Jordanian Kaolinite with TiO2 for Improving Solar Light Harvesting Used in Dye Removal
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of TiO2-JK Nanocomposites
2.2. Photocatalytic Degradation Efficiency
2.3. Reusability of TiO2-JK Photocatalyst
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis and Characterization of TiO2-JK NC
3.3. Photodegradation of CR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Izati, S.; Mashuri, S.; Ibrahim, M.L.; Kasim, M.F.; Mastuli, M.S.; Rashid, U.; Abdullah, A.H.; Islam, A.; Mijan, N.A.; Tan, Y.H.; et al. Photocatalysis for Organic Wastewater Treatment: From the Basis to Current Challenges for Society. Catalysts 2020, 10, 1260. [Google Scholar]
- Rai, A.; Sirotiya, V.; Mourya, M.; Khan, M.J.; Ahirwar, A.; Sharma, A.K.; Kawatra, R.; Marchand, J.; Schoefs, B.; Varjani, S.; et al. Sustainable treatment of dye wastewater by recycling microalgal and diatom biogenic materials: Biorefinery perspectives. Chemosphere 2022, 305, 135371. [Google Scholar] [CrossRef]
- Zou, J.P.; Chen, Y.; Zhu, M.; Wang, D.; Luo, X.B.; Luo, S.L. Semiconductor-Based Nanocomposites for Photodegradation of Organic Pollutants; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128148389. [Google Scholar]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Raizada, P.; Kumar Thakur, V.; Saini, A.K.; Mittal, D.; Nguyen, V.H.; Ahamad, T.; Chien Nguyen, C.; Young Kim, S.; et al. Synergistic photocatalytic dye mitigation and bacterial disinfection using carbon quantum dots decorated dual Z-scheme Manganese Indium Sulfide/Cuprous Oxide/Silver oxide heterojunction. Mater. Lett. 2022, 313, 131716. [Google Scholar] [CrossRef]
- Al Jabri, H.; Feroz, S. Studies on the Effect of TiO2 Nano Photocatalysis in the Pretreatment of Seawater Reverse Osmosis Desalination. Int. J. Environ. Sci. Dev. 2015, 6, 543–546. [Google Scholar] [CrossRef]
- Li, X.; Simon, U.; Bekheet, M.F.; Gurlo, A. Mechanisms and Environmental Applications. Energies 2022, 15, 5607. [Google Scholar]
