Production and Anti-Inflammatory Performance of PVA Hydrogels Loaded with Curcumin Encapsulated in Octenyl Succinic Anhydride Modified Schizophyllan as Wound Dressings
Abstract
1. Introduction
2. Results and Discussion
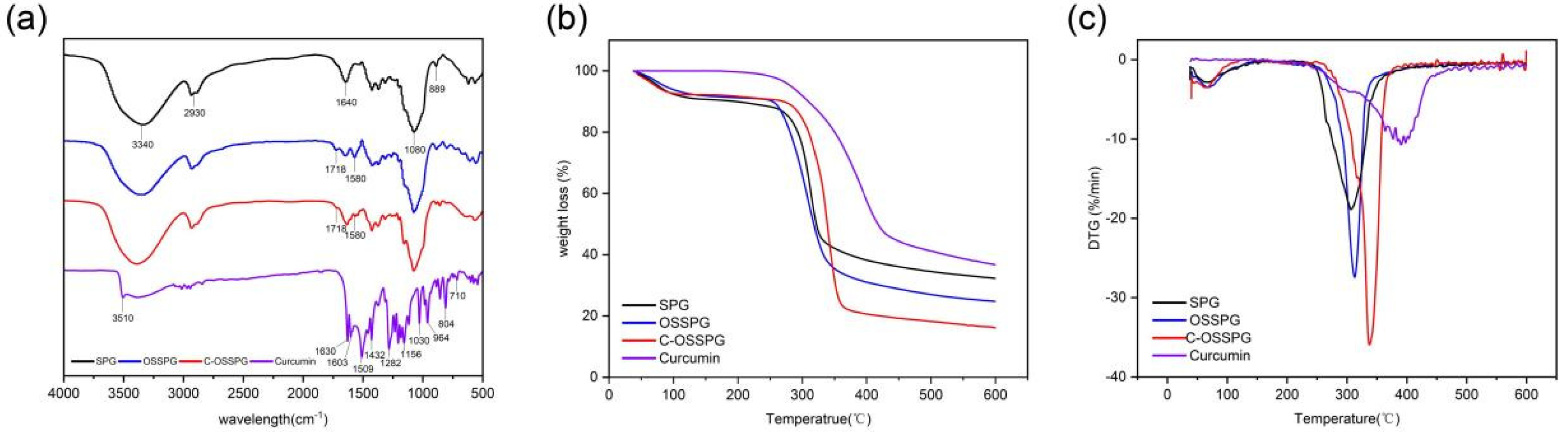
2.1. FT-IR Analysis
2.2. TG Analysis
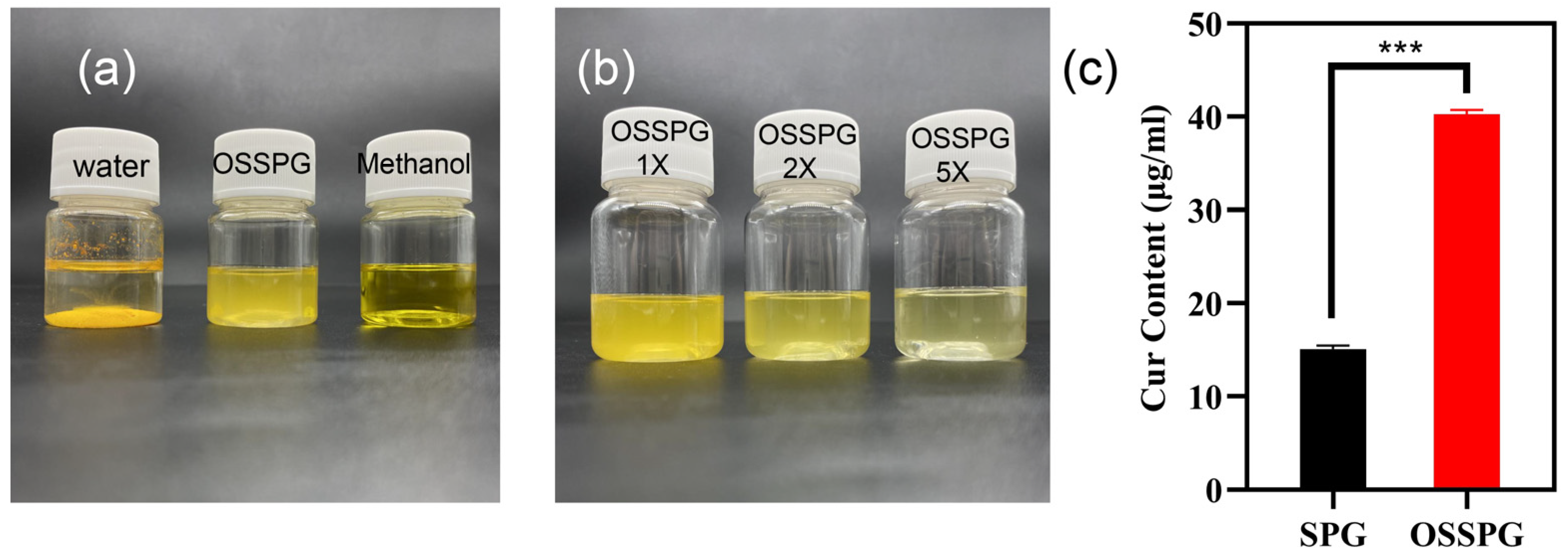
2.3. Loading Capacity (LC) of Curcumin
2.4. Properties of Hydrogel
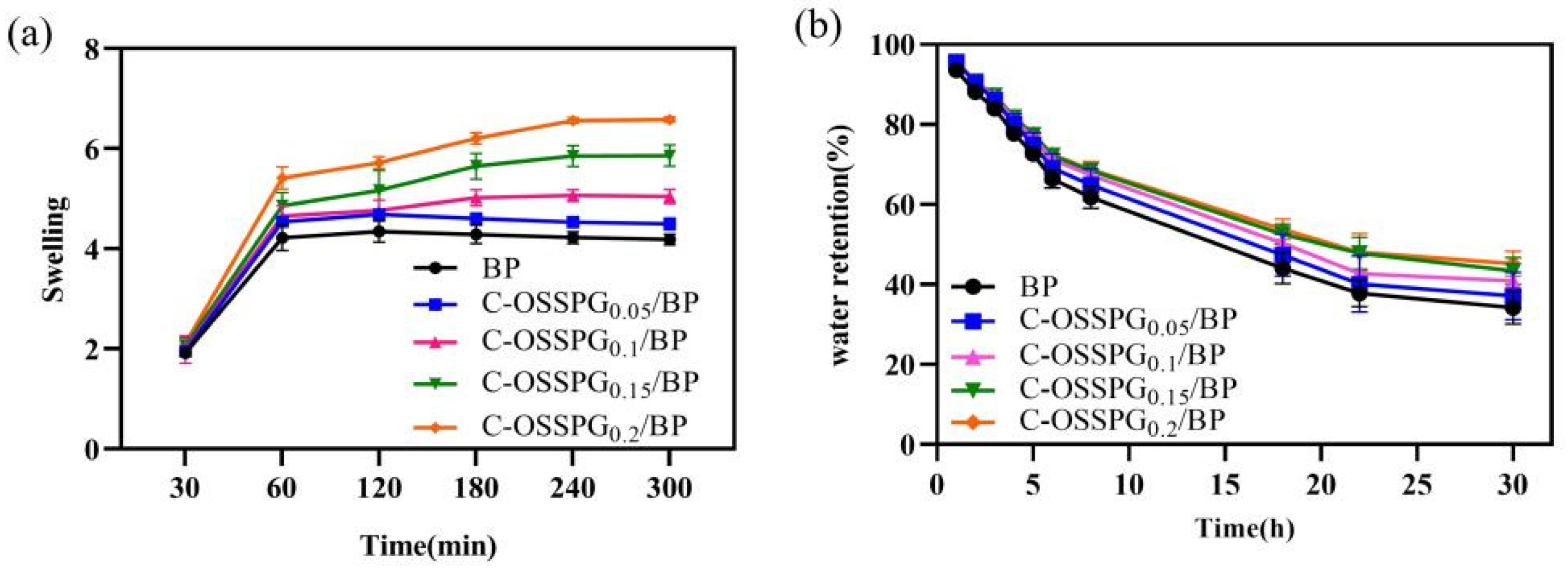
2.5. Swelling Abilities
2.6. Water Retention
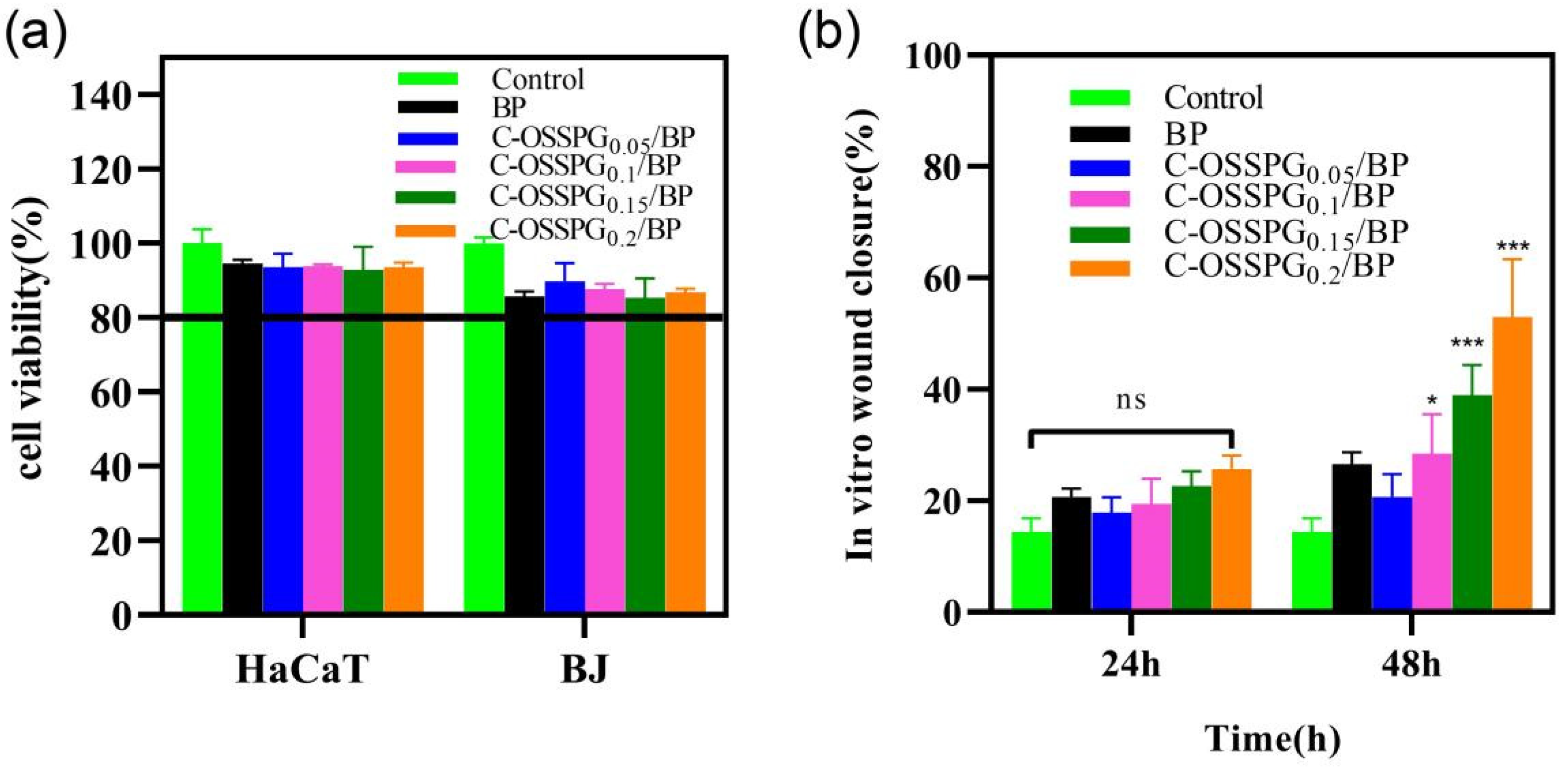
2.7. Cytotoxicity of Hydrogels
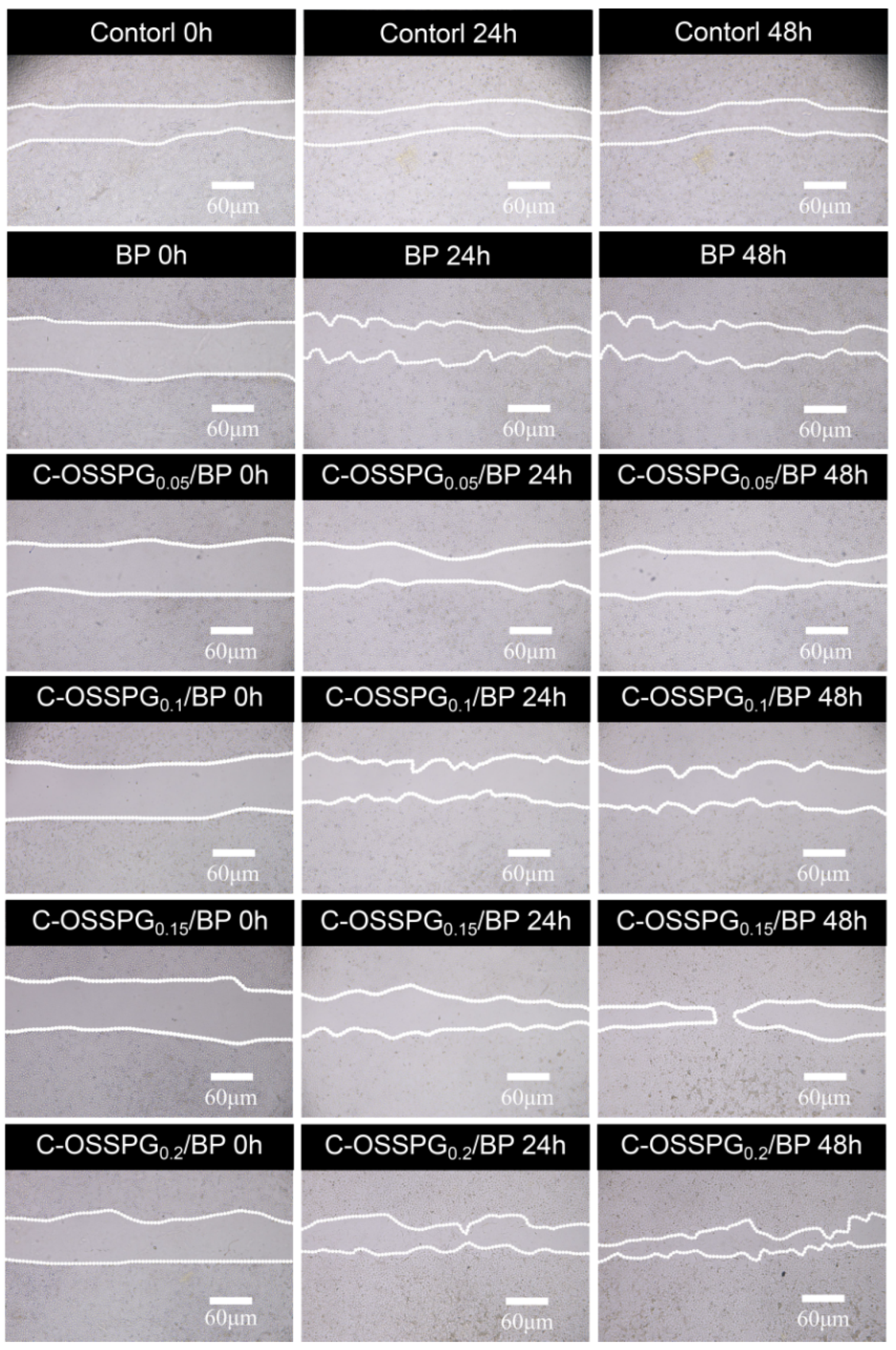
2.8. In Vitro Wound-Healing Assay
2.9. Anti-Inflammatory
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Octenyl Succinic Schizophyllan Synthesis
4.3. Substitution Degree of OSSPG
4.4. Preparation of the OSSPG/Cur Inclusion Complex
4.5. Loading Capacity
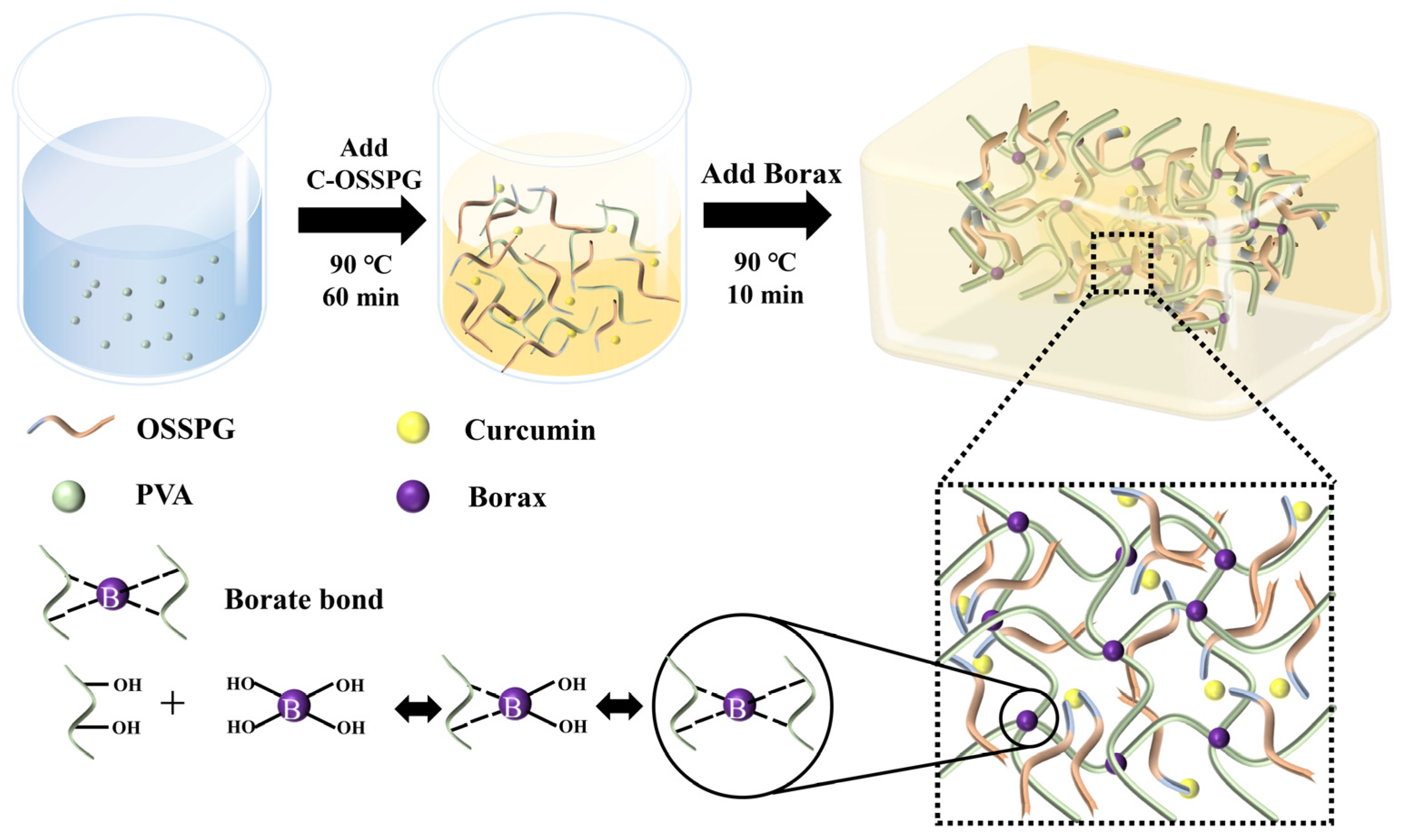
4.6. Synthesis of Hydrogels
4.7. Characterization Analysis
4.8. Macroscopic Self-Healing Test of C-OSSPG0.2/BP Hydrogel
4.9. Swelling
4.10. Water Evaporation Rate
4.11. Cell Cytotoxicity Assay
4.12. In Vitro Wound-Healing Assay
4.13. Anti-Inflammatory
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SPG | Schizophyllan |
| OSA | Ocenyl succinate anhydride |
| OSSPG | Octenylsuccinic schizophyllan |
| C-OSSPG | Curcumin loaded octenylsuccinic schizophyllan |
| DS | Substitution degree |
| HPLC | High Performance Liquid Chromatography |
| FT-IR | Fourier Transform Infrared spectroscopy |
| TG | ThermoGravimetry |
| TGA | Thermogravimetric Analysis |
| SEM | Scanning Electron Microscope |
| LC | Loading capacity |
| BP | Hydorgel containing Borax and PVA |
| C-OSSPGx/BP | Hydorgel containing Curcumin loaded octenylsuccinic schizophyllan, Borax, and PVA, x represent the concentration of curcumin loaded octenylsuccinic schizophyllan |
| BJ | Fibroblast cell line |
| HaCaT | HaCat cell line |
| DMEM | Dulbecco’s modified eagle medium |
| FBS | Fetal Bovine Serum |
| RAW 264.7 | leukemia cells in mouse macrophage |
| LPS | Lipopolysaccharide |
| TNF-α | tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
References
- Zhu, J.; Zhu, J.; Zhang, M.; Gao, Y.; Qin, X.; Zhang, T.; Cui, W.; Mao, C.; Xiao, D.; Lin, Y. Tetrahedral framework nucleic acids promote scarless healing of cutaneous wounds via the AKT-signaling pathway. Signal Transduct. Target Ther. 2020, 5, 120. [Google Scholar] [CrossRef]
