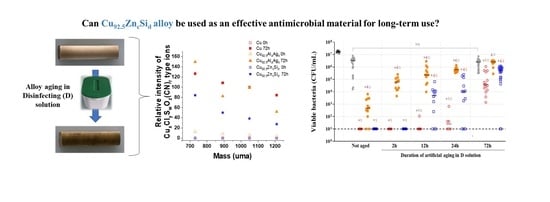
Effects of Ageing in Disinfectant Solution on the Corrosion Resistance and Antimicrobial Behavior of Copper Alloys
Abstract
1. Introduction
2. Results and Discussions
2.1. Corrosion Tests
2.1.1. Open Circuit Potential Measurements
2.1.2. Electrochemical Impedance
2.2. XPS Study
2.2.1. Elementary and Quantitative Analysis
2.2.2. Chemical Forms of Detected Elements
- Carbon: Peak C 1s
- Oxygen: Peak O1s
- Copper: Cu 2p3/2 and Cu LMM peaks
- Nitrogen: Peak N1s
- Chlorine: Peak Cl2p
- Sulfur: Peak S2p
2.3. ToF-SIMS
2.3.1. ToF-SIMS Analysis of D Solution Deposited on an Aluminum Substrate
2.3.2. ToF-SIMS Analysis of Copper Based Materials before and after Immersion in D Solution
ToF-SIMS Analysis of Polished Copper (Copper 0 h) Sample
ToF-SIMS Analysis of Polished Former Alloy 0 h
ToF-SIMS Analysis of Polished New Alloy 0 h
2.3.3. Surface Composition of Copper, Former Alloy and New Alloy after 72 h Immersion in D Solution
2.4. Antimicrobial Performance of the Copper Surfaces
2.5. Correlation between EIS, XPS, ToF-SIMS Results and Antibacterial Activity
3. Materials and Methods
3.1. Chosen Copper Alloys and Material Preparation for Corrosion Tests
3.2. Electrochemical System/Corrosion Tests
3.3. Surface Analysis
3.3.1. X-ray Photoelectron Spectroscopy (XPS) Test
3.3.2. Time of Flight Secondary Ion Mass Spectroscopy (ToF-SIMS)
3.4. Antibacterial Tests
3.4.1. Metal Preparation for Antibacterial Tests
3.4.2. Artificial Aging of Metal Samples
3.4.3. Preparation of MRSA
3.4.4. Antibacterial Assays
3.4.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- European Centre for Disease Prevention and Control. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals: 2011–2012.; The Publications Office of the European Union: Luxembourg, Luxembourg, 2013. [Google Scholar]
- Savey, A.; Machut, A.; Barreto, C. Enquête Nationale de Prévalence Des Infections Associées Aux Soins et Des Traitements Antibiotiques En Établissements d’hébergement Pour Personnes Âgées Dépendantes; Résultats Nationaux 2016. (National Prevalence Survey of Healthcare-Associated Infections and Antibiotic Use in Nursing Homes; 2016 national outcomes). St. Publique Fr. 2017, 67. (In French) [Google Scholar]
- Colin, M.; Klingelschmitt, F.; Charpentier, E.; Josse, J.; Kanagaratnam, L.; De Champs, C.; Gangloff, S. Copper Alloy Touch Surfaces in Healthcare Facilities: An Effective Solution to Prevent Bacterial Spreading. Materials 2018, 11, 2479. [Google Scholar] [CrossRef] [PubMed]
- Colin, M.; Carré, G.; Klingelschmitt, F.; Reffuveille, F.; Gangloff, S.C. Copper alloys to prevent bacterial biofilm formation on touch surfaces. Mater. Lett. 2021, 305, 130712. [Google Scholar] [CrossRef]
- Schmidt, M.G.; Attaway, H.H.; Sharpe, P.A.; John, J.; Sepkowitz, K.A.; Morgan, A.; Fairey, S.E.; Singh, S.; Steed, L.L.; Cantey, J.R.; et al. Sustained Reduction of Microbial Burden on Common Hospital Surfaces through Introduction of Copper. J. Clin. Microbiol. 2012, 50, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Inkinen, J.; Mäkinen, R.; Keinänen-Toivola, M.M.; Nordström, K.; Ahonen, M. Copper as an antibacterial material in different facilities. Lett. Appl. Microbiol. 2017, 64, 19–26. [Google Scholar] [CrossRef]
