Abstract
Fifteen push-pull dyes comprising the tetracyclic polyaromatic pyrene have been designed and synthesized. The optical properties of the fifteen dyes have been examined in twenty-two solvents of different polarities. Surprisingly, contrarily to what is classically observed for push-pull dyes of D-π-A structures, a negative solvatochromism could be found for numerous dyes. The photoluminescence and thermal properties of the dyes were also examined. Theoretical calculations were carried out to support the experimental results.
1. Introduction
During the last few decades, push-pull dyes have been extensively studied due to the facile tunability of their optical properties [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. Among them, dyes with D-π-A structures, where D and A stand for electron donors and electron acceptors, respectively, and π for a π-conjugated spacer, are the most widely studied [8,17,18,19,20,21,22,23,24,25,26]. Indeed, for a given series of electron acceptors, an electron donor can be used as a reference donor to examine the influence of the electron-accepting group on the photophysical properties. However, the opposite situation is also true and the same electron acceptor can be used for the design of a series of dyes, variation occurring this time on the electron donor. Using these two strategies, different series of dyes have been prepared, comprising 2-(3-cyano-4,5,5-trimethylfuran-2(5H)-ylidene)malononitrile (TCF) [27,28,29], 2,4,5,7-tetranitrofluorene (TNF) [30,31] or 1H-cyclopenta[b]naphthalene-1,3(2H)-dione [32] as the electron acceptors, and Michler’s aldehyde [33], ferrocene [34], the 4,4-bis(4-methoxyphenyl)butadienyl donor [35] or the 4-(9-ethyl-9H-carbazol-3-yl)-4-phenylbuta-1,3-dienyl group [36] as the electron donors. By improvement the electron-donating ability of a donor, a red-shift of the intramolecular charge transfer band can be obtained so that dyes absorbing in the near-infrared range could be obtained, notably by using TNF as the electron acceptor [30,31]. Investigation of the optical properties of push-pull dyes is notably justified by the number of applications requiring push-pull dyes. Thus, push-pull dyes have been extensively used in photopolymerization [37,38,39,40,41,42,43], non-linear optics [4,5,44,45,46,47,48,49], light-to-energy conversion [6,50,51,52,53,54,55,56,57], biological labelling [58,59,60,61,62,63,64], light-emitting diodes [65,66,67] or cell nucleus staining [68,69]. Among electron donors that have only been scarcely used for the design of push-pull dyes, pyrene is one example. This polycyclic aromatic hydrocarbon composed of four fused aromatic rings exhibits a strong tendency to form dimers in solution [70,71,72], but also long-living excited states, so that pyrene is extensively used as a building block for the design of visible light photoinitiators of polymerization for multicomponent systems [73,74,75,76,77]. By its long-living excited state, the excited pyrene can efficiently interact with the different additives introduced into the photocurable resin. Recently, pyrene has also been used in an emerging research field, i.e., photoredox catalysis and photosynthetic systems enabling various chemical transformations were obtained [78]. As a drawback, pyrene is also considered as a pollutant whose mineralization is extensively studied [79,80,81,82]. Considering the difficulty of decomposing this polyaromatic structure, the fate of pyrene in the environment is the focus of numerous works [83,84,85,86]. Meanwhile, regarding pyrene-based push-pull dyes, examples of structures reported in the literature remain scarce. As shown in Figure 1, only twelve dyes have been reported in the literature [68,69,87,88,89,90,91,92]. Moreover, comparisons of their optical properties are difficult, the optical properties of these dyes being recorded in different solvents. Additionally, solvatochromism has not been investigated for all these dyes. Thus, their thermal properties have not been examined for all dyes. Therefore, a study in which all dyes have been investigated in similar conditions is missing. It has to be noted that the solvatochromic properties have been examined in detail for one compound, namely 6-pentafluorostyryl-1-dimethylaminopyrene, exhibiting a polarity- and a viscosity-dependent emission [93,94]. In this work, a series of fifteen pyrene-based push-pull dyes have been prepared and their optical properties examined in twenty-two solvents of different polarities (see Figure 2). The thermal properties of the different dyes were also examined. To obtain a deeper insight into the optical properties, theoretical calculations were also carried out.
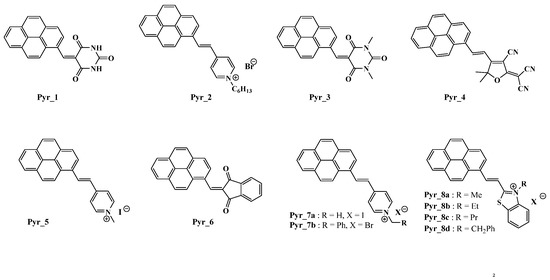
Figure 1.
Chemical structures of push-pull dyes Pyr_1–Pyr_6 previously reported in the literature: Pyr_1 [92], Pyr_2 [91], Pyr_3 [90], Pyr_4 [89], Pyr_5 [88], Pyr_6 [87], Pyr_7 [69], Pyr_8 [68].
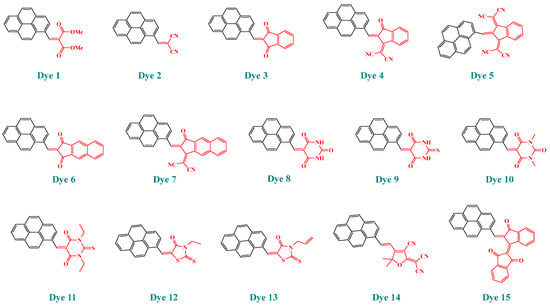
Figure 2.
Chemical structures of the pyrene-based dyes Dye 1–Dye 15 examined in this work.
2. Results and Discussion
2.1. Synthesis of Dye 1–Dye 15
Two distinct synthetic strategies were developed to access the different structures, depending on the electron acceptors. Except for Dye 5 and Dye 15, the different compounds Dye 1–Dye 4, Dye 6–Dye 14 were obtained by a Knoevenagel reaction in basic conditions, using piperidine as the base. Conversely, for EA5 and EA15, for which anions are highly stable and unreactive in basic conditions, acidic conditions had to be used and acetic anhydride was selected as the appropriate solvent [7,95,96]. Upon reflux of the solutions for two hours for Dye 5 or heating at 90 °C overnight for Dye 15, Dye 5 and Dye 15 could be obtained, with reaction yields ranging from 86% for Dye 5 to 88% for Dye 15 (see Scheme 1). In turn, the fifteen dyes could be obtained in reasonable yields, ranging from 72% for Dye 4 to 88% for Dye 15.
Scheme 1.
Synthetic routes to obtain Dye 1–Dye 15.
2.2. Optical Properties
Pyrene-based dyes are highly polyaromatic structures, so that the determination of a common solvent in which all dyes could be soluble was not possible. Interestingly, almost all dyes were soluble in N,N-dimethylformamide (DMF), except three dyes, i.e., Dye 5, Dye 7 and Dye 15, for which absorption spectra were recorded in dioxane. For these three dyes, dioxane was used as the appropriate solvent for examining their optical properties. In these conditions, the optical properties of almost of the dyes could be compared in DMF. As shown in Figure 3, all dyes showed strong absorption centered in the visible range. Considering that pyrene is a weak electron donor, all dyes showed an intense absorption band in the 350–500 nm region corresponding to the intramolecular charge transfer (ICT) band. The most red-shifted absorption was found for Dye 7 (λmax = 549 nm), comprising 2-(3-oxo-2,3-dihydro-1H-cyclopenta[b]naphthalen-1-ylidene) malononitrile EA7 as the electron acceptor. As shown in Figure 3a, the charge transfer band of Dye 7 is broad and extends from 450 to 700 nm. Following Dye 7, Dye 5, comprising 2,2′-(1H-Indene-1,3(2H)-diylidene)dimalononitrile EA5 as the acceptor, exhibited the second most red-shifted absorption maximum at 549 nm, outperforming all the other dyes (see Figure 3c).
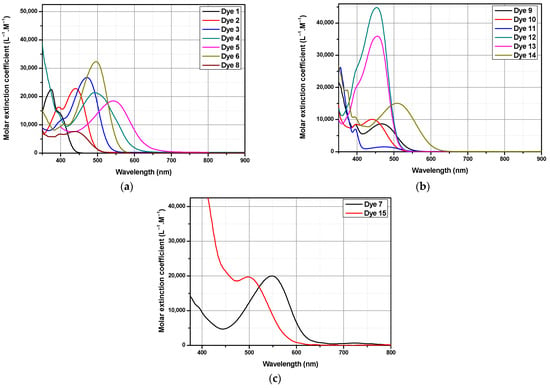
Figure 3.
UV–visible absorption spectra of Dye 1-Dye 4, Dye 6 and Dye 8-Dye 14 in DMF (a,b), and Dye 5, Dye 7 and Dye 15 in dioxane (c).
These results are consistent with previous works reported in the literature evidencing the remarkable electron-withdrawing ability of this group [32,97]. As anticipated, the bluest-shifted absorption was found for Dye 1, comprising dimethyl malonate as the electron acceptor. Indeed, EA1 is the weakest electron acceptor of the series. In this last case, the maximum absorption located at 374 nm could be determined. Moreover, if the absorption of this dye is strongly UV-centered, absorption could, however, be found in the visible range thanks to the long tail extending to 450 nm. The highest molar extinction coefficient of the series was determined for Dye 12, peaking at 44,800 L−1.M−1. A slightly lower molar extinction coefficient (ε = 35,950 L−1.M−1) was determined for Dye 13, also bearing a rhodanine-based electron acceptor (see Table 1). Theoretical studies were also carried out to investigate the energy levels as well as the molecular orbital (M.O.) compositions of the different dyes. DFT calculations were performed for all dyes at the wb97xd/6-311g(d,p) level of theory using the Gaussian 09 program to determine the transitions involved in the different absorption peaks. DMF was used as the solvent model with a polarizable continuum model (PCM) [98,99,100,101,102,103,104]. Theoretical UV–visible absorption spectra were obtained by TD-DFT calculations and the different spectra are presented in Figure 4. Optical characteristics are summarized in Table 1. The position of the absorption maxima was determined from the theoretical spectra. As shown in Table 1, for all dyes, the positions of the theoretical absorption maxima were determined in DMF as the solvent. Noticeably, all theoretical absorption maxima were blue-shifted compared to the experimental ones. In fact, the PCM model only allows us to create an electrostatic field corresponding to the dielectric nature of the solvent around the molecule. In no case does this model allow us to take into account specific interactions between the molecule and the solvent. Notably, it does not allow us to take into account a protic effect of a solvent, for instance. In fact, no solvate model in DFT allows us to take into account easily the molecule/solvent interactions. As a consequence of this, a mismatch between the theoretical and the experimental positions of the absorption maxima is found.

Table 1.
Optical characteristics of the different compounds in DMF, dioxane and dichloromethane and theoretically determined.
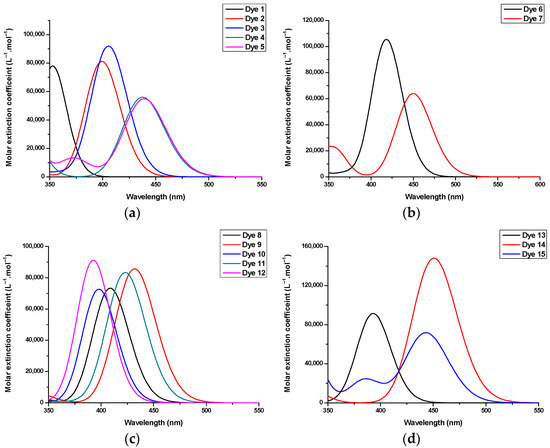
Figure 4.