- Hu, M.S.; Borrelli, M.R.; Hong, W.X.; Malhotra, S.; Cheung, A.T.M.; Ransom, R.C.; Rennert, R.C.; Morrison, S.D.; Lorenz, H.P.; Longaker, M.T. Embryonic skin development and repair. Organogenesis 2018, 14, 46–63. [Google Scholar] [CrossRef]
- Dai, T.; Kharkwal, G.B.; Tanaka, M.; Huang, Y.Y.; Bil, D.A.V.; Hamblin, M.R. Animal models of external traumatic wound infections. Virulence 2011, 2, 296–315. [Google Scholar] [CrossRef]
- Stojko, M.; Włodarczyk, J.; Sobota, M.; Karpeta-Jarząbek, P.; Pastusiak, M.; Janeczek, H.; Dobrzyński, P.; Starczynowska, G.; Orchel, A.; Stojko, J.; et al. Biodegradable Electrospun Nonwovens Releasing Propolis as a Promising Dressing Material for Burn Wound Treatment. Pharmaceutics 2020, 12, 883. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Horng, H.; Yeh, C.; Chen, Y. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, X.; Xiao, L.; Wang, L.; Qiang, S. The Role of microRNA in the Inflammatory Response of Wound Healing. Front. Immunol. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Ip, M.; Lui, S.L.; Poon, V.K.M.; Lung, I.; Burd, A. Antimicrobial activities of silver dressings: An in vitro comparison. J. Med. Microbiol. 2006, 55, 59–63. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review Collagen-Based Biomaterials for Wound Healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- López-Muñoz, H.A.; Lopez-Romero, M.; Franco-Molina, M.A.; Manzano-Ramirez, A.; Velasquillo, C.; España-Sanchez, B.L.; Martinez-Hernandez, A.L.; Vergara-Castañeda, H.; Giraldo-Betancur, A.; Favela, S.; et al. Chitosan-G-Glycidyl Methacrylate/Au Nanocomposites Promote Accelerated Skin Wound Healing. Pharmaceutics 2022, 14, 1855. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, J.; Cao, L.; Jiao, Q.; Zhou, J.; Yang, L.; Zhang, H.; Wei, Y. Antifouling Antioxidant Zwitterionic Dextran Hydrogels as Wound Dressing Materials with Excellent Healing Activities. ACS Appl. Mater. Interfaces 2021, 13, 7060–7069. [Google Scholar] [CrossRef] [PubMed]
- Aduba, D.; Yang, H. Polysaccharide Fabrication Platforms and Biocompatibility Assessment as Candidate Wound Dressing Materials. Bioengineering 2017, 4, 1. [Google Scholar] [CrossRef]
