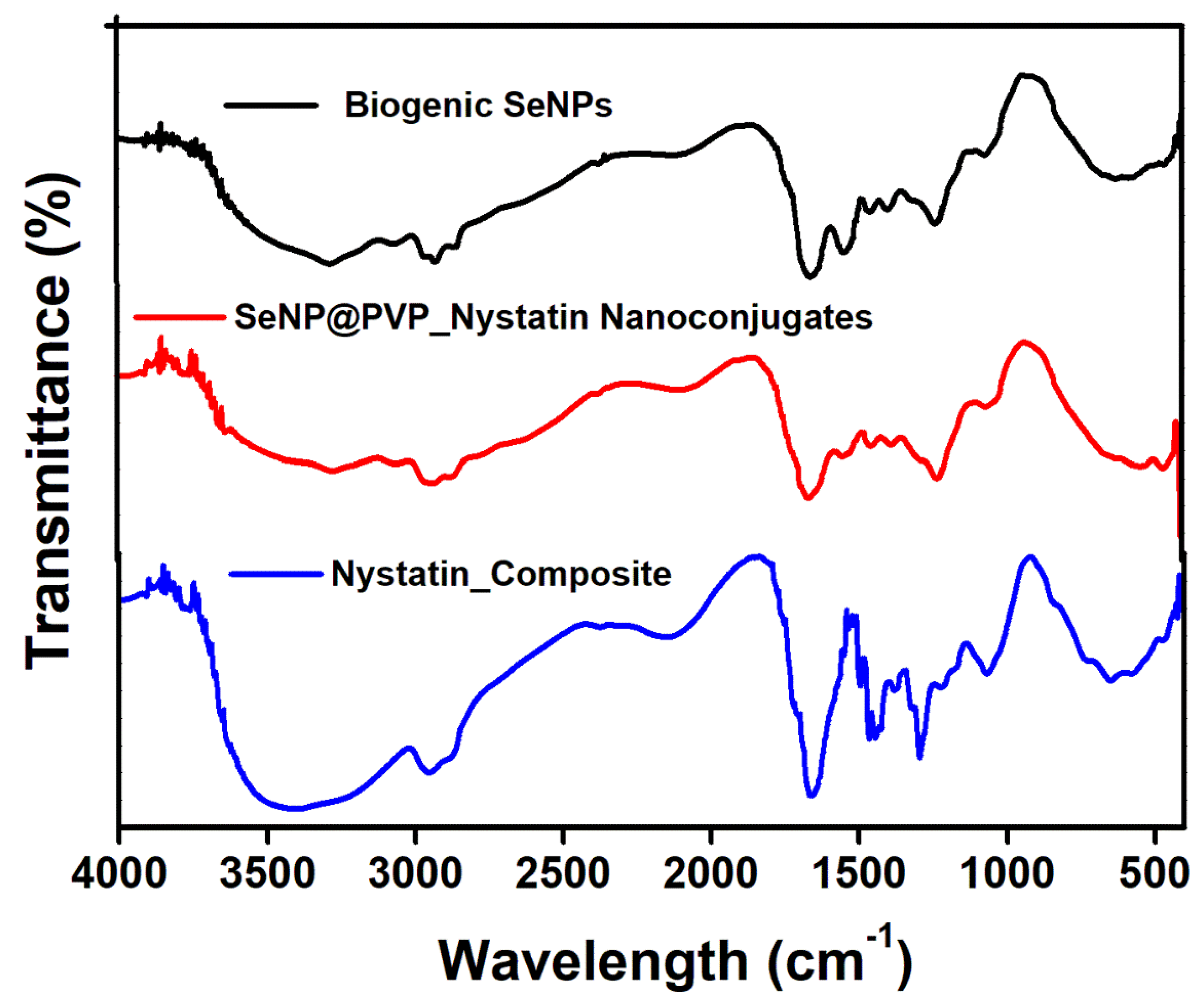
Before characterization, the biogenic SeNPs were separated from the culture broth by centrifugation. After centrifugation, the pellets obtained contained cells and NPs, which were first rinsed repeatedly with distilled water, followed by octanol, and then suspended in octanol for 24 h, to separate the NPs from the cells [48]. The nanoconjugates were first characterized by X-ray diffraction (XRD) analysis. Figure 2 shows the XRD analysis of biogenic SeNPs and nanoconjugates. Sharp diffraction peaks at 2θ (degrees) of 23.57°, 29.73°, 41.28°, 43.68°, 45.43°, 51.72°, 56.07°, 61.62°, 65.24°, and 71.60°, respectively, have been indexed as the (100), (101), (110), (102), (111), (201), (112), (103), (210), and (113) planes of Se. All the diffraction peaks can be indexed for the trigonal phase of selenium, which are in good agreement with the reported data (JCPDS card No. 06-362) [49,50]. In nanocomposites, the presence of nystatin over biogenic SeNPs was studied by Fourier Transform Infrared Spectroscopy (FTIR). Figure 3 shows an FTIR analysis of nanoconjugates. The FTIR analysis showed the characteristic peaks for the amide bonds at 1654 cm−1 and 1540 cm−1, indicating the presence of proteins (from P. terreus) for the stabilization of the biogenic NPs. A C=O peak at 1665 cm−1 can be attributed to the PVP. In FTIR, the nystatin could be ascertained from a broad peak at 3367 cm−1, which could be assigned to O-H stretching vibration [51]. The peak located at 2937 cm−1 could be attributed to the CH2 stretch vibration. The peaks at 1709 and 1620 cm−1 could be attributed to C=O stretching vibrations in the carboxylic group and C=C asymmetric stretching, respectively [52]. Furthermore, the peaks at 1069, 848, and 795 cm−1 could be attributed to -CH2, and -C-H stretch vibrations [13]. As nystatin was functionalized over the surface of biogenic SeNPs, a significant increase in the band intensity of the C-H bond could be detected at 2922 cm−1.
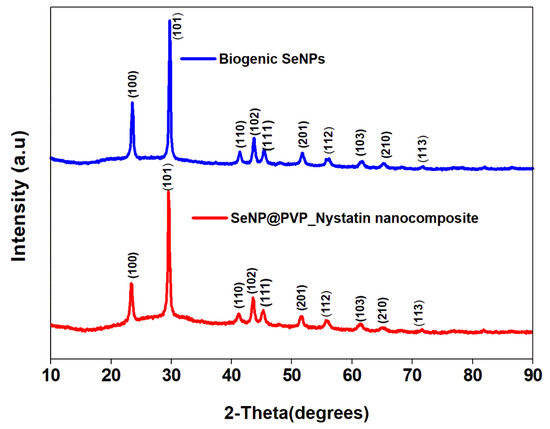
Figure 2.
XRD analysis of biogenic SeNPs and SeNP@PVP_Nystatin nanocomposites, showing characteristic 2θ values indexed at the (100), (101), (110), (102), (111), (201), (112), (103), (210), and (113) planes.
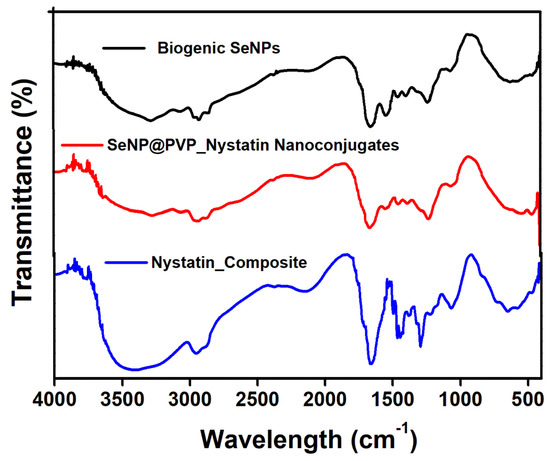
Figure 3.
FTIR analysis of Biogenic SeNPs, Nystatin composite, and SeNP@PVP_Nystatin nanoconjugates.