Theoretical UV–visible absorption spectra of Dye 1–Dye 15 in DMF. (a) Dye 1–Dye 5; (b) Dye 6, Dye 7; (c) Dye 8–Dye12; (d) Dye 13–Dye 15.
As anticipated, the minor variations of the substitution pattern of Dye 12 and Dye 13 did not modify the UV–visible absorption spectra, as experimentally observed. Examination of the contour plots of the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO) revealed a classical electronic distribution for the two orbitals. As shown in Figure 5, logically, the HOMO orbital is located on the electron donor, whereas the LUMO level is centered on the electron-accepting one.
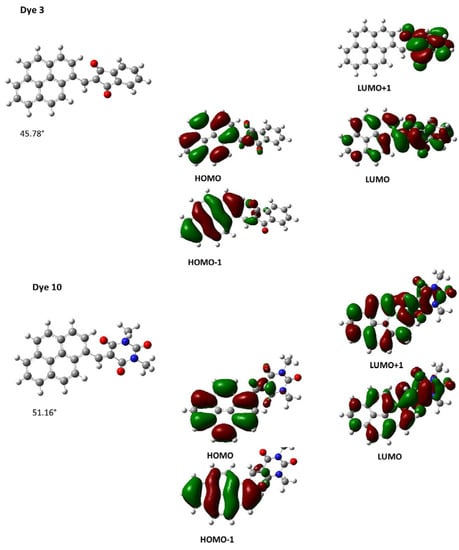
Figure 5.
Contour plots of the HOMO and LUMO orbitals of Dye 3 (top) and Dye 10 (bottom).
To obtain a deeper insight into the different transitions involved in the intramolecular charge transfer bands of the different dyes, theoretical calculations were carried out and the different data are summarized in Table 2. Notably, for Dye 5, which comprises the bulkiest electron acceptor, the intramolecular charge transfer band was determined as being an admixture of HOMO => LUMO, HOMO => LUMO + 1 and HOMO − 1 => LUMO transitions. Optimization of the geometry for Dye 5 also revealed this dye to exhibit strong internal torsion with a dihedral angle of 43.05° due to the steric hindrance generated by the electron acceptor (see Figure 6). Moreover, despite this internal torsion, a HOMO energy level extending over the pyrene moiety and the dicyanomethylene groups of the acceptor could be determined (see Figure 6). Similarly, the LUMO energy level of Dye 5 is mainly located on the electron acceptor but also extends over the pyrene moiety, demonstrating an electronic communication that is maintained between the donor and the acceptor. A similar distribution of both the HOMO and LUMO energy levels can be evidenced for Dye 7 and Dye 15, also comprising sterically hindered electron acceptors (see Figure 7). In these cases, torsion angles of 45.65° and 58.64° were determined between the donors and acceptors in Dye 7 and Dye 15, respectively.

Table 2.
Summary of the simulated absorption characteristics in dilute DMF of synthetized compounds. Data were obtained in DMF solution.
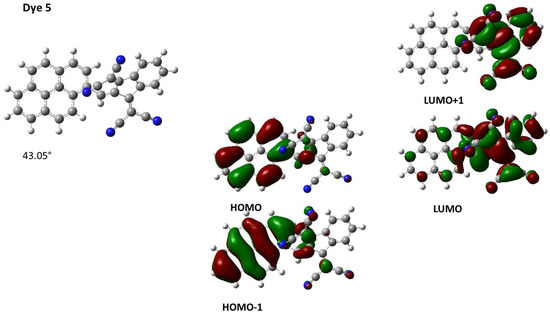
Figure 6.
Three-dimensional view of the optimized geometry for Dye 5 and contour plots of the HOMO and LUMO orbitals.
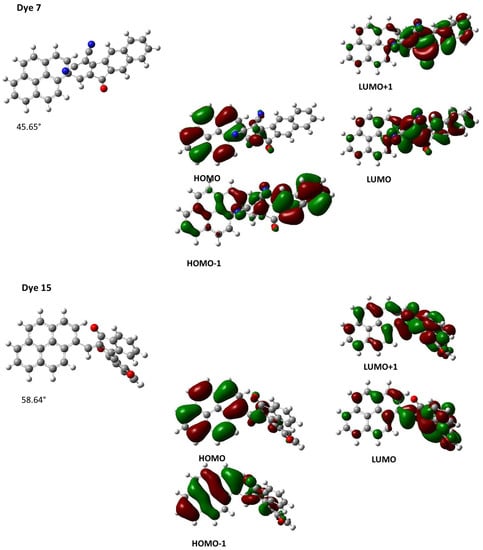
Figure 7.
Contour plots of the HOMO and LUMO orbitals of Dye 7 (top) and Dye 15 (bottom).
For all dyes, the position of the theoretical absorption maximum was close to the position determined for the HOMO => LUMO transition by TD-DFT, since a difference of a few nanometers was found. Therefore, the main transition involved at the absorption maximum corresponds to a HOMO => LUMO transition. Moreover, the exciton binding energy of the different dyes, which is defined as the difference between the electrochemical and optical bandgaps, could not be determined [105,106], the dyes being not sufficiently soluble to determine with accuracy the positions of the HOMO and LUMO energy levels by electrochemistry. Based on the position of the ICT bands, the different electron acceptors could be classified according to the order presented in Scheme 2. Among all electron acceptors, EA7 and EA5 were determined as exhibiting the highest electron-withdrawing abilities of the series of 15 electron acceptors, consistent with the ordering previously reported in the literature [33]. The weakest electron acceptors were determined as being EA1 and EA2 [107,108]. Notably, EA1 is rarely used for the design of push-push dyes due to the weak electronic delocalization that this electron acceptor involves [87,109,110,111].
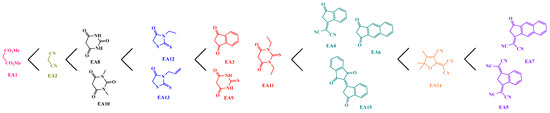
Scheme 2.
Classification of the electron acceptors based on the position of the ICT bands.
2.3. Solvatochromism
Despite the polyaromatic nature of the electron donor and the low solubility of the different push-pull dyes in numerous solvents, the solvatochromism of the fifteen dyes could, however, be examined in a wide range of solvents differing by their polarities. A summary of the optical properties of the fifteen dyes, Dye 1–Dye 15, is provided in Table 3. Solvatochromism corresponds to a charge redistribution upon excitation, and, in this field, several empirical solvent polarity scales have been developed over the years. Among these, Kamlet–Taft’s [112], Catalan’s [113], Kawski–Chamma Viallet’s [114], Lippert–Mataga’s [115], McRae–Suppan’s [116], Dimroth–Reichardt’s [117], and Bakhshiev’s [118] scales have been developed by various research groups in order to rationalize the solvatochromism observed experimentally. As the main parameters governing the solvatochromism, the polarity and the polarizability of the solvents can be cited as the main parameters governing the modification of the optical bandgaps. Classically, a reduction in the HOMO–LUMO gap (where HOMO and LUMO denote the Highest Occupied Molecular Orbital and Lowest Unoccupied Molecular Orbital, respectively) is observed upon the increase in the solvent polarity. In the present case, linear correlations could be obtained with only two scales, i.e., the Kamlet–Taft and the Catalan scales. Two distinct behaviors could be clearly identified. Thus, for dyes such as Dye 1, Dye 2, Dye 5, Dye 6, Dye 12 and Dye 14, a positive solvatochromism could be determined, notably using the Kamlet–Taft polarity scale (See Figure 8). This behavior is observed for numerous dyes of D-π-A structures and is indicative of an excited state more polar than the ground one. However, the opposite situation could also be found for numerous dyes, as exemplified for Dye 3, Dye 4, Dye 8–Dye 11 and Dye 13, and a negative solvatochromism could be clearly evidenced using the same polarity scales (see Figure 9). Noticeably, Dye 8 and Dye 9, which are the non-alkylated versions of Dye 10 and Dye 11, exhibited a similar solvatochromism to that observed for Dye 10 and Dye 11, for which electron acceptors are alkylated. Moreover, in the case of Dye 8 and Dye 9, a different behavior of the electron acceptors can be anticipated compared to Dye 10 and Dye 11. Indeed, in the case of Dye 8 and Dye 9, electron-withdrawing groups EA8 and EA9 can switch between the enol and the keto forms depending on the environment [119]. As a result of this equilibrium, the electron-withdrawing abilities of EA8 and EA9 differ from one solvent to another, complicating the solvatochromism of Dye 8 and Dye 9. Based on the literature, the negative solvatochromism detected for Dye 8 and Dye 9 is not unusual for (thio)barbituric dyes. Indeed, negative solvatochromisms for (thio)barbituric dyes comprising the enolizable electron acceptors EA8 or EA9 have already been reported in the literature [119,120]. If non-enolizable, EA10 and EA11 are nonetheless able to generate hydrogen bonds between molecules, generating complex supramolecular structures in solution [121]. In particular, these supramolecular structures can be strongly impacted by the hydrogen-bonding ability of the solvent so that a complex solvatochromism can also be evidenced for these structures. In turn, a negative solvatochromism was determined for Dye 8–Dye 11.

Table 3.
Summary of the optical properties of the fifteen dyes in 23 different solvents recorded at room temperature.
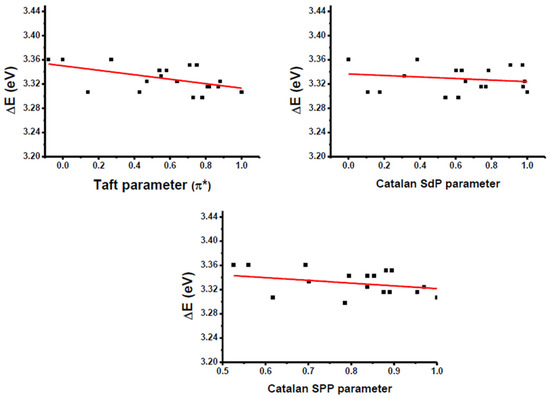
Figure 8.
Linear regressions obtained using different polarity scales for Dye 1.
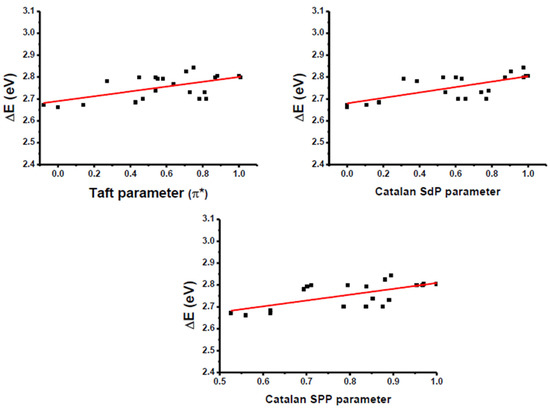
Figure 9.
Linear regressions obtained using different polarity scales for Dye 10.