- Kony, D.B.; Damm, W.; Stoll, S.; van Gunsteren, W.F.; Hünenberger, P.H. Explicit-Solvent Molecular Dynamics Simulations of the Polysaccharide Schizophyllan in Water. Biophys. J. 2007, 93, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Takeuchi, S.; Yajima, A.; Akiya, K.; Kasamatsu, T.; Tomoda, Y.; Ozawa, M.; Sekiba, K.; Sugimori, H.; Hashimoto, S.; et al. Clinical Effect of Sizofiran Combined with Irradiation in Cervical Cancer Patients: A Randomized Controlled Study. Jpn. J. Clin. Oncol. 1992, 22, 17–25. [Google Scholar] [PubMed]
- Majtan, J.; Kumar, P.; Koller, J.; Dragunova, J.; Gabriz, J. Induction of metalloproteinase 9 secretion from human keratinocytes by pleuran (beta-glucan from Pleurotus ostreatus). Z. Naturforsch. C. J. Biosci. 2009, 64, 597–600. [Google Scholar] [CrossRef]
- Fusté, N.P.; Guasch, M.; Guillen, P.; Anerillas, C.; Cemeli, T.; Pedraza, N.; Ferrezuelo, F.; Encinas, M.; Moralejo, M.; Garí, E. Barley β-glucan accelerates wound healing by favoring migration versus proliferation of human dermal fibroblasts. Carbohydr. Polym. 2019, 210, 389–398. [Google Scholar] [CrossRef]
- Seo, G.; Hyun, C.; Choi, S.; Kim, Y.M.; Cho, M. The wound healing effect of four types of beta-glucan. Appl. Biol. Chem. 2019, 62, 1–9. [Google Scholar] [CrossRef]
- RuItoh, W. Augmentation of protective immune responses against viral infection by oral administration of schizophyllan. Mediat. Inflamm. 1997, 6, 267–269. [Google Scholar]
- Deng, Y.; Liu, H.; Huang, Q.; Tu, L.; Hu, L.; Zheng, B.; Sun, H.; Lu, D.; Guo, C.; Zhou, L. Mechanism of Longevity Extension of Caenorhabditis elegans Induced by Schizophyllum commune Fermented Supernatant with Added Radix Puerariae. Front. Nutr. 2022, 9, 847064. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, Q.; Hu, L.; Liu, T.; Zheng, B.; Lu, D.; Guo, C.; Zhou, L. Enhanced exopolysaccharide yield and antioxidant activities ofSchizophyllum commune fermented products by the addition of Radix Puerariae. RSC Adv. 2021, 11, 38219–38234. [Google Scholar] [CrossRef]
- Pontes-Quero, G.M.; Benito-Garzón, L.; Pérez Cano, J.; Aguilar, M.R.; Vázquez-Lasa, B. Amphiphilic polymeric nanoparticles encapsulating curcumin: Antioxidant, anti-inflammatory and biocompatibility studies. Mater. Sci. Eng. C 2021, 121, 111793. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, A.; Yazdian, F.; Shahmoradi, S.; Ghaderi, L.; Hemati, M.; Amoabediny, G. Curcumin-loaded polysaccharide nanoparticles: Optimization and anticariogenic activity against Streptococcus mutans. Mater. Sci. Eng. C 2017, 75, 1259–1267. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Xu, J.; Gao, Z.; Wu, J.; Zhu, L.; Zhan, X. Novel amphiphilic carboxymethyl curdlan-based pH responsive micelles for curcumin delivery. LWT 2022, 153, 112419. [Google Scholar] [CrossRef]
