Thermal and Morphological Analysis of Linear Low-Density Polyethylene Composites Containing d-limonene/β-cyclodextrin for Active Food Packaging
Abstract
1. Introduction
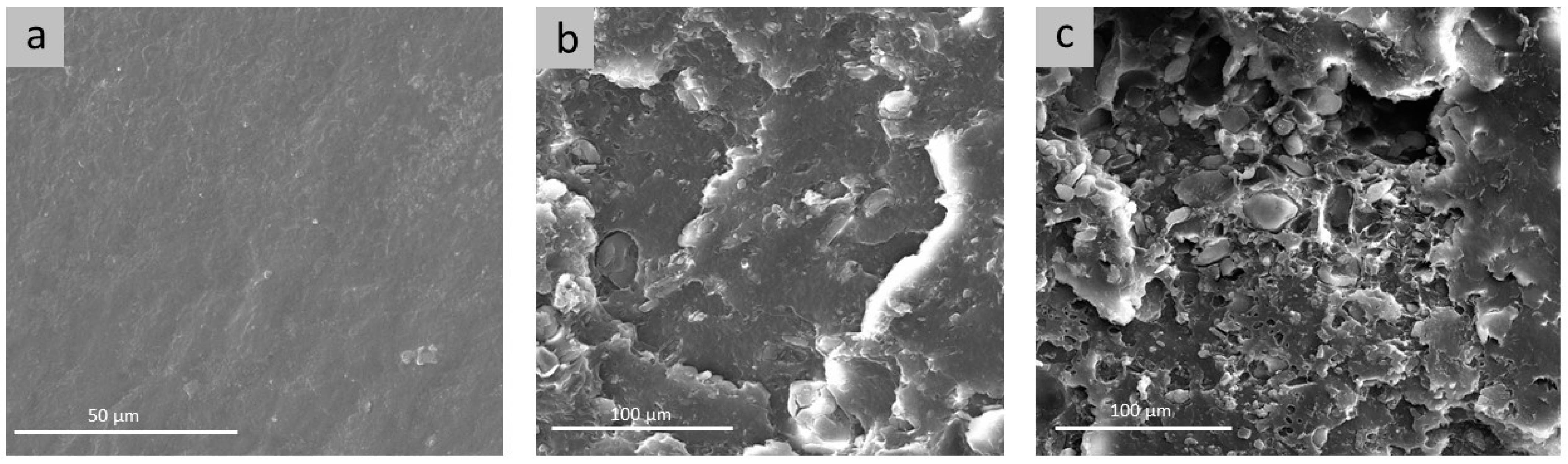
2. Results and Discussion
3. Conclusions
4. Methodology
4.1. Materials
4.2. Preparation of CD-lim Inclusion Complex and Composites
4.3. Methods
4.3.1. Scanning Electron Microscopy (SEM)
4.3.2. Attenuated Total Reflection Fourier-Transform Infrared (FTIR-ATR)
4.3.3. Differential Scanning Calorimetry (DSC)
4.3.4. Thermogravimetric analysis (TGA)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Peres, A.M.; Pires, R.R.; Oréfice, R.L. Evaluation of the Effect of Reprocessing on the Structure and Properties of Low Density Polyethylene/Thermoplastic Starch Blends. Carbohydr. Polym. 2016, 136, 210–215. [Google Scholar] [CrossRef] [PubMed]
- LaChance, A.M.; Hou, Z.; Farooqui, M.M.; Carr, S.A.; Serrano, J.M.; Odendahl, C.E.; Hurley, M.E.; Morrison, T.E.; Kubachka, J.L.; Samuels, N.T.; et al. Polyolefin Films with Outstanding Barrier Properties Based on One-Step Coassembled Nanocoatings. Adv. Compos. Hybrid. Mater. 2022, 5, 1067–1077. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable Polymers and Green-Based Antimicrobial Packaging Materials: A Mini-Review. Advanced Industrial and Engineering Polymer Research 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Sperati, C.A.; Franta, W.A.; Starkweather, H.W. The Molecular Structure of Polyethylene. V. The Effect of Chain Branching and Molecular Weight on Physical Properties 1. J. Am. Chem. Soc. 1953, 75, 6127–6133. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Quiles-Carrillo, L.; Balart, R.; Torres-Giner, S.; Arrieta, M.P. Innovative Solutions and Challenges to Increase the Use of Poly(3-Hydroxybutyrate) in Food Packaging and Disposables. Eur. Polym. J. 2022, 178, 111505. [Google Scholar] [CrossRef]
- El-Wakil, A.E.-A.A.; Moustafa, H.; Youssef, A.M. Antimicrobial Low-Density Polyethylene/Low-Density Polyethylene-Grafted Acrylic Acid Biocomposites Based on Rice Bran with Tea Tree Oil for Food Packaging Applications. J. Thermoplast. Compos. Mater. 2022, 35, 938–956. [Google Scholar] [CrossRef]
- Kolgjini, B.; Schoukens, G.; Kiekens, P. Three-Phase Characterization of Uniaxially Stretched Linear Low-Density Polyethylene. Int. J. Polym. Sci. 2011, 2011, 731708. [Google Scholar] [CrossRef]
- Sajkiewicz, P.; Hashimoto, T.; Saijo, K.; Gradys, A. ‘Intermediate Phase’ in Poly(Ethylene) as Elucidated by the WAXS. Analysis of Crystallization Kinetics. Polymer 2005, 46, 513–521. [Google Scholar] [CrossRef]
- Masayuki, Y. Effect of Molecular Structure in Branched Polyethylene on Adhesion Properties with Polypropylene. J. Polym. Sci. 1998, 70, 457–463. [Google Scholar]