This unexpected behavior was thus observed mostly for the dyes elaborated with the strongest electron-withdrawing groups. Typically, negative solvatochromism is often observed for betaine dyes and assigned to a modification of the relative electrophilicities of both the electron-pair donor and acceptor moieties with the solvent, so that irregular behaviors can be found for this family of dyes [122,123,124]. To the best of our knowledge, such a behavior has never been reported for polyaromatic electron donors. This unexpected behavior can also be tentatively assigned to the formation of pyrene-based dimers with different orientations (cross, g-like, slip or stack), the formation of aggregates of various sizes in solution, affecting the analysis of their solvatochromism. In fact, π–π stacking interactions between polyacene structures have been extensively studied in the literature [125,126,127,128,129,130,131,132,133,134,135,136]. Upon π–π stacking interactions, various orientations can be found between stacked molecules: cross, g-like, slip or stack [137]. Overall, these noncovalent interactions between molecules govern the optical properties of the resulting solutions, which comprise isolated molecules, and the concomitant presence of stacked dyes that adversely impact the rationalization of the solvatochromism [138]. In particular, the negative solvatochromism is observed for the less soluble dyes, supporting this hypothesis of the concomitant presence of isolated molecules and aggregates. Indeed, for highly soluble dyes such as Dye 1 and Dye 2, bearing small electron acceptors, a classical behavior is evidenced for these dyes. In the case of Dye 8 and Dye 9, negative solvatochromism can also be assigned to the hydrogen bond ability of the two electron acceptors (presence of NH groups), affecting the solvatochromism of these dyes. Moreover, in the case of Dye 10 and Dye 11, a negative solvatochromism is also evidenced for these two dyes, despite the alkylation of the electron acceptors.
2.4. Photoluminescence Properties
The photoluminescence of all dyes was examined in dichloromethane and in DMF and the different results are summarized in Table 1. As shown in Figure 10, major differences could be determined for the different dyes. Noticeably, the most blue-shifted emission was determined for Dye 1, bearing the weakest electron acceptor (λmax = 461 nm), whereas the most-redshifted absorption was found for Dye 14, bearing EA14 as the electron acceptor. As shown in Figure 10, an emission extending until the near-infrared range was found for Dye 14. Following Dye 14, the second most red-shifted absorption was determined for Dye 6, comprising EA6. Interestingly, the largest Stokes shift was determined for Dye 14, being 152 nm. Indeed, for this dye, an absorption located at 507 nm and an emission at 659 nm was found in DMF. If this Stokes shift is important, it remains, however, lower than that reported for 6-pentafluorostyryl-1-dimethylaminopyrene, recently reported in the literature and exhibiting a Stokes shift of 247 nm [93]. This exceptional value can be assigned to the presence of the dimethylamino group on pyrene, improving the electronic delocalization between pyrene and the electron acceptor. For the rest of the molecules, Stokes shifts ranging between 80 and 100 nm could be found, except for Dye 7, for which a Stokes shift of only 14 nm was calculated. Dye 14 thus constitutes an excellent candidate as a fluorescent probe for various applications, in light of the large Stokes shift detected for this dye. It has to be noted that comparison of the Stokes shift obtained in dichloromethane and DMF revealed the Stokes shift to be more important in the more polar solvent [94]. This trend is consistent with the previous works reported in the literature mentioning the higher sensitivity of the emission of pyrene-based dyes to the solvent polarity than the absorption and reporting a red shift of the emission maximum with the solvent polarity, which is also observed in this study.
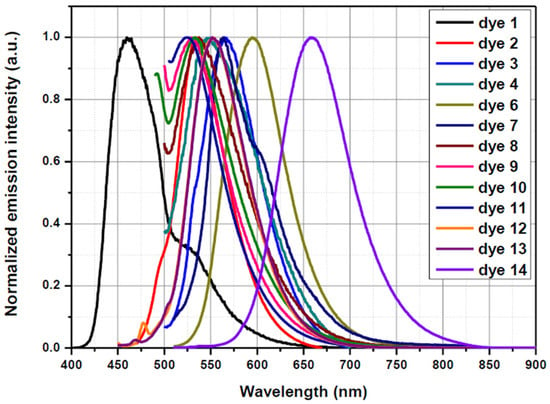
Figure 10.
Fluorescence spectra of Dye 1–Dye 14 recorded in DMF as the solvent.
2.5. Thermal Properties
For numerous applications, the thermal stability of dyes is an important parameter, notably for applications such as solar cells [139,140,141,142]. The thermal properties of the different dyes were examined by thermal gravimetry analyses, and a summary of the decomposition temperatures is given in Table 4 and in Figure 11. Noticeably, despite the presence of the same electron-donating group, major differences could be determined concerning the decomposition temperatures of Dye 1–Dye 15. The lowest one was determined for Dye 3, at 176 °C. Conversely, the highest one was found for Dye 15, determined at 400 °C. Only three dyes showed decomposition temperatures lower than 300 °C, evidencing their remarkable thermal stability.

Table 4.
Decomposition temperatures of the different dyes.
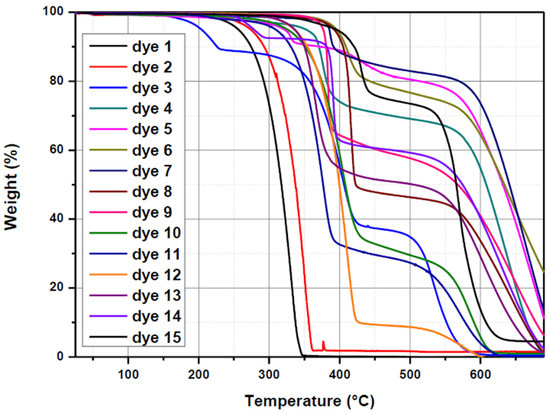
Figure 11.
Thermogravimetric analysis (TGA) thermograms of Dye 1–Dye 15 recorded under nitrogen at a scan rate of 20 °C/min.
3. Materials and Methods
3.1. General Information
All reagents and solvents were purchased from Aldrich, Alfa Aesar or TCI Europe and used as received, without further purification. Mass spectroscopy was performed by the Spectropole of Aix Marseille University. ESI mass spectral analyses were recorded with a 3200 QTRAP (Applied Biosystems SCIEX) mass spectrometer. The HRMS mass spectral analysis was performed with a QStar Elite (Applied Biosystems SCIEX) mass spectrometer. Elemental analyses were recorded with a Thermo Finnigan EA 1112 elemental analysis apparatus driven by the Eager 300 software.
1H and 13C NMR spectra (More details could be found in Supplementary Materials) were determined at room temperature in 5 mm o.d. tubes on a Bruker Avance 400 spectrometer of the Spectropole: 1H (400 MHz) and 13C (100 MHz). The 1H chemical shifts were referenced to the solvent peak CDCl3 (7.26 ppm) and the 13C chemical shifts were referenced to the solvent peak CDCl3 (77 ppm).
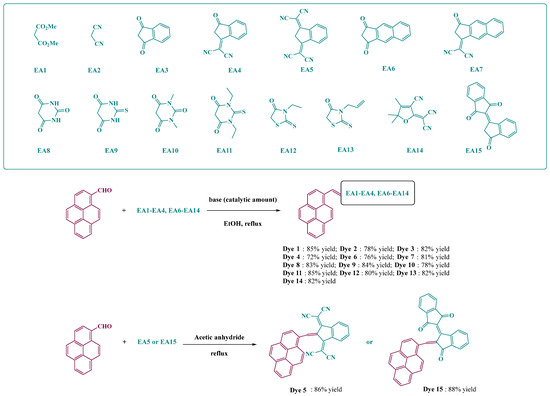
2-(3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile EA4 [143], 2,2′-(1H-indene-1,3(2H)-diylidene)dimalononitrile EA5 [144], 1H-cyclo-penta[b]naphthalene-1,3(2H)-dione EA6 [32,145], 2-(3-oxo-2,3-dihydro-1H-cyclopenta[b]naphthalen-1-ylidene)malononitrile EA7 [145,146], 2-(3-cyano-4,5,5-trimethylfuran-2(5H)-ylidene) malononitrile EA6 [27,29,147,148] and [1,2′]biindenylidene-3,1′,3′-trione EA15 [149] were prepared as previously reported in the literature, without modification and in similar yields.
Thermal properties of the different dyes were investigated by using a TA thermal analyzer (TA Instrument Q50) at a heating rate of 20 °C/min under argon flow. The temperature of thermal degradation (Td) was measured at the point of 5% weight loss. UV–visible absorption spectra were recorded on a Varian Cary 60 UV–vis spectrophotometer with a concentration of 5 × 10−3 M, corresponding to diluted solutions. Fluorescence spectra were recorded on a Jasco spectrofluorometer FP-8350. Melting points were determined with a Buchi Melting Point M-560 at a scan rate of 10 °C/min by varying the temperature between 80 and 350 °C.
3.2. Synthesis of the Dyes
General procedure for the synthesis of all dyes (except Dye 5 and Dye 15): 1-pyrenecarbaldehyde (2 g, 8.68 mmol) and the appropriate electron acceptor (8.68 mmol, 1 eq.) were dissolved in absolute ethanol (50 mL). A few drops of piperidine were added. Immediately, the solution’s color changed. The solution was refluxed and monitoring of the reaction progress was carried out by thin layer chromatography (TLC). After cooling, the solution was concentrated under reduced pressure. Addition of pentane precipitated a solid, which was filtered off and dried under vacuum.
Dimethyl 2-(pyren-1-ylmethylene)malonate (Dye 1)
85% yield. 1H NMR (300 MHz, CDCl3) δ 8.81 (s, 1H), 8.30–7.92 (m, 9H), 3.97 (s, 3H), 3.72 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 167.05 (C=O), 164.57 (C=O), 142.05 (CH=C(CO2Me)2), 132.75, 131.14, 130.63, 129.81, 128.79, 128.71, 127.75, 127.20, 127.18, 126.33, 126.14, 126.05, 125.53, 124.68, 124.59, 124.36, 122.89, 52.79 (OMe), 52.56 (OMe); HRMS (ESI MS) m/z: theor: 344.1049 found: 344.1042 ([M]+. detected); Mp = 146 °C.
2-(Pyren-1-ylmethylene)malononitrile (Dye 2)
78% yield. 1H NMR (300 MHz, DMSO) δ 9.62 (s, 1H), 8.71 (t, J = 9.2 Hz, 2H), 8.54–8.37 (m, 5H), 8.37–8.18 (m, 2H); HRMS (ESI MS) m/z: theor: 278.0844 found: 278.0844 ([M]+. detected); Anal. Calc. for C20H10N2: C, 86.3; H, 3.6; O, 10.1; Found: C, 86.4; H, 3.7; N, 9.7%; Td > 240 °C.
2-(Pyren-1-ylmethylene)-1H-indene-1,3(2H)-dione (Dye 3)
82% yield. 1H NMR (400 MHz, CDCl3) δ 9.34 (d, J = 8.2 Hz, 1H), 9.11 (s, 1H), 8.63 (d, J = 9.3 Hz, 1H), 8.31–8.28 (m, 2H), 8.28–8.23 (m, 2H), 8.21 (d, J = 8.9 Hz, 1H), 8.12 (d, J = 8.9 Hz, 1H), 8.10–8.03 (m, 3H), 7.87–7.81 (m, 2H); HRMS (ESI MS) m/z: theor: 358.0994 found: 358.0996 ([M]+. detected); Anal. Calc. for C26H14O2: C, 87.1; H, 3.9; O, 8.9; Found: C, 87.4; H, 3.7; N, 8.7%; Td > 140 °C.
2-(3-Oxo-2-(pyren-1-ylmethylene)-2,3-dihydro-1H-inden-1-ylidene)malononitrile (Dye 4)
72% yield. HRMS (ESI MS) m/z: theor: 406.1106 found: 406.1103 ([M]+ detected); Anal. Calc. for C29H14N2 O: C, 85.7; H, 3.5; O, 3.9; Found: C, 85.4; H, 3.7; N, 3.7%; Td > 200 °C.
