Synthesis of Multi-Stimuli Responsive Fe3O4 Coated with Diamonds Nanocomposite for Magnetic Assisted Chemo-Photothermal Therapy
Abstract
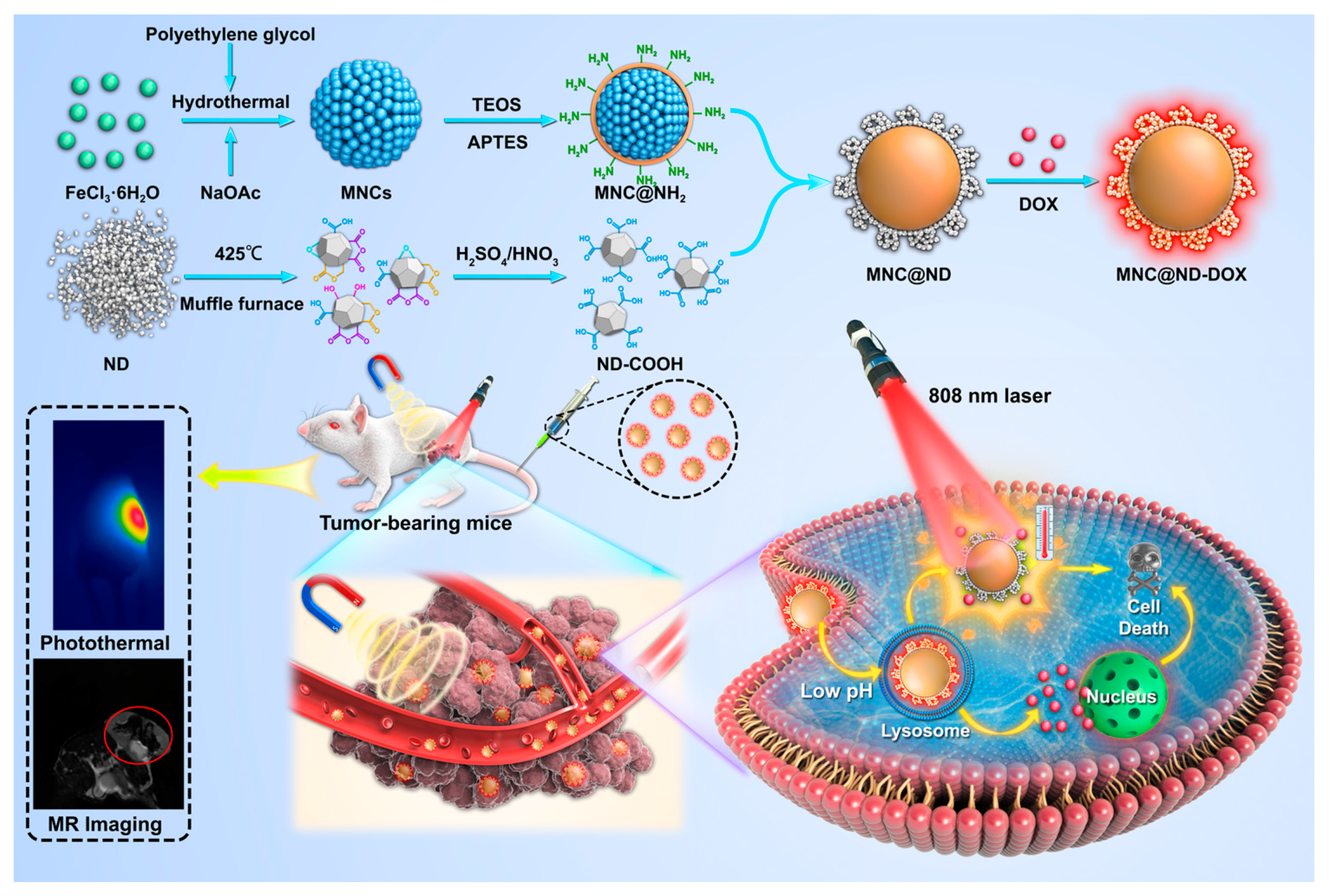
1. Introduction
2. Results and Discussion
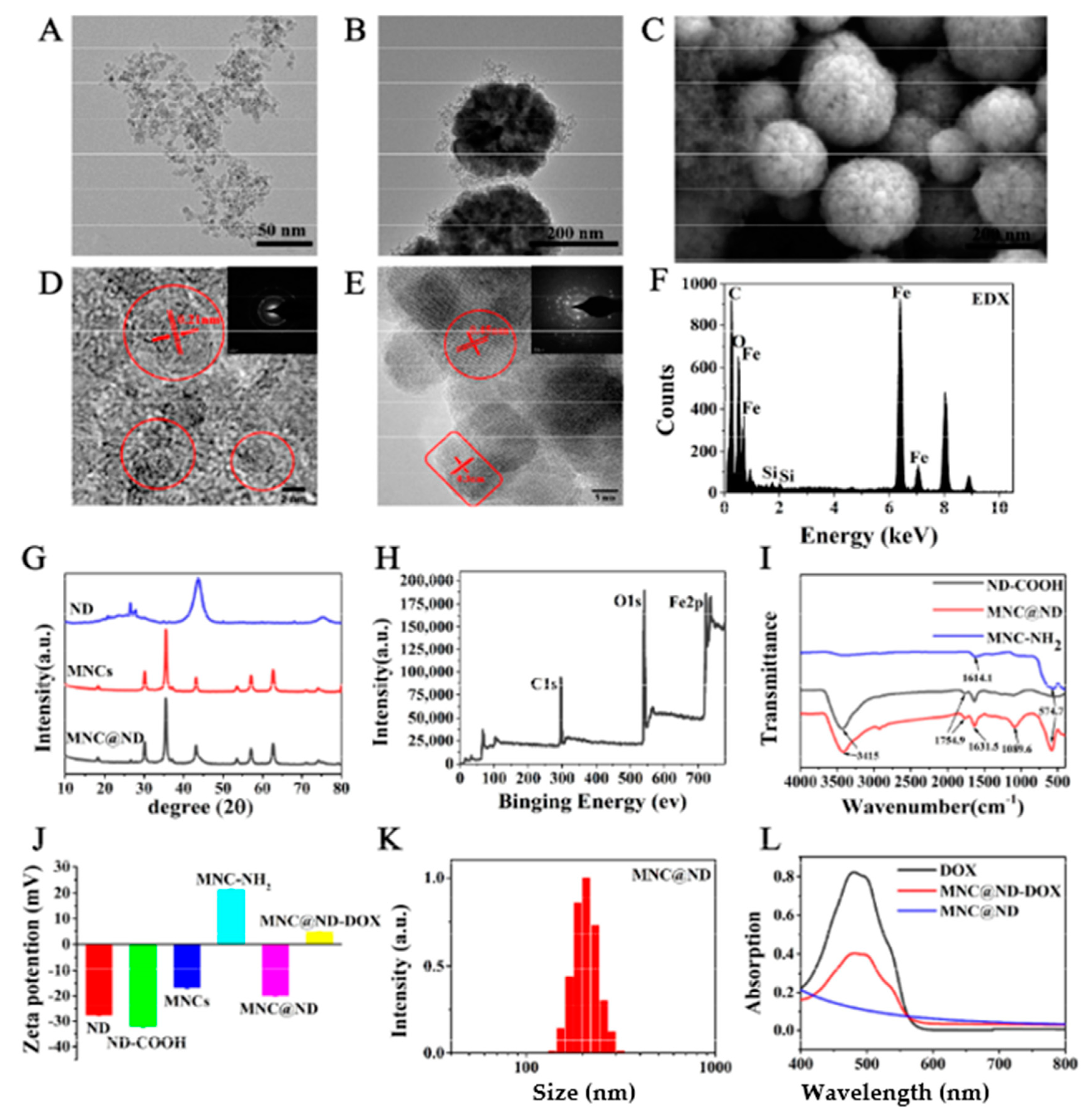
2.1. Preparation and Characterization of MNCs
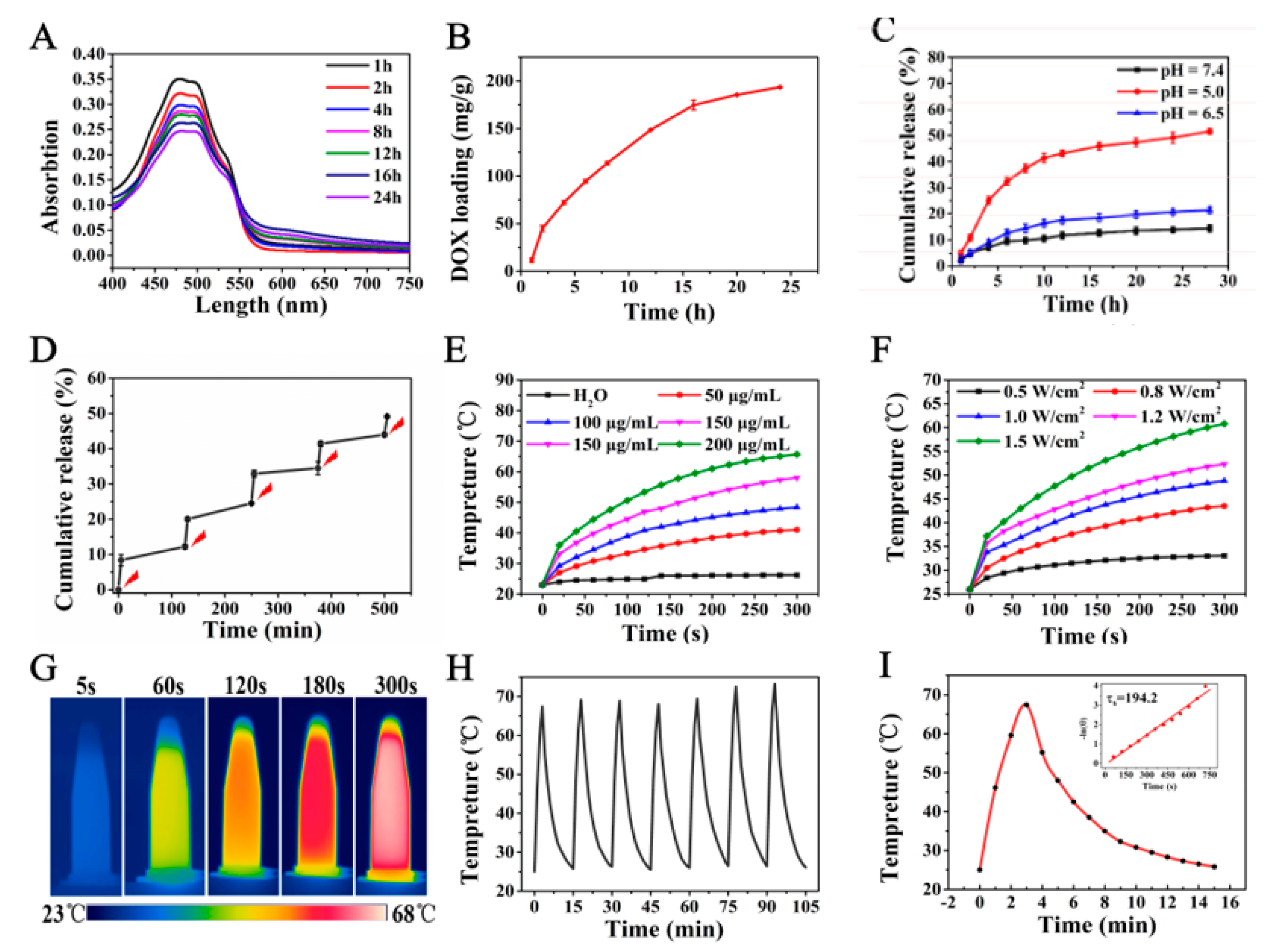
2.2. DOX Loading and In Vitro Release
2.3. In Vitro Photothermal Effect
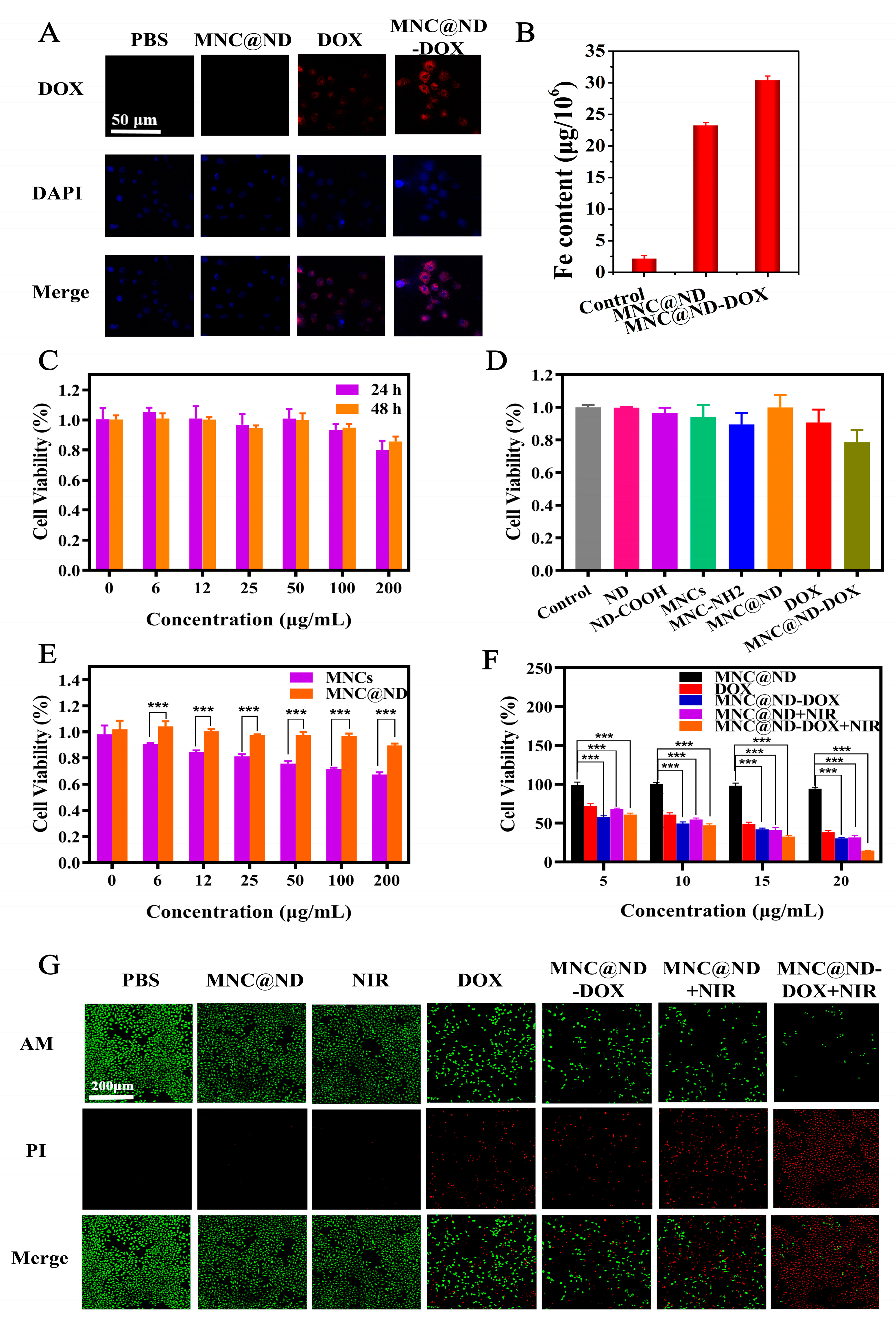
2.4. Cellular Uptake
2.5. Biocompatibility and Cytotoxicity
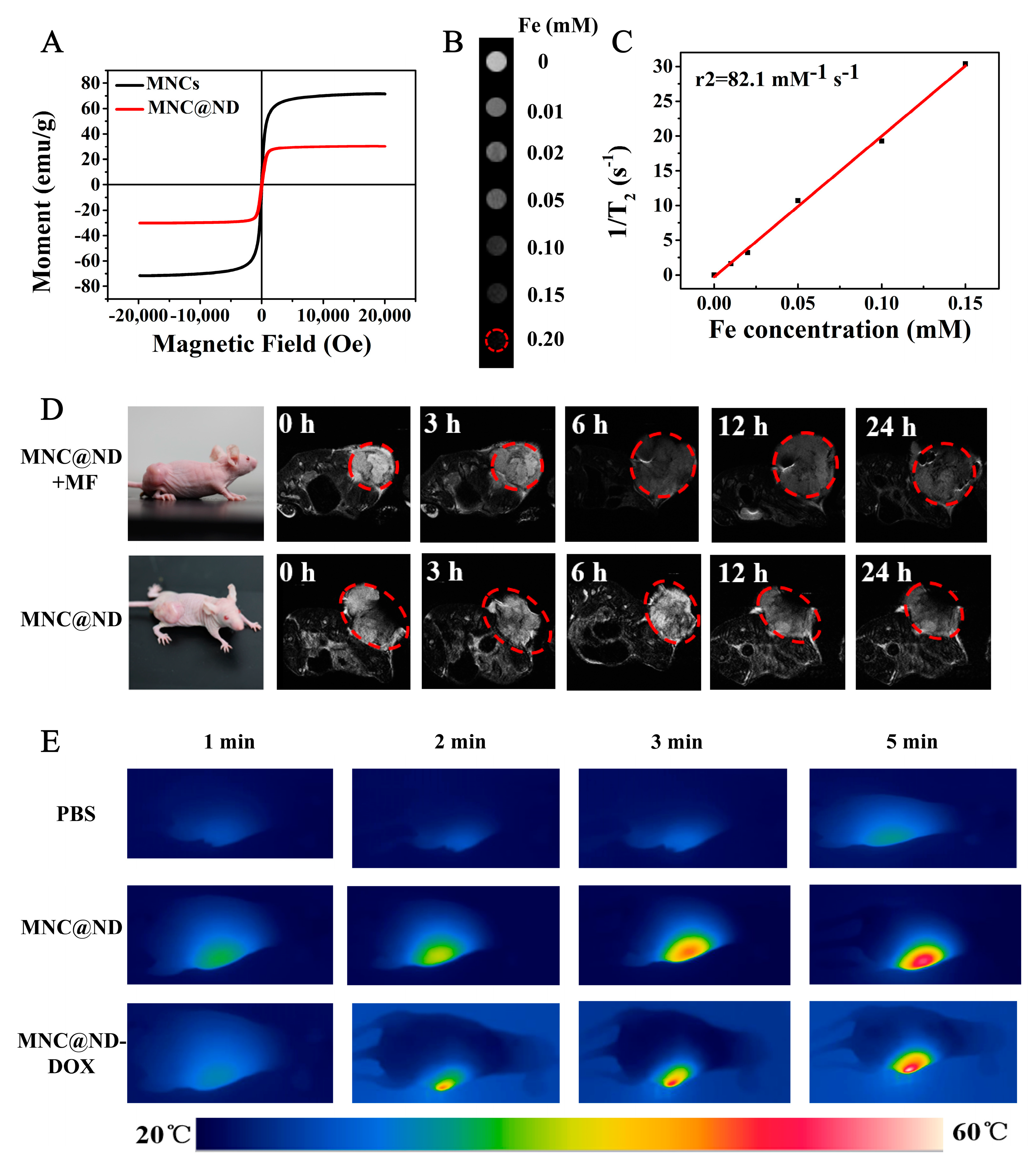
2.6. In Vivo MR Imaging
2.7. In Vivo Photothermal Effect
3. Materials and Methods
3.1. Materials
3.2. Celland Animals Model
3.3. Preparation of ND-COOH
3.4. Preparation of MNCs Nanoparticles
3.5. Preparation of MNCs@SiO2-NH2 Nanoparticles
3.6. Synthesis of MNCs@NDs
3.7. Characterization
3.8. DOX Loading
3.9. Stimuli-Responsive DOX Release from MNCs@ND-DOX
3.10. Photothermal Performance of MNCs@ND
3.11. Cellar Uptake
3.12. In Vitro Cytotoxicity Assay
3.13. In Vitro Living-Dead Staining
3.14. In Vivo MR Imaging
3.15. In Vivo Photothermal Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, M.W.; Secomb, T.W. Transport of drugs from blood vessels to tumour tissue. Nat. Rev. Cancer 2017, 17, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Rebanda, M.M.; Bettini, S.; Blasi, L.; Gaballo, A.; Ragusa, A.; Quarta, A.; Piccirillo, C. Poly(l-lactide-co-caprolactone-co-glycolide)-based nanoparticles as delivery platform: Effect of the surfactants on characteristics and delivery efficiency. Nanomaterials 2022, 12, 1550. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liang, Y.; Zhang, Z.; Ren, G.; Liu, Y.; Wu, S.; Shen, J. Ag@Fe3O4@c nanoparticles for multi-modal imaging-guided chemo-photothermal synergistic targeting for cancer therapy. Anal. Chim. Acta 2019, 1086, 122–132. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, M.; Lu, H.; Liang, D.; Huang, Y.; Xia, Y.; Hu, Y.; Hu, S.; Wang, J.; Yi, X.; et al. Triple stimuli-responsive magnetic hollow porous carbon-based nanodrug delivery system for magnetic resonance imaging-guided synergistic photothermal/chemotherapy of cancer. ACS Appl. Mater. Interfaces 2018, 10, 21939–21949. [Google Scholar] [CrossRef]
- Wang, X.; Low, X.C.; Hou, W.; Abdullah, L.N.; Toh, T.B.; Mohd Abdul Rashid, M.; Ho, D.; Chow, E.K. Epirubicin-adsorbed nanodiamonds kill chemoresistant hepatic cancer stem cells. ACS Nano 2014, 8, 12151–12166. [Google Scholar] [CrossRef]
- Whitlow, J.; Pacelli, S.; Paul, A. Multifunctional nanodiamonds in regenerative medicine: Recent advances and future directions. J. Control. Release 2017, 261, 62–86. [Google Scholar] [CrossRef]
- Moore, L.; Yang, J.; Lan, T.T.H.; Osawa, E.; Lee, D.; Johnson, W.D.; Xi, J.; Chow, E.K.; Ho, D. Biocompatibility assessment of detonation nanodiamond in non-human primates and rats using histological, hematologic, and urine analysis. ACS Nano 2016, 10, 7385–7400. [Google Scholar] [CrossRef]
