Natural Agents as Novel Potential Source of Proteasome Inhibitors with Anti-Tumor Activity: Focus on Multiple Myeloma
Abstract
1. Introduction
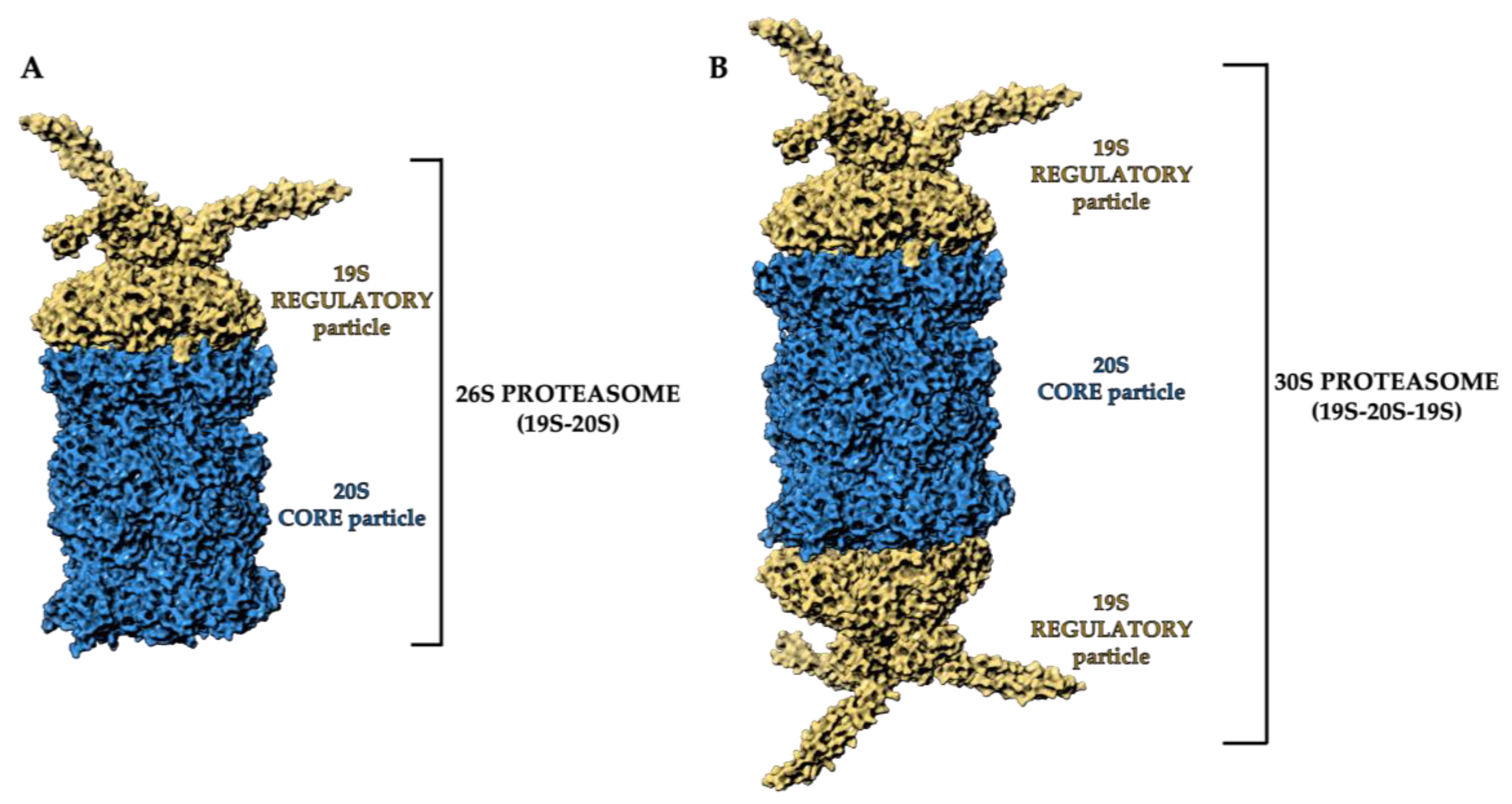
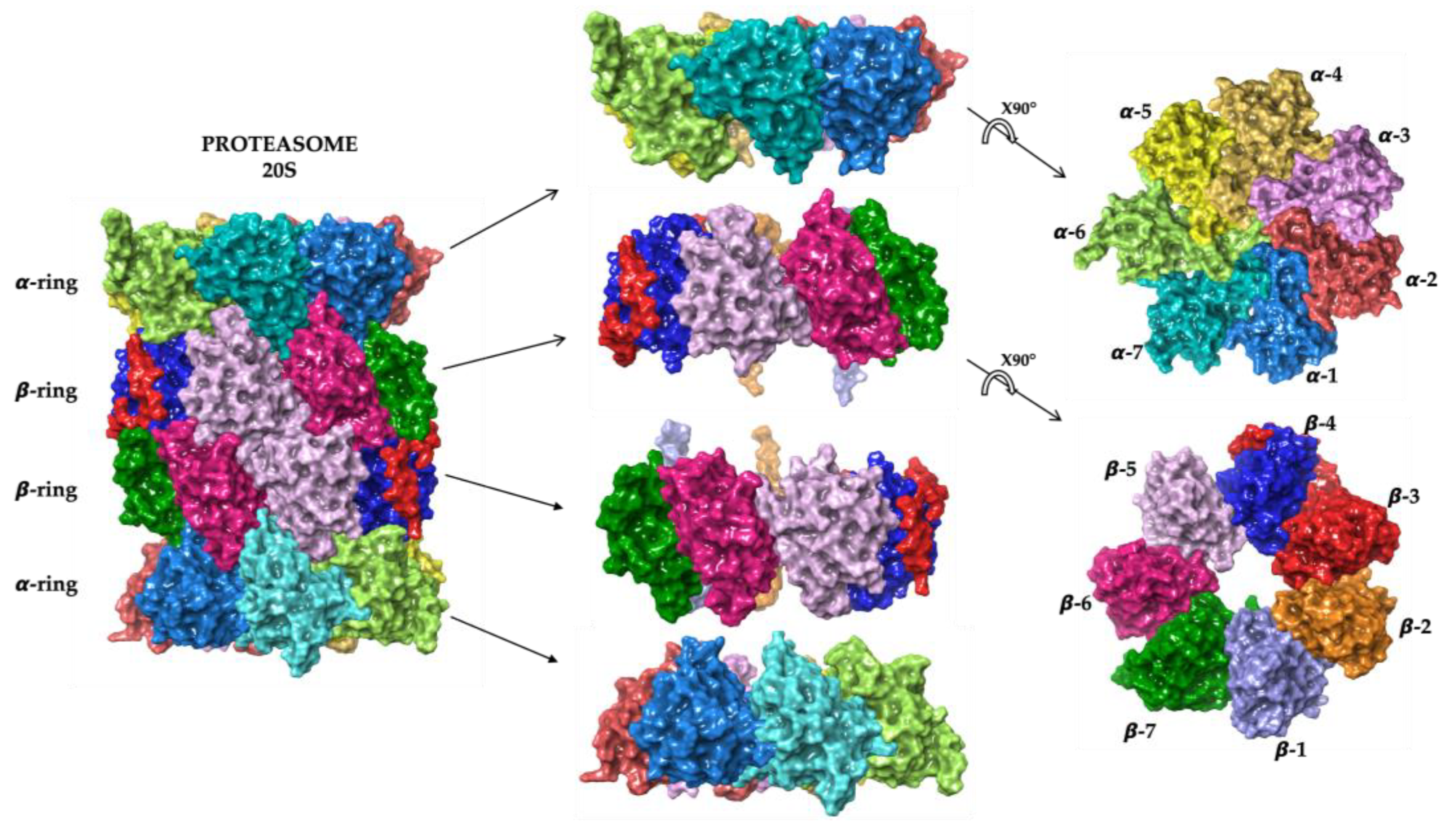
2. Proteasome Structure and Function
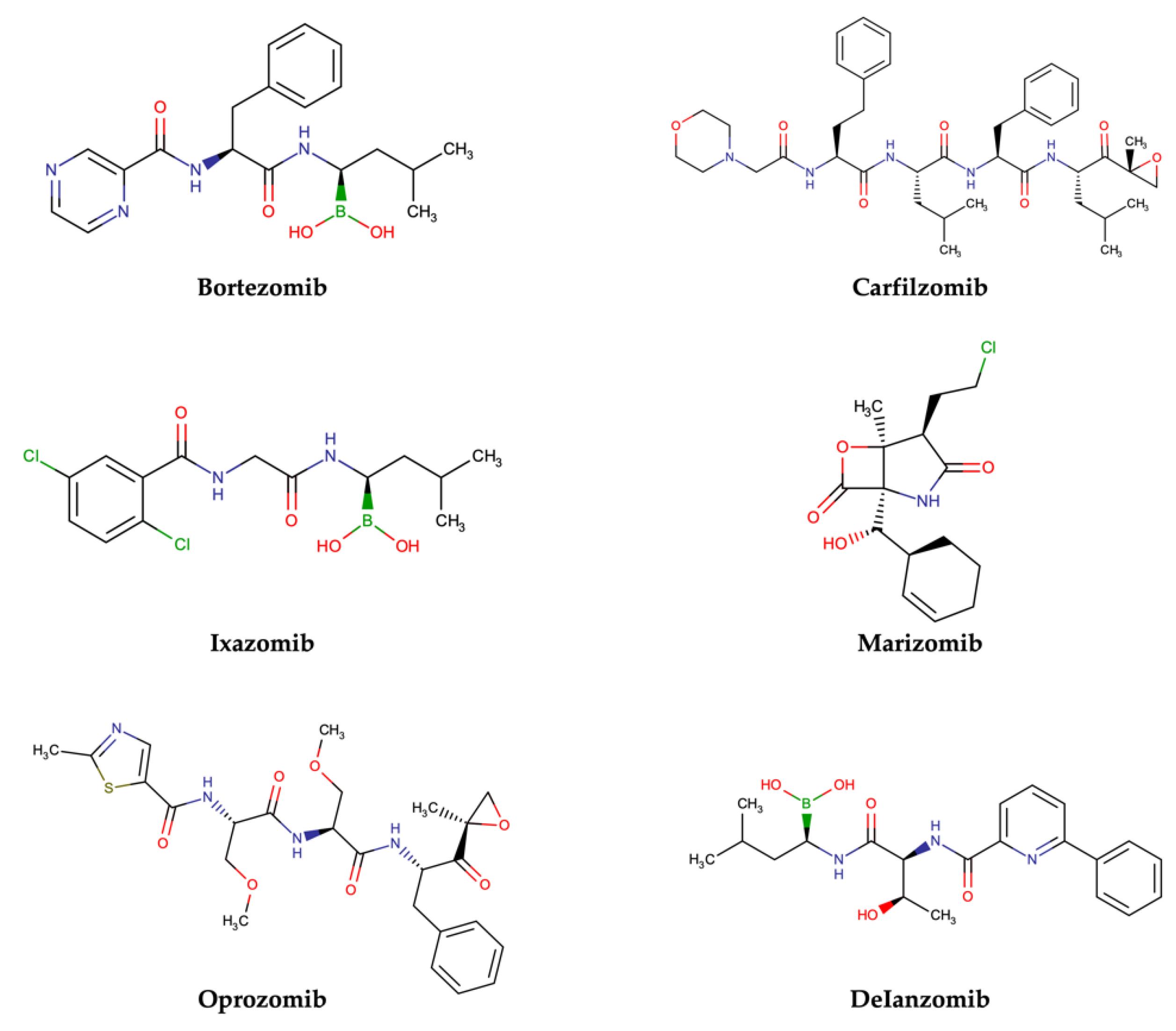
Approved and Investigational PIs for MM Treatment
- Bortezomib
- Carfilzomib
- Ixazomib
- Marizomib
- Oprozomib
- Delanzomib
| Compound | Pharmacophoric Moiety | Binding Kinetics | Proteasome Subunit |
|---|---|---|---|
| Bortezomib | Boronate | Reversible | β5 > β1 |
| Carfilzomib | Epoxyketone | Irreversible | β5 |
| Ixazomib | Boronate | Reversible | β5 > β1 |
| Marizomib | β-lactone | Irreversible | β5 > β1 > β2 |
| Oprozomib | Epoxyketone | Irreversible | β5 |
| Delanzomib | Boronate | Reversible | β5 > β1 |
3. Natural PIs in MM
3.1. Tea Polyphenols
3.2. Hib-Ester and Hib-Carbaldehyde
3.3. Celastrol
3.4. Syrbactins
3.5. Tyropeptins
3.6. Pristimerin
3.7. Ursolic Acid
| Name | 2D Structure | Natural Source | Biological Effects | |
|---|---|---|---|---|
| In Vitro Studies | In Vivo Studies | |||
| Theaflavin |  | Black tea |
| n.a. |
| Theaflavin-3-gallate |  | |||
| Theaflavin-3′-gallate | 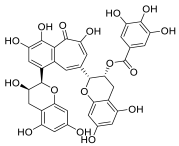 | |||
| Theaflavin-3, 3′-digallate | 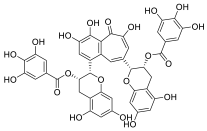 | |||
| Hib-ester | 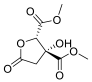 | Hibiscus sabdariffa | n.a. | n.a. |
| Hib-carbaldehyde | 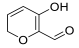 | |||
| Celastrol |  | Tripterygium wilfordii |
|
|
| Syringolin |  | Pseudomonas syringae pv. Syringae |
|
|
| TIR-199 |  | – | ||
| Tyropeptin A | 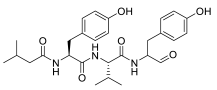 | Actinomycete strain MK993-dF2 | n.a. | |
| AS-06 | 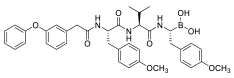 | - |
|
|
| AS-29 | 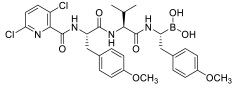 | - | ||
| Pristimerin |  | Myatenus, Crossopetalum, Celastrus, Cheiloclinium, Mortonia |
