Abstract
Central nervous system disorders, especially neurodegenerative diseases, are a public health priority and demand a strong scientific response. Various therapy procedures have been used in the past, but their therapeutic value has been insufficient. The blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier is two of the barriers that protect the central nervous system (CNS), but are the main barriers to medicine delivery into the CNS for treating CNS disorders, such as brain tumors, Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease. Nanotechnology-based medicinal approaches deliver valuable cargos targeting molecular and cellular processes with greater safety, efficacy, and specificity than traditional approaches. CNS diseases include a wide range of brain ailments connected to short- and long-term disability. They affect millions of people worldwide and are anticipated to become more common in the coming years. Nanotechnology-based brain therapy could solve the BBB problem. This review analyzes nanomedicine’s role in medication delivery; immunotherapy, chemotherapy, and gene therapy are combined with nanomedicines to treat CNS disorders. We also evaluated nanotechnology-based approaches for CNS disease amelioration, with the intention of stimulating the immune system by delivering medications across the BBB.
1. Introduction
Nanomedicine is a new field that utilizes nano-scale resources for applications in the diagnosis and treatment of diseases [1]. Several nano-scale materials are used in nanomedicine: organic, inorganic, carbon-based, polymeric, liposomes, extracellular vesicles, and metals [2,3,4]. Boron-containing compounds (BCCs) have a wide range of medicinal effects [5,6,7]. Currently, only a few BCCs, such as vaborbactam, tavaborole, crisaborole, and bortezomib, are permitted for use in people. However, many other BCCs are demonstrating promising results as prospective therapies for human disorders, particularly neurological conditions. Consequently, the interest in BCCs on models of neuronal disorders is rising. These include studies that reveal beneficial activity on multiple targets, those that demonstrate a pro-survival impact on human-derived SH-SY5Y cells, such as an A-toxicity model, and those that demonstrate ameliorative abilities in both in vitro and in vivo models of AD [8,9,10,11]. The relationship between nanoscience and pharmaceutical science is broad and there are emerging applications in various fields of disease diagnosis and therapeutics [12,13,14]. Tumor cells are passively targeted by nanomedicines through enhanced retention and permeability (EPR) [15]. The improved permeability and retention are used to target the lymphatic and microvascular systems in the tumor’s interior [16,17,18]. Alternatively, nanomedicine approaches in active and functional targeting are widely examined to overcome particular challenges in tumor theragnostic [19].
The term “central nervous system (CNS) disorders” refers to a broad range of significant neurological conditions, the majority of which have no effective treatments [20]. Gliomas and glioblastomas, two types of brain cancer, are inherited diseases that originate from cells known as neuroglial ancestor cells [21]. Glioblastoma, which makes up 15% of primary brain tumors and 50% of all gliomas, is the most typical primary CNS tumor in adults. Only temozolomide (TMZ) and the antiangiogenic medicine bevacizumab have been approved by the US Food and Drug Administration (FDA) in recent years for the treatment of gliomas [22]. The median survival rate for people with glioblastoma is less than 2 years despite recent treatment advancements [23]. The more common neurodegenerative disorders Parkinson’s disease (PD) and Alzheimer’s disease (AD) affect millions of people worldwide [24]. There are several drugs for AD that have been licensed by the FDA, such as cholinesterase inhibitors (e.g., rivastigmine and donepezil) as well as NMDA receptor antagonists (e.g., memantine), However, they have limited benefits on severe cognitive impairment and cannot reverse the course of the condition [25,26,27]. The only drug approved to treat Parkinson’s disease is Xadago (safinamide) that reduces motor symptoms without addressing the underlying pathophysiology of the disease [28,29]. Preventing disease development and treating the symptoms and pathology after a late-stage diagnosis are the major challenges in this profession. A new wave of treatment strategies has emerged as a result of these challenges. Currently, radiation, chemotherapy, gene therapy, immunotherapy, and surgery are used to treat CNS illnesses; however, every therapeutic approach has advantages and disadvantages [30,31]. Immunotherapy uses the immune system of the host to target disease cells by enhancing or suppressing innate immune responses [32]. Active immunotherapy and passive immunotherapy are the two categories of immunotherapeutic techniques [33]. Nanomedicines, tumor vaccines, and non-specific immune stimulants are all examples of active immunotherapeutic techniques that aim to elicit an immune response [34,35,36]. Through administering lymphocytes or antibodies to patients, passive immunotherapy promotes anticancer effects [37]. Therefore, while implementing immunotherapeutic techniques, it is important to take into account the distinct immunological milieu of the CNS [38,39]. The presence of complement components, the expression of Toll-like receptors, and the existence of microglia, astrocytes, and pericytes as antigen-presenting cells (APCs) demonstrate that the CNS immune system exists [40]. Even with a disrupted blood-brain barrier (BBB), certain invading cancers contain no enhancing areas, which restricts therapeutic medication interaction despite the immune system’s dynamic and adaptive characteristics [41]. Additionally, the delivery of medicines across the BBB is a substantial problem due to the tremendous adaptive characteristics of glioblastoma, in addition to its comparatively low immunogenicity, development of an inhibiting tumor microenvironment (TME), and intertumoral heterogeneity. In fact, many scientists have proposed that the CNS may indeed be regarded as an “immunologically inactive” location, providing a distinct and balanced environment that favors the predominance of immunosuppressive mediator production [42].
The fundamental challenge that restricts the efficacy of immunotherapies is the engagement of surrogate immunosuppression by brain tumors via several pathways. Overexpressed markers (CD4+, CD25+, and FOXP3+) on regulatory T cells (Tregs) in the sick state influence the immunologically cold TME [43]. Glioblastomas are a particular kind of immunoprivileged tumor since they are sometimes referred to as “cold TMEs” and are only peripherally penetrated by immune cells. A cold TME may also be influenced by immune-suppressive cytokines like interleukins IL-6 and IL-10, as well as immune-suppressive cytokines like transforming growth factor-beta (TGF-β) [44]. PD-1 (programmed cell death protein 1) is overexpressed by inactive Tregs, which aids tumors in evading CNS immune responses and results in a cold TME [45,46]. As a result, a number of tightly controlled checkpoints maintain brain immunity. Immunotherapy has undergone several changes as a result of training the immune system to detect disease locations [47]. One is immune checkpoint inhibition, in which medications (typically antibodies) block immunological checkpoints that tumors have overexpressed in order to reveal cancer cells and ultimately activate immune responses against the tumors (e.g., melanoma) [48]. Alternatively, immune responses can be triggered by genomically altered targeted therapeutics (such as chimeric antigen receptor T cells (CAR T cells)), which have been altered to detect and cure the patient’s malignancy [49]. Immunotherapy for the treatment of CNS illnesses continues to be a significant therapeutic issue despite decades of scientific study. For instance, immunological checkpoints and CAR T-cell therapy are less effective in treating nonresponsive cancers that have fewer mutations and neoantigens [50]. Immunotherapy can be particularly difficult due to the location and morphologic similarity of nonmalignant cells with neuroglial cells [51]. A significant barrier to obtaining a better therapeutic effect for CNS illnesses is the difficulty in deliver therapeutically appropriate dosages to the disease region.
Drug clearance via the kidneys, drug circulation time in the blood, medication penetration through the blood–brain barrier (BBB) and blood–brain tumor barrier (BBTB) are further obstacles to drug delivery into the central nervous system (CNS) [52]. The payload’s ability to enter the CNS is frequently constrained by a natural defense mechanism of efflux pumps, such as multidrug-resistant protein [53] and convection-enhanced diffusion [54]. In order to overcome these difficulties, CNS treatments are being developed using an active and functionally focused nanomedicine-based strategy [55]. The emerging field of nanomedicine uses nanoscale materials for a wide range of purposes in diseases diagnosis and treatment [1]. The BBB needs to be crossed by effective nanomedicines for CNS illnesses, and different parameters need to be tuned (e.g., shape, size, functional surface chemistry, circulating half-life, structural stability, permeability, and extravasation) [56]. Additionally, many receptor-facilitated contacts are required to maintain a high degree of surface conjugation chemistry and the related targeting capabilities. One of the primary mechanisms for BBB penetration is receptor-mediated transcytosis (RMT) [57], Transcytosis mediated by adsorption [58] and cell-mediated transport by immune cells, macrophages, and monocytes [59,60] are further examples. Extracellular vesicles, liposomes, and red blood cell membranes are just a few examples of the various nanoscale materials that have been used as nanomedicines thus far [61,62], in addition to metal nanostructures [3]. Drug-carrying nanostructures significantly enhance the pharmacokinetics and biodistribution of pharmaceuticals in the CNS when compared to free drugs [18,63].
Neurodegenerative diseases adversely affect the structure and function of neurons, which are a building block of the nervous system, and are frequently involved in neuronal death [64]. Mechanistically, the factors underlying the pathogenesis and propagation of neurodegenerative disorders include genetic mutations [65], mitochondrial dysfunction [66], damage to DNA or organelle membranes, protein misfolding and aggregation, autophagic as well as apoptotic cell death, and transglutaminase binding [67]. With a million people affected by Alzheimer’s disease [68], it is irreparable, though the available treatments consist of pharmaceutical, behavioral, social, and caregiving treatments. Pharmaceutical treatments include donepezil, rivastigmine, galantamine, tacrine (acetylcholinesterase (Ache) inhibitors), and memantine (N-methyl-aspartate (NMDA) receptor antagonist) [69]. The treatment of Parkinson’s disease (PD) is similarly critical, with 10 million people suffering worldwide. Treatments such as medicines, surgery, and physical therapy have been confirmed to increase quality of life. The primary treatment for extenuating motor symptoms is levodopa, used in combination with a dopa-decarboxylase inhibitor and other inhibitors such as dopamine agonists, catechol-o-methyltransferase (COMT) inhibitors, and monoamine oxidase-B (MOA-B) inhibitors [70]. Nanomedicine is unarguably moving with the anticipated pace to conquer neurodegenerative disorders and their associated consequences. Incidentally, nanomaterials have been manipulated to attain appropriate physicochemical properties, for instance the type of matrix, size, charge, surface chemistry, polarity, etc., to enable the crossing of the BBB and targeting the CNS to release their cargo [71]. Current intriguing advances in various kinds of polymeric nanoparticles, metal nanoparticles, nanoparticles (NPs), namely liposomes, etc., for the cure and identification of numerous neurodegenerative diseases are reviewed here.
This review presents collected information about different therapies, such as gene therapy, chemotherapy, and especially immunotherapy, based on nanomedicine for the treatment of CNS diseases. The diseases under study include brain cancer and the neurodegenerative Alzheimer’s and Parkinson’s diseases. The data are hypothesized from the experimental models of the oriented propagation hypothesis for glioma, tauopathies, amyloid-β, and α-synucleinopathy Lewy bodies. This study evaluates a role in disease progression, the latest information about different therapies, especially immunology, that have a role in disease progression, outlining the current advances and shedding light on some problems. Finally, the information may surprise researchers as to whether these selective targeting strategies and the elimination of symptoms engage the immunotherapy approaches among various pathological hypotheses associated with CNS diseases.
2. The Immune System in the Brain
Since the CNS lacks a lymphatic system and early destructive T-cell responses are driven by the parenchyma, it is usually viewed as an immune-privileged system [35]. According to the results of a recent study, despite the presence of a functional meningeal system, particles were able to move into subterranean cervical lymphatic nodes due to the functioning meninges [72]. Lymphatic endothelial cells were intensely studied for their configuration that expresses all their molecular markers [73,74], and, nowadays, they are generally recognized as the glymphatic system. The particular range of the system (Figure 1) is still unknown [75]. According to another study, the cerebrospinal fluid and interstitial fluid periarterial space containing macromolecules and solutes are continuously exchanged within the system of perivascular tunnels, basement membrane, and astroglia cells [76,77]. Additional research studies revealed a specific perivascular section for small lipid transport and glial communication signaling [78].
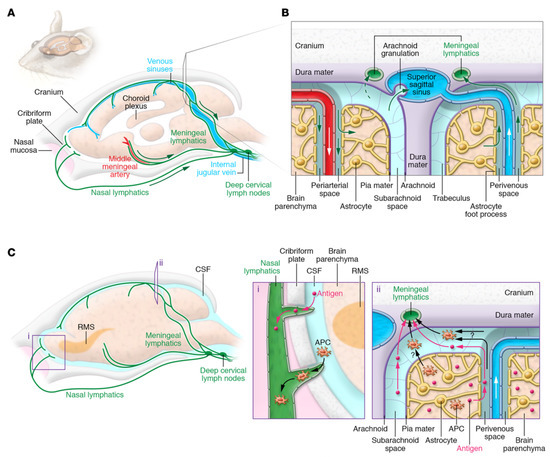
Figure 1.
The cervical lymph nodes through the CNS’s lymphatic and lymphatic drainage pathways. (A) The meningeal lymphatic vascular system is depicted schematically in the mouse brain. Along with the venous sinuses, arteries, and cranial nerves, the dural lymphatic vessels leave the cranium through the foramina in alignment with the dural blood vessels and cranial nerves. Along with the olfactory nerves, certain lymphatic veins may be seen crisscrossing the cribriform plate. Tracers injected into the SAS or brain parenchyma drain into the dcLNs adjacent to the jugular vein through the dural lymphatic arteries. (B) ISF and CSF circulation up close. CSF and solutes are transported into the brain via the perivascular glymphatic drainage system through the periarterial pathway, whereas ISF and solutes are transported out of the brain by the perivenous glymphatic pathway. CSF macromolecules and immune cells are mostly carried by the dural lymphatic channels into the lymph nodes and extracranial systemic circulation, and CSF can reach the venous system via arachnoid granulations. (C) Routes for antigens and antigen-presenting cells to exit the central nervous system (APCs). Dendritic cells, in particular, may migrate along the rostral migratory stream (RMS) to enter the lymphatics via the olfactory bulb’s SAS. Alternatively, antigens and APCs are proposed to leave the CNS via the glymphatic pathway (as demonstrated for antigens), reaching the SAS and entering the meningeal lymphatic vasculature via SAS and trafficking to the dcLNs. APCs in the meningeal spaces may also go to the dcLNs through meningeal lymphatic channels. It is still unknown how much each mechanism contributes to cell and antigen outflow. Reproduced with permission from [47].
CNS immune treatment was partially credited to the absence of a standard lymphatic system. The parenchyma of the brain was able to maintain and potentiate the immune response induced by allografts that were recognized by an immune response in peripheral organs [79]. As previously mentioned, the brain parenchyma and meningeal part of the brain shows a wide variety of properties when compared to the BBB, starting from the fact that the meningeal blood barrier is more permissive than the BBB, so immune cells are allowed to flow separately through the meningeal spaces even under physiological conditions [73]. Even though studies suggested that a model in which a selective barrier, for example, the BBB, does not characterize the individual static structure capable of separating immune cells outside the organs, nonetheless they are slightly permissive entrances that control the passage of cells in particular circumstances, such as in some specific phenotypes. In this circumstance, the limit of the CNS is not the authority for elimination. As a result, the capacity to communicate with the active immune system is limited [80].
Through an internal recirculation mechanism connecting the cerebrospinal fluid with intestinal fluid, antigens pass from the brain to deep cervical lymph nodes through meningeal lymphatic vessels [75]. Nevertheless, the response is low; therefore, it is required for a considerable number of antigens or secondary signaling to generate this response. Alternatively, the cervical lymph nodes may possess the ability to moderate immune responses to CNS antigens and tolerance or reactivity in response to these antigens [73].
3. Challenges in Nanomedicine-Based Immunotherapy in the Brain
Human brains are among the best-protected organs in the body. The skull, meninges, and cerebrospinal fluid provide shields of protection: the BBB and BBTB [81]. All these layers protect the brain from injuries and prevent diseases. However, the protective layers lessen the entrance of therapeutic agents to the brain in a diseased state.
Despite research on many drugs or medicines, only a few drugs, such as rivastigmine, memantine, galantamine, tacrine, and donepezil [82], are currently used to support the clinical treatment of neurodegenerative disorders [1]. The CNS is the most complex and sensitive structure in the human body, securely packed and sealed by the BBB and BCFB [83,84]. Numerous transporter systems are the critical factors for regulating the CNS’s internal environment; they are located at the BBB and are capable of shipping elements through the BBB. For instance, the multidrug resistance protein named P-glycoprotein (Pgp) is a vital transporter belonging to the ATP-binding cassette transporter family [85].
Due to its multifaceted nature, there are numerous limitations in delivering targeted medicine from the blood to the CNS. There is a lack of data about the role of medicines, the half-life of medicines, or bio-presences for brain cells, their effects on the CNS, or the irregular and uncertain relationships between off-target medicines and receptors and enzymes. The complicated pharmacology of some medicines, neurodegenerative symptoms, the lengthy latency period, and incompetence of medicines, the subsequent development of infections, an incorrect number of medicines, the patient’s genotype, and diverse reactions to medicines constitute the instability index of assessed medications. In addition, with concerns related to the brain, the difficulties become even more difficult [86,87,88]. Here, the three main obstacles inhibiting drug transport into the brain are discussed.
3.1. The BBB
The CNS is a good site in regards to immunity due to being protected by the BBB, which plays a critical defensive role in the transportation of molecules, adjuvants, and immune modulators from the bloodstream to the brain [89]. Nevertheless, the CNS has immature T cells in blurred form, while T lymphocytes in active form circulate and can do so during their movement [38]. Moreover, animals that bear tumors and glioma patients’ normal infiltration process of cytotoxic T cells should be considered [90]. During neuroinflammation of the CNS, the brain uses microglia and pericytes as antigen-presenting cells (APCs), which often triggers neurological disorders [40]. Despite BBB disturbance, some non-enhancing regions of the infiltrating tumor where the BBB is unbroken limit therapeutic drug contact [41].
The presence of the BBB and BBTB protective shells around brain tumors is a significant obstacle to delivering therapeutic agents (Figure 2). The BBB, acting as a barrier, forms from a tight restriction of cerebral capillary endothelium, astrocytes, pericytes, basal membranes with infrequent transcytosis, and endocytosis [52]. Due to the overexpression of existing P glycoproteins on cerebral endothelial cells, utmost active efflux process penetration occurs [41]. Moreover, macromolecules of all kinds and large parts of smaller molecules, including anticancer drugs, cannot pass through the tiny and tight holes present in the connective cells of the CNS [41]. Thus, modern chemotherapies fail to reach their target owing to the BBB.
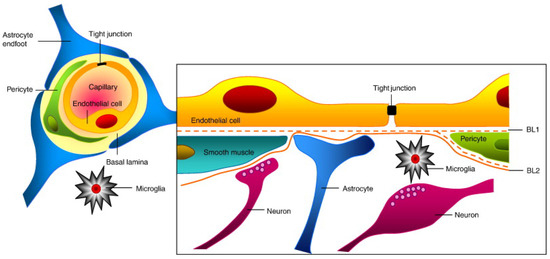
Figure 2.