- European Food Safety Authority (EFSA). Guidelines on submission of a dossier for safety evaluation by the EFSA of active or intelligent substances present in active and intelligent materials and articles intended to come into contact with food. EFSA J. 2009, 7, 1208. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial Agents and Packaging Systems in Antimicrobial Active Food Packaging: An Overview of Approaches and Interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Moeini, A.; van Reenen, A.; van Otterlo, W.; Cimmino, A.; Masi, M.; Lavermicocca, P.; Valerio, F.; Immirzi, B.; Santagata, G.; Malinconico, M.; et al. α-Costic Acid, a Plant Sesquiterpenoid from Dittrichia Viscosa, as Modifier of Poly (Lactic Acid) Properties: A Novel Exploitation of the Autochthone Biomass Metabolite for a Wholly Biodegradable System. Ind. Crops Prod. 2020, 146, 112134. [Google Scholar] [CrossRef]
- Falleh, H.; ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric Antimicrobial Food Packaging and Its Applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Ul-Islam, M.; Ahmed, A.A.Q.; Ullah, M.W.; Yang, G. Development and Characterization of Plant Oil-Incorporated Carboxymethyl Cellulose/Bacterial Cellulose/Glycerol-Based Antimicrobial Edible Films for Food Packaging Applications. Adv. Compos. Hybrid Mater. 2022, 5, 973–990. [Google Scholar] [CrossRef]
- Boro, U.; Priyadarsini, A.; Moholkar, V.S. Synthesis and Characterization of Poly(Lactic Acid)/Clove Essential Oil/Alkali-Treated Halloysite Nanotubes Composite Films for Food Packaging Applications. Int. J. Biol. Macromol. 2022, 216, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-Limonene Blends for Food Packaging Applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Mallardo, S.; de Vito, V.; Malinconico, M.; Volpe, M.G.; Santagata, G.; Di Lorenzo, M.L. Poly(Butylene Succinate)-Based Composites Containing β-Cyclodextrin/d-Limonene Inclusion Complex. Eur. Polym. J. 2016, 79, 82–96. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Knitter, M.; Mallardo, S.; Del Barone, M.C.; Santagata, G.; Di Lorenzo, M.L. Thermal and Thermo-Mechanical Properties of Poly(L-Lactic Acid) Biocomposites Containing β-Cyclodextrin/d-Limonene Inclusion Complex. Materials 2021, 14, 2569. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska-Mizera, M.; Knitter, M.; Szymanowska, D.; Mallardo, S.; Santagata, G.; Di Lorenzo, M.L. Optical, Mechanical, and Antimicrobial Properties of Bio-based Composites of Poly(L-lactic Acid) and D-limonene/Β-cyclodextrin Inclusion Complex. J. Appl. Polym. Sci. 2022, 139, 52177. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Moreno, J.A.; Garcia-Garcia, G.; Arjandas, S.; Calero, M. Life Cycle Assessment of Mechanical Recycling of Post-Consumer Polyethylene Flexible Films Based on a Real Case in Spain. J. Clean Prod. 2022, 365, 132625. [Google Scholar] [CrossRef]
- Canales, D.; Montoille, L.; Rivas, L.M.; Ortiz, J.A.; Yañez-S, M.; Rabagliati, F.M.; Ulloa, M.T.; Alvarez, E.; Zapata, P.A. Fungicides Films of Low-Density Polyethylene (LDPE)/Inclusion Complexes (Carvacrol and Cinnamaldehyde) Against Botrytis Cinerea. Coatings 2019, 9, 795. [Google Scholar] [CrossRef]
