Abstract
Herbal drugs have been attracting much scientific interest in the last few decades and nowadays, phytoconstituents-based research is in progress to disclose their unidentified medicinal potential. Daidzein (DAI) is the natural phytoestrogen isoflavone derived primarily from leguminous plants, such as the soybean and mung bean, and its IUPAC name is 4′,7-dihydroxyisoflavone. This compound has received great attention as a fascinating pharmacophore with remarkable potential for the therapeutic management of several diseases. Certain pharmacokinetic properties of DAI such as less aqueous solubility, low permeability, and poor bioavailability are major obstacles restricting the therapeutic applications. In this review, distinctive physicochemical characteristics and pharmacokinetics of DAI has been elucidated. The pharmacological applications in treatment of several disorders like oxidative stress, cancer, obesity, cardiovascular, neuroprotective, diabetes, ovariectomy, anxiety, and inflammation with their mechanism of action are explained. Furthermore, this review article comprehensively focuses to provide up-to-date information about nanotechnology-based formulations which have been investigated for DAI in preceding years which includes polymeric nanoparticles, solid lipid nanoparticles, nanostructured lipid carrier, polymer-lipid nanoparticles, nanocomplexes, polymeric micelles, nanoemulsion, nanosuspension, liposomes, and self-microemulsifying drug delivery systems.
1. Introduction
Therapies based on compounds derived from plants that grow in nature have always been a symbol of the extraordinary phenomenon of symbiosis in the body. Moreover, herbal medicines have their ancestry in every culture all over the world [1,2]. The severe adverse effects of allopathic treatments on individual health have encouraged the emergence of natural remedies as potential therapeutics for acute and chronic disorders. The thousands of medicinal plants are utilized around the planet, which is attributed to their contribution towards potent therapeutic agents with numerous potential therapeutic effects, without producing serious side effects and, therefore, herbal medicines, by implicating plant-based compounds, have been accepted as being useful a remedy for numerous diseases [3,4,5].
Additionally, medicinal plants offer a rich source of beneficial phytoconstituents that could be investigated for the development of novel drug delivery systems [6]. Alkaloids, tannins, flavonoids, and phenolic compounds are significant bioactive plant components which play an explicit role in the mitigation of a range of health issues as well as chronic diseases [7,8]. The investigations of secondary plant products have progressed comprehensively in the last few decades. Flavonoids are a group of secondary plant metabolites with polyphenolic structure and are generally found in fruits, vegetables, and certain drinks [9]. Flavonoids are classified into subgroups such as catechins, anthocyanins, chalcones, flavones, flavonols, flavanones, flavanonols, and flavanols [10,11]. Numerous studies have revealed that flavonoids have potential to avert several bacterial and viral infections [12], cancer [13], arthritis [14], osteoporosis [15], diabetes [16], skin disorders [17], cardiovascular disease [18], and other age-related illnesses [19].
Daidzein (DAI) belongs to the isoflavone class of flavonoids which are commonly consumed by Western populations such as North Americans and Europeans in relatively modest amounts and in relatively high concentrations by Asian populations such as the Chinese and Japanese [20]. DAI is a type of naturally occurring, non-steroidal isoflavone that is typically derived from leguminous plants such as the soybean and mung bean [21]. Food products from soy, such as tofu, tempeh, miso, textured soy protein, soy flour, and soy protein isolates, contain DAI. The amount of DAI in a cup of soy milk is 7 mg, a half-cup of miso is 22 mg, three ounces of tempeh is 15 mg, and three ounces of tofu is 8 mg [22].
The physicochemical properties and pharmacokinetics profile of DAI has been revealed in the current review. This article summarizes the pharmacological applications and mechanisms of action of DAI in the management of many disease conditions, including oxidative stress, cancer, obesity, cardiovascular, neuroprotective, diabetes, ovariectomy, anxiety, and inflammation. In addition, the objective of the current review article is to provide up-to-date knowledge on the nanotechnology-based approaches investigated in the past to increase the solubility and permeability of DAI. These approaches include polymeric nanoparticles, solid lipid nanoparticles, nanostructured lipid carriers, polymer-lipid nanoparticles, nanocomplexes, polymeric micelles, nanoemulsion, nanosuspension, liposomes, and self-microemulsifying drug delivery system. For this purpose, a comprehensive literature survey was conducted using the Google Scholar, PubMed, and ScienceDirect databases. The research and review papers published in peer-reviewed journals between the years 2000 and 2022 served as the basis for the literature review.
2. Physicochemical Properties and Pharmacokinetics Profile of Daidzein
DAI has the IUPAC name 4′, 7-dihydroxylisoflavone and is a water-insoluble isoflavone, existing as pale yellow crystalline prisms and having a partition coefficient of 2.55; its solubility in the different solvents was in the sequence like propanone > methanol > ethyl ethanoate > hexane > trichloromethane > water [23], and in aqueous buffer (with pH 6.0) was found 18.76 nmol/mL [24].
Some of DAI’s unfavorable physicochemical characteristics (poor solubility, low partition coefficient, and high intestine and hepatic metabolism) lead to low oral bioavailability. According to animal model studies, absolute bioavailability of DAI suspension after oral administration to rats was 6.1%, which seems to be the greatest constraint limiting therapeutic and pharmacological uses. There have been numerous approaches investigated to make DAI more bioavailable by derivatizing ionizable groups (such as sulfation, phosphating, or glycosylation of DAI) to more water-soluble forms [25,26,27,28,29,30].
DAI was administered to healthy premenopausal women, and the results showed that it has a low bioavailability and non-linear pharmacokinetics with higher intakes, showing that its absorption is rate-limited and saturated [31,32].
Intestinal microbiota has a significant effect on the metabolism and bioavailability of isoflavones, and it has been discovered that isoflavones cannot be absorbed without microbiota [33]. The bioavailability and absorption of isoflavones may be influenced by the bacterial flora of the stomach. Some isoflavones are ingested in their chemically modified form because the stomach may convert relatively weak molecules into stronger forms. DAI can be converted by intestinal microbiota into a number of substances, such as odesmethylangolensin, dihydrodaidzein, and 7-hydroxyisoflavan [34].
The blood–brain barrier allows daizein-8-C-apiosyl-(1-6)-glycoside to enter the brain quickly, and it may be detected in the brain within an hour of administration [35,36].
The diet of the human population is largely composed of soy products. As opposed to less than 2 mg in Western countries, the Asian population can consume up to 50 mg of isoflavones per day, although this number may be higher in menopausal women [20].
DAI has a 336.25 L volume of distribution, a 30.09 L/h clearance rate, and a 7.75 H half-life, respectively [37,38]. Absolute and relative bioavailability of DAI suspension (20 mg/kg i.v. vs. 50 mg/kg i.p.) and complexed form (0.54 mg/kg i.v. vs. 1.35 mg/kg i.p.) were evaluated. DAI complexed was absorbed more quickly (tmax = 15 min) and to a greater extent (Cmax = 615 vs. 173 ng/mL) following intraperitoneal administration than DAI in suspension (tmax = 45 min). DAI’s i.v. half-life was longer in the complex of DAI when compared to DAI in suspension (t0.5 = 80 min vs. 230 min) [31].
The physicochemical characteristics and pharmacokinetic profile of DAI have been summarized in Table 1 and Figure 1 depicts the chemical structure of DAI and its various analogs [21].

Table 1.
Description of physicochemical characteristics and pharmacokinetic profile of Daidzein (DAI).
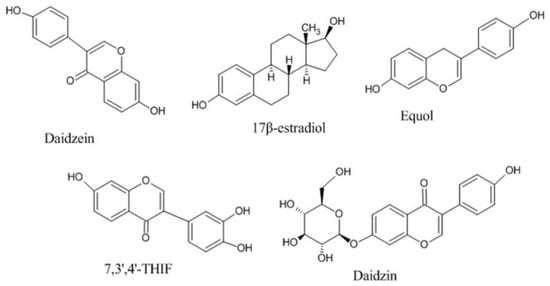
Figure 1.
Chemical structures of Daidezin and its various analogs.
3. Mechanism of Action and Pharmacological Applications of Daidzein
Numerous pharmacological effects of DAI include anti-carcinogenesis [42], anti-inflammatory [43], antioxidant [44], anti-diabetic [45], cholesterol-lowering [46], and cardiovascular activity [47,48].
DAI imitates human estrogen, which has a substantial impact on the prevention of osteoporosis, cancer, and postmenopausal disorders. Soy products are highly recommended for cancer prevention due to high content of anticarcinogens in them [49,50,51].
Matrix metalloproteinase-2 activity is inhibited by DAI to produce an anticancer effect, and its non-toxic concentration is also extensively used to modulate Hedgehog signaling to prevent tumor necrosis factor-induced migration and the invasion of human breast cancer cells [52].
