Creation of a Composite Bioactive Coating with Antibacterial Effect Promising for Bone Implantation
Abstract
1. Introduction
2. Results and Discussions
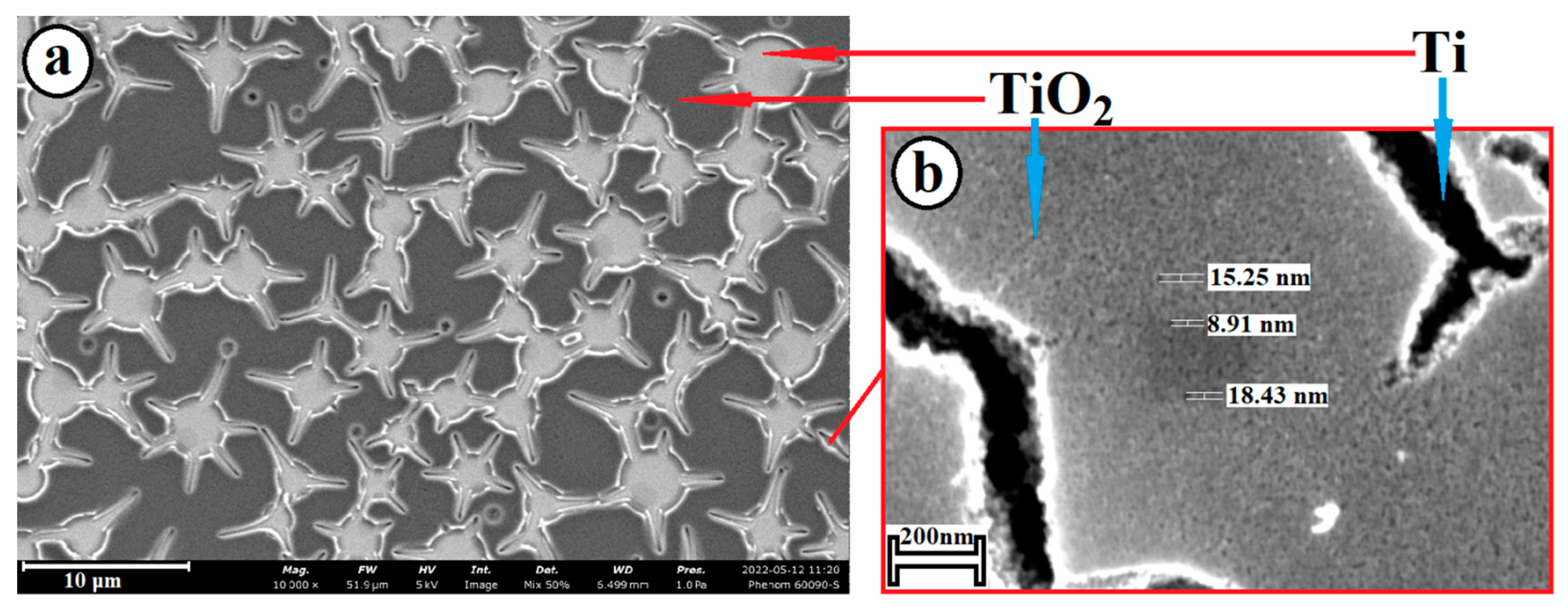
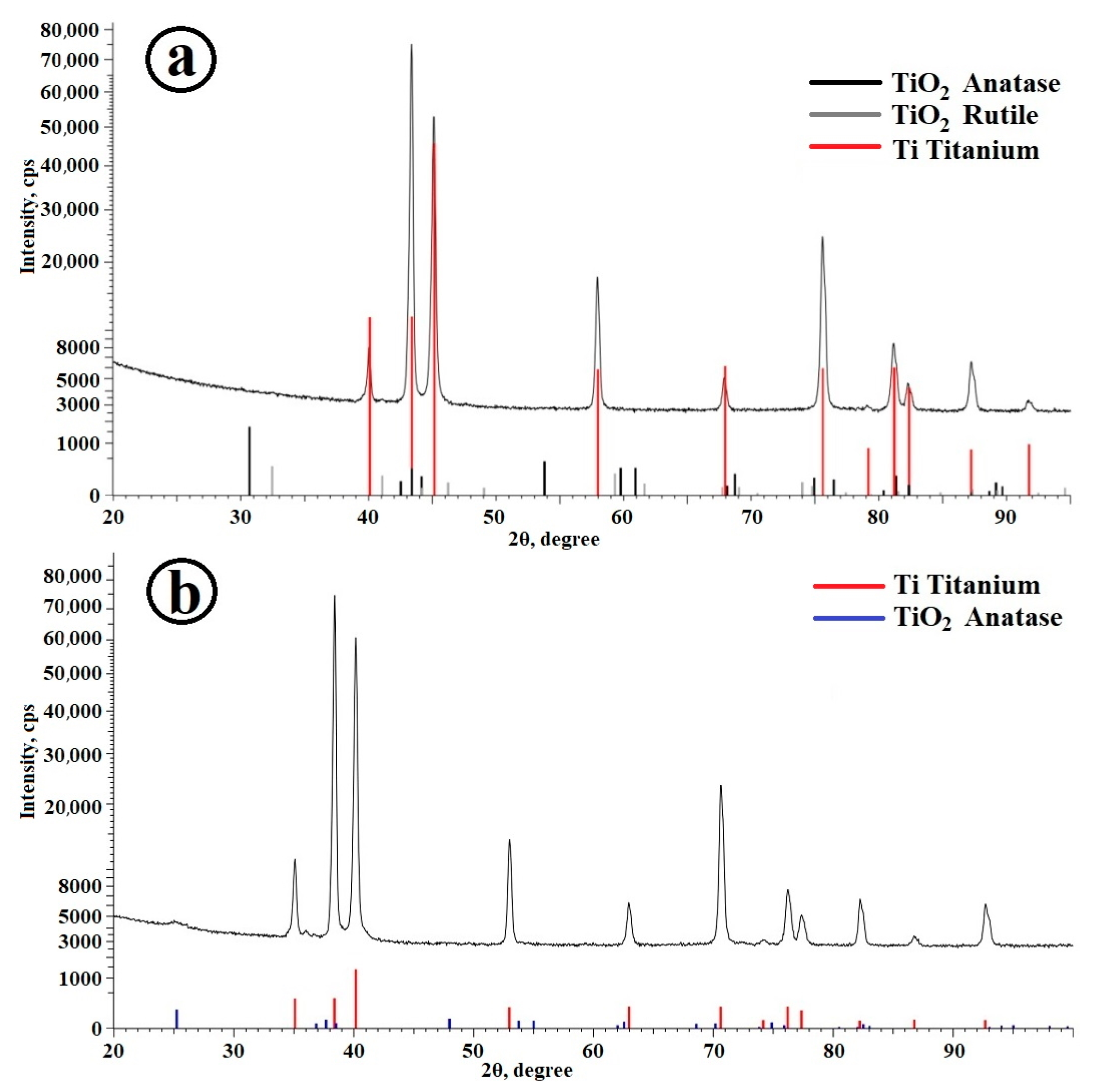
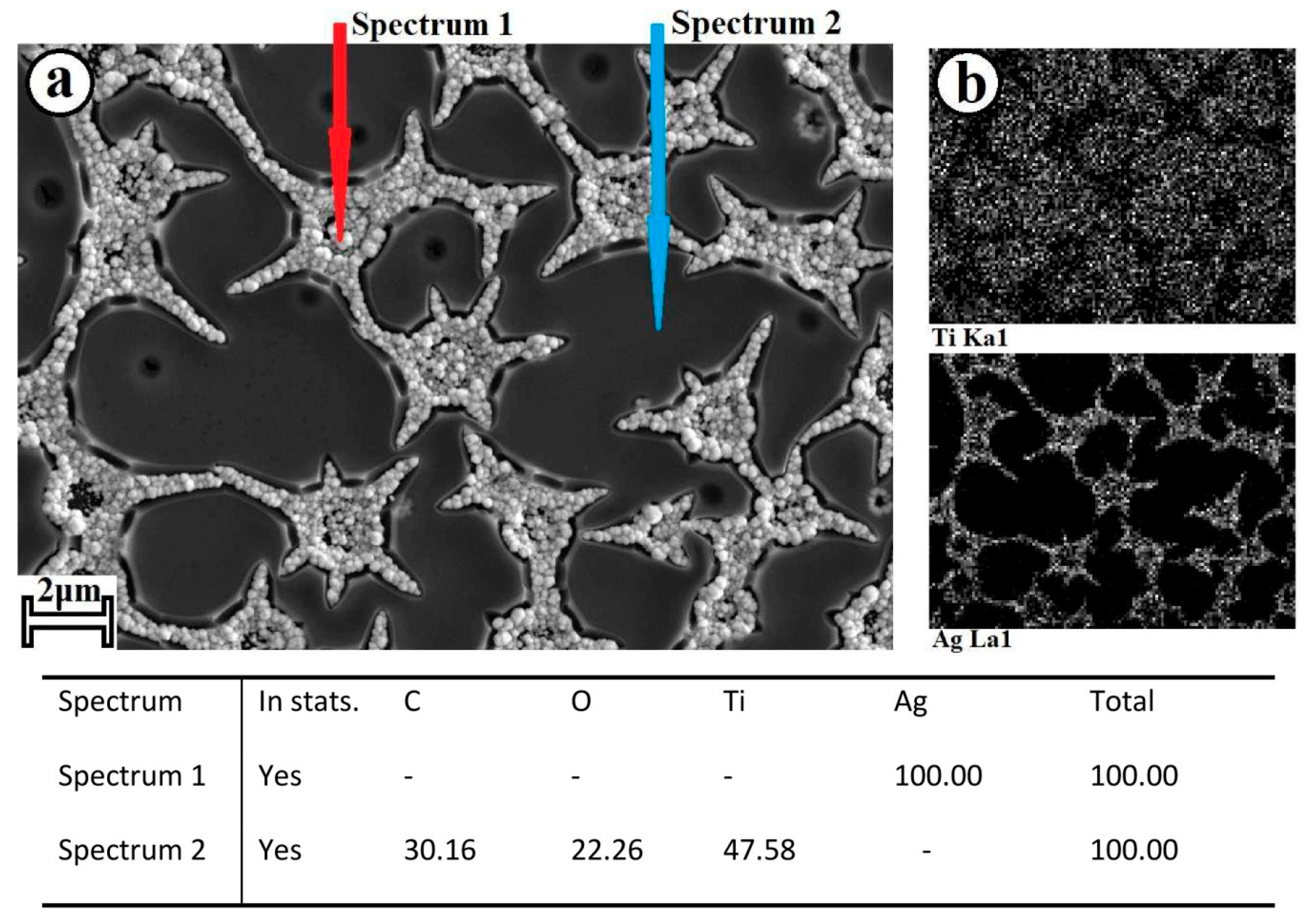
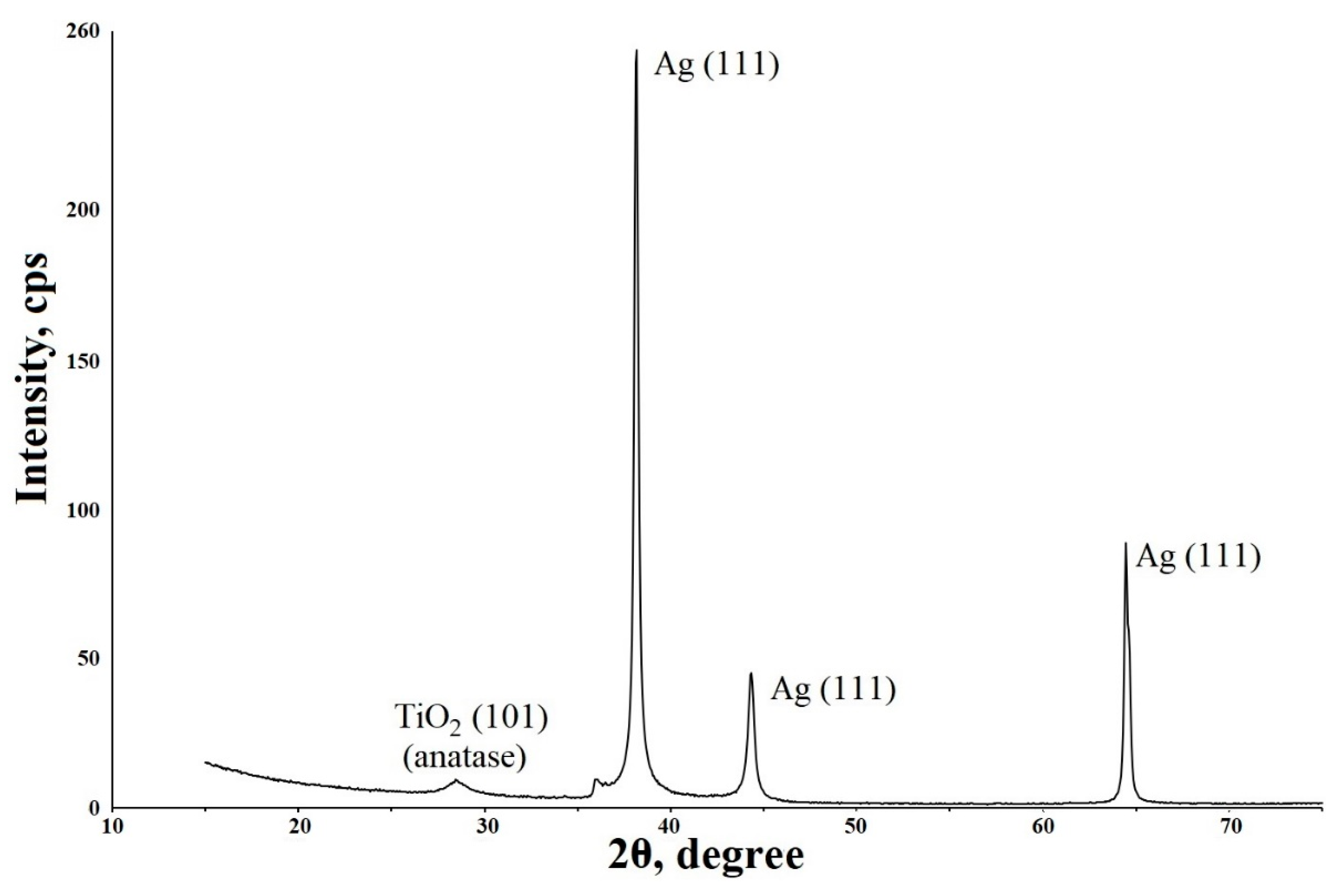
2.1. Preparation of Composite Coating TiO2/Ag/HAp on Titanium
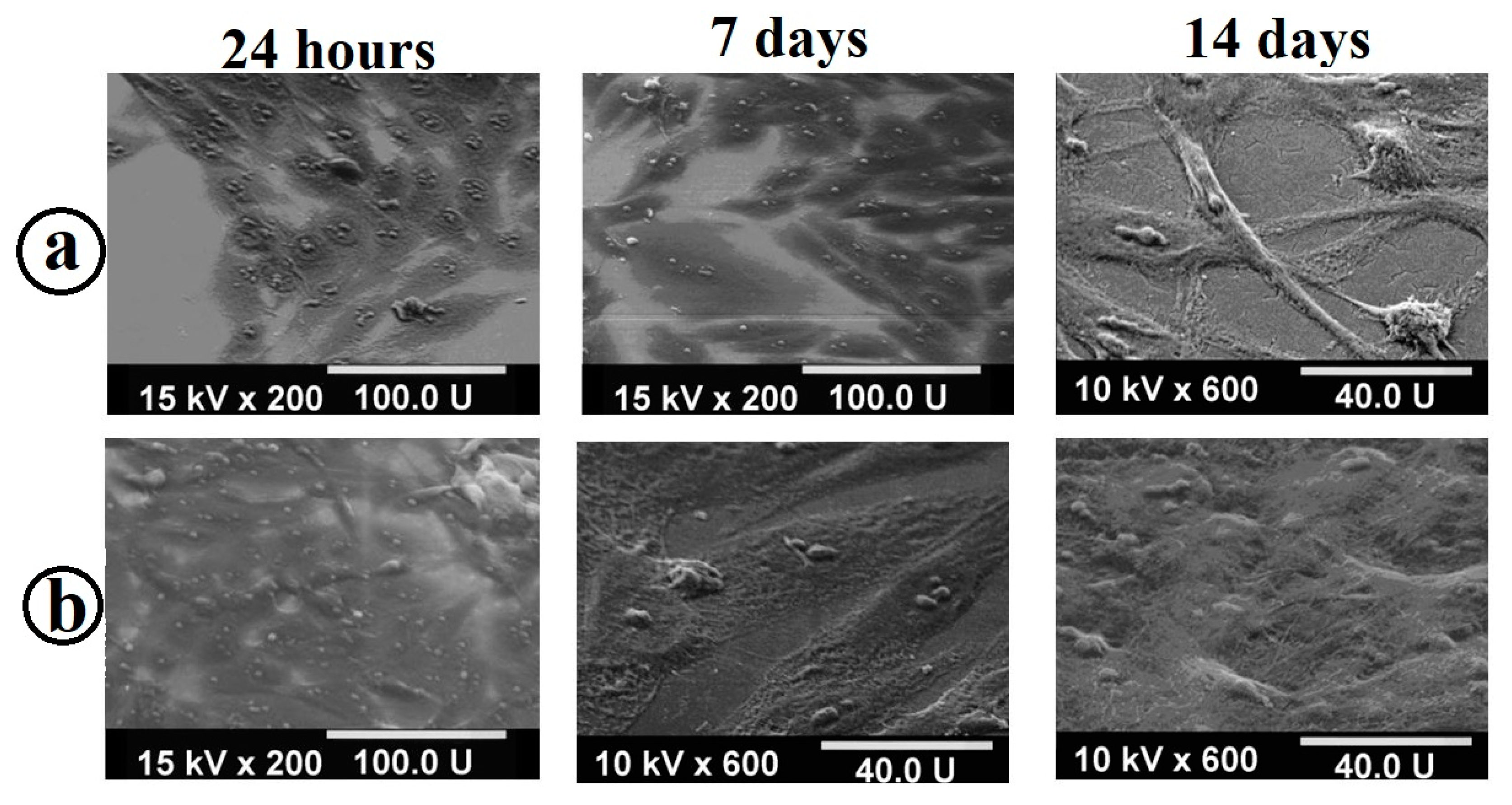
2.2. Cytological Studies of Samples
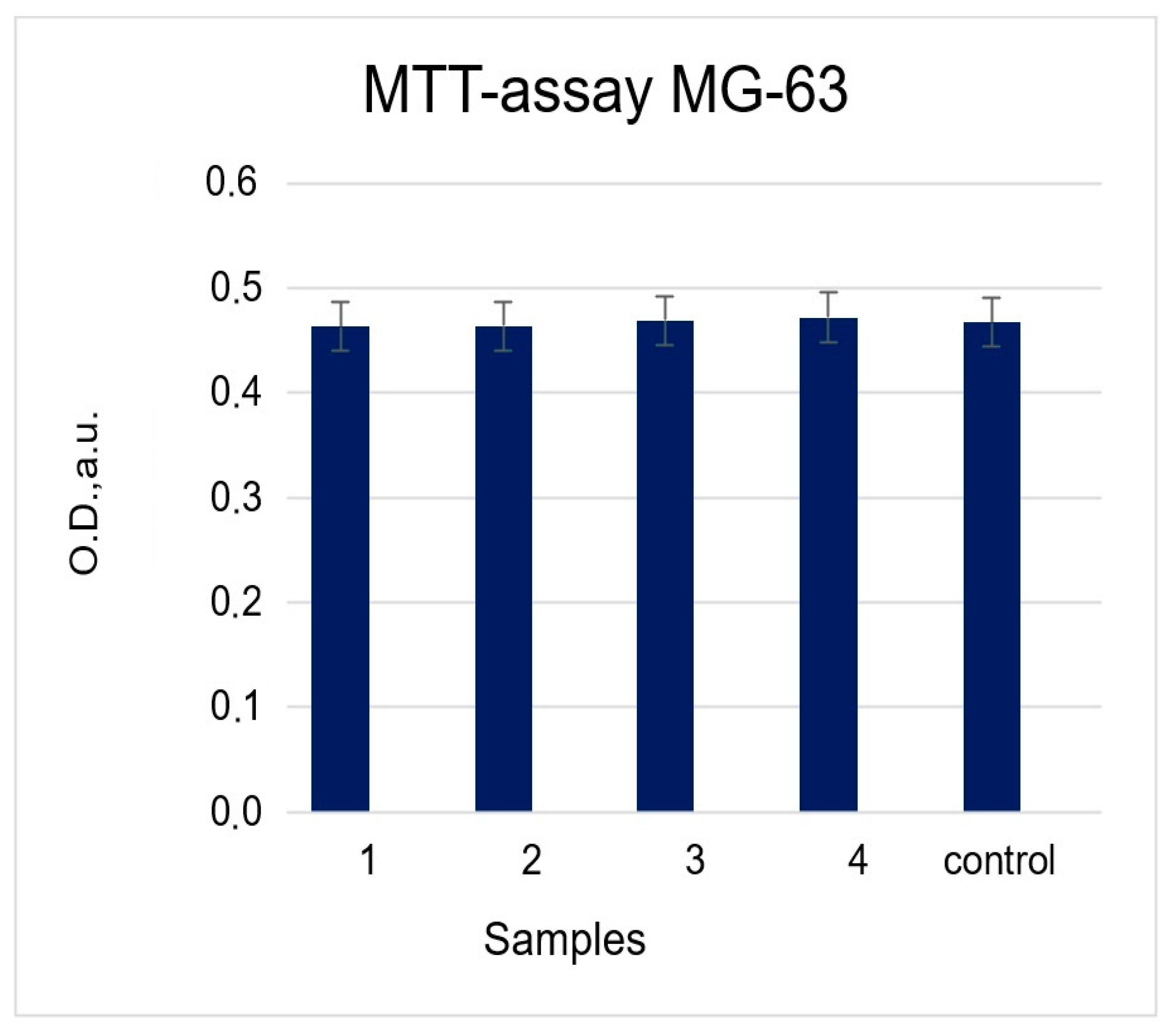
2.2.1. MTT Test (Cytotoxicity Assessment)
2.2.2. Estimation of Alkaline Phosphatase (ALP) Content
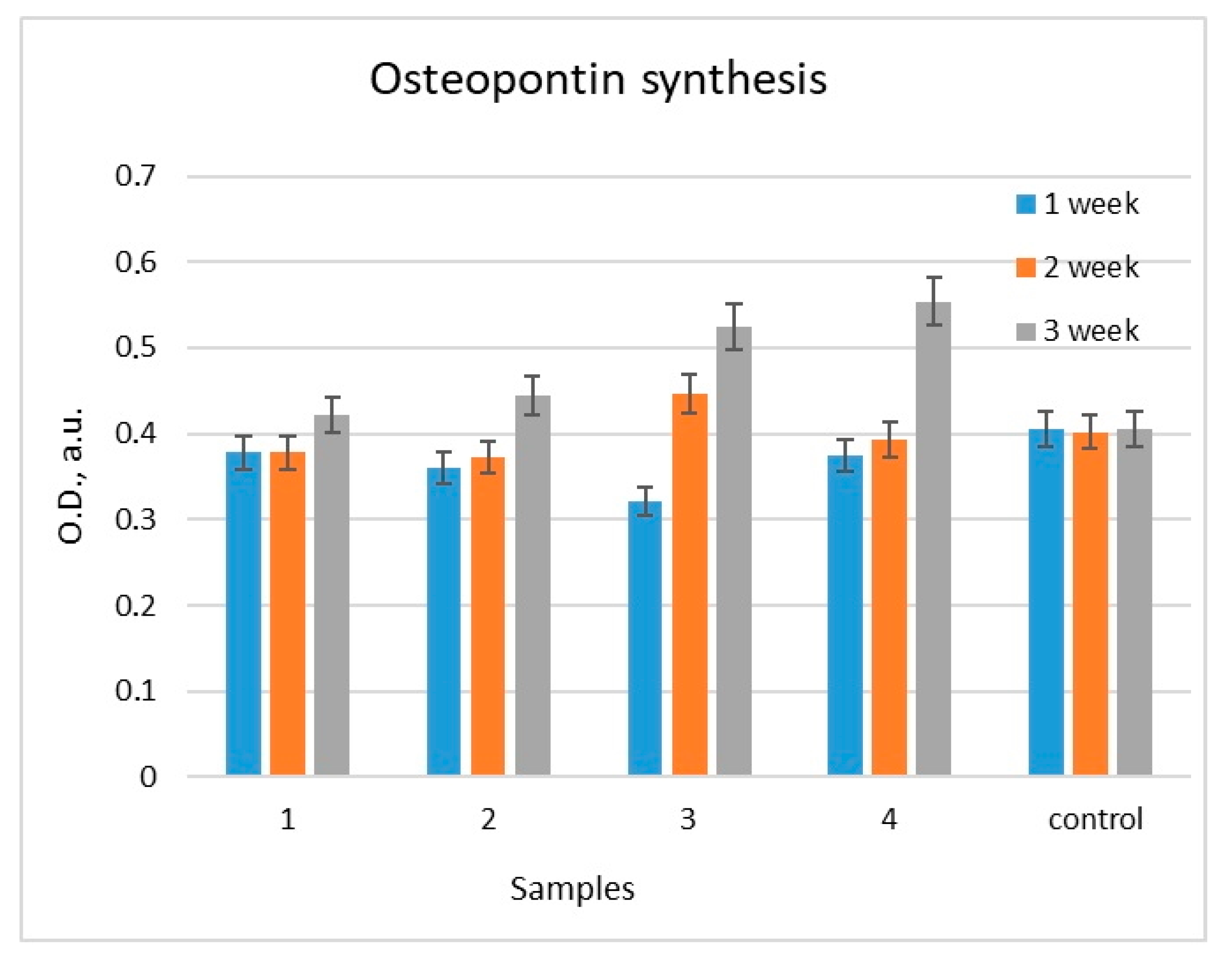
2.2.3. Estimation of Osteopontin (OPN) Content
2.3. The Antibacterial Activity of Samples with a Composite Coating TiO2/Ag/HAp
3. Materials and Methods
3.1. Preparation of TiO2/Ag/HAp Coating on Ti Surface
3.2. Evaluation of the Formation of a Cellular Monolayer of MC3T3-E1 Osteoblasts on Titanium Samples with Composite Coating TiO2/Ag/HAp
3.3. MTT Test (Cytotoxicity Assessment)
3.4. Evaluation of Cell Differentiation in the Osteogenic Direction
3.5. Studies of the Antibacterial Activity of the Surface of the TiO2/Ag/HAp Composite Coating
3.6. Characterization of the Composite Coating TiO2/Ag/HAp
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Murr, L.E. Strategies for creating living, additively manufactured, open-cellular metal and alloy implants by promoting osseointegration, osteoinduction and vascularization: An overview. J. Mater. Sci. Technol. 2019, 35, 231–241. [Google Scholar] [CrossRef]