2-(Pyren-1-ylmethylene)-1H-cyclopenta[b]naphthalene-1,3(2H)-dione (Dye 6)
76% yield. HRMS (ESI MS) m/z: theor: 408.1150 found: 408.1157 ([M]+. detected); Anal. Calc. for C30H16O2: C, 88.2; H, 3.9; O, 7.8; Found: C, 88.4; H, 3.9; N, 8.1%; Mp = 314 °C.
2-(3-Oxo-2-(pyren-1-ylmethylene)-2,3-dihydro-1H-cyclo-penta[b]naphthalen-1-ylidene)malononitrile (Dye 7)
81% yield. HRMS (ESI MS) m/z: theor: 456.1263 found: 456.1256 ([M]+. detected); Anal. Calc. for C33H16N2O: C, 86.8; H, 3.5; O, 3.5; Found: C, 86.8; H, 3.6; N, 3.6 %; Td > 200 °C.
5-(Pyren-1-ylmethylene)pyrimidine-2,4,6(1H,3H,5H)-trione (Dye 8)
83% yield. 1H NMR (400 MHz, DMSO) δ 11.50 (s, 1H), 11.21 (s, 1H), 9.11 (s, 1H), 8.42–8.11 (m, 9H); HRMS (ESI MS) m/z: theor: 340.0848 found: 340.0842 ([M]+. detected); Anal. Calc. for C21H12N2O3: C, 74.1; H, 3.5; O, 14.1; Found: C, 74.4; H, 3.7; N, 14.2%; Td > 200 °C.
5-(Pyren-1-ylmethylene)-2-thioxodihydropyrimidine-4,6(1H, 5H)-dione (Dye 9)
84% yield. 1H NMR (400 MHz, DMSO) δ 12.57 (s, 1H), 12.33 (s, 1H), 9.14 (s, 1H), 8.42 (dd, J = 12.8, 7.9 Hz, 3H), 8.31 (dd, J = 15.2, 8.6 Hz, 3H), 8.23 (dd, J = 9.0, 3.4 Hz, 2H), 8.14 (t, J = 7.6 Hz, 1H); HRMS (ESI MS) m/z: theor: 356.0619 found: 356.0619 ([M]+. detected); Anal. Calc. for C21H12N2O2S: C, 70.8; H, 3.4; O, 9.0; Found: C, 70.6; H, 3.3; N, 9.2%; Td > 200 °C.
1,3-Dimethyl-5-(pyren-1-ylmethylene)pyrimidine-2,4,6(1H,3H, 5H)-trione (Dye 10)
78% yield. 1H NMR (400 MHz, CDCl3) δ 9.56 (s, 1H), 8.47 (d, J = 8.2 Hz, 1H), 8.26 (d, J = 7.6 Hz, 2H), 8.21–8.14 (m, 4H), 8.11–8.01 (m, 2H), 3.51 (s, 3H), 3.35 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 162.44 (C=O), 160.05 (C=O), 157.60 (C=O), 151.47 (=CH vinyl), 134.27, 131.08, 131.04, 130.56, 129.65, 129.32, 128.75, 127.38, 127.22, 126.75, 126.66, 126.36, 124.37, 123.75, 123.34, 118.56, 29.03 (N-Me), 28.40 (N-Me); HRMS (ESI MS) m/z: theor: 368.1161 found: 368.1165 ([M]+. detected); Td > 200 °C.
1,3-Diethyl-5-(pyren-1-ylmethylene)-2-thioxodihydro-pyrimi-dine-4,6(1H,5H)-dione (Dye 11)
85% yield. 1H NMR (400 MHz, CDCl3) δ 9.57 (s, 1H), 8.55 (d, J = 8.2 Hz, 1H), 8.27 (dd, J = 15.9, 6.5 Hz, 4H), 8.20 (t, J = 7.7 Hz, 2H), 8.13–8.04 (m, 2H), 4.66 (q, J = 6.9 Hz, 2H), 4.53 (q, J = 6.9 Hz, 2H), 1.41 (t, J = 7.0 Hz, 3H), 1.29 (t, J = 7.0 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 179.12 (C=S), 160.73 (C=O), 158.29 (C= O), 158.14 (=CH vinyl), 134.65, 131.44, 131.04, 130.52, 129.93, 129.47, 129.03, 127.39, 127.32, 126.93, 126.84, 126.41, 124.32, 124.30, 123.81, 123.36, 118.88, 44.15 (OCH2CH3), 43.62 (OCH2CH3), 12.53 (OCH2CH3), 12.47 (OCH2CH3); HRMS (ESI MS) m/z: theor: 412.1245 found: 412.1242 ([M]+. detected); Td > 200 °C.
3-Ethyl-5-(pyren-1-ylmethylene)-2-thioxothiazolidin-4-one (Dye 12)
80% yield. 1H NMR (300 MHz, CDCl3) δ 8.76 (s, 1H), 8.42 (d, J = 9.3 Hz, 1H), 8.28–8.11 (m, 5H), 8.05 (t, J = 6.9 Hz, 3H), 4.27 (q, J = 7.1 Hz, 2H), 1.36 (t, J = 7.1 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 193.66 (C=S), 167.40 (C=O), 133.03 (=CH vinyl), 131.27, 130.97, 130.66, 130.01, 129.53, 129.24, 127.25, 126.91, 126.60, 126.56, 126.43, 125.66, 125.09, 125.01, 124.91, 124.34, 122.35, 39.84 (NCH2CH3), 12.32 (NCH2CH3); HRMS (ESI MS) m/z: theor: 373.05955 found: 373.0590 ([M]+. detected); Mp = 215 °C
3-Allyl-5-(pyren-1-ylmethylene)-2-thioxothiazolidin-4-one (Dye 13)
82% yield. 1H NMR (400 MHz, CDCl3) δ 8.81 (s, 1H), 8.46 (d, J = 9.3 Hz, 1H), 8.29–8.04 (m, 8H), 5.99–5.88 (m, 1H), 5.34 (ddd, J = 13.7, 11.4, 1.2 Hz, 2H), 4.83 (dt, J = 5.9, 1.3 Hz, 2H); HRMS (ESI MS) m/z: theor: 385.0595 found: 385.0598 ([M]+. detected); Anal. Calc. for C23H15NOS2: C, 71.7; H, 3.9; O, 4.1; Found: C, 71.4; H, 3.7; N, 4.4%; Mp = 195 °C.
2-(3-Cyano-5,5-dimethyl-4-(2-(pyren-1-yl)vinyl)furan-2(5H)-ylidene)malononitrile (Dye 14)
82% yield. Due to its insolubility, no 1H NMR spectrum could be acquired. HRMS (ESI MS) m/z: theor: 411.1372 found: 411.1373 ([M]+. detected); Anal. Calc. for C28H17N3O: C, 81.7; H, 4.2; O, 3.9; Found: C, 81.4; H, 3.9; N, 4.1%; Td > 200 °C.
Synthesis of 2,2′-(2-(pyren-1-ylmethylene)-1H-indene-1,3(2H)-diylidene) dimalononitrile (Dye 5)
2,2′-(1H-Indene-1,3(2H)-diylidene)dimalononitrile (1.22 g, 5.02 mmol, M = 242.24 g/mol) and pyrene-1-carbaldehyde (1.15 g, 5.02 mmol, M = 230.27 g/mol) were dissolved in acetic anhydride (20 mL) and the solution was refluxed for two hours. After cooling, the solvent was removed under reduced pressure. Addition of ether followed by pentane precipitated a blue solid that was filtered off, washed several times with pentane and dried under vacuum (1.96 g, 86% yield).
1H NMR (400 MHz, CDCl3) δ 9.56 (s, 1H), 8.74 (dd, J = 5.9, 3.0 Hz, 1H), 8.65 (dd, J = 5.9, 2.9 Hz, 1H), 8.37 (s, 2H), 8.33 (t, J = 7.9 Hz, 2H), 8.25 (d, J = 8.9 Hz, 1H), 8.17 (d, J = 8.1 Hz, 1H), 8.11 (dd, J = 12.7, 5.1 Hz, 2H), 7.94 (d, J = 8.1 Hz, 1H), 7.91 (dd, J = 6.6, 2.9 Hz, 2H); HRMS (ESI MS) m/z: theor: 454.1218 found: 454.1218 ([M]+. detected); Anal. Calc. for C32H14N4: C, 84.6; H, 3.1; N, 12.3; Found: C, 84.4; H, 2.7; N, 12.4%; Td > 200 °C
Synthesis of 2-(pyren-1-ylmethylene)-[1,2′-biindenylidene]-1′,3,3′(2H)-trione (Dye 15)
To a mixture of [1,2′]-biindenylidene-3,1′,3′-trione (0.5 g, 1.28 mmol, M = 274.28 g/mol) and 2-pyrenecarbaldehyde (0.42 g, 1.28 mmol, 230.27 g/mol) was added acetic anhydride so that the powder was covered by the liquid. The reaction mixture was heated at 90 °C overnight. After cooling, ether was added. A red precipitate formed. It was filtered off, washed several times with ether and dried under vacuum to remove the traces of acetic anhydride (0.55 g, 88% yield).
1H NMR (400 MHz, CDCl3) δ 9.39 (d, J = 7.5 Hz, 1H), 9.30 (dd, J = 17.0, 7.9 Hz, 2H), 8.05 (d, J = 7.4 Hz, 1H), 7.89–7.79 (m, 3H), 7.75 (dt, J = 19.1, 6.1 Hz, 3H), 7.68 (d, J = 7.7 Hz, 1H), 7.61 (t, J = 7.1 Hz, 1H), 7.58–7.49 (m, 2H), 7.39 (t, J = 7.4 Hz, 1H), 7.28 (s, 3H); HRMS (ESI MS) m/z: theor: 486.1256 found: 486.1251 ([M]+. detected); Anal. Calc. for C35H18O3: C, 86.4; H, 3.7; O, 9.9; Found: C, 86.4; H, 3.9; N, 10.1%; Td > 200 °C.
4. Conclusions
To conclude, a series of fifteen dyes based on pyrene as the electron donor have been designed and synthesized. Noticeably, all dyes could be prepared using standard synthetic procedures, enabling us to obtain the different compounds in reasonable yields. Examination of their solvatochromic properties in 22 solvents of different polarities revealed the most soluble dyes to exhibit a positive solvatochromism. On the contrary, for the less soluble dyes, the opposite trend was found, and a negative solvatochromism was detected for these dyes. This unexpected behavior was assigned to the formation of aggregates of different sizes and shapes in solution, the formation of hydrogen bonds between molecules for all dyes possessing electron-withdrawing groups comprising NH or C=O groups affecting in turn the examination of their solvatochromic properties, as the isolated molecules were concomitantly present with aggregates and hydrogen-bonded molecules. All dyes also showed high thermal stability, and a decomposition temperature higher than 300 °C was determined for most of the dyes. Considering that all dyes strongly absorb in the visible range and that pyrene derivatives are excellent photoinitiators of polymerization, future works will consist of examining their photoinitiating abilities during the free radical polymerization of acrylates upon visible light irradiation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031489/s1, Figure S1. chemical structures of the dyes examined in this work; Figures S2–S20. 1H/13C NMR spectra of the dyes; Figures S21–S35. UV-visible absorption spectra of Dye 1–Dye 15; Figures S36–S49. Position of the absorption maxima of PP1–PP15 in twenty-three solvents of different polarities vs. the Kamlet–Taft parameters π*; Figures S50–S62. Position of the absorption maxima of PP1–PP15 in twenty-three solvents of different polarities vs. the Catalan solvent polarity/polarizability (SPP) scale and the Catalan solvent dipolarity (SdP) scale; Figures S63–S77.Optimized geometries and HOMO LUMO electronic distribution of all compounds. Figures S78–S92. TGA thermograms of the different dyes; Table S1. Summary of the optical properties of Dye 1–Dye 8 in twenty-three solvents, values of the Kamlet and Taft parameters π* and values of the Catalan solvent polarizability (SP), solvent dipolarity (SdP) and solvent polarity/polarizability (SPP) parameters; Table S2. Summary of the optical properties of Dye 9–Dye 15 in twenty-three solvents, values of the Kamlet and Taft parameters π* and values of the Catalan solvent polarizability (SP), solvent dipolarity (SdP) and solvent polarity/polarizability (SPP) parameters; Table S3. Solvent-independent correlation coefficient s of the Kamlet-Taft parameters π*; Table S4. Solvent-independent correlation coefficients a and b of the Catalan parameters SdP and SPP; Table S5. Energy levels of the main orbitals for dyes Dye 1–Dye 15; Table S6. Main transitions observed for dyes Dye 1–Dye 15.
