3-Arylidene-2-oxindoles as Potent NRH:Quinone Oxidoreductase 2 Inhibitors
Abstract
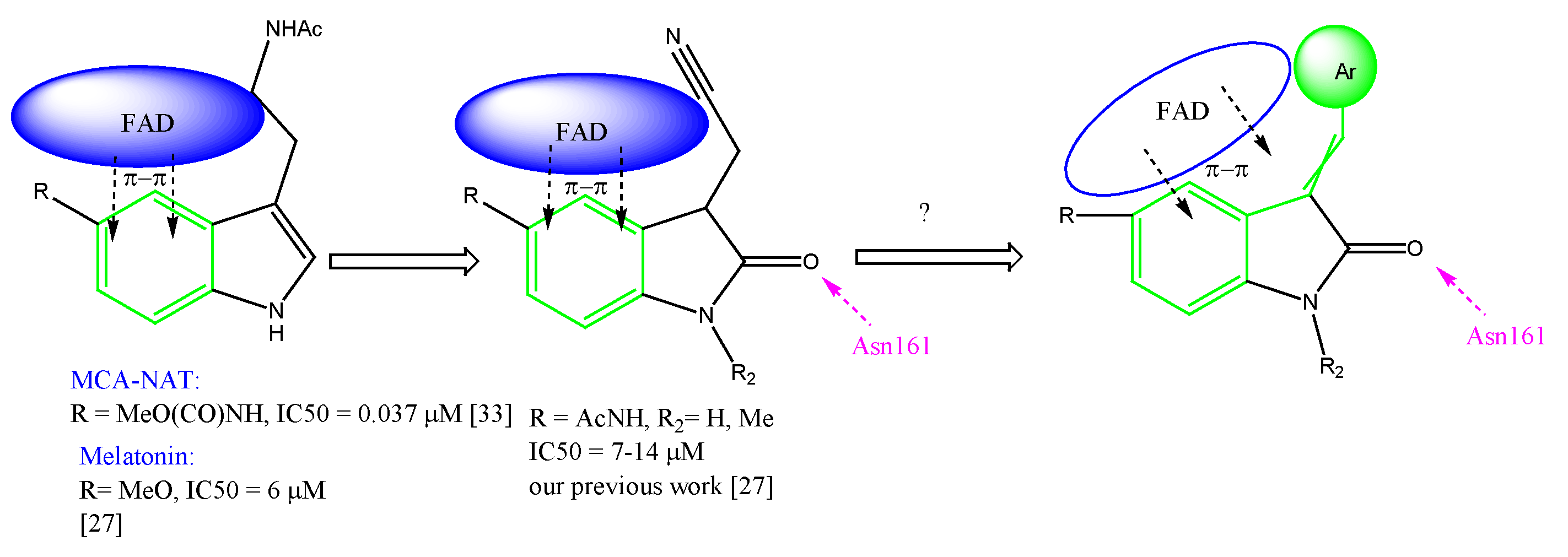
1. Introduction
2. Results and Discussion
2.1. Determining the Configuration of Obtained Compounds
2.1.1. Correlation of 1H NMR Signals for E/Z Isomers of 4′-Substituted Benzylidene-2-Oxindoles
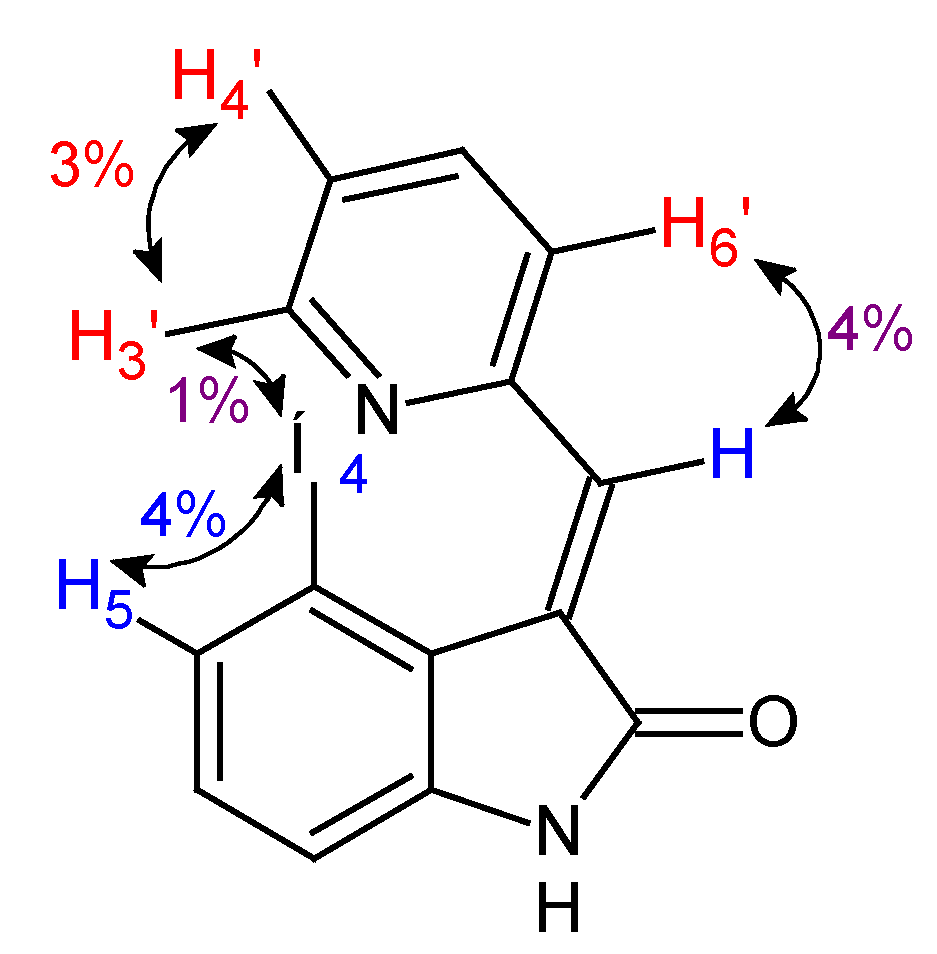
2.1.2. Correlation of 1H NMR Signals for E/Z Isomers of 3-(Pyridin-2-Ylmethylidene)-2-Oxindoles