After functionalizing nystatin over the biogenic SeNPs, both biogenic SeNPs and SeNP@PVP_Nystatin nanoconjugates were characterized for size, shape, size distribution, and zeta potentials. The shape and size of biogenic SeNPs and nanoconjugates were determined by Field Emission Scanning Electron Microscopy (FESEM) (Figure 4). As seen in the FESEM micrograph, almost all the NPs were perfect round spheres, which seemed to be a commonly observed feature of biosynthesized NPs, as was evident in other studies [53,54,55]. There was an accumulation of electron-dense particles around the cells or the outer side of the membrane. When the cells were grown in TSB without selenite, these particles did not appear. In spite of the accumulation of Se0, with a tendency to accumulate particles into aggregates, the outer membrane did not appear distorted or lysed. The biogenic SeNPs appeared to have a spherical shape and a diameter of approximately 200 nm, similar to those found in P. motobuensis [56], Stenotrophomonas maltophilia [57], and Alcaligenes faecalis [58], Duganella sp., Agrobacterium sp. [55], Enterobacter cloacae [59], B. selenitireducens [60], and Azospirillum brasilense [61]. After functionalizing nystatin over the biogenic SeNPs, the size of the biogenic SeNPs increased to 240 nm, indicating the successful formation of nanoconjugates.

Figure 4.
FESEM analysis of Biogenic SeNPs and SeNP@PVP_Nystatin nanoconjugates. Biogenic SeNPs are shown along with cells.
Dynamic light scattering (DLS) analysis was performed to determine the size range analysis of biogenic SeNPs and nanoconjugates. The elemental analysis of biogenic SeNPs and nanoconjugates (Figure S2) by EDX analysis confirmed the characteristic peak for SeLα at 1.37 keV, along with peaks for carbon, oxygen, and nitrogen [35].
As SeNPs were synthesized by using the whole cell mass and later functionalized with nystatin, we were expecting the charges on biogenic SeNPs and nanoconjugates. The charges were determined by measuring the zeta potential on biogenic SeNPs and nanoconjugates. The zeta potential value for biogenic SeNPs and nanoconjugates were, respectively, −37.44 and −37.14 (Table 1). The negative zeta potential value indicates the colloidal stability of the biogenic SeNPs and nanoconjugates, which is very essential for the biological application of biogenic SeNPs and nanoconjugates [62]. The polydispersity indices further support the colloidal stability of biogenic SeNPs, which is due to the presence of proteins [44,63].

Table 1.
DLS, Zeta Potential analysis.
2.3. In Vitro Antifungal Activities of Biogenic SeNPs and SeNP@PVP_Nystatin Nanoconjugates
We studied the in vitro antifungal activities in C. albicans. Three in vitro antifungal activities of biogenic SeNPs and nanoconjugates were studied: Growth inhibition, morphogenesis inhibition, and antibiofilm. Growth inhibiting activity was performed to know if biogenic SeNPs and nanoconjugates inhibited the growth of C. albicans and antibiofilm activity was performed to know if biogenic SeNPs and nanoconjugates inhibit mycelial growth and polysaccharide synthesis. Morphogenesis inhibition was examined to find out whether biogenic SeNPs and nanoconjugates inhibited the transition of yeast to hyphal forms.
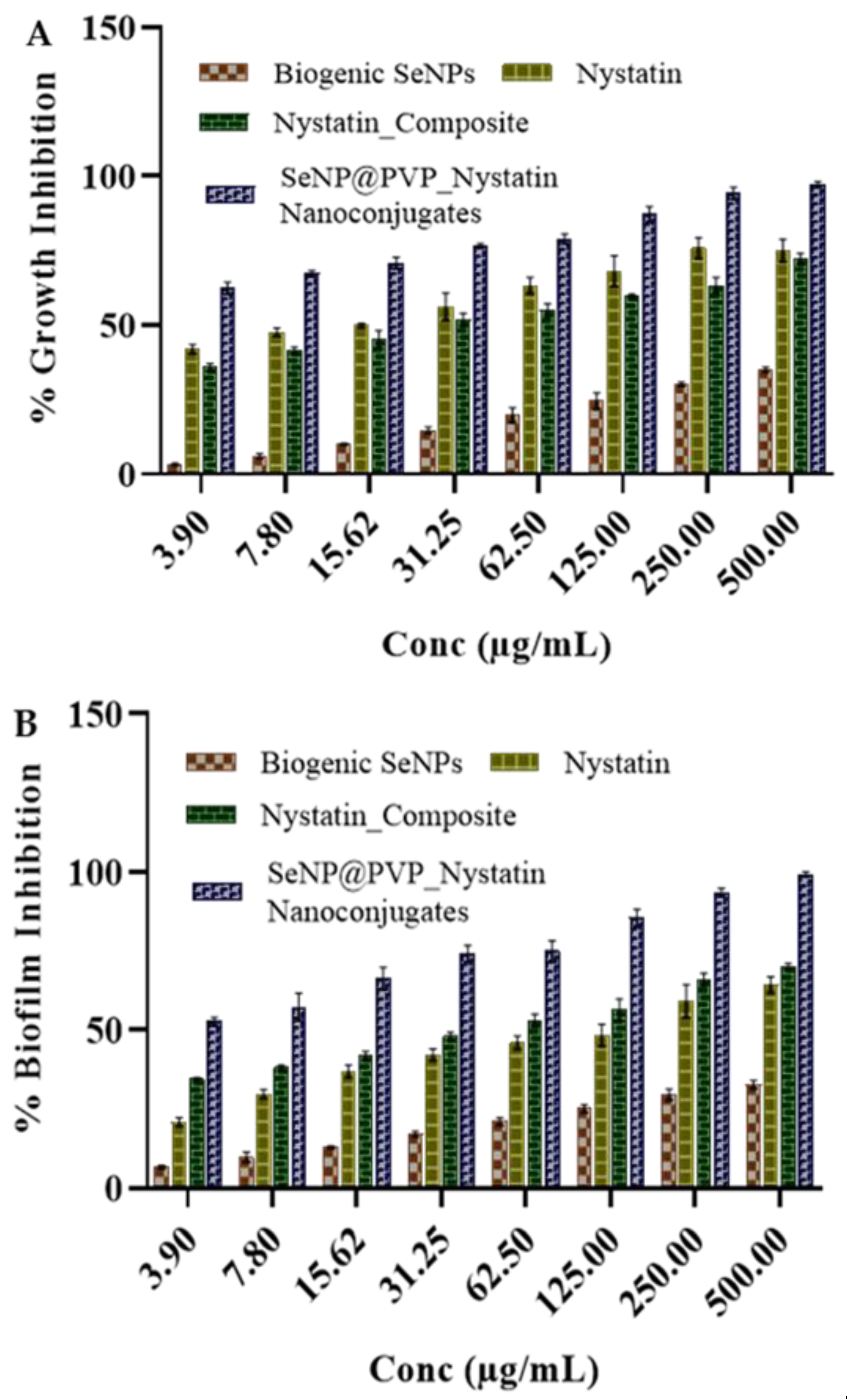
The growth and biofilm inhibition studies were quantitative in nature and performed by measuring the metabolic activities in the yeast and hyphae via the 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay, morphogenesis inhibition studies were qualitative in nature and performed by observing the cellular morphology (yeast and hyphae), under (FESEM). First, we identified whether biogenic SeNPs possessed the aforementioned in vitro antifungal activities. Biogenic SeNPs did not inhibit the growth and biofilm of C. albicans (Figure 5) till 500 µg/mL. This was very surprising as most of the cited literature for the biogenic SeNPs showed moderate-to-good in vitro antifungal activities. At concentrations between 8 and 512 mg/mL, SeNPs synthesized by S. maltophilia and B. mycoides inhibited the growth of Pseudomonas aeruginosa in clinical isolates, but not C. albicans and C. parapsilosis [64]. Similarly, another study [65] demonstrated a strong inhibitory effect of SeNPs (10 μg/mL) on the growth of four Gram-positive pathogens, Staphylococcus aureus, B. cereus, methicillin-resistant S. aureus, and S. agalactiae.