- Farahani, Z.K.; Mousavi, M.; Seyedain Ardebili, S.M.; Bakhoda, H. Modification of sodium alginate by octenyl succinic anhydride to fabricate beads for encapsulating jujube extract. Curr. Res. Food Sci. 2022, 5, 157–166. [Google Scholar] [CrossRef]
- Toumi, S.; Yahoum, M.M.; Lefnaoui, S.; Hadjsadok, A. Synthesis and physicochemical evaluation of octenylsuccinated kappa-carrageenan: Conventional versus microwave heating. Carbohydr. Polym. 2022, 286, 119310. [Google Scholar] [CrossRef]
- Mortenson, M.A.; Labuza, T.P.; Reineccius, G.A. Moisture Sorption Isotherms for Un-Modified and Osan-Substituted Dextrin and Gum Acacia Used as Carrier Materials for Spray Dried Encapsulation of Flavoring Materials. Int. J. Food Prop. 2010, 13, 992–1001. [Google Scholar] [CrossRef]
- Abiddin, N.F.Z.; Yusoff, A.; Ahmad, N. Effect of octenylsuccinylation on physicochemical, thermal, morphological and stability of octenyl succinic anhydride (OSA) modified sago starch. Food Hydrocoll. 2018, 75, 138–146. [Google Scholar] [CrossRef]
- Fang, Y.; Takahashi, R.; Nishinari, K. Protein/Polysaccharide Cogel Formation Based on Gelatin and Chemically Modified Schizophyllan. Biomacromolecules 2005, 6, 3202–3208. [Google Scholar] [CrossRef] [PubMed]
- Rezvan, G.; Pircheraghi, G.; Bagheri, R. Curcumin incorporated PVA-borax dual delivery hydrogels as potential wound dressing materials-Correlation between viscoelastic properties and curcumin release rate. J. Appl. Polym. Sci. 2018, 135, 46734. [Google Scholar] [CrossRef]
- Spoljaric, S.; Salminen, A.; Luong, N.D.; Seppälä, J. Stable, self-healing hydrogels from nanofibrillated cellulose, poly(vinyl alcohol) and borax via reversible crosslinking. Eur. Polym. J. 2014, 56, 105–117. [Google Scholar] [CrossRef]
- Ai, J.; Li, K.; Li, J.; Yu, F.; Ma, J. Super flexible, fatigue resistant, self-healing PVA/xylan/borax hydrogel with dual-crosslinked network. Int. J. Biol. Macromol. 2021, 172, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jayamanohar, J.; Devi, P.B.; Kavitake, D.; Rajendran, S.; Priyadarisini, V.B.; Shetty, P.H. Characterization of α-D-glucan produced by a probiont Enterococcus hirae KX577639 from feces of south Indian Irula tribals. Int. J. Biol. Macromol. 