Glutamine Deprivation Synergizes the Anticancer Effects of Cold Atmospheric Plasma on Esophageal Cancer Cells
Abstract
1. Introduction
2. Results
2.1. CAP Reduced the Viability of Esophageal Cancer
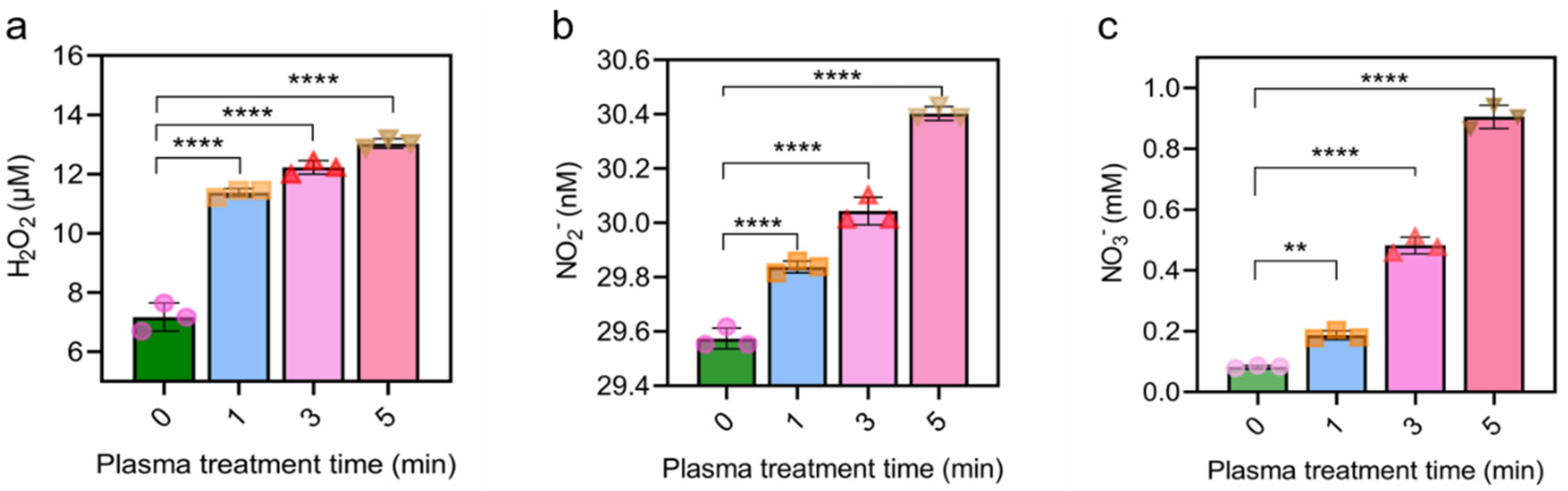
2.2. CAP Notably Increased RONS Level in Cell Culture Medium
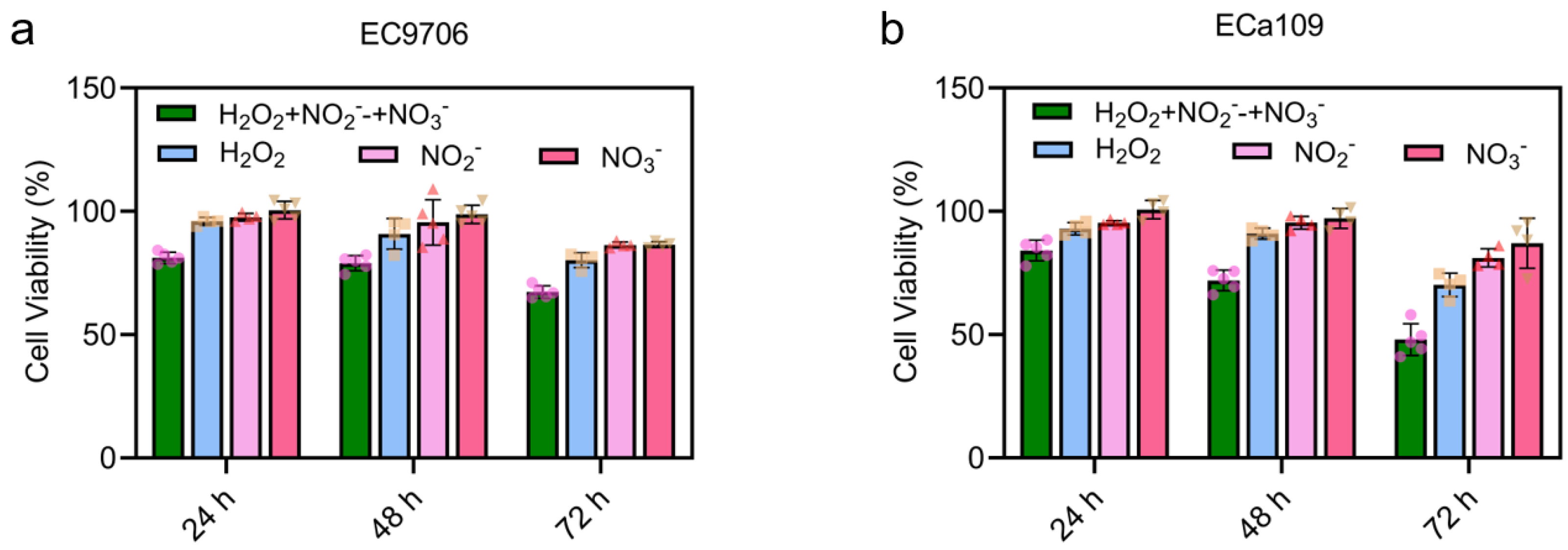
2.3. CAP-Induced Long-Lasting Reactive Species Inhibited the Viability of Esophageal Cancer
2.4. Glutathione Reduced the H2O2 Level in the CAP Activated Medium
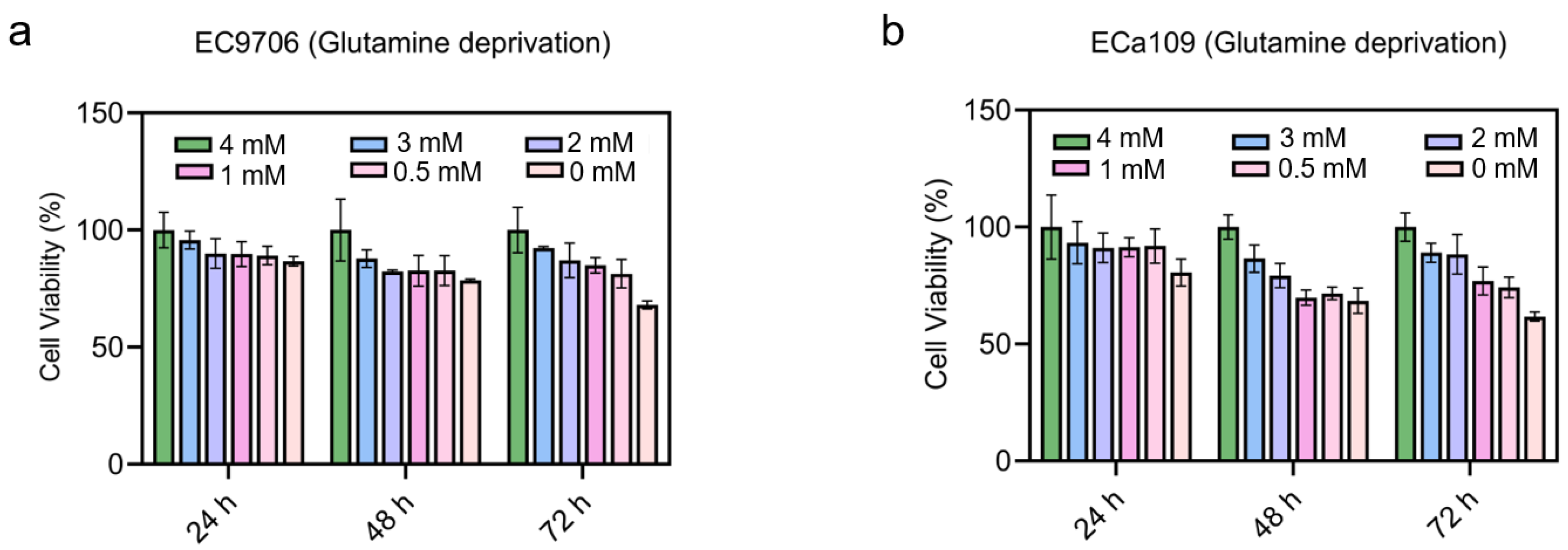
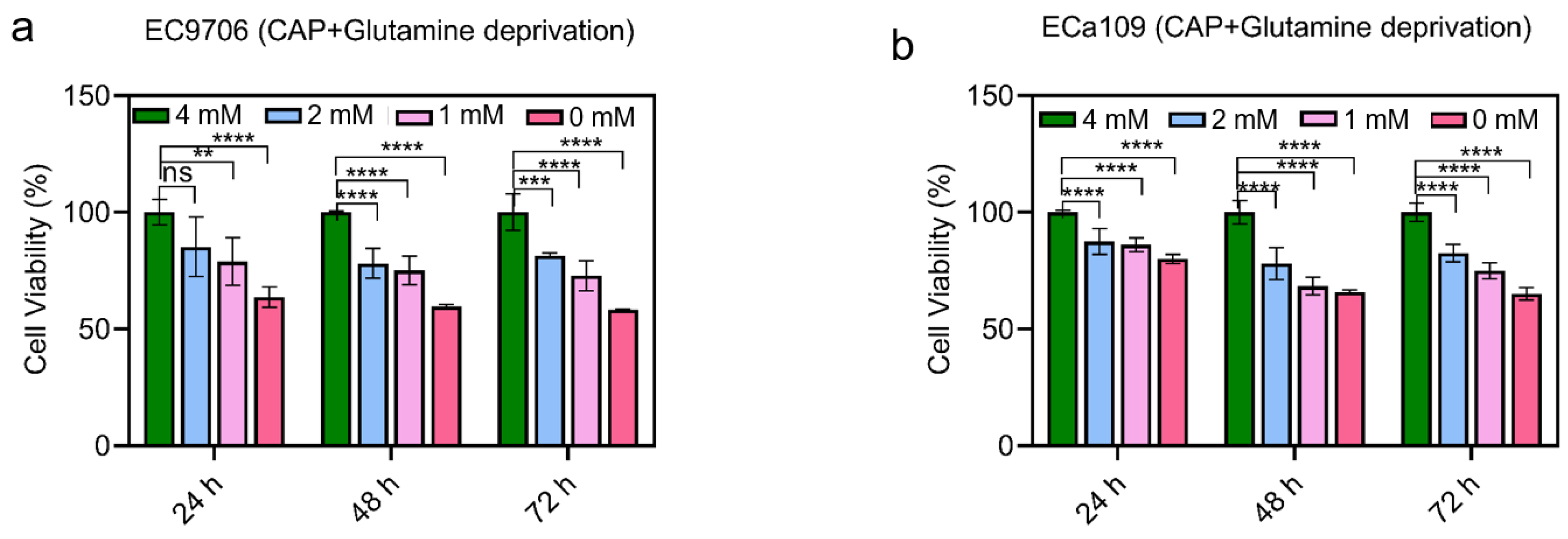
2.5. Cell Viability Decreased after Combined CAP Stimulation and Glutamine Deprivation
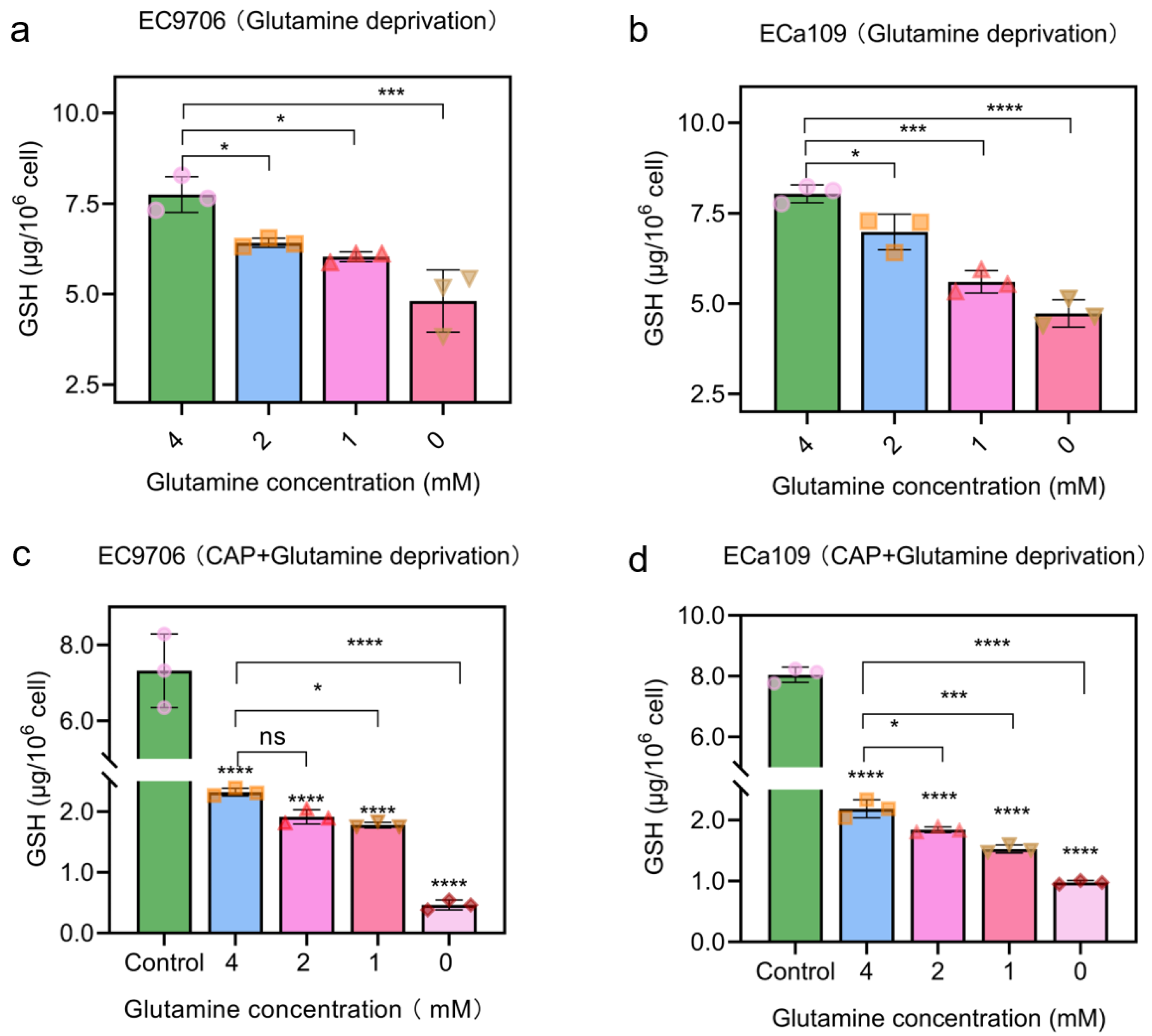
2.6. Combined CAP Stimulation and Glutamine Deprivation Reduced Glutathione Level
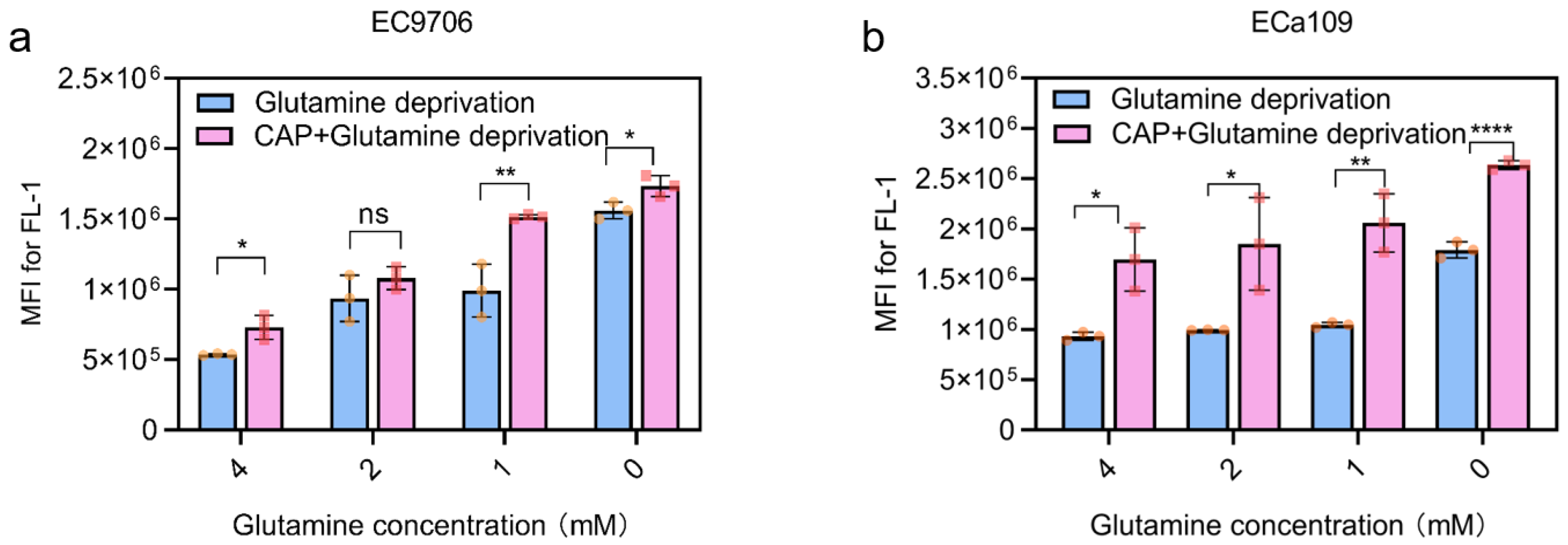
2.7. Combined CAP Stimulation and Glutamine Deprivation Improved ROS Levels
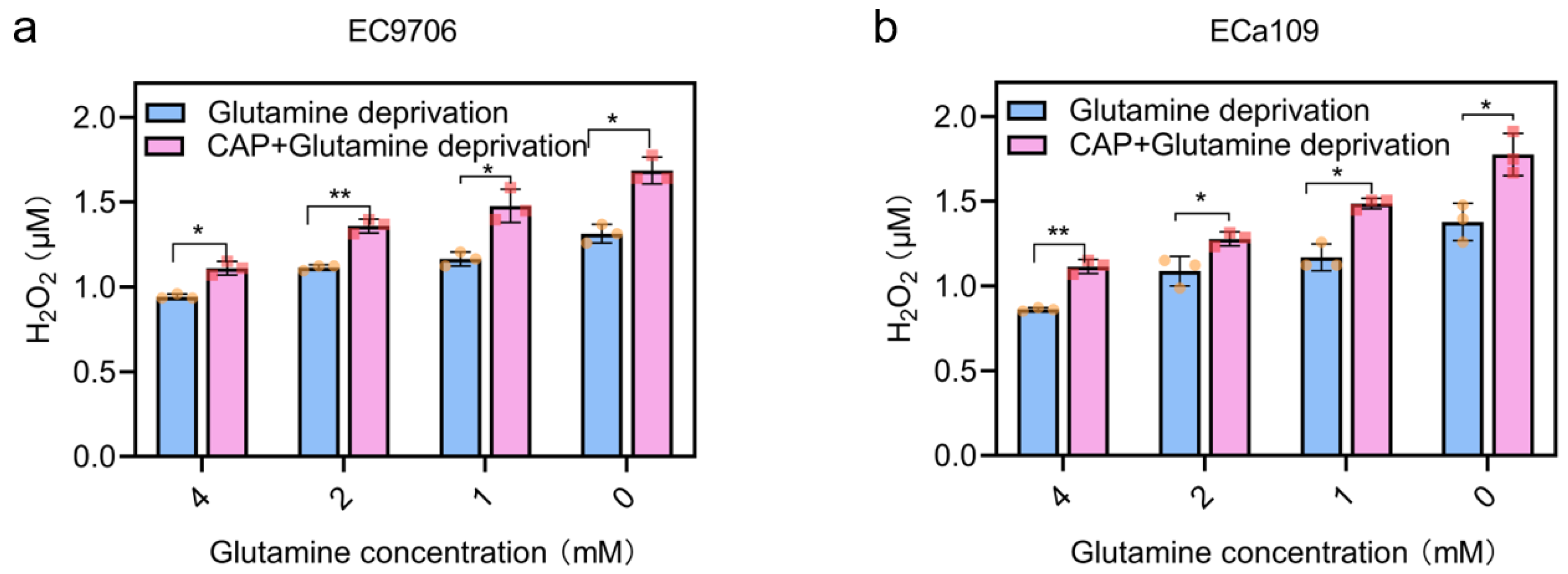
2.8. Intracellular H2O2 Increased after Combined CAP Stimulation and Glutamine Deprivation
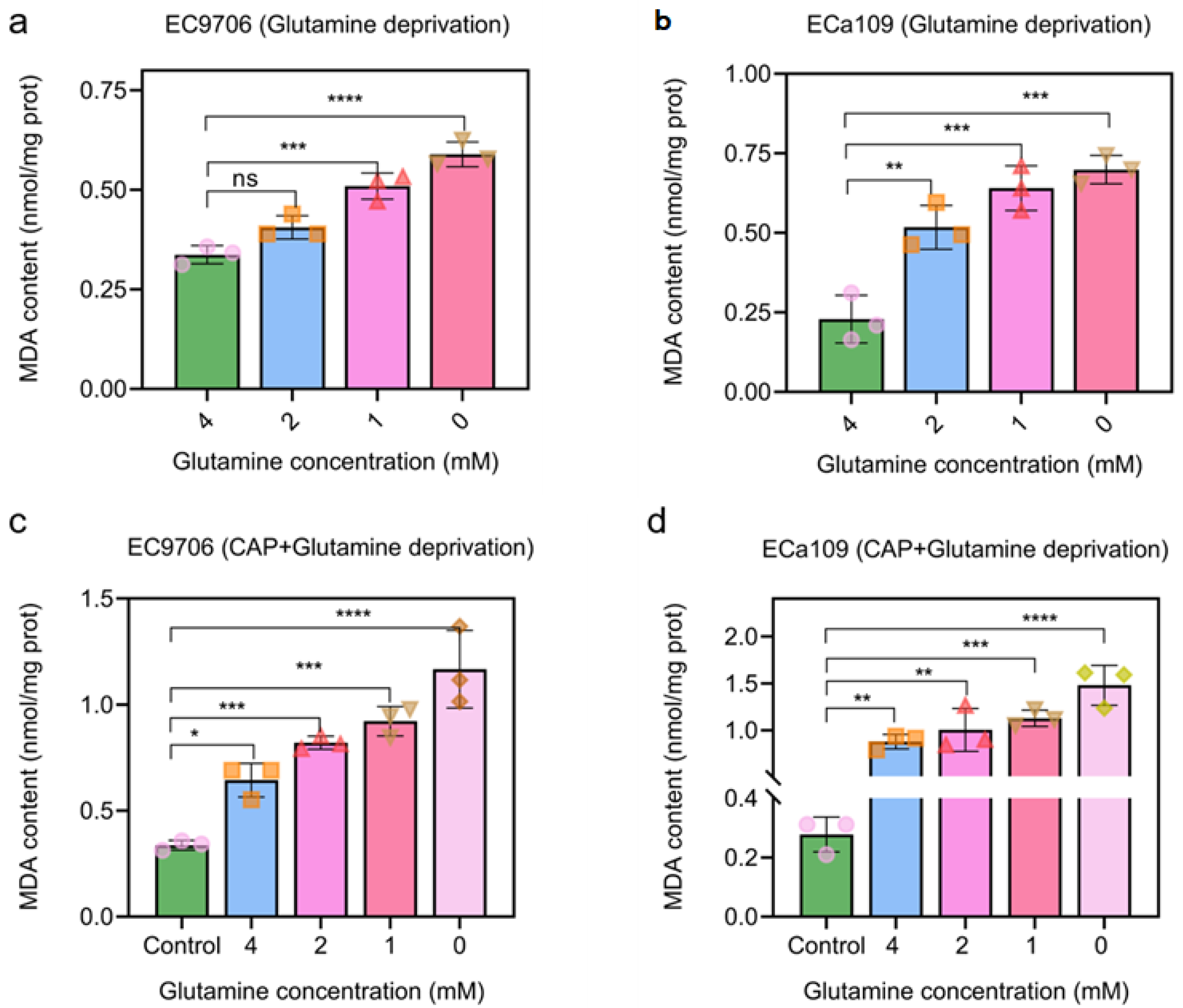
2.9. MAD Increased after CAP Treatment Combined with Glutamine Deprivation
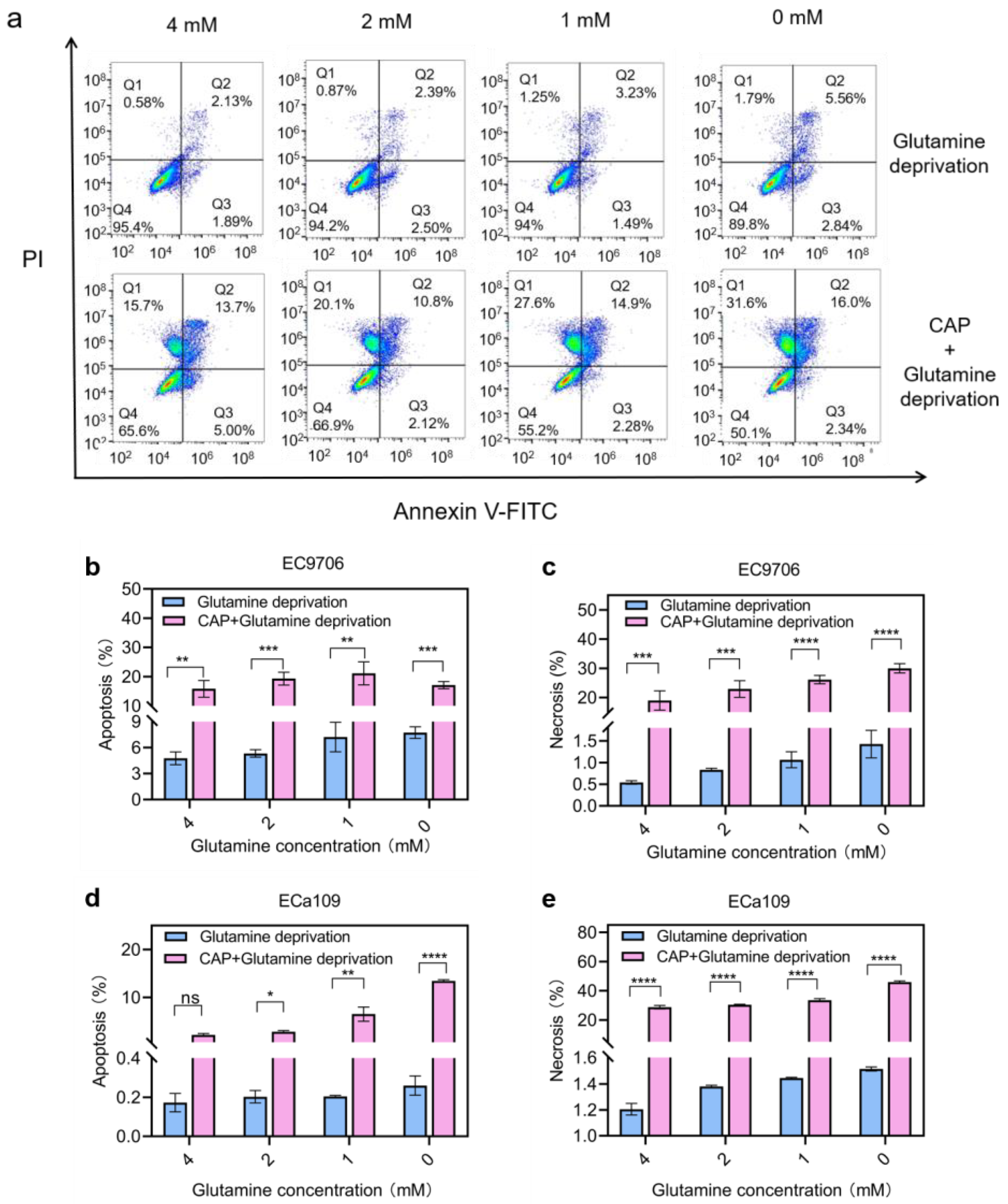
2.10. Apoptosis and Necrosis Increased after Combined Treatment of CAP Stimulation and Glutamine Deprivation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. CAP Treatment
4.3. Cell Viability Assay
4.4. Quantification of NO2−
4.5. Quantification of NO3−
4.6. Quantification of H2O2
4.7. Quantification of Glutathione
4.8. Quantification of Intracellular ROS
4.9. Quantification of MDA
4.10. Apoptosis Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, T.; Fang, P.; Han, C.; Ma, Z.; Xu, W.; Xia, W.; Hu, J.; Xu, Y.; Xu, L.; Yin, R.; et al. Four transcription profile-based models identify novel prognostic signatures in oesophageal cancer. J. Cell. Mol. Med. 2020, 24, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.; Zhuang, W.; Dong, B.; Li, C.; Xu, J.; Wang, G.; Xie, L.; Zhou, Z.; Tian, D.; Chen, G.; et al. Discovery and validation of methylation signatures in circulating cell-free DNA for early detection of esophageal cancer: A case-control study. BMC Med. 2021, 19, 243. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-S.; Yuan, L.-L.; Gao, Y.; Zhou, L.-M.; Yang, J.-W.; Pei, Z.-J. Overexpression of METTL3 associated with the metabolic status on F-18-FDG PET/CT in patients with Esophageal Carcinoma. J. Cancer 2020, 11, 4851–4860. [Google Scholar] [CrossRef]
