NO2 Physical-to-Chemical Adsorption Transition on Janus WSSe Monolayers Realized by Defect Introduction
Abstract
1. Introduction
2. Results and Discussion
2.1. The Physisorption of NO2 on Pristine Janus WSSe Monolayer
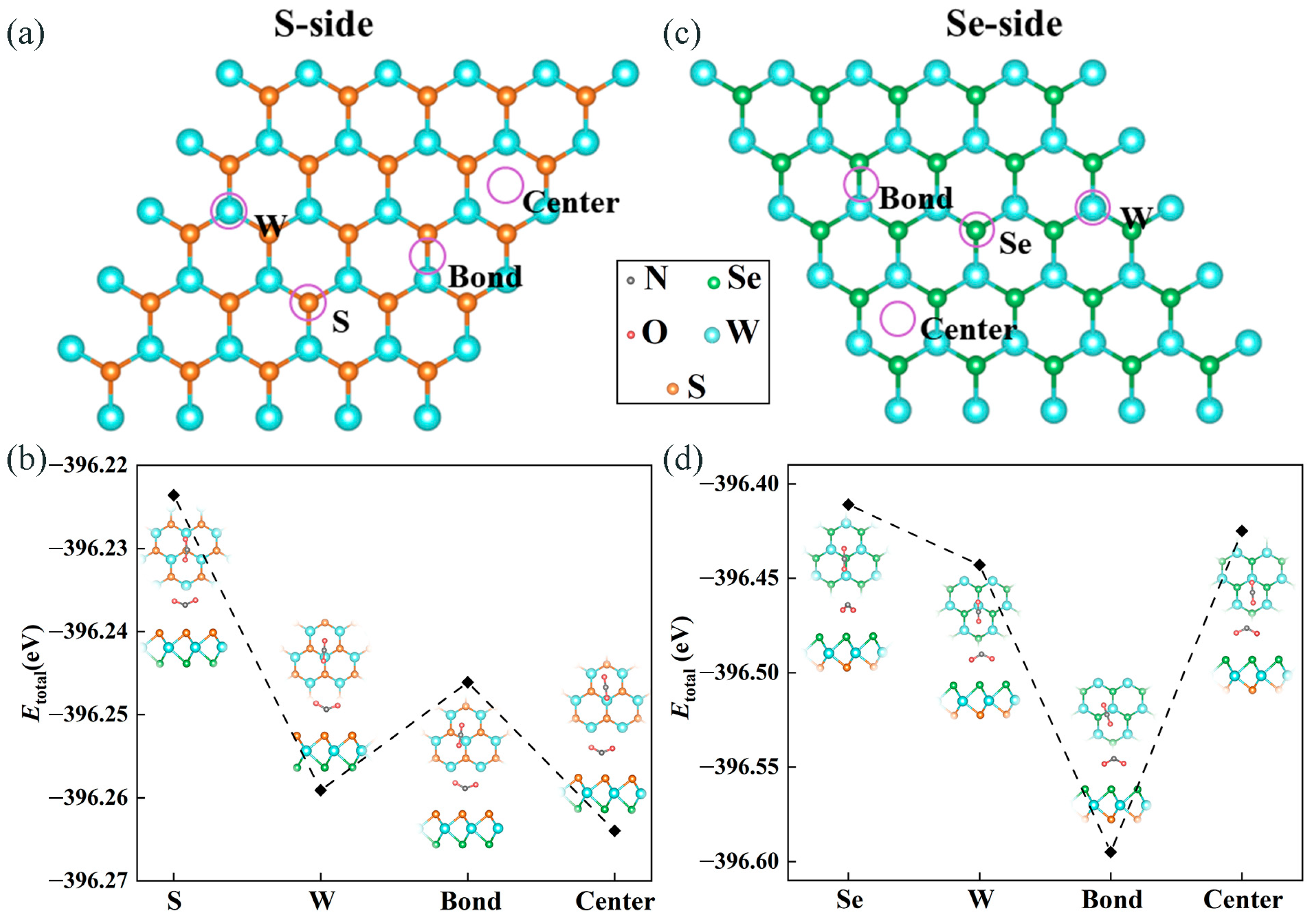
2.1.1. Screening of Adsorption Sites and Adsorption Energy
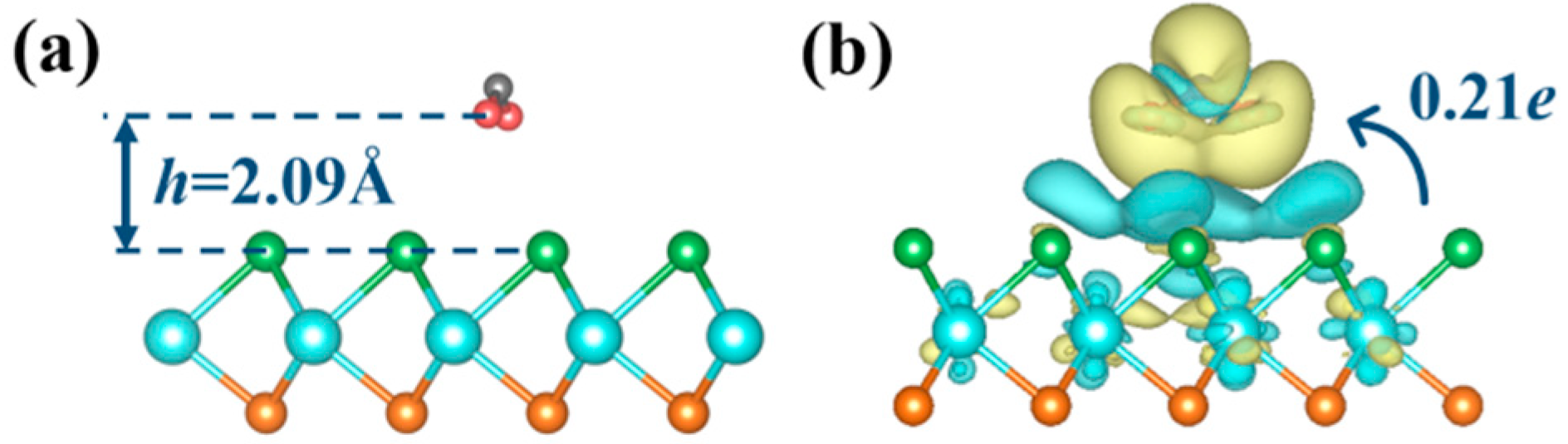
2.1.2. Adsorption Mechanism
2.2. The Chemisorption of NO2 on Defective Janus WSSe Monolayer
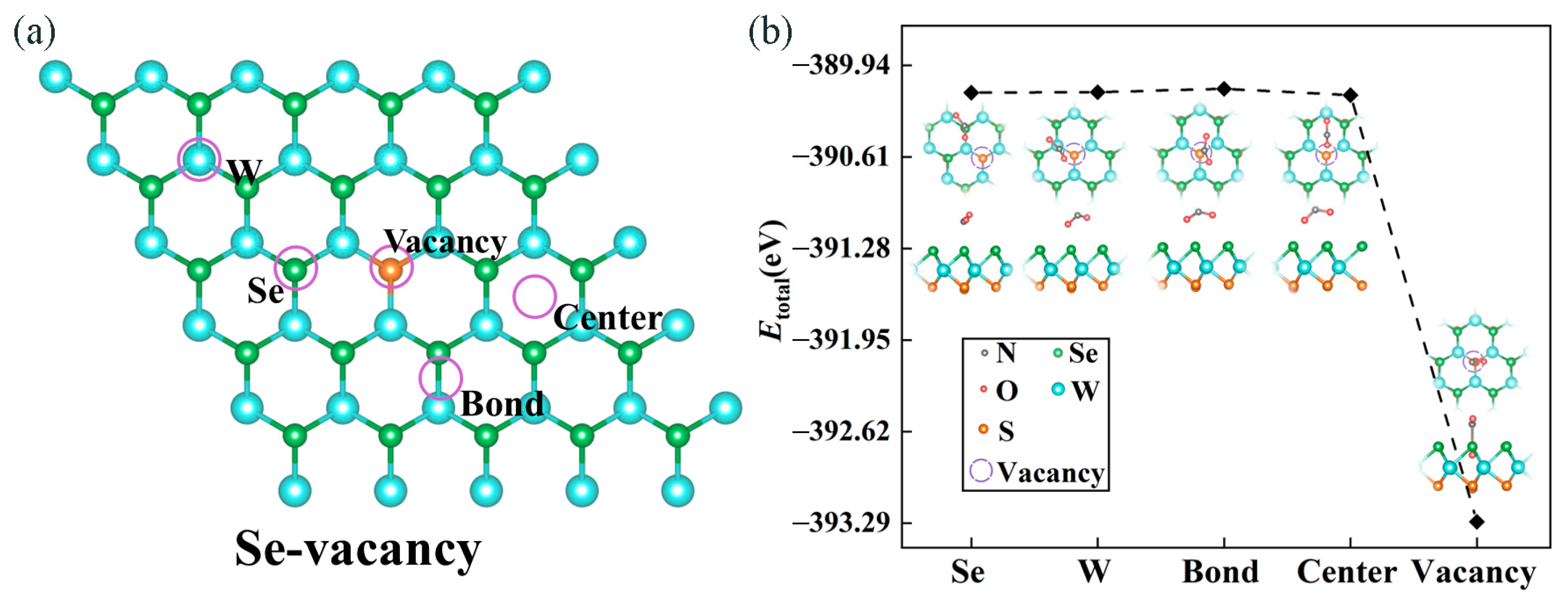
2.2.1. Vacancy Screening
2.2.2. Screening of Adsorption Sites and Adsorption Energy
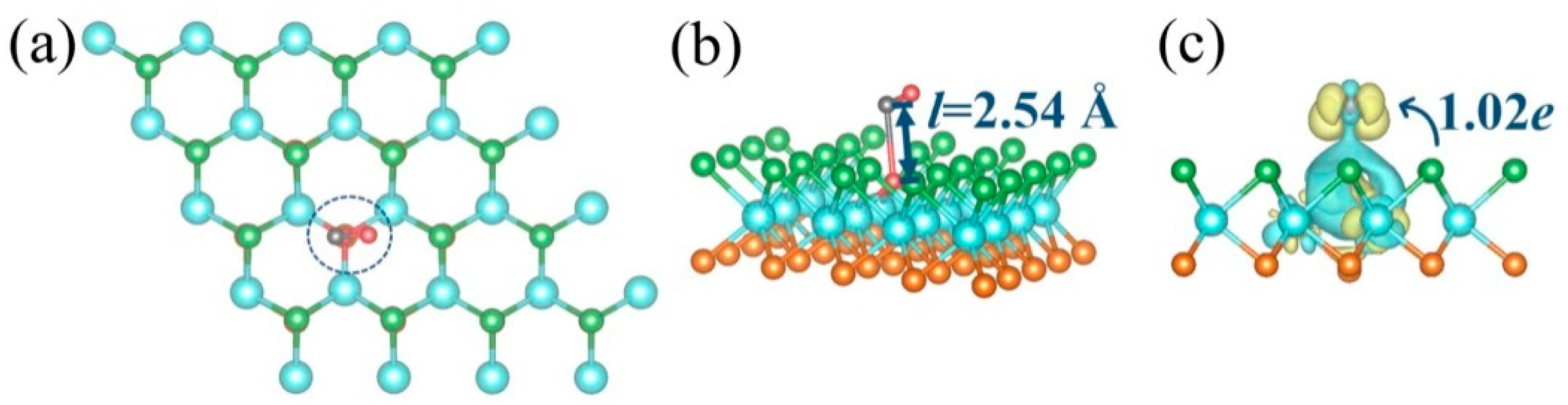
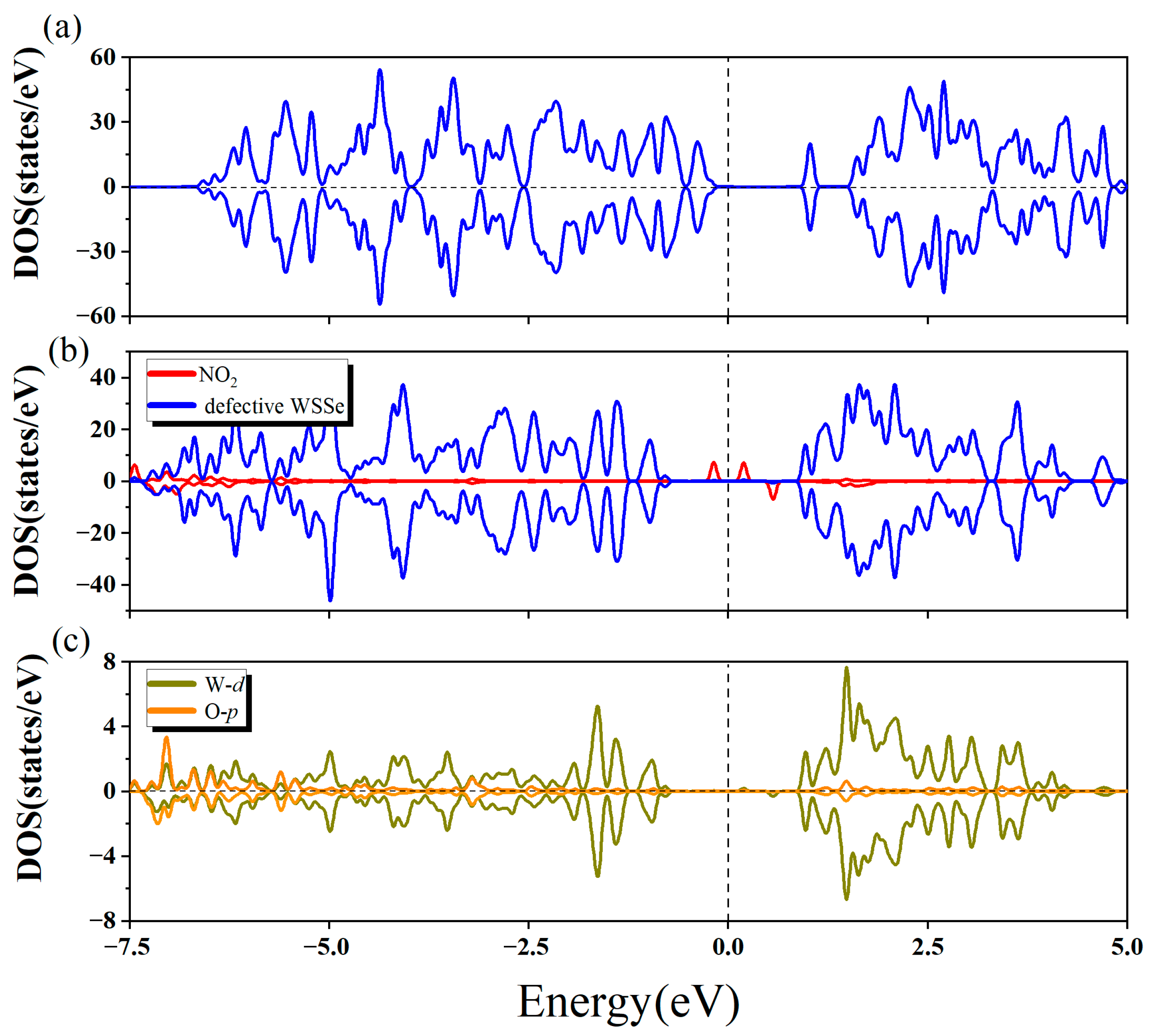
2.2.3. Adsorption Mechanism
2.3. Compression Strain Facilitates Vacancy Formation
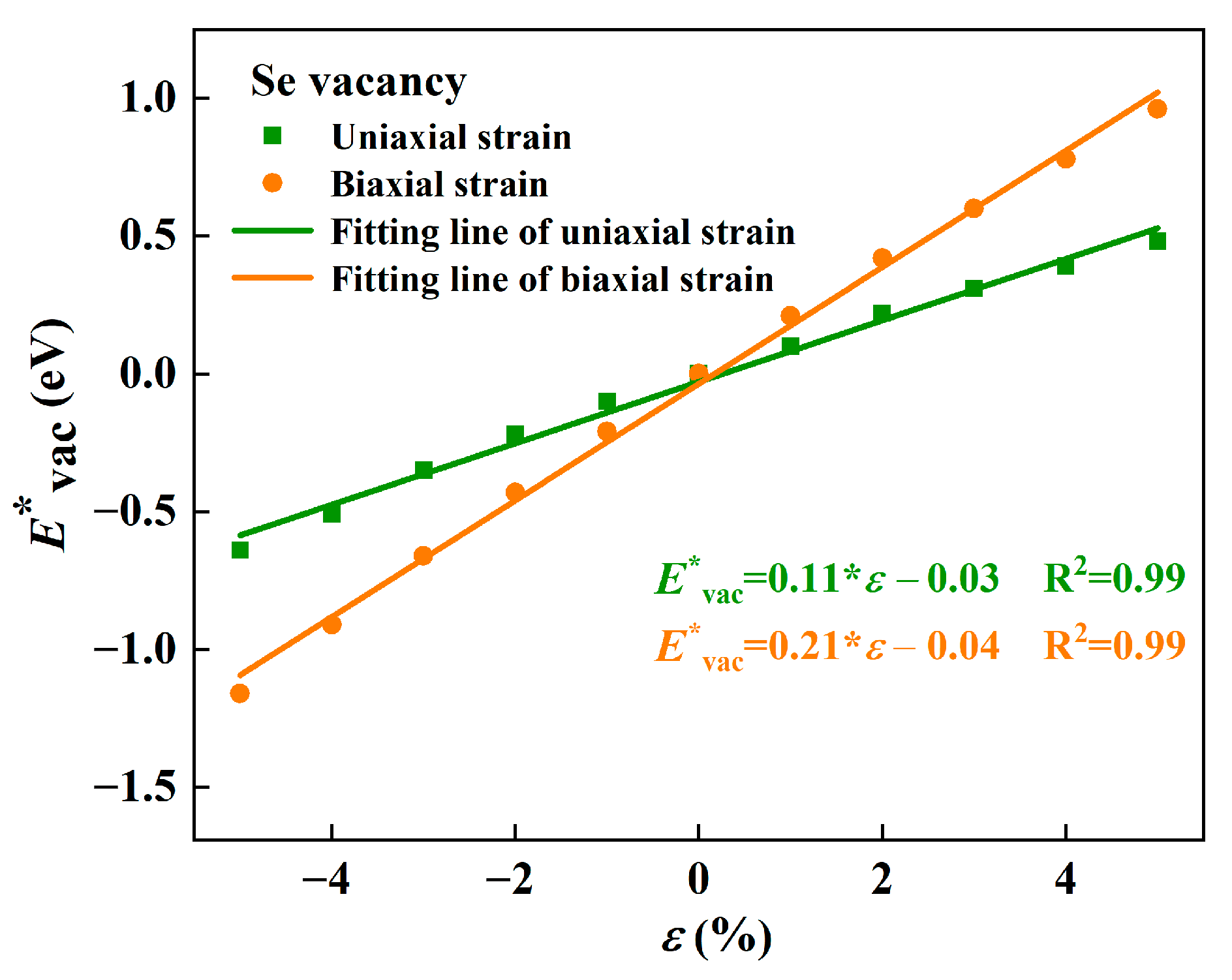
2.3.1. Strain-Dependent Formation Energy
2.3.2. Origin of the Strain-Dependent Vacancy Formation
2.4. Physical-to-Chemical Adsorption Transition
3. Conclusions
4. Computational Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jorres, R.; Nowak, D.; Grimminger, F.; Seeger, W.; Oldigs, M.; Magnussen, H. The effect of 1 ppm nitrogen dioxide on bronchoalveolar lavage cells and inflammatory mediators in normal and asthmatic subjects. Eur. Respir. J. 1995, 8, 416–424. [Google Scholar] [PubMed]
- Last, J.A.; Sun, W.-M.; Witschi, H. Ozone, NO, and NO2: Oxidant air pollutants and more. Environ. Health Persp. 1994, 102 (Suppl. S10), 179–184. [Google Scholar]
- Zhai, L.; Zhang, J.; Wu, M.; Huo, H.; Bi, F.; Wang, B. Balancing good oxygen balance and high heat of formation by incorporating of -C(NO2)2F Moiety and Tetrazole into Furoxan block. J. Mol. Struct. 2020, 1222, 128934. [Google Scholar]