DAI significantly raises high density lipoprotein cholesterol (HDL-C) levels, lowers levels of circulating triglycerides (TGs) and low density lipoprotein cholesterol (LDL-C), and thus, prevents heart attack or stroke [53]. Additionally, it increases the expression of bone morphogenetic protein (BMP) in primary osteoblast cells, promoting the development of osteoblast, which ultimately exhibited anti-osteoporosis activity [54]. Moreover, DAI increases the ratio of glucose transporter-4 (GLUT4) to Na+/K+ ATPase levels, which facilitate in glucose absorption and maintain the proper balance of reactive oxygen species to free radicals [55,56].
Different analogs of DAI (such as equol, 17 β-estradiol, 7, 3′, 4′-THIf and daidzin) exhibited the similar mechanism as DAI by binding with the protein kinase B, estrogen receptors, mitogen-activated protein kinase, and epidermal growth factor receptor kinase, nuclear factor kappa-light-chain-enhancer of activated B cells, and other intracellular signaling mechanisms [30,57]. Figure 2 illustrates the numerous mechanisms through which DAI exerts its therapeutic potential in a variety of essential body organs.
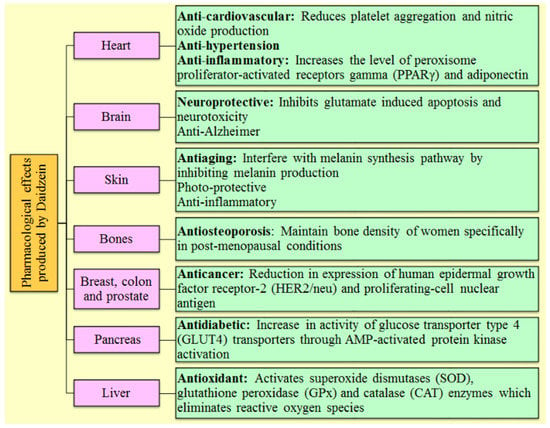
Figure 2.
Representation of several pharmacological activities of Daidzein and their mechanism of action involved in various body organs.
3.1. Anticancer Activity
Polycyclic phenolic phytochemicals known as phytoestrogens have characteristic structures that resemble steroidal estrogen. Their ability to treat and prevent cancer has recently received a lot of attention. Increased consumption of foods and herbal remedies containing phytoestrogens plays a crucial part in lowering estrogen levels and the prevalence of breast cancer [58,59].
By reducing the activity of matrix metalloproteinase-2, DAI prevented the MDA-MB-231 breast cancer cell lines from attacking them, indicating a significant function for DAI in the development of breast cancer [52,60].
TNF-induced nuclear localization of the gene glioma-associated oncogene homologue-1 (Gli1) and genetic expressions into mRNA and protein that inhibited TNF-induced migration and invasion in human breast cancer cells have been studied extensively using DAI to control Hh-signaling [52,61]. DAI inhibited the proliferation of cell lines originated from cancer, and as a result, apoptosis was induced in cancer cells. Depending on the kind of cancer cell, it can be used to increase apoptosis linked to G0/G1 cell cycle arrest. Direct apoptosis is caused by the S- or G2-phase without altering cell distribution [62].
The impact of DAI’s antiproliferative properties on human breast cancer cell lines, i.e., MCF-7 and MDA-MB-453, at dosages ranging from 1 to 100 mM for 24, 48, and 72 h, showed reduced cell proliferation in both types of cells in a dose- and time-dependent manner [63].
Wang et al. demonstrated that 145 mg/kg of DAI administered orally for 22 days causes breast cancer cells to undergo apoptosis via the Fas/FasL-initiated mitochondrial apoptosis signaling pathway in bearing-4T1 mice [64].
Numerous studies have demonstrated that DAI has therapeutic advantages for the treatment of malignancies other than breast cancer. Moreover, it demonstrated anti-proliferative activities in three prostate cancer cell lines (DU 145, LNCaP, and PC-3), modulating the gene expression associated with the cyclin-dependent kinase-related pathway, resulting in cell cycle arrest at the G0/G1 phase, and suppressing angiogenesis. A few of these genes are involved in the angiogenesis process and the DNA damage signaling mechanism, which can lower levels of the epidermal growth factor and insulin-like growth factor and therefore prevent the development of tumors [65].
LoVo cells displayed a tumor-suppressing impact as a consequence of cell cycle arrest at the G0/G1 phase and caspase-3-dependent apoptosis, which had no effect on differentiation. In numerous murine as well as human neuroblastoma cell lines, DAI exhibited its anticancer potential by inhibiting cell growth, arresting the cell cycle during the G2/M phase, and promoting cell death [66].
Due to the biotransformation of DAI, this can be utilized as a chemo-preventive drug in skin cancer despite its lack of effect on cyclooxygenase 2 (COX-2) expression, its metabolite directly binding to tumor progression locus and mitogen-activated protein kinase 4 to block their activity. This significantly lowers the ultraviolet B-induced COX-2 expression and, subsequently, prevents tumor growth, development, and enlargement [57].
3.2. Cardiovascular Diseases
While postmenopausal women have a greater incidence of cardiovascular disorders than premenopausal women [67], males aged 35 to 50 had a higher incidence of cardiovascular diseases than women of equivalent ages [68].
The endothelium’s ability to produce nitric oxide is activated by estrogen receptors, and blood vessels are also relaxed by prostacyclin and hyperpolarizing factor. It is possible that using natural phytoestrogens in small doses has advantages over using synthetic estrogen [69]. Low levels of HDL-C, as well as high levels of TGs and LDL-C, are important risk factors for cardiovascular disease. Six months of DAI therapy in hypercholesterolemic patients can considerably lower triglyceride and uric acid levels in blood, but not in a dose-dependent way [53].
Caveolin, a transmembrane protein, is present in the minute caveolae that project from the plasma membrane. Caveolin-1, a specific marker of caveolae, tends to up-regulate expression in response to conditions such elevated levels of oxidized low density lipoprotein, estrogen deficiency, and hyperglycemia [70]. It functions as a protein that binds to cholesterol and makes it easier for cholesterol to go from the endoplasmic reticulum to the plasma membrane’s endothelial cells via the Golgi apparatus. DAI functions as a caveolin-1 inhibitor, which has the potential to raise endothelial nitric-oxide synthase (eNOS) activity and to improve the vascular endothelium due to an increase in nitric oxide generation and stimulation of eNOS through caveolin-1 inhibition [71].
3.3. Anti-Osteoporosis Activity
Menopause causes the condition of equilibrium in the body to shift in favor of greater resorption, which lowers the bone mineral density and disturbs the bone microarchitecture [72]. The metabolism of bones and the growth of bone mass are influenced by systemic hormones, genetics, and environmental factors [73].
According to the conventional view, osteoporosis is a “breakable bone” disorder that primarily affects post-menopausal Caucasian women and those who consume insufficient levels of calcium and vitamin D [74].
DAI has received the most scientific attention among soy phytoestrogens, and numerous studies have demonstrated that it may have antiosteoporosis potential. DAI stimulates osteoblast formation in mouse osteoblast-like MC3T3-E1 cells via increasing BMP expression in primary osteoblast cells, which in turn promotes cell differentiation and mineralization [75].
DAI treatment prevents bone mass loss in both juvenile and adult ovariectomized rats and appears to promote protein synthesis and alkaline phosphatase in bone development. Phosphatase mineralization, which has been examined after being cultivated in osteoblast-like MC3T3-E1 cells, is an indication of osteoblast-induced matrix maturation [76]. Additionally, DAI greatly increases the activity of alkaline phosphatase, sodium-deoxyribonucleic acid, and calcium in bone tissue [54].
3.4. Antidiabetic Activity
Diabetes is currently posing a challenge upon India because it is progressively acquiring the position of a possible epidemic [77]. The significant mortality and cardiovascular morbidity of diabetes patients also contributes to the rise in demand for bio compounds with antidiabetic characteristics [78].
DAI inhibits the rise in blood glucose levels and promotes glucose absorption in adipocytes and muscle cells. Additionally, it increases the ratio of GLUT4 to Na+/K+ ATPase in the plasma membrane portion of L6 myotubes, indicating that this phytoconstituent may promote glucose absorption by GLUT4 translocation from intracellular micro vesicles [56,79].
DAI has a lower risk of hypoglycemia due to its minimal effect on insulin production and lack of influence on fasting blood sugar levels, significantly decreasing blood sugar levels and raising oral glucose tolerance when administered orally to diabetic mice, which had a significant impact on hyperglycemia. It evidently reduces blood levels of total cholesterol, triglycerides, and LDL-c while modestly raising blood levels of HDL-c. Therefore, this was disclosed that oral administration of DAI is effective in treating hyperglycemia and diabetes-related disorders [80].
3.5. Antioxidant Activity
Soybeans contain large amounts of the isoflavone DAI and is consumed in enormous quantity by Asian populations. Isoflavones have been associated with beneficial health effects as a result of their antioxidant properties due to their ability to cause chelation of toxic metal ions [81].
Dietary DAI is frequently transformed by intestinal bacteria into substances like 3′-OH-daidzein and 6′-OH-daidzein, which have powerful antioxidant potential compared to the parent molecule DAI. The antioxidant effects of DAI-induced antioxidant benefits may be mediated by DAI metabolites generated in the gut [82].