2018, 118, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Gani, A.; Shah, A.; Masoodi, F.A.; Hussain, P.R.; Wani, I.A.; Khanday, F.A. Effect of γ-irradiation on structural, functional and antioxidant properties of β-glucan extracted from button mushroom (Agaricus bisporus). Innov. Food Sci. Emerg. Technol. 2015, 31, 123–130. [Google Scholar] [CrossRef]
- Song, J.; Chen, H.; Wei, Y.; Liu, J. Synthesis of carboxymethylated β-glucan from naked barley bran and its antibacterial activity and mechanism against Staphylococcus aureus. Carbohydr. Polym. 2020, 242, 116418. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Ahmed, Z. Extraction and characterization of β-d-glucan from oat for industrial utilization. Int. J. Biol. Macromol. 2010, 46, 304–309. [Google Scholar] [CrossRef]
- Chen, H.; Chen, F.; Xiao, Q.; Cai, M.; Yang, Q.; Weng, H.; Xiao, A. Structure and physicochemical properties of amphiphilic agar modified with octenyl succinic anhydride. Carbohydr. Polym. 2021, 251, 117031. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, T.; Wang, S.; Copeland, L. Effects of hydrothermal-alkali and freezing-thawing pre-treatments on modification of corn starch with octenyl succinic anhydride. Carbohydr. Polym. 2017, 175, 361–369. [Google Scholar] [CrossRef]
- Araki, K.; Yoshizumi, M.; Kimura, S.; Tanaka, A.; Inoue, D.; Furubayashi, T.; Sakane, T.; Enomura, M. Application of a Microreactor to Pharmaceutical Manufacturing: Preparation of Amorphous Curcumin Nanoparticles and Controlling the Crystallinity of Curcumin Nanoparticles by Ultrasonic Treatment. AAPS PharmSciTech 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Sun, B.; Tian, Y.; Chen, L.; Jin, Z. Linear dextrin as curcumin delivery system: Effect of degree of polymerization on the functional stability of curcumin. Food Hydrocoll. 2018, 77, 911–920. [Google Scholar] [CrossRef]
- Cheng, H.N.; Biswas, A.; Kim, S.; Alves, C.R.; Furtado, R.F. Synthesis and Characterization of Hydrophobically Modified Xylans. Polymers 2021, 13, 291. [Google Scholar] [CrossRef]