Author Contributions
Conceptualization, F.D.; methodology, F.D.; software, F.D.; validation, F.D.; formal analysis, F.D., T.-T.B. and S.P.; investigation, F.D., T.-T.B. and S.P.; resources, F.D., T.-T.B. and S.P.; data curation, F.D., T.-T.B. and S.P.; writing—original draft preparation, F.D., T.-T.B. and S.P.; writing—review and editing, F.D., T.-T.B. and S.P.; visualization, F.D., T.-T.B. and S.P.; supervision, F.D.; project administration, F.D.; funding acquisition, F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Aix Marseille University and the Centre National de la Recherche Scientifique through permanent fundings.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Aix Marseille University and the Centre National de la Recherche Scientifique (CNRS) for the financial support. This work received funding from the CY Initiative of Excellence (grant “Investissements d’Avenir” ANR-16-IDEX-0008) and was developed during Frédéric Dumur’s stay at the CY Advanced Studies, whose support is gratefully acknowledged. Nicolas Giacoletto is acknowledged for his kind help during the TGA measurements performed during his short stay at LPPI. Severine Alfonsi is also acknowledged for her kind help during TGA measurements.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Bureš, F. Fundamental Aspects of Property Tuning in Push–Pull Molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef]
- Kulhánek, J.; Bureš, F.; Pytela, O.; Mikysek, T.; Ludvík, J.; Růžička, A. Push-Pull Molecules with a Systematically Extended π-Conjugated System Featuring 4,5-Dicyanoimidazole. Dye. Pigment. 2010, 85, 57–65. [Google Scholar] [CrossRef]
- Kundu, R.; Kulshreshtha, C. Design, Synthesis and Electronic Properties of Push–Pull–Push Type Dye. RSC Adv. 2015, 5, 77460–77468. [Google Scholar] [CrossRef]
- Huo, F.; Zhang, H.; Chen, Z.; Qiu, L.; Liu, J.; Bo, S.; Kityk, I.V. Novel Nonlinear Optical Push–Pull Fluorene Dyes Chromophore as Promising Materials for Telecommunications. J. Mater. Sci. Mater. Electron. 2019, 30, 12180–12185. [Google Scholar] [CrossRef]
- Raimundo, J.-M.; Blanchard, P.; Gallego-Planas, N.; Mercier, N.; Ledoux-Rak, I.; Hierle, R.; Roncali, J. Design and Synthesis of Push−Pull Chromophores for Second-Order Nonlinear Optics Derived from Rigidified Thiophene-Based π-Conjugating Spacers. J. Org. Chem. 2002, 67, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Parsa, Z.; Naghavi, S.S.; Safari, N. Designing Push–Pull Porphyrins for Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. A 2018, 122, 5870–5877. [Google Scholar] [CrossRef]
- Jeux, V.; Segut, O.; Demeter, D.; Alévêque, O.; Leriche, P.; Roncali, J. Push–Pull Triphenylamine Chromophore Syntheses and Optoelectronic Characterizations. ChemPlusChem 2015, 80, 697–703. [Google Scholar] [CrossRef]
- Dumur, F.; Mayer, C.R.; Dumas, E.; Miomandre, F.; Frigoli, M.; Sécheresse, F. New Chelating Stilbazonium-Like Dyes from Michler’s Ketone. Org. Lett. 2008, 10, 321–324. [Google Scholar] [CrossRef]
- Inoue, K.; Kawakami, R.; Murakami, M.; Nakayama, T.; Yamamoto, S.; Inoue, K.; Tsuda, T.; Sayama, K.; Imamura, T.; Kaneno, D.; et al. Synthesis and Photophysical Properties of a New Push–Pull Pyrene Dye with Green-to-Far-Red Emission and Its Application to Human Cellular and Skin Tissue Imaging. J. Mater. Chem. B 2022, 10, 1641–1649. [Google Scholar] [CrossRef]
- Hosseinnejad, T.; Omrani-Pachin, M. New Designed Push-Pull Organic Dyes Based on the Conjugated π-Spacers for Application in Dye-Sensitized Solar Cells: A Computational Chemistry Study. Bull. Mater. Sci. 2022, 45, 156. [Google Scholar] [CrossRef]
- Niko, Y.; Klymchenko, A.S. Emerging Solvatochromic Push–Pull Dyes for Monitoring the Lipid Order of Biomembranes in Live Cells. J. Biochem. 2021, 170, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Coe, B.J.; Rusanova, D.; Joshi, V.D.; Sánchez, S.; Vávra, J.; Khobragade, D.; Severa, L.; Císařová, I.; Šaman, D.; Pohl, R.; et al. Helquat Dyes: Helicene-like Push–Pull Systems with Large Second-Order Nonlinear Optical Responses. J. Org. Chem. 2016, 81, 1912–1920. [Google Scholar] [CrossRef]
- Gautam, P.; Yu, C.P.; Zhang, G.; Hillier, V.E.; Chan, J.M.W. Pulling with the Pentafluorosulfanyl Acceptor in Push–Pull Dyes. J. Org. Chem. 2017, 82, 11008–11020. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kim, J.O.; Medina, S.; Ramírez, F.J.; Mayorga Burrezo, P.; Wu, S.; Lim, Z.L.; Lambert, C.; Casado, J.; Kim, D.; et al. Push–Pull-Type Polychlorotriphenylmethyl Radicals: New Two-Photon Absorbers and Dyes for Generation of Photo-Charges. Chem. Eur. J. 2017, 23, 7698–7702. [Google Scholar] [CrossRef] [PubMed]
- Hadsadee, S.; Rattanawan, R.; Tarsang, R.; Kungwan, N.; Jungsuttiwong, S. Push-Pull N-Annulated Perylene-Based Sensitizers for Dye-Sensitized Solar Cells: Theoretical Property Tuning by DFT/TDDFT. ChemistrySelect 2017, 2, 9829–9837. [Google Scholar] [CrossRef]
- Pigot, C.; Brunel, D.; Dumur, F. Indane-1,3-Dione: From Synthetic Strategies to Applications. Molecules 2022, 27, 5976. [Google Scholar] [CrossRef]
- Shaya, J.; Fontaine-Vive, F.; Michel, B.Y.; Burger, A. Rational Design of Push–Pull Fluorene Dyes: Synthesis and Structure–Photophysics Relationship. Chem. Eur. J. 2016, 22, 10627–10637. [Google Scholar] [CrossRef]
- Cesaretti, A.; Foggi, P.; Fortuna, C.G.; Elisei, F.; Spalletti, A.; Carlotti, B. Uncovering Structure–Property Relationships in Push–Pull Chromophores: A Promising Route to Large Hyperpolarizability and Two-Photon Absorption. J. Phys. Chem. C 2020, 124, 15739–15748. [Google Scholar] [CrossRef]
- Keerthi, A.; Sriramulu, D.; Liu, Y.; Yuan Timothy, C.T.; Wang, Q.; Valiyaveettil, S. Architectural Influence of Carbazole Push–Pull–Pull Dyes on Dye Sensitized Solar Cells. Dye. Pigment. 2013, 99, 787–797. [Google Scholar] [CrossRef]
- Frigoli, M.; Marrot, J.; Gentili, P.L.; Jacquemin, D.; Vagnini, M.; Pannacci, D.; Ortica, F. P-Type Photochromism of New Helical Naphthopyrans: Synthesis and Photochemical, Photophysical and Theoretical Study. ChemPhysChem 2015, 16, 2447–2458. [Google Scholar] [CrossRef]
- Broman, S.L.; Andersen, C.L.; Jousselin-Oba, T.; Mansø, M.; Hammerich, O.; Frigoli, M.; Nielsen, M.B. Tetraceno[2,1,12,11-Opqra]Tetracene-Extended Tetrathiafulvalene—Redox-Controlled Generation of a Large PAH Core. Org. Biomol. Chem. 2017, 15, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Frigoli, M.; Maurel, F.; Berthet, J.; Delbaere, S.; Marrot, J.; Oliveira, M.M. The Control of Photochromism of [3H]-Naphthopyran Derivatives with Intramolecular CH−π Bonds. Org. Lett. 2012, 14, 4150–4153. [Google Scholar] [CrossRef]
- Jousselin-Oba, T.; Mamada, M.; Wright, K.; Marrot, J.; Adachi, C.; Yassar, A.; Frigoli, M. Synthesis, Aromaticity, and Application of Peri-Pentacenopentacene: Localized Representation of Benzenoid Aromatic Compounds. Angew. Chem. Int. Ed. 2022, 61, e202112794. [Google Scholar] [CrossRef]
- Sbargoud, K.; Mamada, M.; Marrot, J.; Tokito, S.; Yassar, A.; Frigoli, M. Diindeno[1,2-b:2′,1′-n]Perylene: A Closed Shell Related Chichibabin’s Hydrocarbon, the Synthesis, Molecular Packing, Electronic and Charge Transport Properties. Chem. Sci. 2015, 6, 3402–3409. [Google Scholar] [CrossRef] [PubMed]
- Delbaere, S.; Micheau, J.-C.; Frigoli, M.; Mehl, G.H.; Vermeersch, G. Mechanistic Understanding of the Photochromism of a Hybrid Dithienylethene–Naphthopyran System by NMR Spectroscopy. J. Phys. Org. Chem. 2007, 20, 929–935. [Google Scholar] [CrossRef]
- Sbargoud, K.; Mamada, M.; Jousselin-Oba, T.; Takeda, Y.; Tokito, S.; Yassar, A.; Marrot, J.; Frigoli, M. Low Bandgap Bistetracene-Based Organic Semiconductors Exhibiting Air Stability, High Aromaticity and Mobility. Chem. Eur. J. 2017, 23, 5076–5080. [Google Scholar] [CrossRef]
- Noirbent, G.; Pigot, C.; Bui, T.-T.; Péralta, S.; Nechab, M.; Gigmes, D.; Dumur, F. Synthesis, Optical and Electrochemical Properties of a Series of Push-Pull Dyes Based on the 2-(3-Cyano-4,5,5-Trimethylfuran-2(5H)-Ylidene)Malononitrile (TCF) Acceptor. Dye. Pigment. 2021, 184, 108807. [Google Scholar] [CrossRef]
- Birajdar, S.S.; Bhardwaj, K.; Kumar, R.; Kobaisi, M.A.; Bhosale, S.V.; Bhosale, S.V. An Efficient Electron Transport Properties of Fullerene Functionalized with Tricyanovinyldihydrofuran (TCF). Mater. Res. Bull. 2022, 147, 111644. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Qin, J.; Tong, M.; Chen, X.; Liu, T.; Fu, Y.; Wu, S.; Su, Z. Synthesis, Crystal Structures and Nonlinear Optical Properties of Three TCF-Based Chromophores. CrystEngComm 2009, 11, 589–596. [Google Scholar] [CrossRef]
- Noirbent, G.; Pigot, C.; Bui, T.-T.; Péralta, S.; Nechab, M.; Gigmes, D.; Dumur, F. Dyes with Tunable Absorption Properties from the Visible to the near Infrared Range: 2,4,5,7-Tetranitrofluorene (TNF) as a Unique Electron Acceptor. Dye. Pigment. 2021, 189, 109250. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Recent Advances on Nitrofluorene Derivatives: Versatile Electron Acceptors to Create Dyes Absorbing from the Visible to the Near and Far Infrared Region. Materials 2018, 11, 2425. [Google Scholar] [CrossRef] [PubMed]