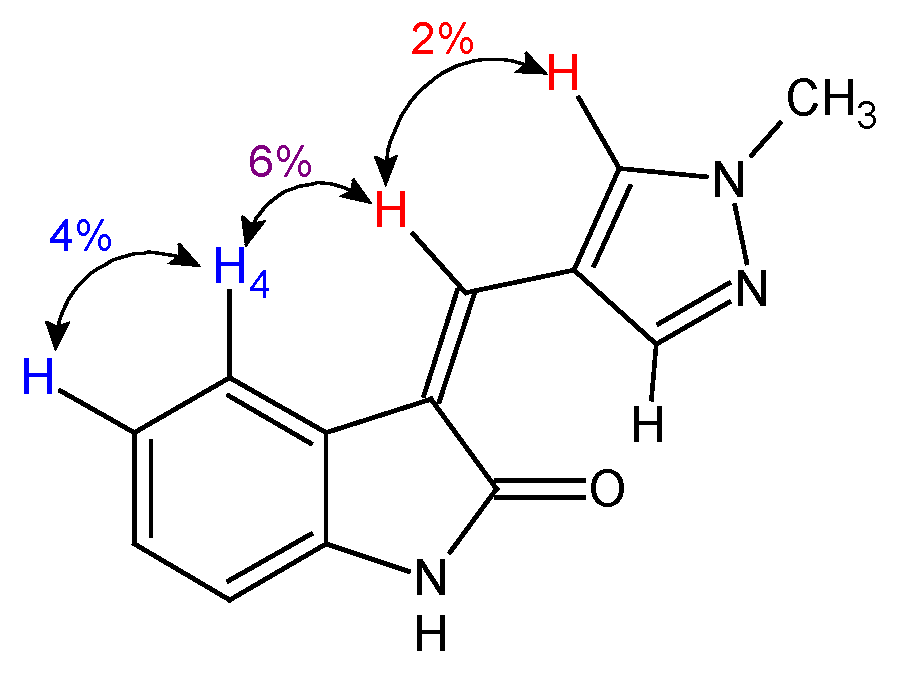
2.1.3. Correlation of 1H NMR Signals for E/Z Isomers of Pyrazole Derivatives
2.2. The Influence of the Reaction Conditions on Isomer Ratio
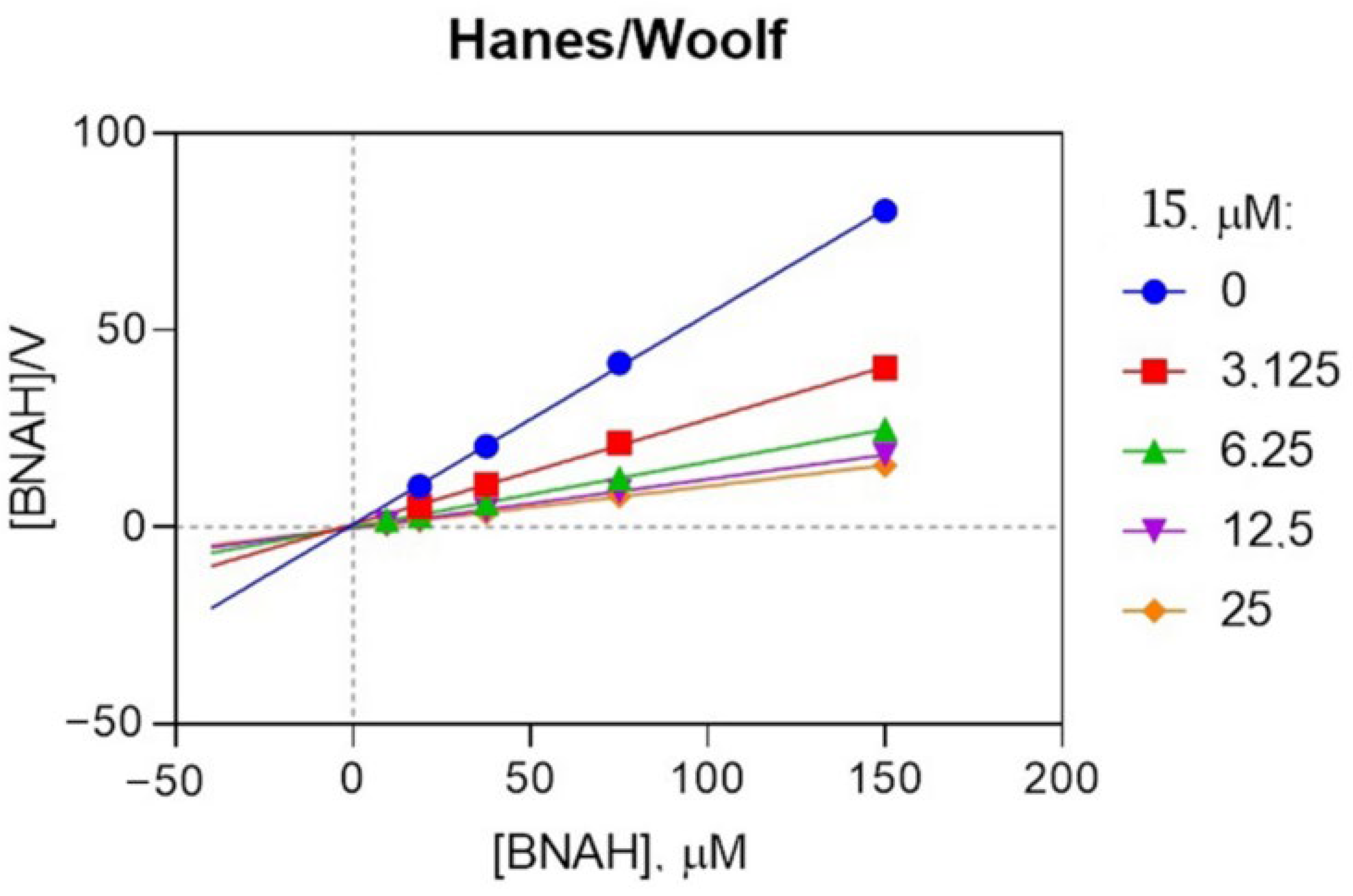
2.3. Biological Activity of the Obtained Compounds
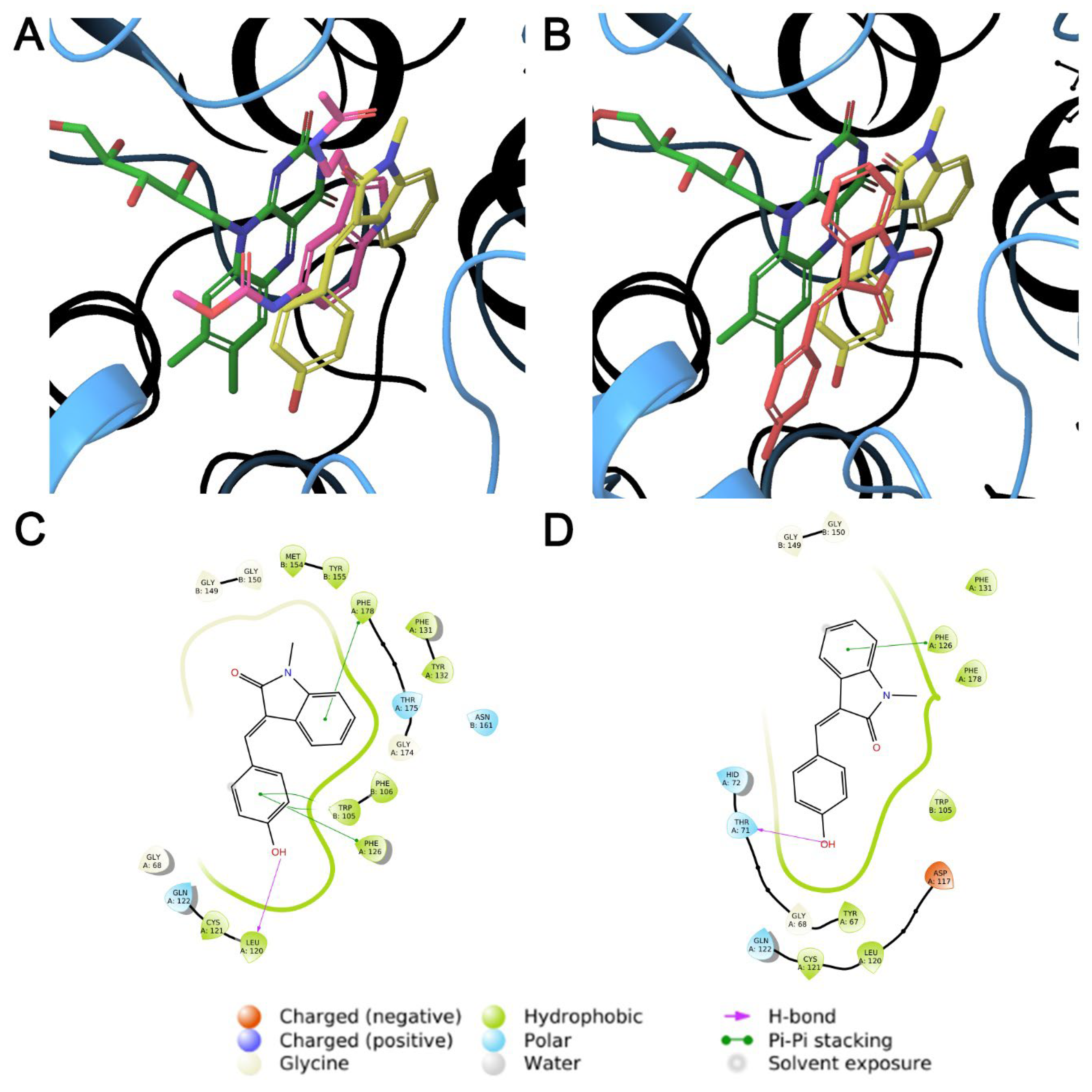
2.4. In Silico Studies
3. Materials and Methods
3.1. Chemistry
