Molecular Recognition of Imidazole-Based Drug Molecules by Cobalt(III)- and Zinc(II)-Coproporphyrins in Aqueous Media
Abstract
1. Introduction
2. Results and Discussion
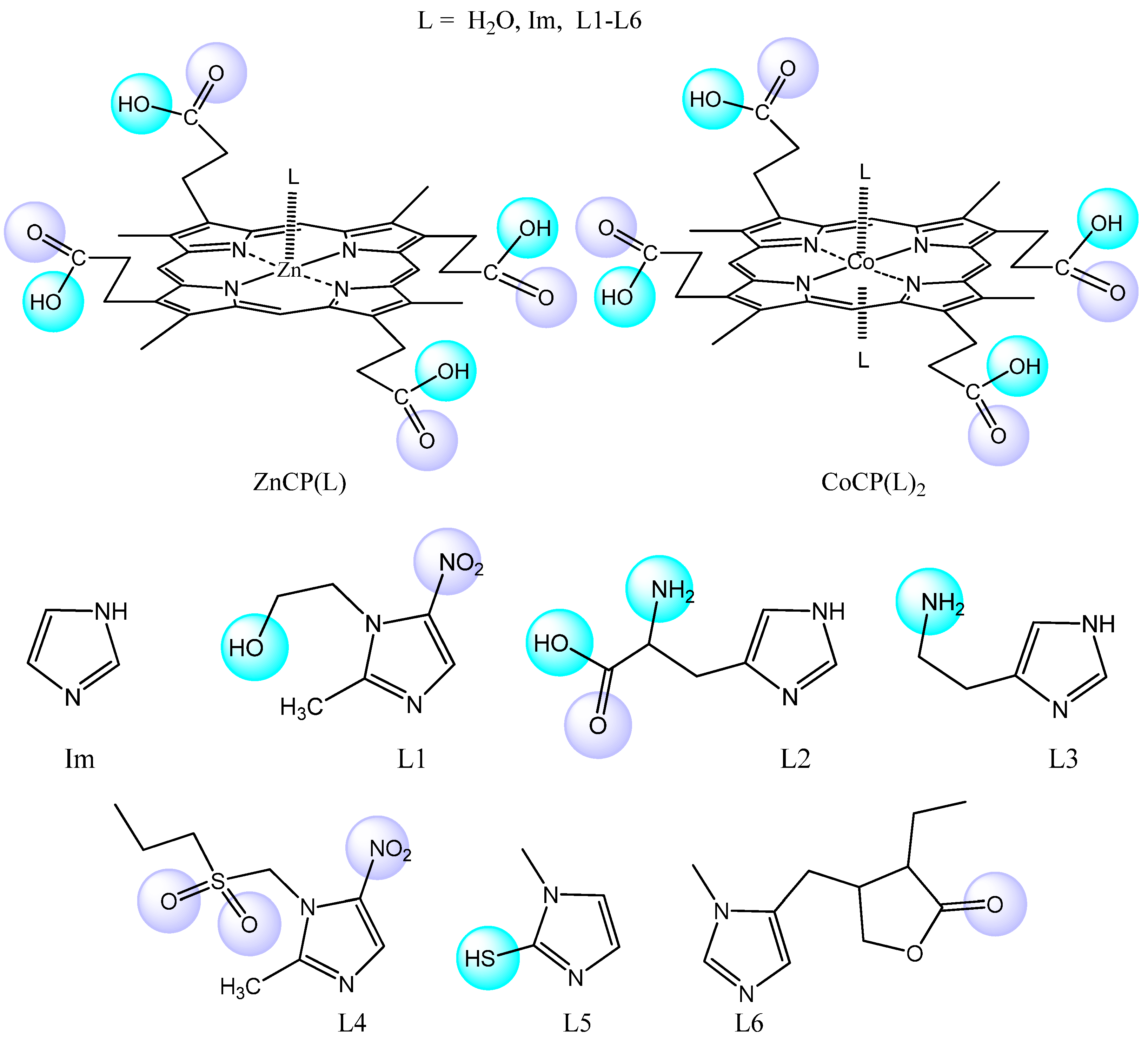
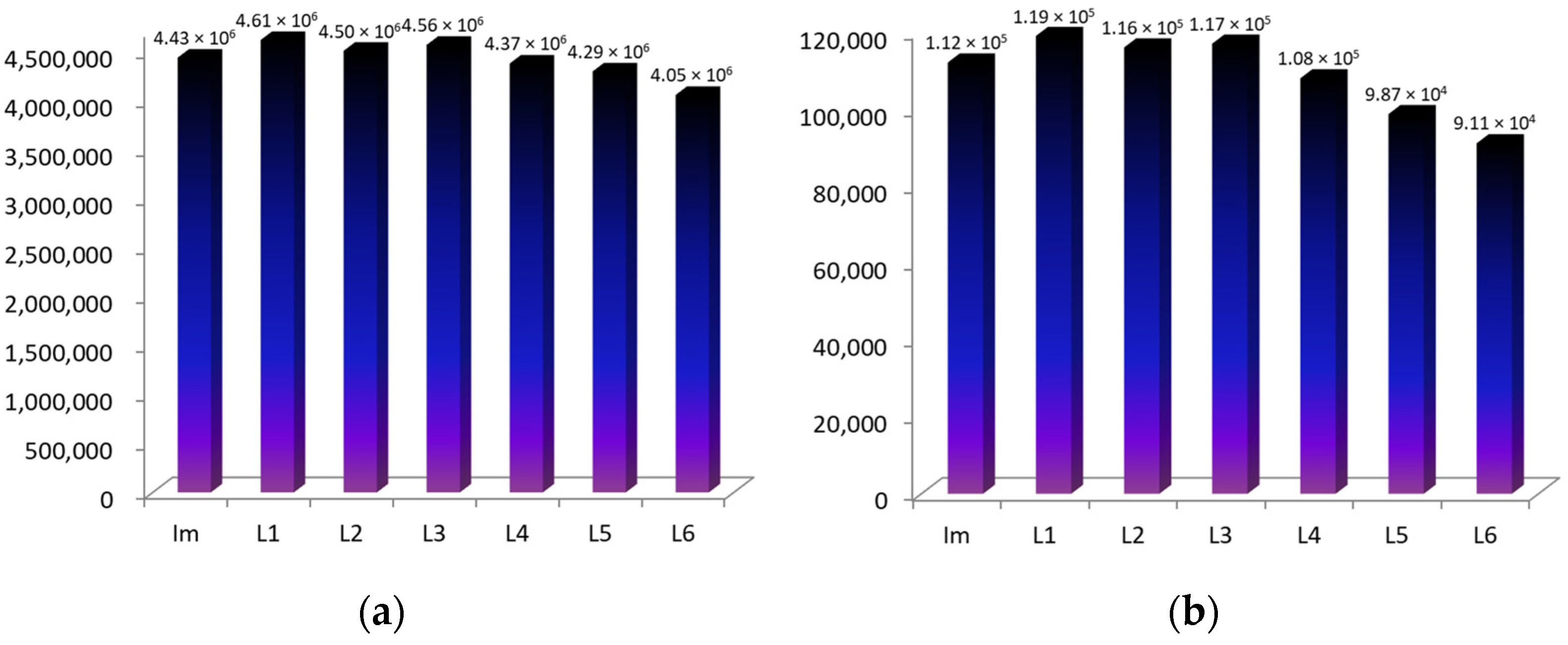
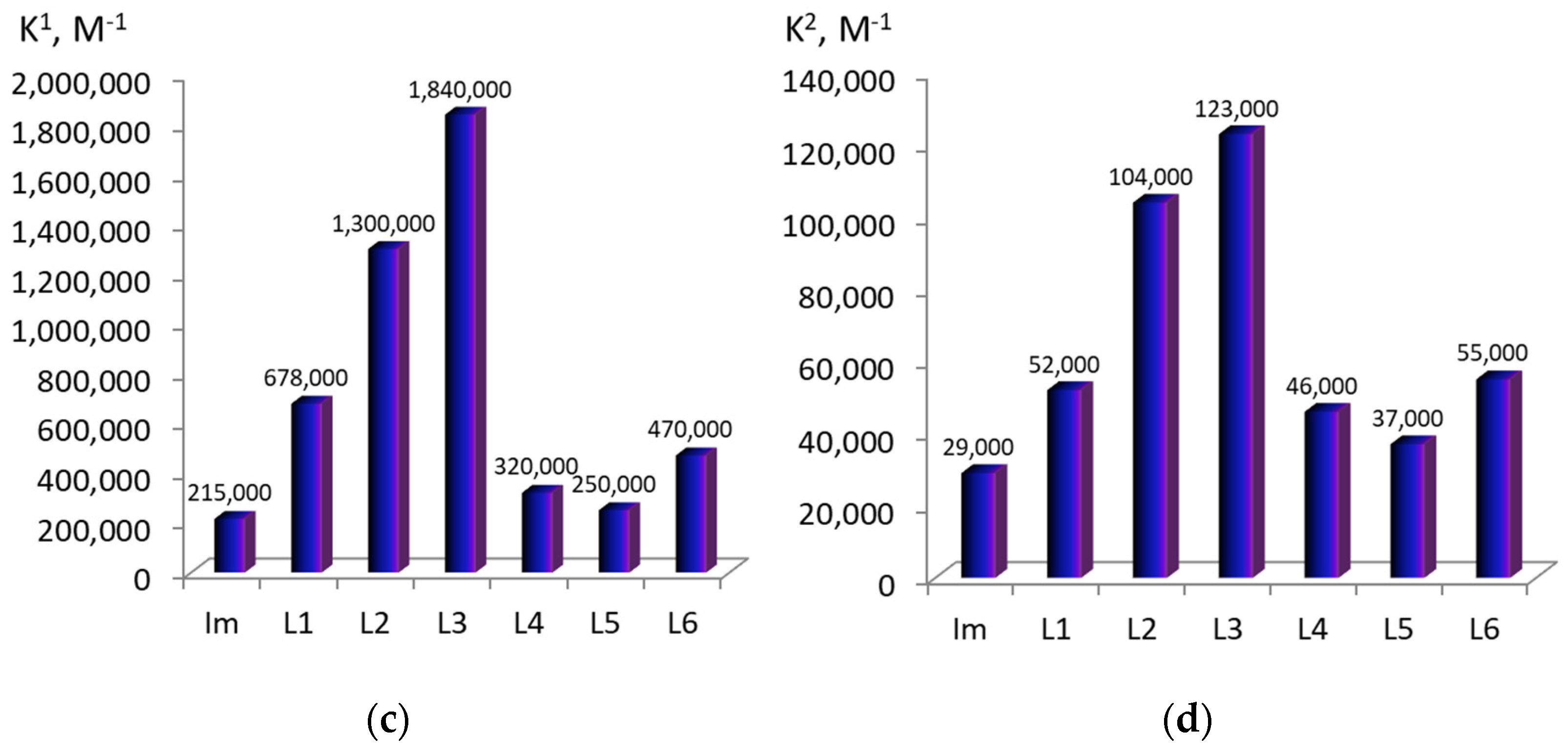
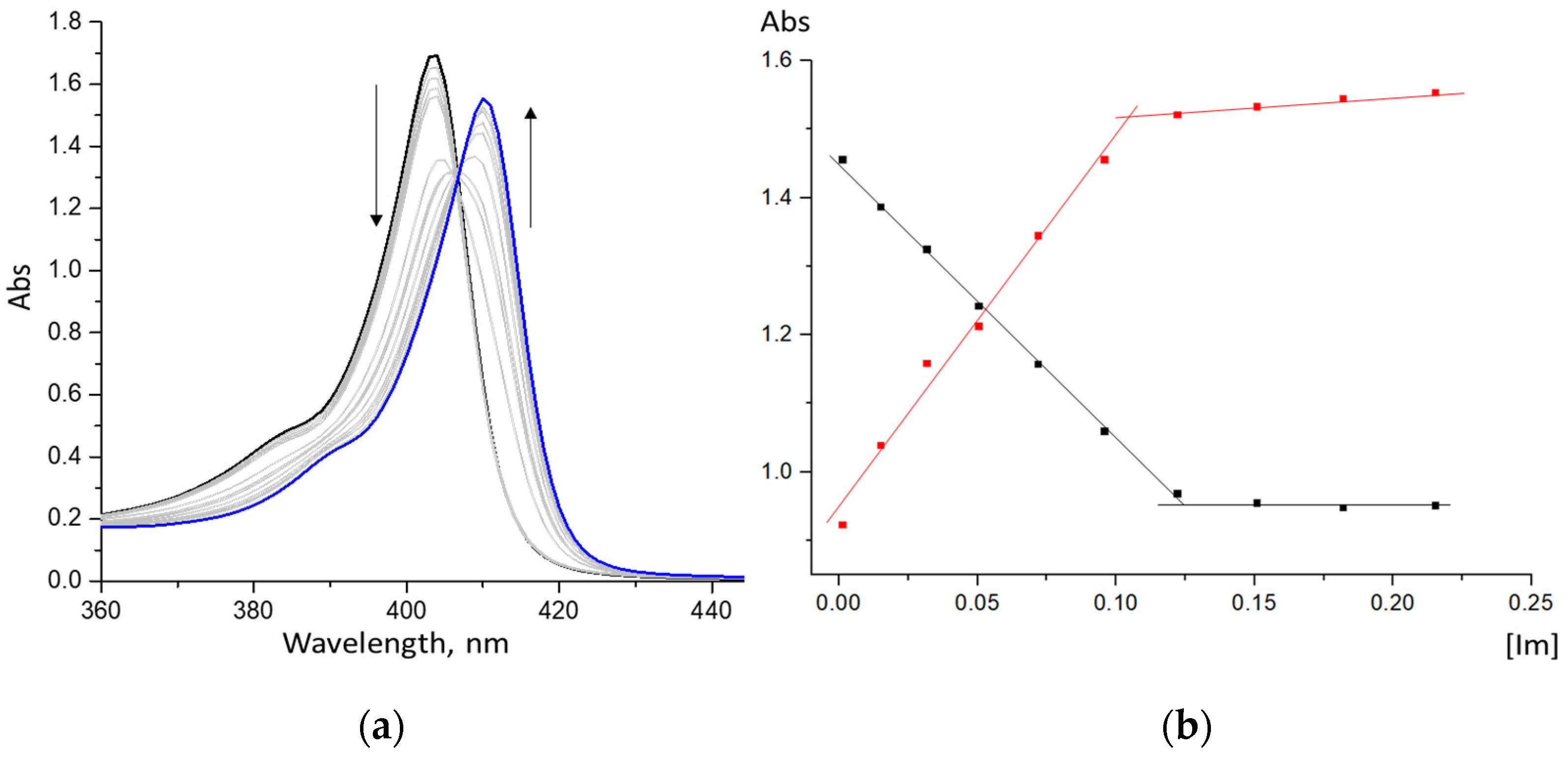
2.1. Formation of Bis-Axial CoCP(H2O)2 Complexes with Imidazole Derivatives
2.2. ZnCP Mono-Axial Complexes with Imidazole Derivatives
2.3. Geometry Optimization of ZnCP(L) and CoCP(L)
2.4. 1H NMR Studies
3. Materials and Methods
3.1. Compounds
3.2. Synthesis and Characterization
3.2.1. Synthesis
3.2.2. Characterization
3.2.3. Details of Quantum Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Watson, C.J. Hematin and Porphyria. N. Engl. J. Med. 1975, 293, 605–607. [Google Scholar] [CrossRef]
- Stein, P.E.; Badminton, M.N.; Rees, D.C. Update Review of the Acute Porphyrias. Br. J. Haematol. 2017, 176, 527–538. [Google Scholar] [CrossRef]
- Wang, B.; Rudnick, S.; Cengia, B.; Bonkovsky, H.L. Acute Hepatic Porphyrias: Review and Recent Progress. Hepatol. Commun. 2019, 3, 193–206. [Google Scholar] [CrossRef]
- Bykhovsky, V.Y.; Zaitseva, N.I.; Mironov, A.F.; Osin, N.S.; Pecherskikh, E.V.; Rumyantseva, V.D.; Sukhin, G.M. Coproporphyrins, Uroporphyrins, and Their Metal Comlexes: Biosynthesis and Application to Immune Analysis and Diagnostic Methods. Appl. Biochem. Microbiol. 2001, 37, 561–568. [Google Scholar] [CrossRef]
- de Haas, R.R.; van Gijlswijk, R.P.M.; van der Tol, E.B.; Zijlmans, H.J.M.A.A.; Bakker–Schut, T.; Bonnet, J.; Verwoerd, N.P.; Tanke, H.J. Platinum Porphyrins as Phosphorescent Label for Time-Resolved Microscopy. J. Histochem. Cytochem. 1997, 45, 1279–1292. [Google Scholar] [CrossRef]
