Abstract
Positron emission tomography (PET) is a molecular imaging technique that makes use of radiolabelled molecules for in vivo evaluation. Carbon-11 is a frequently used radionuclide for the labelling of small molecule PET tracers and can be incorporated into organic molecules without changing their physicochemical properties. While the short half-life of carbon-11 (11C; t½ = 20.4 min) offers other advantages for imaging including multiple PET scans in the same subject on the same day, its use is limited to facilities that have an on-site cyclotron, and the radiochemical transformations are consequently more restrictive. Many researchers have embraced this challenge by discovering novel carbon-11 radiolabelling methodologies to broaden the synthetic versatility of this radionuclide. This review presents new carbon-11 building blocks and radiochemical transformations as well as PET tracers that have advanced to first-in-human studies over the past five years.
1. Introduction
Positron emission tomography (PET) is a molecular imaging technique that utilizes radiotracers for in vivo studies. The radionuclides fluorine-18 and carbon-11 are the most commonly used for labelling PET tracers because of the growing use of organofluorine drugs and as carbon is ubiquitous in nearly every drug or biomolecule. Additionally, their suitable decay characteristics and half-lives match the in vivo pharmacokinetics of small molecules. Consequently, developing new radiochemistry methods for the introduction of these short-lived radionuclides into organic molecules has emerged as one of the greatest challenges in PET radiopharmaceutical chemistry. Our ultimate goal is to radiolabel any molecule for medical imaging—a concept analogous to total synthesis that we introduced as “total radiosynthesis” [1]. Because the radiochemistry of fluorine-18 has been extensively reviewed in recent years [for example see: [2,3,4,5,6,7,8,9,10,11]], the focus of this review is on recent radiochemistry methodologies with carbon-11 and translation of 11C-labelled PET tracers to first-in-human (FIH) PET imaging studies.
Carbon-11 (11C) has a half-life of 20.4 min and is produced in a cyclotron by proton bombardment of nitrogen gas in presence of trace amounts of oxygen (0.1–2%) or hydrogen (5–10%) where it is obtained as [11C]CO2 or [11C]CH4, respectively [12,13]. [11C]CO2 and [11C]CH4 can either be used directly in radiolabelling reactions or further converted to other 11C-building blocks (see Scheme 1) [14]. The most common carbon-11 labelling strategy for PET tracers is 11C-methylation of hydroxy or amino groups using [11C]methyl iodide or [11C]methyl triflate, which are routinely obtained from [11C]CO2 and/or [11C]CH4. The advantages of 11C-methylation are the accessibility of precursors and carbon-11 methylating agents, as well as the general prevalence of methyl groups in pharmaceutical compounds. However, amongst molecules targeting the central nervous system (CNS) the prevalence of such methyl groups is rather low (<35%). Furthermore, metabolic demethylation can lead to cleavage of the radiolabel in vivo [15,16].
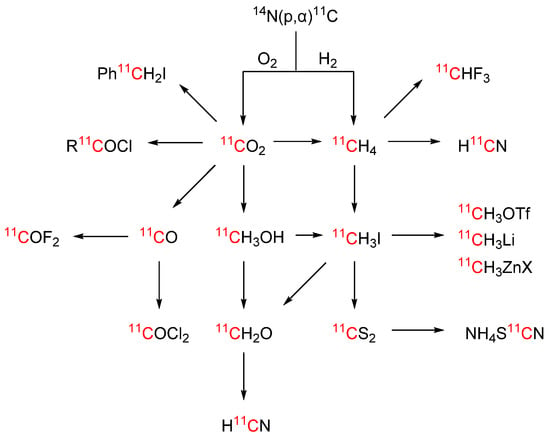
Scheme 1.
Selected carbon-11 labelled building blocks.
Synthetic efforts have been made in recent years to expand the toolbox for 11C-chemistry beyond 11C-methylation (Scheme 1). Particular interest has been paid to the development of [11C]CO and [11C]CO2 chemistry, in order to gain access to 11C-labelled carbonyl-based functional groups. These radiochemistry methods open the door to labelling >75% of the compounds in CNS drug pipelines [15]. And other promising building blocks and synthetic strategies have been developed, such as new reactions with [11C]methyl iodide and related alklylating reagents, [11C]hydrogen cyanide, [11C]fluoroform, [11C]carbonyl difluoride, [11C]carbon disulfide, [11C]thiocyanate and [11C]formaldehyde (vide infra), further broadening the scope of compounds that can be labelled with carbon-11 and paving the way for our ultimate goal of total radiosynthesis.
It should be noted that all yields are reported as they are stated or defined in their original articles (radiochemical yield (RCY), radiochemical conversion (RCC), radiochemical purity (RCP)) and might not necessarily reflect their definition as reported in the nomenclature guidelines [17,18]. The molar activity (Am) depends on several factors including the starting amount of radioactivity and is therefore difficult to compare.
2. Carbon-11 Methodologies
2.1. [11C]Carbon Dioxide
Historically, [11C]CO2 has been a challenging building block for radiochemists to use due to its moderate reactivity and potentially low Am caused by isotopic dilution with atmospheric CO2. The introduction of bulky organic “fixation” bases such as 1,8-diazabicyclo [5.4.0]undec-7-ene (DBU) and 2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine (BEMP) for trapping of [11C]CO2 [19,20,21] was inspired by green chemistry for capturing atmospheric CO2 and represents a major advance for 11C-chemistry: the “fixation” bases allow [11C]CO2 to be easily trapped in a reaction vessel at room temperature and enable access to high oxidation state functional groups such as carbon-11 labelled carboxylic acids, amides, formamides, ureas, carbamates and other functional groups [22]. This methodology has contributed to the accessibility of [11C]CO2 as a building block and, in consequence, a wide array of new [11C]CO2 chemistry applications and PET tracers have emerged over the past decade. This review will focus on novel [11C]CO2 fixation reactions reported within the last five years.
While [11C]CO2 is directly produced in the cyclotron, the irradiated cyclotron target gas contains many undesired chemical and radiochemical entities. To purify [11C]CO2 from carrier gases and other by-products, it is typically trapped using liquid nitrogen or by physical adsorption on porous polymers, such as carbon molecular sieves or polydivinylbenzene copolymers. A new method for purifying [11C]CO2, also inspired by green chemistry literature, has recently been reported by our laboratories which employs chemisorption by solid polyamine-based adsorbents. This method uses small amounts of silica-grafted polyethyleneimine to trap [11C]CO2 at room temperature and quantitatively release it under mild heating (85 °C). Trapping efficiencies (TEs) as high as 79 ± 12% were observed but decreased over multiple cycles, indicating a limited reusability of the capture material. This technology was applied to synthesize a PET tracer by [11C]CO2 fixation reactions, and could potentially be applied for solid phase reactions as well as enable the transportation of carbon isotopes [23].
Traditionally, direct use of [11C]CO2 is achieved by use of Grignard reagents, organolithiums or silanamines to yield 11C-labelled amides or carboxylic acids. However, these reagents are challenging to implement in automated PET tracer production due to their hygroscopic nature, tendency to absorb atmospheric CO2, and corrosiveness [22]. As such, many new methodologies for the preparation of [11C]carboxylic acids have been developed over the past five years by novel [11C]CO2 fixation reactions that employ the aforementioned “fixation” bases. Our laboratory reported the use of aryl and heteroaryl stannanes as precursors which were carboxylated in a copper(I)-mediated reaction with [11C]CO2 (see Scheme 2A) [24]. The method was fully automated and applied for an alternative synthesis of [11C]bexarotene (previously synthesized by reaction of [11C]CO2 with a boronic ester precursor mediated by a copper(I) source [25,26]), and was obtained with a RCY of 32 ± 5% (decay-corrected (dc)) and a Am of 38 ± 23 GBq/µmol. The strategy was also applied by García-Vázquez et al. to the synthesis of 11C-carboxylated tetrazines for the labelling of trans-cyclooctene-functionalized PeptoBrushes [27]. After optimization of the original reaction conditions (CuI instead of CuTC, NMP instead of DMF and addition of TBAT as fluoride ion source), two tetrazines were successfully 11C-carboxylated with RCYs of 10–15% and “clicked” to the TCO-PeptoBrushes. It is noteworthy that Goudou et al. reported the copper-catalyzed radiosynthesis of [11C]carboxylic acids by reaction of [11C]CO2 with terminal alkynes in presence of DBU (see Scheme 2B) [28]. A small library of [11C]propiolic acids was obtained with RCYs between 7 and 28%. A different approach using trimethyl and trialkoxy silanes as precursor has been described by Bongarzone et al. (see Scheme 2C) [29]. In this desilylative carboxylation reaction, aromatic silane precursors were activated by fluoride, forming a pentavalent silicate which was then reacted in a copper-catalyzed reaction with [11C]CO2. [11C]Carboxylic acids were obtained with RCYs of 19–93% and TEs of 21–89%. A more general approach for the synthesis of [11C]carboxylic acids was introduced by Qu et al. (see Scheme 2D) [30]. Sp-, sp2- and sp3-hybridized carbon-attached trimethylsilanes were 11C-carboxylated in a fluoride-mediated desilylation (FMDS) reaction, resulting in a broad substrate scope and high RCYs (up to 98%). The applicability of the method was demonstrated by synthesizing two carboxylic acid PET tracers via the FMDS approach.
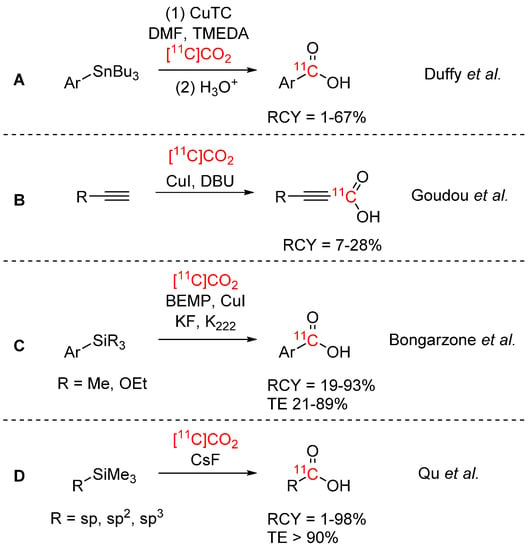
Scheme 2.
New synthetic strategies for [11C]carboxylic acids [24,28,29,30].