- Oliver, J.N.; Su, Y.; Lu, X.; Kuo, P.H.; Du, J.; Zhu, D. Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar] [CrossRef]
- Gulati, K.; Maher, S.; Findlay, D.M.; Losic, D. Titania nanotubes for orchestrating osteogenesis at the bone-implant interface. Nanomedicine 2016, 11, 1847–1864. [Google Scholar] [CrossRef]
- Mendonca, G.; Mendonca, D.B.; Aragao, F.J.; Cooper, L.F. Advancing dental implant surface technology--from micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Zemtsova, E.G.; Yudintceva, N.M.; Morozov, P.E.; Valiev, R.Z.; Smirnov, V.M.; Shevtsov, M.A. Improved osseointegration properties of hierarchical microtopographic/nanotopographic coatings fabricated on titanium implants. Int. J. Nanomed. 2018, 13, 2175–2188. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Jimbo, R.; Tovar, N.; Bonfante, E.A. Osseointegration: Hierarchical designing encompassing the macrometer, micrometer, and nanometer length scales. Dent. Mater. 2015, 31, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral. Maxillofac. Implant. 2010, 25, 63–74. [Google Scholar]
- Arbenin, A.Y.; Zemtsova, E.G.; Orekhov, E.V.; Smirnov, V.M. Features of the sol-gel synthesis of TiO2 nanolayers with calcium phosphate structures on titanium surface. Russ. J. Gen. Chem. 2017, 87, 340–341. [Google Scholar] [CrossRef]
- Nazarov, D.V.; Smirnov, V.M.; Zemtsova, E.G.; Yudintceva, N.M.; Shevtsov, M.A.; Valiev, R.Z. Enhanced Osseointegrative Properties of Ultra-Fine-Grained Titanium Implants Modified by Chemical Etching and Atomic Layer Deposition. ACS Biomater. Sci. Eng. 2018, 4, 3268–3281. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef]