BBB’s structure and composition. The brain endothelium, astrocytes, extracellular matrix, and endothelial cells form tight junctions, which make up the majority of the BBB. Image reproduced with permission from [91].
3.2. Blood–Cerebrospinal Fluid Barrier
After overcoming the BBB, the next obstacle is the blood–cerebrospinal fluid barrier (BCFB) that is assembled by the epithelial cells of the choroid plexus (CP), which systemically deals with drug molecules [88]. The choroid plexus epithelium (CPE) is a secretary epithelium that is one of the most effective tissue types based on secretory mechanisms involved in balancing cellular transport mechanisms [92]. Similar to the BBB, this epithelial barrier system separates the blood and CSF. The CPE is tightly preserved throughout form-adjacent cells found next to the CSF-facing surface. The CPE comprises multi-specific efflux transport proteins and detoxifying enzymes, both preventing entry of potentially lethal compounds into the CNS [93]. Several plans for drug delivery have been developed, most of which target the microvascular endothelium and are suitable for the BBB. In addition to the BBB, targeting the BCFB shaped by the choroid epithelium is important for the curing of CNS diseases [93].
3.3. Multidrug Resistance Proteins
In recent times, the BBB was considered a static lipid membrane barrier. Physical feature selectivity to a wide variety of circulating compounds by the BBB membrane was due to factors such as cerebral endothelial cells containing tight junctions, deficiency of vesicles or caveolae, and high electrical resistance. However, immunocytochemistry, molecular biology, and biochemistry experiments on BBB transport pathways, have established that cerebral endothelial cells acquire a wide range of metabolic enzymes (e.g., glutathione transferases, alkaline phosphatase, and cytochrome P450 enzyme) and efflux transport proteins (energy-dependent), including P-glycoprotein and multidrug resistance proteins arranged irregularly and serving as a barrier. P-glycoprotein (P-gp) (170–180 kDa) is a plasma membrane-associated protein and is one of the essential ATP-dependent efflux transporters in the BBB. P-glycoprotein is a multidrug resistance protein encoded by the MDR1 gene [94], that limits the access of various drugs and preventing them from achieving their precise therapeutic objectives. In comparison, when P-gp expression is suppressed, the drug quantity arriving in the CNS was increased. Another experiment suggested that inhibitors and inducers of P-gp have an apparent effect on brucine pharmacokinetics and pharmacodynamics [94].
The challenge in pharmaceutical research is discovering devices and methods for allowing efficient and effective drug distribution into the CNS [95]. Immunotherapy is an approach of clinical oncology with significant development for effective therapies against numerous cancers [96]. However, the microscopic immunological environment in the CNS requires appropriate precautions to engage in immunotherapeutic strategies for gliomas. Immunotherapy has the potential for the treatment of glioblastoma (GBM), although researchers agree that combination regimens are required to attain a robust response. Owing to its powerful adaptive capabilities, relative lack of immunogenicity, immunosuppressive tumor microenvironment, and intra-tumoral heterogeneity, the amelioration of GBM across the BBB is still challenging. It is believed that the CNS has a unique, activated, distinctive, and adaptive immune system, with microglia cells specified for antigen presentation, expression of Toll-like receptors (TLRs), and complement components [97,98]. The CNS is also considered an “immunologically inactive” site with an environment employed to express immunosuppressive mediators [42]. Based on these facts, CNS disorders are a significant cause of delayed or hampered immune responses.
The engagement of multiple mechanisms to induce immunosuppression provides an obstacle limiting the value of immune therapies for glioma. The gloom remains as immunologically cold tumors with a suppressed TME are insufficiently infiltrated by functional immune effector cells. The key factors that modulate the immunologically cold microenvironment for T cell regulation, also called “Tregs”, in gliomas, are due to highly expressed markers such as CD25+, CD4+, and FOXP3+. Moreover, tumor cell-derived cytokines act as immune inhibitors, for example IL-6, IL-10, and transforming growth factor-beta (TGF-β) [44]. Glioma immune responses are due to the Tregs in both tumor-resistant or glioma-grade increased programmed cell death protein-1 (PD-1) expression [43,46]. Although there are existing tumor-infiltrating lymphocytes in the TME, it still has a very low response rates because of immunosuppressive signals from accumulation of exhaustion [45].
4. Nanomedicines for Targeting CNS Diseases
Neurodegenerative disorders such as Alzheimer’s (AD), Parkinson’s (PD), and amyotrophic lateral sclerosis (ALS) are characterized by a protracted pathological degradation process that causes incalculable suffering to patients and their relatives. With the maturation of nanotechnology since the 1990s, the technical barriers to nanomaterial research have disappeared. Nanomaterials have steadily entered the area of view of neurodegenerative disease experts. For research purposes, numerous nanomaterials, including lipid-based nanomaterials (e.g., liposomes and solid lipid nanoparticle (SLN)), polymeric nanomaterials (e.g., micelles, dendrimers, nanocapsules, and nanospheres), and inorganic nanoparticles, have been utilized. Nanoparticle-based therapeutic strategies in CNS-associated diseases are mainly focused on and sustain local release and targeted delivery of curative agents at the brain’s affected zone after bypassing the BBB [99]. The hallmark feature of AD is amyloid-β peptides combining to form amyloid plaques. Existing AD treatments include cholinesterase inhibitors like donepezil, rivastigmine, galantamine, and N-methyl-d-aspartate receptor antagonists (memantine) [100]. With the help of polymeric nanoparticles, lipid-based nanoparticles, and non-polymeric quantum dots, passage through the BBB is permitted and side effects of free drug usage are decreased by reformulating clinically used drugs [101]. Concerning the nano delivery method, numerous neuroprotective compounds such as metal chelators and several NMDA antagonists of anti-amyloids improved the passage through the BBB and reduced Aβ aggregate formation [102,103].
PD’s treatment based on dopamine replacement is the most used approach; later on, treatments for the effects on motor neurons and attenuating disease development were developed, although these drugs’ effects on behavior and perception are still under investigation [104]. Recent studies focused on curative nanoparticles in various approaches, for example, targeting dopamine delivery by using polymeric nanoparticles or liposomes [105]. Many studies use different drugs such as bromocriptine, apomorphine, mitoapoacynin, and ropinirole encapsulated with liposomes or polymeric nanoparticles to advance the sustained discharge of drugs and to decrease unwanted outcomes of predictable PD therapy [106,107,108]. Anti-inflammatory therapeutics have been established via PEGylated liposomes or polymeric nanoparticles to inhibit neuronal cell death in PD [82,83,84] and increased dopamine levels [109,110]. Alternatively, numerous groups proposed nano-systems for the transfer of hereditary material such as nucleic acid (DNA and RNA) and oligo-nucleotides to prevent abnormal gene expression or production of beneficial proteins in targeted cells [111]. Significant progress in clinical signs was noted in progressive PD patients using gene therapy, which is still a contradictive matter considering PD’s heterogenic pathology [112].
Recently, nanomedicines based on GBM therapeutics have received important considerations [101,113]. In the case of generally directed free drugs, a minute proportion passes through the BBB in a nonspecific manner in off-target tissues, resulting in severe undesirable outcomes. Therefore, nanoparticle use for drug delivery to the brain can increase the percentage of drugs crossing the BBB and decrease nonspecific drug accumulation in other tissues [15]. For instance, gadolinium-loaded nanocarrier systems improved drug penetration and targeting ability to 100-fold higher levels than free gadolinium. NPs with a ligand-modified surface have facilitated imaging of brain tumors [114,115]. In another study, the PEGylation of nanoparticles was used in drug delivery to protect nanoparticles from blood protein interactions on the reticuloendothelial system (RES) [102,103]. Similarly, the PEGylated NPs thoroughly avoided interfacing with protein in the plasma. Studies have found, however, that PEGylating proteins does not completely prevent their interaction with nanoparticles in the blood [116].
Nanomedicine-based approaches have the promising potential to overcome these restrictions and advance the treatment of CNS disorders. The uses of nanoparticles as drugs and drug-delivery carriers have been extensively examined in preclinical studies and are currently being applied in the clinical setting for certain CNS diseases (Table 1) [117,118,119,120,121]. A variety of nanoformulations have demonstrated that they significantly improve CNS pharmacokinetics and distribution in brain areas when compared to free drugs [122,123]. In addition to overcoming the BBB, surface decoration with specific ligands allows ‘active targeting’ to different brain cell types [101]. Targeting neuronal cells in CNS pathology markers is a crucial task in nanomedicine and neuroscience. Presently, there is only a limited number of nanomedicines in clinical use or in the pipeline to cure CNS disorders, such as glatiramer acetate [124], peginterferon-1a for sclerosis [125], gold nanocrystals (CNM-Au8) for lateral sclerosis and PD [126], APH-1105 for AD [119,127], and nanocurcumin for lateral sclerosis [128,129]. These nanomedicines are mainly based on polymeric-based therapeutics, gold nanocrystals, and curcumin encapsulated in nanomedicine in nano micelles, or NPs containing an inhibitor of α-secretase to treat Alzheimer’s disease. Although these nano-formulations were not precisely made to target neurons, their systematic features and the changes required to enhance their clinical results, are still poorly known. The low number of nanomedicines used to target the CNS in clinical trials is the main difficulty facing the strategy and advancement of these methodologies. Thus, a comprehensive preclinical confirmation is mandatory. Therefore, in recent years, numerous struggles have concentrated on the advancement and testing of nanomedicines in models of CNS disorders that can advance the clinical usage of these NPs in the CNS.

Table 1.
List of nanomedicines targeting CNS diseases.
5. Surface Modification of Nanoparticles
The nanomedicine system used for CNS diseases must be compatible and able to cross the BBB efficiently. While designing nanocarriers, the following parameters require optimization: size, shape, structural stability, functional surface chemistry, permeability, circulating half-life of the tumor, extravasations, and targeting capability to enter the tumor using receptor- or transporter-facilitated interactions. In this aspect, surface-conjugated chemistry may play a pivotal role in overcoming the significant challenges traversing the BBB. The BBB is a group of highly specialized, closely connected cells arranged in a group. The BBB can protect the brain from many potential threats and it has a pivotal role in the brain’s homeostasis and physiology. The BBB’s structural features primarily consist of highly impenetrable brain capillary endothelial cells (BCECs) enclosed with the wrapping of perivascular end feet of astrocytes and basal lamina pericytes (as shown in Figure 1A) [56]. The epithelial cells possess tight junctions (TJs) that strictly restrict the permeability of water-insoluble molecules via diffusion. Thus, the BBB acts as a barrier resisting nearly all (98%) small-sized adjuvants and drugs, whereas the large-molecule neuro-therapeutics are completely blocked [156].
Luminal and abluminal epithelial cells act as transport sites for substrates into the brain [56,156]. Lipophilic molecules (≤400 Da), alcohol, nicotine, steroid hormones, CO2, and O2, can take a transcellular lipophilic pathway to cross the BBB via diffusion. Detailed routes are shown in the schematic in Figure 1B. Moreover, carrier-mediated transcytosis (CMT) is the primary method to cross the BBB that is used by the many essential nutrients and ions, such as amino acids, glucose, nucleosides, vitamins, and electrolytes. Glucose transporter-1 (GLUT1) is another essential receptor that favors bi-directional glucose diffusion via a concentration gradient [57]. Furthermore, another pathway includes receptor-mediated transcytosis (RMT), which includes some specific receptors, such as the receptors for low-density lipoprotein [157], insulin, and transferrin, as natural targets for nanomedicines [158]. Meanwhile, adsorptive-mediated transcytosis (AMT) used to transport molecules including cationic proteins or cell-penetrating peptides (CPPs) [58], can interact with anionic sites on the surface of the epithelial cell membrane through electrostatic interactions to transport the payload. Moreover, transport across the intact BBB can be achieved in a cell-mediated manner, including stem cells and immune cells, such as monocytes and macrophages [59,60]. In some cases, the payload gets into the CNS. The nanomedicine-based delivery system may enter the endothelium by efflux pumps but the drugs may be squeezed back into the circulatory system. This type of system is one of the protective mechanisms that occur naturally in the brain and are responsible for avoiding exposure to foreign molecules or materials. There are two major transport carriers, including multidrug resistance proteins (MRPs), such as adenosine triphosphate (ATP)-binding cassette and P-glycoprotein (P-gp) [53], and convection-enhanced diffusion (CED), which have been the most extensively exploited for clinical studies based on convection for drug delivery with the aid of constant hydrostatic pressure gradients [54].
6. Applications of Nanotechnology in CNS Disorders
The development of treatments for neurological disorders also includes experts in nanotechnology. The field gives new techniques to treat PD, HD, AD, stroke, brain tumors, and epilepsy. Molecules are designed in such a way that they are able to cross the BBB; specific cells are targeted or used as a pathway for the signaling process. The designed molecules are used as carriers to deliver genes. In addition, experts in nanotechnology have started work on delivering radio-contrast objects to aid in diagnosing diseases through imaging.
6.1. Glioblastoma
Glioblastoma and gliomas are inborn brain tumors that arise from the ancestor cells of neuroglial [21]. Due to their localization, morphologic resemblances with non-malignant neuroglial cells, and propagation, immunotherapy is extremely challenging for individual brain cells [159]. Due to these facts, this type of disease might be included in a diverse range of CNS cancers, including astrocytomas, oligodendrogliomas, ependymomas, neuroblastomas, and glioblastomas [159,160,161]. GBM is the most common CNS tumor in adults, comprising 50% of all gliomas and 15% of primary brain tumors [23]. The overall median survival rate is less than two years, even with antagonistic therapy [21]. The U.S. Food and Drug Administration (FDA) has approved a wide range of oncology drugs for treating different types of cancers in recent decades; nevertheless, only two drugs have successfully passed the clinical trials for glioma therapy, temozolomide and the anti-angiogenic drug, bevacizumab [22].
Many researchers have tried to develop new nanotherapeutic approaches for treating gliomas. Nano-formulations were developed that deliver drugs across the BBB [162]. One nano-formulation that involves PBCA mixed with methotrexate [163], and another using temozolomide [152], produced significantly improved results for delivering drugs into the brain compared free drugs. Similarly, in vitro results using SLNs containing paclitaxel [153] showed an increase in inhibitory effects on the proliferation of glioma cells [164]. It was also demonstrated that transferrin receptor-targeted nanoparticles can enhance cellular internalization and cytotoxicity of docetaxel with enhanced pharmacokinetics [165,166]. Dendrimers are used to transfer antineoplastic treatments into the brain. A conjugated molecule made of polyether-copolyester (PEPE) dendrimers and methotrexate confirmed improvements in cytotoxicity in cultured U343 and U87 cancer cell lines. The nano-formulation was able to overcome resistance from drugs [167]. The conformation from PEGylated PAMAM dendrimers with doxorubicin (DOX) opens a new therapeutic window by hindering C6 glioma spheroid proliferation. There was little cytotoxicity in opposition to brain micro-vascular endothelial cells in vitro [168].
A contrast agent NP that offers detailed cellular and molecular imaging can aid in the surgical removal of gliomas. In a study conducted by Hernandez-Pedro et al., gadolinium oxide crystals of a size less than 5 nm in diameter were used to label glioma cells [169]. In another study, researchers used an antineoplastic drug encapsulated in PEG-coated hexadecyl cyanoacrylate NPs in a mouse model of gliosarcoma to study the precise drug-release kinetics and found higher diffusion of the drug through the BBB, with regard to bulk drugs [170].
Over the last several decades of research on understanding malignant brain tumors’ etiology, there has only been limited successes in the development of treatments. Typically, glioma patients are treated with surgical re-section of the tumor site, chemotherapy, and radiation, which can result in severe side effects [171]. Due to the CNS’ structure, the tumor’s location reduces the efficiency and preciseness of therapeutic interventions to cure the disease entirely; therefore, recurrence is inevitable. In 2005, temozolomide was widely implemented for chemotherapy of newly diagnosed glioblastoma, yet uncertainty has been found in the survival of glioblastoma patients [172,173]. Newly diagnosed GBM with full resection of the tumor is treated with temozolomide and radiotherapy [23]. In most patients, GBM resulted in a worsening effect and did not ensure a healthy life after relapse [174]. Therefore, the light treatment of patients with GBM are considered and significant interest is being directed to develop new therapy techniques for this disease.
6.1.1. Cellular Immunology for Malignant Gliomas
To date, even with decades of extensive experimental work, only a modest increase in patient life expectancy has resulted, even in the most promising clinical trials. The mechanisms underlying these small developments are still poorly understood. Interestingly, GBM possesses unique immunosuppression mechanisms; for instance, GBM infrequently shows metastasis to extracranial sites, even though circulating tumor cells (CTCs) have been detected in GBM patients [175,176,177]. Glioma grows surrounded by the BBB and BBTB that provides a reliable shielding mechanism from the immune system. Hence, it is also challenging for the transmigration of immune cells [178]. However, when T cells are in an inactivated state, they can cross an intact BBB and BBTB [179]. Furthermore, the BBB is compromised in GBM because of amplified fenestrations, disruptions in tight junctions, and low BBB-connected pericytes [180,181]. Therefore, the success rate of immunotherapy of glioma was limited until now. Gliomas with reduced anti-glioma immune responses expressed interleukin-10 (IL-10) and TGF-β [182]. For instance, TGF-β constrains the proliferation pathways and T cell activation, suppresses lytic enzymes production, and initiates the expansion of naive T cells into regulatory T cells [183,184]. Under physiological conditions, regulatory T cells are needed to shield against autoimmune diseases. Nonetheless, Tregs have been suggested to be the foremost contributor to depressed cellular immunity in glioma patients [185]. Furthermore, vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) are considered indispensable mediators in glioma angiogenesis and can induce expression of the inhibitors for cell adhesion molecules [22] in non-glioma cells in both animal models and endothelial cells extracted from normal tissues [186,187,188,189]. Dendritic cells (DCs), which originate from myeloid cells, are the most potent APCs and usually represent tumor-derived epitope peptides. Furthermore, these cells play an essential role in producing histocompatibility-complex (MHC) peptides. When MCH peptides can activate T cells, which then expand clonally and travel to the tumor-containing organs where they recognize antigen epitopes on tumor cells in an MHC/peptide complex similar to the TCR [98]. Thus, tumor cells are guided to activated T cells, which can release preformed cytotoxic molecules such as granzyme and perforin [190]. A more detailed schematic illustration is shown in Figure 3.