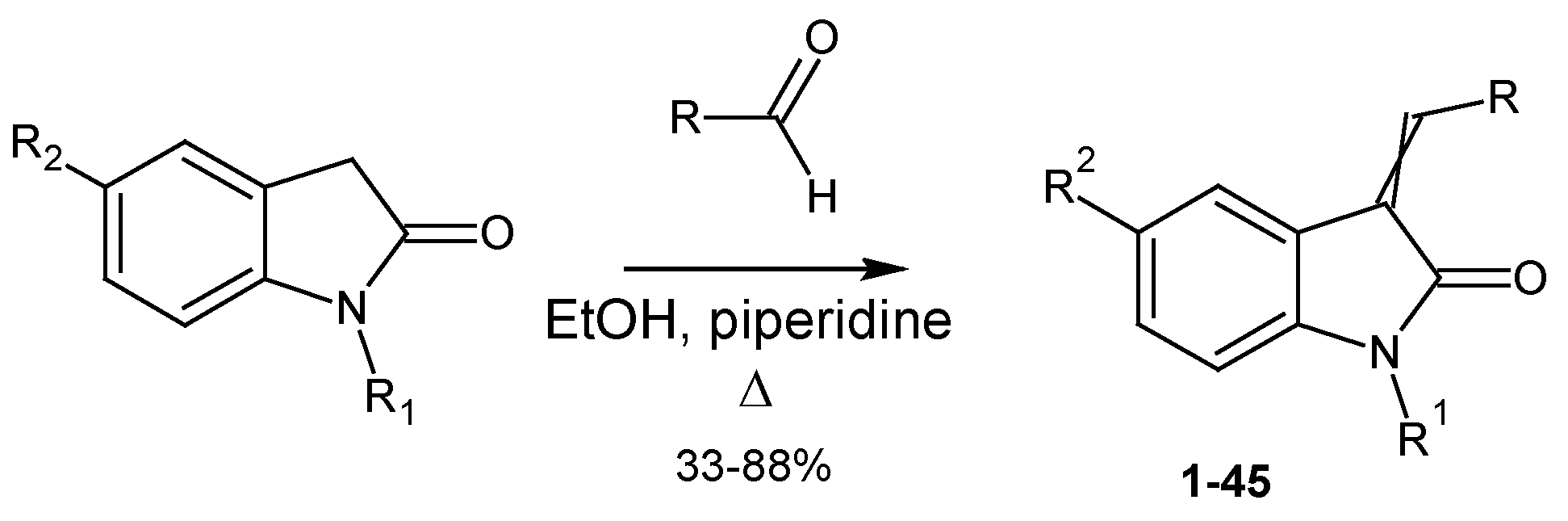
3.1.1. General Procedure for Synthesis of 3-Arylidene-2-Oxindoles
- (E,Z)-3-(2-Pyridinylmethylidene)-5-acetamido-2-oxindole 5
- From 0.18 g (0.94 mmol) of 5-acetamido-2-oxindole, 87 µL (0.94 mmol) of 2-pyridinecarboxaldehyde and 75 µL (0.88 mmol) of piperidine with ethanol as solvent the reddish-brown powder (0.156 g, 59%) was obtained as a mixture of two isomers. According to 1H NMR E/Z stereoisomer ratio is 7.15:1, respectively.