| n.a. |
| Ursolic acid | 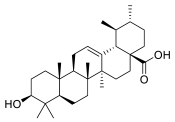 | Apple peel |
| n.a. |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Primers 2017, 3, 17046. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, S.; Laubach, J.P.; Hideshima, T.; Chauhan, D.; Anderson, K.C.; Richardson, P.G. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017, 36, 561–584. [Google Scholar] [CrossRef] [PubMed]
- Manier, S.; Salem, K.Z.; Park, J.; Landau, D.A.; Getz, G.; Ghobrial, I.M. Genomic complexity of multiple myeloma and its clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Raimondi, L.; De Luca, A.; Giavaresi, G.; Raimondo, S.; Gallo, A.; Taiana, E.; Alessandro, R.; Rossi, M.; Neri, A.; Viglietto, G.; et al. Non-Coding RNAs in Multiple Myeloma Bone Disease Pathophysiology. Noncoding RNA 2020, 6, 37. [Google Scholar] [CrossRef]
- Taiana, E.; Gallo Cantafio, M.E.; Favasuli, V.K.; Bandini, C.; Viglietto, G.; Piva, R.; Neri, A.; Amodio, N. Genomic Instability in Multiple Myeloma: A “Non-Coding RNA” Perspective. Cancers 2021, 13, 2127. [Google Scholar] [CrossRef]
- Amodio, N.; D’Aquila, P.; Passarino, G.; Tassone, P.; Bellizzi, D. Epigenetic modifications in multiple myeloma: Recent advances on the role of DNA and histone methylation. Expert. Opin. Ther. Targets 2017, 21, 91–101. [Google Scholar] [CrossRef]
- Raimondi, L.; De Luca, A.; Morelli, E.; Giavaresi, G.; Tagliaferri, P.; Tassone, P.; Amodio, N. MicroRNAs: Novel Crossroads between Myeloma Cells and the Bone Marrow Microenvironment. Biomed. Res. Int. 2016, 2016, 6504593. [Google Scholar] [CrossRef]
- Morelli, E.; Gullà, A.; Rocca, R.; Federico, C.; Raimondi, L.; Malvestiti, S.; Agosti, V.; Rossi, M.; Costa, G.; Giavaresi, G.; et al. The Non-Coding RNA Landscape of Plasma Cell Dyscrasias. Cancers 2020, 12, 320. [Google Scholar] [CrossRef]