- Kadono, T.; Yamamoto, S.; Kato, K. Current perspectives of the Japanese Esophageal Oncology Group on the development of immunotherapy for esophageal cancer. Jpn. J. Clin. Oncol. 2022, 52, 1089–1096. [Google Scholar] [CrossRef]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef]
- Tavares-da-Silva, E.; Pereira, E.; Pires, A.S.; Neves, A.R.; Braz-Guilherme, C.; Marques, I.A.; Abrantes, A.M.; Goncalves, A.C.; Caramelo, F.; Silva-Teixeira, R.; et al. Cold Atmospheric Plasma, a Novel Approach against Bladder Cancer, with Higher Sensitivity for the High-Grade Cell Line. Biology 2021, 10, 41. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. Rev. Sect. Phys. Lett. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Jawaid, P.; Rehman, M.U.; Zhao, Q.-L.; Misawa, M.; Ishikawa, K.; Hori, M.; Shimizu, T.; Saitoh, J.-I.; Noguchi, K.; Kondo, T. Small size gold nanoparticles enhance apoptosis-induced by cold atmospheric plasma via depletion of intracellular GSH and modification of oxidative stress. Cell Death Discov. 2020, 6, 83. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef]
- Ratovitski, E.A.; Cheng, X.; Yan, D.; Sherman, J.H.; Canady, J.; Trink, B.; Keidar, M. Anti-Cancer Therapies of 21st Century: Novel Approach to Treat Human Cancers Using Cold Atmospheric Plasma. Plasma Process. Polym. 2014, 11, 1128–1137. [Google Scholar] [CrossRef]
- Biscop, E.; Lin, A.; Boxem, W.V.; Loenhout, J.V.; Backer, J.; Deben, C.; Dewilde, S.; Smits, E.; Bogaerts, A.A. Influence of Cell Type and Culture Medium on Determining Cancer Selectivity of Cold Atmospheric Plasma Treatment. Cancers 2019, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chung, T.H. Cold atmospheric plasma jet-generated RONS and their selective effects on normal and carcinoma cells. Sci. Rep. 2016, 6, 20332. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Xu, W.; Yao, X.; Lin, L.; Sherman, J.H.; Keidar, M. The Cell Activation Phenomena in the Cold Atmospheric Plasma Cancer Treatment. Sci. Rep. 2018, 8, 15418. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, R.; Rehman, M.U.; Zhao, Q.L.; Jawaid, P.; Takeda, K.; Ishikawa, K.; Hori, M.; Tomihara, K.; Noguchi, K.; Kondo, T.; et al. Cold atmospheric helium plasma causes synergistic enhancement in cell death with hyperthermia and an additive enhancement with radiation. Sci. Rep. 2017, 7, 11659. [Google Scholar] [CrossRef]
- Li, Y.; Kang, M.H.; Uhm, H.S.; Lee, G.J.; Choi, E.H.; Han, I. Effects of atmospheric-pressure non-thermal bio-compatible plasma and plasma activated nitric oxide water on cervical cancer cells. Sci. Rep. 2017, 7, 45781. [Google Scholar] [CrossRef]
- Cui, H.; Jiang, M.; Zhou, W.; Gao, M.; He, R.; Huang, Y.; Chu, P.K.; Yu, X.F. Carrier-Free Cellular Transport of CRISPR/Cas9 Ribonucleoprotein for Genome Editing by Cold Atmospheric Plasma. Biology 2021, 10, 1038. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Christodoulou, A.M.; Tachliabouri, M.; Meropoulis, S.; Christopoulou, M.E.; Karalis, T.T.; Chatzopoulos, A.; Skandalis, S.S. Cold Atmospheric Plasma Attenuates Breast Cancer Cell Growth Through Regulation of Cell Microenvironment Effectors. Front. Oncol. 2021, 11, 826865. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Verloy, R.; Cardenas Delahoz, E.; Lin, A.; Vanlanduit, S.; Smits, E.; Bogaerts, A. Cold Atmospheric Plasma Does Not Affect Stellate Cells Phenotype in Pancreatic Cancer Tissue in Ovo. Int. J. Mol. Sci. 2022, 23, 1954. [Google Scholar] [CrossRef]
- Yusupov, M.; Yan, D.; Cordeiro, R.M.; Bogaerts, A. Atomic scale simulation of H2O2 permeation through aquaporin: Toward the understanding of plasma cancer treatment. J. Phys. D-Appl. Phys. 2018, 51, 125401. [Google Scholar] [CrossRef]
- Yan, D.; Xiao, H.; Zhu, W.; Nourmohammadi, N.; Zhang, L.G.; Bian, K.; Keidar, M. The role of aquaporins in the anti-glioblastoma capacity of the cold plasma-stimulated medium. J. Phys. D-Appl. Phys. 2017, 50, 055401. [Google Scholar] [CrossRef]