- Meirelles, L.; Uzumaki, E.T.; Lima, J.H.; Muller, C.A.; Albrektsson, T.; Wennerberg, A.; Lambert, C.S. A novel technique for tailored surface modification of dental implants—A step wise approach based on plasma immersion ion implantation. Clin. Oral. Implant. Res. 2013, 24, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dubey, A.K. Various Biomaterials and Techniques for Improving Antibacterial Response. ACS Appl. Bio Mater. 2018, 1, 3–20. [Google Scholar] [CrossRef]
- Saidin, S.; Jumat, M.A.; Mohd Amin, N.A.A.; Saleh Al-Hammadi, A.S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 118, 111382. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Tian, B.; Chen, W.; Yu, D.; Lei, Y.; Ke, Q.; Guo, Y.; Zhu, Z. Fabrication of silver nanoparticle-doped hydroxyapatite coatings with oriented block arrays for enhancing bactericidal effect and osteoinductivity. J. Mech. Behav. Biomed. Mater. 2016, 61, 345–359. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhu, Y.; Lu, D.Z.; Dong, W.; Bi, W.J.; Feng, X.J.; Wen, L.M.; Sun, H.; Qi, M.C. Evaluation of osteogenic and antibacterial properties of strontium/silver-containing porous TiO2 coatings prepared by micro-arc oxidation. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 505–516. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, B.; Wang, Y.; Zhou, X.; Weng, J.; Qu, S.; Feng, B.; Watari, F.; Ding, Y.; Leng, Y. Nano-Ag-loaded hydroxyapatite coatings on titanium surfaces by electrochemical deposition. J. R. Soc. Interface 2011, 8, 529–539. [Google Scholar] [CrossRef]