- E isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 2.02 (s, 3H, CH3), 6.76 (d, J = 8.4 Hz, 1H, H7), 7.42–7.48 (m, 1H), 7.49–7.55 (m, 2H), 7.82 (d, J= 7.7 Hz, 1H), 7.92 (td, J1 = 7.7 Hz, J2 = 1.8 Hz, 1H), 8.90 (d, J = 3.7 Hz, 1H, H3′), 9.30 (d, J = 2.0 Hz, 1H, H4), 9.89 (br.s, 1H, NH), 10.53 (br. s, 1H, NH).
- Selected peaks of Z isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 1.96 (s, 3H, CH3), 6.85 (d, J = 7.9 Hz, 1H, H7), 7.66–7.71 (m, 1H), 8.00–8.05 (m, 1H), 8.80 (d, J = 4.8 Hz, 1H, H3′).
- 13C NMR (100.6 MHz, DMSO-d6, ppm): 24.35, 109.61, 120.54, 121.91, 122.44, 124.45, 128.47, 130.18, 133.79, 134.19, 137.61, 139.66, 150.16, 153.59, 168.25, 169.77.
- HRMS (ESI), m/z: (M+H) found 280.1073, C16H14N3O2 requires 280.1086, (M + Na) found 302.0891, C16H13N3O2Na requires 302.0905.