- de Haas, R.R.; van Gijlswijk, R.P.M.; van der Tol, E.B.; Veuskens, J.; van Gijssel, H.E.; Tijdens, R.B.; Bonnet, J.; Verwoerd, N.P.; Tanke, H.J. Phosphorescent Platinum/Palladium Coproporphyrins for Time-Resolved Luminescence Microscopy. J. Histochem. Cytochem. 1999, 47, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ramzan, M.; Qureshi, A.; Khan, M.; Tariq, M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ma, Z.; Zhang, D. Synthesis of Imidazole-Based Medicinal Molecules Utilizing the van Leusen Imidazole Synthesis. Pharmaceuticals 2020, 13, 37. [Google Scholar] [CrossRef]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 978-0-470-51233. [Google Scholar]
- Grunenberg, J. Complexity in Molecular Recognition. Phys. Chem. Chem. Phys. 2011, 13, 10136–10146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Landry, M.P.; Barone, P.W.; Kim, J.-H.; Lin, S.; Ulissi, Z.W.; Lin, D.; Mu, B.; Boghossian, A.A.; Hilmer, A.J.; et al. Molecular Recognition Using Corona Phase Complexes Made of Synthetic Polymers Adsorbed on Carbon Nanotubes. Nat. Nanotechnol. 2013, 8, 959–968. [Google Scholar] [CrossRef]
- Kuroda, Y.; Ogoshi, H. Molecular Recognition of Modified Porphyrins. Synlett 1994, 1994, 319–324. [Google Scholar] [CrossRef]
- Ogoshi, H.; Hatakeyama, H.; Kotani, J.; Kawashima, A.; Kuroda, Y. New Mode of Porphyrin Complexation with Nucleobase. J. Am. Chem. Soc. 1991, 113, 8181–8183. [Google Scholar] [CrossRef]