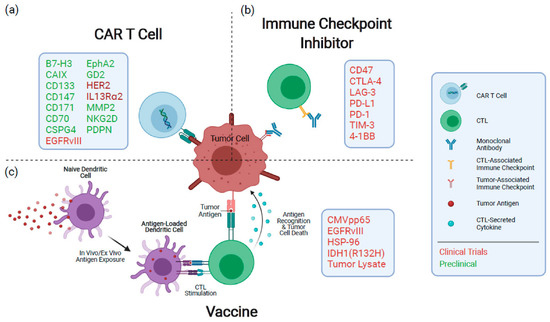
Figure 3.
An overview of the immunotherapeutic techniques currently under investigation for the treatment of GBM. (a) CAR T cells identify antigens via a genetically designed extracellular receptor that, following antigen binding, induces intracellular T cell activation and degranulation. (b) Immunological checkpoint protein inhibitors limit the dampening of immune responses during activation and exhaustion. (c) Vaccines expose antigen-presenting cells to tumor antigens, inducing an immune response specific to the target antigens. Therapeutic targets or mediators being pursued for each modality are denoted in the boxes. CAR: chimeric antigen receptor; CTL: cytotoxic T cell. Reproduced with permission from [191].
6.1.2. Vaccine-Based Immunotherapy of Glioblastomas
Numerous vaccine-based immunotherapy techniques, such as altering glioma cells, dendritic cell application, peptide-based vaccinations, and combination approaches with various therapeutic modalities, have been explored extensively in rodent models [192]. The most prevalent targets in glioblastomas are mutant IDH, EGFRvIII, a panel of antigens, or personally selected antigens. In forty percent of glioblastoma cases, the EGFR gene is amplified, and in more than fifty percent of cases, exon 2–7 is deleted or mutated [192,193]. The mutated form of the protein lacks a ligand-binding domain, resulting in constitutive activation, which in turn promotes cancer. Likewise, the mutant receptor can be activated by a variety of kinases, including Src family kinase [193]. The altered amino acid sequence has been revealed to be immunogenic. A vaccine called rindopepimut, generated using the mutated peptide sequence, was developed to prompt the immune system [192]. The early clinical trials indicated benefits from the vaccine. However, the subsequent phase III clinical trials failed to indicate benefits in a general population [157]. In another clinical trial, named ReACT, patients with recurring glioblastoma received a control or rindopepimut. The vaccine presented benefits over the control, as the medium survival rate was 12 months versus 8.8 months, respectively [194]. Utilizing NPs can increase the efficiency of the vaccine. They can protect the vaccine against degradation and boost the absorption by APCs. Kuai et al. created nanodiscs using lipids and peptides derived from high-density lipoprotein. Antigen peptides and cholesterol were attached to the surface at that time. In mice with melanoma tumors, the nanodiscs were more effective in priming T cells and the tumors took longer to manifest. In tumor-bearing mouse models, nanodiscs plus anti-PD-1 treatment inhibited the progression of cancer in 88% of animals, which is much greater than for either treatment alone [195]. Vaccines can also be administered as mRNA. Liu et al. created lipid/calcium/phosphate nanoparticles with MUC1 mRNA. The immunizations were subsequently administered alongside the anti-CLTA-4 antibody to mice with triple-negative breast cancer. The dual treatment provided a superior response than either treatment separately [196].
6.1.3. Cargo-Loaded NP-Based Immunotherapy of Glioma
The delivery of therapeutic agents based on cargoes that induce immune responses for glioma treatment is fascinating. An extended narration highlighting this topic is given in the recent online article “Glioblastoma is ‘hot’ for personalized vaccines” [197]. In these aspects, nanotechnology may perhaps play a vital role in engaging immune cells. The current barrier in glioma therapy can be tackled with nanomedicines based on immune-therapy using nanoparticles [198]. This study has critical shortcomings, lacking the induction of cytotoxic anti-tumor responses from T cells, rather than merely drug delivery in tumor diseases. Therefore, a practical, precise cancer vaccine could be developed via administrating immune-modulatory antigens and agents. Moreover, the cancer vaccines capable of targeting and activating T cells directly are biological compounds and molecular cargoes such as immune checkpoint inhibitors, suppressors of TME modulation, RNAi, nucleic acids, and adjuvants [199,200,201]. So far, different nanostructure materials have been used as cargo-loaded adjuvants and drugs (presented in Figure 3). Herein, various targeting steps of nanomedicines are described using different strategies for activating the immune process in the cell-mediated immunity targeting glioblastomas and other CNS tumors.
Babak et al. [202] developed nano-tubes with multiple layers of carbon. The tube delivers siRNA and DNA cargoes into GL261 glioma and BV2 microglia. With an inert nature, the nanoplatform is also biodegradable and non-toxic and has advantages for brain tumor immunogenetic therapy. Moreover, APCs can efficiently internalized nanoparticles to develop an immune-stimulatory cascade in the brain’s cancerous cells [203].
Recently, many therapeutic approaches have been designed to synergistically treat gliomas. For instance, Qiao et al. [204] have replicated the nano-theragnostic response of reactive oxygen species (ROS) polymers of nanoparticles. The compounds loaded with Angio pep LipoPCB (TMZ+BAP/siTGF-β) (ALBTA), which is composed of iron oxide NPs (IONPs), TMZ drug, and siRNA targeting TGF-β, an immunosuppressive cytokine. For crossing the BBB, Angiopep-2 was changed on the surface of the NPs. The overall endurance of mice was increased by improving the immunosuppressive micro-environment. The frequent correlation of expression of vascular laminin-411 (α4β1γ1) with a tumor with a higher expression of cancer stem cell markers (e.g., Notch, CD133, nestin, c-Myc) shortens the lifespan of GBM patients. A nano-bioconjugate that inhibited laminin-411 crossing the BBB, inhibited markers of stem cells, and targeted the TME resulted in the increased survival of mice with cephalic cancer [205]. On the other hand, in immunotherapy, the low accruement of glioma antigens by APCs acts as a barrier. Recently, to address this issue, a “cluster bomb” nano-vaccine was developed with a high-loading-capacity antigen carrier with zinc oxide and triblock-copolymer nanoparticles on the surface [206]. The main chain reduction-sensitive polymer MPSDP can react with dithiopyridine in the Polydopamine (PDA) that is blocked by the sulfhydryl group; the self-assembled MPSDP polymers resemble “cluster bomb” nano vaccines, which are then spoiled by the hydrophilic–hydrophobic interaction. Due to the existence of multiple interactions in the nano-vaccines, the adjuvant to cellular and humoral immunity is promoted. The nano-vaccine with a three-fold reduction response can trigger the vaccine to bomb in antigen-presenting cells. Cytotoxic T lymphocytes and antibody responses to cytokine secretion is promoted by CD8+ vigorously. A multi-dimensional platform used for nanoparticles improves the accumulation of drugs targeting the immune system at the cellular and humoral levels, which improved the survival of mice with tumors [206]. The combination of T-cell activators that have anti-tumor activity, such as α-galactosyl ceramide, with C6 glioma-derived exosomes to treat rat models with GBM was investigated by Liu et al. [207]. The induction of an amenable immune response, including expression of IFN-γ and TNF-α, was increased after vaccine-based immunotherapy.
The nanotechnology approach is combined with therapy using chimeric antigen receptor (CAR)-T cells. This approach is the cutting edge of treatments of solid tumors. For instance, Zhang et al. [208] proposed that the TME assists iRGD-lipid nanoparticles in upgrading the perseverance and function of transferred CAR-T cells in glioma patients. An objective of lipid nanocarriers is to induce therapeutics in glioma tumors. On one hand, it can remove the protumor cell populations (“releasing the brakes”), while on another, it can stimulate the essential antitumor effector cells (“stepping on the gas”). Recently, Shi et al. [209] developed polymersomes coated with Angiopep-2 and anti-PLK1 siRNA payloads. In mouse studies, it has not only improved BBB permeability, but also greatly stimulated anti-GBM activity. In addition, another study demonstrated that folate-targeted polymeric micelles can deliver TMZ and anti-BCL2 siRNA in rat models with orthotopic glioma. Intracerebral administration of this nanocarrier-based combination therapy inhibited tumor development and prolonged survival [210].
Recently, Galstyan et al. [211] demonstrated the utilization of a poly (-L-malic acid) natural biopolymer scaffold to which a-CTLA-4 or a-PD-1 nanoscale immunoconjugate targeting moiety was covalently bonded (NICs). NICs are utilized to systemically transfer payloads across the BBB and to stimulate local anti-tumor immune responses in the brain. NICs were used to treat intracranial GL261 glioblastoma (GBM) with an increase in CD8+ T cells, NK cells, and macrophages, and a decrease in regulatory T cells (Tregs) in the microenvironment of the brain tumor. On the one hand, the tumor-targeted polymer-conjugated NICs function as checkpoint inhibitors as a prospective GBM treatment by stimulating the systemic and local brain tumor immune responses, which are associated with increased survival in mouse models. Therefore, the proliferation of tumor cells is attenuated, resulting in enhanced survival rates.
6.1.4. Nanomedicine-Based Combination Therapy
The complex immunosuppressive tumor microenvironment (TME) in glioblastoma overwhelms endogenous antitumor immune activity, resulting in increased immunological tolerance [212]. Recently, immune-stimulating non-methylated oligonucleotides (such as CpG) have been created to tackle this issue, boosting long-term immunity against resistant tumors [213]. By preventing long-term relapses, Lollo et al. have achieved the highest therapeutic index against GBM by combining chemotherapy with immune-stimulating CpG-mediated immunotherapy [214]. PTX/CpG co-loaded lipid nanocapsules dramatically increased the survival rate of orthotopic GL261 glioma-bearing mice compared to single-loaded PXT in the same system without CpG. Consequently, they noticed a greater anti-glioma efficacy with the combined chemotherapy and immunotherapy than with chemotherapy alone.
Recent studies have also shown that specific tumor cells escape the immune system’s elimination through modulation of immune checkpoint pathways [215]. indoleamine 2,3-dioxygenase (IDO) is abundantly expressed in brain tumors and is recognized as one of the most important immune checkpoint receptors. It is a key immunotherapeutic target for numerous brain cancers [216,217]. Recently, 1-methyltryptophan (1MT), which is a specific competitive inhibitor of IDO, has been shown to inhibit IDO expression and slow down tumor cell growth [218]. However, to date, 1MT has not been proven as an efficient targeting agent. Therefore, few research groups have combined 1MT with other drugs for effective targeting [219]. For instance, Kuang et al. [220] developed a nano-strategy of co-delivery of 1MT with DOX on mesoporous silica NPs (MSNPs) modified with tumor-targeting/penetrating peptide CRGDK/RGPD/EC (iRGD) for therapeutic application to orthotopic gliomas.
Recently, Kadiyala et al. [221] created nanodiscs modified with a high-density lipoprotein-mimicking nano-formulation (called sHDL nanodiscs) that could not only carry glioma tumor-specific antigens, but also operate as a vehicle for the administration of adjuvants and bioactive compounds. To target glioma both in vitro and in vivo, sHDL nanodiscs were loaded with CpG deoxynucleotides, a Toll-like receptor 9 (TLR9) agonist, and a chemotherapeutic, namely docetaxel (DTX). The combined chemo-immunotherapy delivery system utilizing DTXsHDL–CpG nanodiscs not only targeted the drugs to the tumor site, but also activated antitumor immune responses and prevented the recurrence of the tumor due to the increased delivery of bioactive compounds to immune cells surrounding the glioma. The delivery of payloads in DTXsHDL–CpG nandiscs into the tumor mass induced tumor relapse and improved antitumor CD8+ T cell responses in the immunosuppressive TME of the brain. The standard of care for glioblastoma multiforme (GBM) combined with the DTX-sHDL-CpG treatment led to tumor degradation and considerable improvement in the median survival of glioma-bearing mice up to 80%. In addition, galectin-1-targeting siRNA encapsulated in an intranasal chitosan nanoparticle is a promising option for enhancing the efficacy and dependability of chemotherapy and immune checkpoint suppression in order to increase the life expectancy of mice with tumors [222]. This intranasal siGal-1 delivery induced a dramatic alteration in the composition of the tumor microenvironment (TME), including a decrease in myeloid suppressor cells and regulatory T cells, and an increase in CD4+ and CD8+ T cells, during the progression of GBM. A therapy combining siGal-1 with TMZ or an immunotherapeutic strategy (such as dendritic cell immunization and PD-1 blockade) exhibits synergistic effects.
6.1.5. Gene Therapy for Glioma
Gene therapy appeared as a new treatment for many human cancers. It could be specially used to control the oncogenes in anti-tumor treatments [223,224]. The adenovirus-facilitated gene therapy with sitimagene, ceradenovec, and ganciclovir, and later resection, improved the survival time of patients with recently identified glioblastoma multiforme [225]. Therefore, gene therapy was commonly offered as a valuable support for recent glioblastoma treatments [226]. The combination of gene therapy with immunotherapy must overcome the full consequences of glioblastoma cure [227,228]. For example, Maria-Carmela Speranza et al. used a non-replicating adenovirus with the HSV TK gene to potentiate the anti-PD-1 effect in a syngeneic glioblastoma mouse model. The HSV TK, also known as AdV-tk, increased PD-L1 levels and cytotoxic CD8+ T cells were induced to localize in the tumors. This combination therapy increases the survival of animals from 30–50% to 88% [229]. Although a possible gene therapy, the basic features of this gene restricted the effective transfer to cancer locations and delayed their development for clinical uses [230,231].
Transport across the BBB presents an additional obstacle for anti-glioma therapies. Nanoparticles can compensate for gene deficits and carry gene and immunotherapeutic drugs to brain tumors safely. Gulsah et al. developed a cyclic peptide iRGD ornate solid–lipid nanoparticle to transport siRNA against EGFR and PD-L1 for combination therapy. The siRNA-targeting nanoparticles decreased EGFR levels by 54.7% and PD-L1 by 58.6%. Additionally, the average lifespan of mice treated with f(SLN)-iRGD: siRNA by radiation increased to 38 days. These three combinations significantly increased the survival rate of mice.
Gene therapy was frequently used to modulate immunosuppressive signals and enhance systemic therapy in glioma treatments, according to the available evidence. These could increase the immune response to immunotherapy in a synergistic manner. However, there are few published studies on this strategy. Current anti-glioma treatments typically include gene therapy and immunotherapy as supportive therapies. Moreover, the paucity of genes and antigens remains the most significant hurdles to an effective therapy.
6.1.6. Chemotherapy
Historically, Egyptians were the first to use chemotherapy, and proper exploration of chemotherapy started when some soldiers in World War II who were exposed to some chemicals started showing unusual reactions. Currently, chemotherapy is one of the most established clinical therapies, which is due to its convenience and several chemotherapeutic drugs are FDA-approved [232]. Chemotherapeutic drugs disrupt cellular function at one or all phases of the cell cycle. Some of the key chemotherapy agent categories are: antimetabolites, plant alkaloids, alkylating agents, and anti-tumor antibiotics. As cancer is a complex disease, multiple complicated mechanisms are involved, such as the growth, progression, and invasion processes [233].
CNS tumors are neoplasms arising from numerous kinds of cells within the CNS, which account for 2% of all cancers. Every year, out of a hundred thousand, about nineteen individuals are diagnosed with primary brain tumors and CNS tumors, worldwide. For example, GBM is one of the utmost violent and common cancers occurring in the central nervous system. In 2005, a study reported a 27.2% survival rate with oral temozolomide (TMZ; an alkylating cytotoxic agent) and concurrent radiotherapy, while the survival rate was only 10.9% with radiotherapy alone [234].
However, accumulating data suggest that, in the case of single curative strategy, it results in drug resistance and gives rise to tumor cell tolerance, leading to tumor recurrence and metastasis. Considering the associated complications, combination chemotherapy is being used in several cancers. Therefore, therapeutic strategies combined with various agents should be able to overcome these problems. Still, these therapeutic agents have some shortcomings, such as insufficient accumulation, controlled transportation, and release at the tumor site and short half-life in circulation [235].
In GBM treatment, to overcome physiological barriers and potentiate the therapeutic effects, nanotechnology has been investigated as a new approach. For instance, delivery systems that have been investigated include: liposomes, lipopolymer nanoparticles, dendrimer nanoparticles, polymer nanoparticles, and hybrid nanoparticles [236]. In the case of GBM, nano-delivery system transport of multiple therapeutic agents across the BBB mediated by adsorptive- or receptor-mediated endocytosis or carrier-mediated transport, can improve tumor targeting in the brain and reduce side effects [237]. In addition, the introduction of stimuli-sensitive responses into delivery systems can ensure the maximal drug retention at the desired sites [238]. In the last few decades, intensive progress has revealed effective and promising results in cancer therapy, but only a small amount of them have been applied in clinical trials.
6.2. Alzheimer’s Disease (AD)
The most common neurodegenerative diseases are AD and PD, from which millions of people are suffering worldwide [239]. Regardless of all the scientific advancements, the currently available therapeutics have low efficiency in the amelioration of these diseases. The significant hurdles come with halting disease progression and late-stage diagnosis. In contrast, the only symptomatic treatment without modifying these diseases’ progress is the current state-of-the-art therapeutics. The causes of AD are known to be either extracellular amyloid-beta (Aβ) peptides deposition in senile plaques or the formation of neurofibrillary tangles with phosphorylated tau proteins [158,240]. Another pathological hallmark includes the tauopathies caused by hyperphosphorylation of tau protein deposits and its insoluble aggregates inside neurons in CNS disorders [241]. As a result, the AD-suffering patient may progressively lose memory, develop problems with proper functioning in a physical environment, fail to make decisions, and develop language difficulties as the most domineering clinical hallmarks of this disease [242].
Many nano-formulations have produced positive results in their impact on AD patients. The conformation using PEG stabilization of nano-micelles comprised of lipids with phosphate lessens the aggregation of Aβ, devalues neurotoxicity induced by Aβ in human SHSY-5Y cells, and an in vitro neuroblastoma cell line [243]. An experiment in mice showed meagre bioavailability, even though the phytochemical curcumin was able to lessen cytotoxicity and oligomerization [244]. The bioavailability enhanced, but did not harm, Aβ’s aggregation ability by non-liposomal deviation of curcumin [245]. Excess metal ion-like copper usage plays a role in the pathology of AD; thus, chelating agent usage is another technique for managing AD [246]. Microemulsion of nanoparticles with copper d-penicillamine as a chelating agent showed a profound ability to cross the BBB and soften Aβ aggregates in vitro [247]. Furthermore, in the pathology of AD, oxidative damage is another key factor that suggests the application of antioxidants in the management of AD. It has a neuroprotective effect against glutamate receptors that induce excitotoxicity. This can be achieved using derivatives of fullerene with the ability to act as scavengers of free radicals. In addition, neuroprotection by fullerene against Aβ toxicity has not yet been proved. However, an ability to inhibit Aβ peptide fibrillization and prevent Aβ from inducing cognitive injury after intraventricular administration suggests a beneficial role for fullerene for AD treatment [248].