- Rousseau, A.; Bertolotti, A. Regulation of proteasome assembly and activity in health and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 697–712. [Google Scholar] [CrossRef]
- Nagel, D.; Vincendeau, M.; Eitelhuber, A.C.; Krappmann, D. Mechanisms and consequences of constitutive NF-κB activation in B-cell lymphoid malignancies. Oncogene 2014, 33, 5655–5665. [Google Scholar] [CrossRef]
- Paradzik, T.; Bandini, C.; Mereu, E.; Labrador, M.; Taiana, E.; Amodio, N.; Neri, A.; Piva, R. The Landscape of Signaling Pathways and Proteasome Inhibitors Combinations in Multiple Myeloma. Cancers 2021, 13, 1235. [Google Scholar] [CrossRef]
- Amodio, N.; Bellizzi, D.; Leotta, M.; Raimondi, L.; Biamonte, L.; D’Aquila, P.; Di Martino, M.T.; Calimeri, T.; Rossi, M.; Lionetti, M.; et al. miR-29b induces SOCS-1 expression by promoter demethylation and negatively regulates migration of multiple myeloma and endothelial cells. Cell Cycle 2013, 12, 3650–3662. [Google Scholar] [CrossRef]
- Amodio, N.; Leotta, M.; Bellizzi, D.; Di Martino, M.T.; D’Aquila, P.; Lionetti, M.; Fabiani, F.; Leone, E.; Gullà, A.M.; Passarino, G.; et al. DNA-demethylating and anti-tumor activity of synthetic miR-29b mimics in multiple myeloma. Oncotarget 2012, 3, 1246–1258. [Google Scholar] [CrossRef]
- Amodio, N.; Di Martino, M.T.; Neri, A.; Tagliaferri, P.; Tassone, P. Non-coding RNA: A novel opportunity for the personalized treatment of multiple myeloma. Expert. Opin. Biol. Ther. 2013, 13 (Suppl. S1), S125–S137. [Google Scholar] [CrossRef]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T.; Taskforce, I.N.P.S. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug. Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- Capalbo, A.; Lauritano, C. Multiple Myeloma: Possible Cure from the Sea. Cancers 2022, 14, 2965. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, L.; De Luca, A.; Giavaresi, G.; Barone, A.; Tagliaferri, P.; Tassone, P.; Amodio, N. Impact of Natural Dietary Agents on Multiple Myeloma Prevention and Treatment: Molecular Insights and Potential for Clinical Translation. Curr. Med. Chem. 2020, 27, 187–215. [Google Scholar] [CrossRef] [PubMed]