- Mamardashvili, G.; Kaigorodova, E.; Dmitrieva, O.; Koifman, O.; Mamardashvili, N. Molecular Recognition of Imidazole Derivatives by Co(III)-Porphyrins in Phosphate Buffer (PH = 7.4) and Cetylpyridinium Chloride Containing Solutions. Molecules 2021, 26, 868. [Google Scholar] [CrossRef] [PubMed]
- Ashley, K.R.; Leipoldt, J.G. Kinetic and Equilibrium Study of the Reaction of (Meso-Tetrakis(p-SuIfonatophenyI)Porphyrinato)Diaquocobaltate(III) with Pyridine in Aqueous Solution. Inorg. Chem. 1981, 20, 2326–2333. [Google Scholar] [CrossRef]
- Hambright, P.; Langley, R. Cyanide Scavengers: Kinetics of the Reactions of Cyanide with a Water Soluble Cobalt(III) Porphyrin. J. Inorg. Biochem. 1988, 32, 197–205. [Google Scholar] [CrossRef]
- Oku, M. X-Ray Photoelectron Spectrum of Low-Spin Co(III) in LiCoO2. J. Solid State Chem. 1978, 23, 177–185. [Google Scholar] [CrossRef]
- Kaigorodova, E.Y.; Mamardashvili, G.M.; Mamardashvili, N.Z. Axial Coordination of Pyridine- and Imidazole-Based Drug Molecules to Co(III)-Tetra(4-Carboxyphenyl)Porphyrin. Russ. J. Inorg. Chem. 2018, 63, 1192–1198. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Kaigorodova, E.Y.; Khodov, I.A.; Scheblykin, I.; Mamardashvili, N.Z.; Koifman, O.I. Micelles Encapsulated Co(III)-Tetra(4-Sulfophenyl)Porphyrin in Aqueous CTAB Solutions: Micelle Formation, Imidazole Binding and Redox Co(III)/Co(II) Processes. J. Mol. Liq. 2019, 293, 111471. [Google Scholar] [CrossRef]
- D’Souza, F.; Deviprasad, G.R.; Zandler, M.E. Aggregation and Axial Ligand Exchange Behavior of Water-Soluble Pyrrole-β Brominated Porphyrins. J. Chem. Soc. Dalton Trans. 1997, 93, 3699–3704. [Google Scholar] [CrossRef]
- Mamardashvili, G.; Mamardashvili, N.; Koifman, O. Macrocyclic Receptors for Identification and Selective Binding of Substrates of Different Nature. Molecules 2021, 26, 5292. [Google Scholar] [CrossRef]