- Morris, G.V.; Kozdryk, J.; Gregory, J.; Jeys, L. The use of silver-coated orthopaedic implants: Are all silvers the same? Curr. Orthop. Pract. 2017, 28, 532–536. [Google Scholar] [CrossRef]
- Liang, R.; Xu, Y.; Zhao, M.; Han, G.; Li, J.; Wu, W.; Dong, M.; Yang, J.; Liu, Y. Properties of silver contained coatings on CoCr alloys prepared by vacuum plasma spraying. Mater. Sci. Eng. C—Mater. Biol. Appl. 2020, 106, 110156. [Google Scholar] [CrossRef]
- He, Y.; Kang, Z.; Chen, D. Integrating nanodiamonds to improve anticorrosion property and conductivity simultaneously of Ag composite films via layer by layer spin-spray deposition. Appl. Surf. Sci. 2020, 505, 144643. [Google Scholar] [CrossRef]
- Varghese, S.; Elfakhri, S.; Sheel, D.W.; Sheel, P.; Bolton, F.J.; Foster, H.A. Novel antibacterial silver-silica surface coatings prepared by chemical vapour deposition for infection control. J. Appl. Microbiol. 2013, 115, 1107–1116. [Google Scholar] [CrossRef]
- Fu, C. Synthesis and Characterization of Hydroxyapatite Coatings for Medical Applications. Ph.D. Thesis, University of Rochester, Rochester, NY, USA, 2016. [Google Scholar]
- Chatakun, P.; Nunez-Toldra, R.; Diaz Lopez, E.J.; Gil-Recio, C.; Martinez-Sarra, E.; Hernandez-Alfaro, F.; Ferres-Padro, E.; Giner-Tarrida, L.; Atari, M. The effect of five proteins on stem cells used for osteoblast differentiation and proliferation: A current review of the literature. Cell Mol. Life Sci. 2014, 