[11C]Carboxylic acids can also be synthesized by isotopic exchange reactions. Destro et al. reported the isotopic exchange reaction of cesium salt precursors with 13C, 14C, and a few selected examples of 11C (see Scheme 3A) [31]. While good yields were obtained for [13C]CO2 and [14C]CO2, yields were low for [11C]CO2 (3–50%) due to low TEs. Another take on this strategy was demonstrated by Kong et al., who employed photoredox catalysis and obtained similar results (see Scheme 3B) [32]. In both cases, Am was low, as expected (<0.2 GBq/µmol). A very recent addition to the portfolio of carboxylic acid labelling strategies by isotopic exchange was presented by Bsharat et al. [33]. These authors developed an aldehyde-catalyzed carboxylate exchange reaction in α-amino acids (see Scheme 3C) with 13C and 11C. For the 11C-reactions, imine carboxylates were pre-formed by condensation of α-amino acids with aryl aldehydes and subsequently subjected to the carboxylate exchange reaction with [11C]CO2. An array of α-amino acids was labelled with RCYs of 4–24%, and the modest yields were also attributed to low TEs of the [11C]CO2. Phenylalanine was isolated by this reaction with a Am of 8.4 GBq/mmol.
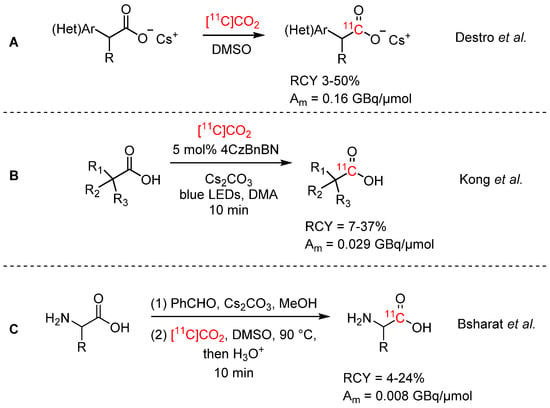
Scheme 3.
Synthesis of [11C]carboxylic acids via isotopic exchange [31,32,33].
Scheme 4 gives an overview of the proposed mechanism of [11C]CO2 fixation with fixation bases such as BEMP and DBU and formation of the [11C]isocyanate, as well as the 11C-labelled functional groups that can be obtained via this pathway [18]. While early works focused on the synthesis of carbamates, the scope of 11C-labelled functional groups has broadened immensely over time.
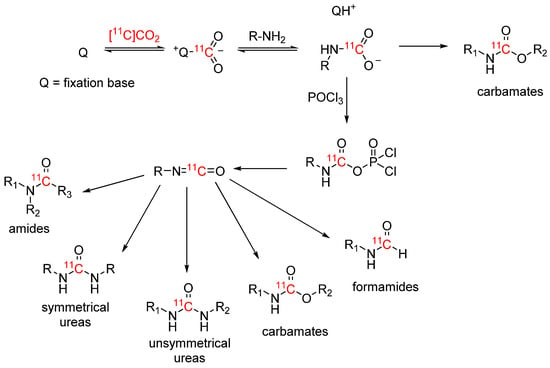
Scheme 4.
[11C]CO2 fixation, [11C]isocyanate formation and 11C-products.
The efficient syntheses of carbon-11 labelled amides, ureas, and formamides have been a longstanding goal in PET radiochemistry and have seen an emergence of interest in recent years. Bongarzone et al. reported a rapid one-pot synthesis of amides via a Mitsunobu reaction (see Scheme 5A) [34]. [11C]CO2 was trapped with DBU, converted to [11C]isocyanate (or an [11C]oxyphosphonium intermediate) using Mitsunobu reagents and subsequently reacted with a Grignard reagent to form the respective amide. RCYs of up to 50% were obtained. The substrate scope was not investigated for [11C]CO2, but [11C]melatonin was synthesized to demonstrate the applicability of this method to biologically relevant compounds. Mair et al. used organozinc iodides as alternatives to Grignard reagents in a rhodium-catalyzed addition to [11C]isocyanates (see Scheme 5B) [35]. The isocyanates were generated similarly to the previous method and reacted with the organozinc iodides in presence of a rhodium catalyst with RCYs of 5–99%. One model compound was isolated with a RCY of 12% and Am of 267 GBq/µmol to demonstrate suitability for PET tracer production. In order to develop an efficient synthesis strategy for the benzimidazolone PET tracer (S)-[11C]CGP12177, Horkka et al. reported a BEMP/Mitsunobu-based strategy for the synthesis of cyclic aromatic ureas: ortho-Phenylenediamines were reacted with [11C]CO2 in presence of BEMP as fixation base. Mitsunobu reagents (DBAD, nBu3P) were added to form the [11C]isocyanate intermediates which then reacted intramolecularly to yield the respective 11C-labelled urea (see Scheme 5C) [36]. The strategy was also applied to cyclic carbamates and thiocarbamates, as well as the tracer (S)-[11C]CGP12177, which was obtained in 23% RCY (dc) with a Am of 14 GBq/µmol. Luzi et al. reported the synthesis of [11C]formamides (see Scheme 5D) [37]. [11C]CO2 was trapped with BEMP in diglyme and was reacted with aromatic and aliphatic primary amines to form the respective [11C]isocyanates, which were subsequently reduced to the [11C]formamides with sodium borohydride. The method performed better for aliphatic amines compared to aromatic amines.
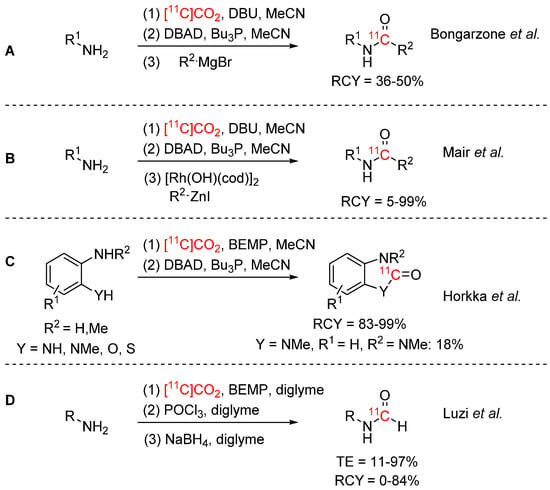
Scheme 5.
Synthesis of [11C]amides and [11C]formamides via [11C]isocyanates [34,35,36,37].
In an attempt to make [11C]CO2 fixation with BEMP and DBU more widely accessible and amenable to automation, two strategies of “in-loop” [11C]CO2 fixation have been developed. While our laboratory developed this method using a standard stainless-steel HPLC loop for [11C]CO2 fixation, Downey et al. applied a disposable ethylene tetrafluoroethylene loop [38,39]. In both cases, [11C]CO2 was captured in the loop in the presence of an amine precursor and fixation base, prior to reaction with a model substrate. The “in-loop” fixation has been applied to synthesize 11C-labelled carbamates, unsymmetrical, and symmetrical ureas.
A different approach to access ureas and carbamates via [11C]isocyanate was presented by Audisio and co-workers (see Scheme 6). The [11C]isocyanate intermediates were generated through a Staudinger aza-Wittig reaction from the respective azide, then reacted either intramolecularly to form cyclic [11C]ureas [40] and [11C]carbamates [41] or intermolecularly with an amine to form linear ureas [42]. All three strategies were applied for the synthesis of 13C-, 14C- and 11C-labelled compounds. RCYs of the isolated 11C-compounds generally ranged between 20 to 50%.
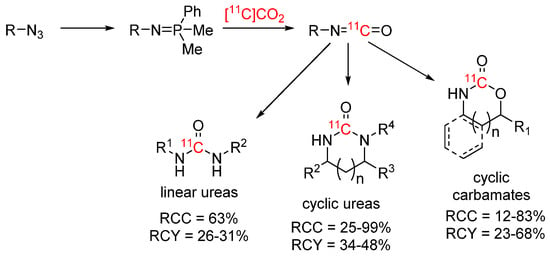
Scheme 6.
11C-labelled ureas and carbamates via the Staudinger aza-Wittig reaction [40,41,42].
To avoid the multi-step syntheses and limited substrate scope of previously reported methods, Liger et al. reported a novel radiolabelling strategy for benzimidazoles and benzothiazoles (see Scheme 7). In this work, [11C]CO2 was reacted with aromatic diamines and aminobenzenethiols in presence of 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene (IPr), zinc chloride, and phenylsilane as reducing reagent to obtain various benzimidazoles and benzothiazoles [43].
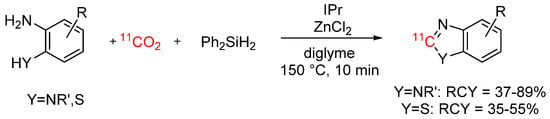
Scheme 7.
Synthesis of carbon-11 labelled benzimidazoles and benzothiazoles [43].
Previously, the synthesis of [11C]carbonates could only be achieved using the esoteric building block [11C]phosgene, which is technically challenging to prepare and requires specialized apparatus. To access this functional group directly from [11C]CO2, Dheere et al. developed a procedure involving an alkyl chloride, an alcohol, TBAI and base in DMF (see Scheme 8) [44]. The procedure was used for the synthesis of one model compound, and resulted in either moderate RCY (31 ± 2%) and higher Am (10–20 GBq/µmol; low amounts of 11C), or high RCY (up to 82%) and lower Am (2 GBq/µmol), depending on the base.
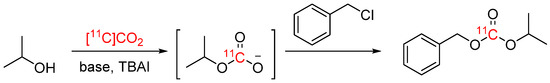
Scheme 8.
Synthesis of a [11C]carbonate from [11C]CO2 (RCY and Am are base-dependent) [44].
A novel method for ring-opening non-activated aziridines with [11C]CO2 using DBU/DBN halide ionic liquids was developed by our laboratory (see Scheme 9) [45]. [11C]CO2 was introduced to a pre-activated mixture of benzyl aziridine and the ionic liquid giving 4-benzyl [11C]oxazolidine-2-one with 77% radiochemical conversion (RCC) and 78% TE. The method was applied to radiolabel an array of [11C]oxazolidinones (RCCs 5–95%) as well as a MAO-B inhibitor, [11C]toloxatone, as a proof of concept.
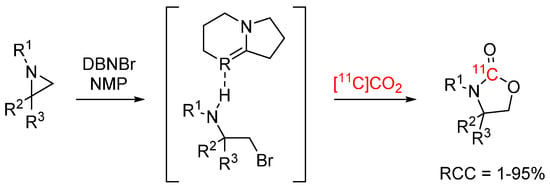
Scheme 9.
Ring-opening of non-activated aziridines with [11C]CO2 [45].