- Maltceva, O.; Mamardashvili, G.; Khodov, I.; Lazovskiy, D.; Khodova, V.; Krest’yaninov, M.; Mamardashvili, N.; Dehaen, W. Molecular Recognition of Nitrogen—Containing Bases by Zn[5,15-Bis-(2,6-Dodecyloxyphenyl)]Porphyrin. Supramol. Chem. 2017, 29, 360–369. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A Microporous 2D Cobalt-Based MOF with Pyridyl Sites and Open Metal Sites for Selective Adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Wu, J.; Li, F.-M.; Liu, W.-C.; Li, B.-H.; Wang, J.; Li, Q.-L.; Yadav, R.; Kumar, A. Luminescent Sensing from a New Zn(ii) Metal–Organic Framework. RSC Adv. 2016, 6, 31161–31166. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Luo, Z.; Wang, J.; Li, Y.; Han, Y.; Liu, J. Fluorescence Detection of Mn2+, Cr2O72− and Nitroexplosives and Photocatalytic Degradation of Methyl Violet and Rhodamine B Based on Two Stable Metal–Organic Frameworks. RSC Adv. 2017, 7, 10415–10423. [Google Scholar] [CrossRef]
- Sonti, R.; Gopi, H.N.; Muddegowda, U.; Ragothama, S.; Balaram, P. A Designed Three-Stranded β-Sheet in an α/β Hybrid Peptide. Chem.-A Eur. J. 2013, 19, 5955–5965. [Google Scholar] [CrossRef]
- Nikolić, A.; Jović, B.; Csanady, S.; Petrović, S. N–H⋯O Hydrogen Bonding: FT IR, NIR and 1H NMR Study of N-Methylpropionamide—Cyclic Ether Systems. J. Mol. Struct. 2007, 834–836, 249–252. [Google Scholar] [CrossRef]
- Yamauchi, K.; Kuroki, S.; Ando, I. The Amide Proton NMR Chemical Shift and Hydrogen-Bonded Structure of Glycine-Containing Peptides and Polypeptides in the Solid State as Studied by Multi-Pulse-Associated High-Speed MAS 1H NMR. J. Mol. Struct. 2002, 602–603, 9–16. [Google Scholar] [CrossRef]