- Raja, I.S.; Song, S.; Kang, M.S.; Lee, Y.B.; Kim, B.; Hong, S.W.; Jeong, S.J.; Lee, J.; Han, D. Toxicity of zero- and one-dimensional carbon nanomaterials. Nanomaterials 2019, 9, 1214. [Google Scholar] [CrossRef]
- Basu, S.; Pacelli, S.; Wang, J.; Paul, A. Adoption of nanodiamonds as biomedical materials for bone repair. Nanomedicine 2017, 12, 2709–2713. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Neuman, K.C. Surface modification of fluorescent nanodiamonds for biological applications. Nanomaterials 2021, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lee, M.; Lin, H.; Lin, Y.; Lai, W.; Chien, Y.; Huo, T.; Lo, W.; Lan, Y.; Chen, Y.; et al. Nanodiamond-based microrna delivery system promotes pluripotent stem cells toward myocardiogenic reprogramming. J. Chin. Med. Assoc. 2021, 84, 177–182. [Google Scholar] [CrossRef]
- Long, W.; Ouyang, H.; Wan, W.; Yan, W.; Zhou, C.; Huang, H.; Liu, M.; Zhang, X.; Feng, Y.; Wei, Y. “two in one”: Simultaneous functionalization and dox loading for fabrication of nanodiamond-based ph responsive drug delivery system. Mater. Sci. Eng. C 2020, 108, 110411–110413. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.C.; Shukla, S.; Pandey, G.; Narayan, R.J. Nanostructured diamond for biomedical applications. Nanotechnology 2021, 32, 132001–132023. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (epr) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Mo, S.; Carlisle, R.; Laga, R.; Myers, R.; Graham, S.; Cawood, R.; Ulbrich, K.; Seymour, L.; Coussios, C. Increasing the density of nanomedicines improves their ultrasound-mediated delivery to tumours. J. Control. Release 2015, 210, 10–18. [Google Scholar] [CrossRef]
- Park, K. Facing the truth about nanotechnology in drug delivery. ACS Nano 2013, 7, 7442–7447. [Google Scholar] [CrossRef]
- Li, D.; Chen, X.; Wang, H.; Liu, J.; Zheng, M.; Fu, Y.; Yu, Y.; Zhi, J. Cetuximab-conjugated nanodiamonds drug delivery system for enhanced targeting therapy and 3d raman imaging. J. Biophotonics 2017, 10, 1636–1646. [Google Scholar] [CrossRef]
- Liao, W.; Ho, Y.; Lin, Y.; Naveen Raj, E.; Liu, K.; Chen, C.; Zhou, X.; Lu, K.; Chao, J. Targeting egfr of triple-negative breast cancer enhances the therapeutic efficacy of paclitaxel- and cetuximab-conjugated nanodiamond nanocomposite. Acta Biomater. 2019, 86, 395–405. [Google Scholar] [CrossRef]
- Slegerova, J.; Hajek, M.; Rehor, I.; Sedlak, F.; Stursa, J.; Hruby, M.; Cigler, P. Designing the nanobiointerface of fluorescent nanodiamonds: Highly selective targeting of glioma cancer cells. Nanoscale 2015, 7, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Lam, R.; Xu, X.; Chow, E.K.; Kim, H.J.; Ho, D. Multimodal nanodiamond drug delivery carriers for selective targeting, imaging, and enhanced chemotherapeutic efficacy. Adv. Mater. 2011, 23, 4770–4775. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, M.; Zhang, J.; Hou, W.; Chen, H.; Zeng, D.; Wang, Z. Ultrasound-enhanced delivery of doxorubicin-loaded nanodiamonds from pullulan-all-trans-retinal nanoparticles for effective cancer therapy. ACS Appl. Mater. Interfaces 2019, 11, 20341–20349. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zeng, D.; Wang, Z.; Fang, L.; Li, F.; Wang, Z. Ultrasound-enhanced delivery of doxorubicin/all-trans retinoic acid-loaded nanodiamonds into tumors. Nanomedicine 2018, 13, 981–996. [Google Scholar] [CrossRef]
- Saadat, M.; Manshadi, M.K.D.; Mohammadi, M.; Zare, M.J.; Zarei, M.; Kamali, R.; Sanati-Nezhad, A. Magnetic particle targeting for diagnosis and therapy of lung cancers. J. Control. Release 2020, 328, 776–791. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, F.; Li, K.; Xu, J.; Li, P.; Fan, Y. Ph-responsive mesoporous Fe2O3–au nanomedicine delivery system with magnetic targeting for cancer therapy. Med. Nov. Technol. Devices 2022, 15, 100127. [Google Scholar] [CrossRef]
- Bai, C.; Hu, P.; Liu, D.; Chen, Y.; Ma, M.; Gu, N.; Zhang, Y. A novel method to construct dual-targeted magnetic nanoprobes by modular assembling. Colloids Surf. A Physicochem. Eng. Asp. 2020, 605, 125339–125346. [Google Scholar] [CrossRef]
- Mayorova, O.A.; Sindeeva, O.A.; Lomova, M.V.; Gusliakova, O.I.; Tarakanchikova, Y.V.; Tyutyaev, E.V.; Pinyaev, S.I.; Kulikov, O.A.; German, S.V.; Pyataev, N.A.; et al. Endovascular addressing improves the effectiveness of magnetic targeting of drug carrier. Comparison with the conventional administration method. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102184–102197. [Google Scholar] [CrossRef]