The potential of DAI to chelate copper ions results in its antioxidant activity. The Cu2+ has a propensity to stimulate lipoprotein oxidation in serum, which causes the LDL particles to aggregate and fuse. The chelation of Cu2+ has an antioxidant effect and protects against the oxidative transformation of LDL [83].
3.6. Anti-Inflammatory Activity
Inflammation is a biological response triggered upon by infections, damaged cells, and irritants [84]. Anti-inflammatory drugs, whether steroidal or nonsteroidal, are frequently used to treat inflammation, but they frequently have several adverse side effects. Recent studies have demonstrated that polyphenols derived from plants, in particular flavonoids, have potent anti-inflammatory activities [85].
Chronic/acute intestinal inflammation are both correlated with abnormal mucosal immune responses. Inflammatory bowel disease and increased pro-inflammatory chemical production are typically the two main pathogenic factors in chronic inflammatory diseases [86].
An imbalance between the synthesis of reactive oxygen species and antioxidant activity is known as oxidative stress which causes tissue damage. DAI 100 μM decreased interleukin-1β, interleukin-6, and tumor necrosis factor-α expression by 73.8 ± 5.3%, 58.8 ± 9.0% and 55.5 ± 7.2%, respectively. Through the downregulation of Kelch-like ECH-associated protein 1 and the upregulation of nuclear factor erythroid 2-related factor 2 expression, it also decreased the formation of reactive oxygen species caused by lipopolysaccharide by 23.9 ± 7.8% and enhanced superoxide dismutase activity by 88.4 ± 18.9% [43]. Oxidative stress is a condition that is often brought on by an increase in free radicals and reactive oxygen species [55,87].
In order to prevent human diseases and maintain proper health conditions by avoiding oxidative stress, an increase in antioxidant intake is required. DAI’s gut microbial metabolites O-desmethylangolensin (O-DMA), equol, and daidzin have antioxidant properties in the following sequence: DAI > equol > O-DMA > daidzin [88].
3.7. Neuroprotective Activity
DAI can prevent the progression of neurodegenerative diseases. Beta-secretase and cholinesterase are scientifically identified targets of Alzheimer’s disease, and both have benefited significantly from bio compounds. Given that Alzheimer’s disease is a serious public health issue, it requires the use of multiple-targeted drugs to be treated [89].
Stroke has a high morbidity rate globally, and there are currently no viable treatments for this disease [90]. Strokes are known to be associated with brain damage that permanently harms the body, while DAI aids in neuroprotection and functional recovery following a stroke [91].
DAI has neuroprotective effects in stroke conditions and has shown peroxisome proliferator-activated receptor gamma (PPAR-γ)-dependent therapeutic effects in brain cells and has huge potential to improve synaptic functioning in cultured neurons. An experimental study found that DAI increased PPAR-γ transcriptional activity while suppressing selective PPAR-γ antagonist [92].
In ischemic, neurodegenerative, and inflammatory brain disorders, PPAR activity assists in preventing neuronal death [93]. DAI treatment produced an anxiolytic effect in treated males by significantly increasing locomotor activity, improving harmonious behavior, reducing hostility, and reducing sexual behavior during social interaction [94].
Table 2 provides a systematic summary of pre-clinical investigations carried over the past few decades exploring the pharmacological applications of DAI in conditions such as oxidative stress, cancer, obesity, cardiovascular, neuroprotective, diabetes, ovariectomy, anxiety, and inflammation. It also includes information on animal models used and study outcomes.

Table 2.
Recapitulation of the outcomes of DAI’s pharmacological studies in several pre-clinical investigations for various disease conditions.
4. Outline of Nanotechnological Aspects Explored for Daidzein in Therapeutics
DAI has limited clinical applications because of poor aqueous solubility and less permeability which causes low oral bioavailability. In the light of available information, the development of nanoparticles is a suitable strategy to address issues of low solubility, permeability, and bioavailability.
A significant role of nanomedicine in the treatment of many disorders has been demonstrated in research conducted in this field. Utilizing nanotechnology enables early diagnosis and more effective drug administration. Nanomaterials range in diameters between 1 and 1000 nm and have a large surface area to volume ratio. According to their structural properties, nanomaterials can be classified as either nanostructured or nanocrystalline. Nanostructured materials can be divided into three categories: lipid-based, polymer-based, and non-polymer-based depending on the type of material used [115,116,117].
The numerous applications of nanotechnology in the pharmaceutical sector have been demonstrated in areas such as targeted diagnostics, therapy, delaying drug release, enhancing drug solubility and bioavailability, reducing drug adverse effects, and overcoming barriers in the human body [118].
Table 3 summarizes the recent advancements in the field of nanotechnology-based drug delivery systems of DAI which has been investigated to improve solubility and bioavailability (Table 3).

Table 3.
Review of up-to-date progression in development of nanocarriers based drug delivery of DAI for solubility and bioavailability enhancement.
There are several methods for encapsulating DAI nanoparticles, including the solvent evaporation method [26], antisolvent method [119], emulsion solvent diffusion [120], hot homogenization [121], film homogenization [29], media milling [27], ultrasonication/lipid film hydration method [129], and emulsification [123].
The experimental study showed that poly(lactic-co-glycolic acid) (PLGA) and PLGA-Gelucire nanoparticles loaded with DAI were used to treat glioblastoma multiforme, and it led to the conclusion that the formulation used was effective for sustained delivery, reducing neurotoxic effects, and maintaining cytotoxic effects against cancer cells [120].
DAI is a very useful medication for the treatment of cardio-cerebrovascular illnesses, but it is not as effective as it might be because of its poor oral absorption and bioavailability. A group of researchers prepared solid lipid nanoparticles for treatment of cardio-cerebrovascular diseases. The prepared solid lipid nanoparticles released the drug in a sustained manner and demonstrated over 90% release within 120 h [121].
The structural composition of several nanocarriers investigated for innovative delivery of DAI is shown in Figure 3.
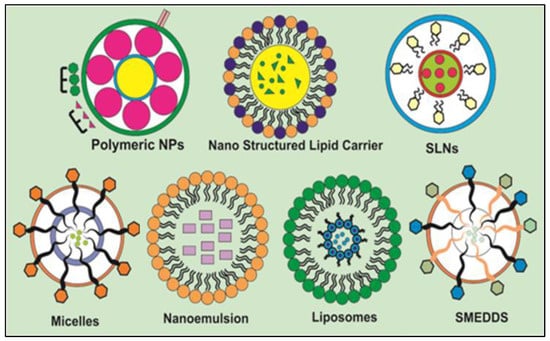
Figure 3.
Structural composition of various nanocarriers explored for encapsulation of DAI regarding solubility and bioavailability enhancement.
4.1. Polymeric Nanoparticles
Polymeric nanoparticles (PNPs) are a type of particle with sizes ranging from 1 to 1000 nm, that are comprised of active compounds that have been entrapped inside the polymeric core or surface-adsorbed onto the polymeric core [130].
PNPs’ ability to protect drugs and their potential for controlled release can increase drugs’ bioavailability and therapeutic index [131]. PNPs, which contain a variety of therapeutic compounds, are produced with biodegradable materials such as poly-(D, L-lactic acid), PLGA, polycaprolactone, and its copolymers such as polyethylene glycol [132].
PNPs can be synthesized from two methods, i.e., (i) dispersion of performed polymers and (ii) polymerization of monomers. Dialysis, nanoprecipitation, solvent evaporation, supercritical fluid technology, emulsification, solvent diffusion, and salting out are the methods utilized to disperse the performed polymers. Another method for producing PNPs using microemulsion polymerization, controlled radical polymerization, and interfacial polymerization involves the polymerization of monomers [133,134,135].
A group of researchers formulated DAI PLGA nanoparticles using the emulsion-solvent evaporation method, and relative bioavailability was enhanced about 5.57- and 8.85-fold, respectively, in comparison to the control group [26]. By employing the anti-solvent approach, Zou and Gu synthesized TPGS 1000 emulsified zein nanoparticles, and they discovered that nanoparticles had increased Cmax of DAI by 2.64-fold and are under the curve (AUC) (0–12 h) by 2.4-fold compared to free drug [119].
4.2. Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLNs) are efficient colloidal carriers which have fascinating characteristics such as small size, large surface zone, high drug entrapment, and the capacity to improve the therapeutic performance of pharmaceuticals [136,137].
Aqueous surfactant is coated over a solid core of high melting point lipid in SLNs. Triglycerides, acyl glycerol, glyceryl monostearate, waxes, cetyl palmitate, soy lecithin, and egg lecithin are among the several lipid types employed in the production of SLNs [138,139].
A number of techniques are employed to prepare SLNs, including double emulsion (w/o/w), ultrasound dispersion, high shear homogenization, solvent emulsification-diffusion, solvent injection, and high pressure homogenization (cold and hot homogenization) [140,141,142]. A group of researchers developed DAI solid lipid nanoparticles using hot homogenization method and it demonstrated sustained drug release with cumulative release over 90% within 120 h [121].