2.2. [11C]Carbon Monoxide
[11C]Carbon monoxide has gained much interest in recent years. Novel 11CO-chemistry will not be covered within this review but we refer to recent comprehensive reviews of [11C]CO production methods and 11C-carbonylation chemistry [46,47,48,49,50]. Although many straightforward routes for [11C]CO production have been established, and a diverse portfolio of [11C]carbonylation reactions has been developed, this branch of carbon-11 chemistry is still heavily underrepresented in PET tracer synthesis. In fact, of the 100+ labelled compounds synthesized from [11C]CO, only four are reported for human use to our knowledge [51]. One likely reason for the hampered translation of [11C]CO radiochemistry to the clinic can be attributed to the historic lack of commercially available automated synthesis units for [11C]CO. This has now been overcome with systems such as the TracerMakerTM which is used by our laboratories for the syntheses of N-[11C]acrylamide PET tracers for imaging Bruton’s tyrosine kinase via a palladium-NiXantphos-mediated carbonylation using [11C]CO [51,52]. The synthesis of the same class of compounds has also recently been automated as “in-loop” procedure using the GE TracerLab synthesis modules [53]. Prior to this recent work, 11C-labelled N-acrylamides were synthesized from [11C]acrylic acid or [11C]acryloyl chloride (formed by carboxylation of Grignard or organolithium reagents with [11C]CO2) and were not suitable for human translation.
2.3. [11C]Methyl Iodide and Other 11C-Alkylation Agents
[11C]Methyl iodide and [11C]methyl triflate have been known for many decades [54,55,56,57] and are by far the most commonly used 11C-labelling agents. Their widespread use is attributed to their routine radiosyntheses and high reactivity. Both [11C]methyl iodide and [11C]methyl triflate can be easily synthesized from the primary cyclotron products (i.e., [11C]CH4 or [11C]CO2) using the classical wet-chemistry approach with lithium aluminium hydride and HI or the gas-phase method involving I2, and dedicated synthesis devices with fully automated procedures are commercially available [58]. Mostly, [11C]methyl iodide and [11C]methyl triflate are employed in 11C-methylation reactions of hydroxyl, amine or thiol precursors, but also many different 11C-C coupling reactions have been established, including Suzuki, Stille, and Negishi couplings. For an overview of 11C-C cross-coupling strategies, we refer the reader to a comprehensive review from H. Doi [59]. Recent progress in the field has been made by Rokka et al., who systematically studied the reaction of various organoborane precursors with [11C]methyl iodide in two different reaction media, DMF(/water) and THF/water, to determine the best precursor and solvent for Suzuki-type cross coupling reactions in 11C-chemistry [60]. These authors found that for their model compound (1-[11C]methylnaphthalene), the boronic acid and pinacol ester precursors gave the highest yields, while the solvent mixture THF/water was equal or superior in any tested reaction. Recent work focused on broadening the substrate scope to diversify 11C-methylation chemistry. Pipal et al. reported the 11C-methylation of aromatic and aliphatic bromides via metallaphotoredox catalysis (see Scheme 10A) [61]. The applicability of this labelling strategy was demonstrated by synthesizing 11 11C-labelled biologically active compounds, including the PET tracers [11C]UCB-J and [11C]PHNO, in RCYs of 13–72% for proof of concept. Qu et al. extended their fluoride-mediated desilylation of organosilanes, initially developed for [11C]CO2 fixation (vide supra), to [11C]methyl iodide and succeeded in labelling a diverse library of silane substrates with RCYs of up to 93% (see Scheme 10B) [25].
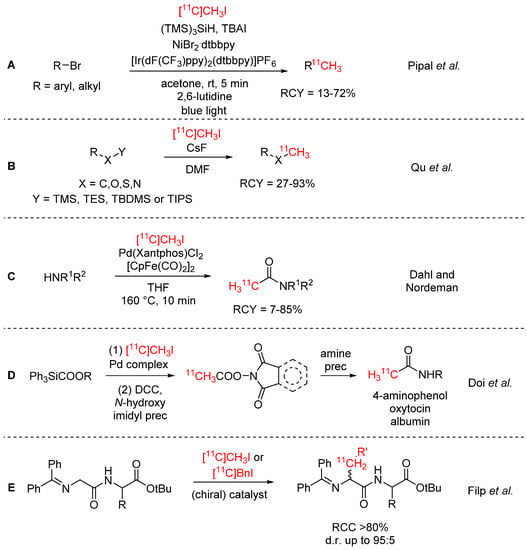
Scheme 10.
Recent progress in [11C]CH3I chemistry [25,61,62,63,64].
As an alternative to [11C]CO or [11C]acetyl chloride chemistry, Dahl and Nordeman developed a procedure for 11C-acetylation of amines with [11C]methyl iodide (see Scheme 10C) [62]. Bis(cyclopentadienyldicarbonyliron) was used as the CO source in the Pd-mediated reaction. The reaction was established for a range of primary amine precursors, including three biologically relevant compounds, and a few examples of secondary amines. A different approach to the same functional group was presented by Doi et al., whereby [11C]acetic acid was synthesized in a palladium-mediated cross-coupling reaction from [11C]methyl iodide and carboxytriphenylsilane, then converted to the [11C]acetic acid phthalimidyl ester or succinimidyl ester (see Scheme 10D) [63]. The imidyl esters were subsequently employed in a 11C-acetylation reaction with small, medium-sized, and large molecules.
In an effort to develop a stereoselective 11C-alkylation procedure for diastereomerically enriched dipeptides, Filp et al. investigated the use of various quaternary ammonium salts as chiral phase-transfer catalysts in the 11C-alkylation of N-terminal glycine Schiff bases (see Scheme 10E) [64]. Next to [11C]methyl iodide, the procedure was also applied to [11C]benzyl iodide. RCCs of >80% and high diastereomeric ratios (d.r.) of up to 95:5 were obtained. A similar strategy has been used by Pekošak et al. for the stereoselective 11C-labelling of the tetrapeptide Phe-D-Trp-Lys-Thr with [11C]benzyl iodide [65]. [11C]Phe-D-Trp-Lys-Thr was synthesized over five steps starting from [11C]CO2 and isolated with high stereoselectivity (94% de), RCYs of 9–10% (dc), and Am of 15–35 GBq/µmol.
To address the shortcomings of current cross-coupling strategies with [11C]methyl iodide, Helbert et al. developed a new cross-coupling procedure with [11C]methyllithium. [11C]Methyllithium was developed as a more reactive alternative for [11C]methyl iodide and can be synthesized by reaction of [11C]methyl iodide with n-butyllithium [66,67]. In the procedure of Herbert et al., [11C]methyllithium was added without intermediate purification to the aryl bromide precursors and a selection of relevant PET tracers was labelled by palladium-mediated 11C-C cross-coupling with RCYs of 33–57% (see Scheme 11) [68].
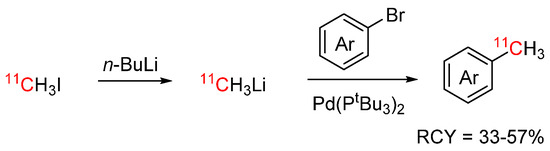
Scheme 11.
11C-C cross-coupling with [11C]methyllithium [68].
2.4. [11C]Hydrogen Cyanide
Since its inception in the 1960s [69], [11C]HCN has developed into a versatile building block for the 11C-labelling of neurotransmitters, amino acids, and other molecules. This is mainly due to its versatility: It can function as nucleophile as well as electrophile, and [11C]cyanide incorporation generates many different functionalities, such as nitriles, hydantoins, (thio)cyanates and, through subsequent reaction, carboxylic acids, aldehydes, amides and amines. Two extensive reviews on [11C]hydrogen cyanide have been recently published, therefore this 11C-building block will not be discussed in detail herein [70,71]. Since [11C]hydrogen cyanide is one of the few 11C-building blocks used for FIH PET tracers in recent years (vide infra), we will provide a brief summary of recent work that has not been covered by other reviews.
[11C]Hydrogen cyanide is typically produced by reacting [11C]CH4 with NH3 gas on a platinum catalyst at 1000 °C. While fully automated production systems are commercially available, [11C]HCN is not widely used. In an effort to make [11C]hydrogen cyanide more accessible, Kikuchi et al. developed a novel synthesis strategy from widely available [11C]methyl iodide (see Scheme 12) [72]. This method involves passing [11C]methyl iodide over a heated reaction column, in which it is first converted to [11C]formaldehyde and subsequently to [11C]hydrogen cyanide. The [11C]hydrogen cyanide is obtained fast and with RCYs comparable to the traditional method (50–60% at EOB), without the need for specialized equipment.
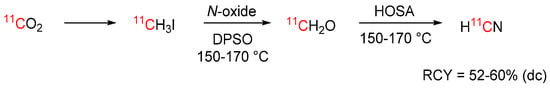
Scheme 12.
Production of [11C]hydrogen cyanide from [11C]methyl iodide [72].
2.5. [11C]Fluoroform
Due to the prevalence of CF3 groups in drugs and other biologically active compounds, there has been much interest in labelling this group with carbon-11 and fluorine-18. Haskali et al. published a synthesis procedure for carbon-11 labelled fluoroform in 2017, where cyclotron-produced [11C]methane was fluorinated by passing it over a CoF3 column at elevated temperatures (270 °C) [73]. [11C]Fluoroform was obtained with RCYs of ~60%. The process was not only fast and reproducible, but the developed system also required very little maintenance. [11C]Fluoroform was reacted with various model compounds (see Scheme 13), in addition to three biologically active compounds.
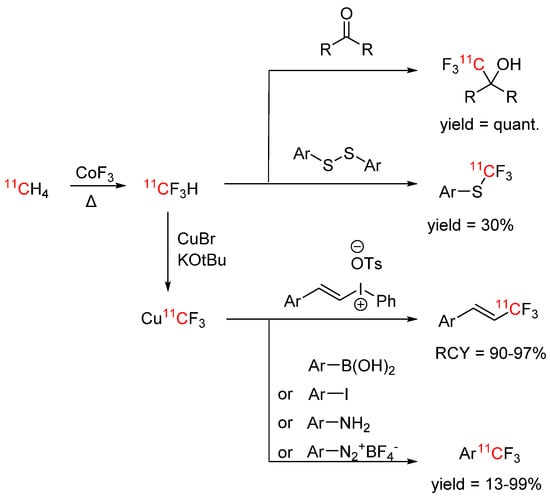
Scheme 13.
[11C]Fluoroform chemistry [73,74,75].