- Bel Hadjltaief, H.; Galvez, M.E.; Ben Zina, M.; Da Costa, P. TiO2/clay as a heterogeneous catalyst in photocatalytic/photochemical oxidation of anionic reactive blue 19. Arab. J. Chem. 2019, 12, 1454–1462. [Google Scholar] [CrossRef]
- Annan, E.; Agyei-Tuffour, B.; Bensah, Y.D.; Konadu, D.S.; Yaya, A.; Onwona-Agyeman, B.; Nyankson, E. Application of clay ceramics and nanotechnology in water treatment: A review. Cogent Eng. 2018, 5, 1476017. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Abdalla, M.A.; Ahamad, T.; Abdullah ALOthman, Z.; Alshehri, S.M.; Ghfar, A.A. Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: A study of adsorption parameters and interaction mechanism. J. Clean. Prod. 2017, 156, 426–436. [Google Scholar] [CrossRef]
- Awwad, A.M.; Amer, M.W.; Al-aqarbeh, M.M. TiO2-kaolinite nanocomposite prepared from the Jordanian Kaolin clay: Adsorption and thermodynamics of Pb(II) and Cd(II) ions in aqueous solution. Chem. Int. 2020, 6, 168–178. [Google Scholar] [CrossRef]
- Djellabi, R.; Fouzi Ghorab, M.; Smara, A.; Bianchi, C.L.; Cerrato, G.; Zhao, X.; Yang, B. Titania-Montmorillonite for the Photocatalytic Removal of Contaminants from Water: Adsorb & Shuttle Process. In Green Materials for Wastewater Treatment; Springer: Cham, Switzerland, 2020; pp. 291–319. [Google Scholar] [CrossRef]
- Ediputra, K.; Aziz, H. Photoreactor Design by Clay Pottery Modification with TiO2 Coating in Peat Water Purification. KnE Eng. 2019, 1, 171. [Google Scholar] [CrossRef]
- Khoury, H.N. Geochemistry of Surficial Uranium Deposits from Central Jordan. Jordan J. Earth Environ. Sci. 2014, 6, 11–22. [Google Scholar]
- Al-Momani, T.M.; Khoury, H.N. Processing and treatment of Hiswa clay deposits, South Jordan using hydrocyclone separation and high iIntensity wet magnetic separation. Dirasat Pure Sci. 2010, 37, 36–47. [Google Scholar]
- Khoury, H.N. Review of clays and clay minerals in Jordan. Arab. J. Geosci. 2019, 12, 706. [Google Scholar] [CrossRef]