- Krylov, I.B.; Budnikov, A.S.; Lopat’eva, E.R.; Nikishin, G.I.; Terent’ev, A.O. Mild Nitration of Pyrazolin-5-ones by a Combination of Fe(NO3)3 and NaNO2: Discovery of a New Readily Available Class of Fungicides, 4-Nitropyrazolin-5-ones. Chem. Eur. J. 2019, 25, 5922–5933. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, R.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K. Cold atmospheric plasma activated water as a prospective disinfectant: The crucial role of peroxynitrite. Green Chem. 2018, 20, 5276–5284. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Z.; Zhang, J.; Pu, J.; Lin, Y.; Xu, S.; Shen, L.; Chen, Q.; Shi, W. Fully Printed, Rapid-Response Sensors Based on Chemically Modified Graphene for Detecting NO2 at Room Temperature. ACS Appl. Mater. Interfaces 2014, 6, 7426–7433. [Google Scholar] [CrossRef]
- Tang, H.; Lau, T.; Brassard, B.; Cool, W. A new all-season passive sampling system for monitoring NO2 in air. Field Anal. Chem. Technol. 1999, 3, 338–345. [Google Scholar]
- Palmes, E.D.; Gunnison, A.F.; DiMattio, J.; Tomczyk, C. Personal sampler for nitrogen dioxide. Am. Ind. Hyg. Assoc. J. 1976, 37, 570–577. [Google Scholar]
- Colombo, M.; Nova, I.; Tronconi, E. Detailed kinetic modeling of the NH3–NO/NO2 SCR reactions over a commercial Cu-zeolite catalyst for Diesel exhausts after treatment. Catal. Today 2012, 197, 243–255. [Google Scholar]
- Zhang, W.-J.; Bagreev, A.; Rasouli, F. Reaction of NO2 with Activated Carbon at Ambient Temperature. Ind. Eng. Chem. Res. 2008, 47, 4358–4362. [Google Scholar]
- Lee, G.; Yoo, D.K.; Ahmed, I.; Lee, H.J.; Jhung, S.H. Metal-organic frameworks composed of nitro groups: Preparation and applications in adsorption and catalysis. Chem. Eng. J. 2023, 451, 138538. [Google Scholar]