Another feature of the pathology of AD is the obvious scarcity of the neurotransmitter, acetylcholine (ACh). ACh breaks down promptly in the blood if it is directly administration to the body. An approach using nanotechnology is to deliver ACh directly to the brain to balance the ACh levels. Nanotubes filled with ACh significantly restored cognitive function to pre-AD levels in a kainic acid mouse model compared to free Ach [249].
Generally, Aβ peptide is produced by several CNS cells, including neurons, as the result of the proteolysis of amyloid precursor protein (APP) [250]. The β- and γ-secretases are responsible for cleaving APP to form Aβ fragments with differing numbers of amino acids, ranging from 36 to 42 [251]. Still, the exact origin of the formation of Aβ peptides, which can accumulate in the brain, is not understood [252]. However, some transport molecules can be transported across the BBB with the help of the apolipoprotein family (Apo-E2/3 and Apo-E4) and two efflux pumps [253,254]. Moreover, the LRP and the very-low-density lipoprotein receptor (VLDLR) [255] are the primary transport methods to cross the BBB.
The available approved drugs for AD treatment include donepezil, galantamine, and rivastigmine, which can target metabolic shortfalls [256]. Nevertheless, these drugs are also associated with brain function impairment. Typically, these drugs may be classified as either targeting the acetyl-cholinesterase inhibitors (AChEI) or N-methylD-aspartate (NMDA) receptor [257]. The deposition of Aβ triggers a hypothetically distinct pathological immune response in AD [258]. Interestingly, the microglial cells have natural machinery for removing protein aggregates and debris from the brain [259]. Several routes play a role in the clearance of amyloid-β from the resident microglial cells. The activation of microglial cells might clear the amyloid-β after immunization, as after a stroke or amyloid-β injection [260]. The clearance of amyloid-β is associated with higher microglial cell activity [261]. Recently, reports suggest that, the mice that overexpressed transforming growth factor-β (TGFβ1) were crossed with APP-transgenic mice to produce offspring with reduced amyloid loads and amplified microglial cell activation [262]. Moreover, blocking of complement evasion or activation of pathways to decrease microglial cell activity has also been observed in APP transgenic mice [263]. A detailed description of how immune modulation may play an important role in degrading and clearing NFTs is well addressed by Winner et al. [264].
Recently, Liu et al. reported applying a zwitterionic poly(carboxybetaine) (PCB)-based nanoparticle co-loaded with fingolimod, siSTAT3, and zinc oxide into the polymeric NPs named as MCPZFS NPs [265]. The NPs inhibited microglia and Aβ recruitment for the effective treatment of AD. The MCPZFS NPs significantly improved microglia priming by reducing the level of proinflammatory mediators and promoting the secretion of BDNF (as shown in Figure 4). Notably, PCB-based NPs can increase the recruitment of Aβ into microglia, which can significantly improve Aβ phagocytosis, and when Aβ is degraded, NPs can enter the proteasomal pathway. Numerous major pro-inflammatory cytokines, including IL-1, interferon-γ (IFN-γ), IL-6, and interleukin 17A (IL-17A), were increased in the brains of APP/PS1 mice following administration of the nanomedicine system. The APPswe/PS1dE9 animals demonstrated a reduction in Aβ load, neuronal injury, cognitive impairments, and neuro-inflammation in the brain. Thus, the MCPZFS NPs have excellent potential to function as an “Aβ cleanser”, offering a fresh perspective on therapeutic strategies for AD treatment. Zhang et al. developed a glycosylated “triple-interaction” stabilized polymeric siRNA nanomedicine (Gal-NP@siRNA) that targets BACE1 in an APP/PS1 transgenic AD mice model. Gal-NP@siRNA demonstrated superior blood stability and efficiently crossed the blood–brain barrier (BBB) via a glycemia-controlled glucose transporter-1 (Glut1)-mediated transport, suggesting that siRNAs reduce BACE1 expression and modify metabolic pathways. Remarkably, Gal-NP@siBACE1 reversed the loss in cognitive ability in mice with Alzheimer’s disease without any significant adverse effects. This Trojan horse strategy validates the efficacy of RNA interference therapy for neurodegenerative disorders [130]. See Figure 5 and Figure 6.
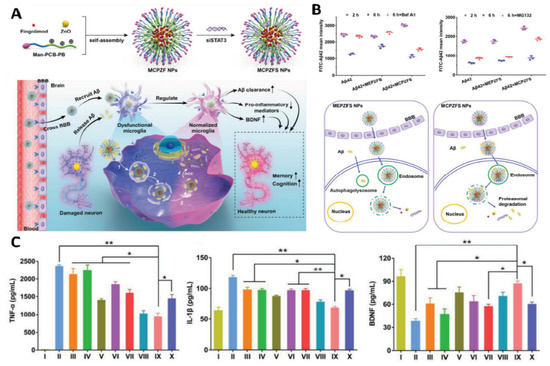
Figure 4.
Immune-modulating effect of a PCB-based zwitterionic nanoparticle for AD therapy. (A) MCPZFS NPs for AD therapy: structure, preparation, and mechanism. MCPZFS NPs can penetrate the BBB and endocytose into microglia cells to normalized malfunctioning microglia. Pro-inflammatory mediators were reduced. Microglia’s phagocytic capacity was restored when BDNF production rose. The injured neuron is then healed in numerous ways. (1) NPs endocytosed amyloid-β into microglia. (2) After perturbing the endosome/lysosome membrane, NPs leaked into the cytoplasm. (4) Finally, ROS-mediated release of fingolimod, siSTAT3, ZnO, and amyloid-β. (B) The effect of NPs on microglial phagocytosis and Aβ degradation after 2 h co-incubation with FITC-A42 and NPs. Flow cytometry was utilized to detect the degradation of A42 of BV2 in BafA1 or MG132 (top right) and the phagocytosis and degradation behavior mediated by MEPZFS NPs and MCPZFS NPs. (C) Representing the effect of NPs on the inflammatory regulation of microglia via the ELISA method was applied to determine the levels of TNF-α, IL-1β, and BDNF in the supernatants. Samples: (I) PBS, II) Aβ42, (III) Aβ42 + fingolimod, (IV) Aβ42 + siSTAT3, (V) Aβ42 + CPFS, (VI) Aβ42 + CPZS, (VII) Aβ42 + CPZF-siNC, (VIII) Aβ42 + CPZFS, (IX) Aβ42 + MCPZFS, and (X) Aβ42 + MEPZFS. Copyright, 2019, Wiley and Sons IncReproduced with permission from [265]. * p < 0.05, ** p < 0.01.
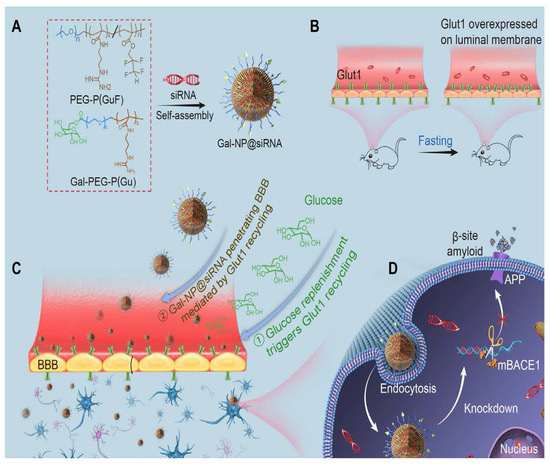
Figure 5.
Illustration of glycosylated “triple-interaction” stabilized siRNA nanomedicine (Gal-NP@siRNA) and strategy of treating AD pathology in APP/PS1 transgenic mice. (A) Schematic of Gal NP@siRNA manufacturing. (B,C) How Gal-NP@siRNA enters the brain and accumulates. 24 h fasting increases BBB luminal Glut1 expression. After treatment with Gal-NP@siRNA, glucose replenishment in fasting mice leads to Glut1 recycling from the BBB luminal to the abluminal membrane. (D) Gal-NP@siRNA-mediated BACE1 mRNA knockdown reduces amyloid plaques. Adapted with permission from [266].
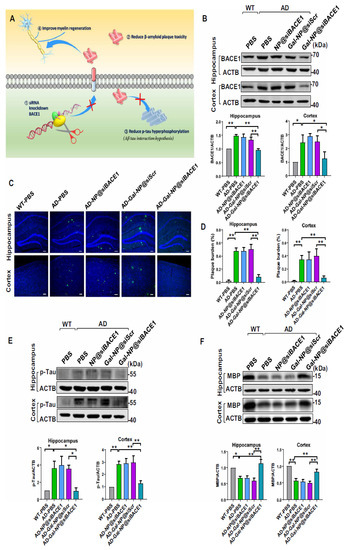
Figure 6.
Gal-NP@siBACE1 treatment modulates AD hallmarks in APP/PS1 mice. (A) A mechanism for siBACE1′s actions. (B) BACE1 protein expression in the hippocampus and cortex of nanocarrier-treated APP/PS1 mice, control APP/PS1 groups, and WT mice. BACE1 expression was quantified relative to actin (n = 3, mean ± SEM, * p < 0.05, ** p < 0.01). CLSM imaging data to assess amyloid plaque load. A plaque (green) in APP/PS1 transgenic and WT mice hippocampus and cortex. DAPI staining nuclei (blue), 100 µm scale bars. (C) Representative confocal laser scanning microscopy (CLSM) imaging data are assessing the amyloid plaque burden. Immunofluorescence of Aβ plaques (green) in the hippocampus and cortex from APP/PS1 transgenic and WT mice. Nuclei were stained by DAPI (blue). Scale bars, 100 µm. (D) Amyloid plaques were measured in the hippocampus (left) and cortex [181] (n = 4, ** p < 0.01; mean ± SEM). (E) p-tau and (F) MBP expression in the hippocampus and cortex for nanocarrier-treated APP/PS1 mice, control APP/PS1 groups, and WT mice (top). Quantification of Western blotting analysis was relative to β-actin (bottom) (n = 3, mean with SEM, * p < 0.05, ** p < 0.01). All samples were collected after 10 administrations of nanomedicine. Adapted with permission from [266].
There are two immunization strategies, named active and passive immunization-based immunotherapy, for AD [267]. Active immunization is long-lasting, as it induces the immunological memory of the patients [268]. Active vaccines are the most straightforward way to administer vaccines (involving different routes), but their production is not cost-efficient [269]. Immunization produced a polyclonal response; multiple epitopes on the target protein surface with diverse affinity and avidity could be recognized and accessed through antibodies [270]. Alternatively, the immune response also strongly depends on the host immune system; therefore, various patients’ antibody responses may vary from individual to individual [271].
6.3. Parkinson’s Disease (PD)
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorder characterized by motor and non-motor (psychiatric, cognitive, sleep, and autonomic) features, affecting over 10 million people, worldwide [272]. The etiology of PD is mostly attributed to the genetics of individual patients or environmental factors [273]. However, the aggregates of α-synuclein protein (α-syn) form intracellular inclusions known as Lewy bodies, which are considered the neuropathological hallmark of PD [274]. Based on these facts, PD is also called an synucleinopathy and, similarly, includes multiple system atrophy (MSA), pure autonomic failure [41], and Lewy body dementia (LBD) [275]. Nanomedicine-based immunotherapies may offer a novel and alternative strategy for enhancing the immune response against synucleinopathies and Lewy body dementia [276]. Cell-based therapies are the recent research frontier, which has provided an enhanced understanding of the disease, and, possibly, some induced pluripotent stem cell (iPSC) therapies could be used for personalized therapy [277,278].
The technique for gene delivery is also used in regards to PD. The common methods for gene delivery involve vectors of a virus to induce toxicity and immunogenicity. Conversely, an approach using nanotechnology is free from such problems [279]. The complexes composed of polyethyleneimine nanogels and PEG with antisense oligonucleotide efficiently crossed the BBB in vitro, when a functional gel with insulin or Tf molecules was injected into a vein [280]. In another study in a rat model of PD, using 6-hydroxydopamine (6-OHDA) tyrosine hydroxylase, and Tf receptor antibodies combined with PEGylated liposomes reversed impairment after a single administration into the veins. A recent investigation clarified that growth factor for nerves binds with PBCA nanoparticles [281], and L-Dopa-capsulated nanoparticles crossed the BBB to treat prominent basic signs and symptoms of PD [282]. Nanoparticles such as mPEG PLGA with a size of 70 nm, e.g., Schisantherin A (SA), were used to combat Parkinson’s diseases (PD) in the larvae of zebrafish. The SA encapsulated in nanoparticles increased in circulation and uptake by the brain to provide an efficient treatment for PD. SA delivery is more effective than a suspension of SA alone. In conclusion, SA NPs play a neuroprotective role in zebrafish and cellular models of PD.
Recently, PD’s effects on the immune system and microglia have been characterized, which showed that microglia are the major scavenger for extracellular α-syn aggregates, resulting in an increased inflammatory response. These responses were mediated by proinflammatory cytokines including interleukin 1-β (IL 1-β), interleukin-6 (IL-6), interferon γ (INF-γ), and tumor necrosis factor-alpha (TNF-α) [283]. Currently, the gold standard for PD treatment continues to be orally administered dopamine agonists, such as levodopa [284]. Numerous neuro-inflammatory effects, including microgliosis, astrocytosis, and infiltration of T leukocytes, have been shown in the midbrain of PD patients and rat models [285]. A considerably high amount of pro-inflammatory cytokines (TNF-α, IL-1β, and interferon-γ) are released, and oxidative stress and proinflammatory markers such as reactive oxygen species (ROS) and nitric oxide (NO) are produced; in PD, activated astrocytes and microglia are usually linked with BBB impairment [286].
Currently, there is no therapeutic strategy available to treat PD. However, some tentative treatment strategies have been proposed including reducing α-syn expression with either small interfering RNAs (siRNA) or anti-sense RNA and reducing α-syn aggregation using small molecules. In addition, increasing the clearance of α-syn with drugs that promote autophagy prevents the seeding and prion-like spreading of α-syn [287,288]. On the other hand, some immunotherapy studies have shown that vaccination against α-syn reduced α-syn accumulation through activation of autophagy [289] or microglial pathways [290]. Furthermore, specific passive or active immunization strategies such as those with monoclonal antibodies can recognize the epitopes of non-amyloid β component (NAC) and C-terminus of α-syn ameliorated the behavioral deficits and α-syn accumulation in neurons [291] and glial cells [292]. Toll-like receptors (TLRs) initiate innate immune responses through mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-ҝB) signaling pathways. Microglial cells with strong TLR-induced α-syn stimulate responses, such as TLR2/1- and TLR7-mediated responses, by supplementing the excretion of IL-6 and chemokine immune checkpoint proteins [293].
Recently, several studies used fluorescent quantum dots to inhibit amyloidosis [68]. Graphene quantum dots (GQDs) have shown potential in inhibiting α-synuclein and amyloid pathogenesis [294]; the GQDs efficiently penetrated the BBB due to their small size and antibodies on their surfaces. After a treatment lasting only 7 days, the aggregation and dissembling process has abolished. Moreover, the use of GQDs has a protective role against dopamine neuron loss that is caused by α-synuclein fibril or aggregation formation. The protection of dopaminergic neurons exposed to alpha-synuclein inhibited the formation of fibrils. These GQDs can interact with alpha synuclein fibrils in vitro, to stop their development and even promote their disaggregation. Therefore, if it is considered safe, this kind of nanomedicine could be the magic bullet for PD and other neurogenerative diseases.
7. Huntington’s Disease
Progressive motor, cognitive, and psychiatric symptoms describe Huntington’s disease (HD), which is characterized by neuronal loss in the striatum and other brain regions. A mutation in exon 1 of the Huntington gene causes polyglutamine expansion (poly Q), as well as misfolding and accumulation of the Huntington protein (HTT) in the brain [295]. Numerous simulations have characterized the function of astrocytes in HD. Mutant HTT accumulates in striatal astrocytes in the brains of HD patients and HD mouse models, leading to age-dependent HD-like illness and early mortality. There are indicated and preventative treatment options for HD, but none of them are effective enough to entirely cure the disease. Tetra-benazine is currently the only FDA-approved medication for HD treatment [296].
A probable connection between oxidative stress and neurodegenerative disorders, including HD, has been revealed in the literature [297]. Therefore, antioxidant treatment can work in HD to avert oxidative stress. Fullerenes have the capability to clear ROS and decrease oxidative load in cells. Jin et al. described their aggressive behavior on glutamate receptors and, consequently, they can be used for neuroprotective purposes [298]. Nitrendipine is a calcium channel blocker that reduces the incidence of dementia in HD patients by up to 50% over a two-year period. Due to its hydrophilic nature, this medication has absorption issues and, therefore, cannot efficiently cross the BBB. SLNs of nitrendipine were labeled, and a comparison was made between the uptake of bulk medication and nano-formulations. The results reveal that the drug was absorbed more efficiently when encapsulated in SLNs. In addition, short-interfering RNA (siRNA)-encapsulated cyclodextrin nanoparticles that inhibit HTT mRNA expression in vivo and in vitro were investigated [299].
Solid–lipid NPs conjugated with curcumin were used to target mitochondrial imperfections (affecting complex II activity) as a treatment for HD. In the same experiment, 3-nitropropionic acid upregulation of Nrf2 mRNA levels was also established [300]. Another method conjugated rosmarinic acid (RA) with solid–lipid NPs targeting Huntington’s disease, based on RA’s brain-targeting efficacy [301]. Recently, in a Caenorhabditis elegans model of HD, Cong et al. used selenium (Se) as a targeted delivery system. Se and selenoproteins were observed to act as neuro-protective compounds and neurodegenerative pathway regulators. Selenium deficiency in the brain confirmed direct correlation with mutant Huntington aggregation, an increase in oxidized glutathione levels, and brain dysfunction [302]. Se is known to reduce oxidative stress in tissues and reduced levels or deficiency of Se is associated with many neurodegenerative disorders, including HD; thus, Se can be considered a therapeutic agent in the future, specifically in a particular formulation. At the present time, many investigations are ongoing to identify new therapeutic targets for the treatment of HD, which is an urgent requirement as Huntington’s disease poses an enigmatic threat to patients.