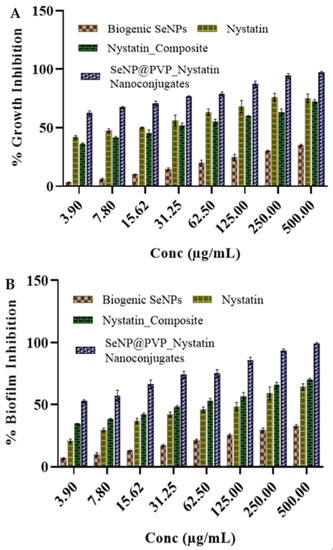
Figure 5.
Antifungal activity of biogenic SeNPs, Nystatin_Composites, and SeNP@PVP_Nystatin nanoconjugates on C. albicans. Nanoconjugates showed better antifungal activity (inhibition of growth (A) and biofilm (B)) than composites, and biogenic SeNPs did not show growth inhibition activity up to 500 μg/mL.
Interestingly, other Gram-negative bacteria (P. aeruginosa, Enterobacter sp., Enterococcus sp., Proteus mirabilis, Klebsiella sp., Salmonella enteritidis, and S. maltophilia) and C. albicans were not inhibited at this concentration. However, higher concentrations of SeNPs (400 μg/mL) that were synthesized by using B. subtilis BSN313 showed potential antimicrobial activity on St. mutans, B. cereus, Escherichia coli, and C. albicans [36]. A larger MIC concentration of biogenic SeNP (>500 g/mL) on C. albicans is comparable to other reports [66]. As biogenic SeNPs have not inhibited the growth and biofilm, we decided to prepare SeNP@PVP_Nystatin nanoconjugates by coating nystatin, an antifungal drug, over the biogenic SeNPs, to use against C. albicans. Nystatin is a known antifungal molecule often used to treat C. albicans infections. Nystatin is coated over the biogenic SeNPs as a composite (Nystatin_Composites), by using a water-soluble polymer, PVP, made from the monomer N-vinylpyrrolidone. PVP polymers are non-cytotoxic, biodegradable, and do not possess antifungal activity. The purpose of preparing a nanocomposite is to create extended-release carriers either to enhance the delivery or uptake of nystatin into the target cells or to reduce the toxicity of the free nystatin.
The growth and biofilm inhibition of the biogenic nystatin, SeNPs, nystatin-PVP, and nanocomposite are shown in Figure 5. Nystatin has shown antifungal activity, and the activity increases with increasing concentrations of nystatin. Similarly, the Nystatin_PVP composite has also shown antifungal activity comparable to nystatin. However, nanocomposites have shown improved antifungal activity against C. albicans. The calculated MIC50 value for the growth inhibition for nystatin and PVP_Nystatin was between 7.8 to 15.6 µg/mL. Similarly, the calculated MIC50 value for the antibiofilm activity of nystatin was 31.2 µg/mL. The MIC50 value for the growth inhibition and antibiofilm activity for nanoconjugates had decreased to less than 3.9 µg/mL. A possible explanation for these increased in vitro antifungal activities of nanoconjugates may be because of the greater surface area of NPs, which enhances nystatin’s solubility and availability [67]. Another reason could be the localization of nanocomposites over the surfaces of cells. By directly contacting the surface of cells, nanocomposites may effectively recruit the nystatin molecules to enhance the antifungal activity.
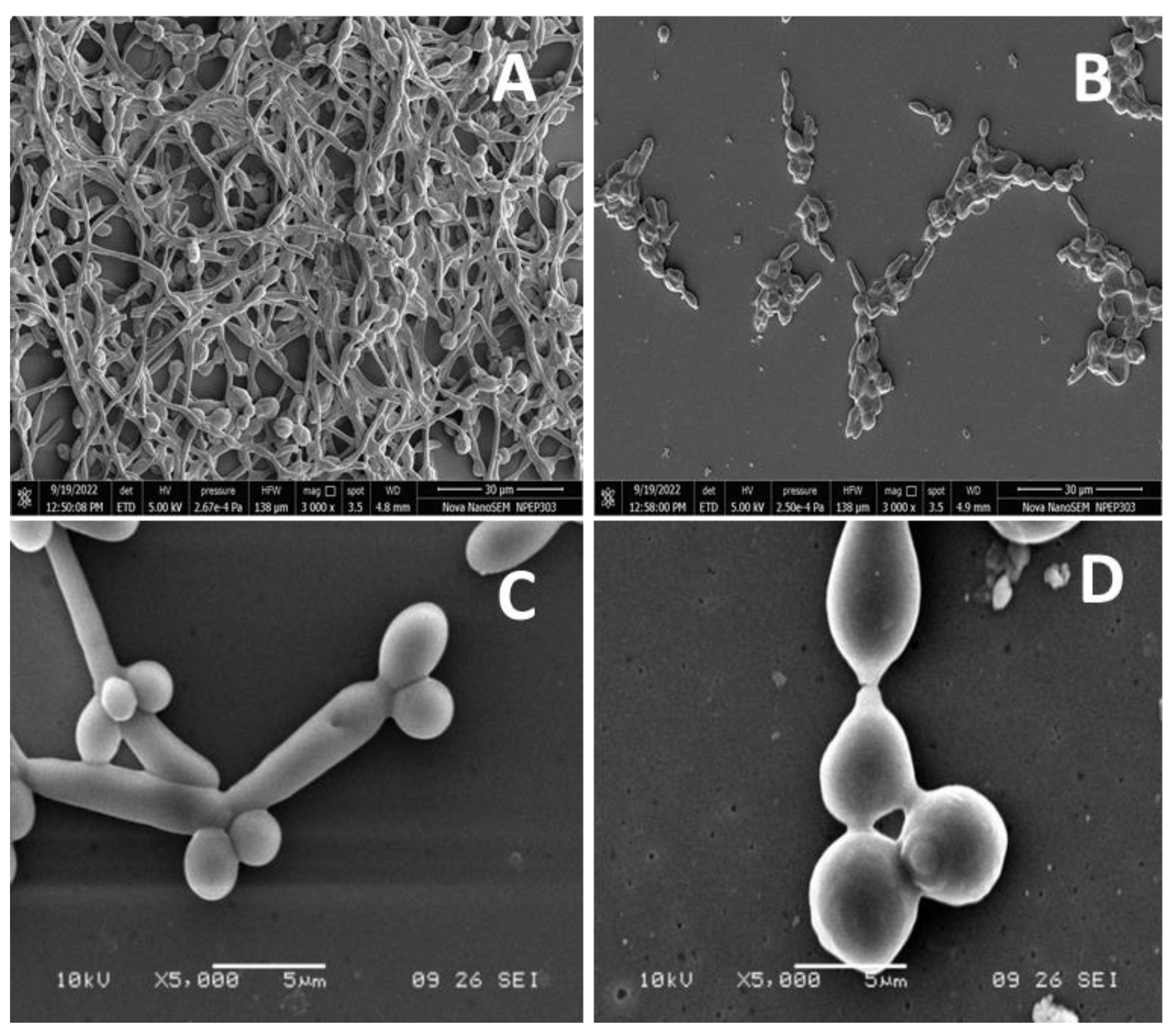
As a result of the intercalation of nystatin into ergosterol-containing membranes of the fungal cells, it forms channels that prevent ion transfer in the cell, causing the leakage of cytoplasmic contents and eventually cell death. The biodegradable nature of PVP further assists the antifungal activity by the slow release of nystatin molecules on or near the surface of cells, which is well-documented in the literature [68,69]. The nystatin that functionalized over the surface magnetic NPs was shown to possess more fungicidal activity than unbound nystatin, due to the enhanced ability of the magnetic NPs to improve nystatin penetration, thus improving their killing properties and exerting a rapid effect on the Candida cells [70]. The nystatin coated on iron NPs and the chitosan composite [71], as also the nystatin-conjugated iron oxide nanocomposite [72] have also shown improved antifungal activity over unbound nystatin. The antifungal activity of nanocomposites has become more evident following studies on morphogenesis inhibition. At 3.9 µg/mL, nanoconjugates inhibited the morphogenesis of the yeast form to the hyphae form (Figure 6B,D). Control samples (without nanocomposites) showed the transition of the yeast form to the hyphal form.