4.3. Nanostructured Lipid Carriers
Lipid-based formulations, such as nanostructured lipid carriers (NLCs), are regarded to be superior to conventional lipid-based nanocarriers, which have a rigid matrix at room temperature. NLCs are created by combining liquid lipid and solid lipid in such a way that prevents the oil molecules from contributing to the crystalline structure [143]. In order to overcome the drawbacks of SLNs, NLCs have been developed which demonstrated better loading capacity for active chemicals as compared to SLN. Moreover, there is less possibility of drug discharge from NLCs during storage [144].
The methods that are typically employed for the production of NLCs include film-ultrasonic, evaporation-low temperature solidification, high-pressure homogenization, microemulsion, supercritical fluid, membrane contactor, solvent dispersion, microchannel, and microtubes [145].
By using emulsification and low-temperature solidification technique, Song and his colleague produced DAI-loaded nanostructured lipid carriers for transdermal application. Researchers found that the permeation rate was 3.78 times higher than that of pure DAI solution [122].
4.4. Polymeric Micelles
Polymeric micelles are amphiphilic co-polymers that have formed into nanoscale colloidal particles with sizes between 5 and 100 nm above the critical micelle concentration. The aqueous media is used for the production of micellar core–shell structure in order to reduce hydrophobic segment’s interaction with the single chains of polymers [146].
Additionally, polymeric micelles have a unique core–shell structure with an inner core that serves as a nanocontainer for hydrophobic drugs and an outer shell that is surrounded by hydrophilic polymer shell. Numerous advantages of polymeric micelles include ease of production, efficient drug loading without chemical alteration of the parent molecule, and controlled drug release [147].
Researchers synthesized DAI micelles using the anti-solvent technique and demonstrated that the AUC0-t was 20 times higher than it was for the free drug [124].
4.5. Nanosuspension
The colloidal dispersion containing drug particles with a submicron size is known as nanosuspension. A pharmaceutical nanosuspension is comprised of colloidal biphasic particles that are stabilized by surfactants and polymers and are free of matrix components. According to research, nanosuspension boosts bioavailability and absorption, which results in a dose reduction for oral dosage forms [148,149].
Homogenization, wet milling, emulsification, solvent evaporation, precipitation or microprecipitation are common methods for manufacturing nanosuspensions [150,151].
The stability of the particles created by the nanosuspension depends on their size. When compared to other delivery methods, nanosuspensions have the benefit of being simpler and have the ability to overcome concerns with poorly lipid- and water-soluble compounds [152].
A group of researchers synthesized DAI nanosuspension by precipitation high-pressure homogenization and concluded that oral bioavailability increased by 1.63–2.19 times greater than that of crude DAI [125].
4.6. Nanoemulsion
Nanoemulsions are colloidal particle systems with submicron sizes (10–1000 nm) that serve as drug carriers. Solid spheres with an amorphous, lipophilic, and negatively charged surface constitute these carriers. These usually improve drug delivery systems by increasing the therapeutic potency of drugs and minimizing their adverse effects [153]. The primary applications of nanoemulsions include the treatment of reticuloendothelial system infections, liver enzyme replacement therapy, cancer treatment, and vaccination [154]. The phase inversion method, sonication method, and high pressure homogenization are the techniques utilized to create nanoemulsions [155].
Drugs that are poorly water soluble can have their bioavailability increased by using oil-in-water nanoemulsion. However, the difficulties in reducing droplet size and the requirement for specialized equipment and manufacturing procedures make the development of nanoemulsion an expensive operation [156].
Researchers formulated a nanoemulsion of DAI using high-pressure homogenization, and a study revealed that it significantly increased cell death as compared to pure DAI [127].
4.7. Liposomes
Liposomes are spherical, uni lamellar or multilamellar vesicles that are used to deliver drugs into cells through the cell membrane, which is made up of cholesterol and a phospholipid bilayer [157].
Hand shaking techniques, sonication techniques employing probe or bath sonicators, reverse phase evaporation techniques, and freeze dried rehydration techniques are all used to produce liposomes [158,159].
Liposomes are effective for intracellular delivery of deoxyribonucleic acid, ribosome, proteins, and peptides. Targeted drug delivery to diseased sites is facilitated by the long circulation residence times of liposomes. Compared to free complements, liposomal drugs are more efficacious and have reduced toxicities [160,161]. Researchers prepared DAI-loaded liposomes using ultrasonication and lipid film hydration and found that the t1/2, mean residence time0-t and AUC0-t of DAI in the liposomes were 1.8, 1.6, and 2.5 times higher than those in free DAI [128].
4.8. Self-Micro Emulsifying Drug Delivery System (SMEDDS)
The Self-Micro Emulsifying Drug Delivery System (SMEDDS) refers to isotropic compositions of synthetic or natural oils, liquid or solid surfactants, or hydrophilic solvents/co-solvents which possess the remarkable ability to generate fine oil-in-water (o/w) microemulsions on gentle agitation accompanied by dilution in an aqueous environment such as gastrointestinal fluids [162].
SMEDDS is a cutting-edge method for making lipophilic drugs more soluble in water, which eventually increases their bioavailability. SMEDDS is an ideal carrier that has great potential for producing drug delivery across intestinal aqueous boundary and consequently tends to improve the bioavailability because it can carry out drug delivery to the gastrointestinal tract (GIT) in the form of globules with sizes ranging from 1 to 100 nm and enormous specific surface area. Peptides that are susceptible to enzymatic hydrolysis may be transported to the GIT via SMEDDS. To obtain sustained drug release, polymer can be added to the SMEDDS formulation [163].
The main advantages that distinguish SMEDDS from other nanocarriers when compared to other drug delivery systems are its simplicity in manufacturing and scaling up. For large-scale production, SMEDDS requires relatively low-cost manufacturing equipment, such as a conventional mixer with agitator and volumetric liquid filling machinery [164].
Researchers synthesized SMEDDS of DAI using the emulsification process, and the results showed that the bioavailability was increased by about 2.5 times when compared to the control group [129].
5. Clinical Status of Daidzein
On the official website of ClinicalTrials.gov, a search was conducted for the clinical trials including DAI and its medicinal uses that have been completed to date. According to research, DAI has undergone four successful clinical studies. Table 4 summarizes study tile, sponsor condition, study type/allocation/intervention model, and number of clinical trials (NCT) [165].

Table 4.
The state-of-the-art about clinical trial status related to DAI and its therapeutic applications.
6. Conclusions and Future Perspectives
DAI, an isoflavone flavonoid, has attracted a lot of attention in recent years due to its wide range of therapeutic benefits on oxidative stress, cancer, obesity, cardiovascular disease, neuroprotection, diabetes, ovariectomy, anxiety, and inflammation. Despite the wide range of biological activities that this phytoconstituent exhibits, there are certain limitations to DAI’s administration, including its poor water solubility, slow absorption, and limited oral bioavailability.
This review revealed a number of nanocarriers that have been investigated for the delivery of DAI, including polymeric nanoparticles, solid lipid nanoparticles, nanostructured lipid carriers, polymeric micelles, nanocomplexes, nanosuspension, nanoemulsion, liposomes, and self-micro emulsifying drug delivery systems. Additionally, our paper highlighted the results of several studies that focused into generating nanocarrier-based DAI to increase its pharmacological potential, and it ultimately showed that nanotechnology might be quite helpful in resolving solubility and permeability challenges faced by phytoconstituents in therapeutic applications. The use of soy products has expanded over the last few years due to DAI’s vital role in therapeutic applications.
On the other hand, a long-term high soy product diet could reduce the secretion of serum testosterone and, therefore, can cause complications in male fertility. Additionally, research is required to examine a novel extraction technique to produce DAI analogues with a greater bioavailability.
Nano formulations present a tremendous opportunity for investigating the effectiveness and bioavailability of DAI because of their small particle size, high specific surface area, increased surface reactivity, and superior adsorption capacity.