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins—New players in cancer biology. J. Mol. Med. Jmm. 2008, 86, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Chuang, C.C.; Wu, S.; Zuo, L. Reactive oxygen species in redox cancer therapy. Cancer Lett. 2015, 367, 18–25. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, S.H.; Kim, S.Z.; Park, W.H. Carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) as an O2(*−) generator induces apoptosis via the depletion of intracellular GSH contents in Calu-6 cells. Lung Cancer 2009, 63, 201–209. [Google Scholar] [CrossRef]
- Ju, E.; Dong, K.; Chen, Z.; Liu, Z.; Liu, C.; Huang, Y.; Wang, Z.; Pu, F.; Ren, J.; Qu, X. Copper(II)-Graphitic Carbon Nitride Triggered Synergy: Improved ROS Generation and Reduced Glutathione Levels for Enhanced Photodynamic Therapy. Angew. Chem. Int. Ed. 2016, 55, 11467–11471. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G.; Sersenová, D.; Graves, D.B.; Machala, Z. Dynamics of Singlet Oxygen-Triggered, RONS-Based Apoptosis Induction after Treatment of Tumor Cells with Cold Atmospheric Plasma or Plasma-Activated Medium. Sci. Rep. 2019, 9, 13931. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H. MAPK inhibitors, particularly the JNK inhibitor, increase cell death effects in H2O2-treated lung cancer cells via increased superoxide anion and glutathione depletion. Oncol. Rep. 2018, 39, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Boysen, G.; Jamshidi-Parsian, A.; Davis, M.A.; Siegel, E.R.; Simecka, C.M.; Kore, R.A.; Dings, R.P.M.; Griffin, R.J. Glutaminase inhibitor CB-839 increases radiation sensitivity of lung tumor cells and human lung tumor xenografts in mice. Int. J. Radiat. Biol. 2019, 95, 436–442. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef]
- Saito, K.; Asai, T.; Fujiwara, K.; Sahara, J.; Koguchi, H.; Fukuda, N.; Suzuki-Karasaki, M.; Soma, M.; Suzuki-Karasaki, Y. Tumor-selective mitochondrial network collapse induced by atmospheric gas plasma-activated medium. Oncotarget 2016, 7, 19910–19927. [Google Scholar] [CrossRef]
- Bandebuche, S.; Melinkeri, R.R. Oxidative Stress and Antioxidant Status in Patients of Ovarian Cancer. Biomed. Res. India 2011, 22, 193–197. [Google Scholar]