- Karpanen, T.J.; Casey, A.L.; Lambert, P.A.; Cookson, B.D.; Nightingale, P.; Miruszenko, L.; Elliott, T.S.J. The Antimicrobial Efficacy of Copper Alloy Furnishing in the Clinical Environment: A Crossover Study. Infect. Control Hosp. Epidemiol. 2012, 33, 3–9. [Google Scholar] [CrossRef]
- Neelly, A.N.; Maley, M.P. Survival of Enterococci and Staphylococci on Hospital Fabrics and Plastic. AORN J. 2001, 73, 515. [Google Scholar] [CrossRef]
- Neely, A.N. A Survey of Gram-Negative Bacteria Survival on Hospital Fabrics and Plastics. J. Burn Care Rehabil. 2000, 21, 523–527. [Google Scholar] [CrossRef]
- Abad, X.F.; Pinto, R.F.; Bosch, A. Survival of enteric viruses on environmental fomites. Appl. Environ. Microbiol. Novemb. 1994, 60, 3704–3710. [Google Scholar] [CrossRef]
- Clay, S.; Maherchandani, S.; Malik, Y.S.; Goyal, S.M. Survival on uncommon fomites of feline calicivirus, a surrogate of noroviruses. Am. J. Infect. Control 2006, 34, 41–43. [Google Scholar] [CrossRef]
- Otter, J.A.; Yezli, S.; Salkeld, J.A.G.; French, G.L. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control 2013, 41, S6–S11. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Anderson, D.; Rutala, W.A. The role of the surface environment in healthcare-associated infections. Curr. Opin. Infect. Dis. 2013, 26, 338–344. [Google Scholar] [CrossRef]
- Rampling, A.; Wiseman, S.; Davis, L.; Hyett, A.P.; Walbridge, A.N.; Payne, G.C.; Cornaby, A.J. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 2001, 49, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Rutala, W.A. Self-disinfecting surfaces: Review of current methodologies and future prospects. Am. J. Infect. Control 2013, 41, S31–S35. [Google Scholar] [CrossRef] [PubMed]
- Augustin, A.; Huilgol, P.; Udupa, K.R.; Bhat K, U. Effect of current density during electrodeposition on microstructure and hardness of textured Cu coating in the application of antimicrobial Al touch surface. J. Mech. Behav. Biomed. Mater. 2016, 63, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Xi, T.; Shahzad, M.B.; Xu, D.; Sun, Z.; Zhao, J.; Yang, C.; Qi, M.; Yang, K. Effect of copper addition on mechanical properties, corrosion resistance and antibacterial property of 316L stainless steel. Mater. Sci. Eng. C 2017, 71, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Kang, E.-T.; Neoh, K.G. Antimicrobial Copper-Based Materials and Coatings: Potential Multifaceted Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 21159–21182. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial applications of copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012, 235–236, 265–270. [Google Scholar] [CrossRef]
- Mehtar, S.; Wiid, I.; Todorov, S.D. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: An in-vitro study. J. Hosp. Infect. 2008, 68, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Chyderiotis, S.; Legeay, C.; Verjat-Trannoy, D.; Le Gallou, F.; Astagneau, P.; Lepelletier, D. New insights on antimicrobial efficacy of copper surfaces in the healthcare environment: A systematic review. Clin. Microbiol. Infect. 2018, 24, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, D.; Krans, T.; Santo, C.E.; Elowsky, C.G.; Domaille, D.W.; Chang, C.J.; Grass, G. Mechanisms of Contact-Mediated Killing of Yeast Cells on Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2011, 77, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials. mBio 2015, 6, e01697-15. [Google Scholar] [CrossRef] [PubMed]