- Niazmand, R.; Razavizadeh, B.M. Active Polyethylene Films Incorporated with β-Cyclodextrin/Ferula Asafoetida Extract Inclusion Complexes: Sustained Release of Bioactive Agents. Polym. Test. 2021, 95, 107113. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Huber, T.; Müssig, J. Fibre Matrix Adhesion of Natural Fibres Cotton, Flax and Hemp in Polymeric Matrices Analyzed with the Single Fibre Fragmentation Test. Compos. Interfaces 2008, 15, 335–349. [Google Scholar] [CrossRef]
- Bredács, M.; Barretta, C.; Castillon, L.F.; Frank, A.; Oreski, G.; Pinter, G.; Gergely, S. Prediction of Polyethylene Density from FTIR and Raman Spectroscopy Using Multivariate Data Analysis. Polym. Test. 2021, 104, 107406. [Google Scholar] [CrossRef]
- Partal Ureña, F.; Moreno, J.R.A.; López González, J.J. Conformational Study of (R)-(+)-Limonene in the Liquid Phase Using Vibrational Spectroscopy (IR, Raman, and VCD) and DFT Calculations. Tetrahedron Asymmetry 2009, 20, 89–97. [Google Scholar] [CrossRef]
- Marcuzzo, E.; Debeaufort, F.; Sensidoni, A.; Tat, L.; Beney, L.; Hambleton, A.; Peressini, D.; Voilley, A. Release Behavior and Stability of Encapsulated <scp>d</Scp> -Limonene from Emulsion-Based Edible Films. J. Agric. Food Chem. 2012, 60, 12177–12185. [Google Scholar] [CrossRef]
- Cava, D.; Catala, R.; Gavara, R.; Lagaron, J.M. Testing Limonene Diffusion through Food Contact Polyethylene by FT-IR Spectroscopy: Film Thickness, Permeant Concentration and Outer Medium Effects. Polym. Test. 2005, 24, 483–489. [Google Scholar] [CrossRef]
- Derdar, H.; Belbachir, M.; Harrane, A. A Green Synthesis of Polylimonene Using Maghnite-H+, an Exchanged Montmorillonite Clay, as Eco-Catalyst. Bulletin of Chemical Reaction Engineering & Catalysis 2019, 14, 69. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, X.; Miller, J.B.; Huber, G.W. The Chemistry and Kinetics of Polyethylene Pyrolysis: A Process to Produce Fuels and Chemicals. ChemSusChem 2020, 13, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Dubdub, I.; Al-Yaari, M. Pyrolysis of Low Density Polyethylene: Kinetic Study Using TGA Data and ANN Prediction. Polymers 2020, 12, 891. [Google Scholar] [CrossRef]
- Aboulkas, A.; el harfi, K.; el Bouadili, A. Thermal Degradation Behaviors of Polyethylene and Polypropylene. Part I: Pyrolysis Kinetics and Mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Kaci, M.; Cimmino, S.; di Lorenzo, M.L.; Silvestre, C.; Sadoun, T. Effect of The Thermo-Oxidation and Natural Weather on The Structure, Morphology, and Properties of Unstabilized and Hals-Stabilized LDPE Films. J. Macromol. Sci. Part A 1999, 36, 253–274. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal Degradation Kinetics of Plastics and Model Selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
- Niu, B.; Chen, J.-B.; Chen, J.; Ji, X.; Zhong, G.-J.; Li, Z.-M. Crystallization of Linear Low Density Polyethylene on an in Situ Oriented Isotactic Polypropylene Substrate Manipulated by an Extensional Flow Field. CrystEngComm 2016, 18, 77–91. [Google Scholar] [CrossRef]