8. Ongoing Clinical Trials and Approved Nanomedicines
The more than 250 US FDA-approved nanodrugs are available on the market is evidence of the success of NPs in clinical trials. There are several interesting drugs for treating multiple myeloma, schizophrenia, lymphomatous meningitis, and MS, including Doxil (doxorubicin HCL liposome injection), Invega Sustenna (paliperidone palmitate), DepoCyt (liposomal cytarabine), and Plegridy (PEGylated interferon B-1a) [303]. Over 33% of the drugs available on the market are in the form of liposomal formulations, which have been identified as the most commonly used nanodrugs [304]. Furthermore, NMs have been studied in a number of clinical trials to establish their relevance in therapeutic settings. In prior research, magnetic nanoparticles and decreased radiation were found to enhance overall survival in glioblastoma patients when compared to traditionally treated peers [305]. Furthermore, nanoparticles play an important role in reducing the toxicity caused by conventional drugs. The delivery capability and distribution of chemotherapeutics (e.g., temozolomide) in intracranial tumor regions in dogs using magnetic NPs should also be mentioned [306]. The most current research confirmed that treating migraine sufferers with curcumin nanoparticles and omega-3 fatty acids considerably lowered inflammation by inhibiting the production of TNF-a, ICAM-1, and COX-2/inducible nitric oxide synthase (Table 2) [307,308,309]. The evidence presented above suggests that NPs are capable of facilitating drug delivery, generating synergistic effects, and reducing the toxicities of drugs in brain diseases by enhancing drug delivery and inducing synergistic effects.

Table 2.
Treatment trials using NPs for brain diseases and disorders.
9. Conclusions
Recent breakthroughs in medicine, biochemistry, protein engineering, and materials science have led to nanoscale targeting techniques that can transform CNS-based therapeutics. Despite developments in nanotherapeutics, treatments for CNS disorders are not used in clinical practice. Conventional glioblastoma treatment encounters substantial tumor resistance, resulting in poor clinical outcomes. Many researchers are developing new therapeutic techniques to target tumor resistance and improve clinical outcomes. Nanobiotechnology may improve the delivery of immunological therapeutics for CNS-related disorders, such as antibodies, cytotoxic medicines, vaccination antigens, and siRNAs. Nanotechnology has helped immunotherapy reduce tumor or disease progression and improve patient survival. Some nanotechnologies convey synergistic medication combinations and improve innate and adaptive immune responses to fight glioblastoma. These approaches are more effective when using rationally designed nanoparticles with stimuli-sensitive nanoscale materials (i.e., pH and temperature) or inside water-soluble hydrogels and matrices, which can dynamically target immune cells to release the immunotherapeutic once it reaches a certain concentration in the TME. Few studies have examined the role of nano-delivery systems in stimulating innate and adaptive cellular immune responses in glioblastoma patients.
Cancer treatment requires stimulating the immune system without unmanageable side effects. Nanomedicines for glioblastoma immunotherapy require careful design. First, unique nano-immunomodulators are needed to activate macrophages and DCs. Nanocarriers with selective targeting ligands (proteins, peptides, and aptamers) or cell membrane-derived vesicles are designed to actively penetrate barriers and approach APCs. This glioma-specific design differs from standard drug carriers, which are changed (e.g., PEGylation) to avoid phagocytes. Glioblastoma is highly diverse, making it difficult to analyze clinical trial therapy efficacy. Understanding GBM pathogenesis is important when building nanomedicines.
Due to AD’s multiple physiological components and complexity, standard therapy techniques are ineffective. AD has limited treatment techniques despite long-term treatment and research efforts. Nanotechnology offers a helpful alternative to advance research by modulating pathways in specified regions. Specific amyloid antibodies mitigate toxicity and activate clearance pathways. Effective amyloid clearance relies on antibody-mediated or antibody-independent methods that target T cells or microglial cells with amyloid immunotherapy. Indirect activation of microglial innate immune receptors may elicit amyloid clearance without T- or B-cell responses.
Reducing amyloid levels can also entail non-immune strategies, including proteases that degrade toxic amyloid peptides. AD immunotherapy may be paired with nanomedicines or vaccinations. Current nanomedicines or vaccines are hazardous. More biocompatible nanoscale materials are needed to carry AD therapy from lab to bedside. To pass clinical trials, nanotechnology’s pharmacokinetics and toxicology must be improved.
Therapeutic approaches that inhibit α-syn production, toxicity, and aggregation make PD preclinical trials difficult. Synucleinopathies are hallmarks of PD disorders, but their complexity and structural variation make nanomedicine-based immunotherapy problematic. Syn may change conformation. Developing nanomedicine-based immunotherapy is difficult. Due to syn’s structural diversity based on environmental factors or patient variation, more careful designs for immunotherapy are needed. Small nanoparticle-based nanomedicines are reported to decrease PD progression; however, biosafety problems have prevented clinical studies.
Author Contributions
S.K. conceived the study. Z.-H.G., S.K. and M.A.R. conducted this study. S.K., M.A.R., M.A.A., M.N.A., S.R. and D.-D.W. drafted the manuscript. S.K., M.A.R., C.-Y.Y., D.-D.W. and X.-Y.J. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81802718, U1504817), the Foundation of Science and Technology Department of Henan Province, China (Nos. 192102310151, 202102310480), and the Training Program for Young Backbone Teachers of Institutions of Higher Learning in Henan Province, China (No. 2020GGJS038).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lammers, T.; Aime, S.; Hennink, W.E.; Storm, G.; Kiessling, F. Theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Tao, W.; Zou, Y.; Farokhzad, O.C.; Shi, B. Nanotechnology-based strategies for siRNA brain delivery for disease therapy. Trends Biotechnol. 2018, 36, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Tayyba, T. Role of extracellular vesicles in human diseases. Biomed. Lett. 2019, 5, 67–68. [Google Scholar]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Song, S.; Gao, P.; Sun, L.; Kang, D.; Kongsted, J.; Poongavanam, V.; Zhan, P.; Liu, X. Recent developments in the medicinal chemistry of single boron atom-containing compounds. Acta Pharm. Sin. B 2021, 11, 3035–3059. [Google Scholar] [CrossRef]
- Fernandes, G.F.S.; Denny, W.A.; Santos, J.L.D. Boron in drug design: Recent advances in the development of new therapeutic agents. Eur. J. Med. Chem. 2019, 179, 791–804. [Google Scholar] [CrossRef]
- Soriano-Ursúa, M.A.; Farfán-García, E.D.; Geninatti-Crich, S. Turning fear of boron toxicity into boron-containing drug design. Curr. Med. Chem. 2019, 26, 5005–5018. [Google Scholar] [CrossRef] [PubMed]
- Ritacca, A.G.; Ritacco, I.; Dabbish, E.; Russo, N.; Mazzone, G.; Sicilia, E. A Boron-Containing Compound Acting on Multiple Targets Against Alzheimer’s Disease. Insights from Ab Initio and Molecular Dynamics Simulations. J. Chem. Inf. Model. 2021, 61, 3397–3410. [Google Scholar] [CrossRef]
- Jiménez-Aligaga, K.; Bermejo-Bescós, P.; Martín-Aragón, S.; Csákÿ, A.G. Discovery of alkenylboronic acids as neuroprotective agents affecting multiple biological targets involved in Alzheimer’s disease. Bioorganic Med. Chem. Lett. 2013, 23, 426–429. [Google Scholar] [CrossRef]
- Lu, C.-J.; Hu, J.; Wang, Z.; Xie, S.; Pan, T.; Huang, L.; Li, X. Discovery of boron-containing compounds as Aβ aggregation inhibitors and antioxidants for the treatment of Alzheimer’s disease. MedChemComm 2018, 9, 1862–1870. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Burch, Z.N.; Flaherty, D.B.; Larkin, J.D.; Dunbar, G.L. Ameliorative Properties of Boronic Compounds in In Vitro and In Vivo Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6664. [Google Scholar] [CrossRef] [PubMed]
- Amiji, M.M. Nanotechnology for Cancer Therapy; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Athar, M.; Das, A.J. Therapeutic nanoparticles: State-of-the-art of nanomedicine. Adv. Mater. Rev 2014, 1, 25–37. [Google Scholar]
- Rehman, F.U. Nanomedicine: Why it still taking long from “bench to bedside”? Biomed. Lett. 2018, 4, 1373–1378. [Google Scholar]
- Chapman, C.D.; Frey, W.H.; Craft, S.; Danielyan, L.; Hallschmid, M.; Schiöth, H.B.; Benedict, C. Intranasal Treatment of Central Nervous System Dysfunction in Humans. Pharm. Res. 2013, 30, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Pridgen, E.M.; Langer, R.; Farokhzad, O.C. Biodegradable, polymeric nanoparticle delivery systems for cancer therapy. Nanomedicine 2007, 2, 669–680. [Google Scholar] [CrossRef]
- Wagner, V.; Dullaart, A.; Bock, A.-K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Ozdemir-Kaynak, E.; Qutub, A.A.; Yesil-Celiktas, O. Advances in Glioblastoma Multiforme Treatment: New Models for Nanoparticle Therapy. Front. Physiol. 2018, 9, 170. [Google Scholar] [CrossRef]
- Lie, D.C.; Song, H.; Colamarino, S.A.; Ming, G.-l.; Gage, F.H. Neurogenesis IN THE Adult Brain: New. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 399–421. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. New England J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef]
- Zanganeh, S.; Georgala, P.; Corbo, C.; Arabi, L.; Ho, J.Q.; Javdani, N.; Sepand, M.R.; Cruickshank, K.; Campesato, L.F.; Weng, C.H. Immunoengineering in glioblastoma imaging and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2019, 11, e1575. [Google Scholar] [CrossRef]
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.; Halliday, G.; Brundin, P.; Volkmann, J.; Schrag, A.; Lang, A. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef]
- Hanif, S.; Muhammad, P.; Chesworth, R.; Rehman, F.U.; Qian, R.-j.; Zheng, M.; Shi, B.-y. Nanomedicine-based immunotherapy for central nervous system disorders. Acta Pharmacol. Sin. 2020, 41, 936–953. [Google Scholar] [CrossRef] [PubMed]
- Behrens, S.; Rattinger, G.B.; Schwartz, S.; Matyi, J.; Sanders, C.; DeBerard, M.S.; Lyketsos, C.G.; Tschanz, J.T. Use of FDA approved medications for Alzheimer’s disease in mild dementia is associated with reduced informal costs of care. Int. Psychogeriatr. 2018, 30, 1499–1507. [Google Scholar] [CrossRef]
- Kelly, C.B.; Milligan, J.A.; Tilley, L.J.; Sodano, T.M. Bicyclobutanes: From curiosities to versatile reagents and covalent warheads. Chem. Sci. 2022, 13, 11721–11737. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.M.; Fell, M.J. Current approaches to the treatment of Parkinson’s Disease. Bioorganic Med. Chem. Lett. 2017, 27, 4247–4255. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kiss, L.; Mei, H.; Remete, A.M.; Ponikvar-Svet, M.; Sedgwick, D.M.; Roman, R.; Fustero, S.; Moriwaki, H.; Soloshonok, V.A. Chemical aspects of human and environmental overload with fluorine. Chem. Rev. 2021, 121, 4678–4742. [Google Scholar] [CrossRef]
- Madajewicz, S.; Chowhan, N.; Tfayli, A.; Roque, C.; Meek, A.; Davis, R.; Wolf, W.; Cabahug, C.; Roche, P.; Manzione, J. Therapy for patients with high grade astrocytoma using intraarterial chemotherapy and radiation therapy. Cancer 2000, 88, 2350–2356. [Google Scholar] [CrossRef]
- Buglioni, L.; Raymenants, F.; Slattery, A.; Zondag, S.D.; Noël, T. Technological innovations in photochemistry for organic synthesis: Flow chemistry, high-throughput experimentation, scale-up, and photoelectrochemistry. Chem. Rev. 2021, 122, 2752–2906. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Till, S.J.; Francis, J.N.; Nouri-Aria, K.; Durham, S.R. Mechanisms of immunotherapy. J. Allergy Clin. Immunol. 2004, 113, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, W.; Nam, J.; Moon, J.J.; Kim, B.Y. Immunomodulating nanomedicine for cancer therapy. Nano Lett. 2018, 18, 6655–6659. [Google Scholar] [CrossRef]
- Cifuentes-Rius, A.; Desai, A.; Yuen, D.; Johnston, A.P.; Voelcker, N.H. Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat. Nanotechnol. 2021, 16, 37–46. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordāo, M.J.; Joyce, J.A. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef]
- Brody, D.L.; Holtzman, D.M. Active and passive immunotherapy for neurodegenerative disorders. Annu. Rev. Neurosci. 2008, 31, 175. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Gao, C.; Wang, P.; Huang, J.; Qian, Y.; Guo, L.; Zhang, J.; Jiang, R. Correlation of circulating T lymphocytes and intracranial hypertension in intracerebral hemorrhage. World Neurosurg. 2017, 107, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Gunn, M.D.; Fecci, P.E.; Ashley, D.M. Brain immunology and immunotherapy in brain tumours. Nat. Rev. Cancer 2020, 20, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Rustenhoven, J.; Jansson, D.; Smyth, L.C.; Dragunow, M. Brain pericytes as mediators of neuroinflammation. Trends pharmacolo. Sci. 2017, 38, 291–304. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K. Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncol. 2018, 20, 184–191. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Julien, J.-P.; Rivest, S. Innate immunity: The missing link in neuroprotection and neurodegeneration? Nat. Rev. Neurosci. 2002, 3, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Lowther, D.E.; Hafler, D.A. Regulatory T cells in the central nervous system. Immunol. Rev. 2012, 248, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Wucherpfennig, K.; Chiocca, E.A. Immunotherapy for glioblastoma: On the sidelines or in the game? Discov. Med. 2017, 24, 201–208. [Google Scholar] [PubMed]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Farber, S.H.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in GlioblastomaT-Cell Exhaustion Signatures in Glioblastoma. Clin. Cancer Res. 2018, 24, 4175–4186. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Louveau, A.; Plog, B.A.; Antila, S.; Alitalo, K.; Nedergaard, M.; Kipnis, J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin. Invest. 2017, 127, 3210–3219. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Jackson, C.M.; Lim, M.; Drake, C.G. Immunotherapy for Brain Cancer: Recent Progress and Future PromiseImmunotherapy for Brain Cancer. Clin. Cancer Res. 2014, 20, 3651–3659. [Google Scholar] [CrossRef]
- Johnson, D.B.; Sullivan, R.J.; Menzies, A.M. Immune checkpoint inhibitors in challenging populations. Cancer 2017, 123, 1904–1911. [Google Scholar] [CrossRef]
- Tomitaka, A.; Kaushik, A.; Kevadiya, B.D.; Mukadam, I.; Gendelman, H.E.; Khalili, K.; Liu, G.; Nair, M. Surface-engineered multimodal magnetic nanoparticles to manage CNS diseases. Drug Discov. Today 2019, 24, 873–882. [Google Scholar] [CrossRef]
- Chakroun, R.W.; Zhang, P.; Lin, R.; Schiapparelli, P.; Quinones-Hinojosa, A.; Cui, H. Nanotherapeutic systems for local treatment of brain tumors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2018, 10, e1479. [Google Scholar] [CrossRef]
- Rauf, M.A.; Rehman, F.U.; Zheng, M.; Shi, B. The strategies of nanomaterials for traversing blood-brain barrier. In Nanomedicine in Brain Diseases; Springer: Berlin/Heidelberg, Germany, 2019; pp. 29–57. [Google Scholar]
- Lonser, R.R.; Sarntinoranont, M.; Morrison, P.F.; Oldfield, E.H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 2015, 122, 697–706. [Google Scholar] [CrossRef]
- Zhao, M.; van Straten, D.; Broekman, M.L.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Banks, W.A. From blood–brain barrier to blood–brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Oller-Salvia, B.; Sánchez-Navarro, M.; Giralt, E.; Teixidó, M. Blood–brain barrier shuttle peptides: An emerging paradigm for brain delivery. Chem. Soc. Rev. 2016, 45, 4690–4707. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Ali, I.U.; . Chen, X. Penetrating the blood–brain barrier: Promise of novel nanoplatforms and delivery vehicles. ACS Nano 2015, 9, 9470–9474. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, Y.; Feng, C.; Lee, A.; Yin, J.; Chung, R.; Park, J.B.; Rizos, H.; Tao, W.; Zheng, M. Charge conversional biomimetic nanocomplexes as a multifunctional platform for boosting orthotopic glioblastoma RNAi therapy. Nano Lett. 2020, 20, 1637–1646. [Google Scholar] [CrossRef]
- He, W.; Zou, Y.; Zheng, M.; Shi, B. Cell-derived biomimetic drug delivery systems for cancer therapy. Sci. Sin. Chim. 2019, 49, 1203–1212. [Google Scholar]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef]
- Rubinsztein, D.C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 2006, 443, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.L.; Lukacsovich, T.; Thompson, L.M. Animal models of polyglutamine diseases and therapeutic approaches. J. Biol. Chem. 2009, 284, 7431–7435. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Madamsetty, V.S.; Bhattacharya, D.; Roy Chowdhury, S.; Paul, M.K.; Mukherjee, A. Recent advancements of nanomedicine in neurodegenerative disorders theranostics. Adv. Funct. Mater. 2020, 30, 2003054. [Google Scholar] [CrossRef]