Author Contributions
Conceptualization, S.S., N.S. and T.B.; methodology, N.S., S.S. and S.G. (Sonam Grewal); investigation, S.S. and T.B.; resources, T.B. and A.B.; data curation, S.S., M.K.A. and T.B.; writing original draft preparation, T.B., S.G. (Sonam Grewal), C.V.-D.-L.-C. and N.S.; writing—review and editing, S.S., S.M., S.G. (Sumeet Gupta) and A.B.; visualization, S.S.; supervision, T.B. and S.G.B. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the University of Oradea through an internal project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Department of Pharmaceutics, MM College of Pharmacy, Maharishi Markandeshwar (Deemed to be University), Mullana-Ambala, Haryana, India 133207, and the School of Health Science, University of Petroleum and Energy Studies, Dehradun, Uttarakhand, India, for providing the facilities for the completion of this review. The authors would like to express their gratitude to the University of Oradea for supporting the APC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karunamoorthi, K.; Jegajeevanram, K.; Vijayalakshmi, J.; Mengistie, E. Traditional medicinal plants: A source of phytotherapeutic modality in resource-constrained health care settings. J. Evid. Based. Complement. Altern. Med. 2013, 18, 67–74. [Google Scholar] [CrossRef]
- Verma, S.; Singh, S.P. Current and future status of herbal medicines. Vet. World 2008, 1, 347. [Google Scholar] [CrossRef]
- Foghis, M.; Bungau, S.G.; Bungau, A.F.; Vesa, C.M.; Purza, A.L.; Tarce, A.G.; Tit, D.M.; Pallag, A.; Behl, T.; Hassan, S.S.U.; et al. Plants-based medicine implication in the evolution of chronic liver diseases. Biomed. Pharmacother. 2023, 158, 114207. [Google Scholar] [CrossRef]
- Patle, D.; Vyas, M.; Khatik, G.L. A review on natural products and herbs used in the management of diabetes. Curr. Diabetes Rev. 2021, 17, 186–197. [Google Scholar] [PubMed]
- Gitea, M.A.; Bungau, S.G.; Gitea, D.; Pasca, B.M.; Purza, A.L.; Radu, A.-F. Evaluation of the Phytochemistry–Therapeutic Activity Relationship for Grape Seeds Oil. Life 2023, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, R.; Ravindhran, R. Preliminary comparative phytochemical screening of root extracts of Diospyrus ferrea (Wild.) Bakh and Aerva lanata (L.) Juss. Ex Schultes. Asian J. Plant Sci. Res. 2011, 2, 581–587. [Google Scholar]
- Nandagoapalan, V.; Doss, A.; Marimuthu, C. Phytochemical analysis of some traditional medicinal plants. Biosci. Discov. 2016, 7, 17–20. [Google Scholar]
- Yadav, M.; Chatterji, S.; Gupta, S.K.; Watal, G. Preliminary phytochemical screening of six medicinal plants used in traditional medicine. Int. J. Pharm. Pharm. Sci. 2014, 6, 539–542. [Google Scholar]
- Pal, D.; Verma, P. Flavonoids: A powerful and abundant source of antioxidants. Int. J. Pharm. Pharm. Sci. 2013, 5, 95–98. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.-S.; Ng, M.-S.; Cheng, S.-S.; Lo, A.W.-Y.; Xiao, Z.; Shin, T.-S.; Chung, G.; Lam, H.-M. Understanding the composition, biosynthesis, accumulation and transport of flavonoids in crops for the promotion of crops as healthy sources of flavonoids for human consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Nair, R.M.K.; Rahdar, A.; Bungau, S.; Zaha, D.C.; Aleya, L.; Tit, D.M. Overview of the anticancer activity of withaferin A, an active constituent of the Indian ginseng Withania somnifera. Environ. Sci. Pollut. Res. Int. 2020, 27, 26025–26035. [Google Scholar] [CrossRef] [PubMed]
- Khuntia, A.; Martorell, M.; Ilango, K.; Bungau, S.G.; Radu, A.-F.; Behl, T.; Sharifi-Rad, J. Theoretical evaluation of Cleome species’ bioactive compounds and therapeutic potential: A literature review. Biomed. Pharmacother. 2022, 151, 113161. [Google Scholar] [CrossRef]
- Bellavia, D.; Dimarco, E.; Costa, V.; Carina, V.; De Luca, A.; Raimondi, L.; Fini, M.; Gentile, C.; Caradonna, F.; Giavaresi, G. Flavonoids in bone erosive diseases: Perspectives in osteoporosis treatment. Trends Endocrinol. Metab. 2021, 32, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Caro-Ordieres, T.; Marín-Royo, G.; Opazo-Ríos, L.; Jiménez-Castilla, L.; Moreno, J.A.; Gómez-Guerrero, C.; Egido, J. The coming age of flavonoids in the treatment of diabetic complications. J. Clin. Med. 2020, 9, 346. [Google Scholar] [CrossRef]
- Bungau, S.; Vesa, C.M.; Abid, A.; Behl, T.; Tit, D.M.; Purza, A.L.; Pasca, B.; Todan, L.M.; Endres, L. Withaferin A—A Promising Phytochemical Compound with Multiple Results in Dermatological Diseases. Molecules 2021, 26, 2407. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef]
- Domaszewska-Szostek, A.; Puzianowska-Kuźnicka, M.; Kuryłowicz, A. Flavonoids in skin senescence prevention and treatment. Int. J. Mol. Sci. 2021, 22, 6814. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. 2005, 230, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-Y.; Ye, Y.; Xiao, L.; Rahman, K.; Xia, W.; Zhang, H. Daidzein: A review of pharmacological effects. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 117–132. [Google Scholar] [CrossRef]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M. Therapeutic potential of isoflavones with an emphasis on daidzein. Oxid. Med. Cell. Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef] [PubMed]
- Nan, G.; Shi, J.; Huang, Y.; Sun, J.; Lv, J.; Yang, G.; Li, Y. Dissociation constants and solubilities of daidzein and genistein in different solvents. J. Chem. Eng. Data 2014, 59, 1304–1311. [Google Scholar] [CrossRef]
- Huang, Z.-R.; Hung, C.-F.; Lin, Y.-K.; Fang, J.-Y. In vitro and in vivo evaluation of topical delivery and potential dermal use of soy isoflavones genistein and daidzein. Int. J. Pharm. 2008, 364, 36–44. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, X.; Song, B.; Zhong, D.; Liu, C. Influence of dosage forms on pharmacokinetics of daidzein and its main metabolite daidzein-7-O-glucuronide in rats. Acta Pharmacol. Sin. 2005, 26, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhao, X.; Li, J.; Shen, Q. The comparison of different daidzein-PLGA nanoparticles in increasing its oral bioavailability. Int. J. Nanomed. 2012, 7, 559. [Google Scholar]
- Kaplan, A.B.U.; Öztürk, N.; Çetin, M.; Vural, I.; Özer, T.Ö. The nanosuspension formulations of daidzein: Preparation and in vitro characterization. Turk. J. Pharm. Sci. 2022, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, Y.; Chadha, K.; Chadha, R.; Karan, M. Daidzein cocrystals: An opportunity to improve its biopharmaceutical parameters. Heliyon 2019, 5, e02669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, Y.; Gao, F.; Bu, H.; Gu, W.; Li, Y. Daidzein–phospholipid complex loaded lipid nanocarriers improved oral absorption: In vitro characteristics and in vivo behavior in rats. Nanoscale 2011, 3, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Alwasel, S.H.; Harrath, A.H. The influence of plant isoflavones daidzein and equol on female reproductive processes. Pharmaceuticals 2021, 14, 373. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, A.; Ruda-Kucerova, J.; Kamiński, K.; Babinska, Z.; Popiołek, I.; Szczubiałka, K.; Nowakowska, M.; Walczak, M. Improved pharmacokinetics and tissue uptake of complexed daidzein in rats. Pharmaceutics 2020, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Amawi, H.; Ashby, C.R.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef]
- Rafii, F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites 2015, 5, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Choue, R. Plasma pharmacokinetics and urinary excretion of isoflavones after ingestion of soy products with different aglycone/glucoside ratios in South Korean women. Nutr. Res. Pract. 2013, 7, 393–399. [Google Scholar] [CrossRef]
- Ahmed, T.; Javed, S.; Tariq, A.; Onofrio, G.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Daidzein and its effects on brain. Curr. Med. Chem. 2017, 24, 365–375. [Google Scholar]
- Xiao, B.-X.; Feng, L.; Cao, F.-R.; Pan, R.-L.; Liao, Y.-H.; Liu, X.-M.; Chang, Q. Pharmacokinetic profiles of the five isoflavonoids from Pueraria lobata roots in the CSF and plasma of rats. J. Ethnopharmacol. 2016, 184, 22–29. [Google Scholar] [CrossRef]
- Laddha, A.P.; Murugesan, S.; Kulkarni, Y.A. In-vivo and in-silico toxicity studies of daidzein: An isoflavone from soy. Drug Chem. Toxicol. 2022, 45, 1408–1416. [Google Scholar] [CrossRef]