- Malacrida, A.; Cavalloro, V.; Martino, E.; Costa, G.; Ambrosio, F.A.; Alcaro, S.; Rigolio, R.; Cassetti, A.; Miloso, M.; Collina, S. Anti-Multiple Myeloma Potential of Secondary Metabolites from. Molecules 2021, 26, 6596. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulou, M.; Ioannou, E.; Roussis, V.; Chondrogianni, N. Modulation of the ubiquitin-proteasome system by marine natural products. Redox Biol. 2021, 41, 101897. [Google Scholar] [CrossRef]
- Budenholzer, L.; Cheng, C.L.; Li, Y.; Hochstrasser, M. Proteasome Structure and Assembly. J. Mol. Biol. 2017, 429, 3500–3524. [Google Scholar] [CrossRef]
- Sahu, I.; Mali, S.M.; Sulkshane, P.; Xu, C.; Rozenberg, A.; Morag, R.; Sahoo, M.P.; Singh, S.K.; Ding, Z.; Wang, Y.; et al. The 20S as a stand-alone proteasome in cells can degrade the ubiquitin tag. Nat. Commun. 2021, 12, 6173. [Google Scholar] [CrossRef]
- Jung, T.; Grune, T. The proteasome and the degradation of oxidized proteins: Part I-structure of proteasomes. Redox Biol. 2013, 1, 178–182. [Google Scholar] [CrossRef]
- Tanaka, K. The proteasome: Overview of structure and functions. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 2009, 85, 12–36. [Google Scholar] [CrossRef]
- Wehmer, M.; Rudack, T.; Beck, F.; Aufderheide, A.; Pfeifer, G.; Plitzko, J.M.; Förster, F.; Schulten, K.; Baumeister, W.; Sakata, E. Structural insights into the functional cycle of the ATPase module of the 26S proteasome. Proc. Natl. Acad. Sci. USA 2017, 114, 1305–1310. [Google Scholar] [CrossRef]
- Schrödinger Release 2018-1: Maestro, Schrödinger, LLC, New York, NY. 2018. Available online: https://www.schrodinger.com/releases/release-2018-1 (accessed on 26 December 2022).