- No, J.; Shin, M. Preparation and characteristics of octenyl succinic anhydride-modified partial waxy rice starches and encapsulated paprika pigment powder. Food Chem. 2019, 295, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Miskeen, S.; An, Y.S.; Kim, J. Application of starch nanoparticles as host materials for encapsulation of curcumin: Effect of citric acid modification. Int. J. Biol. Macromol. 2021, 183, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, Q. Enhanced in vitro anti-cancer activity of curcumin encapsulated in hydrophobically modified starch. Food Chem. 2010, 119, 669–674. [Google Scholar] [CrossRef]
- Huang, S.; Shuyi, S.; Gan, H.; Linjun, W.; Lin, C.; Danyuan, X.; Zhou, H.; Lin, X.; Qin, Y. Facile fabrication and characterization of highly stretchable lignin-based hydroxyethyl cellulose self-healing hydrogel. Carbohydr. Polym. 2019, 223, 115080. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Luo, J.; Zhang, W.; Sun, J.; Wang, J.; Qin, C.; Zhuo, Q.; Dai, L. A Novel Polyvinyl Alcohol-Based Hydrogel with Ultra-Fast Self-Healing Ability and Excellent Stretchability Based on Multi Dynamic Covalent Bond Cross-Linking. Macromol. Mater. Eng. 2022, 2200525. [Google Scholar] [CrossRef]
- Lim, H.J.; Lee, S.J.; Choi, H.G.; Kim, J.A.; Yong, C.S.; Han, S.S.; Noh, S.K.; Jang, J.; Lyoo, W.S. Preparation of ketoprofen-loaded high-molecular-weight poly(vinyl alcohol) gels. J. Appl. Polym. Sci. 2007, 106, 3268–3272. [Google Scholar] [CrossRef]
- Chen, M.; Gong, G.; Zhou, L.; Zhang, F. Facile fabrication of a magnetic self-healing poly(vinyl alcohol) composite hydrogel. RSC Adv. 2017, 7, 21476–21483. [Google Scholar] [CrossRef]
- Wu, L.; Huang, S.; Zheng, J.; Qiu, Z.; Lin, X.; Qin, Y. Synthesis and characterization of biomass lignin-based PVA super-absorbent hydrogel. Int. J. Biol. Macromol. 2019, 140, 538–545. [Google Scholar] [CrossRef]
- Safaee-Ardakani, M.R.; Hatamian-Zarmi, A.; Sadat, S.M.; Mokhtari-Hosseini, Z.B.; Ebrahimi-Hosseinzadeh, B.; Rashidiani, J.; Kooshki, H. Electrospun Schizophyllan/polyvinyl alcohol blend nanofibrous scaffold as potential wound healing. Int. J. Biol. Macromol. 2019, 127, 27–38. [Google Scholar] [CrossRef]
- Junker, J.P.E.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef]
- Lin, S.; Lo, K.; Tseng, T.; Liu, J.; Shih, T.; Cheng, K. Evaluation of PVA/dextran/chitosan hydrogel for wound dressing. Cell. Polym. 2019, 38, 15–30. [Google Scholar] [CrossRef]
- Hamedi, S.; Shojaosadati, S.A. Preparation of antibacterial ZnO NP-containing schizophyllan/bacterial cellulose nanocomposite for wound dressing. Cellulose 2021, 28, 9269–9282. [Google Scholar] [CrossRef]