- Sarasquete, C.; Úbeda-Manzanaro, M.; Ortiz-Delgado, J.B. Toxicity and non-harmful effects of the soya isoflavones, genistein and daidzein, in embryos of the zebrafish, Danio rerio. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 211, 57–67. [Google Scholar] [CrossRef]
- Foti, P.; Erba, D.; Spadafranca, A.; Ciappellano, S.; Bresciani, J.; Testolin, G. Daidzein is absorbed by passive transport in isolated small intestine of rats. Nutr. Res. 2006, 26, 284–288. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Zhao, X.; Shoaf, S.E.; Ragland, K. The pharmacokinetics of S-(-) equol administered as SE5-OH tablets to healthy postmenopausal women. J. Nutr. 2009, 139, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Clerici, C. Equol: Pharmacokinetics and biological actions. J. Nutr. 2010, 140, 1363S–1368S. [Google Scholar] [CrossRef]
- Chu, H.; Li, J.; Liu, T.; Miao, N.; Zhang, W. Anticancer effects of Daidzein against the human melanoma cell lines involves cell cycle arrest, autophagy and deactivation of PI3K/AKT signalling pathways. J. BUON Off. J. Balk. Union Oncol. 2021, 26, 290. [Google Scholar]
- Yu, Z.; Yang, L.; Deng, S.; Liang, M. Daidzein ameliorates LPS-induced hepatocyte injury by inhibiting inflammation and oxidative stress. Eur. J. Pharmacol. 2020, 885, 173399. [Google Scholar] [CrossRef]
- Inoue, Y.; Yoshida, M.; Ezawa, T.; Tanikawa, T.; Arce, F.; See, G.L.; Tomita, J.; Suzuki, M.; Oguchi, T. Inclusion Complexes of Daidzein with Cyclodextrin-Based Metal–Organic Framework-1 Enhance Its Solubility and Antioxidant Capacity. AAPS PharmSciTech 2022, 23, 2. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. Daidzein ameliorates diabetic retinopathy in experimental animals. Life Sci. 2021, 265, 118779. [Google Scholar] [CrossRef] [PubMed]
- Herwana, E.; Pusparini, P.; Graciela, A. High dietary daidzein intake lowers cholesterol levels among post-menopausal women. Universa Med. 2020, 39, 47–54. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. Daidzein mitigates myocardial injury in streptozotocin-induced diabetes in rats. Life Sci. 2021, 284, 119664. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Liu, S.; Su, Y.; Li, Y. Cardiotonic Activity of Daidzein Against Doxorubicin-lnduced Congestive Cardiac Failure in Rats. Curr. Top. Nutraceutical Res. 2022, 20, 106–113. [Google Scholar]
- Rawat, S.; Pathak, S.; Gupta, G.; Singh, S.K.; Singh, H.; Mishra, A.; Gilhotra, R. Recent updates on daidzein against oxidative stress and cancer. EXCLI J. 2019, 18, 950. [Google Scholar]
- Amaral, C.; Toloi, M.R.T.; Vasconcelos, L.D.; Fonseca, M.J.V.; Correia-da-Silva, G.; Teixeira, N. The role of soybean extracts and isoflavones in hormone-dependent breast cancer: Aromatase activity and biological effects. Food Funct. 2017, 8, 3064–3074. [Google Scholar] [CrossRef]
- Meng, H.; Fu, G.; Shen, J.; Shen, K.; Xu, Z.; Wang, Y.; Jin, B.; Pan, H. Ameliorative effect of daidzein on cisplatin-induced nephrotoxicity in mice via modulation of inflammation, oxidative stress, and cell death. Oxid. Med. Cell. Longev. 2017, 2017, 3140680. [Google Scholar] [CrossRef]
- Magee, P.J.; Allsopp, P.; Samaletdin, A.; Rowland, I.R. Daidzein, R-(+) equol and S-(−) equol inhibit the invasion of MDA-MB-231 breast cancer cells potentially via the down-regulation of matrix metalloproteinase-2. Eur. J. Nutr. 2014, 53, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Shu, F.; Zeng, Y.; Meng, X.; Wang, B.; Diao, L.; Wang, L.; Wan, J.; Zhu, J.; Wang, J. Daidzein supplementation decreases serum triglyceride and uric acid concentrations in hypercholesterolemic adults with the effect on triglycerides being greater in those with the GA compared with the GG genotype of ESR-β Rsa I. J. Nutr. 2014, 144, 49–54. [Google Scholar] [CrossRef]
- Sun, J.; Sun, W.J.; Li, Z.Y.; Li, L.; Wang, Y.; Zhao, Y.; Wang, C.; Yu, L.R.; Li, L.Z.; Zhang, Y.L. Daidzein increases OPG/RANKL ratio and suppresses IL-6 in MG-63 osteoblast cells. Int. Immunopharmacol. 2016, 40, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Q.U.; Ali, A.H.M.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Sabere, A.S.M.; Nawi, M.S.M.; Khatib, A.; Siddiqui, M.J.; Umar, A. Medicinal potential of isoflavonoids: Polyphenols that may cure diabetes. Molecules 2020, 25, 5491. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Lee, K.W.; Byun, S.; Jung, S.K.; Song, N.; Lim, S.H.; Heo, Y.-S.; Kim, J.E.; Kang, N.J.; Kim, B.Y. 7,3′,4′-Trihydroxyisoflavone, a metabolite of the soy isoflavone daidzein, suppresses ultraviolet B-induced skin cancer by targeting Cot and MKK4. J. Biol. Chem. 2011, 286, 14246–14256. [Google Scholar] [CrossRef]
- Velentzis, L.S.; Woodside, J.V.; Cantwell, M.M.; Leathem, A.J.; Keshtgar, M.R. Do phytoestrogens reduce the risk of breast cancer and breast cancer recurrence? What clinicians need to know. Eur. J. Cancer 2008, 44, 1799–1806. [Google Scholar] [CrossRef]
- Liu, M.M.; Huang, Y.; Wang, J. Developing phytoestrogens for breast cancer prevention. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Agents) 2012, 12, 1306–1313. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- Bao, C.; Namgung, H.; Lee, J.; Park, H.-C.; Ko, J.; Moon, H.; Ko, H.W.; Lee, H.J. Daidzein suppresses tumor necrosis factor-α induced migration and invasion by inhibiting hedgehog/Gli1 signaling in human breast cancer cells. J. Agric. Food Chem. 2014, 62, 3759–3767. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agarwal, R. Natural flavonoids targeting deregulated cell cycle progression in cancer cells. Curr. Drug Targets 2006, 7, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, G.-H. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine 2008, 15, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, H.; Hu, X.; Ma, W.; Zhang, J.; Li, Y.; Yu, M.; Zhang, Y.; Li, X.; Ye, X. Synergetic inhibition of daidzein and regular exercise on breast cancer in bearing-4T1 mice by regulating NK cells and apoptosis pathway. Life Sci. 2020, 245, 117387. [Google Scholar] [CrossRef] [PubMed]
- Rabiau, N.; Kossaï, M.; Braud, M.; Chalabi, N.; Satih, S.; Bignon, Y.-J.; Bernard-Gallon, D.J. Genistein and daidzein act on a panel of genes implicated in cell cycle and angiogenesis by polymerase chain reaction arrays in human prostate cancer cell lines. Cancer Epidemiol. 2010, 34, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.M.; Xiao, B.X.; Liu, D.H.; Grant, M.; Zhang, S.; Lai, Y.F.; Guo, Y.B.; Liu, Q. Biphasic effect of daidzein on cell growth of human colon cancer cells. Food Chem. Toxicol. 2004, 42, 1641–1646. [Google Scholar] [CrossRef]
- Gheorghe, G.; Toth, P.P.; Bungau, S.; Behl, T.; Ilie, M.; Stoian, A.P.; Bratu, O.G.; Bacalbasa, N.; Rus, M.; Diaconu, C.C. Cardiovascular Risk and Statin Therapy Considerations in Women. Diagnostics 2020, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Sex steroids, cardiovascular disease, and hypertension: Unanswered questions and some speculations. Hypertension 2005, 45, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.L.; Serock, M.R.; Khalil, R.A. Experimental benefits of sex hormones on vascular function and the outcome of hormone therapy in cardiovascular disease. Curr. Cardiol. Rev. 2008, 4, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Guo, W.; Han, J.; Li, X.-A. Role of caveolin-1 and caveolae signaling in endotoxemia and sepsis. Life Sci. 2013, 93, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, F.R.; van Meer, G. Cholesterol, the central lipid of mammalian cells. Curr. Opin. Cell Biol. 2010, 22, 422–429. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Hampson, G. The pathogenesis, diagnosis, investigation and management of osteoporosis. J. Clin. Pathol. 2011, 64, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Tit, D.M.; Bungau, S.; Iovan, C.; Cseppento, D.C.N.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.C.; Wolber, F.M. Osteoporosis: Modern paradigms for last century’s bones. Nutrients 2016, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yu, B.; Tang, D.; Li, S.; Wu, Y. Daidzein promotes osteoblast proliferation and differentiation in OCT1 cells through stimulating the activation of BMP-2/Smads pathway. Genet. Mol. Res. 2016, 15, gmr.15028792. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Sugimoto, E. Stimulatory effect of genistein and daidzein on protein synthesis in osteoblastic MC3T3-E1 cells: Activation of aminoacyl-tRNA synthetase. Mol. Cell. Biochem. 2000, 214, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kaveeshwar, S.A.; Cornwall, J. The current state of diabetes mellitus in India. Australas. Med. J. 2014, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Iorga, R.A.; Bacalbasa, N.; Carsote, M.; Bratu, O.G.; Stanescu, A.M.A.; Bungau, S.; Pantis, C.; Diaconu, C.C. Metabolic and cardiovascular benefits of GLP-1 agonists, besides the hypoglycemic effect (Review). Exp. Ther. Med. 2020, 20, 2396–2400. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Sarkar, S.; Bordoloi, J.; Wann, S.B.; Kalita, J.; Manna, P. Daidzein, its effects on impaired glucose and lipid metabolism and vascular inflammation associated with type 2 diabetes. Biofactors 2018, 44, 407–417. [Google Scholar] [CrossRef]