- Caccamo, D.; Currò, M.; Condello, S.; Ferlazzo, N.; Ientile, R. Critical role of transglutaminase and other stress proteins during neurodegenerative processes. Amino Acids 2010, 38, 653–658. [Google Scholar] [CrossRef]
- Aderibigbe, B.; Naki, T.; Owonubi, S. Graphene-Based Materials for Brain Targeting. In Handbook of Graphene Set, I-VIII; Wiley: Hoboken, NJ, USA, 2019; 225p. [Google Scholar]
- Ellermann, C.; Coenen, A.; Niehues, P.; Leitz, P.; Kochhäuser, S.; Dechering, D.G.; Fehr, M.; Eckardt, L.; Frommeyer, G. Proarrhythmic Effect of Acetylcholine-Esterase Inhibitors Used in the Treatment of Alzheimer’s Disease: Benefit of Rivastigmine in an Experimental Whole-Heart Model. Cardiovasc. Toxicol. 2020, 20, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Schenkman, M.; Moore, C.G.; Kohrt, W.M.; Hall, D.A.; Delitto, A.; Comella, C.L.; Josbeno, D.A.; Christiansen, C.L.; Berman, B.D.; Kluger, B.M. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol. 2018, 75, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Carradori, D.; Balducci, C.; Re, F.; Brambilla, D.; Le Droumaguet, B.; Flores, O.; Gaudin, A.; Mura, S.; Forloni, G.; Ordoñez-Gutierrez, L. Antibody-functionalized polymer nanoparticle leading to memory recovery in Alzheimer’s disease-like transgenic mouse model. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 609–618. [Google Scholar] [CrossRef]
- Sahebjam, S.; Sharabi, A.; Lim, M.; Kesarwani, P.; Chinnaiyan, P. Immunotherapy and radiation in glioblastoma. J. Neuro-Oncol. 2017, 134, 531–539. [Google Scholar] [CrossRef]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, ra111–ra147. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Bechet, N.B.; Battistella, R.; Pavan, C.; Xavier, A.L.; Nedergaard, M.; Lundgaard, I. Cisterna magna injection in rats to study glymphatic function. In Astrocytes; Springer: Berlin/Heidelberg, Germany, 2019; pp. 97–104. [Google Scholar]
- Morris, A.W.; Sharp, M.M.; Albargothy, N.J.; Fernandes, R.; Hawkes, C.A.; Verma, A.; Weller, R.O.; Carare, R.O. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 2016, 131, 725–736. [Google Scholar] [CrossRef]
- Thrane, V.R.; Thrane, A.S.; Plog, B.A.; Thiyagarajan, M.; Iliff, J.J.; Deane, R.; Nagelhus, E.A.; Nedergaard, M. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci. Rep. 2013, 3, 2582. [Google Scholar] [CrossRef]
- Medawar, P. Immunity to homologous grafted skin. II. The relationship between the antigens of blood and skin. Br. J. Exp. Pathol. 1946, 27, 15. [Google Scholar]
- Shechter, R.; London, A.; Schwartz, M. Orchestrated leukocyte recruitment to immune-privileged sites: Absolute barriers versus educational gates. Nat. Rev. Immunol. 2013, 13, 206–218. [Google Scholar] [CrossRef]
- Gutkin, A.; Cohen, Z.R.; Peer, D. Harnessing nanomedicine for therapeutic intervention in glioblastoma. Expert Opin. Drug Deliv. 2016, 13, 1573–1582. [Google Scholar] [CrossRef]
- Birks, J.S.; Evans, J.G. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2015, 4. [Google Scholar] [CrossRef]
- Furtado, D.; Björnmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the blood–brain barrier: The role of nanomaterials in treating neurological diseases. Adv. Mater. 2018, 30, 1801362. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Blood–brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2013, 33, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- van Assema, D.M.; Lubberink, M.; Boellaard, R.; Schuit, R.C.; Windhorst, A.D.; Scheltens, P.; Lammertsma, A.A.; van Berckel, B.N. P-glycoprotein function at the blood–brain barrier: Effects of age and gender. Mol. Imaging Biol. 2012, 14, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Fernández, T.; Martínez-Serrano, A.; Cussó, L.; Desco, M.; Ramos-Gómez, M. Functionalization and characterization of magnetic nanoparticles for the detection of ferritin accumulation in Alzheimer’s disease. ACS Chem. Neurosci. 2018, 9, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Chen, J.; Gao, J. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: Focus on recent advances. Asian J. Pharm. Sci. 2019, 14, 480–496. [Google Scholar] [CrossRef]
- Sharma, U.; Badyal, P.N.; Gupta, S. Polymeric nanoparticles drug delivery to brain: A review. Int. J. Pharmacol. 2015, 2, 60–69. [Google Scholar]
- Krol, S.; Macrez, R.; Docagne, F.; Defer, G.; Laurent, S.; Rahman, M.; Hajipour, M.J.; Kehoe, P.G.; Mahmoudi, M. Therapeutic benefits from nanoparticles: The potential significance of nanoscience in diseases with compromise to the blood brain barrier. Chem. Rev. 2012, 113, 1877–1903. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.F.; Meng, Y. Recent advances in immunotherapy for human glioma. Curr. Opin. Oncol. 2006, 18, 631–636. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards improvements for penetrating the blood–brain barrier—recent progress from a material and pharmaceutical perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Damkier, H.H.; Brown, P.D.; Praetorius, J. Cerebrospinal fluid secretion by the choroid plexus. Physiolo. Rev. 2013, 93, 1847–1892. [Google Scholar] [CrossRef] [PubMed]
- Strazielle, N.; Ghersi-Egea, J.-F. Potential pathways for CNS drug delivery across the blood-cerebrospinal fluid barrier. Curr. Pharm. Des. 2016, 22, 5463–5476. [Google Scholar] [CrossRef]
- Xu, D.-H.; Yan, M.; Fang, P.-F.; Liu, Y.-W. Influence of P-glycoprotein on brucine transport at the in vitro blood–brain barrier. Eur. J. Pharmacol. 2012, 690, 68–76. [Google Scholar] [CrossRef]
- Machtoub, L.; Kasugai, Y. Amyotrophic Lateral Sclerosis: Advances and Perspectives of Neuronanomedicine; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Rosenberg, S.A.; Dudley, M.E.; Restifo, N.P. Cancer immunotherapy. N. Engl. J. Med. 2008, 359, 1072. [Google Scholar]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107. [Google Scholar] [CrossRef]
- Lin, Y.; Okada, H. Cellular immunotherapy for malignant gliomas. Expert Opin. Biol. Ther. 2016, 16, 1265–1275. [Google Scholar] [CrossRef]
- Liu, H.-L.; Fan, C.-H.; Ting, C.-Y.; Yeh, C.-K. Combining Microbubbles and Ultrasound for Drug Delivery to Brain Tumors: Current Progress and Overview. Theranostics 2014, 4, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Klafki, H.-W.; Staufenbiel, M.; Kornhuber, J.; Wiltfang, J. Therapeutic approaches to Alzheimer’s disease. Brain 2006, 129, 2840–2855. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nature Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Machaidze, R.; Kaluzova, M.; Wang, L.; Schuette, A.J.; Chen, H.; Wu, X.; Mao, H. EGFRvIII Antibody–Conjugated Iron Oxide Nanoparticles for Magnetic Resonance Imaging–Guided Convection-Enhanced Delivery and Targeted Therapy of GlioblastomaEGFRvIII-Targeted Therapy of GBM by IONPs after CED. Cancer Res. 2010, 70, 6303–6312. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.A.; MacDonald, A.A.; Seergobin, K.N.; Tamjeedi, R.; Ganjavi, H.; Provost, J.-S.; Monchi, O. The effect of dopamine therapy on ventral and dorsal striatum-mediated cognition in Parkinson’s disease: Support from functional MRI. Brain 2011, 134, 1447–1463. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Nanotechnology-mediated nose to brain drug delivery for Parkinson’s disease: A mini review. J. Drug Target. 2015, 23, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Azeem, A.; Talegaonkar, S.; Negi, L.M.; Ahmad, F.J.; Khar, R.K.; Iqbal, Z. Oil based nanocarrier system for transdermal delivery of ropinirole: A mechanistic, pharmacokinetic and biochemical investigation. Int. J. Pharm. 2012, 422, 436–444. [Google Scholar] [CrossRef]
- Md, S.; Khan, R.A.; Mustafa, G.; Chuttani, K.; Baboota, S.; Sahni, J.K.; Ali, J. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: Pharmacodynamic, Pharmacokinetic and Scintigraphy study in mice model. Eur. J. Pharm. Sci. 2013, 48, 393–405. [Google Scholar] [CrossRef]
- Gendelman, H.E.; Anantharam, V.; Bronich, T.; Ghaisas, S.; Jin, H.; Kanthasamy, A.G.; Liu, X.; McMillan, J.; Mosley, R.L.; Narasimhan, B.; et al. Nanoneuromedicines for degenerative, inflammatory, and infectious nervous system diseases. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 751–767. [Google Scholar] [CrossRef]
- Huang, R.; Ke, W.; Liu, Y.; Wu, D.; Feng, L.; Jiang, C.; Pei, Y. Gene therapy using lactoferrin-modified nanoparticles in a rotenone-induced chronic Parkinson model. J. Neurol. Sci. 2010, 290, 123–130. [Google Scholar] [CrossRef]
- Konishi, M.; Kawamoto, K.; Izumikawa, M.; Kuriyama, H.; Yamashita, T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J. Gene Med. 2008, 10, 610–618. [Google Scholar] [CrossRef]
- Davis, S.S. Biomedical applications of nanotechnology—Implications for drug targeting and gene therapy. Trends Biotechnol. 1997, 15, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Yurek, D.M.; Flectcher, A.M.; Kowalczyk, T.H.; Padegimas, L.; Cooper, M.J. Compacted DNA nanoparticle gene transfer of GDNF to the rat striatum enhances the survival of grafted fetal dopamine neurons. Cell Transplant. 2009, 18, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Wu, X.Y.; Bendayan, R. Nanotechnological advances for the delivery of CNS therapeutics. Adv. Drug Deliv. Rev. 2012, 64, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.L.; Kim, P.E.; Law, M.; Liu, C.Y.; Apuzzo, M.L. Visualizing the future: Enhancing neuroimaging with nanotechnology. World Neurosurg. 2011, 75, 626–637. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjugate Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Downs, M.E.; Buch, A.; Karakatsani, M.E.; Konofagou, E.E.; Ferrera, V.P. Blood-brain barrier opening in behaving non-human primates via focused ultrasound with systemically administered microbubbles. Sci. Rep. 2015, 5, 15076. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lin, Y.-A.; Kannan, S.; Kannan, R.M. Targeting specific cells in the brain with nanomedicines for CNS therapies. J. Control. Release 2016, 240, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Harilal, S.; Jose, J.; Parambi, D.G.T.; Kumar, R.; Mathew, G.E.; Uddin, M.S.; Kim, H.; Mathew, B. Advancements in nanotherapeutics for Alzheimer’s disease: Current perspectives. J. Pharm. Pharmacol. 2019, 71, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Cutler, E.G.; Cho, H. Therapeutic nanoplatforms and delivery strategies for neurological disorders. Nano Converg. 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef]
- Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv. Drug Deliv. Rev. 2014, 71, 2–14. [Google Scholar] [CrossRef]
- Duncan, R.; Vicent, M.J. Polymer therapeutics-prospects for 21st century: The end of the beginning. Adv. Drug Deliv. Rev. 2013, 65, 60–70. [Google Scholar] [CrossRef]
- Kolb-Mäurer, A.; Sunderkötter, C.; Kukowski, B.; Meuth, S.G. An update on Peginterferon beta-1a Management in Multiple Sclerosis: Results from an interdisciplinary Board of German and Austrian Neurologists and dermatologists. BMC Neurol. 2019, 19, 130. [Google Scholar] [CrossRef]
- Robinson, A.P.; Zhang, J.Z.; Titus, H.E.; Karl, M.; Merzliakov, M.; Dorfman, A.R.; Karlik, S.; Stewart, M.G.; Watt, R.K.; Facer, B.D.; et al. Nanocatalytic activity of clean-surfaced, faceted nanocrystalline gold enhances remyelination in animal models of multiple sclerosis. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 272–293. [Google Scholar] [CrossRef] [PubMed]
- Dolati, S.; Ahmadi, M.; Aghebti-Maleki, L.; Nikmaram, A.; Marofi, F.; Rikhtegar, R.; Ayromlou, H.; Yousefi, M. Nanocurcumin is a potential novel therapy for multiple sclerosis by influencing inflammatory mediators. Pharmacol. Rep. 2018, 70, 1158–1167. [Google Scholar] [CrossRef]
- Ahmadi, M.; Agah, E.; Nafissi, S.; Jaafari, M.R.; Harirchian, M.H.; Sarraf, P.; Faghihi-Kashani, S.; Hosseini, S.J.; Ghoreishi, A.; Aghamollaii, V. Safety and efficacy of nanocurcumin as add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: A pilot randomized clinical trial. Neurotherapeutics 2018, 15, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhai, M.; Chen, Z.; Han, X.; Yu, F.; Li, Z.; Xie, X.; Han, C.; Yu, L.; Yang, Y. Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv. 2017, 24, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Madhankumar, A.; Slagle-Webb, B.; Mintz, A.; Sheehan, J.M.; Connor, J.R. Interleukin-13 receptor–targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol. Cancer Ther. 2006, 5, 3162–3169. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, H.; Kasai, T.; Khayrani, A.C.; Asakura, M.; Oo, A.K.K.; Du, J.; Vaidyanath, A.; El-Ghlban, S.; Mizutani, A.; Seno, A. Targeting glioblastoma cells expressing CD44 with liposomes encapsulating doxorubicin and displaying chlorotoxin-IgG Fc fusion protein. Int. J. Mol. Sci. 2018, 19, 659. [Google Scholar] [CrossRef]
- Jain, N.K.; Rana, A.; Jain, S.K. Brain drug delivery system bearing dopamine hydrochloride for effective management of parkinsonism. Drug Dev. Ind. Pharm. 1998, 24, 671–675. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, Q.; Liang, L.; Wang, X.; Wang, J.; Zhang, X.; Pu, X.; Zhang, Q. Chlorotoxin-modified stealth liposomes encapsulating levodopa for the targeting delivery against the Parkinson’s disease in the MPTP-induced mice model. J. Drug Target. 2012, 20, 67–75. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Zhang, S.; Deng, Z.-B.; Grizzle, W.; Miller, D.; Zhang, H.-G. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv. Drug Deliv. Rev. 2013, 65, 342–347. [Google Scholar] [CrossRef]
- Dixit, S.; Novak, T.; Miller, K.; Zhu, Y.; Kenney, M.E.; Broome, A.-M. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 2015, 7, 1782–1790. [Google Scholar] [CrossRef]
- Lee, C.; Hwang, H.S.; Lee, S.; Kim, B.; Kim, J.O.; Oh, K.T.; Lee, E.S.; Choi, H.G.; Youn, Y.S. Rabies virus-inspired silica-coated gold nanorods as a photothermal therapeutic platform for treating brain tumors. Adv. Mater. 2017, 29, 1605563. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, H.; Madsen, S.J. Cell mediated photothermal therapy of brain tumors. J. Neuroimmune Pharmacol. 2017, 12, 99–106. [Google Scholar] [CrossRef]
- Hu, K.; Chen, X.; Chen, W.; Zhang, L.; Li, J.; Ye, J.; Zhang, Y.; Zhang, L.; Li, C.-H.; Yin, L. Neuroprotective effect of gold nanoparticles composites in Parkinson’s disease model. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Qu, X. Gold-Nanoparticle-Based Multifunctional Amyloid-β Inhibitor against Alzheimer’s Disease. Chem.–A Eur. J. 2015, 21, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Qian, J.; Cao, S.; Yang, Z.; Pang, Z.; Pan, S.; Fan, L.; Xi, Z.; Jiang, X.; Zhang, Q. Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. Biomaterials 2012, 33, 5115–5123. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gao, X.; Su, L.; Xia, H.; Gu, G.; Pang, Z.; Jiang, X.; Yao, L.; Chen, J.; Chen, H. Aptamer-functionalized PEG–PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 2011, 32, 8010–8020. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Chaudhari, K.; Dantuluri, P.; Murthy, R.; Das, S. Paclitaxel-loaded PLGA nanoparticles surface modified with transferrin and Pluronic® P85, an in vitro cell line and in vivo biodistribution studies on rat model. J. Drug Target. 2009, 17, 533–542. [Google Scholar] [CrossRef]
- Wang, B.; Lv, L.; Wang, Z.; Jiang, Y.; Lv, W.; Liu, X.; Wang, Z.; Zhao, Y.; Xin, H.; Xu, Q. Improved anti-glioblastoma efficacy by IL-13Rα2 mediated copolymer nanoparticles loaded with paclitaxel. Sci. Rep. 2015, 5, 16589. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Jiang, X.; Gu, J.; Sha, X.; Chen, L.; Law, K.; Chen, Y.; Wang, X.; Jiang, Y.; Fang, X. Angiopep-conjugated poly (ethylene glycol)-co-poly (ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials 2011, 32, 4293–4305. [Google Scholar] [CrossRef] [PubMed]
- Jafarieh, O.; Md, S.; Ali, M.; Baboota, S.; Sahni, J.; Kumari, B.; Bhatnagar, A.; Ali, J. Design, characterization, and evaluation of intranasal delivery of ropinirole-loaded mucoadhesive nanoparticles for brain targeting. Drug Dev. Ind. Pharm. 2015, 41, 1674–1681. [Google Scholar] [CrossRef]
- Gambaryan, P.; Kondrasheva, I.; Severin, E.; Guseva, A.; Kamensky, A. Increasing the Efficiency of Parkinson’s Disease Treatment Using a poly (lactic-co-glycolic acid)(PLGA) Based L-DOPA Delivery System. Exp. Neurobiol. 2014, 23, 246148. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.N.; Agarwal, S.; Bhatnagar, P.; Singhal, N.K.; Tiwari, S.K.; Kumar, P.; Chauhan, L.K.S.; Patel, D.K.; Chaturvedi, R.K.; Singh, M.P. Nicotine-encapsulated poly (lactic-co-glycolic) acid nanoparticles improve neuroprotective efficacy against MPTP-induced parkinsonism. Free Radic. Biol. Med. 2013, 65, 704–718. [Google Scholar] [CrossRef]
- Hasadsri, L.; Kreuter, J.; Hattori, H.; Iwasaki, T.; George, J.M. Functional protein delivery into neurons using polymeric nanoparticles. J. Biol. Chem. 2009, 284, 6972–6981. [Google Scholar] [CrossRef] [PubMed]
- Mulik, R.S.; Monkkonen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. ApoE3 mediated poly (butyl) cyanoacrylate nanoparticles containing curcumin: Study of enhanced activity of curcumin against beta amyloid induced cytotoxicity using in vitro cell culture model. Mol. Pharm. 2010, 7, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.V.; Roney, C.A.; Antich, P.P.; Bonte, F.J.; Raghu, A.V.; Aminabhavi, T.M. Quinoline-n-butylcyanoacrylate-based nanoparticles for brain targeting for the diagnosis of Alzheimer’s disease. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2010, 2, 35–47. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 705. [Google Scholar] [CrossRef]