- Colin, M.; Charpentier, E.; Klingelschmitt, F.; Bontemps, C.; De Champs, C.; Reffuveille, F.; Gangloff, S.C. Specific antibacterial activity of copper alloy touch surfaces in five long-term care facilities for older adults. J. Hosp. Infect. 2020, 104, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Souli, M.; Antoniadou, A.; Katsarolis, I.; Mavrou, I.; Paramythiotou, E.; Papadomichelakis, E.; Drogari-Apiranthitou, M.; Panagea, T.; Giamarellou, H.; Petrikkos, G.; et al. Reduction of Environmental Contamination with Multidrug-Resistant Bacteria by Copper-Alloy Coating of Surfaces in a Highly Endemic Setting. Infect. Control Hosp. Epidemiol. 2017, 38, 765–771. [Google Scholar] [CrossRef]
- Casey, A.L.; Adams, D.; Karpanen, T.J.; Lambert, P.A.; Cookson, B.D.; Nightingale, P.; Miruszenko, L.; Shillam, R.; Christian, P.; Elliott, T.S.J. Role of copper in reducing hospital environment contamination. J. Hosp. Infect. 2010, 74, 72–77. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial Killing by Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef]
- Róza´nska, A.; Chmielarczyk, A.; Romaniszyn, D.; Sroka-Oleksiak, A.; Bulanda, M.; Walkowicz, M.; Osuch, P.; Knych, T. Antimicrobial Properties of Selected Copper Alloys on Staphylococcus aureus and Escherichia coli in Different Simulations of Environmental Conditions: With vs. without Organic Contamination. Int. J. Environ. Res. Public Health 2017, 14, 813. [Google Scholar] [CrossRef]
- Cornette, P.; Zanna, S.; Seyeux, A.; Costa, D.; Marcus, P. The native oxide film on a model aluminium-copper alloy studied by XPS and ToF-SIMS. Corros. Sci. 2020, 174, 108837. [Google Scholar] [CrossRef]
- Li, S.; Teague, M.T.; Doll, G.L.; Schindelholz, E.J.; Cong, H. Interfacial corrosion of copper in concentrated chloride solution and the formation of copper hydroxychloride. Corros. Sci. 2018, 141, 243–254. [Google Scholar] [CrossRef]
- Hutchison, M.J.; Zhou, P.; Ogle, K.; Scully, J.R. Enhanced Electrochemical Cu Release from Commercial Cu-Sn Alloys: Fate of the Alloying Elements in Artificial Perspiration. Electrochim. Acta 2017, 241, 73–88. [Google Scholar] [CrossRef]
- Ter-Ovanessian, B.; Alemany-Dumont, C.; Normand, B. Single frequency electrochemical impedance investigation of zero charge potential for different surface states of Cu–Ni alloys. J. Appl. Electrochem. 2014, 44, 399–410. [Google Scholar] [CrossRef]
- Moulder, J.; Stickle, W.; Sobol, P.; Bomben, K. Handbook of X-ray Photoelectron Spectroscopy; Pekin-Elmer Corporation: Eden Prairie, MN, USA, 1992; p. 260. [Google Scholar]
- Available online: http://www.lasurface.com/database/liaisonxps.php (accessed on 29 November 2022).
- Aniosurf Premium. Available online: https://procomed.ch/wp-content/uploads/2017/08/ANIOSURF-PREMIUM-FT-1916-FR.pdf (accessed on 29 November 2022).