- Almond, J.B.; Cohen, G.M. The proteasome: A novel target for cancer chemotherapy. Leukemia 2002, 16, 433–443. [Google Scholar] [CrossRef]
- Martinez-Fonts, K.; Davis, C.; Tomita, T.; Elsasser, S.; Nager, A.R.; Shi, Y.; Finley, D.; Matouschek, A. The proteasome 19S cap and its ubiquitin receptors provide a versatile recognition platform for substrates. Nat. Commun. 2020, 11, 477. [Google Scholar] [CrossRef]
- Schrader, J.; Henneberg, F.; Mata, R.A.; Tittmann, K.; Schneider, T.R.; Stark, H.; Bourenkov, G.; Chari, A. The inhibition mechanism of human 20S proteasomes enables next-generation inhibitor design. Science 2016, 353, 594–598. [Google Scholar] [CrossRef]
- Köhler, A.; Cascio, P.; Leggett, D.S.; Woo, K.M.; Goldberg, A.L.; Finley, D. The axial channel of the proteasome core particle is gated by the Rpt2 ATPase and controls both substrate entry and product release. Mol. Cell 2001, 7, 1143–1152. [Google Scholar] [CrossRef]
- Guedes, R.A.; Serra, P.; Salvador, J.A.; Guedes, R.C. Computational Approaches for the Discovery of Human Proteasome Inhibitors: An Overview. Molecules 2016, 21, 927. [Google Scholar] [CrossRef]
- Crawford, L.J.; Walker, B.; Irvine, A.E. Proteasome inhibitors in cancer therapy. J. Cell Commun. Signal. 2011, 5, 101–110. [Google Scholar] [CrossRef]
- Thibaudeau, T.A.; Smith, D.M. A Practical Review of Proteasome Pharmacology. Pharmacol. Rev. 2019, 71, 170–197. [Google Scholar] [CrossRef]
- Chen, D.; Frezza, M.; Schmitt, S.; Kanwar, J.; Dou, Q.P. Bortezomib as the first proteasome inhibitor anticancer drug: Current status and future perspectives. Curr. Cancer Drug. Targets 2011, 11, 239–253. [Google Scholar] [CrossRef]
- Herndon, T.M.; Deisseroth, A.; Kaminskas, E.; Kane, R.C.; Koti, K.M.; Rothmann, M.D.; Habtemariam, B.; Bullock, J.; Bray, J.D.; Hawes, J.; et al. U.S. Food and Drug Administration approval: Carfilzomib for the treatment of multiple myeloma. Clin. Cancer Res. 2013, 19, 4559–4563. [Google Scholar] [CrossRef]
- Moreau, P.; Richardson, P.G.; Cavo, M.; Orlowski, R.Z.; San Miguel, J.F.; Palumbo, A.; Harousseau, J.L. Proteasome inhibitors in multiple myeloma: 10 years later. Blood 2012, 120, 947–959. [Google Scholar] [CrossRef]
- Shirley, M. Ixazomib: First Global Approval. Drugs 2016, 76, 405–411. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Q.; Dou, Q.P.; Yang, H. Discovery of Natural Proteasome Inhibitors as Novel Anticancer Therapeutics: Current Status and Perspectives. Curr. Protein. Pept. Sci. 2018, 19, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Fricker, L.D. Proteasome Inhibitor Drugs. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Okazuka, K.; Ishida, T. Proteasome inhibitors for multiple myeloma. Jpn J. Clin. Oncol. 2018, 48, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Jöhrer, K.; Ҫiҫek, S.S. Multiple Myeloma Inhibitory Activity of Plant Natural Products. Cancers 2021, 13, 2678. [Google Scholar] [CrossRef]
- Chen, D.; Milacic, V.; Chen, M.S.; Wan, S.B.; Lam, W.H.; Huo, C.; Landis-Piwowar, K.R.; Cui, Q.C.; Wali, A.; Chan, T.H.; et al. Tea polyphenols, their biological effects and potential molecular targets. Histol. Histopathol. 2008, 23, 487–496. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Mukhtar, H. Tea antioxidants in cancer chemoprevention. J. Cell Biochem. Suppl. 1997, 27, 59–67. [Google Scholar] [CrossRef]
- Yang, G.Y.; Liao, J.; Kim, K.; Yurkow, E.J.; Yang, C.S. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis 1998, 19, 611–616. [Google Scholar] [CrossRef]
- Kundu, T.; Dey, S.; Roy, M.; Siddiqi, M.; Bhattacharya, R.K. Induction of apoptosis in human leukemia cells by black tea and its polyphenol theaflavin. Cancer Lett. 2005, 230, 111–121. [Google Scholar] [CrossRef]