Whereas flourine-18 labelled fluoroform generally suffers from low molar activities (≤1 GBq/µmol) and only few examples of higher molar activities are known, high molar activities of >200 GBq/µmol were easily obtained with carbon-11 labelled fluoroform. In later works, the substrate scope of reactions with [11C]fluoroform was broadened from aryl boronates, aryl iodides, ketones, diazonium salts, and diarylsulfanes to aryl amines and arylvinyl iodonium tosylates (see Scheme 13) [74,75].
2.6. [11C]Carbonyl Difluoride
As an alternative strategy to access carbon-11 labelled ureas, carbamates, and thiocarbamates, Jakobsson et al. presented the [11C]carbonyl group transfer agent [11C]carbonyl difluoride [76]. [11C]Carbonyl difluoride was synthesized quantitatively by passing [11C]CO over a AgF2 column at room temperature. The building block was subsequently reacted with diamines, aminoalcohols, and aminothiols to form the corresponding cyclic azolidin-2-ones (see Scheme 14) under mild conditions with very low precursor quantities, and even in presence of water. The same laboratory expanded their procedure to linear unsymmetrical ureas and established reaction conditions for a broad scope of aryl and aliphatic amines [77]. For the aryl amines, [11C]carbonyl fluoride was trapped in a solution with the aryl amine precursor and subsequently reacted with another amine. For the aliphatic amines, alkylammonium tosylate precursors were used in the first step to lower the reactivity of the amine and prevent symmetrical urea formation. Pyridine was used to improve [11C]carbonyl fluoride trapping. Suitability for PET tracer synthesis was demonstrated by labelling the epoxide hydrolase inhibitor [11C]AR-9281, which was obtained after optimization in high RCYs of 80%.
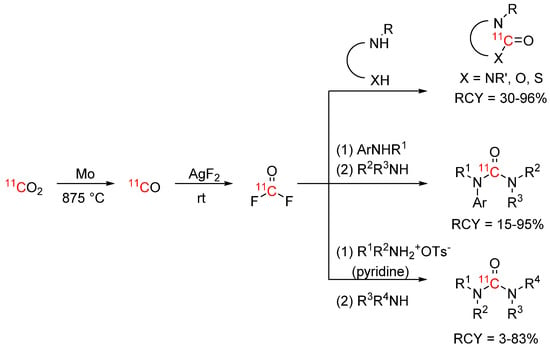
Scheme 14.
[11C]Carbonyl difluoride synthesis and subsequent reaction to cyclic products and linear unsymmetrical ureas [76,77].
2.7. [11C]Carbon Disulfide
[11C]Carbon disulfide, the sulfur analog of [11C]carbon dioxide, is an interesting 11C-building block for the synthesis of organosulfur compounds. It has first been described in 1984, and had limited utility until a decade ago when Miller and Bender proposed a new synthesis strategy, which was further improved by Haywood et al. [78,79,80]. It can now be readily obtained through the reaction of [11C]CH3I with elemental sulfur and has been used to synthesize [11C]thioureas, thiocarbamates and related structures. Cesarec et al. recently published a procedure for the synthesis of late transition metal complexes with [11C]dithiocarbamate ligands [81]. To this end, [11C]carbon disulfide was reacted with diethyl amine or dibenzyl amine to form the respective ammonium [11C]dithiocarbamate salt and subsequently reacted with Au(I), Au(III), Pd(II) or Pt(II) complexes to form the respective complexes in RCYs > 70% (see Scheme 15).
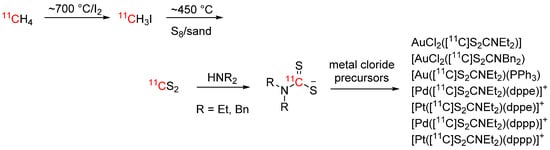
Scheme 15.
Synthesis of [11C]carbon disulfide and formation of [11C]dithiocarbamate transition metal complexes [81].
2.8. [11C]Thiocyanate
[11C]Thiocyanate is an interesting 11C-building block because of its reactivity and potential to give access to a wide range of organosulfur derivatives. Up until recently, its production relied on the use of [11C]HCN [82]. Haywood et al. presented a new way to synthesize this 11C-building block by reacting [11C]carbon disulfide with ammonia at 90 °C to form ammonium [11C]thiocyanate in near quantitative RCC [83]. The ammonium [11C]thiocyanate was subsequently reacted with benzyl bromide, a range of α-ketobromides, and mannose triflate in high RCYs of ≥75% (see Scheme 16). The α-[11C]thiocyanatophenones could also be cyclized in the presence of sulfuric and acetic acid to 11C-thiazolones.
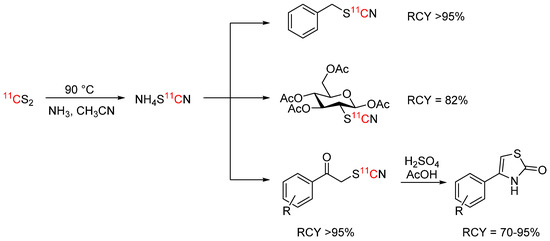
Scheme 16.
Synthesis of and reactions with ammonium [11C]thiocyanate [83].
2.9. [11C]Formaldehyde
[11C]Formaldehyde is an established and versatile building block for carbon-11 chemistry (see [84] and references therein). Many different synthetic strategies have been proposed, traditionally involving reduction in cyclotron-produced [11C]CO2 to [11C]CH3OH and subsequent oxidation to [11C]formaldehyde. Nader et al. recently proposed the use of XeF2 as an oxidizing agent (see Scheme 17) [85]. [11C]Formaldehyde was obtained in non-decay corrected RCYs of 54 ± 5% starting from [11C]CO2 and was used in a proof-of principle synthesis to form α-(N-[11C]methylamino)isobutyric acid via reductive 11C-methylation.
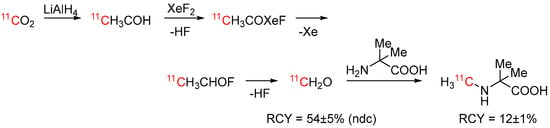
Scheme 17.
Synthesis of [11C]formaldehyde using XeF2 as oxidizing agent and reaction to [11C]Me-AIB [85].
3. First-in-Human Translation
Despite the short physical half-life and the need for an on-site cyclotron, 11C continues to be a favoured radionuclide for small molecule PET tracers. As discussed in this review, innovations continue in 11C-radiolabelling strategies for applications in 11C-tracer development. Within the past five years, to our knowledge at least 27 novel 11C-labelled PET tracers have been translated for FIH PET studies (see Figure 1) [86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109]. Unsurprisingly, the vast majority of these PET tracers were designed to image targets within the CNS (see Table 1). Carbon-11 is ideal for CNS PET because the substitution of naturally occurring 12C with 11C does not change the physicochemical properties of the compound, thereby enabling imaging with isotopologues of the molecules of interest for accurate determination of brain penetrance, target affinity, pharmacokinetics, or pharmacodynamics of the molecule, and multiple scans can be performed in the same subject in the same day. Interestingly, many of the 11C-labelled PET tracers for FIH use focused on imaging markers of neuroinflammation, a critical component in the etiology and pathology of several neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) [110,111,112,113,114]. The remaining PET tracers translated for FIH studies that were reported in the past five years strived to image non-CNS targets, including bacterial infection and lung inflammation.
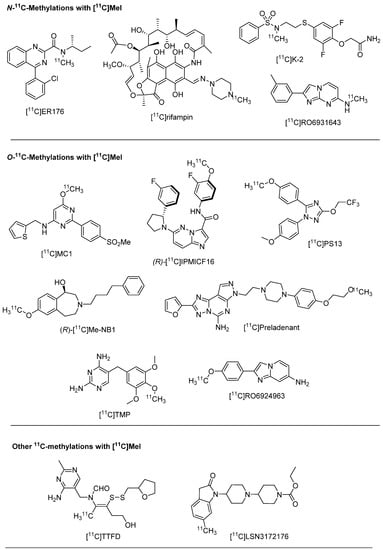
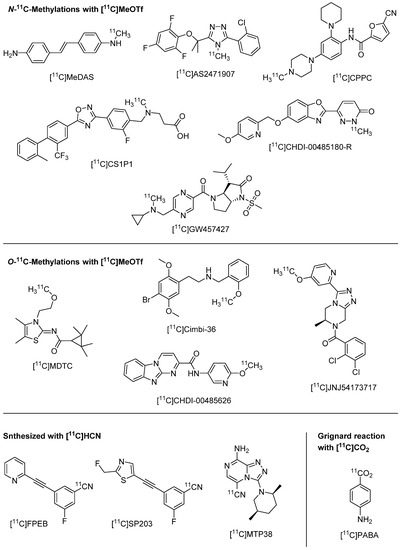
Figure 1.
Chemical structures of the majority of first-in-human PET tracers labelled with 11C since 2017. Targets, publication years and references of the tracers are listed in Table 1.

Table 1.
First-in-human 11C PET tracers and their targets reported since 2017.
When sorting the tracers according to the labelling method, it becomes immediately apparent that the predominant synthetic strategy remains 11C-methylation of hydroxy or amino precursors: more than ¾ of all tracers were synthesized via this strategy, either using [11C]methyl iodide or [11C]methyl triflate as the 11C-buidling block (see Figure 2). This can be attributed to the accessibility of these building blocks from cyclotron-produced [11C]CO2 or [11C]CH4 and the availability of commercial synthesis devices (vide supra) [58]. Other tracers have been synthesized by alternate 11C-labelling strategies, using [11C]HCN or Grignard reactions with [11C]CO2. The latest developments in 11C-chemistry are not represented among the FIH tracers, which is not surprising since it usually takes time for a new method to be implemented by the broader community. However, many of the existing 11C-building blocks have not been introduced in the past few years but have been around for decades and should, therefore, be available for clinical application. As indicated in some cases (e.g., [11C]CO chemistry), it may be the historic lack of specialized or commercially available radiosynthesis equipment that hampers FIH translation of new PET tracers. Other reasons could be that new 11C-methodologies are often only developed up to the point of proof-of-principle and not optimized for automated tracer production. Rather than further broadening the scope of 11C-chemistry, future efforts should focus on closing the gap between new method development and clinical translation.
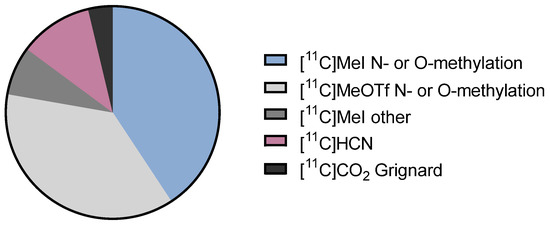
Figure 2.