- Pigot, C.; Noirbent, G.; Bui, T.-T.; Péralta, S.; Gigmes, D.; Nechab, M.; Dumur, F. Push-Pull Chromophores Based on the Naphthalene Scaffold: Potential Candidates for Optoelectronic Applications. Materials 2019, 12, 1342. [Google Scholar] [CrossRef] [PubMed]
- Pigot, C.; Péralta, S.; Bui, T.-T.; Nechab, M.; Dumur, F. Push-Pull Dyes Based on Michler’s Aldehyde: Design and Characterization of the Optical and Electrochemical Properties. Dye. Pigment. 2022, 202, 110278. [Google Scholar] [CrossRef]
- Noirbent, G.; Brunel, D.; Bui, T.-T.; Péralta, S.; Aubert, P.-H.; Gigmes, D.; Dumur, F. D–A Dyads and A–D–A Triads Based on Ferrocene: Push–Pull Dyes with Unusual Behaviours in Solution. New J. Chem. 2021, 45, 13475–13498. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Bui, T.-T.; Péralta, S.; Duval, S.; Nechab, M.; Gigmes, D.; Dumur, F. Synthesis, Optical and Electrochemical Properties of a Series of Push-Pull Dyes Based on the 4,4-Bis(4-Methoxy Phenyl)Butadienyl Donor. Dye. Pigment. 2021, 194, 109552. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Bui, T.-T.; Péralta, S.; Duval, S.; Gigmes, D.; Nechab, M.; Dumur, F. Synthesis, and the Optical and Electrochemical Properties of a Series of Push–Pull Dyes Based on the 4-(9-Ethyl-9H-Carbazol-3-Yl)-4-Phenylbuta-1,3-Dienyl Donor. New J. Chem. 2021, 45, 5808–5821. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Bui, T.T.; Goubard, F.; Graff, B.; Morlet-Savary, F.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Panchromatic Photopolymerizable Cationic Films Using Indoline and Squaraine Dye Based Photoinitiating Systems. ACS Macro Lett. 2013, 2, 736–740. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. A Benzophenone-Naphthalimide Derivative as Versatile Photoinitiator of Polymerization under near UV and Visible Lights. J. Polym. Sci. Part Polym. Chem. 2015, 53, 445–451. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Vilà, N.; Graff, B.; Mayer, C.R.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. A Multicolor Photoinitiator for Cationic Polymerization and Interpenetrated Polymer Network Synthesis: 2,7-Di-Tert-Butyldimethyldihydropyrene. Macromol. Rapid Commun. 2013, 34, 1104–1109. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Thirion, D.; Fagour, S.; Vacher, A.; Sallenave, X.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Gigmes, D.; et al. Multicolor Photoinitiators for Radical and Cationic Polymerization: Monofunctional vs Polyfunctional Thiophene Derivatives. Macromolecules 2013, 46, 6786–6793. [Google Scholar] [CrossRef]
- Xiao, P.; Frigoli, M.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Julolidine or Fluorenone Based Push–Pull Dyes for Polymerization upon Soft Polychromatic Visible Light or Green Light. Macromolecules 2014, 47, 106–112. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Push–Pull (Thio)Barbituric Acid Derivatives in Dye Photosensitized Radical and Cationic Polymerization Reactions under 457/473 Nm Laser Beams or Blue LEDs. Polym. Chem. 2013, 4, 3866–3875. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Visible Light Photoinitiators of Polymerization Based on Indane-1,3-Dione and Related Derivatives. Eur. Polym. J. 2021, 143, 110178. [Google Scholar] [CrossRef]
- Mohammed, N.; Wiles, A.A.; Belsley, M.; Fernandes, S.S.M.; Cariello, M.; Rotello, V.M.; Raposo, M.M.M.; Cooke, G. Synthesis and Characterisation of Push–Pull Flavin Dyes with Efficient Second Harmonic Generation (SHG) Properties. RSC Adv. 2017, 7, 24462–24469. [Google Scholar] [CrossRef]
- Yahya, M.; Nural, Y.; Seferoğlu, Z. Recent Advances in the Nonlinear Optical (NLO) Properties of Phthalocyanines: A Review. Dye. Pigment. 2022, 198, 109960. [Google Scholar] [CrossRef]
- Han, W.; Shi, Y.; Xue, T.; Wang, T. Synthesis and Electrochemical, Linear and Third-Order Nonlinear Optical Properties of Ferrocene-Based D-π-A Dyes as Novel Photoredox Catalysts in Photopolymerization under Visible LED Irradiations. Dye. Pigment. 2019, 166, 140–148. [Google Scholar] [CrossRef]
- Han, P.; Wang, D.; Gao, H.; Zhang, J.; Xing, Y.; Yang, Z.; Cao, H.; He, W. Third-Order Nonlinear Optical Properties of Cyanine Dyes with Click Chemistry Modification. Dye. Pigment. 2018, 149, 8–15. [Google Scholar] [CrossRef]
- Sun, J.; Wang, G.; Liu, C.; Shi, Y.; Zhao, M. Synthesis of Four Pyrene-Containing Chalcone Derivatives: Achieving Excellent Third-Order Nonlinear Optical Properties by Optimizing Halopyridines. Opt. Laser Technol. 2019, 109, 600–607. [Google Scholar] [CrossRef]
- Centore, R.; Fusco, S.; Peluso, A.; Capobianco, A.; Stolte, M.; Archetti, G.; Kuball, H.-G. Push–Pull Azo-Chromophores Containing Two Fused Pentatomic Heterocycles and Their Nonlinear Optical Properties. Eur. J. Org. Chem. 2009, 2009, 3535–3543. [Google Scholar] [CrossRef]
- Farré, Y.; Raissi, M.; Fihey, A.; Pellegrin, Y.; Blart, E.; Jacquemin, D.; Odobel, F. Synthesis and Properties of New Benzothiadiazole-Based Push-Pull Dyes for p-Type Dye Sensitized Solar Cells. Dye. Pigment. 2018, 148, 154–166. [Google Scholar] [CrossRef]
- Yella, A.; Mai, C.-L.; Zakeeruddin, S.M.; Chang, S.-N.; Hsieh, C.-H.; Yeh, C.-Y.; Grätzel, M. Molecular Engineering of Push–Pull Porphyrin Dyes for Highly Efficient Dye-Sensitized Solar Cells: The Role of Benzene Spacers. Angew. Chem. Int. Ed. 2014, 53, 2973–2977. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Alsaleh, A.; Trinh, O.; D’Souza, F.; Wang, H. β-Functionalized Push–Pull Opp-Dibenzoporphyrins as Sensitizers for Dye-Sensitized Solar Cells: The Push Group Effect. J. Mater. Chem. A 2021, 9, 27692–27700. [Google Scholar] [CrossRef]
- Qian, X.; Yan, R.; Shao, L.; Li, H.; Wang, X.; Hou, L. Triindole-Modified Push–Pull Type Porphyrin Dyes for Dye-Sensitized Solar Cells. Dye. Pigment. 2016, 134, 434–441. [Google Scholar] [CrossRef]
- Lu, J.; Liu, S.; Wang, M. Push-Pull Zinc Porphyrins as Light-Harvesters for Efficient Dye-Sensitized Solar Cells. Front. Chem. 2018, 6, 541. [Google Scholar] [CrossRef] [PubMed]
- Boschloo, G. Improving the Performance of Dye-Sensitized Solar Cells. Front. Chem. 2019, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Nalzala Thomas, M.R.; Kanniyambatti Lourdusamy, V.J.; Dhandayuthapani, A.A.; Jayakumar, V. Non-Metallic Organic Dyes as Photosensitizers for Dye-Sensitized Solar Cells: A Review. Environ. Sci. Pollut. Res. 2021, 28, 28911–28925. [Google Scholar] [CrossRef]
- Zdyb, A.; Krawczak, E. Organic Dyes in Dye-Sensitized Solar Cells Featuring Back Reflector. Energies 2021, 14, 5529. [Google Scholar] [CrossRef]
- Thooft, A.M.; Cassaidy, K.; VanVeller, B. A Small Push–Pull Fluorophore for Turn-on Fluorescence. J. Org. Chem. 2017, 82, 8842–8847. [Google Scholar] [CrossRef]
- Karpenko, I.A.; Niko, Y.; Yakubovskyi, V.P.; Gerasov, A.O.; Bonnet, D.; Kovtun, Y.P.; Klymchenko, A.S. Push–Pull Dioxaborine as Fluorescent Molecular Rotor: Far-Red Fluorogenic Probe for Ligand–Receptor Interactions. J. Mater. Chem. C 2016, 4, 3002–3009. [Google Scholar] [CrossRef]
- Ayyavoo, K.; Velusamy, P. Pyrene Based Materials as Fluorescent Probes in Chemical and Biological Fields. New J. Chem. 2021, 45, 10997–11017. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, D.; Li, M.; Yang, Y.; Wang, Y.; Yin, H.; Liu, J.; Jia, B.; Wu, X. A Simple Pyrene-Based Fluorescent Probe for Highly Selective Detection of Formaldehyde and Its Application in Live-Cell Imaging. Anal. Chim. Acta 2018, 1033, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Z.; Huang, Z.; Lei, X.; Wang, Y.; Li, L.; Yang, L.; Liu, H.; Sun, F.; Ma, L.-J. A Pyrene-Based PH Fluorescence Probe with Continuous Multiple Responses under Acidic Conditions and Its Application for Environmental Water Systems and Cells. J. Photochem. Photobiol. Chem. 2021, 418, 113438. [Google Scholar] [CrossRef]
- Chao, J.; Li, M.; Zhang, Y.; Yin, C.; Huo, F. A Fluorescent Probe Based on Pyrene Ring for Detecting Cys and Its Application in Biology. J. Fluoresc. 2019, 29, 1241–1248. [Google Scholar] [CrossRef]
- Yu, C.; Yang, M.; Cui, S.; Ji, Y.; Zhang, J. A Ratiometric Selective Fluorescent Probe Derived from Pyrene for Cu2+ Detection. Chemosensors 2022, 10, 207. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, Z.; Tan, J.-H.; Chen, W.-C.; Zhou, P.; Xing, L.; Ji, S.; Qin, Y.; Zhao, Z.; Huo, Y. Deep-Blue Organic Light-Emitting Diodes Based on Push-Pull π-Extended Imidazole-Fluorene Hybrids. Dye. Pigment. 2021, 184, 108754. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Kvashnin, Y.A.; Bogdanov, P.I.; Medvedeva, M.V.; Svalova, T.S.; Kozitsina, A.N.; Samsonova, L.G.; Degtyarenko, K.M.; Grigoryev, D.V.; Kurtcevich, A.E.; et al. The Effect of Molecular Structure on the Efficiency of 1,4-Diazine–Based D–(π)–A Push-Pull Systems for Non-Doped OLED Applications. Dye. Pigment. 2021, 187, 109124. [Google Scholar] [CrossRef]