- Baltrusaitis, J.; Jayaweera, P.M.; Grassian, V.H. XPS study of nitrogen dioxide adsorption on metal oxide particle surfaces under different environmental conditions. Phys. Chem. Chem. Phys. 2009, 11, 8295–8305. [Google Scholar] [PubMed]
- Haubrich, J.; Quiller, R.G.; Benz, L.; Liu, Z.; Friend, C.M. In Situ Ambient Pressure Studies of the Chemistry of NO2 and Water on Rutile TiO2(110). Langmuir 2010, 26, 2445–2451. [Google Scholar]
- Sivachandiran, L.; Thevenet, F.; Gravejat, P.; Rousseau, A. Investigation of NO and NO2 adsorption mechanisms on TiO2 at room temperature. Appl. Catal. B-Environ. 2013, 142–143, 196–204. [Google Scholar] [CrossRef]
- Dalton, J.S.; Janes, P.A.; Jones, N.G.; Nicholson, J.A.; Hallam, K.R.; Allen, G.C. Photocatalytic oxidation of NOx gases using TiO2: A surface spectroscopic approach. Environ. Pollut. 2002, 120, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Epling, W.S.; Yezerets, A.; Currier, N.W. The effects of regeneration conditions on NOX and NH3 release from NOX storage/reduction catalysts. Appl. Catal. B-Environ. 2007, 74, 117–129. [Google Scholar]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar]
- He, Q.; Zeng, Z.; Yin, Z.; Li, H.; Wu, S.; Huang, X.; Zhang, H. Fabrication of Flexible MoS2 Thin-Film Transistor Arrays for Practical Gas-Sensing Applications. Small 2012, 8, 2994–2999. [Google Scholar]
- Wehling, T.O.; Novoselov, K.S.; Morozov, S.V.; Vdovin, E.E.; Katsnelson, M.I.; Geim, A.K.; Lichtenstein, A.I. Molecular Doping of Graphene. Nano Lett. 2008, 8, 173–177. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Khan, A.F.; Brownson, D.A.C.; Randviir, E.P.; Smith, G.C.; Banks, C.E. 2D Hexagonal Boron Nitride (2D-hBN) Explored for the Electrochemical Sensing of Dopamine. Anal. Chem. 2016, 88, 9729–9737. [Google Scholar] [CrossRef]