- Amiri, N.; Golin, A.P.; Jalili, R.B.; Ghahary, A. Roles of cutaneous cell-cell communication in wound healing outcome: An emphasis on keratinocyte-fibroblast crosstalk. Exp. Dermatol. 2022, 31, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Yussof, S.J.M.; Omar, E.; Pai, D.R.; Sood, S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2019, 45, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, S.; Zhou, J.; Shen, Y.; Huang, W.; Zhang, C.; Rahaman, M.N.; Wang, D. Evaluation of borate bioactive glass scaffolds as a controlled delivery system for copper ions in stimulating osteogenesis and angiogenesis in bone healing. J. Mater. Chem. B 2014, 2, 8547–8557. [Google Scholar] [CrossRef] [PubMed]
- Myrna, K.E.; Pot, S.A.; Murphy, C.J. Meet the corneal myofibroblast: The role of myofibroblast transformation in corneal wound healing and pathology. Vet Ophthalmol. 2009, 12 (Suppl. S1), 25–27. [Google Scholar] [CrossRef]
- Jiang, F.; Ding, Y.; Tian, Y.; Yang, R.; Quan, M.; Tong, Z.; Zhang, X.; Luo, D.; Chi, Z.; Liu, C. Hydrolyzed low-molecular-weight polysaccharide from Enteromorpha prolifera exhibits high anti-inflammatory activity and promotes wound healing. Biomater. Adv. 2022, 133, 112637. [Google Scholar] [CrossRef]
- Gao, F.; Lei, J.; Zhang, Z.; Yang, Y.; You, H. Curcumin alleviates LPS-induced inflammation and oxidative stress in mouse microglial BV2 cells by targeting miR-137-3p/NeuroD1. RSC Adv. 2019, 9, 38397–38406. [Google Scholar] [CrossRef]
- Shlar, I.; Droby, S.; Choudhary, R.; Rodov, V. The mode of antimicrobial action of curcumin depends on the delivery system: Monolithic nanoparticles vs. supramolecular inclusion complex. RSC Adv. 2017, 7, 42559–42569. [Google Scholar] [CrossRef]
- Pawar, R.S.; Toppo, F.A.; Mandloi, A.S.; Shaikh, S. Exploring the role of curcumin containing ethanolic extract obtained from Curcuma longa (rhizomes) against retardation of wound healing process by aspirin. Indian J. Pharmacol. 2015, 2, 160–166. [Google Scholar] [CrossRef]
- Yen, Y.; Pu, C.; Liu, C.; Chen, Y.; Chen, Y.; Liang, C.; Hsieh, J.; Huang, H.; Chen, Y. Curcumin accelerates cutaneous wound healing via multiple biological actions: The involvement of TNF-α, MMP-9, α-SMA, and collagen. Int. Wound J. 2018, 15, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Dai, L.; Qin, Y.; Xiong, L.; Sun, Q. Preparation and Characterization of Octenyl Succinic Anhydride Modified Taro Starch Nanoparticles. PLoS ONE 2016, 11, e0150043. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Bai, Y.; Shi, Y. Identification of isomers and determination of octenylsuccinate in modified starch by HPLC and mass spectrometry. Food Chem. 2012, 135, 665–671. [Google Scholar] [CrossRef]