71, 113–142. [Google Scholar] [CrossRef]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009, 5, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, X.; Huang, Y.; Ding, Q.; Pang, X. Antibacterial and bioactivity of silver substituted hydroxyapatite/TiO 2 nanotube composite coatings on titanium. Appl. Surf. Sci. 2014, 314, 348–357. [Google Scholar] [CrossRef]
- Pratap Reddy, M.; Venugopal, A.; Subrahmanyam, M. Hydroxyapatite-supported Ag-TiO2 as Escherichia coli disinfection photocatalyst. Water Res. 2007, 41, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Chen, W.; Dong, Y.; Marymont, J.V.; Lei, Y.; Ke, Q.; Guo, Y.; Zhu, Z. Silver nanoparticle-loaded hydroxyapatite coating: Structure, antibacterial properties, and capacity for osteogenic induction in vitro. RSC Adv. 2016, 6, 8549–8562. [Google Scholar] [CrossRef]
- Arbenin, A.Y.; Zemtsova, E.G.; Ermakov, S.S.; Gaskov, A.M.; Baburova, P.I.; Sokolova, D.N.; Yaroshenko, S.V.; Smirnov, V.M. Three-component working electrode micron-sized Ag particles/TiO2 layer/Ti: Template electrochemical synthesis and potential use as electrochemical sensor for glutathione detection. Mater. Res. Express 2020, 7, 035401. [Google Scholar] [CrossRef]
- Orekhov, E.V.; Arbenin, A.Y.; Zemtsova, E.G.; Sokolova, D.N.; Ponomareva, A.N.; Shevtsov, M.A.; Yudintceva, N.M.; Smirnov, V.M. Template Electrochemical Synthesis of Hydroxyapatite on a Titania–Silver Composite Surface for Potential Use in Implantology. Coatings 2022, 12, 266. [Google Scholar] [CrossRef]