Graphical representation of the proportions of 11C-labelling strategies used for FIH PET tracers since 2017.
Author Contributions
Conceptualization, A.P. and N.V.; writing—original draft preparation, A.P.; writing—review and editing, M.C., A.L. and N.V.; visualization, A.P.; supervision, N.V. All authors have read and agreed to the published version of the manuscript.
Funding
A.P. is supported by the CAMH Discovery Fund. M.C. was supported by the Canadian Institute for Health Research with a Canadian Graduate Scholarship (CGS-M), as well as a Mitacs Accelerate Internship award provided by the Structural Genomics Consortium (SGC). N.V. is supported by the Azrieli Foundation, the Canada Research Chairs Program, Canada Foundation for Innovation and the Ontario Research Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no relevant conflict of interest.
Abbreviations
| 4CzBnBN | (2,3,4,6)-3-Benzyl-2,4,5,6-tetra(9H-carbazol-9-yl)benzonitrile |
| 5-HT2A receptor | 5-Hydroxy-tryptamine 2A receptor |
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| Am | Molar activity |
| AMPA receptor | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| BEMP | 2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine |
| CB2 receptor | Cannabinoid receptor type 2 |
| CNS | Central nervous system |
| COX-1, -2 | Cyclooxygenase-1, -2 |
| CSF1 | colony stimulating factor 1 |
| CuTC | Copper(I) thiophene-2-carboxylate |
| DBAD | Di-tert-butyl azodicarboxylate |
| DBN | 1,5-Diazabicyclo [4.3.0]non-5-ene |
| DBU | 1,8-diazabicyclo [5.4.0]undec-7-ene |
| dc | Decay-corrected |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| de | Diastereomeric excess |
| DMA | Dimethylacetamide |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| DPSO | Diphenyl sulfoxide |
| d.r. | Diastereomeric ratio |
| dtbbpy | 4,4’-Di-tert-butyl-2,2’-bipyridine |
| FIH | First-in-human |
| FMDS | Fluoride-mediated desilylation |
| HOSA | Hydroxylamine-O-sulfonic acid |
| HPLC | High-performance liquid chromatography |
| IPr | 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) |
| K222 | 4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo [8.8.8]hexacosane |
| MAO-B | Monoamine oxidase B |
| mGluR5 | Metabotropic glutamate receptor 5 |
| mHTT | Mutant huntingtin protein |
| NMDA | N-methyl-D-aspartate |
| NMP | N-Methyl-2-pyrrolidone |
| PD | Parkinson’s disease |
| PDE7 | Phosphodiesterase 7 |
| PET | Positron emission tomography |
| ppy | 2-Phenylpyridine |
| prec | precursor |
| quant. | quantitative |
| RCC | Radiochemical conversion |
| RCP | Radiochemical purity |
| RCY | Radiochemical yield |
| Ref. | Reference |
| rt | Room temperature |
| t1/2 | Half-life |
| TBAT | Tetrabutylammonium difluorotriphenylsilicate |
| TBAI | Tetra-n-butylammonium iodide |
| TE | Trapping efficiency |
| THF | Tetrahydrofuran |
| TMEDA | Tetramethylethylenediamine |
| TMS | Trimetylsilyl |
| TrkB/C | Tropomyosin receptor kinase B/C |
| TSPO | Translocator protein |
References
- Liang, S.H.; Vasdev, N. Total Radiosynthesis: Thinking Outside ‘the Box’. Aust. J. Chem. 2015, 68, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Rong, J.; Wang, L.; Vasdev, N.; Zhang, L.; Josephson, L.; Liang, S.H. Chemistry for Positron Emission Tomography: Recent Advances in 11C-, 18F-, 13N-, and 15O-Labeling Reactions. Angew. Chem. Int. Ed. 2019, 58, 2580–2605. [Google Scholar] [CrossRef]
- Ajenjo, J.; Destro, G.; Cornelissen, B.; Gouverneur, V. Closing the Gap between 19F and 18F Chemistry. EJNMMI Radiopharm. Chem. 2021, 6, 33. [Google Scholar] [CrossRef]
- Van der Born, D.; Pees, A.; Poot, A.J.; Orru, R.V.A.; Windhorst, A.D.; Vugts, D.J. Fluorine-18 Labelled Building Blocks for PET Tracer Synthesis. Chem. Soc. Rev. 2017, 46, 4709–4773. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Gauthier, V.; Lepage, M.L.; Waengler, B.; Bailey, J.J.; Liang, S.H.; Perrin, D.M.; Vasdev, N.; Schirrmacher, R. Recent Advances in 18F Radiochemistry: A Focus on B-18F, Si-18F, Al-18F, and C-18F Radiofluorination via Spirocyclic Iodonium Ylides. J. Nucl. Med. 2018, 59, 568–572. [Google Scholar] [CrossRef]
- Goud, N.S.; Joshi, R.K.; Bharath, R.D.; Kumar, P. Fluorine-18: A Radionuclide with Diverse Range of Radiochemistry and Synthesis Strategies for Target Based PET Diagnosis. Eur. J. Med. Chem. 2020, 187, 111979. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Q.; Shi, H.; Cheng, D. Fluorine-18: Radiochemistry and Target-Specific PET Molecular Probes Design. Front. Chem. 2022, 10, 884517. [Google Scholar] [CrossRef]
- Bratteby, K.; Shalgunov, V.; Herth, M.M. Aliphatic 18F-Radiofluorination: Recent Advances in the Labeling of Base-Sensitive Substrates. ChemMedChem 2021, 16, 2612–2622. [Google Scholar] [CrossRef]
- Francis, F.; Wuest, F. Advances in [18F]Trifluoromethylation Chemistry for PET Imaging. Molecules 2021, 26, 6478. [Google Scholar] [CrossRef]
- Wright, J.S.; Kaur, T.; Preshlock, S.; Tanzey, S.S.; Winton, W.P.; Sharninghausen, L.S.; Wiesner, N.; Brooks, A.F.; Sanford, M.S.; Scott, P.J.H. Copper-Mediated Late-Stage Radiofluorination: Five Years of Impact on Preclinical and Clinical PET Imaging. Clin. Transl. Imaging 2020, 8, 167–206. [Google Scholar] [CrossRef]
- Bui, T.T.; Kim, H. Recent Advances in Photo-mediated Radiofluorination. Chem. Asian J. 2021, 16, 2155–2167. [Google Scholar] [CrossRef]
- Goud, N.S.; Bhattacharya, A.; Joshi, R.K.; Nagaraj, C.; Bharath, R.D.; Kumar, P. Carbon-11: Radiochemistry and Target-Based PET Molecular Imaging Applications in Oncology, Cardiology, and Neurology. J. Med. Chem. 2021, 64, 1223–1259. [Google Scholar] [CrossRef] [PubMed]
- Boscutti, G.; Huiban, M.; Passchier, J. Use of Carbon-11 Labelled Tool Compounds in Support of Drug Development. Drug Discov. Today Technol. 2017, 25, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Dahl, K.; Halldin, C.; Schou, M. New Methodologies for the Preparation of Carbon-11 Labeled Radiopharmaceuticals. Clin. Transl. Imaging 2017, 5, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, B.H.; Liang, S.H.; Placzek, M.S.; Hooker, J.M.; Gee, A.D.; Dollé, F.; Wilson, A.A.; Vasdev, N. 11C=O Bonds Made Easily for Positron Emission Tomography Radiopharmaceuticals. Chem. Soc. Rev. 2016, 45, 4708–4726. [Google Scholar] [CrossRef]
- Pike, V.W. PET Radiotracers: Crossing the Blood–Brain Barrier and Surviving Metabolism. Trends Pharmacol. Sci. 2009, 30, 431–440. [Google Scholar] [CrossRef]
- Coenen, H.H.; Gee, A.D.; Adam, M.; Antoni, G.; Cutler, C.S.; Fujibayashi, Y.; Jeong, J.M.; Mach, R.H.; Mindt, T.L.; Pike, V.W.; et al. Consensus Nomenclature Rules for Radiopharmaceutical Chemistry—Setting the Record Straight. Nucl. Med. Biol. 2017, 55, v–xi. [Google Scholar] [CrossRef]
- Herth, M.M.; Ametamey, S.; Antuganov, D.; Bauman, A.; Berndt, M.; Brooks, A.F.; Bormans, G.; Choe, Y.S.; Gillings, N.; Häfeli, U.O.; et al. On the Consensus Nomenclature Rules for Radiopharmaceutical Chemistry—Reconsideration of Radiochemical Conversion. Nucl. Med. Biol. 2021, 93, 19–21. [Google Scholar] [CrossRef]
- Hooker, J.M.; Reibel, A.T.; Hill, S.M.; Schueller, M.J.; Fowler, J.S. One-Pot, Direct Incorporation of [11C]CO2 into Carbamates. Angew. Chem. Int. Ed. 2009, 48, 3482–3485. [Google Scholar] [CrossRef]
- Wilson, A.A.; Garcia, A.; Houle, S.; Sadovski, O.; Vasdev, N. Synthesis and Application of Isocyanates Radiolabeled with Carbon-11. Chem. Eur. J. 2011, 17, 259–264. [Google Scholar] [CrossRef]
- Wilson, A.A.; Garcia, A.; Houle, S.; Vasdev, N. Direct Fixation of [11C]CO2 by Amines: Formation of [11C-Carbonyl]-Methylcarbamates. Org. Biomol. Chem. 2010, 8, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, B.H.; Liang, S.H.; Holland, J.P.; Collier, T.L.; Hooker, J.M.; Wilson, A.A.; Vasdev, N. 11CO2 Fixation: A Renaissance in PET Radiochemistry. Chem. Commun. 2013, 49, 5621–5629. [Google Scholar] [CrossRef]