- Rémond, M.; Hwang, J.; Kim, J.; Kim, S.; Kim, D.; Bucher, C.; Bretonnière, Y.; Andraud, C.; Kim, E. Push–Pull Dyes for Yellow to NIR Emitting Electrochemical Cells. Adv. Funct. Mater. 2020, 30, 2004831. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Wijesinghe, K.J.; Stahelin, R.V.; Pang, Y. Red-Emitting Pyrene–Benzothiazolium: Unexpected Selectivity to Lysosomes for Real-Time Cell Imaging without Alkalinizing Effect. Chem. Commun. 2019, 55, 3469–3472. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Wijesinghe, K.J.; Stahelin, R.V.; Pang, Y. Bright Red-Emitting Pyrene Derivatives with a Large Stokes Shift for Nucleus Staining. Chem. Commun. 2017, 53, 5886–5889. [Google Scholar] [CrossRef]
- Chakraborty, D.; Lischka, H.; Hase, W.L. Dynamics of Pyrene-Dimer Association and Ensuing Pyrene-Dimer Dissociation. J. Phys. Chem. A 2020, 124, 8907–8917. [Google Scholar] [CrossRef]
- Sabbah, H.; Biennier, L.; Klippenstein, S.J.; Sims, I.R.; Rowe, B.R. Exploring the Role of PAHs in the Formation of Soot: Pyrene Dimerization. J. Phys. Chem. Lett. 2010, 1, 2962–2967. [Google Scholar] [CrossRef]
- Kholghy, M.R.; Kelesidis, G.A.; Pratsinis, S.E. Reactive Polycyclic Aromatic Hydrocarbon Dimerization Drives Soot Nucleation. Phys. Chem. Chem. Phys. 2018, 20, 10926–10938. [Google Scholar] [CrossRef] [PubMed]
- Tehfe, M.-A.; Dumur, F.; Contal, E.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New Insights into Radical and Cationic Polymerizations upon Visible Light Exposure: Role of Novel Photoinitiator Systems Based on the Pyrene Chromophore. Polym. Chem. 2013, 4, 1625–1634. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Design of New Type I and Type II Photoinitiators Possessing Highly Coupled Pyrene–Ketone Moieties. Polym. Chem. 2013, 4, 2313–2324. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Faury, T.; Graff, B.; Tehfe, M.-A.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New Core-Pyrene π Structure Organophotocatalysts Usable as Highly Efficient Photoinitiators. Beilstein J. Org. Chem. 2013, 9, 877–890. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Pyrene-Based Photoinitiators of Polymerization. Eur. Polym. J. 2020, 126, 109564. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Brunel, D.; Dumur, F. Recent Advances on Push–Pull Organic Dyes as Visible Light Photoinitiators of Polymerization. Eur. Polym. J. 2020, 133, 109797. [Google Scholar] [CrossRef]
- Ghosh, I.; Shaikh, R.S.; König, B. Sensitization-Initiated Electron Transfer for Photoredox Catalysis. Angew. Chem. Int. Ed. 2017, 56, 8544–8549. [Google Scholar] [CrossRef]
- Kazunga, C.; Aitken, M.D. Products from the Incomplete Metabolism of Pyrene by Polycyclic Aromatic Hydrocarbon-Degrading Bacteria. Appl. Environ. Microbiol. 2000, 66, 1917–1922. [Google Scholar] [CrossRef]
- Bömmel, H.; Li-Weber, M.; Serfling, E.; Duschl, A. The Environmental Pollutant Pyrene Induces the Production of IL-4. J. Allergy Clin. Immunol. 2000, 105, 796–802. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Kumar, V.; Vlaskin, M.S.; Nanda, M. Impact of Pyrene (Polycyclic Aromatic Hydrocarbons) Pollutant on Metabolites and Lipid Induction in Microalgae Chlorella Sorokiniana (UUIND6) to Produce Renewable Biodiesel. Chemosphere 2021, 285, 131482. [Google Scholar] [CrossRef] [PubMed]
- Defois, C.; Ratel, J.; Denis, S.; Batut, B.; Beugnot, R.; Peyretaillade, E.; Engel, E.; Peyret, P. Environmental Pollutant Benzo[a]Pyrene Impacts the Volatile Metabolome and Transcriptome of the Human Gut Microbiota. Front. Microbiol. 2017, 8, 1562. [Google Scholar] [CrossRef] [PubMed]
- Jouanneau, Y.; Willison, J.C.; Meyer, C.; Krivobok, S.; Chevron, N.; Besombes, J.-L.; Blake, G. Stimulation of Pyrene Mineralization in Freshwater Sediments by Bacterial and Plant Bioaugmentation. Environ. Sci. Technol. 2005, 39, 5729–5735. [Google Scholar] [CrossRef] [PubMed]
- Child, R.; Miller, C.D.; Liang, Y.; Sims, R.C.; Anderson, A.J. Pyrene Mineralization by Mycobacterium Sp. Strain KMS in a Barley Rhizosphere. J. Environ. Qual. 2007, 36, 1260–1265. [Google Scholar] [CrossRef]
- Ute, S.; Fritsche, W. Enhancement of Pyrene Mineralization in Soil by Wood-Decaying Fungi. FEMS Microbiol. Ecol. 1997, 22, 77–83. [Google Scholar] [CrossRef]
- Haderlein, A.; Legros, R.; Ramsay, B.A. Pyrene Mineralization Capacity Increases with Compost Maturity. Biodegradation 2006, 17, 293–302. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Blue-to-Red Light Sensitive Push–Pull Structured Photoinitiators: Indanedione Derivatives for Radical and Cationic Photopolymerization Reactions. Macromolecules 2013, 46, 3332–3341. [Google Scholar] [CrossRef]
- Cao, X.; Yi, H.; Li, L.; Zhang, S.; Pan, H.; Chen, J.; Xu, J. Using a Fluorescent 1-Methyl-4-(2-Pyren-1-Yl-Vinyl)-Pyridinium Iodide to Characterize Solvent Polarities. J. Appl. Spectrosc. 2018, 84, 939–947. [Google Scholar] [CrossRef]
- Planells, M.; Pizzotti, M.; Nichol, G.S.; Tessore, F.; Robertson, N. Effect of Torsional Twist on 2nd Order Non-Linear Optical Activity of Anthracene and Pyrene Tricyanofuran Derivatives. Phys. Chem. Chem. Phys. 2014, 16, 23404–23411. [Google Scholar] [CrossRef]
- Samanta, D.; Mukherjee, P.S. Multicomponent Self-Sorting of a Pd7 Molecular Boat and Its Use in Catalytic Knoevenagel Condensation. Chem. Commun. 2013, 49, 4307–4309. [Google Scholar] [CrossRef]
- Tonga, M.; Lahti, P.M. Designing Conjugation-Extended Viologens for High Molar Absorptivity with Longer Wavelength Absorption. Synth. Met. 2019, 254, 75–84. [Google Scholar] [CrossRef]
- Thetford, D.; Chorlton, A.P.; Hardman, J. Synthesis and Properties of Some Polycyclic Barbiturate Pigments. Dye. Pigment. 2003, 59, 185–191. [Google Scholar] [CrossRef]
- Ludwanowski, S.; Samanta, A.; Loescher, S.; Barner-Kowollik, C.; Walther, A. A Modular Fluorescent Probe for Viscosity and Polarity Sensing in DNA Hybrid Mesostructures. Adv. Sci. 2021, 8, 2003740. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Manna, A.K. Origins of Large Stokes Shifts in a Pyrene–Styrene-Based Push–Pull Organic Molecular Dyad in Polar Solvents and Large Electron Mobility in the Crystalline State: A Theoretical Perspective. J. Phys. Chem. C 2022, 126, 423–433. [Google Scholar] [CrossRef]
- Gudeika, D.; Zilinskaite, V.; Grazulevicius, J.V.; Lytvyn, R.; Rutkis, M.; Tokmakov, A. 4-(Diethylamino)Salicylaldehyde-Based Twin Compounds as NLO-Active Materials. Dye. Pigment. 2016, 134, 244–250. [Google Scholar] [CrossRef]
- Zilinskaite, V.; Gudeika, D.; Grazulevicius, J.V.; Hladka, I. Synthesis and Cationic Polymerization of Oxyranyl-Functionalized Indandiones. Polym. Bull. 2016, 1, 229–239. [Google Scholar] [CrossRef]
- Sun, K.; Liu, S.; Pigot, C.; Brunel, D.; Graff, B.; Nechab, M.; Gigmes, D.; Morlet-Savary, F.; Zhang, Y.; Xiao, P.; et al. Novel Push–Pull Dyes Derived from 1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione as Versatile Photoinitiators for Photopolymerization and Their Related Applications: 3D Printing and Fabrication of Photocomposites. Catalysts 2020, 10, 1196. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B. GAUSSIAN 09, Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. A New Mixing of Hartree–Fock and Local Density-functional Theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF Version of the PCM Solvation Method: An Overview of a New Method Addressed to Study Molecular Solutes at the QM Ab Initio Level. J. Mol. Struct. THEOCHEM 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous Surface Charge Polarizable Continuum Models of Solvation. I. General Formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef] [PubMed]
- O’boyle, N.M.; Tenderholt, A.L.; Langner, K.M. Cclib: A Library for Package-Independent Computational Chemistry Algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
- Nakayama, K.; Okura, T.; Okuda, Y.; Matsui, J.; Masuhara, A.; Yoshida, T.; White, M.S.; Yumusak, C.; Stadler, P.; Scharber, M.; et al. Single-Component Organic Solar Cells Based on Intramolecular Charge Transfer Photoabsorption. Materials 2021, 14, 1200. [Google Scholar] [CrossRef]
- Leenaers, P.J.; Maufort, A.J.L.A.; Wienk, M.M.; Janssen, R.A.J. Impact of π-Conjugated Linkers on the Effective Exciton Binding Energy of Diketopyrrolopyrrole–Dithienopyrrole Copolymers. J. Phys. Chem. C 2020, 124, 27403–27412. [Google Scholar] [CrossRef]
- Mokbel, H.; Dumur, F.; Telitel, S.; Vidal, L.; Xiao, P.; Versace, D.-L.; Tehfe, M.-A.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; et al. Photoinitiating Systems of Polymerization and in Situ Incorporation of Metal Nanoparticles into Polymer Matrices upon Exposure to Visible Light: Push–Pull Malonate and Malononitrile Based Dyes. Polym. Chem. 2013, 4, 5679–5687. [Google Scholar] [CrossRef]
- Haenle, J.C.; Bruchlos, K.; Ludwigs, S.; Köhn, A.; Laschat, S. Rigidified Push–Pull Dyes: Using Chromophore Size, Donor, and Acceptor Units to Tune the Ground State between Neutral and the Cyanine Limit. ChemPlusChem 2017, 82, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Klikar, M.; Jelínková, V.; Růžičková, Z.; Mikysek, T.; Pytela, O.; Ludwig, M.; Bureš, F. Malonic Acid Derivatives on Duty as Electron-Withdrawing Units in Push–Pull Molecules. Eur. J. Org. Chem. 2017, 2017, 2764–2779. [Google Scholar] [CrossRef]