- Soo, J.Z.; Chai, L.C.; Ang, B.C.; Ong, B.H. Enhancing the Antibacterial Performance of Titanium Dioxide Nanofibers by Coating with Silver Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 5743–5751. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T.; Jimbo, R. Implant Surfaces and Their Biological and Clinical Impact, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2015; p. 182. [Google Scholar]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Jones, F. Teeth and bones: Applications of surface science to dental materials and related biomaterials. Surf. Sci. Rep. 2001, 42, 75–205. [Google Scholar] [CrossRef]
- Li, B.E.; Li, Y.; Min, Y.; Hao, J.Z.; Liang, C.Y.; Li, H.P.; Wang, G.C.; Liu, S.M.; Wang, H.S. Synergistic effects of hierarchical hybrid micro/nanostructures on the biological properties of titanium orthopaedic implants. RSC Adv. 2015, 5, 49552–49558. [Google Scholar] [CrossRef]
- Jiang, N.; Zhu, S.; Li, J.; Zhang, L.; Liao, Y.; Hu, J. Development of a novel biomimetic micro/nano-hierarchical interface for enhancement of osseointegration. RSC Adv. 2016, 6, 49954–49965. [Google Scholar] [CrossRef]
- Seddiki, O.; Harnagea, C.; Levesque, L.; Mantovani, D.; Rosei, F. Evidence of antibacterial activity on titanium surfaces through nanotextures. Appl. Surf. Sci. 2014, 308, 275–284. [Google Scholar] [CrossRef]
- Zemtsova, E.G.; Orehhov, J.V.; Arbenin, A.Y.; Valiev, R.Z.; Smirnov, V.M. The creation of nanocoatings of various morphology on the basis of titanium dioxide on a titanium matrix for bone implant. Mater. Phys. Mech. 2016, 29, 138–144. [Google Scholar]