- Finšgar, M. Surface analysis by gas cluster ion beam XPS and ToF-SIMS tandem MS of 2-mercaptobenzoxazole corrosion inhibitor for brass. Corros. Sci. 2021, 182, 109269. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Carrière, C.; Seyeux, A.; Marcus, P.; Singh, A. Electrochemical, ToF-SIMS and computational studies of 4-amino-5-methyl-4H-1,2,4-triazole-3-thiol as a novel corrosion inhibitor for copper in 3.5% NaCl. J. Mol. Liq. 2019, 289, 111113. [Google Scholar] [CrossRef]
- Barbouth, N.; Pagetti, J.; Oudar, J. Influence de l’adsorption chimique du soufre sur le comportement electrochimique du cuivre dans une solution d’acide nitrique. Electrochim. Acta 1972, 17, 2105–2115. [Google Scholar] [CrossRef]
- Dilimon, V.S.; Denayer, J.; Delhalle, J.; Mekhalif, Z. Electrochemical and Spectroscopic Study of the Self-Assembling Mechanism of Normal and Chelating Alkanethiols on Copper. Langmuir 2012, 28, 6857–6865. [Google Scholar] [CrossRef]






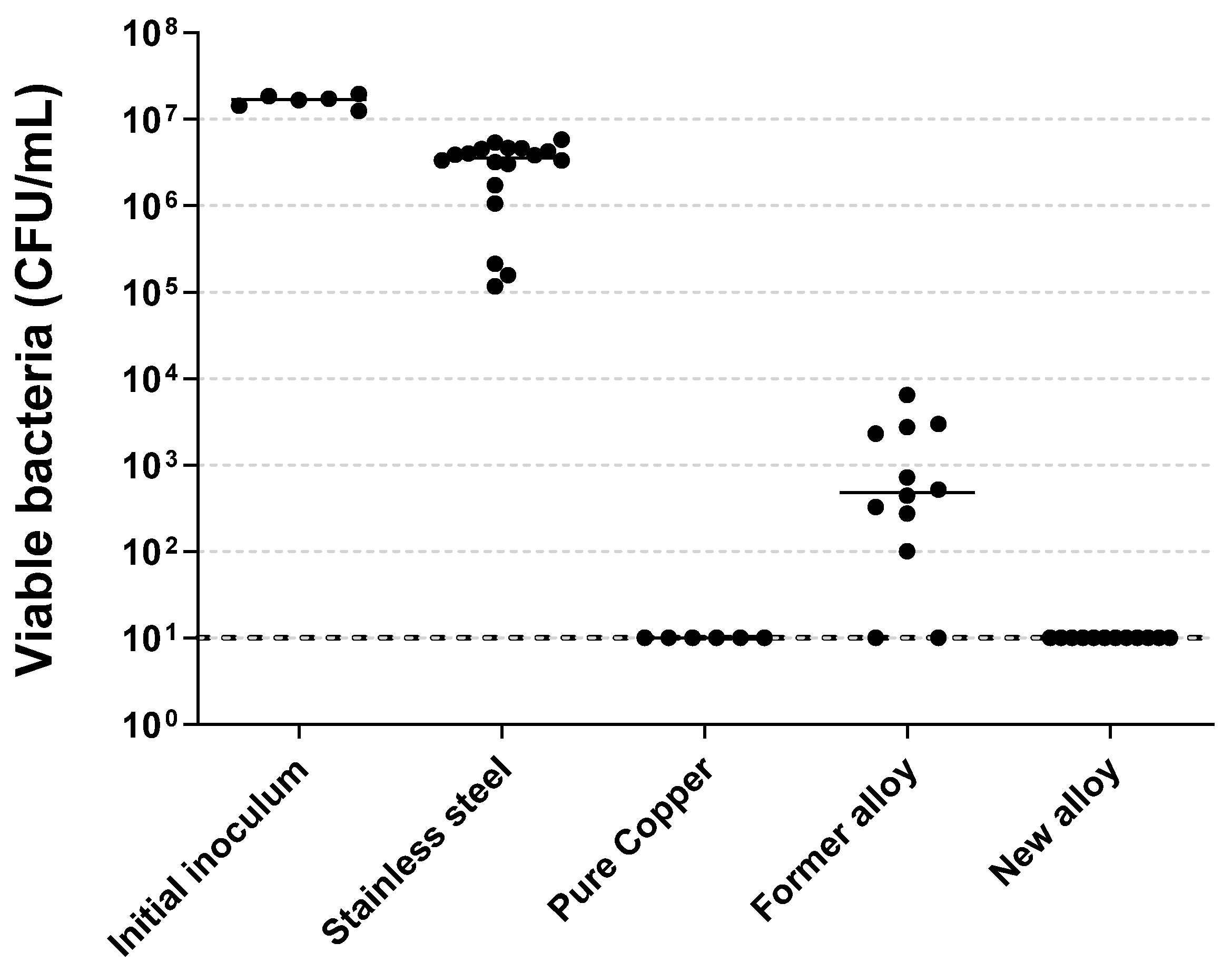
 Stainless steel.