- Garcion, E.; Lamprecht, A.; Heurtault, B.; Paillard, A.; Aubert-Pouessel, A.; Denizot, B.; Menei, P.; Benoît, J.P. A new generation of anticancer, drug-loaded, colloidal vectors reverses multidrug resistance in glioma and reduces tumor progression in rats. Mol. Cancer Therap. 2006, 5, : 1710–1722. [Google Scholar] [CrossRef]
- Sibov, T.T.; Pavon, L.F.; Miyaki, L.A.; Mamani, J.B.; Nucci, L.P.; Alvarim, L.T.; Silveira, P.H.; Marti, L.C.; Gamarra, L. Umbilical cord mesenchymal stem cells labeled with multimodal iron oxide nanoparticles with fluorescent and magnetic properties: Application for in vivo cell tracking. Int. J. Nanomed. 2014, 9, 337. [Google Scholar]
- Sillerud, L.O.; Solberg, N.O.; Chamberlain, R.; Orlando, R.A.; Heidrich, J.E.; Brown, D.C.; Brady, C.I.; Vander Jagt, T.A.; Garwood, M.; Vander Jagt, D.L. SPION-enhanced magnetic resonance imaging of Alzheimer’s disease plaques in AβPP/PS-1 transgenic mouse brain. J. Alzheimer’s Dis. 2013, 34, 349–365. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Riemenschneider, M.; Wagenpfeil, S.; Vanderstichele, H.; Otto, M.; Wiltfang, J.; Kretzschmar, H.; Vanmechelen, E.; Förstl, H.; Kurz, A. Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt–Jakob disease from other dementias. Mol. Psychiatry 2003, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.B.S. The 2017 World Health Organization classification of tumors of the pituitary gland: A summary. Acta Neuropathol. 2017, 134, 521–535. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Huse, J.T. 2016 World Health Organization classification of central nervous system tumors. Contin. Lifelong Learn. Neurol. 2017, 23, 1531–1547. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, C.; Li, Y.; Yi, X.; Wang, R.; Lee, S.M.-Y.; Zheng, Y. Small-sized mPEG–PLGA nanoparticles of Schisantherin A with sustained release for enhanced brain uptake and anti-parkinsonian activity. ACS Appl. Mater. Interfaces 2017, 9, 9516–9527. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Jiang, X. Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Int. J. Pharm. 2006, 310, 213–219. [Google Scholar] [CrossRef]
- Lamprecht, A.; Benoit, J.-P. Etoposide nanocarriers suppress glioma cell growth by intracellular drug delivery and simultaneous P-glycoprotein inhibition. J. Control. Release 2006, 112, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Singh, R.P.; Kumari, L.; Sharma, G.; Koch, B.; Rajesh, C.V.; Mehata, A.K.; Singh, S.; Pandey, B.L.; Muthu, M.S. TPGS-chitosan cross-linked targeted nanoparticles for effective brain cancer therapy. Mater. Sci. Eng. C 2017, 74, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Singh, R.P.; Sharma, G.; Mehata, A.K.; Singh, S.; Rajesh, C.V.; Pandey, B.L.; Koch, B.; Muthu, M.S. Bioadhesive micelles of d-α-tocopherol polyethylene glycol succinate 1000: Synergism of chitosan and transferrin in targeted drug delivery. Colloids Surf. B: Biointerfaces 2017, 152, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Dhanikula, R.S.; Argaw, A.; Bouchard, J.-F.; Hildgen, P. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: Enhanced efficacy and intratumoral transport capability. Mol. Pharm. 2008, 5, 105–116. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; Jia, X.-R.; Du, J.; Ying, X.; Lu, W.-L.; Lou, J.-N.; Wei, Y. PEGylated Poly (amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials 2011, 32, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pedro, N.Y.; Rangel-López, E.; Magaña-Maldonado, R.; de la Cruz, V.P.; Santamaría del Angel, A.; Pineda, B.; Sotelo, J. Application of nanoparticles on diagnosis and therapy in gliomas. BioMed Res. Int. 2013, 2013, 351031. [Google Scholar] [CrossRef]
- Brigger, I.; Morizet, J.; Aubert, G.; Chacun, H.; Terrier-Lacombe, M.-J.; Couvreur, P.; Vassal, G. Poly (ethylene glycol)-coated hexadecylcyanoacrylate nanospheres display a combined effect for brain tumor targeting. J. Pharmacol. Exp. Ther. 2002, 303, 928–936. [Google Scholar] [CrossRef]
- Berendsen, S.; Broekman, M.; Seute, T.; Snijders, T.; van Es, C.; de Vos, F.; Regli, L.; Robe, P. Valproic acid for the treatment of malignant gliomas: Review of the preclinical rationale and published clinical results. Expert Opin. Investig. Drugs 2012, 21, 1391–1415. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.E.; Folkert, M.; Zumsteg, Z.S.; Chan, T.A.; Beal, K.; Gutin, P.H.; Pentsova, E.; Yamada, Y. Temporal relationship of post-operative radiotherapy with temozolomide and oncologic outcome for glioblastoma. J. Neuro-Oncol. 2014, 116, 357–363. [Google Scholar] [CrossRef]
- Prah, M.; Stufflebeam, S.; Paulson, E.; Kalpathy-Cramer, J.; Gerstner, E.; Batchelor, T.; Barboriak, D.; Rosen, B.; Schmainda, K. Repeatability of standardized and normalized relative CBV in patients with newly diagnosed glioblastoma. Am. J. Neuroradiol. 2015, 36, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.D.; Drappatz, J.; Wen, P.Y. Antiangiogenic therapies for high-grade glioma. Nat. Rev. Neurol. 2009, 5, 610. [Google Scholar] [CrossRef]
- Schweitzer, T.; Vince, G.; Herbold, C.; Roosen, K.; Tonn, J.-C. Extraneural metastases of primary brain tumors. J. Neuro-Oncol. 2001, 53, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, A.; Riebensahm, C.; Mohme, M.; Joosse, S.; Velthaus, J.-L.; Berger, L.; Bernreuther, C.; Glatzel, M.; Loges, S.; Lamszus, K. Frequency of Circulating Tumor Cells (CTC) in Patients with Brain Metastases: Implications as a Risk Assessment Marker in Oligo-Metastatic Disease. Cancers 2018, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.P.; Nahed, B.V.; Madden, M.W.; Oliveira, S.M.; Springer, S.; Bhere, D.; Chi, A.S.; Wakimoto, H.; Rothenberg, S.M.; Sequist, L.V. Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer Discov. 2014, 4, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.R.; Calzascia, T.; Dietrich, P.Y. All in the head: Obstacles for immune rejection of brain tumours. Immunology 2002, 107, 28–38. [Google Scholar] [CrossRef]
- Engelhardt, B.; Ransohoff, R.M. The ins and outs of T-lymphocyte trafficking to the CNS: Anatomical sites and molecular mechanisms. Trends Immunol. 2005, 26, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Blood–brain barrier breakdown in septic encephalopathy and brain tumours. J. Anat. 2002, 200, 639–646. [Google Scholar] [CrossRef]
- Rascher, G.; Fischmann, A.; Kröger, S.; Duffner, F.; Grote, E.-H.; Wolburg, H. Extracellular matrix and the blood-brain barrier in glioblastoma multiforme: Spatial segregation of tenascin and agrin. Acta Neuropathol. 2002, 104, 85–91. [Google Scholar] [CrossRef]
- Prins, R.M.; Liau, L.M. Cellular immunity and immunotherapy of brain tumors. Front. Biosci. 2004, 9, 3124–3136. [Google Scholar] [CrossRef]
- Fantini, M.C.; Becker, C.; Monteleone, G.; Pallone, F.; Galle, P.R.; Neurath, M.F. Cutting edge: TGF-β induces a regulatory phenotype in CD4+ CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 2004, 172, 5149–5153. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.-K.L.; Flavell, R.A. Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Fecci, P.E.; Mitchell, D.A.; Whitesides, J.F.; Xie, W.; Friedman, A.H.; Archer, G.E.; Herndon, J.E.; Bigner, D.D.; Dranoff, G.; Sampson, J.H. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006, 66, 3294–3302. [Google Scholar] [CrossRef]
- Jensen, R.L.; Ragel, B.T.; Whang, K.; Gillespie, D. Inhibition of hypoxia inducible factor-1α (HIF-1α) decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. J. Neuro-Oncol. 2006, 78, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.; Ratliff, T.; Huppertz, A.; Ge, Y.; Dictus, C.; Ahmadi, R.; Grau, S.; Hiraoka, N.; Eckstein, V.; Ecker, R.C. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin. Cancer Res. 2011, 17, 4296–4308. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Hu, G.; Chen, Y.; Liu, Y.; Tu, W.; Lu, Y.; Wu, L.; Xu, G. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur. Rev. Med. Pharm. Sci. 2017, 21, 959–972. [Google Scholar]
- Caffo, M.; Barresi, V.; Caruso, G.; La Fata, G.; Pino, M.A.; Raudino, G.; Alafaci, C.; Tomasello, F. Gliomas Biology: Angiogenesis and Invasion. In Evolution of the Molecular Biology of Brain Tumors and the Therapeutic Implications; IntechOpen: London, UK, 2013. [Google Scholar]
- Girardi, M.; Oppenheim, D.E.; Steele, C.R.; Lewis, J.M.; Glusac, E.; Filler, R.; Hobby, P.; Sutton, B.; Tigelaar, R.E.; Hayday, A.C. Regulation of cutaneous malignancy by γδ T cells. Science 2001, 294, 605–609. [Google Scholar] [CrossRef]
- Chokshi, C.R.; Brakel, B.A.; Tatari, N.; Savage, N.; Salim, S.K.; Venugopal, C.; Singh, S.K. Advances in immunotherapy for adult glioblastoma. Cancers 2021, 13, 3400. [Google Scholar] [CrossRef]
- Weller, M.; Roth, P.; Preusser, M.; Wick, W.; Reardon, D.A.; Platten, M.; Sampson, J.H. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat. Rev. Neurol. 2017, 13, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Chekhonin, I.V.; Chekhonin, V.P. The EGFR variant III mutant as a target for immunotherapy of glioblastoma multiforme. Eur. J. Pharmacol. 2017, 810, 70–82. [Google Scholar] [CrossRef]
- Reardon, D.A.; Schuster, J.; Tran, D.D.; Fink, K.L.; Nabors, L.B.; Li, G.; Bota, D.A.; Lukas, R.V.; Desjardins, A.; Ashby, L.S. ReACT: Overall Survival from a Randomized Phase II Study of Rindopepimut (CDX-110) Plus Bevacizumab in Relapsed Glioblastoma; American Society of Clinical Oncology: Alexandria, VA, USA, 2015. [Google Scholar]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Miao, L.; Liu, Q.; Musetti, S.; Li, J.; Huang, L. Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol. Ther. 2018, 26, 45–55. [Google Scholar] [CrossRef]
- Baratta, M.G. Glioblastoma is ‘hot’for personalized vaccines. Nat. Rev. Cancer 2019, 19, 129. [Google Scholar] [CrossRef]
- Lyon, J.G.; Mokarram, N.; Saxena, T.; Carroll, S.L.; Bellamkonda, R.V. Engineering challenges for brain tumor immunotherapy. Adv. Drug Deliv. Rev. 2017, 114, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Zhang, L.; Zheng, G. Advancing Cancer Immunotherapies with Nanotechnology. Adv. Ther. 2019, 2, 1800128. [Google Scholar] [CrossRef]
- Mahjub, R.; Jatana, S.; Lee, S.E.; Qin, Z.; Pauli, G.; Soleimani, M.; Madadi, S.; Li, S.-D. Recent advances in applying nanotechnologies for cancer immunotherapy. J. Control. Release 2018, 288, 239–263. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Kateb, B.; Van Handel, M.; Zhang, L.; Bronikowski, M.J.; Manohara, H.; Badie, B. Internalization of MWCNTs by microglia: Possible application in immunotherapy of brain tumors. NeuroImage 2007, 37, S9–S17. [Google Scholar] [CrossRef] [PubMed]
- Krishnamachari, Y.; Geary, S.M.; Lemke, C.D.; Salem, A.K. Nanoparticle delivery systems in cancer vaccines. Pharm. Res. 2011, 28, 215–236. [Google Scholar] [CrossRef]
- Qiao, C.; Yang, J.; Shen, Q.; Liu, R.; Li, Y.; Shi, Y.; Chen, J.; Shen, Y.; Xiao, Z.; Weng, J.; et al. Traceable Nanoparticles with Dual Targeting and ROS Response for RNAi-Based Immunochemotherapy of Intracranial Glioblastoma Treatment. Adv. Mater. 2018, 30, 1705054. [Google Scholar] [CrossRef]
- Sun, T.; Patil, R.; Galstyan, A.; Klymyshyn, D.; Ding, H.; Chesnokova, A.; Cavenee, W.K.; Furnari, F.B.; Ljubimov, V.A.; Shatalova, E.S.; et al. Blockade of a Laminin-411–Notch Axis with CRISPR/Cas9 or a Nanobioconjugate Inhibits Glioblastoma Growth through Tumor-Microenvironment Cross-talk. Cancer Res. 2019, 79, 1239–1251. [Google Scholar] [CrossRef]
- Shen, Q.; Yang, J.; Liu, R.; Liu, L.; Zhang, J.; Shen, S.; Zhang, X. Hybrid ‘clusterbombs’ as multifunctional nanoplatforms potentiate brain tumor immunotherapy. Mater. Horiz. 2019, 6, 810–816. [Google Scholar] [CrossRef]
- Liu, H.; Chen, L.; Liu, J.; Meng, H.; Zhang, R.; Ma, L.; Wu, L.; Yu, S.; Shi, F.; Li, Y. Co-delivery of tumor-derived exosomes with alpha-galactosylceramide on dendritic cell-based immunotherapy for glioblastoma. Cancer Lett. 2017, 411, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Stephan, S.B.; Ene, C.I.; Smith, T.T.; Holland, E.C.; Stephan, M.T. Nanoparticles that reshape the tumor milieu create a therapeutic window for effective t-cell therapy in solid malignancies. Cancer Res. 2018, 78, 3718–3730. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, Y.; Cao, J.; Yang, W.; Zhang, J.; Meng, F.; Zhong, Z. Boosting RNAi therapy for orthotopic glioblastoma with nontoxic brain-targeting chimaeric polymersomes. J. Control. Release 2018, 292, 163–171. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, J.; Xiao, H.; Wu, T.; Shuai, X. Codelivery of temozolomide and siRNA with polymeric nanocarrier for effective glioma treatment. Int. J. Nanomed. 2018, 13, 3467. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, A.; Markman, J.L.; Shatalova, E.S.; Chiechi, A.; Korman, A.J.; Patil, R.; Klymyshyn, D.; Tourtellotte, W.G.; Israel, L.L.; Braubach, O. Blood–brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat. Commun. 2019, 10, 3850. [Google Scholar] [CrossRef]
- Lollo, G.; Vincent, M.; Ullio-Gamboa, G.; Lemaire, L.; Franconi, F.; Couez, D.; Benoit, J.-P. Development of multifunctional lipid nanocapsules for the co-delivery of paclitaxel and CpG-ODN in the treatment of glioblastoma. Int. J. Pharm. 2015, 495, 972–980. [Google Scholar] [CrossRef]
- Badie, B.; Berlin, J.M. The future of CpG immunotherapy in cancer. Immunotherapy 2013, 5, 1–3. [Google Scholar] [CrossRef]
- Bastiancich, C.; Bozzato, E.; Luyten, U.; Danhier, F.; Bastiat, G.; Préat, V. Drug combination using an injectable nanomedicine hydrogel for glioblastoma treatment. Int. J. Pharm. 2019, 559, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, D.A.; Balyasnikova, I.V.; Chang, A.L.; Ahmed, A.U.; Moon, K.S.; Auffinger, B.; Tobias, A.L.; Han, Y.; Lesniak, M.S. IDO Expression in Brain Tumors Increases the Recruitment of Regulatory T Cells and Negatively Impacts SurvivalIDO Regulates Treg Infiltration in Brain Tumors. Clinic. Cancer Res. 2012, 18, 6110–6121. [Google Scholar] [CrossRef] [PubMed]
- Löb, S.; Königsrainer, A.; Rammensee, H.G.; Opelz, G.; Terness, P. Inhibitors of indoleamine-2, 3-dioxygenase for cancer therapy: Can we see the wood for the trees? Nat. Rev. Cancer 2009, 9, 445–452. [Google Scholar] [CrossRef]
- Hou, D.-Y.; Muller, A.J.; Sharma, M.D.; DuHadaway, J.; Banerjee, T.; Johnson, M.; Mellor, A.L.; Prendergast, G.C.; Munn, D.H. Inhibition of indoleamine 2, 3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007, 67, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.J.; DuHadaway, J.B.; Donover, P.S.; Sutanto-Ward, E.; Prendergast, G.C. Inhibition of indoleamine 2, 3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 2005, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Song, W.; Yin, J.; Zeng, X.; Han, S.; Zhao, Y.P.; Tao, J.; Liu, C.J.; He, X.H.; Zhang, X.Z. iRGD Modified Chemo-immunotherapeutic Nanoparticles for Enhanced Immunotherapy against Glioblastoma. Adv. Funct. Mater. 2018, 28, 1800025. [Google Scholar] [CrossRef]
- Kadiyala, P.; Li, D.; Nuñez, F.M.; Altshuler, D.; Doherty, R.; Kuai, R.; Yu, M.; Kamran, N.; Edwards, M.; Moon, J.J. High-Density Lipoprotein-Mimicking Nanodiscs for Chemo-immunotherapy against Glioblastoma Multiforme. ACS Nano 2019, 13, 1365–1384. [Google Scholar] [CrossRef]
- Van Woensel, M.; Mathivet, T.; Wauthoz, N.; Rosière, R.; Garg, A.D.; Agostinis, P.; Mathieu, V.; Kiss, R.; Lefranc, F.; Boon, L. Sensitization of glioblastoma tumor micro-environment to chemo-and immunotherapy by Galectin-1 intranasal knock-down strategy. Sci. Rep. 2017, 7, 1217. [Google Scholar] [CrossRef]
- Chou, S.-T.; Patil, R.; Galstyan, A.; Gangalum, P.R.; Cavenee, W.K.; Furnari, F.B.; Ljubimov, V.A.; Chesnokova, A.; Kramerov, A.A.; Ding, H. Simultaneous blockade of interacting CK2 and EGFR pathways by tumor-targeting nanobioconjugates increases therapeutic efficacy against glioblastoma multiforme. J. Control. Release 2016, 244, 14–23. [Google Scholar] [CrossRef]
- Kozielski, K.L.; Ruiz-Valls, A.; Tzeng, S.Y.; Guerrero-Cázares, H.; Rui, Y.; Li, Y.; Vaughan, H.J.; Gionet-Gonzales, M.; Vantucci, C.; Kim, J. Cancer-selective nanoparticles for combinatorial siRNA delivery to primary human GBM in vitro and in vivo. Biomaterials 2019, 209, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood–brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef]
- Lozada-Delgado, E.L.; Grafals-Ruiz, N.; Vivas-Mejía, P.E. RNA interference for glioblastoma therapy: Innovation ladder from the bench to clinical trials. Life Sci. 2017, 188, 26–36. [Google Scholar] [CrossRef]
- Candolfi, M.; Kroeger, K.M.; Muhammad, A.; Yagiz, K.; Farrokhi, C.; Pechnick, R.N.; Lowenstein, P.R.; Castro, M.G. Gene therapy for brain cancer: Combination therapies provide enhanced efficacy and safety. Curr. Gene Ther. 2009, 9, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Harford, J.B.; Moghe, M.; Slaughter, T.; Doherty, C.; Chang, E.H. A tumor-targeting nanomedicine carrying the p53 gene crosses the blood–brain barrier and enhances anti-PD-1 immunotherapy in mouse models of glioblastoma. Int. J. Cancer 2019, 145, 2535–2546. [Google Scholar] [CrossRef] [PubMed]