- Abbas, A.N.; Liu, B.; Chen, L.; Ma, Y.; Cong, S.; Aroonyadet, N.; Köpf, M.; Nilges, T.; Zhou, C. Black Phosphorus Gas Sensors. ACS Nano 2015, 9, 5618–5624. [Google Scholar] [PubMed]
- Shukla, V.; Wärnå, J.; Jena, N.K.; Grigoriev, A.; Ahuja, R. Toward the Realization of 2D Borophene Based Gas Sensor. J. Phys. Chem. C 2017, 121, 26869–26876. [Google Scholar] [CrossRef]
- Cui, S.; Pu, H.; Wells, S.A.; Wen, Z.; Mao, S.; Chang, J.; Hersam, M.C.; Chen, J. Ultrahigh sensitivity and layer-dependent sensing performance of phosphorene-based gas sensors. Nat. Commun. 2015, 6, 8632. [Google Scholar] [PubMed]
- Ju, L.; Bie, M.; Tang, X.; Shang, J.; Kou, L. Janus WSSe Monolayer: An Excellent Photocatalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 29335–29343. [Google Scholar] [CrossRef]
- Ju, L.; Bie, M.; Zhang, X.; Chen, X.; Kou, L. Two-dimensional Janus van der Waals heterojunctions: A review of recent research progresses. Front. Phys. 2021, 16, 13201. [Google Scholar]
- Ju, L.; Qin, J.; Shi, L.; Yang, G.; Zhang, J.; Sun, L. Rolling the WSSe Bilayer into Double-Walled Nanotube for the Enhanced Photocatalytic Water-Splitting Performance. Nanomaterials 2021, 11, 705. [Google Scholar]
- Zhang, J.; Tang, X.; Chen, M.; Ma, D.; Ju, L. Tunable Photocatalytic Water Splitting Performance of Armchair MoSSe Nanotubes Realized by Polarization Engineering. Inorg. Chem. 2022, 61, 17353–17361. [Google Scholar] [CrossRef]
- Yue, Q.; Shao, Z.; Chang, S.; Li, J. Adsorption of gas molecules on monolayer MoS2 and effect of applied electric field. Nanoscale Res. Lett. 2013, 8, 425. [Google Scholar]