- Huang, R.; Chen, H.; Liang, J.; Li, Y.; Yang, J.; Luo, C.; Tang, Y.; Ding, Y.; Liu, X.; Yuan, Q.; et al. Dual Role of Reactive Oxygen Species and their Application in Cancer Therapy. J. Cancer 2021, 12, 5543–5561. [Google Scholar] [CrossRef]
- Wang, M.; Holmes, B.; Cheng, X.; Zhu, W.; Keidar, M.; Zhang, L.G. Cold atmospheric plasma for selectively ablating metastatic breast cancer cells. PLoS ONE 2013, 8, e73741. [Google Scholar] [CrossRef]
- Kvam, E.; Davis, B.; Mondello, F.; Garner, A.L. Nonthermal Atmospheric Plasma Rapidly Disinfects Multidrug-Resistant Microbes by Inducing Cell Surface Damage. Antimicrob. Agents Chemother. 2012, 56, 2028–2036. [Google Scholar] [CrossRef]
- Ji, C.; Li, H.; Zhang, L.; Wang, P.; Lv, Y.; Sun, Z.; Tan, J.; Yuan, Q.; Tan, W. Ferrocene-Containing Nucleic Acid-Based Energy-Storage Nanoagent for Continuously Photo-Induced Oxidative Stress Amplification. Angew. Chem. Int. Ed. Engl. 2022, 61, e202200237. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-W.; Liu, X.-H.; Fan, J.-X.; Peng, S.-Y.; Wang, J.-W.; Wang, X.-N.; Zhang, C.; Liu, C.-J.; Zhang, X.-Z. Self-Mineralized Photothermal Bacteria Hybridizing with Mitochondria-T argeted Metal–Organic Frameworks for Augmenting Photothermal T umor Therapy. Adv. Funct. Mater. 2020, 30, 1909806. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J. Effects of Non-Thermal Plasma on Mammalian Cells. PLoS ONE 2011, 6, e16270. [Google Scholar] [CrossRef]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Hori, M.; Kikkawa, F. Selective cytotoxicity of indirect nonequilibrium atmospheric pressure plasma against ovarian clear-cell carcinoma. Springerplus 2014, 3, 398. [Google Scholar] [CrossRef] [PubMed]
- Tornin, J.; Mateu-Sanz, M.; Rodríguez, A.; Labay, C.; Rodríguez, R.; Canal, C. Pyruvate Plays a Main Role in the Antitumoral Selectivity of Cold Atmospheric Plasma in Osteosarcoma. Sci. Rep. 2019, 9, 10681. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.; Judée, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017, 7, 4562. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, D.; Wang, B.; Chen, C.; Chen, Z.; Li, D.; Yang, Y.; Chen, H.; Kong, M.G. In Situ OH Generation from O2− and H2O2 Plays a Critical Role in Plasma-Induced Cell Death. PLoS ONE 2015, 10, e0128205. [Google Scholar] [CrossRef] [PubMed]