- Lu, J.; Ho, C.T.; Ghai, G.; Chen, K.Y. Differential effects of theaflavin monogallates on cell growth, apoptosis, and Cox-2 gene expression in cancerous versus normal cells. Cancer Res. 2000, 60, 6465–6471. [Google Scholar]
- Mujtaba, T.; Dou, Q.P. Black tea polyphenols inhibit tumor proteasome activity. In Vivo 2012, 26, 197–202. [Google Scholar]
- Golden, E.B.; Lam, P.Y.; Kardosh, A.; Gaffney, K.J.; Cadenas, E.; Louie, S.G.; Petasis, N.A.; Chen, T.C.; Schönthal, A.H. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood 2009, 113, 5927–5937. [Google Scholar] [CrossRef]
- Mohamed, R.; Fernández, J.; Pineda, M.; Aguilar, M. Roselle (Hibiscus sabdariffa) seed oil is a rich source of gamma-tocopherol. J. Food Sci. 2007, 72, S207–S211. [Google Scholar] [CrossRef]
- Listro, R.; Malacrida, A.; Ambrosio, F.A.; Rossino, G.; Di Giacomo, M.; Cavalloro, V.; Garbagnoli, M.; Linciano, P.; Rossi, D.; Cavaletti, G.; et al. From Nature to Synthetic Compounds: Novel 1(N),2,3 Trisubstituted-5-oxopyrrolidines Targeting Multiple Myeloma Cells. Int. J. Mol. Sci. 2022, 23, 13061. [Google Scholar] [CrossRef]
- Zhong, Y.L.; Xu, G.J.; Huang, S.; Zhao, L.; Zeng, Y.; Xiao, X.F.; An, J.L.; Liu, J.; Yang, T. Celastrol induce apoptosis of human multiple myeloma cells involving inhibition of proteasome activity. Eur. J. Pharmacol. 2019, 853, 184–192. [Google Scholar] [CrossRef]
- Amrein, H.; Makart, S.; Granado, J.; Shakya, R.; Schneider-Pokorny, J.; Dudler, R. Functional analysis of genes involved in the synthesis of syringolin A by Pseudomonas syringae pv. syringae B301 D-R. Mol. Plant. Microbe Interact. 2004, 17, 90–97. [Google Scholar] [CrossRef]
- Groll, M.; Schellenberg, B.; Bachmann, A.S.; Archer, C.R.; Huber, R.; Powell, T.K.; Lindow, S.; Kaiser, M.; Dudler, R. A plant pathogen virulence factor inhibits the eukaryotic proteasome by a novel mechanism. Nature 2008, 452, 755–758. [Google Scholar] [CrossRef]
- Bachmann, A.S.; Opoku-Ansah, J.; Ibarra-Rivera, T.R.; Yco, L.P.; Ambadi, S.; Roberts, C.C.; Chang, C.E.; Pirrung, M.C. Syrbactin Structural Analog TIR-199 Blocks Proteasome Activity and Induces Tumor Cell Death. J. Biol Chem 2016, 291, 8350–8362. [Google Scholar] [CrossRef]
- Pierce, M.R.; Robinson, R.M.; Ibarra-Rivera, T.R.; Pirrung, M.C.; Dolloff, N.G.; Bachmann, A.S. Syrbactin proteasome inhibitor TIR-199 overcomes bortezomib chemoresistance and inhibits multiple myeloma tumor growth in vivo. Leuk Res. 2020, 88, 106271. [Google Scholar] [CrossRef]
- Tandon, V.; Vala, R.M.; Chen, A.; Sah, R.L.; Patel, H.M.; Pirrung, M.C.; Banerjee, S. Syrbactin-class dual constitutive- and immuno-proteasome inhibitor TIR-199 impedes myeloma-mediated bone degeneration in vivo. Biosci. Rep. 2022, 42, BSR20212721. [Google Scholar] [CrossRef]
- Momose, I.; Watanabe, T. Tyropeptins, proteasome inhibitors produced by Kitasatospora sp. MK993-dF2. J. Antibiot. 2017, 70, 542–550. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Yokosawa, H. Inhibition of the ubiquitin-proteasome system by natural products for cancer therapy. Planta Med. 2010, 76, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Momose, I.; Sekizawa, R.; Hirosawa, S.; Ikeda, D.; Naganawa, H.; Iinuma, H.; Takeuchi, T. Tyropeptins A and B, new proteasome inhibitors produced by Kitasatospora sp. MK993-dF2. II. Structure determination and synthesis. J. Antibiot. 2001, 54, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Momose, I.; Sekizaw, R.; Hashizume, H.; Kinoshita, N.; Homma, Y.; Hamada, M.; Linuma, H.; Takeuchi, T. Tyropeptins A and B, new proteasome inhibitors produced by Kitasatospora sp. MK993-dF2. I. Taxonomy, isolation, physico-chemical properties and biological activities. J. Antibiot. 2001, 54, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Momose, I.; Abe, H.; Watanabe, T.; Ohba, S.; Yamazaki, K.; Dan, S.; Yamori, T.; Masuda, T.; Nomoto, A. Antitumor effects of tyropeptin-boronic acid derivatives: New proteasome inhibitors. Cancer Sci. 2014, 105, 1609–1615. [Google Scholar] [CrossRef]