- Speranza, M.-C.; Passaro, C.; Ricklefs, F.; Kasai, K.; Klein, S.R.; Nakashima, H.; Kaufmann, J.K.; Ahmed, A.-K.; Nowicki, M.O.; Obi, P. Preclinical investigation of combined gene-mediated cytotoxic immunotherapy and immune checkpoint blockade in glioblastoma. Neuro-Oncol. 2018, 20, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, V.; Nowicki, M.O.; Idriss, M.; Jimenez, M.A.; Lugli, G.; Hayes, J.L.; Mahmoud, A.B.; Zane, R.E.; Passaro, C.; Ligon, K.L. The functional synergism of microRNA clustering provides therapeutically relevant epigenetic interference in glioblastoma. Nat. Commun. 2019, 10, 442. [Google Scholar] [CrossRef]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Zhao, C.-Y.; Cheng, R.; Yang, Z.; Tian, Z.-M. Nanotechnology for cancer therapy based on chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Blanco, J.; Sanz-Arriazu, L.; Lorenzoni, R.; Blanco-Prieto, M.J. Glioblastoma chemotherapeutic agents used in the clinical setting and in clinical trials: Nanomedicine approaches to improve their efficacy. Int. J. Pharm. 2020, 581, 119283. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shi, Z.; Liu, R.; Wu, Y.; Zhang, X. Combined-therapeutic strategies synergistically potentiate glioblastoma multiforme treatment via nanotechnology. Theranostics 2020, 10, 3223–3239. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, C.; Fitch, S.; Yang, F. Targeting tumor hypoxia using nanoparticle-engineered CXCR4-overexpressing adipose-derived stem cells. Theranostics 2018, 8, 1350. [Google Scholar] [CrossRef]
- Khan, A.R.; Yang, X.; Fu, M.; Zhai, G. Recent progress of drug nanoformulations targeting to brain. J. Control. Release 2018, 291, 37–64. [Google Scholar] [CrossRef]
- Ganipineni, L.P.; Danhier, F.; Préat, V. Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J. Control. Release 2018, 281, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Prim. 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Hampel, H.; Zetterberg, H. Biomarkers in amyloid-β immunotherapy trials in Alzheimer’s disease. Neuropsychopharmacology 2014, 39, 189. [Google Scholar] [CrossRef] [PubMed]
- Buée, L.; Bussière, T.; Buée-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef]
- Foidl, B.M.; Humpel, C. Can mouse models mimic sporadic Alzheimer’s disease? Neural Reg. Res. 2020, 15, 401. [Google Scholar]
- Pai, A.S.; Rubinstein, I.; Önyüksel, H. PEGylated phospholipid nanomicelles interact with β-amyloid (1–42) and mitigate its β-sheet formation, aggregation and neurotoxicity in vitro. Peptides 2006, 27, 2858–2866. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Taylor, M.; Moore, S.; Mourtas, S.; Niarakis, A.; Re, F.; Zona, C.; La Ferla, B.; Nicotra, F.; Masserini, M.; Antimisiaris, S.G. Effect of curcumin-associated and lipid ligand-functionalized nanoliposomes on aggregation of the Alzheimer’s Aβ peptide. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 541–550. [Google Scholar] [CrossRef]
- Lovell, M.; Robertson, J.; Teesdale, W.; Campbell, J.; Markesbery, W. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Cui, Z.; Lockman, P.R.; Atwood, C.S.; Hsu, C.-H.; Gupte, A.; Allen, D.D.; Mumper, R.J. Novel D-penicillamine carrying nanoparticles for metal chelation therapy in Alzheimer’s and other CNS diseases. Eur. J. Pharm. Biopharm. 2005, 59, 263–272. [Google Scholar] [CrossRef]
- Podolski, I.Y.; Podlubnaya, Z.; Kosenko, E.; Mugantseva, E.; Makarova, E.; Marsagishvili, L.; Shpagina, M.; Kaminsky, Y.G.; Andrievsky, G.; Klochkov, V. Effects of hydrated forms of C60 fullerene on amyloid β-peptide fibrillization in vitro and performance of the cognitive task. J. Nanosci. Nanotechnol. 2007, 7, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Y.; Yang, Y.; Sun, L.; Han, D.; Li, H.; Wang, C. Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 427–441. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Annaert, W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci 2000, 113, 1857–1870. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. The cell biology of β-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Bio. 1998, 8, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Verdile, G.; Fuller, S.; Atwood, C.S.; Laws, S.M.; Gandy, S.E.; Martins, R.N. The role of beta amyloid in Alzheimer’s disease: Still a cause of everything or the only one who got caught? Pharmacol. Res. 2004, 50, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, C.; Shackleton, B.; Ojo, J.; Paris, D.; Mullan, M.; Crawford, F. Apolipoprotein E isoform-specific effects on lipoprotein receptor processing. NeuroMol. Med. 2014, 16, 686–696. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, C.-C.; Qiao, W.; Bu, G. Apolipoprotein E, Receptors, and Modulation of Alzheimer’s Disease. Biol Psychiatry 2018, 83, 347–357. [Google Scholar] [CrossRef]
- Sagare, A.P.; Deane, R.; Zlokovic, B.V. Low-density lipoprotein receptor-related protein 1: A physiological Aβ homeostatic mechanism with multiple therapeutic opportunities. Pharmacol. Ther. 2012, 136, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.K.; Farlow, M.R.; Greig, N.H.; Sambamurti, K. Current drug targets for Alzheimer’s disease treatment. Drug Dev. Res. 2002, 56, 267–281. [Google Scholar] [CrossRef]
- Andrieux, K.; Couvreur, P. Nanomedicine as a promising approach for the treatment and diagnosis of brain diseases: The example of Alzheimer’s disease. Ann. Pharm. Fr. 2013, 71, 225–233. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Inflammation, autotoxicity and Alzheimer disease. Neurobio. Aging 2001, 22, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, J.A.; Wilkinson, D.; Holmes, C.; Steart, P.; Markham, H.; Weller, R.O. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: A case report. Nat. Med. 2003, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; McGeer, P.L. Specificity of mechanisms for plaque removal after Aβ immunotherapy for Alzheimer disease. Nat. Med. 2004, 10, 117. [Google Scholar] [CrossRef]
- Schenk, D.; Barbour, R.; Dunn, W.; Gordon, G.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K.; Khan, K. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999, 400, 173. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Lin, C.; Yan, F.; Yu, G.-Q.; Rohde, M.; McConlogue, L.; Masliah, E.; Mucke, L. TGF-β1 promotes microglial amyloid-β clearance and reduces plaque burden in transgenic mice. Nat. Med. 2001, 7, 612. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Yan, F.; Lin, A.H.-T.; Lambris, J.D.; Alexander, J.J.; Quigg, R.J.; Masliah, E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc. Natl Acad. Sci. USA 2002, 99, 10837–10842. [Google Scholar] [CrossRef] [PubMed]
- Weiner, H.L.; Frenkel, D. Immunology and immunotherapy of Alzheimer’s disease. Nat. Rev. Immunol. 2006, 6, 404. [Google Scholar] [CrossRef]
- Liu, R.; Yang, J.; Liu, L.; Lu, Z.; Shi, Z.; Ji, W.; Shen, J.; Zhang, X. An “Amyloid-β Cleaner” for the Treatment of Alzheimer’s Disease by Normalizing Microglial Dysfunction. Adv. Sci. 2019, 7, 1901555. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, F.; Liu, Y.; Zheng, M.; Wang, Y.; Zhang, D.; Anraku, Y.; Zou, Y.; Li, J.; Wu, H. Blood-brain barrier–penetrating siRNA nanomedicine for Alzheimer’s disease therapy. Sci. Adv. 2020, 6, eabc7031. [Google Scholar] [CrossRef]
- Gu, H.; Dodel, R.; Farlow, M.; Du, Y. Advances in the development of antibody-based immunotherapy against prion disease. Antib. Technol. J. 2014, 4, 45. [Google Scholar]
- Winblad, B.; Graf, A.; Riviere, M.-E.; Andreasen, N.; Ryan, J.M. Active immunotherapy options for Alzheimer’s disease. Alzheimer’s Res. Ther. 2014, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.; Avila, J.; Schöll, M.; Kovacs, G.G.; Kövari, E.; Skrabana, R.; Evans, L.D.; Kontsekova, E.; Malawska, B.; de Silva, R. A walk through tau therapeutic strategies. Acta Neuropathol. Commun. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.; Mairet-Coello, G.; Danis, C.; Lieger, S.; Caillierez, R.; Carrier, S.; Skrobala, E.; Landrieu, I.; Michel, A.; Schmitt, M. Prevention of tau seeding and propagation by immunotherapy with a central tau epitope antibody. Brain 2019, 142, 1736–1750. [Google Scholar] [CrossRef] [PubMed]
- De Genst, E.; Messer, A.; Dobson, C.M. Antibodies and protein misfolding: From structural research tools to therapeutic strategies. Biochim. Biophys. Acta 2014, 1844, 1907–1919. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Bellucci, A.; Mercuri, N.B.; Venneri, A.; Faustini, G.; Longhena, F.; Pizzi, M.; Missale, C.; Spano, P. Parkinson’s disease: From synaptic loss to connectome dysfunction. Neuropathol. Appl. Neurobiol. 2016, 42, 77–94. [Google Scholar] [CrossRef]
- Dehay, B.; Bourdenx, M.; Gorry, P.; Przedborski, S.; Vila, M.; Hunot, S.; Singleton, A.; Olanow, C.W.; Merchant, K.M.; Bezard, E. Targeting α-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet Neurol. 2015, 14, 855–866. [Google Scholar] [CrossRef]
- Spencer, B.; Potkar, R.; Trejo, M.; Rockenstein, E.; Patrick, C.; Gindi, R.; Adame, A.; Wyss-Coray, T.; Masliah, E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in α-synuclein models of Parkinson’s and Lewy body diseases. J. Neurosci. 2009, 29, 13578–13588. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Bae, E.-J.; Lee, S.-J. Extracellular α-synuclein—A novel and crucial factor in Lewy body diseases. Nat. Rev. Neurol. 2014, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Stoddard-Bennett, T.; Reijo Pera, R. Treatment of Parkinson’s Disease through Personalized Medicine and Induced Pluripotent Stem Cells. Cells 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Ng, H.H.; Takahashi, R.; Tan, E.-K. Induced pluripotent stem cells in Parkinson’s disease: Scientific and clinical challenges. J Neurol Neurosurg Psychiatry 2016, 87, 697–702. [Google Scholar] [CrossRef]
- Witt, J.; Marks, W.J. An update on gene therapy in Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2011, 11, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.V.; Batrakova, E.V.; Kabanov, A.V. Nanogels for oligonucleotide delivery to the brain. Bioconjugate Chem. 2004, 15, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Kurakhmaeva, K.B.; Djindjikhashvili, I.A.; Petrov, V.E.; Balabanyan, V.U.; Voronina, T.A.; Trofimov, S.S.; Kreuter, J.; Gelperina, S.; Begley, D.; Alyautdin, R.N. Brain targeting of nerve growth factor using poly (butyl cyanoacrylate) nanoparticles. J. Drug Target. 2009, 17, 564–574. [Google Scholar] [CrossRef]
- Mohanraj, K.; Sethuraman, S.; Krishnan, U.M. Development of poly (butylene succinate) microspheres for delivery of levodopa in the treatment of parkinson’s disease. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2013, 101, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, T.; Xia, Y.; Wan, F.; Ma, K.; Guo, X.F.; Kou, L.; Yin, S.; Han, C.; Liu, L. New perspectives on roles of alpha-synuclein in Parkinson’s disease. Front. Aging Neurosci. 2018, 10, 370. [Google Scholar] [CrossRef]
- Olanow, C.W.; Obeso, J.A.; Stocchi, F. Drug insight: Continuous dopaminergic stimulation in the treatment of Parkinson’s disease. Nat. Rev. Neurol. 2006, 2, 382. [Google Scholar] [CrossRef] [PubMed]
- Moloney, K.K. Age and IFN-Gamma Deficiency Modulate the Impact of Repeated Paraquat Exposure in a Mouse Model of Parkinson’s Disease; Carleton University: ottawa, ON, Canada, 2011. [Google Scholar]
- Heinemann, U.; Kaufer, D.; Friedman, A. Blood-brain barrier dysfunction, TGFβ signaling, and astrocyte dysfunction in epilepsy. Glia 2012, 60, 1251–1257. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurol. 2013, 14, 38. [Google Scholar] [CrossRef]
- Valera, E.; Masliah, E. Therapeutic approaches in Parkinson’s disease and related disorders. J. Neurochem. 2016, 139, 346–352. [Google Scholar] [CrossRef]
- Mandler, M.; Valera, E.; Rockenstein, E.; Weninger, H.; Patrick, C.; Adame, A.; Santic, R.; Meindl, S.; Vigl, B.; Smrzka, O. Next-generation active immunization approach for synucleinopathies: Implications for Parkinson’s disease clinical trials. Acta Neuropathol. 2014, 127, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Mandler, M.; Valera, E.; Rockenstein, E.; Mante, M.; Weninger, H.; Patrick, C.; Adame, A.; Schmidhuber, S.; Santic, R.; Schneeberger, A. Active immunization against alpha-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol. Neurodegener. 2015, 10, 10. [Google Scholar] [CrossRef]
- Masliah, E.; Rockenstein, E.; Mante, M.; Crews, L.; Spencer, B.; Adame, A.; Patrick, C.; Trejo, M.; Ubhi, K.; Rohn, T.T. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS ONE 2011, 6, e19338. [Google Scholar] [CrossRef]
- Bae, E.-J.; Lee, H.-J.; Rockenstein, E.; Ho, D.-H.; Park, E.-B.; Yang, N.-Y.; Desplats, P.; Masliah, E.; Lee, S.-J. Antibody-aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J. Neurosci. 2012, 32, 13454–13469. [Google Scholar] [CrossRef]
- Roodveldt, C.; Labrador-Garrido, A.; Gonzalez-Rey, E.; Lachaud, C.C.; Guilliams, T.; Fernandez-Montesinos, R.; Benitez-Rondan, A.; Robledo, G.; Hmadcha, A.; Delgado, M. Preconditioning of microglia by α-synuclein strongly affects the response induced by toll-like receptor (TLR) stimulation. PLoS ONE 2013, 8, e79160. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yoo, J.M.; Hwang, H.; Lee, J.; Lee, S.H.; Yun, S.P.; Park, M.J.; Lee, M.; Choi, S.; Kwon, S.H.; et al. Graphene quantum dots prevent α-synucleinopathy in Parkinson’s disease. Nat. Nanotechnol. 2018, 13, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Bates, G.; Dorsey, R.; Gusella, J. Nat Rev Dis Primers. 2015.
- Bradford, J.; Shin, J.-Y.; Roberts, M.; Wang, C.-E.; Li, X.-J.; Li, S. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc. Natl. Acad. Sci. USA 2009, 106, 22480–22485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxidative Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Chen, W.; Tang, X.; Chiang, L.; Yang, C.; Schloss, J.; Wu, J. Polyhydroxylated C60, fullerenols, as glutamate receptor antagonists and neuroprotective agents. J. Neurosci. Res. 2000, 62, 600–607. [Google Scholar] [CrossRef]
- Godinho, B.M.; Ogier, J.R.; Darcy, R.; O’Driscoll, C.M.; Cryan, J.F. Self-assembling modified β-cyclodextrin nanoparticles as neuronal siRNA delivery vectors: Focus on Huntington’s disease. Mol. Pharm. 2013, 10, 640–649. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. Neuromolecular Med. 2014, 16, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.; Singh, D.; Prakash, A.; Mishra, N. Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington’s disease. Drug Deliv. 2015, 22, 931–939. [Google Scholar] [CrossRef]
- Cong, W.; Bai, R.; Li, Y.-F.; Wang, L.; Chen, C. Selenium nanoparticles as an efficient nanomedicine for the therapy of Huntington’s disease. ACS Appl. Mater. Interfaces 2019, 11, 34725–34735. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742. [Google Scholar]
- D’Mello, S.R.; Cruz, C.N.; Chen, M.-L.; Kapoor, M.; Lee, S.L.; Tyner, K.M. The evolving landscape of drug products containing nanomaterials in the United States. Nat. Nanotechnol. 2017, 12, 523–529. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Young, J.S.; Bernal, G.; Polster, S.P.; Nunez, L.; Larsen, G.F.; Mansour, N.; Podell, M.; Yamini, B. Convection-enhanced delivery of polymeric nanoparticles encapsulating chemotherapy in canines with spontaneous supratentorial tumors. World Neurosurg. 2018, 117, e698–e704. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, M.; Jafarieh, A.; Sarraf, P.; Sedighiyan, M.; Yousefi, A.; Tafakhori, A.; Abdollahi, H.; Salehinia, F.; Djalali, M. The neuromodulatory effects of ω-3 fatty acids and nano-curcumin on the COX-2/iNOS network in migraines: A clinical trial study from gene expression to clinical symptoms. Endocr. Metab. Immune Disord. -Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2019, 19, 874–884. [Google Scholar] [CrossRef]
- Abdolahi, M.; Tafakhori, A.; Togha, M.; Okhovat, A.A.; Siassi, F.; Eshraghian, M.R.; Sedighiyan, M.; Djalali, M.; Mohammadzadeh Honarvar, N.; Djalali, M. The synergistic effects of ω-3 fatty acids and nano-curcumin supplementation on tumor necrosis factor (TNF)-α gene expression and serum level in migraine patients. Immunogenetics 2017, 69, 371–378. [Google Scholar] [CrossRef]
- Soveyd, N.; Abdolahi, M.; Djalali, M.; Hatami, M.; Tafakhori, A.; Sarraf, P.; Honarvar, N.M. The combined effects of ω-3 fatty acids and nano-curcumin supplementation on intercellular adhesion molecule-1 (ICAM-1) gene expression and serum levels in migraine patients. CNS Neurol. Disord. -Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2017, 16, 1120–1126. [Google Scholar] [CrossRef]
- Lackner, P.; Beer, R.; Broessner, G.; Helbok, R.; Galiano, K.; Pleifer, C.; Pfausler, B.; Brenneis, C.; Huck, C.; Engelhardt, K. Efficacy of silver nanoparticles-impregnated external ventricular drain catheters in patients with acute occlusive hydrocephalus. Neurocritical Care 2008, 8, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Grauer, O.; Jaber, M.; Hess, K.; Weckesser, M.; Schwindt, W.; Maring, S.; Wölfer, J.; Stummer, W. Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients. J. Neuro-Oncol. 2019, 141, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Schroeter, M.; Ringelstein, A.; Hartung, H.-P.; Siebler, M.; Mödder, U.; Jander, S. Iron oxide particle-enhanced MRI suggests variability of brain inflammation at early stages after ischemic stroke. Stroke 2007, 38, 2733–2737. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).