- Chassé, M.; Sen, R.; Goeppert, A.; Prakash, G.K.S.; Vasdev, N. Polyamine Based Solid CO2 Adsorbents for [11C]CO2 Purification and Radiosynthesis. J. CO2 Util. 2022, 64, 102137. [Google Scholar] [CrossRef]
- Duffy, I.R.; Vasdev, N.; Dahl, K. Copper(I)-Mediated 11C-Carboxylation of (Hetero)Arylstannanes. ACS Omega 2020, 5, 8242–8250. [Google Scholar] [CrossRef]
- Riss, P.J.; Lu, S.; Telu, S.; Aigbirhio, F.I.; Pike, V.W. CuI-Catalyzed 11C Carboxylation of Boronic Acid Esters: A Rapid and Convenient Entry to 11C-Labeled Carboxylic Acids, Esters, and Amides. Angew. Chem. Int. Ed. 2012, 51, 2698–2702. [Google Scholar] [CrossRef]
- Rotstein, B.H.; Hooker, J.M.; Woo, J.; Collier, T.L.; Brady, T.J.; Liang, S.H.; Vasdev, N. Synthesis of [11C]Bexarotene by Cu-Mediated [11C]Carbon Dioxide Fixation and Preliminary PET Imaging. ACS Med. Chem. Lett. 2014, 5, 668–672. [Google Scholar] [CrossRef]
- García-Vázquez, R.; Battisti, U.M.; Shalgunov, V.; Schäfer, G.; Barz, M.; Herth, M.M. [11C]Carboxylated Tetrazines for Facile Labeling of Trans-Cyclooctene-Functionalized PeptoBrushes. Macromol. Rapid Commun. 2022, 43, 2100655. [Google Scholar] [CrossRef]
- Goudou, F.; Gee, A.D.; Bongarzone, S. Carbon-11 Carboxylation of Terminal Alkynes with [11C]CO2. J. Label. Compd. Radiopharm. 2021, 64, 237–242. [Google Scholar] [CrossRef]
- Bongarzone, S.; Raucci, N.; Fontana, I.C.; Luzi, F.; Gee, A.D. Carbon-11 Carboxylation of Trialkoxysilane and Trimethylsilane Derivatives Using [11C]CO2. Chem. Commun. 2020, 56, 4668–4671. [Google Scholar] [CrossRef]
- Qu, W.; Hu, B.; Babich, J.W.; Waterhouse, N.; Dooley, M.; Ponnala, S.; Urgiles, J. A General 11C-Labeling Approach Enabled by Fluoride-Mediated Desilylation of Organosilanes. Nat. Commun. 2020, 11, 1736. [Google Scholar] [CrossRef]
- Destro, G.; Horkka, K.; Loreau, O.; Buisson, D.; Kingston, L.; Del Vecchio, A.; Schou, M.; Elmore, C.S.; Taran, F.; Cantat, T.; et al. Transition-Metal-Free Carbon Isotope Exchange of Phenyl Acetic Acids. Angew. Chem. 2020, 132, 13592–13597. [Google Scholar] [CrossRef]
- Kong, D.; Munch, M.; Qiqige, Q.; Cooze, C.J.C.; Rotstein, B.H.; Lundgren, R.J. Fast Carbon Isotope Exchange of Carboxylic Acids Enabled by Organic Photoredox Catalysis. J. Am. Chem. Soc. 2021, 143, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- Bsharat, O.; Doyle, M.G.J.; Munch, M.; Mair, B.A.; Cooze, C.J.C.; Derdau, V.; Bauer, A.; Kong, D.; Rotstein, B.H.; Lundgren, R.J. Aldehyde-Catalysed Carboxylate Exchange in α-Amino Acids with Isotopically Labelled CO2. Nat. Chem. 2022, 14, 1367–1374. [Google Scholar] [CrossRef]
- Bongarzone, S.; Runser, A.; Taddei, C.; Dheere, A.K.H.; Gee, A.D. From [11C]CO2 to [11C]Amides: A Rapid One-Pot Synthesis via the Mitsunobu Reaction. Chem. Commun. 2017, 53, 5334–5337. [Google Scholar] [CrossRef]
- Mair, B.A.; Fouad, M.H.; Ismailani, U.S.; Munch, M.; Rotstein, B.H. Rhodium-Catalyzed Addition of Organozinc Iodides to Carbon-11 Isocyanates. Org. Lett. 2020, 22, 2746–2750. [Google Scholar] [CrossRef]
- Horkka, K.; Dahl, K.; Bergare, J.; Elmore, C.S.; Halldin, C.; Schou, M. Rapid and Efficient Synthesis of 11C-Labeled Benzimidazolones Using [11C]Carbon Dioxide. ChemistrySelect 2019, 4, 1846–1849. [Google Scholar] [CrossRef]
- Luzi, F.; Gee, A.D.; Bongarzone, S. Rapid One-Pot Radiosynthesis of [Carbonyl-11C]Formamides from Primary Amines and [11C]CO2. EJNMMI Radiopharm. Chem. 2020, 5, 20. [Google Scholar] [CrossRef]
- Dahl, K.; Collier, T.L.; Cheng, R.; Zhang, X.; Sadovski, O.; Liang, S.H.; Vasdev, N. “In-Loop” [11C]CO2 Fixation: Prototype and Proof of Concept. J. Label. Compd. Radiopharm. 2018, 61, 252–262. [Google Scholar] [CrossRef]
- Downey, J.; Bongarzone, S.; Hader, S.; Gee, A.D. In-Loop Flow [11C]CO2 Fixation and Radiosynthesis of N,N′-[11C]Dibenzylurea. J. Label. Compd. Radiopharm. 2018, 61, 263–271. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Caillé, F.; Chevalier, A.; Loreau, O.; Horkka, K.; Halldin, C.; Schou, M.; Camus, N.; Kessler, P.; Kuhnast, B.; et al. Late-Stage Isotopic Carbon Labeling of Pharmaceutically Relevant Cyclic Ureas Directly from CO2. Angew. Chem. 2018, 130, 9892–9896. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Talbot, A.; Caillé, F.; Chevalier, A.; Sallustrau, A.; Loreau, O.; Destro, G.; Taran, F.; Audisio, D. Carbon Isotope Labeling of Carbamates by Late-Stage [11C], [13C] and [14C]Carbon Dioxide Incorporation. Chem. Commun. 2020, 56, 11677–11680. [Google Scholar] [CrossRef]
- Babin, V.; Sallustrau, A.; Loreau, O.; Caillé, F.; Goudet, A.; Cahuzac, H.; Del Vecchio, A.; Taran, F.; Audisio, D. A General Procedure for Carbon Isotope Labeling of Linear Urea Derivatives with Carbon Dioxide. Chem. Commun. 2021, 57, 6680–6683. [Google Scholar] [CrossRef] [PubMed]
- Liger, F.; Cadarossanesaib, F.; Iecker, T.; Tourvieille, C.; Le Bars, D.; Billard, T. 11C-Labeling: Intracyclic Incorporation of Carbon-11 into Heterocycles: 11C-Labeling: Intracyclic Incorporation of Carbon-11 into Heterocycles. Eur. J. Org. Chem. 2019, 2019, 6968–6972. [Google Scholar] [CrossRef]
- Haji Dheere, A.K.; Bongarzone, S.; Shakir, D.; Gee, A. Direct Incorporation of [11C]CO2 into Asymmetric [11C]Carbonates. J. Chem. 2018, 2018, 7641304. [Google Scholar] [CrossRef]
- Lindberg, A.; Vasdev, N. Ring-Opening of Non-Activated Aziridines with [11C]CO2 via Novel Ionic Liquids. RSC Adv. 2022, 12, 21417–21421. [Google Scholar] [CrossRef] [PubMed]
- Taddei, C.; Pike, V.W. [11C]Carbon Monoxide: Advances in Production and Application to PET Radiotracer Development over the Past 15 Years. EJNMMI Radiopharm. Chem. 2019, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Antoni, G.; Långström, B.; Itsenko, O. The Development of 11C-Carbonylation Chemistry: A Systematic View. Nucl. Med. Biol. 2021, 92, 115–137. [Google Scholar] [CrossRef]
- Nielsen, D.U.; Neumann, K.T.; Lindhardt, A.T.; Skrydstrup, T. Recent Developments in Carbonylation Chemistry Using [13C]CO, [11C]CO, and [14C]CO. J. Label. Compd. Radiopharm. 2018, 61, 949–987. [Google Scholar] [CrossRef]
- Taddei, C.; Gee, A.D. Recent Progress in [11C]Carbon Dioxide ([11C]CO2) and [11C]Carbon Monoxide ([11C]CO) Chemistry. J. Label. Compd. Radiopharm. 2018, 61, 237–251. [Google Scholar] [CrossRef]
- Shegani, A.; Kealey, S.; Luzi, F.; Basagni, F.; Machado, J.D.M.; Ekici, S.D.; Ferocino, A.; Gee, A.D.; Bongarzone, S. Radiosynthesis, Preclinical, and Clinical Positron Emission Tomography Studies of Carbon-11 Labeled Endogenous and Natural Exogenous Compounds. Chem. Rev. 2023, 123, 105–229. [Google Scholar] [CrossRef] [PubMed]
- Dahl, K.; Turner, T.; Vasdev, N. Radiosynthesis of a Bruton’s Tyrosine Kinase Inhibitor, [11C]Tolebrutinib, via Palladium-NiXantphos-mediated Carbonylation. J. Label. Compd. Radiopharm. 2020, 63, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, A.; Boyle, A.J.; Tong, J.; Harkness, M.B.; Garcia, A.; Tran, T.; Zhai, D.; Liu, F.; Donnelly, D.J.; Vasdev, N. Radiosynthesis of [11C]Ibrutinib via Pd-Mediated [11C]CO Carbonylation: Preliminary PET Imaging in Experimental Autoimmune Encephalomyelitis Mice. Front. Nucl. Med. 2021, 1, 772289. [Google Scholar] [CrossRef]
- Donnelly, D.J.; Preshlock, S.; Kaur, T.; Tran, T.; Wilson, T.C.; Mhanna, K.; Henderson, B.D.; Batalla, D.; Scott, P.J.H.; Shao, X. Synthesis of Radiopharmaceuticals via “In-Loop” 11C-Carbonylation as Exemplified by the Radiolabeling of Inhibitors of Bruton’s Tyrosine Kinase. Front. Nucl. Med. 2022, 1, 820235. [Google Scholar] [CrossRef]
- Langstrom, B.; Lundqvist, H. The Preparation of 11C-Methyl Iodide and Its Use in the Synthesis of 11C-Methyl-Methionine. Int. J. Appl. Radiat. Isot. 1976, 27, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Comar, D.; Cartron, J.-C.; Maziere, M.; Marazano, C. Labelling and Metabolism of Methionine-Methyl-11C. Eur. J. Nucl. Med. 1976, 1, 11–14. [Google Scholar] [CrossRef]
- Marazano, C.; Maziere, M.; Berger, G.; Comar, D. Synthesis of Methyl Iodide-11C and Formaldehyde-11C. Int. J. Appl. Radiat. Isot. 1977, 28, 49–52. [Google Scholar] [CrossRef]