- Coluccini, C.; Terraneo, G.; Pasini, D. Synthesis of Binaphthyl-Based Push-Pull Chromophores with Supramolecularly Polarizable Acceptor Ends. J. Chem. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, S.; Zhu, H.; Xie, G.; Liu, R.; Zhu, H. Dimethyl Malonate Based Organic Compounds Bearing Different Aromatic Substituents: Synthesis, Photophysics and Application in Anti-Blue Light Lenses. Opt. Mater. 2022, 126, 112183. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear Solvation Energy Relationships. 23. A Comprehensive Collection of the Solvatochromic Parameters, π*, α, and β, and Some Methods for Simplifying the Generalized Solvatochromic Equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Catalán, J. On the ET (30), π*, Py, S‘, and SPP Empirical Scales as Descriptors of Nonspecific Solvent Effects. J. Org. Chem. 1997, 62, 8231–8234. [Google Scholar] [CrossRef] [PubMed]
- Kawski, A. Der Wellenzahl von Elecktronenbanden Lumineszierenden Molecule. Acta Phys Pol. 1966, 29, 507–518. [Google Scholar]
- Lippert, E. Dipolmoment Und Elektronenstruktur von Angeregten Molekülen. Z. Für Nat. A 1955, 10, 541–545. [Google Scholar] [CrossRef]
- Suppan, P. Solvent Effects on the Energy of Electronic Transitions: Experimental Observations and Applications to Structural Problems of Excited Molecules. J. Chem. Soc. Inorg. Phys. Theor. 1968, 0, 3125–3133. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Bakshiev, N.G. Universal Intermolecular Interactions and Their Effect on the Position of the Electronic Spectra of Molecules in Two Component Solutions. Opt. Spektrosk. 1964, 16, 821–832. [Google Scholar]
- Schade, A.; Schreiter, K.; Rüffer, T.; Lang, H.; Spange, S. Interactions of Enolizable Barbiturate Dyes. Chem. Eur. J. 2016, 22, 5734–5748. [Google Scholar] [CrossRef]
- Ding, S.; Yao, B.; Schobben, L.; Hong, Y. Barbituric Acid Based Fluorogens: Synthesis, Aggregation-Induced Emission, and Protein Fibril Detection. Molecules 2020, 25, 32. [Google Scholar] [CrossRef]
- Seifert, S.; Seifert, A.; Brunklaus, G.; Hofmann, K.; Rüffer, T.; Lang, H.; Spange, S. Probing the Surface Polarity of Inorganic Oxides Using Merocyanine-Type Dyes Derived from Barbituric Acid. New J. Chem. 2012, 36, 674–684. [Google Scholar] [CrossRef]
- Rezende, M.C.; Aracena, A. A General Framework for the Solvatochromism of Pyridinium Phenolate Betaine Dyes. Chem. Phys. Lett. 2013, 558, 77–81. [Google Scholar] [CrossRef]
- Jacques, P.; Graff, B.; Diemer, V.; Ay, E.; Chaumeil, H.; Carré, C.; Malval, J.-P. Negative Solvatochromism of a Series of Pyridinium Phenolate Betaine Dyes with Increasing Steric Hindrance. Chem. Phys. Lett. 2012, 531, 242–246. [Google Scholar] [CrossRef]
- Ooyama, Y.; Asada, R.; Inoue, S.; Komaguchi, K.; Imae, I.; Harima, Y. Solvatochromism of Novel Donor–π–Acceptor Type Pyridinium Dyes in Halogenated and Non-Halogenated Solvents. New J. Chem. 2009, 33, 2311–2316. [Google Scholar] [CrossRef]
- Grimme, S. Accurate Description of van Der Waals Complexes by Density Functional Theory Including Empirical Corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Lim, E.C. Evaluation of the Hartree−Fock Dispersion (HFD) Model as a Practical Tool for Probing Intermolecular Potentials of Small Aromatic Clusters: Comparison of the HFD and MP2 Intermolecular Potentials. J. Phys. Chem. A 2003, 107, 10105–10110. [Google Scholar] [CrossRef]
- Schuetz, C.A.; Frenklach, M. Nucleation of Soot: Molecular Dynamics Simulations of Pyrene Dimerization. Proc. Combust. Inst. 2002, 29, 2307–2314. [Google Scholar] [CrossRef]
- Zreid, M.; Tabasi, Z.A.; Zhao, Y. Comparative Studies of the Noncovalent Interactions in the Single-Crystal Packing of Pyrene, Pyrene-4,5-Dione, and Pyrene-4,5,9,10-Tetraone. J. Phys. Org. Chem. 2021, 34, e4192. [Google Scholar] [CrossRef]
- King, N.J.; Brown, A. Intermolecular Interactions of Pyrene and Its Oxides in Toluene Solution. J. Phys. Chem. A 2022, 126, 4931–4940. [Google Scholar] [CrossRef]
- Lee, N.K.; Kim, S.K. Ab Initio-Based Intermolecular Carbon–Carbon Pair Potentials for Polycyclic Aromatic Hydrocarbon Clusters. J. Chem. Phys. 2005, 122, 031102. [Google Scholar] [CrossRef]
- Podeszwa, R.; Szalewicz, K. Physical Origins of Interactions in Dimers of Polycyclic Aromatic Hydrocarbons. Phys. Chem. Chem. Phys. 2008, 10, 2735–2746. [Google Scholar] [CrossRef]
- Herdman, J.D.; Miller, J.H. Intermolecular Potential Calculations for Polynuclear Aromatic Hydrocarbon Clusters. J. Phys. Chem. A 2008, 112, 6249–6256. [Google Scholar] [CrossRef]
- Elvati, P.; Violi, A. Thermodynamics of Poly-Aromatic Hydrocarbon Clustering and the Effects of Substituted Aliphatic Chains. Proc. Combust. Inst. 2013, 34, 1837–1843. [Google Scholar] [CrossRef]
- Silva, N.J.; Machado, F.B.C.; Lischka, H.; Aquino, A.J.A. π–π Stacking between Polyaromatic Hydrocarbon Sheets beyond Dispersion Interactions. Phys. Chem. Chem. Phys. 2016, 18, 22300–22310. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H. Structures, Stability, and Growth Sequence Patterns of Small Homoclusters of Naphthalene, Anthracene, Phenanthrene, Phenalene, Naphthacene, and Pyrene. Clust. Dimers Nanoparticles 2013, 1021, 84–90. [Google Scholar] [CrossRef]
- Hoche, J.; Schmitt, H.-C.; Humeniuk, A.; Fischer, I.; Mitrić, R.; Röhr, M.I.S. The Mechanism of Excimer Formation: An Experimental and Theoretical Study on the Pyrene Dimer. Phys. Chem. Chem. Phys. 2017, 19, 25002–25015. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro-Lago, E.M.; Rodríguez-Otero, J. On the Nature of σ–σ, σ–π, and π–π Stacking in Extended Systems. ACS Omega 2018, 3, 9348–9359. [Google Scholar] [CrossRef]
- Singh, A.; Raj, P.; Dubowski, J.J.; Singh, N. ATP Induced Modulation in π–π Stacking Interactions in Pyrene Based Zinc Complexes: Chemosensor Study and Quantitative Investigation of Apyrase Activity. Cryst. Growth Des. 2018, 18, 4320–4333. [Google Scholar] [CrossRef]
- Jiang, N.; Sumitomo, T.; Lee, T.; Pellaroque, A.; Bellon, O.; Milliken, D.; Desilvestro, H. High Temperature Stability of Dye Solar Cells. Sol. Energy Mater. Sol. Cells 2013, 119, 36–50. [Google Scholar] [CrossRef]
- Matsui, H.; Okada, K.; Kitamura, T.; Tanabe, N. Thermal Stability of Dye-Sensitized Solar Cells with Current Collecting Grid. Sol. Energy Mater. Sol. Cells 2009, 93, 1110–1115. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Wu, Y.; Tan, S. An Efficient and Thermally Stable Dye-Sensitized Solar Cell Based on a Lamellar Nanostructured Thiolate/Disulfide Liquid Crystal Electrolyte and Carbon/PEDOT Composite Nanoparticle Electrode. RSC Adv. 2019, 9, 35924–35930. [Google Scholar] [CrossRef]
- Mohammadnezhad, M.; Selopal, G.S.; Wang, Z.M.; Stansfield, B.; Zhao, H.; Rosei, F. Towards Long-Term Thermal Stability of Dye-Sensitized Solar Cells Using Multiwalled Carbon Nanotubes. ChemPlusChem 2018, 83, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Wen, Y.; Li, S.; Du, S.; He, X.; Cai, L.; Li, Y.; Yang, L.; Gao, H.; Song, Y. A Triphenylamine-Containing Donor−Acceptor Molecule for Stable, Reversible, Ultrahigh Density Data Storage. J. Am. Chem. Soc. 2007, 129, 11674–11675. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.R.; Frazer, A.; Woodward, A.W.; Ahn-White, H.-Y.; Fonari, A.; Tongwa, P.; Timofeeva, T.; Belfield, K.D. Design, Synthesis, and Structural and Spectroscopic Studies of Push–Pull Two-Photon Absorbing Chromophores with Acceptor Groups of Varying Strength. J. Org. Chem. 2013, 78, 1014–1025. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Peralta, S.; Duval, S.; Bui, T.-T.; Aubert, P.-H.; Nechab, M.; Gigmes, D.; Dumur, F. New Push-Pull Dyes Based on 2-(3-Oxo-2,3-Dihydro-1H-Cyclopenta[b]Naphthalen-1-Ylidene)Malononitrile: An Amine-Directed Synthesis. Dye. Pigment. 2020, 175, 108182. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Peralta, S.; Duval, S.; Nechab, M.; Gigmes, D.; Dumur, F. Unprecedented Nucleophilic Attack of Piperidine on the Electron Acceptor during the Synthesis of Push-Pull Dyes by a Knoevenagel Reaction. Helv. Chim. Acta 2019, 102, e1900229. [Google Scholar] [CrossRef]
- Schmidt, K.; Barlow, S.; Leclercq, A.; Zojer, E.; Jang, S.-H.; Marder, S.R.; Jen, A.K.-Y.; Brédas, J.-L. Efficient Acceptor Groups for NLO Chromophores: Competing Inductive and Resonance Contributions in Heterocyclic Acceptors Derived from 2-Dicyanomethylidene-3-Cyano-4,5,5-Trimethyl-2,5-Dihydrofuran. J. Mater. Chem. 2007, 17, 2944–2949. [Google Scholar] [CrossRef]
- Liu, F.; Xu, H.; Zhang, H.; Chen, L.; Liu, J.; Bo, S.; Zhen, Z.; Liu, X.; Qiu, L. Synthesis of Julolidine-Containing Nonlinear Optical Chromophores: Achieving Excellent Electro-Optic Activity by Optimizing the Bridges and Acceptors. Dye. Pigment. 2016, 134, 358–367. [Google Scholar] [CrossRef]
- Bürckstümmer, H.; Tulyakova, E.V.; Deppisch, M.; Lenze, M.R.; Kronenberg, N.M.; Gsänger, M.; Stolte, M.; Meerholz, K.; Würthner, F. Efficient Solution-Processed Bulk Heterojunction Solar Cells by Antiparallel Supramolecular Arrangement of Dipolar Donor–Acceptor Dyes. Angew. Chem. Int. Ed. 2011, 50, 11628–11632. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).