- Variola, F.; Zalzal, S.F.; Leduc, A.; Barbeau, J.; Nanci, A. Oxidative nanopatterning of titanium generates mesoporous surfaces with antimicrobial properties. Int. J. Nanomed. 2014, 9, 2319–2325. [Google Scholar] [CrossRef]
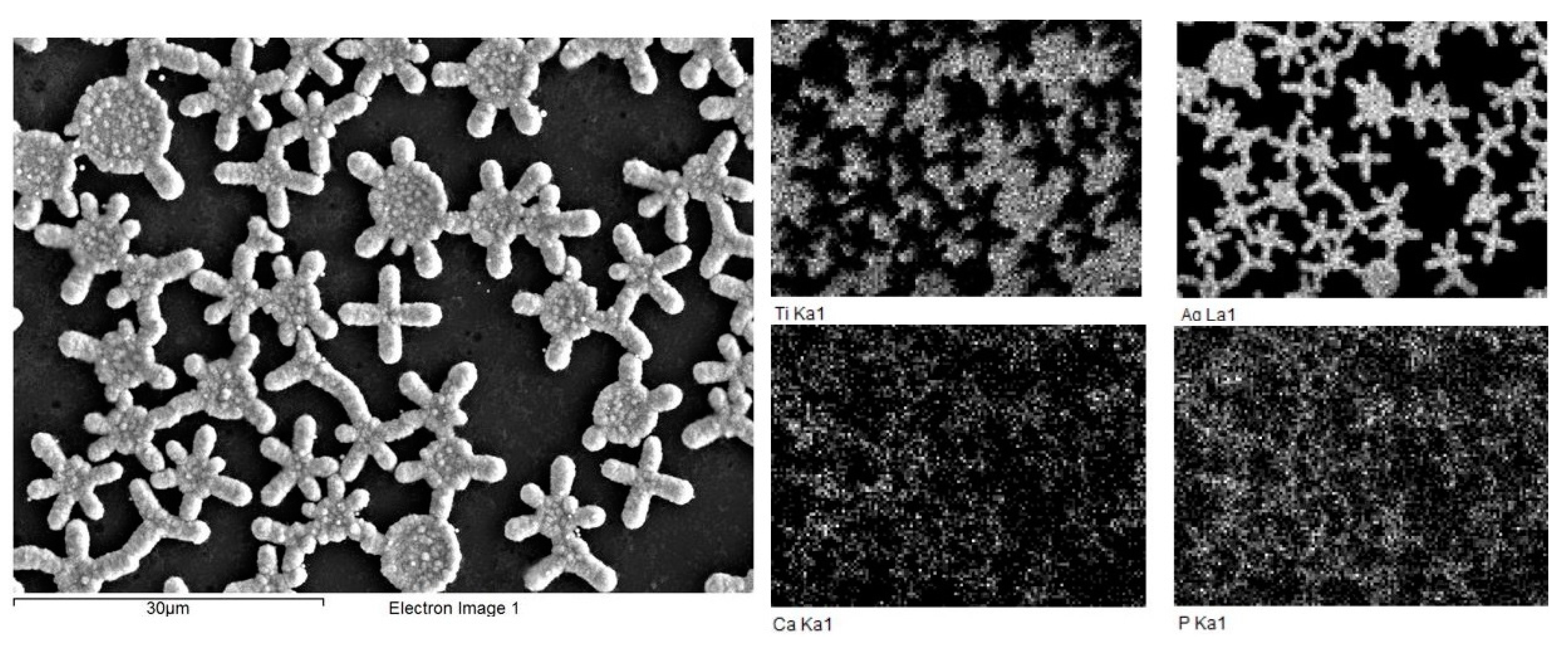
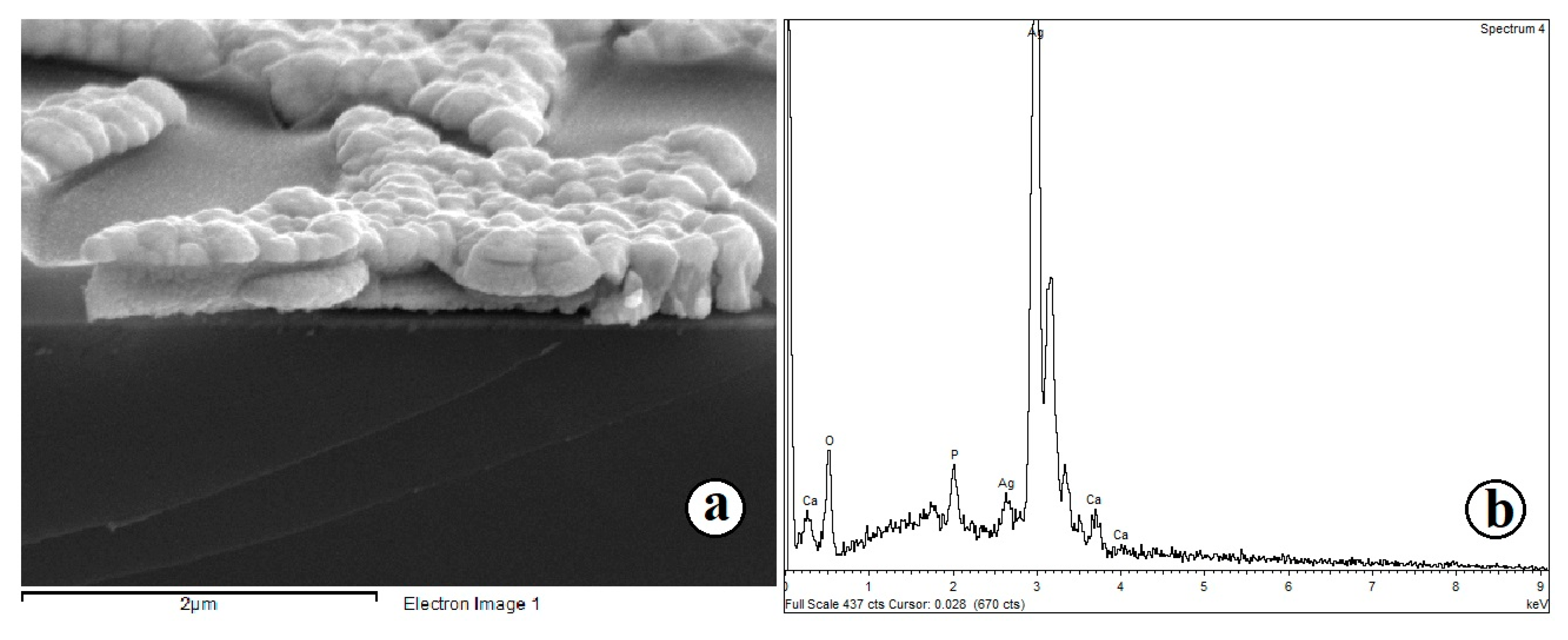
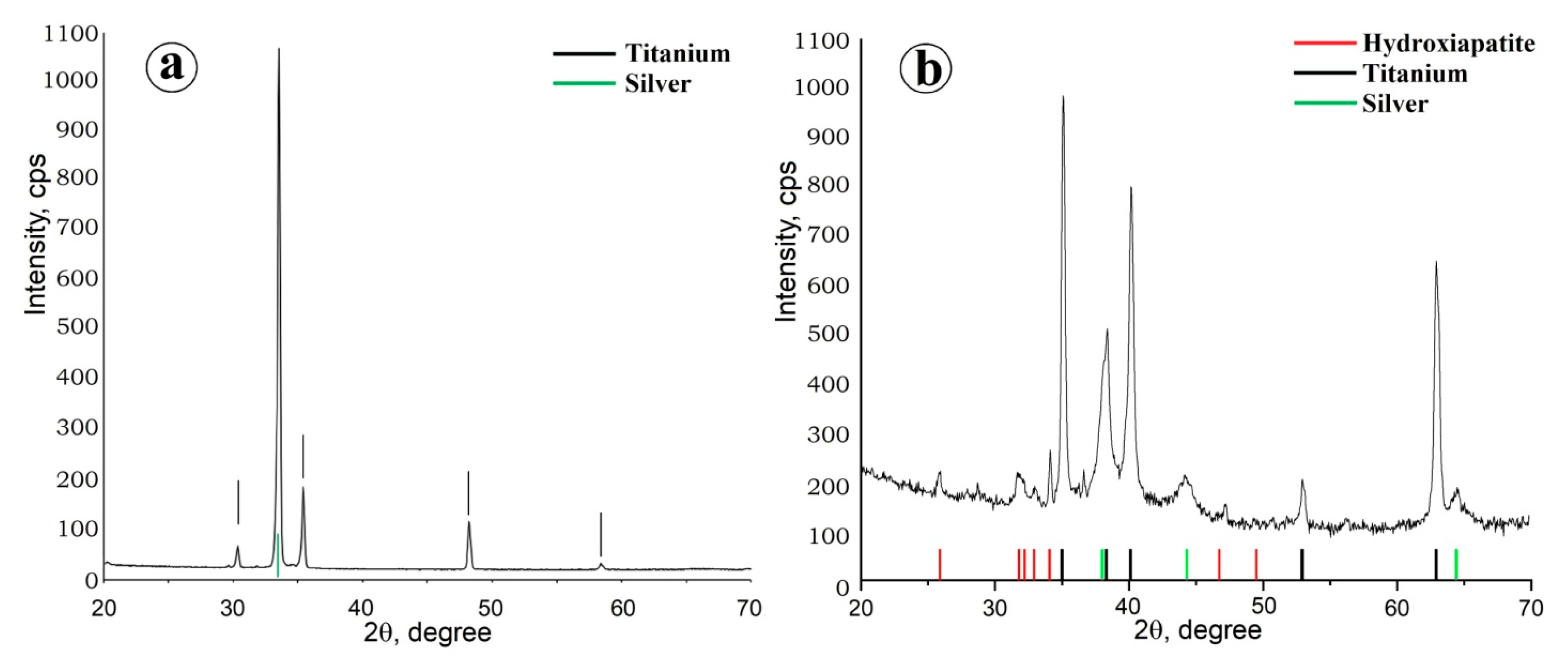


| Sample 1 | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|
| Polished VT 6 titanium | TiO2 on VT 6 titanium | TiO2/Ag on VT 6 titanium | TiO2/Ag/HAp on VT 6 titanium |
| Sample Composition | ||||
|---|---|---|---|---|
| Cultures of Strains | Control Muller—Hinton Agar | Control TiO2 | TiO2/Ag Coating | TiO2/Ag/HAp Composite Coating |
| 1. Enterococcus faecalis 812 (107 CFU/mL) | Microorganisms growth | Microorganisms growth | 100% no growth | 100% no growth |
| 2. Staphylococcus aureus ATCC 25,912 (107 CFU/mL) | Microorganisms growth | Microorganisms growth | 100% no growth | 100% no growth |
| 3. Klebsiella pneumoniae ATCC 19,882 (107 CFU/mL) | Microorganisms growth | Microorganisms growth | 100% no growth | 100% no growth |
| 4. Acinetobacter baumannii 987 (106 CFU/mL) | Microorganisms growth | Microorganisms growth | 100% no growth | 100% no growth |
| 5. Acinetobacter baumannii 987 (107 CFU/mL) | Microorganisms growth | Microorganisms growth | 100% no growth | 100% no growth |
| 6. Pseudomonas aeruginosa ATCC 27,853 (106 CFU/mL) | Microorganisms growth | Microorganisms growth | 100% no growth | 100% no growth |