- Jewett, D.M. A Simple Synthesis of [11C]Methyl Triflate. Appl. Radiat. Isot. 1992, 43, 1383–1385. [Google Scholar] [CrossRef]
- Mock, B. Automated C-11 Methyl Iodide/Triflate Production: Current State of the Art. Curr. Org. Chem. 2013, 17, 2119–2126. [Google Scholar] [CrossRef]
- Doi, H. Pd-Mediated Rapid Cross-Couplings Using [11C]Methyl Iodide: Groundbreaking Labeling Methods in 11C Radiochemistry: Development of Pd-Mediated Rapid C-[11C]Methylations. J. Label. Compd. Radiopharm. 2015, 58, 73–85. [Google Scholar] [CrossRef]
- Rokka, J.; Nordeman, P.; Roslin, S.; Eriksson, J. A Comparative Study on Suzuki-type 11C-methylation of Aromatic Organoboranes Performed in Two Reaction Media. J. Label. Compd. Radiopharm. 2021, 64, 447–455. [Google Scholar] [CrossRef]
- Pipal, R.W.; Stout, K.T.; Musacchio, P.Z.; Ren, S.; Graham, T.J.A.; Verhoog, S.; Gantert, L.; Lohith, T.G.; Schmitz, A.; Lee, H.S.; et al. Metallaphotoredox Aryl and Alkyl Radiomethylation for PET Ligand Discovery. Nature 2021, 589, 542–547. [Google Scholar] [CrossRef]
- Dahl, K.; Nordeman, P. 11C-Acetylation of Amines with [11C]Methyl Iodide with Bis(Cyclopentadienyldicarbonyliron) as the CO Source: 11C-Acetylation of Amines with [11C]Methyl Iodide with Bis(Cyclopentadienyldicarbonyliron) as the CO Source. Eur. J. Org. Chem. 2017, 2017, 5785–5788. [Google Scholar] [CrossRef]
- Doi, H.; Goto, M.; Sato, Y. Pd0 -Mediated Cross-Coupling of [11C]Methyl Iodide with Carboxysilane for Synthesis of [11C]Acetic Acid and Its Active Esters: 11C-Acetylation of Small, Medium, and Large Molecules. Eur. J. Org. Chem. 2021, 2021, 3970–3979. [Google Scholar] [CrossRef]
- Filp, U.; Pekošak, A.; Poot, A.J.; Windhorst, A.D. Stereocontrolled [11C]Alkylation of N-Terminal Glycine Schiff Bases To Obtain Dipeptides: Stereocontrolled [11C]Alkylation of N-Terminal Glycine Schiff Bases To Obtain Dipeptides. Eur. J. Org. Chem. 2017, 2017, 5592–5596. [Google Scholar] [CrossRef]
- Pekošak, A.; Rotstein, B.H.; Collier, T.L.; Windhorst, A.D.; Vasdev, N.; Poot, A.J. Stereoselective 11C Labeling of a “Native” Tetrapeptide by Using Asymmetric Phase-Transfer Catalyzed Alkylation Reactions. Eur. J. Org. Chem. 2017, 2017, 1019–1024. [Google Scholar] [CrossRef]
- Reiffers, S.; Vaalburg, W.; Wiegman, T.; Wynberg, H.; Woldring, M.G. Carbon-11 Labelled Methyllithium as Methyl Donating Agent: The Addition to 17-Keto Steroids. Int. J. Appl. Radiat. Isot. 1980, 31, 535–539. [Google Scholar] [CrossRef]
- Berger, G.; Maziere, M.; Prenant, C.; Sastre, J.; Comar, D. Synthesis of High Specific Activity 11C 17alpha Methyltestosterone. Int. J. Appl. Radiat. Isot. 1981, 32, 811–815. [Google Scholar] [CrossRef]
- Helbert, H.; Antunes, I.F.; Luurtsema, G.; Szymanski, W.; Feringa, B.L.; Elsinga, P.H. Cross-Coupling of [11C]Methyllithium for 11C-Labelled PET Tracer Synthesis. Chem. Commun. 2021, 57, 203–206. [Google Scholar] [CrossRef]
- Dubrin, J.; MacKay, C.; Pandow, M.L.; Wolfgang, R. Reactions of Atomic Carbon with Pi-Bonded Inorganic Molecules. J. lnorg. Nucl. Chem. 1964, 26, 2113–2122. [Google Scholar] [CrossRef]
- Xu, Y.; Qu, W. [11C]HCN Radiochemistry: Recent Progress and Future Perspectives. Eur. J. Org. Chem. 2021, 2021, 4653–4682. [Google Scholar] [CrossRef]
- Zhou, Y.-P.; Makaravage, K.J.; Brugarolas, P. Radiolabeling with [11C]HCN for Positron Emission Tomography. Nucl. Med. Biol. 2021, 102–103, 56–86. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Ogawa, M.; Okamura, T.; Gee, A.D.; Zhang, M.-R. Rapid ‘on-Column’ Preparation of Hydrogen [11C]Cyanide from [11C]Methyl Iodide via [11C]Formaldehyde. Chem. Sci. 2022, 13, 3556–3562. [Google Scholar] [CrossRef] [PubMed]
- Haskali, M.B.; Pike, V.W. [11C]Fluoroform, a Breakthrough for Versatile Labeling of PET Radiotracer Trifluoromethyl Groups in High Molar Activity. Chem. Eur. J. 2017, 23, 8156–8160. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Telu, S.; Yang, B.Y.; Haskali, M.B.; Jakobsson, J.E.; Pike, V.W. Rapid Syntheses of [11C]Arylvinyltrifluoromethanes through Treatment of (E)-Arylvinyl(Phenyl)Iodonium Tosylates with [11C]Trifluoromethylcopper(I). Org. Lett. 2020, 22, 4574–4578. [Google Scholar] [CrossRef] [PubMed]
- Young, N.J.; Pike, V.W.; Taddei, C. Rapid and Efficient Synthesis of [11C]Trifluoromethylarenes from Primary Aromatic Amines and [11C]CuCF3. ACS Omega 2020, 5, 19557–19564. [Google Scholar] [CrossRef]
- Jakobsson, J.E.; Lu, S.; Telu, S.; Pike, V.W. [11C]Carbonyl Difluoride—A New and Highly Efficient [11C]Carbonyl Group Transfer Agent. Angew. Chem. Int. Ed. 2020, 59, 7256–7260. [Google Scholar] [CrossRef]
- Jakobsson, J.E.; Telu, S.; Lu, S.; Jana, S.; Pike, V.W. Broad Scope and High-Yield Access to Unsymmetrical Acyclic [11C]Ureas for Biomedical Imaging from [11C]Carbonyl Difluoride. Chem. Eur. J. 2021, 27, 10369–10376. [Google Scholar] [CrossRef]
- Niisawa, K.; Ogawa, K.; Saito, J.; Taki, K.; Karasawa, T.; Nozaki, T. Production of No-Carrier-Added 11C-Carbon Disulfide and 11C-Hydrogen Cyanide by Microwave Discharge. Int. J. Appl. Radiat. Isot. 1984, 35, 29–33. [Google Scholar] [CrossRef]
- Miller, P.W.; Bender, D. [11C]Carbon Disulfide: A Versatile Reagent for PET Radiolabelling. Chem. Eur. J. 2012, 18, 433–436. [Google Scholar] [CrossRef]
- Haywood, T.; Kealey, S.; Sánchez-Cabezas, S.; Hall, J.J.; Allott, L.; Smith, G.; Plisson, C.; Miller, P.W. Carbon-11 Radiolabelling of Organosulfur Compounds: 11C Synthesis of the Progesterone Receptor Agonist Tanaproget. Chem. Eur. J. 2015, 21, 9034–9038. [Google Scholar] [CrossRef]
- Cesarec, S.; Edgar, F.; Lai, T.; Plisson, C.; White, A.J.P.; Miller, P.W. Synthesis of Carbon-11 Radiolabelled Transition Metal Complexes Using 11 C-Dithiocarbamates. Dalton Trans. 2022, 51, 5004–5008. [Google Scholar] [CrossRef]
- Stone-Elander, S.; Roland, P.; Halldin, C.; Hassan, M.; Seitz, R. Synthesis of [11C]Sodium Thiocyanate for In Vivo Studies of Anion Kinetics Using Positron Emission Tomography (PET). Nucl. Med. Biol. 1989, 16, 741–746. [Google Scholar] [CrossRef]
- Haywood, T.; Cesarec, S.; Kealey, S.; Plisson, C.; Miller, P.W. Ammonium [11C]Thiocyanate: Revised Preparation and Reactivity Studies of a Versatile Nucleophile for Carbon-11 Radiolabelling. Med. Chem. Commun. 2018, 9, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Hooker, J.M.; Schönberger, M.; Schieferstein, H.; Fowler, J.S. A Simple, Rapid Method for the Preparation of [11C]Formaldehyde. Angew. Chem. Int. Ed. 2008, 47, 5989–5992. [Google Scholar] [CrossRef] [PubMed]
- Nader, M.; Oberdorfer, F.; Herrmann, K. Production of [11C]Formaldehyde by the XeF2 Mediated Oxidation of [11C]Methanol and Its Application in the Labeling of α-(N-[11C]Methylamino)Isobutyric Acid. Appl. Radiat. Isot. 2019, 148, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Rischka, L.; Vraka, C.; Pichler, V.; Rasul, S.; Nics, L.; Gryglewski, G.; Handschuh, P.; Murgaš, M.; Godbersen, G.M.; Silberbauer, L.R.; et al. First-in-Humans Brain PET Imaging of the GluN2B-Containing N-Methyl-d-Aspartate Receptor with (R)-11C-Me-NB1. J. Nucl. Med. 2022, 63, 936–941. [Google Scholar] [CrossRef]
- Bernard-Gauthier, V.; Bailey, J.J.; Mossine, A.V.; Lindner, S.; Vomacka, L.; Aliaga, A.; Shao, X.; Quesada, C.A.; Sherman, P.; Mahringer, A.; et al. A Kinome-Wide Selective Radiolabeled TrkB/C Inhibitor for in Vitro and in Vivo Neuroimaging: Synthesis, Preclinical Evaluation, and First-in-Human. J. Med. Chem. 2017, 60, 6897–6910. [Google Scholar] [CrossRef]
- Gallezot, J.-D.; Nabulsi, N.; Henry, S.; Pracitto, R.; Planeta, B.; Ropchan, J.; Lin, S.-F.; Labaree, D.; Kapinos, M.; Shirali, A.; et al. Imaging the Enzyme 11β-Hydroxysteroid Dehydrogenase Type 1 with PET: Evaluation of the Novel Radiotracer 11C-AS2471907 in Human Brain. J. Nucl. Med. 2019, 60, 1140–1146. [Google Scholar] [CrossRef]