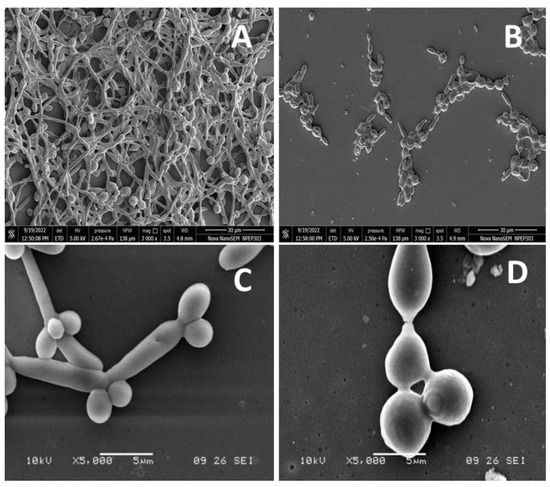
Figure 6.
The anti-morphogenesis activity of SeNP@PVP_Nystatin nanoconjugates. Nanoconjugates inhibited yeast to hyphae transitions (B,D). The control samples (A,C) showed yeast-to-hyphae transitions.
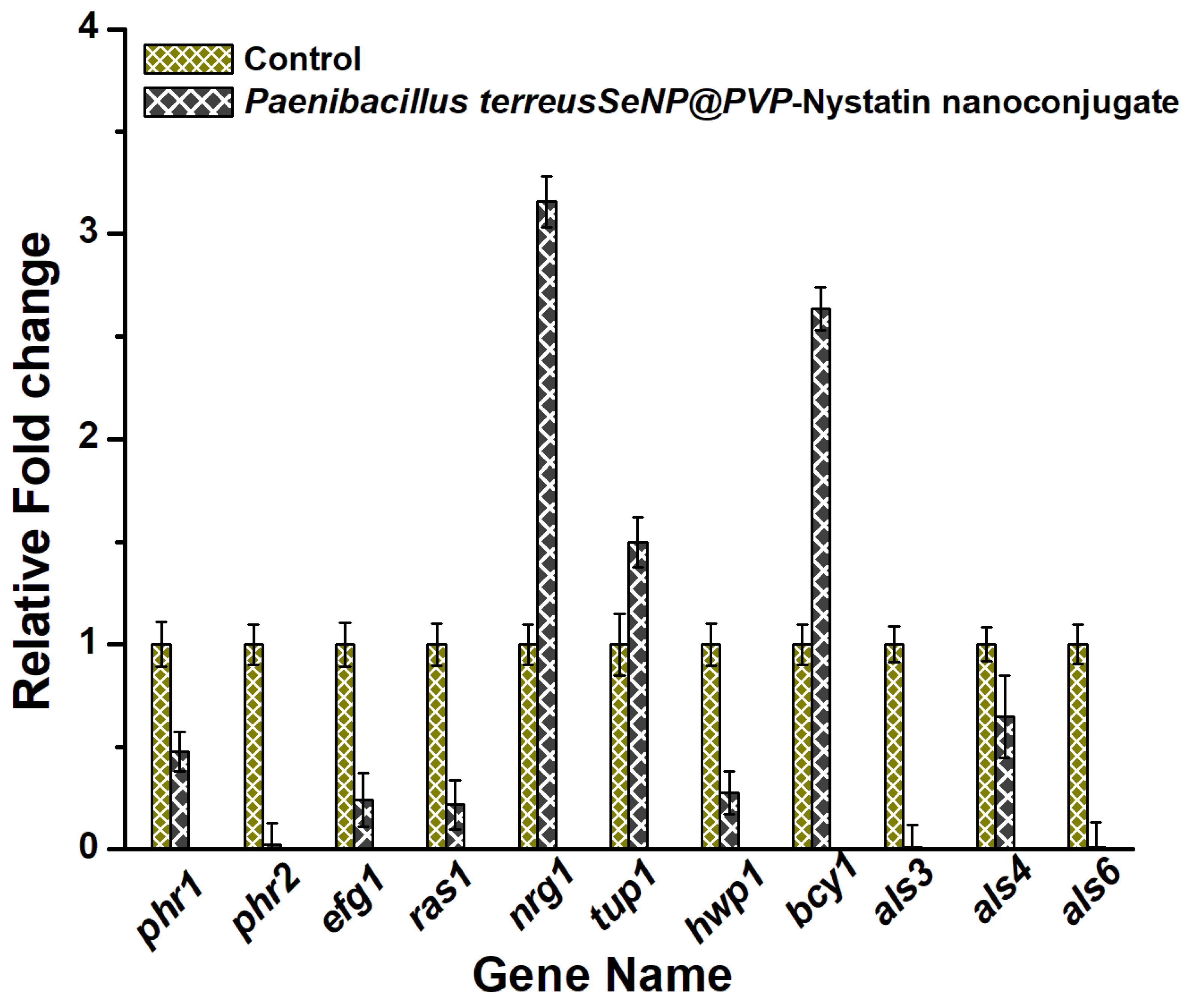
Finally, the mechanism of the antifungal activity of nanoconjugates was studied by measuring the transcript levels of important genes involved in morphogenesis and the biofilm formation in C. albicans, using RT-PCR. Essentially, the RAS/cAMP/PKA signaling pathway was studied, as the RAS/cAMP/PKA pathway played an important role in yeast to hyphae transition and biofilm formation. Adhesion was the first step for the initiation of biofilm formation in C. albicans and was mediated by the expression of Als genes, which encoded the adhesion proteins that helped the cells to adhere to the hydrophobic surfaces or to attach themselves to the endothelial and epithelial cells [73,74,75]. Als3, 4, and 6, and the Hwp1 genes were downregulated in the presence of nanoconjugates (Figure 7). The Hwp1 gene that was involved in hyphal growth and biofilm formation [76] was also downregulated. The Nrg1 and Tup1 genes acted as Hwp1 repressors [77], and both Nrg1 and Tup1 were upregulated. The Efg1 gene played an important role in hyphal growth and biofilm formation, and its expression was related to the Als and Hwp1 gene expressions [78]. The downregulation of the Efg1 gene likely downregulated the expression of the hyphal cell-wall genes, such as Hwp1 and Als3. The Phr1 gene expression was seen to inhibit yeast-to-hyphae transition, and the downregulation of the Phr1 gene likely inhibited the morphogenesis in C. albicans in the presence of nanoconjugates.
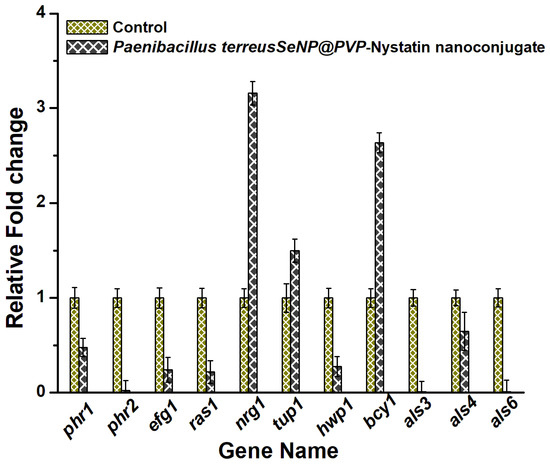
Figure 7.
The anti-morphogenesis mechanism of SeNP@PVP_Nystatin nanoconjugates. The real-time polymerase chain reaction (RT-PCR) of the genes (phr1, phr2, efg11, ras11, tup1, hwp1, als3, als4, and als6) involved in the morphogenesis was downregulated in the presence of the nanocomposites.