- Alibabaei, L.; Wang, M.; Giovannetti, R.; Teuscher, J.; di Censo, D.; Moser, J.-E.; Comte, P.; Pucciarelli, F.; Zakeeruddin, S.M.; Grätzel, M. Application of Cu(ii) and Zn(ii) Coproporphyrins as Sensitizers for Thin Film Dye Sensitized Solar Cells. Energy Environ. Sci. 2010, 3, 956. [Google Scholar] [CrossRef]
- Meites, L. Introduction to Chemical Equilibrium and Kinetics; Pergamon: Oxford, UK, 1981. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision E.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Grimme, S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456. [Google Scholar] [CrossRef] [PubMed]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent Molecular Orbital Methods. XXIII. A Polarization-type Basis Set for Second-row Elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 6.0: Natural Bond Orbital Analysis Program. J. Comput. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Chemcraft-Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 22 December 2022).


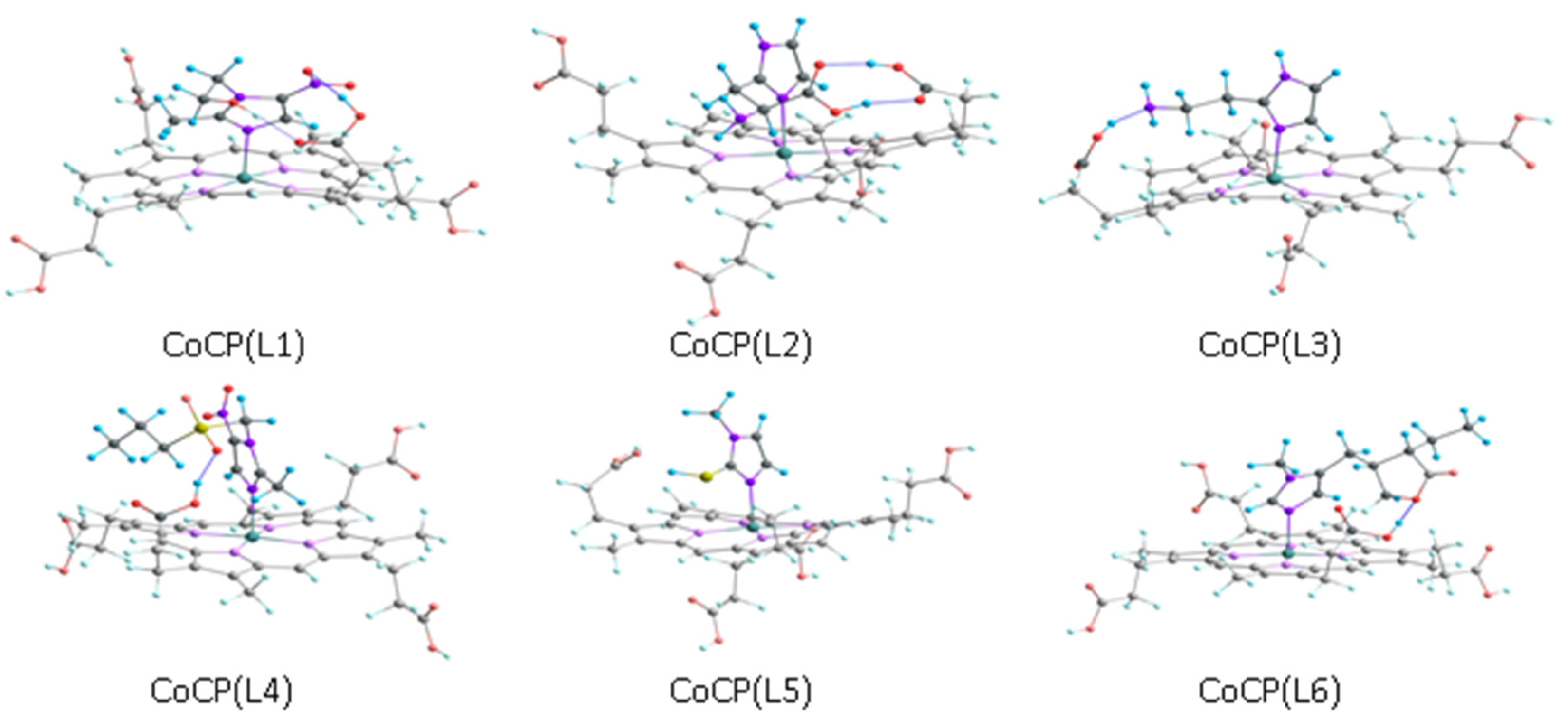





| CoTCPP + L | CoTCPP(L) + L | CoCP + L | CoCP(L) + L | ZnCP + L | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −ΔGg a | −ΔΔGM-N b | −ΔGg | −ΔΔGM-N | −ΔGg | −ΔΔGMN+HB c | −ΔGg | −ΔΔGMN+HB | −ΔGg | −ΔΔGMN+HB | |
| Im | 37.26 | 0 | 28.31 | 0 | 29.90 | 0 | 25.02 | 0 | 15.77 | 0 |
| L1 | 37.36 | 0.10 | 28.46 | 0.15 | 32.69 | 2.27 | 26.44 | 1.42 | 16.21 | 0.44 |
| L2 | 37.30 | 0.04 | 28.40 | 0.09 | 34.28 | 4.38 | 28.13 | 3.11 | 22.29 | 6.52 |
| L3 | 37.33 | 0.07 | 28.41 | 0.10 | 35.12 | 5.22 | 28.54 | 3.52 | 21.58 | 5.81 |