- Kibanova, D.; Cervini-Silva, J.; Destaillats, H. Efficiency of clay-TiO2 nanocomposites on the photocatalytic elimination of a model hydrophobic air pollutant. Environ. Sci. Technol. 2009, 43, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Gougazeh, M.; Buhl, J.C. Synthesis and characterization of zeolite A by hydrothermal transformation of natural Jordanian kaolin. J. Assoc. Arab Univ. Basic Appl. Sci. 2014, 15, 35–42. [Google Scholar] [CrossRef]
- Hussain, M.; Ceccarelli, R.; Marchisio, D.L.; Fino, D.; Russo, N.; Geobaldo, F. Synthesis, characterization, and photocatalytic application of novel TiO2 nanoparticles. Chem. Eng. J. 2010, 157, 45–51. [Google Scholar] [CrossRef]
- Abdennouri, M.; Baâlala, M.; Galadi, A.; El Makhfouk, M.; Bensitel, M.; Nohair, K.; Sadiq, M.; Boussaoud, A.; Barka, N. Photocatalytic degradation of pesticides by titanium dioxide and titanium pillared purified clays. Arab. J. Chem. 2016, 9, S313–S318. [Google Scholar] [CrossRef]
- Velardi, L.; Scrimieri, L.; Serra, A.; Manno, D.; Calcagnile, L. Effect of temperature on the physical, optical and photocatalytic properties of TiO2 nanoparticles. SN Appl. Sci. 2020, 2, 707. [Google Scholar] [CrossRef]
- Liu, J.; Dong, M.; Zuo, S.; Yu, Y. Solvothermal preparation of TiO2/montmorillonite and photocatalytic activity. Appl. Clay Sci. 2009, 43, 156–159. [Google Scholar] [CrossRef]
- Moosavi, S.; Li, R.Y.M.; Lai, C.W.; Yusof, Y.; Gan, S.; Akbarzadeh, O.; Chowhury, Z.Z.; Yue, X.G.; Johan, M.R. Methylene blue dye photocatalytic degradation over synthesised Fe3O4/AC/TiO2 nano-catalyst: Degradation and reusability studies. Nanomaterials 2020, 10, 2360. [Google Scholar] [CrossRef]
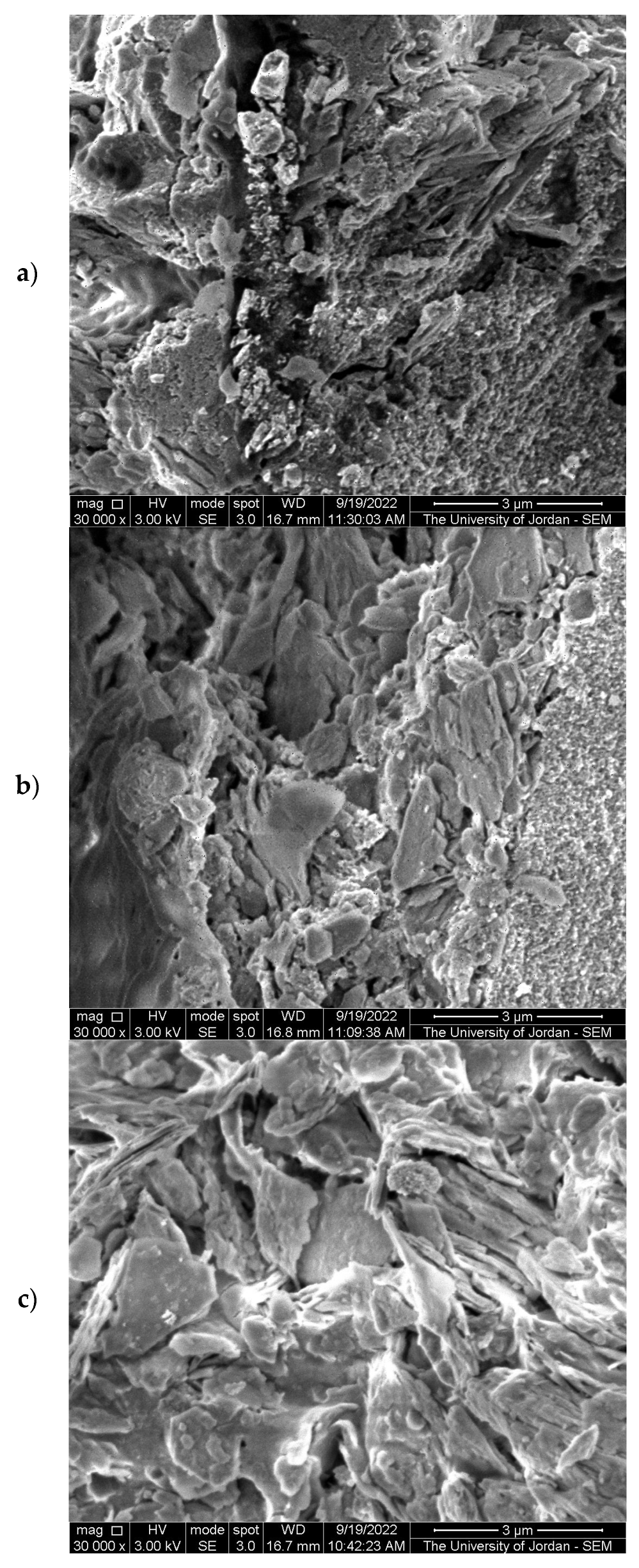
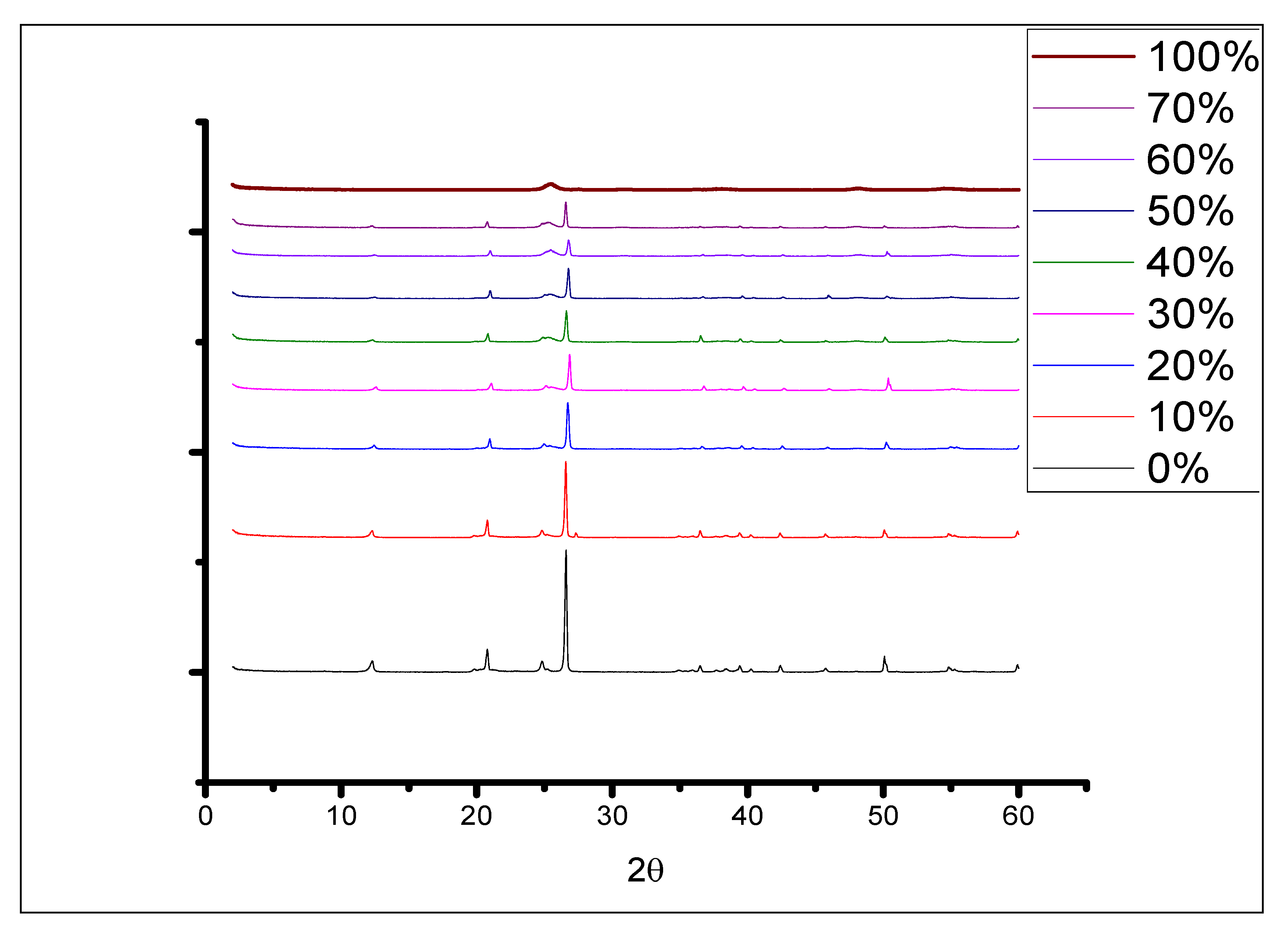
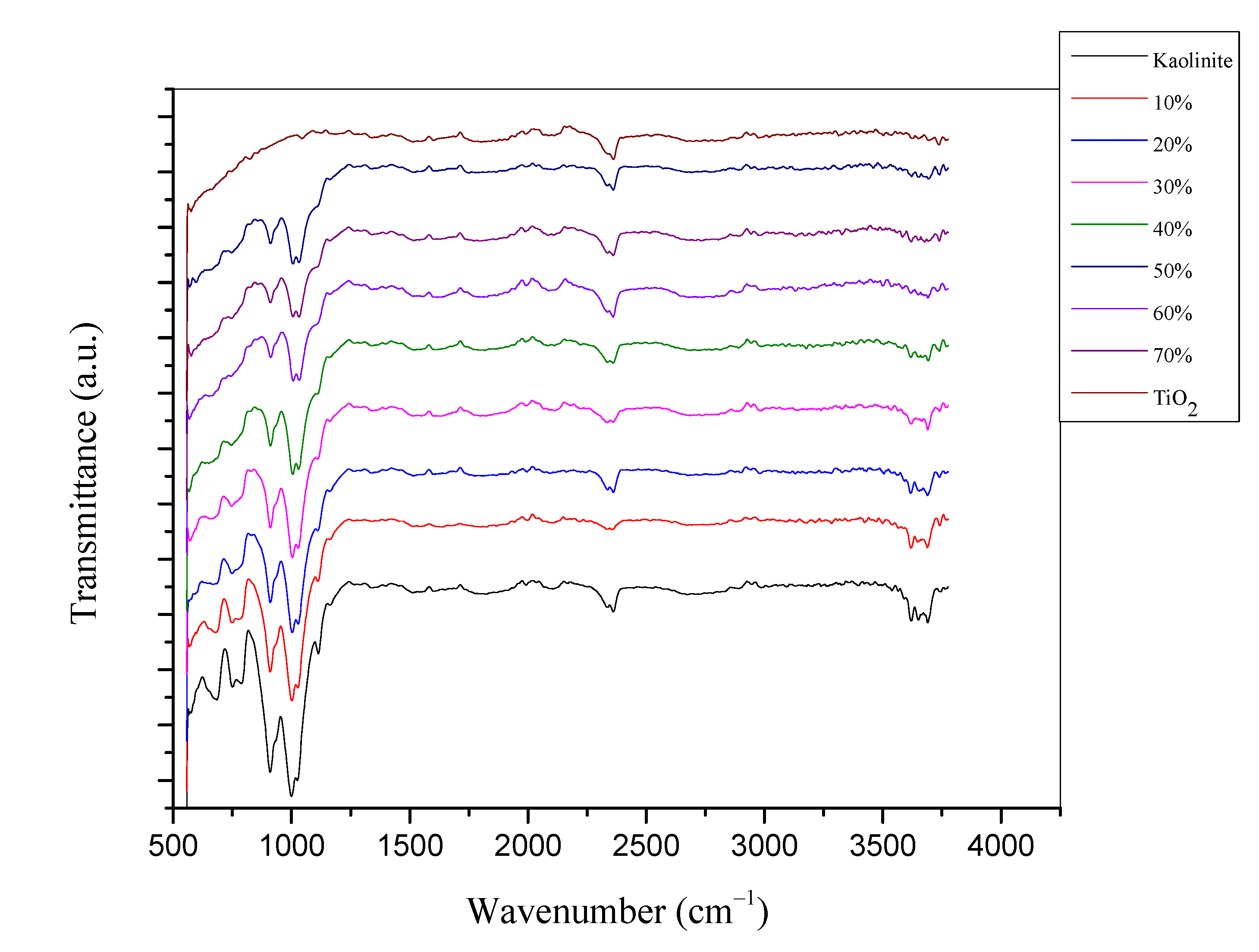
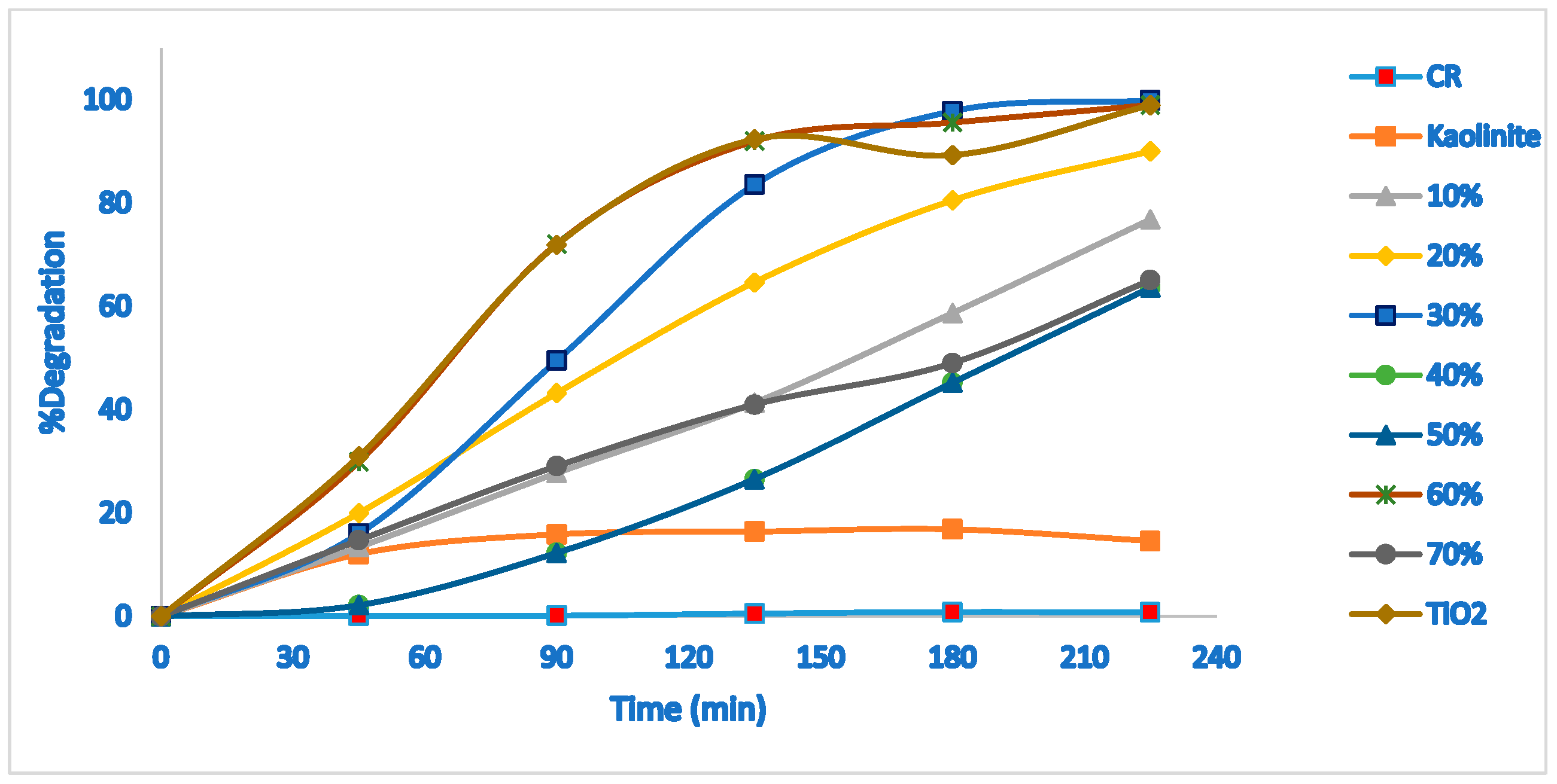
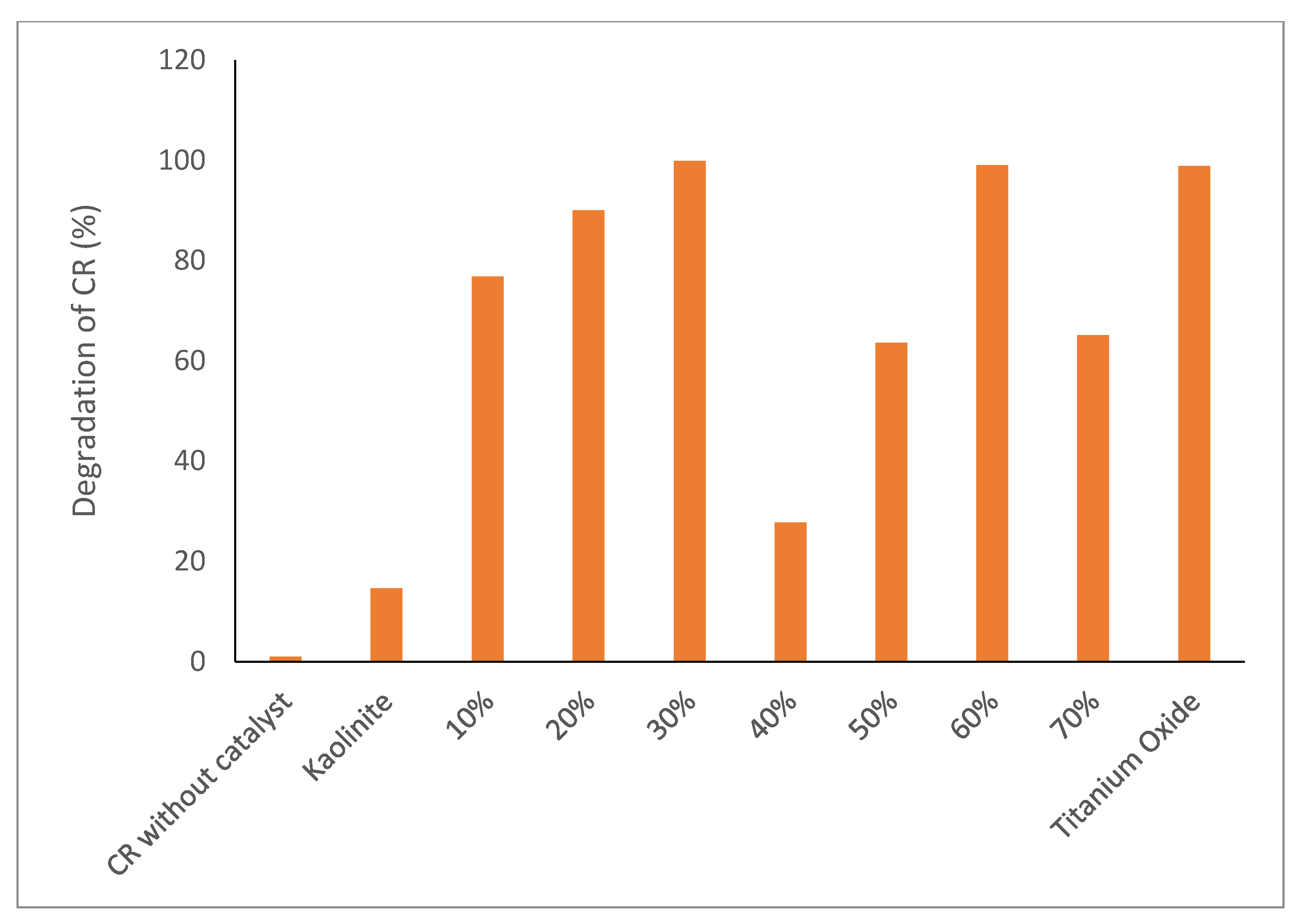


| %TiO2 in TiO2-JK NC | SiO2 | Al2O3 | TiO2 | Fe |
|---|---|---|---|---|
| 20 | 64.6 | 13.8 | 21.0 | 0.6 |
| 30 | 59.6 | 12.6 | 27.1 | 0.7 |
| 60 | 29.3 | 7.5 | 62.9 | 0.2 |