- Tian, D.; Liu, J.; Liu, N.; Wang, R.; Ai, Y.; Jin, L.; Li, F.; Wei, P.; Li, Z.; Wang, C. Daidzin decreases blood glucose and lipid in streptozotocin-induced diabetic mice. Trop. J. Pharm. Res. 2016, 15, 2435–2443. [Google Scholar] [CrossRef]
- Liang, J.; Tian, Y.-X.; Fu, L.-M.; Wang, T.-H.; Li, H.-J.; Wang, P.; Han, R.-M.; Zhang, J.-P.; Skibsted, L.H. Daidzein as an antioxidant of lipid: Effects of the microenvironment in relation to chemical structure. J. Agric. Food Chem. 2008, 56, 10376–10383. [Google Scholar] [CrossRef] [PubMed]
- Kampkötter, A.; Chovolou, Y.; Kulawik, A.; Röhrdanz, E.; Weber, N.; Proksch, P.; Wätjen, W. Isoflavone daidzein possesses no antioxidant activities in cell-free assays but induces the antioxidant enzyme catalase. Nutr. Res. 2008, 28, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Toda, S.; Shirataki, Y. Comparison of antioxidative and chelating effects of daidzein and daidzin on protein oxidative modification by copper in vitro. Biol. Trace Elem. Res. 2001, 79, 83–89. [Google Scholar] [CrossRef]
- Barton, G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-inflammatory benefit and possible caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef]
- Zhang, H.; Kovacs-Nolan, J.; Kodera, T.; Eto, Y.; Mine, Y. γ-Glutamyl cysteine and γ-glutamyl valine inhibit TNF-α signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2015, 1852, 792–804. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G. The antioxidant activity of daidzein metabolites, O-desmethylangolensin and equol, in HepG2 cells. Mol. Med. Rep. 2014, 9, 328–332. [Google Scholar] [CrossRef]
- Sharma, V.; Nath, D.; Gautam, S.; Radu, A.-F.; Behl, T.; Bungau, S.G.; Vesa, C.M. Reviewing the Traditional/Modern Uses, Phytochemistry, Essential Oils/Extracts and Pharmacology of Embelia ribes Burm. Antioxidants 2022, 11, 1359. [Google Scholar] [CrossRef]
- Cheatwood, J.L.; Emerick, A.J.; Kartje, G.L. Neuronal plasticity and functional recovery after ischemic stroke. Top. Stroke Rehabil. 2008, 15, 42–50. [Google Scholar] [CrossRef]
- Stout, J.M.; Knapp, A.N.; Banz, W.J.; Wallace, D.G.; Cheatwood, J.L. Subcutaneous daidzein administration enhances recovery of skilled ladder rung walking performance following stroke in rats. Behav. Brain Res. 2013, 256, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, O.; Ballesteros, I.; Cuartero, M.I.; Moraga, A.; Pradillo, J.M.; Ramírez-Franco, J.; Bartolomé-Martín, D.; Pascual, D.; Torres, M.; Sánchez-Prieto, J. Daidzein has neuroprotective effects through ligand-binding-independent PPARγ activation. Neurochem. Int. 2012, 61, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Bordet, R.; Gelé, P.; Duriez, P.; Fruchart, J.C. PPARs: A new target for neuroprotection. J. Neurol. Neurosurg. Psychiatry 2006, 77, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Tai, F.; Zeng, S.; Zhang, X. Effects of perinatal daidzein exposure on subsequent behavior and central estrogen receptor α expression in the adult male mouse. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 43, 157–167. [Google Scholar] [CrossRef]
- Atiq, A.; Shal, B.; Naveed, M.; Khan, A.; Ali, J.; Zeeshan, S.; Al-Sharari, S.D.; Kim, Y.S.; Khan, S. Diadzein ameliorates 5-fluorouracil-induced intestinal mucositis by suppressing oxidative stress and inflammatory mediators in rodents. Eur. J. Pharmacol. 2019, 843, 292–306. [Google Scholar] [CrossRef]
- Kim, D.H.; Jung, H.A.; Park, S.J.; Kim, J.M.; Lee, S.; Choi, J.S.; Cheong, J.H.; Ko, K.H.; Ryu, J.H. The effects of daidzin and its aglycon, daidzein, on the scopolamineinduced memory impairment in male mice. Arch. Pharm. Res. 2010, 33, 1685–1690. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, G.; Su, X.; Yang, H.; Zhang, J. Antiobesity action of a daidzein derivative on male obese mice induced by a high-fat diet. Nutr. Res. 2009, 29, 656–663. [Google Scholar] [CrossRef]
- Goel, R.; Chaudhary, R. Effect of daidzein on Parkinson disease induced by reserpine in rats. Braz. J. Pharm. Sci. 2020, 56, 1–7. [Google Scholar] [CrossRef]
- Woodman, O.L.; Boujaoude, M. Chronic treatment of male rats with daidzein and 17β-oestradiol induces the contribution of EDHF to endothelium-dependent relaxation. Br. J. Pharmacol. 2004, 141, 322–328. [Google Scholar] [CrossRef]
- Ko, Y.-H.; Kim, S.Y.; Lee, S.-Y.; Jang, C.-G. 6,7,4′-Trihydroxyisoflavone, a major metabolite of daidzein, improves learning and memory via the cholinergic system and the p-CREB/BDNF signaling pathway in mice. Eur. J. Pharmacol. 2018, 826, 140–147. [Google Scholar] [CrossRef]
- Park, M.-H.; Ju, J.-W. Daidzein inhibits carbohydrate digestive enzymes in vitro and alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2013, 712, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, X.; Liu, J.; Li, K. Effects of daidzein sulfates on blood pressure and artery of rats. Basic Clin. Pharmacol. Toxicol. 2006, 99, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, M.; Sharma, P.L. Ameliorative effect of daidzein: A caveolin-1 inhibitor in vascular endothelium dysfunction induced by ovariectomy. Indian J. Exp. Biol. 2012, 50, 28–34. [Google Scholar] [PubMed]
- Choi, E.J. The prooxidant, rather than antioxidant, acts of daidzein in vivo and in vitro: Daidzein suppresses glutathione metabolism. Eur. J. Pharmacol. 2006, 542, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Tai, F.; Zhai, P.; Yuan, A.; Jia, R.; Zhang, X. Effect of daidzein on anxiety, social behavior and spatial learning in male Balb/cJ mice. Pharmacol. Biochem. Behav. 2010, 96, 16–23. [Google Scholar] [CrossRef]
- Farhana, A.; Veeresh, B.; Rao, R. Protective Role of Diadzein in L-Arginine-Induced Acute pancreatitis in Rats. YMER Digit. 2022, 21, 451–460. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Naka, A.; Ohara, N.; Kondo, K.; Iida, K. Daidzein regulates proinflammatory adipokines thereby improving obesity-related inflammation through PPARγ. Mol. Nutr. Food Res. 2014, 58, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xu, J.; Guo, T.L. Isoflavone daidzein regulates immune responses in the B6C3F1 and non-obese diabetic (NOD) mice. Int. Immunopharmacol. 2019, 71, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Alò, R.; Fazzari, G.; Zizza, M.; Avolio, E.; Di Vito, A.; Bruno, R.; Cuda, G.; Barni, T.; Canonaco, M.; Facciolo, R.M. Daidzein Pro-cognitive Effects Coincided with Changes of Brain Neurotensin1 Receptor and Interleukin-10 Expression Levels in Obese Hamsters. Neurotox. Res. 2021, 39, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Crespillo, A.; Alonso, M.; Vida, M.; Pavón, F.J.; Serrano, A.; Rivera, P.; Romero-Zerbo, Y.; Fernández-Llebrez, P.; Martínez, A.; Pérez-Valero, V. Reduction of body weight, liver steatosis and expression of stearoyl-CoA desaturase 1 by the isoflavone daidzein in diet-induced obesity. Br. J. Pharmacol. 2011, 164, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, J.S.; Jung, J.W.; Byun, K.W.; Kang, K.S.; Lee, Y.S. Daidzein supplementation prevents non-alcoholic fatty liver disease through alternation of hepatic gene expression profiles and adipocyte metabolism. Int. J. Obes. 2011, 35, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Mohamad-Shahi, M.; Karandish, M.; Haidari, F.; Omidian, K.; Fatemi-Tabatabayei, S.-R.; Rafiei, H. Effect of daidzein-low-calorie diet on body weight, serum levels of glucose, resistin, and high sensitive C-reactive protein in high fat, high calorie diet induced rats. Saudi. Med. J. 2012, 33, 70–75. [Google Scholar] [PubMed]