- Takashi, Y.; Tomita, K.; Kuwahara, Y.; Roudkenar, M.H.; Roushandeh, A.M.; Igarashi, K.; Nagasawa, T.; Nishitani, Y.; Sato, T. Mitochondrial dysfunction promotes aquaporin expression that controls hydrogen peroxide permeability and ferroptosis. Free Radic. Biol. Med. 2020, 161, 60–70. [Google Scholar] [CrossRef]
- Imai, H.; Matsuoka, M.; Kumagai, T.; Sakamoto, T.; Koumura, T. Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis. Curr. Top. Microbiol. Immunol. 2017, 403, 143–170. [Google Scholar] [PubMed]
- Shaw, P.; Kumar, N.; Privat-Maldonado, A.; Smits, E.; Bogaerts, A. Cold Atmospheric Plasma Increases Temozolomide Sensitivity of Three-Dimensional Glioblastoma Spheroids via Oxidative Stress-Mediated DNA Damage. Cancers 2021, 13, 1780. [Google Scholar] [CrossRef]
- Almeida, N.D.; Klein, A.L.; Hogan, E.A.; Terhaar, S.J.; Kedda, J.; Uppal, P.; Sack, K.; Keidar, M.; Sherman, J.H. Cold Atmospheric Plasma as an Adjunct to Immunotherapy for Glioblastoma Multiforme. World Neurosurg. 2019, 130, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Köritzer, J.; Boxhammer, V.; Schäfer, A.; Shimizu, T.; Klämpfl, T.G.; Li, Y.F.; Welz, C.; Schwenk-Zieger, S.; Morfill, G.E.; Zimmermann, J.L.; et al. Restoration of sensitivity in chemo-resistant glioma cells by cold atmospheric plasma. PLoS ONE 2013, 8, e64498. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.; Adhikari, M.; Lin, L.; Sherman, J.H.; Keidar, M. Theranostic Potential of Adaptive Cold Atmospheric Plasma with Temozolomide to Checkmate Glioblastoma: An In Vitro Study. Cancers 2022, 14, 3116. [Google Scholar] [CrossRef]
- Moniruzzaman, R.; Rehman, M.U.; Zhao, Q.L.; Jawaid, P.; Mitsuhashi, Y.; Imaue, S.; Fujiwara, K.; Ogawa, R.; Tomihara, K.; Saitoh, J.I.; et al. Roles of intracellular and extracellular ROS formation in apoptosis induced by cold atmospheric helium plasma and X-irradiation in the presence of sulfasalazine. Free Radic. Biol. Med. 2018, 129, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.Y.; Liu, X.H.; Chen, Q.W.; Yu, Y.J.; Liu, M.D.; Zhang, X.Z. Harnessing in situ glutathione for effective ROS generation and tumor suppression via nanohybrid-mediated catabolism dynamic therapy. Biomaterials 2022, 281, 121358. [Google Scholar] [CrossRef]