- Chen, M.X.; Wang, D.Y.; Guo, J. 3-Oxo-11β-Hydroxyfriedelane from the Roots of Celastrus Monospermus. J. Chem. Res. 2010, 34, 114–117. [Google Scholar] [CrossRef]
- Taddeo, V.A.; Castillo, U.G.; Martínez, M.L.; Menjivar, J.; Jiménez, I.A.; Núñez, M.J.; Bazzocchi, I.L. Development and Validation of an HPLC-PDA Method for Biologically Active Quinonemethide Triterpenoids Isolated from Maytenus chiapensis. Medicines 2019, 6, 36. [Google Scholar] [CrossRef]
- Alvarenga, N.; Ferro, E.A. Bioactive Triterpenes and Related Compounds from Celastraceae. Stud. Nat. Prod. Chem. 2006, 33, 239–307. [Google Scholar]
- Wu, C.C.; Chan, M.L.; Chen, W.Y.; Tsai, C.Y.; Chang, F.R.; Wu, Y.C. Pristimerin induces caspase-dependent apoptosis in MDA-MB-231 cells via direct effects on mitochondria. Mol. Cancer Ther. 2005, 4, 1277–1285. [Google Scholar] [CrossRef]
- Gao, X.; Liu, Y.; Deeb, D.; Arbab, A.S.; Gautam, S.C. Anticancer activity of pristimerin in ovarian carcinoma cells is mediated through the inhibition of prosurvival Akt/NF-κB/mTOR signaling. J. Exp. Ther. Oncol. 2014, 10, 275–283. [Google Scholar]
- Yousef, B.A.; Hassan, H.M.; Guerram, M.; Hamdi, A.M.; Wang, B.; Zhang, L.Y.; Jiang, Z.Z. Pristimerin inhibits proliferation, migration and invasion, and induces apoptosis in HCT-116 colorectal cancer cells. Biomed. Pharmacother. 2016, 79, 112–119. [Google Scholar] [CrossRef]
- Yang, H.; Landis-Piwowar, K.R.; Lu, D.; Yuan, P.; Li, L.; Reddy, G.P.; Yuan, X.; Dou, Q.P. Pristimerin induces apoptosis by targeting the proteasome in prostate cancer cells. J. Cell Biochem. 2008, 103, 234–244. [Google Scholar] [CrossRef]
- Lu, Z.; Jin, Y.; Chen, C.; Li, J.; Cao, Q.; Pan, J. Pristimerin induces apoptosis in imatinib-resistant chronic myelogenous leukemia cells harboring T315I mutation by blocking NF-kappaB signaling and depleting Bcr-Abl. Mol. Cancer 2010, 9, 112. [Google Scholar] [CrossRef]
- Tiedemann, R.E.; Schmidt, J.; Keats, J.J.; Shi, C.X.; Zhu, Y.X.; Palmer, S.E.; Mao, X.; Schimmer, A.D.; Stewart, A.K. Identification of a potent natural triterpenoid inhibitor of proteosome chymotrypsin-like activity and NF-kappaB with antimyeloma activity in vitro and in vivo. Blood 2009, 113, 4027–4037. [Google Scholar] [CrossRef]
- Liby, K.T.; Sporn, M.B. Synthetic oleanane triterpenoids: Multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol. Rev. 2012, 64, 972–1003. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, R.H.; Wang, M.; Xu, G.B.; Liao, S.G. Prodrugs of triterpenoids and their derivatives. Eur. J. Med. Chem. 2017, 131, 222–236. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef]
- Bittorf, T.; Seiler, J.; Zhang, Z.; Jaster, R.; Brock, J. SHP1 protein tyrosine phosphatase negatively modulates erythroid differentiation and suppression of apoptosis in J2E erythroleukemic cells. Biol. Chem. 1999, 380, 1201–1209. [Google Scholar] [CrossRef]
- Wu, C.; Sun, M.; Liu, L.; Zhou, G.W. The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene 2003, 306, 1–12. [Google Scholar] [CrossRef]
- Pathak, A.K.; Bhutani, M.; Nair, A.S.; Ahn, K.S.; Chakraborty, A.; Kadara, H.; Guha, S.; Sethi, G.; Aggarwal, B.B. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol. Cancer Res. 2007, 5, 943–955. [Google Scholar] [CrossRef]