Stainless steel.  Pure copper.
Pure copper.  Former alloy.
Former alloy.  New alloy. Black lines represent medians. Symbols indicate significant differences compared to: * Not aged stainless steel; £ Pure copper for same aging; $ Former alloy for same aging; ¤ New alloy for same aging. NS: Not significant.
New alloy. Black lines represent medians. Symbols indicate significant differences compared to: * Not aged stainless steel; £ Pure copper for same aging; $ Former alloy for same aging; ¤ New alloy for same aging. NS: Not significant.
 Stainless steel.
Stainless steel.  Pure copper.
Pure copper.  Former alloy.
Former alloy.  New alloy. Black lines represent medians. Symbols indicate significant differences compared to: * Not aged stainless steel; £ Pure copper for same aging; $ Former alloy for same aging; ¤ New alloy for same aging. NS: Not significant.
New alloy. Black lines represent medians. Symbols indicate significant differences compared to: * Not aged stainless steel; £ Pure copper for same aging; $ Former alloy for same aging; ¤ New alloy for same aging. NS: Not significant.
| Samples Name | Qf | Rf [kΩcm2] | Qdl | Rt [kΩcm2] | 10−4χ2 | ||
|---|---|---|---|---|---|---|---|
| 10−6Y01 [S secn1/cm2] | nf | 10−6Y02 [S secn2/cm2] | ndl | ||||
| Copper 12 h | 16.6 | 0.6 | 10.6 | 43.6 | 0.5 | 61.3 | 11.9 |
| Copper 24 h | 10.4 | 0.6 | 2.7 | 66 | 0.4 | 55 | 7.8 |
| Copper 72 h | 0.5 | 0.8 | 2.2 | 30 | 0.4 | 108 | 2 |
| Former alloy 12 h | 12.9 | 0.5 | 1.50 | 37.5 | 0.6 | 122 | 8.1 |
| Former alloy 24 h | 2.1 | 0.7 | 2.1 | 37.9 | 0.5 | 130.2 | 2.9 |
| Former alloy 72 h | 0.5 | 0.8 | 5 | 27.1 | 0.5 | 187 | 1.4 |
| New alloy 12 h | 3.2 | 0.7 | 4.8 | 34.5 | 0.5 | 83 | 8.7 |
| New alloy 24 h | 1.13 | 0.8 | 5.1 | 38.1 | 0.4 | 99.8 | 5.9 |
| New alloy 72 h | 0.26 | 0.9 | 3.3 | 49.9 | 0.4 | 75.7 | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazar, F.S.; Colin, M.; Carré, G.; Bachelard, N.; Chopart, J.-P.; Gangloff, S.C. Effects of Ageing in Disinfectant Solution on the Corrosion Resistance and Antimicrobial Behavior of Copper Alloys. Molecules 2023, 28, 981. https://doi.org/10.3390/molecules28030981
Lazar FS, Colin M, Carré G, Bachelard N, Chopart J-P, Gangloff SC. Effects of Ageing in Disinfectant Solution on the Corrosion Resistance and Antimicrobial Behavior of Copper Alloys. Molecules. 2023; 28(3):981. https://doi.org/10.3390/molecules28030981
Chicago/Turabian StyleLazar, Florica Simescu, Marius Colin, Gaëlle Carré, Nicolas Bachelard, Jean-Paul Chopart, and Sophie C. Gangloff. 2023. "Effects of Ageing in Disinfectant Solution on the Corrosion Resistance and Antimicrobial Behavior of Copper Alloys" Molecules 28, no. 3: 981. https://doi.org/10.3390/molecules28030981
APA StyleLazar, F. S., Colin, M., Carré, G., Bachelard, N., Chopart, J.-P., & Gangloff, S. C. (2023). Effects of Ageing in Disinfectant Solution on the Corrosion Resistance and Antimicrobial Behavior of Copper Alloys. Molecules, 28(3), 981. https://doi.org/10.3390/molecules28030981