| L4 | 37.23 | −0.03 | 28.22 | −0.09 | 30.86 | 0.96 | 26.14 | 1.12 | 17.02 | 1.25 |
| L5 | 37.18 | −0.08 | 28.00 | −0.31 | 30.26 | 0.36 | 25.61 | 0.59 | 16.05 | 0.28 |
| L6 | 37.04 | −0.22 | 27.81 | −0.50 | 32.18 | 2.28 | 26.19 | 1.17 | 17.60 | 1.83 |
| Ligands | −Eint kcal·mol−1 | Zn-L, Å | Est(Zn-L) kcal·mol−1 | qCT (L), e | Est(Zn-Np) kcal·mol−1 | qCT, e | Zn-Np, Å | <N-Zn-N ° |
|---|---|---|---|---|---|---|---|---|
| L1 | −12.97 | 2.268 | 38.26 | 0.103 | 45.55 | 0.150 | 2.074 | 163.5 |
| L2 | −19.57 | 2.183 | 44.66 | 0.120 | 45.07 | 0.148 | 2.088 | 162.9 |
| L3 | −17.31 | 2.197 | 44.47 | 0.116 | 43.91 | 0.144 | 2.071 | 163.7 |
| L4 | −14.93 | 2.253 | 38.62 | 0.101 | 48.14 | 0.158 | 2.074 | 164.5 |
| L5 | −12.42 | 2.256 | 38.33 | 0.113 | 47.50 | 0.184 | 2.074 | 163.2 |
| L6 | −15.19 | 2.166 | 46.18 | 0.123 | 46.93 | 0.150 | 2.079 | 163.5 |
| Ligands | Eint, kcal·mol−1 | r(Co-NIm), Å | ΣEst(LP(NIm)→LP*(Co)), kcal·mol−1 | qst(LP(NIm)→LP*(Co)), e | r(Co-NPyr), Å | ΣEst(LP(NPyr)→LP*(Co)), kcal·mol−1 | qst(LP(NPyr)→LP*(Co)), e | ∠N-Co-N, ° |
|---|---|---|---|---|---|---|---|---|
| Im | 20.44 | 2.1184 | 24.53 | 0.147 | 1.9920 | 34.45 | 0.143 | 173.8 |
| L1 | 34.61 | 2.1992 | 26.9 | 0.124 | 1.9863 | 54.81 | 0.143 | 173.5 |
| L2 | 42.45 | 2.2232 | 28.09 | 0.123 | 1.9755 | 56.31 | 0.143 | 166.9 |
| L3 | 39.48 | 2.2355 | 27.54 | 0.122 | 1.9825 | 55.33 | 0.141 | 171.9 |
| L4 | 34.65 | 2.1498 | 29.67 | 0.131 | 1.9868 | 54.92 | 0.142 | 171.6 |
| L5 | 26.93 | 2.1584 | 30.98 | 0.133 | 1.9756 | 49.13 | 0.149 | 174.8 |
| L6 | 36.76 | 2.1366 | 31.82 | 0.129 | 2.0048 | 58.34 | 0.152 | 174.7 |
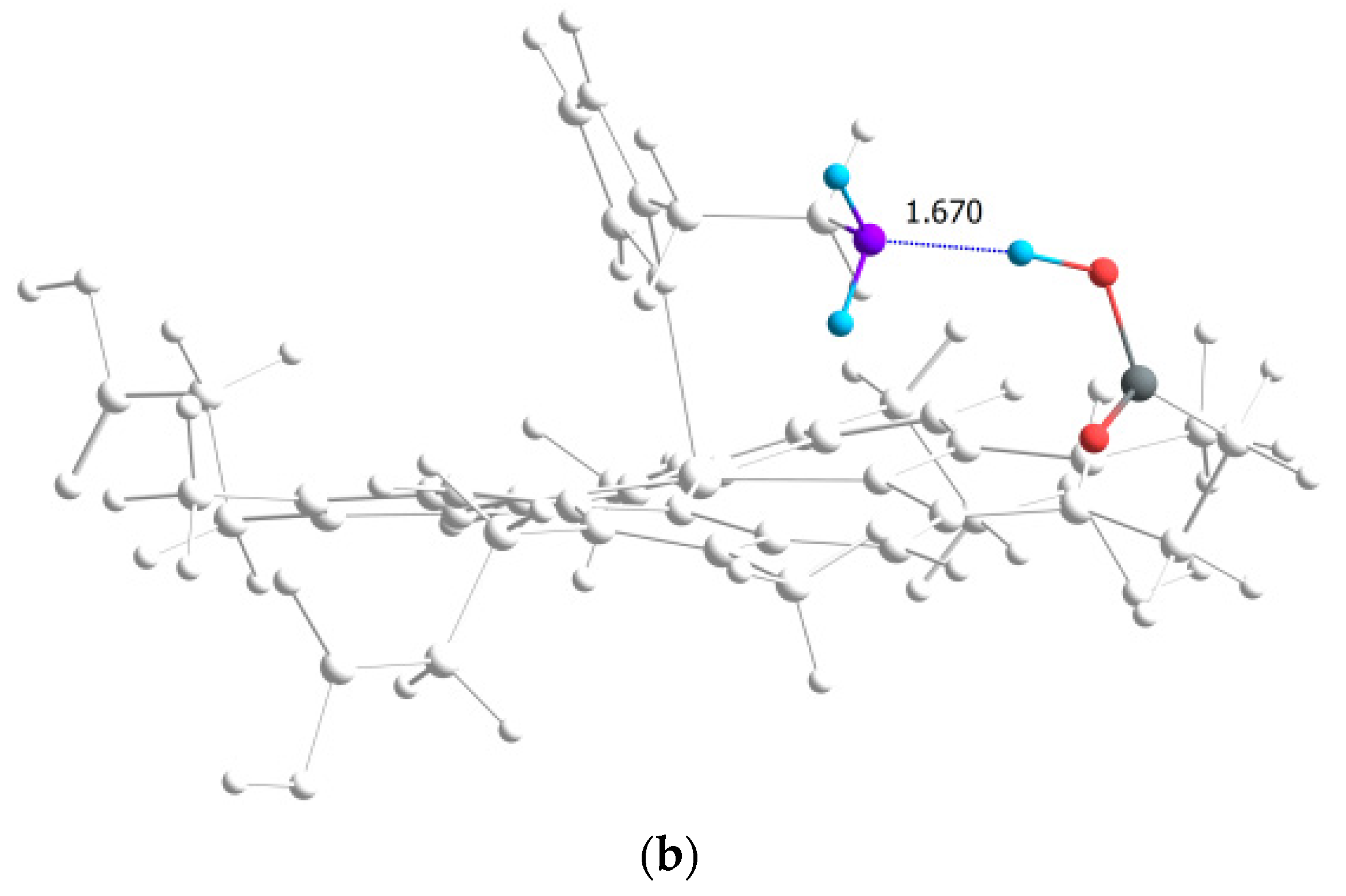
| Ligand | Type | r(H···B), Å | ∠A-H···B, ° | ΣEst, kcal·mol−1 |