- Song, X.; Fu, W.; Cheang, U.K. Immunomodulation and delivery of macrophages using nano-smooth drug-loaded magnetic microrobots for dual targeting cancer therapy. iScience 2022, 25, 104507–104522. [Google Scholar] [CrossRef]
- Karthika, V.; Alsalhi, M.S.; Devanesan, S.; Gopinath, K.; Arumugam, A.; Govindarajan, M. Chitosan overlaid Fe3O4/rGO nanocomposite for targeted drug delivery, imaging, and biomedical applications. Sci. Rep. 2020, 10, 18912. [Google Scholar] [CrossRef]
- Lu, H.; Xu, Y.; Qiao, R.; Lu, Z.; Wang, P.; Zhang, X.; Chen, A.; Zou, L.; Wang, Z. A novel clustered spio nanoplatform with enhanced magnetic resonance t2 relaxation rate for micro-tumor detection and photothermal synergistic therapy. Nano Res. 2020, 13, 2216–2225. [Google Scholar] [CrossRef]
- Taheri-Kafrani, A.; Shirzadfar, H.; Abbasi Kajani, A.; Kudhair, B.K.; Jasim Mohammed, L.; Mohammadi, S.; Lotfi, F. Functionalized graphene oxide/Fe3O4 nanocomposite: A biocompatible and robust nanocarrier for targeted delivery and release of anticancer agents. J. Biotechnol. 2021, 331, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Mukha, I.; Chepurna, O.; Vityuk, N.; Khodko, A.; Storozhuk, L.; Dzhagan, V.; Zahn, D.; Ntziachristos, V.; Chmyrov, A.; Ohulchanskyy, T.Y. Multifunctional magneto-plasmonic Fe3O4/Au nanocomposites: Approaching magnetophoretically-enhanced photothermal therapy. Nanomaterials 2021, 11, 1113. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garnes, M.; Morales, V.; Sanz, R.; Garcia-Munoz, R.A. Cytostatic and cytotoxic effects of hollow-shell mesoporous silica nanoparticles containing magnetic iron oxide. Nanomaterials 2021, 11, 2455. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.C.B.; Jesus, J.R.; Lima, R.J.S.; Moura, K.O.; Almeida, J.M.A.; Duque, J.G.S.; Meneses, C.T. Synthesis and magnetic interaction on concentrated Fe3O4 nanoparticles obtained by the co-precipitation and hydrothermal chemical methods. Ceram. Int. 2020, 46, 11149–11153. [Google Scholar] [CrossRef]
- Ahmed, A.; Mandal, S.; Gines, L.; Williams, O.A.; Cheng, C. Low temperature catalytic reactivity of nanodiamond in molecular hydrogen. Carbon 2016, 110, 438–442. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, T.; Ocsoy, I.; Yasun, E.; Wu, C.; Zhu, G.; You, M.; Han, D.; Jiang, J.; Yu, R.; et al. A cell-targeted, size-photocontrollable, nuclear-uptake nanodrug delivery system for drug-resistant cancer therapy. Nano Lett. 2015, 15, 457–463. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8867. [Google Scholar] [CrossRef]
- Yao, H.; Yan, J.; Shao, P.; Wang, Y.; Liu, T.; Jiang, J.; Liu, T. Co-modification with msc membrane and pda prevents Fe3O4-induced pulmonary toxicity in mice via ampk-ulk1 axis. Toxicol. Lett. 2021, 351, 145–154. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Q.; Qin, S.; Fu, Y.; Liu, R.; Zhi, J.; Shan, C. Nanodiamonds conjugated upconversion nanoparticles for bio-imaging and drug delivery. J. Colloid Interface Sci. 2019, 537, 316–324. [Google Scholar] [CrossRef]
- Dong, S.; Wang, S.; Wang, X.; Zhai, L. Superparamagnetic nanocomposite Fe3O4@SiO2-NH2/CQDs as fluorescent probe for copper (ii) detection. Mater. Lett. 2020, 278, 128404–128416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Kong, J.; Zhao, H.; Liu, Y. Synthesis of Multi-Stimuli Responsive Fe3O4 Coated with Diamonds Nanocomposite for Magnetic Assisted Chemo-Photothermal Therapy. Molecules 2023, 28, 1784. https://doi.org/10.3390/molecules28041784
Li Y, Kong J, Zhao H, Liu Y. Synthesis of Multi-Stimuli Responsive Fe3O4 Coated with Diamonds Nanocomposite for Magnetic Assisted Chemo-Photothermal Therapy. Molecules. 2023; 28(4):1784. https://doi.org/10.3390/molecules28041784
Chicago/Turabian StyleLi, Yang, Jichuan Kong, Huan Zhao, and Yao Liu. 2023. "Synthesis of Multi-Stimuli Responsive Fe3O4 Coated with Diamonds Nanocomposite for Magnetic Assisted Chemo-Photothermal Therapy" Molecules 28, no. 4: 1784. https://doi.org/10.3390/molecules28041784
APA StyleLi, Y., Kong, J., Zhao, H., & Liu, Y. (2023). Synthesis of Multi-Stimuli Responsive Fe3O4 Coated with Diamonds Nanocomposite for Magnetic Assisted Chemo-Photothermal Therapy. Molecules, 28(4), 1784. https://doi.org/10.3390/molecules28041784