- Arndt, J.-H.; Brüll, R.; Macko, T.; Garg, P.; Tacx, J.C.J.F. Characterization of the Chemical Composition Distribution of Polyolefin Plastomers/Elastomers (Ethylene/1-Octene Copolymers) and Comparison to Theoretical Predictions. Polymer 2018, 156, 214–221. [Google Scholar] [CrossRef]
- Androsch, R. Melting and Crystallization of Poly(Ethylene-Co-Octene) Measured by Modulated d.s.c. and Temperature-Resolved X-Ray Diffraction. Polymer 1999, 40, 2805–2812. [Google Scholar] [CrossRef]
- Wunderlich, B. Thermal Analysis; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Hsu, Y.-C.; Truss, R.W.; Laycock, B.; Weir, M.P.; Nicholson, T.M.; Garvey, C.J.; Halley, P.J. The Effect of Comonomer Concentration and Distribution on the Photo-Oxidative Degradation of Linear Low Density Polyethylene Films. Polymer 2017, 119, 66–75. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Knitter, M.; Woźniak-Braszak, A.; Baranowski, M.; Sterzyński, T.; Di Lorenzo, M.L. Poly(l-Lactic Acid)/Pine Wood Bio-Based Composites. Materials 2020, 13, 3776. [Google Scholar] [CrossRef] [PubMed]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee Recommendations for Collecting Experimental Thermal Analysis Data for Kinetic Computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]




| Sample Name | DSC | TGA | ||
|---|---|---|---|---|
| Xcr (%) | Tc,ons (°C) | Td,ons (°C) | d-limonene Content (%) | |
| PE | 54 | 109.7 | 463.5 | - |
| PE/20CD | 64 | 111.2 | 308.5 | - |
| PE/20CD-lim | 63 | 111.2 | 302.5 | 1.1 |
| PE/30CD-lim | 60 | 112.1 | 303.6 | 1.8 |
| Sample Name | Mass Concentration [wt%] | ||
|---|---|---|---|
| LLDPE | CD | CD-lim | |
| PE | 100 | 0 | 0 |
| PE/20CD | 80 | 20 | 0 |
| PE/20CD-lim | 80 | 0 | 20 |
| PE/30CD-lim | 70 | 0 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzyńska-Mizera, M.; Knitter, M.; Piss, M.; Del Barone, C.; Mallardo, S.; Santagata, G.; Di Lorenzo, M.L. Thermal and Morphological Analysis of Linear Low-Density Polyethylene Composites Containing d-limonene/β-cyclodextrin for Active Food Packaging. Molecules 2023, 28, 1220. https://doi.org/10.3390/molecules28031220
Dobrzyńska-Mizera M, Knitter M, Piss M, Del Barone C, Mallardo S, Santagata G, Di Lorenzo ML. Thermal and Morphological Analysis of Linear Low-Density Polyethylene Composites Containing d-limonene/β-cyclodextrin for Active Food Packaging. Molecules. 2023; 28(3):1220. https://doi.org/10.3390/molecules28031220
Chicago/Turabian StyleDobrzyńska-Mizera, Monika, Monika Knitter, Marlena Piss, Cristina Del Barone, Salvatore Mallardo, Gabriella Santagata, and Maria Laura Di Lorenzo. 2023. "Thermal and Morphological Analysis of Linear Low-Density Polyethylene Composites Containing d-limonene/β-cyclodextrin for Active Food Packaging" Molecules 28, no. 3: 1220. https://doi.org/10.3390/molecules28031220
APA StyleDobrzyńska-Mizera, M., Knitter, M., Piss, M., Del Barone, C., Mallardo, S., Santagata, G., & Di Lorenzo, M. L. (2023). Thermal and Morphological Analysis of Linear Low-Density Polyethylene Composites Containing d-limonene/β-cyclodextrin for Active Food Packaging. Molecules, 28(3), 1220. https://doi.org/10.3390/molecules28031220