|---|---|---|---|---|
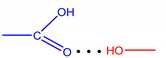
| Im | - | - | - | - |
| L1 |  | 1.83 | 172.5 | 13.47 |
 | 1.98 | 149.4 | ||
| L2 |  | 1.75 | 168.3 | 22.27 |
| 1.74 | 156.8 | |||
| L3 | 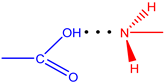 | 1.67 | 159.1 | 36.15 |
| L4 | 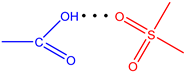 | 1.75 | 162.3 | 5.68 |
| L5 | 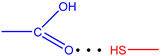 | 2.24 | 147.7 | 3.02 |
| L6 | 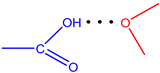 | 1.85 | 166.8 | 6.55 |
| L | 1H NMR-Spectrum of L | 1H NMR-Spectrum of L in the CoCP(L) Complexes |
|---|---|---|
| L1 | 8.027 (s, 1H, Im) 5.3 (br. s, 1H, -OH) 4.36 (t, 2H, -CH2-CH2-OH) 3.67 (t, 2H, -CH2-CH2-OH) 2.46 (s, 3H, -CH3) | 5.86 (br.s, 1H, -OH)-downfield shift 4.23 (t, 2H, -CH2-CH2OH), 3.48 (t, 2H, -CH2-CH2OH), 3.28 (s, 1H, 2-Im) -upfield shift 2.28 (s, 3H, -CH3). |
| L2 | 11.92 (s, 1H, -COOH) 8.24 (s, 1H, Im) 7.43 (s, 1H, Im) 6.13 (br. s. 2H, NH2) 4.9 (br. s, 1H, NH) 4.06 (s, 1H, -CH-) 3.37 (t, 2H, -CH2-) | 12.47 (s, 1H, -COOH) 3.43 (s, 1H, Im) 6.32 (s, 1H, Im) 6.24 (br.s, 2H, NH2) 4.6 (br. s, 1H, NH) 4.15 (s, 1H, -CH-) 3.31 (t, 2H, -CH2-) |
| L3 | 7.54 (s, 1H, Im) 6.80 (s, 1H, Im) 6.25 (br.s. 2H, NH2) 4.98 (br. s, 1H, NH) 2.97 (t, 2H, Im-CH2-CH2-) 2.75 (t, 2H, Im-CH2-CH2-) | 3.58 (s, 1H, Im) 5.73 (s, 1H, Im) 7,12 (br.s, 2H, NH2) 4,90 (br. s, 1H, NH) 2.94 (t, 2H, Im-CH2-CH2-) 2.70 (t, 2H, Im-CH2-CH2-) |
| L4 | 8.027 (s, 1H, Im) 3.48 (s, 2H, -CH2-) 3.26 (t, 2H, -CH2-CH2-CH3) 2.16 (t, 2H, -CH2-CH2-CH3) 1.19 (s, 3H, -CH2-CH2-CH3) 2.46 (s, 3H, -CH3) | 3.61 (s, 1H, Im) 3.32 (s, 2H, -CH2-) 4.12 (t, 2H, -CH2-CH2-CH3) 2.16 (t, 2H, -CH2-CH2-CH3) 1.23 (s, 3H, -CH2-CH2-CH3) 2.51 (s, 3H, -CH3) |