- Delva, A.; Koole, M.; Serdons, K.; Bormans, G.; Liu, L.; Bard, J.; Khetarpal, V.; Dominguez, C.; Munoz-Sanjuan, I.; Wood, A.; et al. Biodistribution and Dosimetry in Human Healthy Volunteers of the PET Radioligands [11C]CHDI-00485180-R and [11C]CHDI-00485626, Designed for Quantification of Cerebral Aggregated Mutant Huntingtin. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 48–60. [Google Scholar] [CrossRef]
- Johansen, A.; Holm, S.; Dall, B.; Keller, S.; Kristensen, J.L.; Knudsen, G.M.; Hansen, H.D. Human Biodistribution and Radiation Dosimetry of the 5-HT2A Receptor Agonist Cimbi-36 Labeled with Carbon-11 in Two Positions. EJNMMI Res. 2019, 9, 71. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Du, Y.; Lesniak, W.G.; Harrington, C.K.; Brosnan, M.K.; O’Toole, R.; Zandi, A.; Sweeney, S.E.; Abdallah, R.; Wu, Y.; et al. First-in-Human Use of 11C-CPPC with Positron Emission Tomography for Imaging the Macrophage Colony-Stimulating Factor 1 Receptor. EJNMMI Res. 2022, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, M.; Lohith, T.G.; Shrestha, S.; Telu, S.; Zoghbi, S.S.; Castellano, S.; Taliani, S.; Da Settimo, F.; Fujita, M.; Pike, V.W.; et al. 11C-ER176, a Radioligand for 18-KDa Translocator Protein, Has Adequate Sensitivity to Robustly Image All Three Affinity Genotypes in Human Brain. J. Nucl. Med. 2017, 58, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Brier, M. Phase 1 Evaluation of 11C-CS1P1 to Assess Safety and Dosimetry in Human Participants. J. Nucl. Med. 2022, 64, 1775–1782. [Google Scholar] [CrossRef]
- Antoni, G.; Lubberink, M.; Sörensen, J.; Lindström, E.; Elgland, M.; Eriksson, O.; Hultström, M.; Frithiof, R.; Wanhainen, A.; Sigfridsson, J.; et al. In Vivo Visualization and Quantification of Neutrophil Elastase in Lungs of COVID-19 Patients—A First-In-Human Positron Emission Tomography Study with 11C-GW457427. J. Nucl. Med. 2022, 64, 263974. [Google Scholar] [CrossRef]
- Van Weehaeghe, D.; Koole, M.; Schmidt, M.E.; Deman, S.; Jacobs, A.H.; Souche, E.; Serdons, K.; Sunaert, S.; Bormans, G.; Vandenberghe, W.; et al. [11C]JNJ54173717, a Novel P2X7 Receptor Radioligand as Marker for Neuroinflammation: Human Biodistribution, Dosimetry, Brain Kinetic Modelling and Quantification of Brain P2X7 Receptors in Patients with Parkinson’s Disease and Healthy Volunteers. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Nakajima, W.; Hatano, M.; Shibata, Y.; Kuroki, Y.; Arisawa, T.; Serizawa, A.; Sano, A.; Kogami, S.; Yamanoue, T.; et al. Visualization of AMPA Receptors in Living Human Brain with Positron Emission Tomography. Nat. Med. 2020, 26, 281–288. [Google Scholar] [CrossRef]
- Naganawa, M.; Nabulsi, N.; Henry, S.; Matuskey, D.; Lin, S.-F.; Slieker, L.; Schwarz, A.J.; Kant, N.; Jesudason, C.; Ruley, K.; et al. First-in-Human Assessment of 11C-LSN3172176, an M1 Muscarinic Acetylcholine Receptor PET Radiotracer. J. Nucl. Med. 2021, 62, 553–560. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, M.-J.; Eldridge, M.; Lehmann, M.L.; Frankland, M.; Liow, J.-S.; Yu, Z.-X.; Cortes-Salva, M.; Telu, S.; Henter, I.D.; et al. PET Measurement of Cyclooxygenase-2 Using a Novel Radioligand: Upregulation in Primate Neuroinflammation and First-in-Human Study. J. Neuroinflammation 2020, 17, 140. [Google Scholar] [CrossRef]
- Du, Y.; Coughlin, J.M.; Brosnan, M.K.; Chen, A.; Shinehouse, L.K.; Abdallah, R.; Lodge, M.A.; Mathews, W.B.; Liu, C.; Wu, Y.; et al. PET Imaging of the Cannabinoid Receptor Type 2 in Humans Using [11C]MDTC. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Van der Weijden, C.W.J.; Meilof, J.F.; van der Hoorn, A.; Zhu, J.; Wu, C.; Wang, Y.; Willemsen, A.T.M.; Dierckx, R.A.J.O.; Lammertsma, A.A.; de Vries, E.F.J. Quantitative Assessment of Myelin Density Using [11C]MeDAS PET in Patients with Multiple Sclerosis: A First-in-Human Study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3492–3507. [Google Scholar] [CrossRef]
- Kubota, M.; Seki, C.; Kimura, Y.; Takahata, K.; Shimada, H.; Takado, Y.; Matsuoka, K.; Tagai, K.; Sano, Y.; Yamamoto, Y.; et al. A First-in-Human Study of 11C-MTP38, a Novel PET Ligand for Phosphodiesterase 7. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Bedoya, C.A.; Ordonez, A.A.; Werner, R.A.; Plyku, D.; Klunk, M.H.; Leal, J.; Lesniak, W.G.; Holt, D.P.; Dannals, R.F.; Higuchi, T.; et al. 11C-PABA as a PET Radiotracer for Functional Renal Imaging: Preclinical and First-in-Human Study. J. Nucl. Med. 2020, 61, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Sakata, M.; Ishibashi, K.; Imai, M.; Wagatsuma, K.; Ishii, K.; Zhou, X.; de Vries, E.F.J.; Elsinga, P.H.; Ishiwata, K.; Toyohara, J. Initial Evaluation of an Adenosine A2A Receptor Ligand, 11C-Preladenant, in Healthy Human Subjects. J. Nucl. Med. 2017, 58, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Lee, J.-H.; Juarez Anaya, F.; Hong, J.; Miller, W.; Telu, S.; Singh, P.; Cortes, M.Y.; Henry, K.; Tye, G.L.; et al. First-in-Human Evaluation of [11C]PS13, a Novel PET Radioligand, to Quantify Cyclooxygenase-1 in the Brain. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3143–3151. [Google Scholar] [CrossRef] [PubMed]
- Tucker, E.W.; Guglieri-Lopez, B.; Ordonez, A.A.; Ritchie, B.; Klunk, M.H.; Sharma, R.; Chang, Y.S.; Sanchez-Bautista, J.; Frey, S.; Lodge, M.A.; et al. Noninvasive 11C-Rifampin Positron Emission Tomography Reveals Drug Biodistribution in Tuberculous Meningitis. Sci. Transl. Med. 2018, 10, 145. [Google Scholar] [CrossRef]
- Lohith, T.G.; Tsujikawa, T.; Siméon, F.G.; Veronese, M.; Zoghbi, S.S.; Lyoo, C.H.; Kimura, Y.; Morse, C.L.; Pike, V.W.; Fujita, M.; et al. Comparison of Two PET Radioligands, [11C]FPEB and [11C]SP203, for Quantification of Metabotropic Glutamate Receptor 5 in Human Brain. J. Cereb. Blood Flow Metab. 2017, 37, 2458–2470. [Google Scholar] [CrossRef]
- Lee, I.K.; Jacome, D.A.; Cho, J.K.; Tu, V.; Young, A.; Dominguez, T.; Northrup, J.D.; Etersque, J.M.; Lee, H.S.; Ruff, A.; et al. Imaging Sensitive and Drug-Resistant Bacterial Infection with [11C]-Trimethoprim. J. Clin. Investig. 2022, 132, e156679. [Google Scholar] [CrossRef]
- Watanabe, Y.; Mawatari, A.; Aita, K.; Sato, Y.; Wada, Y.; Nakaoka, T.; Onoe, K.; Yamano, E.; Akamatsu, G.; Ohnishi, A.; et al. PET Imaging of 11C-Labeled Thiamine Tetrahydrofurfuryl Disulfide, Vitamin B1 Derivative: First-in-Human Study. Biochem. Biophys. Res. Commun. 2021, 555, 7–12. [Google Scholar] [CrossRef]
- Wong, D.F.; Comley, R.A.; Kuwabara, H.; Rosenberg, P.B.; Resnick, S.M.; Ostrowitzki, S.; Vozzi, C.; Boess, F.; Oh, E.; Lyketsos, C.G.; et al. Characterization of 3 Novel Tau Radiopharmaceuticals, 11C-RO-963, 11C-RO-643, and 18F-RO-948, in Healthy Controls and in Alzheimer Subjects. J. Nucl. Med. 2018, 59, 1869–1876. [Google Scholar] [CrossRef]
- Masdeu, J.C.; Pascual, B.; Fujita, M. Imaging Neuroinflammation in Neurodegenerative Disorders. J. Nucl. Med. 2022, 63, 45S–52S. [Google Scholar] [CrossRef]
- Chen, Z.; Haider, A.; Chen, J.; Xiao, Z.; Gobbi, L.; Honer, M.; Grether, U.; Arnold, S.E.; Josephson, L.; Liang, S.H. The Repertoire of Small-Molecule PET Probes for Neuroinflammation Imaging: Challenges and Opportunities beyond TSPO. J. Med. Chem. 2021, 64, 17656–17689. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.; Vugts, D.; Windhorst, A.; Mach, R. PET Imaging of Microglial Activation—Beyond Targeting TSPO. Molecules 2018, 23, 607. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Chaney, A.M.; Carlson, M.L.; Jackson, I.M.; Rao, A.; James, M.L. Neuroinflammation PET Imaging: Current Opinion and Future Directions. J. Nucl. Med. 2020, 61, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswami, V.; Dahl, K.; Bernard-Gauthier, V.; Josephson, L.; Cumming, P.; Vasdev, N. Emerging PET Radiotracers and Targets for Imaging of Neuroinflammation in Neurodegenerative Diseases: Outlook Beyond TSPO. Mol. Imaging 2018, 17, 1536012118792317. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).