- Brunner, T.F.; Probst, F.A.; Troeltzsch, M.; Schwenk-Zieger, S.; Zimmermann, J.L.; Morfill, G.; Becker, S.; Harréus, U.; Welz, C. Primary cold atmospheric plasma combined with low dose cisplatin as a possible adjuvant combination therapy for HNSCC cells-an in-vitro study. Head Face Med. 2022, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, K.N.; DeBerardinis, R.J. Role of glutamine in cancer: Therapeutic and imaging implications. J. Nucl. Med. 2011, 52, 1005–1008. [Google Scholar] [CrossRef]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.; Chandel, N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef]
- Sun, N.; Liang, Y.; Chen, Y.; Wang, L.; Li, D.; Liang, Z.; Sun, L.; Wang, Y.; Niu, H. Glutamine affects T24 bladder cancer cell proliferation by activating STAT3 through ROS and glutaminolysis. Int. J. Mol. Med. 2019, 44, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Schömel, N.; Hancock, S.E.; Gruber, L.; Olzomer, E.M.; Byrne, F.L.; Shah, D.; Hoehn, K.L.; Turner, N.; Grösch, S.; Geisslinger, G.; et al. UGCG influences glutamine metabolism of breast cancer cells. Sci. Rep. 2019, 9, 15665. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.G.; O’Driscoll, C.M.; Bressler, J.; Kaufmann, W.E.; Rojas, C.J.; Slusher, B.S. Small molecule glutaminase inhibitors block glutamate release from stimulated microglia. Biochem. Biophys. Res. Commun. 2014, 443, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Zubor, P.; Wang, Y.; Liskova, A.; Samec, M.; Koklesova, L.; Dankova, Z.; Dørum, A.; Kajo, K.; Dvorska, D.; Lucansky, V.; et al. Cold Atmospheric Pressure Plasma (CAP) as a New Tool for the Management of Vulva Cancer and Vulvar Premalignant Lesions in Gynaecological Oncology. Int. J. Mol. Sci. 2020, 21, 7988. [Google Scholar] [CrossRef]
- Zhou, R.; Pantel, A.R.; Li, S.; Lieberman, B.P.; Ploessl, K.; Choi, H.; Blankemeyer, E.; Lee, H.; Kung, H.F.; Mach, R.H.; et al. F-18 (2S,4R)4-Fluoroglutamine PET Detects Glutamine Pool Size Changes in Triple-Negative Breast Cancer in Response to Glutaminase Inhibition. Cancer Res. 2017, 7, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Susilo, I.; Maulida, H.; Alimsardjono, L.; Fauziah, D.; Pertiwi, H. Apoptosis-Inducing Factor, Protein Expression, and Apoptosis Changes with Glutamine in Podocytes Cells Exposed with Cisplatin. Vet. Med. Int. 2021, 2021, 5599452. [Google Scholar] [CrossRef]