Finally, to confirm the potential application of biogenic SeNPs, cytotoxic studies were performed on HEK-293 cells. The biogenic SeNPs were not cytotoxic on HEK-293 until 125 µg/mL (Figure S3), thus paving the way for its use as a carrier for the effective delivery of the antifungal drug, nystatin, against C. albicans. Thus, the present study utilizes the application of biogenic SeNPs as a vehicle to deliver the effective concentration of nystatin for antifungal activities. The present study is significant as it decreases the effective concentration of the nystatin required for antifungal activities. In the future, biogenic SeNPs can be used as carriers to deliver other antimicrobial molecules.
3. Materials and Methods
The media components used for the experiments were purchased from Hi-Media (Mumbai, India). The chemicals, such as sodium selenite, nystatin, PVP, dimethyl sulfoxide (DMSO), and other reagents used in the experiments were of analytical grade and purchased from Sigma-Aldrich (Bangalore, India). C. albicans ATCC 227 was a kind gift from Dr. Zore, SRTMU, Nanded.
3.1. Screening of Selenium-Tolerant Bacteria
The isolates selected for the screening of selenium-tolerant bacteria were the lab isolates collected from the Western Ghats of Maharashtra, India. To put it briefly, the isolates with 1.0 optical density (O.D) were inoculated into the tryptic soy broth (TSB) containing various concentrations (1 to 75 mM of sodium selenite) at 37 degrees centigrade (°C) and 150 rotation per minute (rpm) with 24 h of incubation. Following the incubation, an aliquot of the samples was spread-plated, and the plates were incubated at 37 °C for 24 h for the colonies to appear. Any spread-plated plate (containing sodium selenite) that did not show any colonies was regarded as having minimum inhibitory concentrations (MIC) of selenium against the tested isolates. The isolate that tolerated the maximum concentrations of sodium selenite was identified by using 16S rRNA sequencing and later used for the synthesis of SeNPs (biogenic SeNPs).
3.2. Synthesis of Biogenic SeNPs
P. terreus was an isolate that was found to be resistant to sodium selenite, and this isolate was used to biosynthesize the SeNPs. In short, 100 mL of the TSB nutrient medium supplemented with 1 mM of sodium selenite in a 250 mL Erlenmeyer flask was inoculated with 1% v/v of P. terreus and grown overnight. The TSB media with 1 mM sodium selenite was used as the control. Flasks were incubated at 37 °C, at 150 rpm. Samples were aseptically withdrawn at different time intervals. SeNPs were separated from cells, purified, and stored in water at 4 °C for further analysis [12].
3.3. Synthesis of SeNP@PVP_Nystatin Nanoconjugates
The synthesis of nanoconjugates was performed by functionalizing nystatin on biogenic SeNPs. An aqueous suspension of 10 mg/mL of the SeNPs solution was prepared and sonicated at 40 Hz for 30 min. Thirty milliliters of 0.5 mg/mL PVP solution was added dropwise into the biogenic SeNP suspension, with stirring conditions at 50 °C for 1.5 h, followed by the dropwise addition of 15 mL of 1 mg/mL of nystatin solution. This total mixture was heated at 50 °C until all the alcohol evaporated (2–3 h), and then it was cooled and centrifuged at 10,000 rpm for 10 min and finally air-dried so the nanoconjugates could be used for further study.
3.4. Characterization of Biogenic SeNPs and SeNP@PVP_Nystatin Nanoconjugates
Crystallographic characterization of biogenic SeNPs and nanoconjugates was performed by XRD analysis. The XRD measurements were performed with the help of the Bruker D8 Advance Diffractometer (Bruker, Germany) (40 kV, 40 mA, Cu-Kα, λ = 1.54 Å) using 2-theta values ranging from 10 to 90. Biogenic SeNPs and nanoconjugates were characterized by UV-Vis spectroscopy (Shimadzu 1800, Kyoto, Japan) and FESEM (FEI Nova NanoSEM 450) for determining the size and shape of the NPs. Elemental composition was performed by using an EDX (Bruker XFlash 6I30) micro-analysis system coupled with the FESEM.
3.5. Antifungal Activity Assay of Biogenic SeNPs and SeNP@PVP_Nystatin Nanoconjugates
The standard broth micro-dilution method was used to assay the antifungal activity (growth inhibition) of biogenic SeNPs and nanoconjugates on the C. albicans in the planktonic form. In short, the concentration of each of the biogenic SeNPs and nanoconjugates in a range of 3.9 to 500 µg/mL was added to 100 µL of the Roswell Park Memorial Institute (RPMI)-1640 medium, containing 1 × 104 cells/mL, in 96-well plates (Costar, Corning Inc., Corning, NY, USA). The plates were incubated at 37 °C for 48 h, and the growth was measured by taking absorbance at 620 nm using a microplate reader (Multiskan EX, Thermo Electron Corp., Waltham, MA, USA). The lowest concentration of each biogenic SeNP and nanoconjugate, which caused a 50% reduction in the absorbance compared to the control, was considered as MIC50. All the experiments were performed in triplicate.
3.6. Anti-Biofilm Assay of Biogenic SeNPs and SeNP@PVP_Nystatin Nanoconjugates
An anti-biofilm assay was performed in tissue culture-treated, 96-well, polystyrene plates. In short, 100 μL of 1 × 107 cells /mL suspensions were added to 96-well plates and allowed to adhere to a solid surface at 37 °C for 90 min. After adhesion, the unbound cells were removed by washing twice with phosphate-buffered saline (0.1 M, pH 7.0). The wells were reconstituted with RPMI-1640 medium (200 μL) along with various concentrations of biogenic SeNPs and nanoconjugates separately, and the plates were incubated for 48 h at 37 °C. The developed biofilms were observed under an inverted light microscope (Metzer, India) and quantified by a 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay. All the experiments were performed in triplicate.
3.7. Anti-Morphogenesis Assay of Biogenic SeNPs and SeNP@PVP_Nystatin Nanoconjugates
An anti-morphogenesis assay of biogenic SeNPs and nanoconjugates was performed in 20% serum. In short, various concentrations of each biogenic SeNP and nanoconjugate were added into 100 μL of 20% serum-containing 1 × 106 cells/mL of C. albicans and incubated for 90 min at 37 °C in an orbital shaker at 200 rpm. After incubation, the cells were observed for the formation of the germ tube (hyphae) under a microscope (Labomed microphotography system (Labomed, India)) at 200 × magnification. The inhibition of 50% hyphae formation was compared with the control and considered as the MIC50 for morphogenesis. All the experiments were performed in triplicate. For observing the morphologies of cells in the presence of MIC50 values of biogenic SeNPs and nanoconjugates, the cells were fixed in 2.5% of glutaraldehyde in 0.1 M PBS (pH 7.2) for 24 h at 4 °C and then dehydrated in a series of graded alcohols. The cells were preserved in 100 μL absolute alcohol. Finally, the cells were layered onto a glass, coated with gold ions, and viewed under FESEM (FEI Nova NanoSEM 450).
3.8. Anti-Morphogenesis Mechanism of SeNP@PVP_Nystatin Nanoconjugates
The anti-morphogenesis mechanism action of nanoconjugates was studied by measuring the differential expression of the hyphae by using RT-PCR. In short, the C. albicans cells (1 × 107 cells/mL) were inoculated in a 20% serum-containing MIC concentration of SeNPs and nanoconjugates and incubated for 90 min at 37 °C. The total RNA was isolated by using the RNeasy® Mini Kit (QIAGEN) and converted to cDNA by using Superscript® III for the first-strand synthesis (Invitrogen, Life Technologies, Carlsbad, CA, USA). The PCR reactions were conducted by using the SYBR Green Real-Time PCR Master Mixes, Thermofisher scientific, Waltham, MA, USA) with preliminary denaturation for 3 min at 95 °C. This was followed by 32 amplification cycles of denaturation at 95 °C for 30 s and annealing at 60 °C for 20 s with primer extension at 72° C for 30 s (CFX 96 Real-time System, Bio-Rad, Hercules, CA, USA). Actin was used as an internal control, and the transcript levels of the selected genes were calculated using the formula 2−ΔΔCT [79]. All the reactions were run in triplicate using biological replicates, and the experiment was repeated thrice. Data were reported as mean ± standard deviation (S.D.). The gene expression was normalized to ACTIN gene levels.