| L5 | 12.2 (br. s, 1H, SH) 7.54 (d, 1H, Im) 6.80 (d, 1H, Im) 2.46 (s, 3H, -CH3) | 12.39 (br.s, 1H, SH) 3.33 (d, 1H, Im) 5.78 (d, 1H, Im), 2.49 (s, 3H, -CH3) |
| L6 | 8.84 (s, 1H, Im) 7.14 (s, 1H, Im) 4.09 (m, 2H, -CH2-) 3.66 (m, 2H, -CH2-) 3.16 (s, 3H, -CH3) 2.98 (s, 1H, -CH<) 2.79 (s, 1H, -CH<) 1.58 (m, 2H, -CH2-CH3) 1.07 (s, 3H, -CH2-CH3) | 3.38 (s, 1H, Im) 7.03 (s, 1H, Im) 4.02 (m, 2H, -CH2-) 3.62 (m, 2H, -CH2-) 3.18 (s, 3H, -CH3) 3.13 (s, 1H, -CH<) 2.97 (s, 1H, -CH<) 1.52 (m, 2H, -CH2-CH3) 1.12(s, 3H, -CH2-CH3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamardashvili, G.; Kaigorodova, E.; Lebedev, I.; Mamardashvili, N. Molecular Recognition of Imidazole-Based Drug Molecules by Cobalt(III)- and Zinc(II)-Coproporphyrins in Aqueous Media. Molecules 2023, 28, 964. https://doi.org/10.3390/molecules28030964
Mamardashvili G, Kaigorodova E, Lebedev I, Mamardashvili N. Molecular Recognition of Imidazole-Based Drug Molecules by Cobalt(III)- and Zinc(II)-Coproporphyrins in Aqueous Media. Molecules. 2023; 28(3):964. https://doi.org/10.3390/molecules28030964
Chicago/Turabian StyleMamardashvili, Galina, Elena Kaigorodova, Ivan Lebedev, and Nugzar Mamardashvili. 2023. "Molecular Recognition of Imidazole-Based Drug Molecules by Cobalt(III)- and Zinc(II)-Coproporphyrins in Aqueous Media" Molecules 28, no. 3: 964. https://doi.org/10.3390/molecules28030964
APA StyleMamardashvili, G., Kaigorodova, E., Lebedev, I., & Mamardashvili, N. (2023). Molecular Recognition of Imidazole-Based Drug Molecules by Cobalt(III)- and Zinc(II)-Coproporphyrins in Aqueous Media. Molecules, 28(3), 964. https://doi.org/10.3390/molecules28030964