- Lin, Y.C.; Liu, C.; Yu, Y.; Zarkadoula, E.; Yoon, M.; Puretzky, A.A.; Liang, L.; Kong, X.; Gu, Y.; Strasser, A.; et al. Low Energy Implantation into Transition-Metal Dichalcogenide Monolayers to Form Janus Structures. ACS Nano 2020, 14, 3896–3906. [Google Scholar] [PubMed]
- Zheng, B.; Ma, C.; Li, D.; Lan, J.; Zhang, Z.; Sun, X.; Zheng, W.; Yang, T.; Zhu, C.; Ouyang, G.; et al. Band Alignment Engineering in Two-Dimensional Lateral Heterostructures. J. Am. Chem. Soc. 2018, 140, 11193–11197. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Jiang, J.; Gao, C.; Dai, F.; An, J.; Wen, Z.; Liu, Y. DFT study of Cu-modified and Cu-embedded WSe2 monolayers for cohesive adsorption of NO2, SO2, CO2, and H2S. Appl. Surf. Sci. 2022, 583, 152522. [Google Scholar]
- Ma, D.; Ju, W.; Li, T.; Zhang, X.; He, C.; Ma, B.; Lu, Z.; Yang, Z. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016, 383, 98–105. [Google Scholar]
- Chaurasiya, R.; Dixit, A.; Pandey, R. Strain-mediated stability and electronic properties of WS2, Janus WSSe and WSe2 monolayers. Superlattices Microstruct. 2018, 122, 268–279. [Google Scholar]
- Jin, C.; Tang, X.; Tan, X.; Smith, S.C.; Dai, Y.; Kou, L. A Janus MoSSe monolayer: A superior and strain-sensitive gas sensing material. J. Mater. Chem. A 2019, 7, 1099–1106. [Google Scholar]
- Ju, L.; Xu, T.; Zhang, Y.; Shi, C.; Sun, L. Ferromagnetism of Na0.5Bi0.5TiO3 (1 0 0) surface with O2 adsorption. Appl. Surf. Sci. 2017, 412, 77–84. [Google Scholar]
- Ju, L.; Dai, Y.; Wei, W.; Li, M.; Huang, B. DFT investigation on two-dimensional GeS/WS2 van der Waals heterostructure for direct Z-scheme photocatalytic overall water splitting. Appl. Surf. Sci. 2018, 434, 365–374. [Google Scholar] [CrossRef]