| 100 | 1.2 | 0.8 | 97.9 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhabbas, M.; Odeh, F.; Alzughoul, K.; Afaneh, R.; Alahmad, W. Jordanian Kaolinite with TiO2 for Improving Solar Light Harvesting Used in Dye Removal. Molecules 2023, 28, 989. https://doi.org/10.3390/molecules28030989
Alkhabbas M, Odeh F, Alzughoul K, Afaneh R, Alahmad W. Jordanian Kaolinite with TiO2 for Improving Solar Light Harvesting Used in Dye Removal. Molecules. 2023; 28(3):989. https://doi.org/10.3390/molecules28030989
Chicago/Turabian StyleAlkhabbas, Manal, Fadwa Odeh, Khitam Alzughoul, Razan Afaneh, and Waed Alahmad. 2023. "Jordanian Kaolinite with TiO2 for Improving Solar Light Harvesting Used in Dye Removal" Molecules 28, no. 3: 989. https://doi.org/10.3390/molecules28030989
APA StyleAlkhabbas, M., Odeh, F., Alzughoul, K., Afaneh, R., & Alahmad, W. (2023). Jordanian Kaolinite with TiO2 for Improving Solar Light Harvesting Used in Dye Removal. Molecules, 28(3), 989. https://doi.org/10.3390/molecules28030989