- Park, S.A.; Choi, M.-S.; Cho, S.-Y.; Seo, J.-S.; Jung, U.J.; Kim, M.-J.; Sung, M.-K.; Park, Y.B.; Lee, M.-K. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006, 79, 1207–1213. [Google Scholar] [CrossRef]
- Tomar, A.; Kaushik, S.; Khan, S.I.; Bisht, K.; Nag, T.C.; Arya, D.S.; Bhatia, J. The dietary isoflavone daidzein mitigates oxidative stress, apoptosis, and inflammation in CDDP-induced kidney injury in rats: Impact of the MAPK signaling pathway. J. Biochem. Mol. Toxicol. 2020, 34, e22431. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, M. Broad-Spectrum Theranostics and Biomedical Application of Functionalized Nanomaterials. Polymers 2022, 14, 1221. [Google Scholar] [CrossRef]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine—Challenge and perspectives. Angew. Chem. Int. Ed. 2009, 48, 872–897. [Google Scholar] [CrossRef] [PubMed]
- Boulaiz, H.; Alvarez, P.J.; Ramirez, A.; Marchal, J.A.; Prados, J.; Rodríguez-Serrano, F.; Perán, M.; Melguizo, C.; Aranega, A. Nanomedicine: Application areas and development prospects. Int. J. Mol. Sci. 2011, 12, 3303–3321. [Google Scholar] [CrossRef] [PubMed]
- Pison, U.; Welte, T.; Giersig, M.; Groneberg, D.A. Nanomedicine for respiratory diseases. Eur. J. Pharmacol. 2006, 533, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Gu, L. TPGS emulsified zein nanoparticles enhanced oral bioavailability of daidzin: In vitro characteristics and in vivo performance. Mol. Pharm. 2013, 10, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Ozakar, R.S.; Cetin, M.; Taghizadehghalehjoughi, A.; Hacimuftuoglu, A. Preparation and In Vitro Evaluation of Daidzein-Loaded Nanoparticulate Systems. Eurasia Proc. Health Environ. Life Sci. 2021, 1, 1–12. [Google Scholar]
- Gao, Y.; Gu, W.; Chen, L.; Xu, Z.; Li, Y. The role of daidzein-loaded sterically stabilized solid lipid nanoparticles in therapy for cardio-cerebrovascular diseases. Biomaterials 2008, 29, 4129–4136. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Fan, X.; Shen, Q. Daidzein-loaded nanostructured lipid carriers-PLGA nanofibers for transdermal delivery. Int. J. Pharm. 2016, 501, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Wusiman, Z.; Sun, L.; Zuo, T.; Yin, Y.; Yang, X.; Shen, Q. Core–shell-type polymer–lipid nanoparticles for the transdermal delivery of daidzein. Micro Nano Lett. 2018, 13, 1363–1366. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Gao, F.; Gao, Z.; Bu, H.; Gu, W.; Li, Y. A self-assembled nanodelivery system enhances the oral bioavailability of daidzein: In vitro characteristics and in vivo performance. Nanomedicine 2011, 6, 1365–1379. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, Y.; Wang, H.; Sang, Z.; Han, X.; Ren, S.; Du, R.; Shi, X.; Xie, Y. Development of daidzein nanosuspensions: Preparation, characterization, in vitro evaluation, and pharmacokinetic analysis. Int. J. Pharm. 2019, 566, 67–76. [Google Scholar] [CrossRef]
- Lv, L.; Fu, C.; Zhang, F.; Wang, S. Thermally-induced whey protein isolate-daidzein co-assemblies: Protein-based nanocomplexes as an inhibitor of precipitation/crystallization for hydrophobic drug. Food Chem. 2019, 275, 273–281. [Google Scholar] [CrossRef]
- Kaplan, A.B.U.; Cetin, M.; Orgul, D.; Taghizadehghalehjoughi, A.; Hacımuftuoglu, A.; Hekimoglu, S. Formulation and in vitro evaluation of topical nanoemulsion and nanoemulsion-based gels containing daidzein. J. Drug Deliv. Sci. Technol. 2019, 52, 189–203. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Wang, J.; Liu, H.; Chen, Y. Preparation and pharmacokinetic study of Daidzein Long-circulating liposomes. Nanoscale Res. Lett. 2019, 14, 321. [Google Scholar] [CrossRef]
- Shen, Q.; Li, X.; Yuan, D.; Jia, W. Enhanced oral bioavailability of daidzein by self-microemulsifying drug delivery system. Chem. Pharm. Bull. 2010, 58, 639–643. [Google Scholar] [CrossRef]
- DeFrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein polymer-based nanoparticles: Fabrication and medical applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Valencia, P.M.; Zhang, L.; Langer, R.; Farokhzad, O.C. Polymeric nanoparticles for drug delivery. In Cancer Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 163–175. [Google Scholar]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Vauthier, C.; Bouchemal, K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef] [PubMed]
- Nagavarma, B.V.N.; Yadav, H.K.S.; Ayaz, A.; Vasudha, L.S.; Shivakumar, H.G. Different techniques for preparation of polymeric nanoparticles-a review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Lingayat, V.J.; Zarekar, N.S.; Shendge, R.S. Solid lipid nanoparticles: A review. Nanosci. Nanotechnol. Res. 2017, 4, 67–72. [Google Scholar]
- Yadav, N.; Khatak, S.; Sara, U.V.S. Solid lipid nanoparticles-a review. Int. J. Appl. Pharm. 2013, 5, 8–18. [Google Scholar]
- Üner, M.; Yener, G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int. J. Nanomed. 2007, 2, 289. [Google Scholar]
- Pizzol, C.D.; Filippin-Monteiro, F.B.; Restrepo, J.A.S.; Pittella, F.; Silva, A.H.; de Souza, P.A.; de Campos, A.M.; Creczynski-Pasa, T.B. Influence of surfactant and lipid type on the physicochemical properties and biocompatibility of solid lipid nanoparticles. Int. J. Environ. Res. Public Health 2014, 11, 8581–8596. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349. [Google Scholar] [CrossRef]
- Patel, D.K.; Tripathy, S.; Nair, S.K.; Kesharwani, R. Nanostructured lipid carrier (Nlc) a modern approach for topical delivery: A review. World J. Pharm. Pharm. Sci. 2013, 2, 921–938. [Google Scholar]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.C.; Cai, Y. A review of the structure, preparation, and application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.; Fathi, H.A.; Eissa, N.G.; Elsabahy, M. Methods for preparation of nanostructured lipid carriers. Methods 2022, 199, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 691–707. [Google Scholar] [CrossRef]
- Nishiyama, N.; Kataoka, K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006, 112, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81. [Google Scholar]
- Krishna, K.B.; Prabhakar, C. A review on nanosuspensions in drug delivery. Int. J. Pharma. Bio Sci. 2011, 2, 549–558. [Google Scholar]
- Lakshmi, P.; Kumar, G.A. Nanosuspension technology: A review. Int. J. Pharm. Pharm. Sci. 2010, 2, 35–40. [Google Scholar]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef]
- Patel, M.; Shah, A.; Patel, N.M.; Patel, M.R.; Patel, K.R. Nanosuspension: A novel approach for drug delivery system. Jpsbr 2011, 1, 1–10. [Google Scholar]
- Patel, R.P.; Joshi, J.R. An overview on nanoemulsion: A novel approach. Int. J. Pharm. Sci. Res. 2012, 3, 4640. [Google Scholar]
- Nikam, T.H.; Patil, M.P.; Patil, S.S.; Vadnere, G.P.; Lodhi, S. Nanoemulsion: A brief review on development and application in Parenteral Drug Delivery. Adv. Pharm. J. 2018, 3, 43–54. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Sharma, N.; Bansal, M.; Visht, S.; Sharma, P.K.; Kulkarni, G.T. Nanoemulsion: A new concept of delivery system. Chron. Young Sci. 2010, 1, 2–6. [Google Scholar]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A review of manufacturing techniques and targeting strategies. Curr. Nanosci. 2011, 7, 436–452. [Google Scholar] [CrossRef]
- Salimi, A. Liposomes as a novel drug delivery system: Fundamental and pharmaceutical application. Asian J. Pharm. 2018, 12, S31–S41. [Google Scholar]
- Gbian, D.L.; Omri, A. Current and novel therapeutic strategies for the management of cystic fibrosis. Expert Opin. Drug Deliv. 2021, 18, 535–552. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Kyatanwar, A.U.; Jadhav, K.R.; Kadam, V.J. Self micro-emulsifying drug delivery system (SMEDDS). J. Pharm. Res. 2010, 3, 75–83. [Google Scholar]
- Patel, D.; Sawant, K.K. Self micro-emulsifying drug delivery system: Formulation development and biopharmaceutical evaluation of lipophilic drugs. Curr. Drug Deliv. 2009, 6, 419–424. [Google Scholar] [CrossRef]
- Gurram, A.K.; Deshpande, P.B.; Kar, S.S.; Nayak, U.Y.; Udupa, N.; Reddy, M.S. Role of components in the formation of self-microemulsifying drug delivery systems. Indian J. Pharm. Sci. 2015, 77, 249. [Google Scholar] [PubMed]
- Clinical Trials Assessing the Medical Role of Daidzein. U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ (accessed on 12 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