- Ju, L.; Liu, C.; Shi, L.; Sun, L. The high-speed channel made of metal for interfacial charge transfer in Z-scheme g–C3N4/MoS2 water-splitting photocatalyst. Mater. Res. Express 2019, 6, 115545. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Luo, X.; Liang, Q.; Wang, Y.; Xie, Q. First-Principles Calculations on Janus MoSSe/Graphene van der Waals Heterostructures: Implications for Electronic Devices. ACS Appl. Nano Mater. 2022, 5, 8371–8381. [Google Scholar]
- Lee, G.-D.; Wang, C.Z.; Yoon, E.; Hwang, N.-M.; Kim, D.-Y.; Ho, K.M. Diffusion, Coalescence, and Reconstruction of Vacancy Defects in Graphene Layers. Phys. Rev. Lett. 2005, 95, 205501. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Hahm, M.G.; Choi, M.; Yoon, J.; Kim, A.R.; Lee, Y.-J.; Park, S.-G.; Kwon, J.-D.; Kim, C.S.; Song, M.; et al. Charge-transfer-based Gas Sensing Using Atomic-layer MoS2. Sci. Rep. 2015, 5, 8052. [Google Scholar] [PubMed]
- Kou, L.; Frauenheim, T.; Chen, C. Phosphorene as a Superior Gas Sensor: Selective Adsorption and Distinct I–V Response. J. Phys. Chem. Lett. 2014, 5, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.; Parker, S.C.; Watson, G.W. Reduction of NO2 on Ceria Surfaces. J. Phys. Chem. B 2006, 110, 2256–2262. [Google Scholar] [CrossRef]
- Zhao, S.; Tang, X.; Li, J.; Zhang, J.; Yuan, D.; Ma, D.; Ju, L. Improving the Energetic Stability and Electrocatalytic Performance of Au/WSSe Single-Atom Catalyst with Tensile Strain. Nanomaterials 2022, 12, 2793. [Google Scholar]
- Hu, H.; Zhang, Z.; Ouyang, G. Transition from Schottky-to-Ohmic contacts in 1T VSe2-based van der Waals heterojunctions: Stacking and strain effects. Appl. Surf. Sci. 2020, 517, 146168. [Google Scholar] [CrossRef]