3.9. Cytotoxicity Study of Biogenic SeNPs and SeNP@PVP_Nystatin Nanoconjugates on HEK-293
The cytotoxicity effects of biogenic SeNPs and nanoconjugates were studied by using the HEK-293 cell line. In short, 100 μL of Dulbecco Modified Eagle Medium (DMEM) was added into 96-well plates with 1 × 103 cells per well. Biogenic SeNPs and nanoconjugates, in the range of 3.9 to 125 µg/mL each, were added separately into the DMEM and incubated at 37 °C with 5% CO2 for 24 h. After incubation, the quantitative analysis of the effect of biogenic SeNPs and nanoconjugates was performed with the help of an MTT assay, as mentioned earlier.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28041836/s1, Figure S1. The growth kinetics of P. terreus in different concentrations of sodium selenite are shown in log cfu (left). Growth of P. terreus is seen in the presence of 1 mM sodium selenite and a corresponding decrease is seen in the concentration of selenium ions after synthesizing the SeNPs (Z-axis). The lower panel indicates the FESEM micrographs showing the size of SeNPs and cells during the synthesis of biogenic SeNPs. Figure S2. DLS analysis of biogenic SeNPs (left) and SeNP@PVP_Nystatin nanoconjugates (right) show the particle size distribution of biogenic SeNPs and nanoconjugates. The average particle sizes of biogenic SeNPs and nanoconjugates were 220 ηm and 242 ηm, respectively. Figure S3. Cytotoxicity study of SeNP@PVP_Nystatin nanoconjugates on the HEK-293 cell line. Table S1: Gene-specific primers for RT-PCR.
Author Contributions
Conceptualization, investigation, methodology, S.H.N. and D.T.; validation, formal analysis, A.S. and K.G.; resources, data curation, J.S.; writing—original draft preparation, W.Z. and E.S.; writing—review and editing, R.P. and G.K.; project administration, funding acquisition, R.P. and G.K.; Revision, S.H.N. and R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Innovative Leading Talents Program for Zhejiang Provincial Universities, National Young Qihuang Scholars Training Program, The Key Science and Technology Projects of Breeding New Varieties of Agriculture in Zhejiang Province (2021C02074-3); the Zhejiang Provincial Ten Thousand Program for Leading Talents of Science and Technology Innovation (2018R52050); the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents; and the Research Project of the Zhejiang Chinese Medical University Research Foundation (2021JKZDZC06).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request from authors.
Acknowledgments
We acknowledge the support from the Research Department Research and Development Grants of the Biotechnology Department of the Savitribai Phule Pune University.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Macias-Paz, I.U.; Pérez-Hernández, S.; Tavera-Tapia, A.; Luna-Arias, J.P.; Guerra-Cárdenas, J.E.; Reyna-Beltrán, E. Candida albicans the main opportunistic pathogenic fungus in humans. Rev. Argent. Microbiol. 2022, 9, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Iyer, K.R.; Robbins, N.; Cowen, L.E. The role of Candida albicans stress response pathways in antifungal tolerance and resistance. iScience 2022, 25, 103953. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Yadav, B. Microbe Profile: Candida albicans: A shape-changing, opportunistic pathogenic fungus of humans. Microbiology 2017, 163, 1145–1147. [Google Scholar] [CrossRef]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef]
- Kornitzer, D. Regulation of Candida albicans Hyphal Morphogenesis by Endogenous Signals. J. Fungi 2019, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Sellam, A.; Whiteway, M. Recent advances on Candida albicans biology and virulence. F1000Research 2016, 5, 2582. [Google Scholar] [CrossRef]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef]
- Costa-de-Oliveira, S.; Rodrigues, A.G. Candida albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal. Microorganisms 2020, 8, 154. [Google Scholar] [CrossRef]
- Lyu, X.; Zhao, C.; Yan, Z.M.; Hua, H. Efficacy of nystatin for the treatment of oral candidiasis: A systematic review and meta-analysis. Drug Des. Dev. Ther. 2016, 10, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Misra, S.R.; Panda, S.; Sokolowski, G.; Mishra, L.; Das, R.; Lapinska, B. Nystatin Effectiveness in Oral Candidiasis Treatment: A Systematic Review & Meta-Analysis of Clinical Trials. Life 2022, 12, 1677. [Google Scholar]
- El-Batal, A.I.; Nada, H.G.; El-Behery, R.R.; Gobara, M.; El-Sayyad, G.S. Nystatin-mediated bismuth oxide nano-drug synthesis using gamma rays for increasing the antimicrobial and antibiofilm activities against some pathogenic bacteria and Candida species. RSC Adv. 2020, 10, 9274–9289. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Joshaghani, H.; Shokohi, T.; Ahmadi, A.; Mehrbakhsh, Z. Antifungal Activity of ZnO Nanoparticles and Nystatin and Downregulation of SAP1–3 Genes Expression in Fluconazole-Resistant Candida albicans Isolates from Vulvovaginal Candidiasis. Infect. Drug Resist. 2020, 13, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Gao, Y.; Liu, L.; Sai, S.; Ding, C. Striking Back against Fungal Infections: The Utilization of Nanosystems for Antifungal Strategies. Int. J. Mol. Sci. 2021, 22, 10104. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.Z.; Khan, S.B. Antimicrobial Efficacy of Silver Nanoparticles against Candida albicans. Materials 2022, 15, 5666. [Google Scholar] [CrossRef]
- Li, B.; Pan, L.; Zhang, H.; Xie, L.; Wang, X.; Shou, J.; Qi, Y.; Yan, X. Recent Developments on Using Nanomaterials to Combat Candida albicans. Front. Chem. 2021, 9, 813973. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. The use of nanoparticles as alternative therapeutic agents against Candida infections: An up-to-date overview and future perspectives. World J. Microbiol. Biotechnol. 2020, 36, 163. [Google Scholar]
- Morozova, O.V. Silver Nanostructures: Limited Sensitivity of Detection, Toxicity and Anti-Inflammation Effects. Int. J. Mol. Sci. 2021, 22, 9928. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic Effects between Metal Nanoparticles and Commercial Antimicrobial Agents: A Review. ACS Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef]
- Carmo, P.; Garcia, M.T.; Figueiredo-Godoi, L.M.A.; Lage, A.C.P.; Silva, N.S.D.; Junqueira, J.C. Metal Nanoparticles to Combat Candida albicans Infections: An Update. Microorganisms 2023, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Khanna, P.; Bisht, N.; Singh, P. Selenium Nanoparticles: A Review on Synthesis and Biomedical Applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar]
- Mosleh-Shirazi, S.; Kasaee, S.R.; Dehghani, F.; Kamyab, H.; Kirpichnikova, I.; Chelliapan, S.; Firuzyar, T.; Akhtari, M.; Amani, A.M. Investigation through the anticancer properties of green synthesized spinel ferrite nanoparticles in present and absent of laser photothermal effect. Ceram. Int. 2022; in press. [Google Scholar] [CrossRef]