| 7. Pseudomonas aeruginosa ATCC 27,853 (107 CFU/mL) | Microorganisms growth | Microorganisms growth | 100% no growth | 100% no growth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemtsova, E.G.; Kozlova, L.A.; Yudintceva, N.M.; Sokolova, D.N.; Arbenin, A.Y.; Ponomareva, A.N.; Korusenko, P.M.; Kraeva, L.A.; Rogacheva, E.V.; Smirnov, V.M. Creation of a Composite Bioactive Coating with Antibacterial Effect Promising for Bone Implantation. Molecules 2023, 28, 1416. https://doi.org/10.3390/molecules28031416
Zemtsova EG, Kozlova LA, Yudintceva NM, Sokolova DN, Arbenin AY, Ponomareva AN, Korusenko PM, Kraeva LA, Rogacheva EV, Smirnov VM. Creation of a Composite Bioactive Coating with Antibacterial Effect Promising for Bone Implantation. Molecules. 2023; 28(3):1416. https://doi.org/10.3390/molecules28031416
Chicago/Turabian StyleZemtsova, Elena G., Lada A. Kozlova, Natalia M. Yudintceva, Daria N. Sokolova, Andrey Yu. Arbenin, Alexandra N. Ponomareva, Petr M. Korusenko, Ludmila A. Kraeva, Elizaveta V. Rogacheva, and Vladimir M. Smirnov. 2023. "Creation of a Composite Bioactive Coating with Antibacterial Effect Promising for Bone Implantation" Molecules 28, no. 3: 1416. https://doi.org/10.3390/molecules28031416
APA StyleZemtsova, E. G., Kozlova, L. A., Yudintceva, N. M., Sokolova, D. N., Arbenin, A. Y., Ponomareva, A. N., Korusenko, P. M., Kraeva, L. A., Rogacheva, E. V., & Smirnov, V. M. (2023). Creation of a Composite Bioactive Coating with Antibacterial Effect Promising for Bone Implantation. Molecules, 28(3), 1416. https://doi.org/10.3390/molecules28031416