- Chen, D.; Lei, X.; Wang, Y.; Zhong, S.; Liu, G.; Xu, B.; Ouyang, C. Tunable electronic structures in BP/MoSSe van der Waals heterostructures by external electric field and strain. Appl. Surf. Sci. 2019, 497, 143809. [Google Scholar] [CrossRef]
- Deng, S.; Li, L.; Rees, P. Graphene/MoXY Heterostructures Adjusted by Interlayer Distance, External Electric Field, and Strain for Tunable Devices. ACS Appl. Nano Mater. 2019, 2, 3977–3988. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar]
- Hohenberg, P.; Kohn, W. Density functional theory (DFT). Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Lunsford, J.H. EPR spectra of radicals formed when NO2 is adsorbed on magnesium oxide. J. Colloid Interf. Sci. 1968, 26, 355–360. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Hassan, A.; Ismail, M.; Reshak, A.H.; Zada, Z.; Khan, A.A.; Rehman, M.F.U.; Arif, M.; Siraj, K.; Zada, S.; Murtaza, G.; et al. Effect of heteroatoms on structural, electronic and spectroscopic properties of polyfuran, polythiophene and polypyrrole: A hybrid DFT approach. J. Mol. Struct. 2023, 1274, 134484. [Google Scholar]
- Yu, H.; Huang, H.; Reshak, A.H.; Auluck, S.; Liu, L.; Ma, T.; Zhang, Y. Coupling ferroelectric polarization and anisotropic charge migration for enhanced CO2 photoreduction. Appl. Catal. B-Environ. 2021, 284, 119709. [Google Scholar] [CrossRef]
- Ullah, R.; Reshak, A.H.; Ali, M.A.; Khan, A.; Murtaza, G.; Al-Anazy, M.; Althib, H.; Flemban, T.H. Pressure-dependent elasto-mechanical stability and thermoelectric properties of MYbF3 (M = Rb, Cs) materials for renewable energy. Int. J. Energy Res. 2021, 45, 8711–8723. [Google Scholar]