- Dehghani, F.; Mosleh-Shirazi, S.; Shafiee, M.; Kasaee, S.R.; Amani, A.M. Antiviral and antioxidant properties of green synthesized gold nanoparticles using Glaucium flavum leaf extract. Appl. Nanosci. 2022, 1–11. [Google Scholar] [CrossRef]
- Mosleh-Shirazi, S.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Kasaee, S.R.; Jangjou, A.; Izadpanah, P.; Amani, A.M. Biosynthesis, simulation, and characterization of Ag/AgFeO2 core–shell nanocomposites for antimicrobial applications. Appl. Phys. A 2021, 127, 857. [Google Scholar] [CrossRef]
- Amani, A.M.; Danaie, P.; Vaez, A.; Gholizadeh, R.; Firuzyar, T.; Dehghani, F.; Mosleh-Shirazi, S. Rutin precursor for the synthesis of superparamagnetic ZnFe2O4 nanoparticles: Experimental and density functional theory. Appl. Phys. A 2022, 128, 696. [Google Scholar] [CrossRef]
- Dehghani, F.; Shahmoradi, S.; Naghizadeh, M.; Firuzyar, T.; Vaez, A.; Kasaee, S.R.; Amani, A.M.; Mosleh-Shirazi, S. Magnetic graphite-ODA@CoFe2O4: Attempting to produce and characterize the development of an innovative nanocomposite to investigate its antimicrobial properties. Appl. Phys. A 2022, 128, 250. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, J.; Xu, J.-F.; Pi, J. The Advancing of Selenium Nanoparticles against Infectious Diseases. Front. Pharmacol. 2021, 12, 682284. [Google Scholar] [CrossRef]
- Song, D.; Li, X.; Cheng, Y.; Xiao, X.; Lu, Z.; Wang, Y.; Wang, F. Aerobic biogenesis of selenium nanoparticles by Enterobacter cloacae Z0206 as a consequence of fumarate reductase mediated selenite reduction. Sci. Rep. 2017, 7, 3239. [Google Scholar] [CrossRef]
- Ashengroph, M.; Hosseini, S.R. A newly isolated Bacillus amyloliquefaciens SRB04 for the synthesis of selenium nanoparticles with potential antibacterial properties. Int. Microbiol. 2021, 24, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, S.; Sundar, K.; Muthukumaran, A. Monodispersed spherical shaped selenium nanoparticles (SeNPs) synthesized by Bacillus subtilis and its toxicity evaluation in zebrafish embryos. Mater. Res. Express 2018, 5, 025020. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Gorain, M.; Banerjee, P.; Shedbalkar, U.U.; Singh, R.; Kundu, G.C.; Chopade, B.A. Green synthesis of selenium nanoparticles using Acinetobacter sp. SW30: Optimization, characterization and its anticancer activity in breast cancer cells. Int. J. Nanomed. 2017, 12, 6841–6855. [Google Scholar] [CrossRef] [PubMed]
- Fesharaki, P.J.; Nazari, P.; Shakibaie, M.; Rezaie, S.; Banoee, M.; Abdollahi, M.; Shahverdi, A.R. Biosynthesis of selenium nanoparticles using Klebsiella pneumoniae and their recovery by a simple sterilization process. Braz. J. Microbiol. 2010, 41, 461–466. [Google Scholar] [CrossRef]
- Ullah, A.; Yin, X.; Wang, F.; Xu, B.; Mirani, Z.A.; Xu, B.; Chan, M.W.H.; Ali, A.; Usman, M.; Ali, N.; et al. Biosynthesis of Selenium Nanoparticles (via Bacillus subtilis BSN313), and Their Isolation, Characterization, and Bioactivities. Molecules 2021, 26, 5559. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bera, S.; Singh, M.; Mondal, D. Agrobacterium-assisted selenium nanoparticles: Molecular aspect of antifungal activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015004. [Google Scholar] [CrossRef]
- Avendaño, R.; Chaves, N.; Fuentes, P.; Sánchez, E.; Jiménez, J.I.; Chavarría, M. Production of selenium nanoparticles in Pseudomonas putida KT2440. Sci. Rep. 2016, 6, 37155. [Google Scholar] [CrossRef]
- Torres, S.K.; Campos, V.L.; León, C.G.; Rodríguez-Llamazares, S.M.; Rojas, S.M.; González, M.; Smith, C.; Mondaca, M.A. Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J. Nanopart. Res. 2012, 14, 1236. [Google Scholar] [CrossRef]
- Xu, C.; Qiao, L.; Ma, L.; Guo, Y.; Dou, X.; Yan, S.; Zhang, B.; Roman, A. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate intestinal epithelial barrier dysfunction caused by oxidative stress via Nrf2 signaling-mediated mitochondrial pathway. Int. J. Nanomed. 2019, 14, 4491–4502. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Zhang, B.; Liu, J. Extracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensor. Colloids Surf. B BioInterfaces 2010, 80, 94–102. [Google Scholar] [CrossRef]
- Tugarova, A.V.; Kamnev, A.A. Proteins in microbial synthesis of selenium nanoparticles. Talanta 2017, 174, 539–547. [Google Scholar] [CrossRef]
- Tugarova, A.V.; Mamchenkova, P.V.; Dyatlova, Y.A.; Kamnev, A.A. FTIR and Raman spectroscopic studies of selenium nanoparticles synthesised by the bacterium Azospirillum thiophilum. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 192, 458–463. [Google Scholar] [CrossRef]
- Tugarova, A.V.; Mamchenkova, P.V.; Khanadeev, V.A.; Kamnev, A.A. Selenite reduction by the rhizobacterium Azospirillum brasilense, synthesis of extracellular selenium nanoparticles and their characterisation. New Biotechnol. 2020, 58, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Shoeibi, S.; Mashreghi, M. Biosynthesis of selenium nanoparticles using Enterococcus faecalis and evaluation of their antibacterial activities. J. Trace Elem. Med. Biol. 2017, 39, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Ramírez, M.C.; Castañeda-Ovando, A.; Pérez-Escalante, E.; Rodríguez-Serrano, G.M.; Ramírez-Moreno, E.; Quintero-Lira, A.; Contreras-López, E.; Añorve-Morga, J.; Jaimez-Ordaz, J.; González-Olivares, L.G. Antimicrobial Activity of Se-Nanoparticles from Bacterial Biotransformation. Fermentation 2021, 7, 130. [Google Scholar] [CrossRef]
- Tavlarakis, P.; Urban, J.J.; Snow, N. Determination of total polyvinylpyrrolidone (PVP) in ophthalmic solutions by size exclusion chromatography with ultraviolet-visible detection. J. Chromatogr. Sci. 2011, 49, 457–462. [Google Scholar] [CrossRef]
- Sonkusre, P.; Nanduri, R.; Gupta, P.; Cameotra, S. Improved Extraction of Intracellular Biogenic Selenium Nanoparticles and their Specificity for Cancer Chemoprevention. J. Nanomed. Nanotechnol. 2014, 5, 1000194. [Google Scholar] [CrossRef]
- Cruz, L.Y.; Wang, D.; Liu, J. Biosynthesis of selenium nanoparticles, characterization and X-ray induced radiotherapy for the treatment of lung cancer with interstitial lung disease. J. Photochem. Photobiol. B 2019, 191, 123–127. [Google Scholar] [CrossRef]
- Ramya, S.; Shanmugasundaram, T.; Balagurunathan, R. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J. Trace Elem. Med. Biol. 2015, 32, 30–39. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, D.; Huang, X. Selenium nanoparticle rapidly synthesized by a novel highly selenite-tolerant strain Proteus penneri LAB-1. iScience 2022, 25, 104904. [Google Scholar] [CrossRef]
- Kamnev, A.A.; Mamchenkova, P.V.; Dyatlova, Y.A.; Tugarova, A.V. FTIR spectroscopic studies of selenite reduction by cells of the rhizobacterium Azospirillum brasilense Sp7 and the formation of selenium nanoparticles. J. Mol. Struct. 2017, 1140, 106–112. [Google Scholar] [CrossRef]