- Al-Gubory, K.H. Mitochondria: Omega-3 in the route of mitochondrial reactive oxygen species. Int. J. Biochem. Cell Biol. 2012, 44, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.S.; Jones, D.P. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in HL60 cells that overexpress Bcl-2. FASEB J. 2002, 16, 1263–1265. [Google Scholar] [CrossRef]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Mukha, A.; Kahya, U.; Linge, A.; Chen, O.; Loeck, S.; Lukiyanchuk, V.; Richter, S.; Alves, T.C.; Peitzsch, M.; Telychko, V.; et al. GLS-driven glutamine catabolism contributes to prostate cancer radiosensitivity by regulating the redox state, stemness and ATG5-mediated autophagy. Theranostics 2021, 11, 7844–7868. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhu, J.; Wang, X.; Yang, C.; Fu, Z. Evaluation of the immunomodulatory effects of C9-13-CPs in macrophages. Acta Biochim. Biophys. Sin. 2021, 53, 1154–1165. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Jing, X.; Wang, T.; Zhang, F. Glutamine Deprivation Synergizes the Anticancer Effects of Cold Atmospheric Plasma on Esophageal Cancer Cells. Molecules 2023, 28, 1461. https://doi.org/10.3390/molecules28031461
Zhao W, Jing X, Wang T, Zhang F. Glutamine Deprivation Synergizes the Anticancer Effects of Cold Atmospheric Plasma on Esophageal Cancer Cells. Molecules. 2023; 28(3):1461. https://doi.org/10.3390/molecules28031461
Chicago/Turabian StyleZhao, Wei, Xumiao Jing, Tao Wang, and Fengqiu Zhang. 2023. "Glutamine Deprivation Synergizes the Anticancer Effects of Cold Atmospheric Plasma on Esophageal Cancer Cells" Molecules 28, no. 3: 1461. https://doi.org/10.3390/molecules28031461
APA StyleZhao, W., Jing, X., Wang, T., & Zhang, F. (2023). Glutamine Deprivation Synergizes the Anticancer Effects of Cold Atmospheric Plasma on Esophageal Cancer Cells. Molecules, 28(3), 1461. https://doi.org/10.3390/molecules28031461