- Li, D.-H.; Li, Q.-M.; Qi, S.-L.; Qin, H.-C.; Liang, X.-Q.; Li, L. Theoretical Study of Hydrogen Production from Ammonia Borane Catalyzed by Metal and Non-Metal Diatom-Doped Cobalt Phosphide. Molecules 2022, 27, 8206. [Google Scholar]
- Liu, X.; Xu, Y.; Sheng, L. Al-Decorated C2N Monolayer as a Potential Catalyst for NO Reduction with CO Molecules: A DFT Investigation. Molecules 2022, 27, 5790. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Chen, Y.-B.; Zhou, K.-G.; Liu, C.-H.; Zeng, J.; Zhang, H.-L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef]


| Vacancy | Synthetic Environment | |
|---|---|---|
| S-Rich | Se-Rich | |
| Se | −0.25 eV | 2.78 eV |
| S | 3.35 eV | 0.32 eV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, L.; Tang, X.; Li, X.; Liu, B.; Qiao, X.; Wang, Z.; Yin, H. NO2 Physical-to-Chemical Adsorption Transition on Janus WSSe Monolayers Realized by Defect Introduction. Molecules 2023, 28, 1644. https://doi.org/10.3390/molecules28041644
Ju L, Tang X, Li X, Liu B, Qiao X, Wang Z, Yin H. NO2 Physical-to-Chemical Adsorption Transition on Janus WSSe Monolayers Realized by Defect Introduction. Molecules. 2023; 28(4):1644. https://doi.org/10.3390/molecules28041644
Chicago/Turabian StyleJu, Lin, Xiao Tang, Xiaoxi Li, Bodian Liu, Xiaoya Qiao, Zhi Wang, and Huabing Yin. 2023. "NO2 Physical-to-Chemical Adsorption Transition on Janus WSSe Monolayers Realized by Defect Introduction" Molecules 28, no. 4: 1644. https://doi.org/10.3390/molecules28041644
APA StyleJu, L., Tang, X., Li, X., Liu, B., Qiao, X., Wang, Z., & Yin, H. (2023). NO2 Physical-to-Chemical Adsorption Transition on Janus WSSe Monolayers Realized by Defect Introduction. Molecules, 28(4), 1644. https://doi.org/10.3390/molecules28041644