- García-Santisteban, I.; Peters, G.J.; Giovannetti, E.; Rodríguez, J.A. USP1 deubiquitinase: Cellular functions, regulatory mechanisms and emerging potential as target in cancer therapy. Mol. Cancer 2013, 12, 91. [Google Scholar] [CrossRef]
- Colland, F.; Formstecher, E.; Jacq, X.; Reverdy, C.; Planquette, C.; Conrath, S.; Trouplin, V.; Bianchi, J.; Aushev, V.N.; Camonis, J.; et al. Small-molecule inhibitor of USP7/HAUSP ubiquitin protease stabilizes and activates p53 in cells. Mol. Cancer Ther. 2009, 8, 2286–2295. [Google Scholar] [CrossRef] [PubMed]
- Morotti, A.; Panuzzo, C.; Crivellaro, S.; Pergolizzi, B.; Familiari, U.; Berger, A.H.; Saglio, G.; Pandolfi, P.P. BCR-ABL disrupts PTEN nuclear-cytoplasmic shuttling through phosphorylation-dependent activation of HAUSP. Leukemia 2014, 28, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Tian, Z.; Nicholson, B.; Kumar, K.G.; Zhou, B.; Carrasco, R.; McDermott, J.L.; Leach, C.A.; Fulcinniti, M.; Kodrasov, M.P.; et al. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell 2012, 22, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, J.; Wu, J.; Cao, P.; Kingsbury, W.D.; McDermott, J.L.; Kodrasov, M.P.; McKelvey, D.M.; Suresh Kumar, K.G.; Goldenberg, S.J.; Mattern, M.R.; et al. Selective Dual Inhibitors of the Cancer-Related Deubiquitylating Proteases USP7 and USP47. ACS Med. Chem. Lett. 2012, 3, 789–792. [Google Scholar] [CrossRef]
- Jing, B.; Liu, M.; Yang, L.; Cai, H.Y.; Chen, J.B.; Li, Z.X.; Kou, X.; Wu, Y.Z.; Qin, D.J.; Zhou, L.; et al. Characterization of naturally occurring pentacyclic triterpenes as novel inhibitors of deubiquitinating protease USP7 with anticancer activity in vitro. Acta Pharmacol. Sin. 2018, 39, 492–498. [Google Scholar] [CrossRef]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Richardson, P.G.; Zimmerman, T.M.; Hofmeister, C.C.; Talpaz, M.; Chanan-Khan, A.A.; Kaufman, J.L.; Laubach, J.P.; Chauhan, D.; Jakubowiak, A.J.; Reich, S.; et al. Phase 1 study of marizomib in relapsed or relapsed and refractory multiple myeloma: NPI-0052-101 Part 1. Blood 2016, 127, 2693–2700. [Google Scholar] [CrossRef]
- Levin, N.; Spencer, A.; Harrison, S.J.; Chauhan, D.; Burrows, F.J.; Anderson, K.C.; Reich, S.D.; Richardson, P.G.; Trikha, M. Marizomib irreversibly inhibits proteasome to overcome compensatory hyperactivation in multiple myeloma and solid tumour patients. Br. J. Haematol. 2016, 174, 711–720. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosio, F.A.; Costa, G.; Gallo Cantafio, M.E.; Torcasio, R.; Trapasso, F.; Alcaro, S.; Viglietto, G.; Amodio, N. Natural Agents as Novel Potential Source of Proteasome Inhibitors with Anti-Tumor Activity: Focus on Multiple Myeloma. Molecules 2023, 28, 1438. https://doi.org/10.3390/molecules28031438
Ambrosio FA, Costa G, Gallo Cantafio ME, Torcasio R, Trapasso F, Alcaro S, Viglietto G, Amodio N. Natural Agents as Novel Potential Source of Proteasome Inhibitors with Anti-Tumor Activity: Focus on Multiple Myeloma. Molecules. 2023; 28(3):1438. https://doi.org/10.3390/molecules28031438
Chicago/Turabian StyleAmbrosio, Francesca Alessandra, Giosuè Costa, Maria Eugenia Gallo Cantafio, Roberta Torcasio, Francesco Trapasso, Stefano Alcaro, Giuseppe Viglietto, and Nicola Amodio. 2023. "Natural Agents as Novel Potential Source of Proteasome Inhibitors with Anti-Tumor Activity: Focus on Multiple Myeloma" Molecules 28, no. 3: 1438. https://doi.org/10.3390/molecules28031438
APA StyleAmbrosio, F. A., Costa, G., Gallo Cantafio, M. E., Torcasio, R., Trapasso, F., Alcaro, S., Viglietto, G., & Amodio, N. (2023). Natural Agents as Novel Potential Source of Proteasome Inhibitors with Anti-Tumor Activity: Focus on Multiple Myeloma. Molecules, 28(3), 1438. https://doi.org/10.3390/molecules28031438