- Nagy, N.Z.; Varga, Z.; Mihály, J.; Domján, A.; Fenyvesi, É.; Kiss, É. Highly Enhanced Curcumin Delivery Applying Association Type Nanostructures of Block Copolymers, Cyclodextrins and Polycyclodextrins. Polymers 2020, 12, 2167. [Google Scholar] [CrossRef]
- Palomino-Durand, C.; Lopez, M.; Cazaux, F.; Martel, B.; Blanchemain, N.; Chai, F. Influence of the SolubleInsoluble Ratios of Cyclodextrins Polymers on the Viscoelastic Properties of Injectable ChitosanBased Hydrogels for Biomedical Application. Polymers 2019, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Yang, T.; Zhang, C.; Peng, X.; Ju, Y.; Li, C.; Zhou, X.; Luo, Y.; Xu, X. Repurposing Napabucasin as an Antimicrobial Agent against Oral Streptococcal Biofilms. BioMed Res. Int. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, L.; Fan, Y.; Deng, Y.; Hu, L.; Sun, H.; Zheng, B.; Lu, D.; Guo, C.; Zhou, L. Production and Anti-Inflammatory Performance of PVA Hydrogels Loaded with Curcumin Encapsulated in Octenyl Succinic Anhydride Modified Schizophyllan as Wound Dressings. Molecules 2023, 28, 1321. https://doi.org/10.3390/molecules28031321
Tu L, Fan Y, Deng Y, Hu L, Sun H, Zheng B, Lu D, Guo C, Zhou L. Production and Anti-Inflammatory Performance of PVA Hydrogels Loaded with Curcumin Encapsulated in Octenyl Succinic Anhydride Modified Schizophyllan as Wound Dressings. Molecules. 2023; 28(3):1321. https://doi.org/10.3390/molecules28031321
Chicago/Turabian StyleTu, Lingyun, Yifeng Fan, Yongfei Deng, Lu Hu, Huaiqing Sun, Bisheng Zheng, Dengjun Lu, Chaowan Guo, and Lin Zhou. 2023. "Production and Anti-Inflammatory Performance of PVA Hydrogels Loaded with Curcumin Encapsulated in Octenyl Succinic Anhydride Modified Schizophyllan as Wound Dressings" Molecules 28, no. 3: 1321. https://doi.org/10.3390/molecules28031321
APA StyleTu, L., Fan, Y., Deng, Y., Hu, L., Sun, H., Zheng, B., Lu, D., Guo, C., & Zhou, L. (2023). Production and Anti-Inflammatory Performance of PVA Hydrogels Loaded with Curcumin Encapsulated in Octenyl Succinic Anhydride Modified Schizophyllan as Wound Dressings. Molecules, 28(3), 1321. https://doi.org/10.3390/molecules28031321