- Bajaj, M.; Schmidt, S.; Winter, J. Formation of Se (0) Nanoparticles by Duganella sp. and Agrobacterium sp. isolated from Se-laden soil of North-East Punjab, India. Microb. Cell Fact. 2012, 11, 64. [Google Scholar] [CrossRef]
- Fernández-Llamosas, H.; Castro, L.; Blázquez, M.L.; Díaz, E.; Carmona, M. Biosynthesis of selenium nanoparticles by Azoarcus sp. CIB. Microb. Cell Fact. 2016, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Debieux, C.M.; Dridge, E.J.; Mueller, C.M.; Splatt, P.; Paszkiewicz, K.; Knight, I.; Florance, H.; Love, J.; Titball, R.W.; Lewis, R.J.; et al. A bacterial process for selenium nanosphere assembly. Proc. Natl. Acad. Sci. USA 2011, 108, 13480–13485. [Google Scholar] [CrossRef]
- Long, Q.; Cui, L.-K.; He, S.-B.; Sun, J.; Chen, Q.-Z.; Bao, H.-D.; Liang, T.-Y.; Liang, B.-Y.; Cui, L.-Y. Preparation, characteristics and cytotoxicity of green synthesized selenium nanoparticles using Paenibacillus motobuensis LY5201 isolated from the local specialty food of longevity area. Sci. Rep. 2023, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Zonaro, E.; Lampis, S.; Turner, R.J.; Qazi, S.J.; Vallini, G. Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front. Microbiol. 2015, 6, 584. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, X.; Zhou, Q.; Fan, T.; Wang, T.; Chen, X.; Li, M.; Ma, Y.; Ni, J.; Hou, J.; et al. Selenite Reduction and the Biogenesis of Selenium Nanoparticles by Alcaligenesfaecalis Se03 Isolated from the Gut of Monochamus alternatus (Coleoptera: Cerambycidae). Int. J. Mol. Sci. 2018, 19, 2799. [Google Scholar] [CrossRef]
- Yee, N.; Ma, J.; Dalia, A.; Boonfueng, T.; Kobayashi, D.Y. Se(VI) reduction and the precipitation of Se(0) by the facultative bacterium Enterobacter cloacae SLD1a-1 are regulated by FNR. Appl. Environ. Microbiol. 2007, 73, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Switzer Blum, J.; Burns Bindi, A.; Buzzelli, J.; Stolz, J.F.; Oremland, R.S. Bacillus arsenicoselenatis, sp. nov., and Bacillus selenitireducens, sp. nov.: Two haloalkaliphiles from Mono Lake, California that respire oxyanions of selenium and arsenic. Arch. Microbiol. 1998, 171, 19–30. [Google Scholar] [CrossRef]
- Tugarova, A.V.; Vetchinkina, E.P.; Loshchinina, E.A.; Burov, A.M.; Nikitina, V.E.; Kamnev, A.A. Reduction of selenite by Azospirillum brasilense with the formation of selenium nanoparticles. Microb. Ecol. 2014, 68, 495–503. [Google Scholar] [CrossRef]
- Buchs, B.; Evangelou, M.W.H.; Winkel, L.H.E.; Lenz, M. Colloidal Properties of Nanoparticular Biogenic Selenium Govern Environmental Fate and Bioremediation Effectiveness. Environ. Sci. Technol. 2013, 47, 2401–2407. [Google Scholar] [CrossRef]
- Srivastava, N.; Mukhopadhyay, M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosyst. Eng. 2015, 38, 1723–1730. [Google Scholar] [CrossRef]
- Cremonini, E.; Zonaro, E.; Donini, M.; Lampis, S.; Boaretti, M.; Dusi, S.; Melotti, P.; Lleo, M.M.; Vallini, G. Biogenic selenium nanoparticles: Characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016, 9, 758–771. [Google Scholar] [CrossRef] [PubMed]
- El-deeb, B.; Al-Talhi, A.; Mostafa, N.; Abou-Assy, R. Biological Synthesis and Structural Characterization of Selenium Nanoparticles and Assessment of Their Antimicrobial Properties. Am. Sci. Res. J. Eng. Technol. Sci. 2018, 45, 135–170. [Google Scholar]
- Kazempour, Z.B.; Yazdi, M.H.; Rafii, F.; Shahverdi, A.R. Sub-inhibitory concentration of biogenic selenium nanoparticles lacks post antifungal effect for Aspergillus niger and Candida albicans and stimulates the growth of Aspergillus niger. Iran J. Microbiol. 2013, 5, 81–85. [Google Scholar] [PubMed]
- Kanugala, S.; Jinka, S.; Puvvada, N.; Banerjee, R.; Kumar, C.G. Phenazine-1-carboxamide functionalized mesoporous silica nanoparticles as antimicrobial coatings on silicone urethral catheters. Sci. Rep. 2019, 9, 6198. [Google Scholar] [CrossRef] [PubMed]
- Tzimogianni, A.; Tzatzarakis, N.E.; Xagorari, A.; Charvalou, K.; Liapi, C.; Galanopoulou, P.; Petrikkou, E.; Karageorgou, A.; Kalkani, E.; Petrikkos, G. Water-soluble nystatin complexes: Antifungal activity against Candida spp. and cellular toxicity. Acta Microbiol. Hell. 2006, 51, 449–454. [Google Scholar]
- Charvalos, E.; Tzatzarakis, M.N.; Van Bambeke, F.; Tulkens, P.M.; Tsatsakis, A.M.; Tzanakakis, G.N.; Mingeot-Leclercq, M.-P. Water-soluble amphotericin β–polyvinylpyrrolidone complexes with maintained antifungal activity against Candida spp. and Aspergillus spp. and reduced haemolytic and cytotoxic effects. J. Antimicrob. Chemother. 2005, 57, 236–244. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Durnaś, B.; Tokajuk, G.; Głuszek, K.; Wilczewska, A.Z.; Misztalewska, I.; Mystkowska, J.; Michalak, G.; Sodo, A.; Wątek, M.; et al. Magnetic nanoparticles as a drug delivery system that enhance fungicidal activity of polyene antibiotics. Nanomedicine 2016, 12, 2395–2404. [Google Scholar] [CrossRef]
- Zomorodian, K.; Veisi, H.; Yazdanpanah, S.; Najafi, S.; Iraji, A.; Hemmati, S.; Karmakar, B.; Veisi, H. Design and In Vitro antifungal activity of Nystatin loaded chitosan-coated magnetite nanoparticles for targeted therapy. Inorg. Nano-Met. Chem. 2021, 51, 1–9. [Google Scholar] [CrossRef]
- Hussein-Al-Ali, S.H.; El Zowalaty, M.E.; Kura, A.U.; Geilich, B.; Fakurazi, S.; Webster, T.J.; Hussein, M.Z. Antimicrobial and controlled release studies of a novel nystatin conjugated iron oxide nanocomposite. BioMed Res. Int. 2014, 2014, 651831. [Google Scholar] [CrossRef] [PubMed]
- Tsang, P.W.; Bandara, H.M.; Fong, W.P. Purpurin suppresses Candida albicans biofilm formation and hyphal development. PLoS ONE 2012, 7, e50866. [Google Scholar] [CrossRef]
- Hogan, D.A.; Sundstrom, P. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future Microbiol. 2009, 4, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Argimón, S.; Wishart, J.A.; Leng, R.; Macaskill, S.; Mavor, A.; Alexandris, T.; Nicholls, S.; Knight, A.W.; Enjalbert, B.; Walmsley, R.; et al. Developmental regulation of an adhesin gene during cellular morphogenesis in the fungal pathogen Candida albicans. Eukaryot. Cell 2007, 6, 682–692. [Google Scholar] [CrossRef]
- Nobile, C.J.; Nett, J.E.; Andes, D.R.; Mitchell, A.P. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot. Cell 2006, 5, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.R.; Head, W.S.; Wang, M.X.; Johnson, A.D. Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics 2000, 156, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.; Urban, C.; Brunner, H.; Rupp, S. EFG1 is a major regulator of cell wall dynamics in Candida albicans as revealed by DNA microarrays. Mol. Microbiol. 2003, 47, 89–102. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).