- (E,Z)-3-(2-Pyridinylmethylidene)-5-benzoylamino-2-oxindole 6
- From 0.100 g (0.4 mmol) of 5-benzoylamino-oxindole, 43 µL (0.4 mmol) of 2-pyridinecarboxaldehyde and 30 µL (0.35 mmol) of piperidine with ethanol as solvent the light brown powder (0.084 g, 62%) was obtained as a mixture of two isomers. According to 1H NMR E/Z stereoisomer ratio is 6:1, respectively.
- E isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 6.84 (d, J = 8.4 Hz, 1H), 7.41–7.58 (m, 5H), 7.61 (dd, J1 = 8.4 Hz, J2 = 2.0 Hz, 1H), 7.84 (d, J = 7.7 Hz, 1H), 7.88–7.97 (m, 4H), 8.88 (d, J = 3.9 Hz, 1H, H3′), 9.46 (d, J = 1.7 Hz, 1H, H4), 10.22 (br. s, 1H, NH), 10.67 (br.s, 1H, NH).
- Selected peaks of Z isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 7.02 (d, J = 8.8 Hz, 1H, H7), 7.19–7.30 (m, 3H), 7.72 (d, J = 7.7 Hz, 1H).
- 13C NMR (100.6 MHz, DMSO-d6, ppm): 109.27, 121.50, 121.68, 123.85, 124.12, 127.69, 128.14, 128.37, 129.74, 131.35, 132.98, 133.93, 135.30, 137.24, 139.97, 149.83, 153.20, 165.37, 169.49.
- HRMS (ESI), m/z: (M + H) found 342.1233, C21H16N3O2 requires 342.1242, (M + Na) found 364.1051, C21H15N3O2Na requires 364.1062, (M+K) found 380.0790, C21H15N3O2K requires 380.0801.
- (E,Z)-3-(4-Hydroxybenzylidene)-5-acetamido-2-oxindole 16
- From 0.192 g (1 mmol) of 5-acetamido-2-oxindole, 0.122 g (1 mmol) of 4-hydroxybenzaldehyde and 80 µL (0.94 mmol) of piperidine with ethanol as solvent the yellow powder (0.189 g, 64%) was obtained as a mixture of two isomers. According to 1H NMR E/Z stereoisomer ratio is 2.6:1 respectively.
- E isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 1.99 (s, 3H, CH3), 6.78 (d, J = 8.3 Hz, 1H, H7), 6.87 (d, J = 8.6 Hz, 2H, H3′,H5′), 7.40 (td, J1 = 8.3 Hz, J2 = 1.9 Hz, 1H, H6), 7.51 (s, 1H, CH=), 7.62 (d, J = 8.7 Hz, 2H, H2′,H6′), 8.12 (d, J = 1.9 Hz, 1H, H4), 9.80 (s, 1H, NH), 10.42 (br.s, 1H, NH).
- Selected peaks of Z isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 2.02 (s, 3H, CH3), 6.70–6.83 (m, 3H), 7.22 (dd, J1 = 8.3 Hz, J2 = 1.9 Hz, 1H, H6), 7.48 (s, 1H, CH=), 7.82 (d, J = 1.9 Hz, 1H, H4), 8.39 (d, J = 8.8 Hz, 2H, H2′,H6′), 9.68 (s, 1H, NH), 10.46 (br.s, 1H, NH).
- 13C NMR (100.6 MHz, DMSO-d6, ppm): 24.29, 24.49, 109.98, 114.47, 116.11, 116.46, 120.83, 121.84, 124.45, 124.58, 132.57, 133.56, 135.58, 137.40, 138.44, 161.08, 168.27, 169.79.
- (E,Z)-3-(4-Methoxybenzylidene)-5-benzoylamino-2-oxindole 21
- From 0.100 g (0.4 mmol) of 5-(benzoylamino)oxindole, 0.055 g (0.4 mmol) of 4-methoxybenzaldehyde and 30 µL (0.35 mmol) of piperidine with ethanol as solvent the greenish grey powder (0.113 g, 77%) was obtained as a mixture of two isomers. According to 1H NMR E/Z stereoisomer ratio is 2:1, respectively.
- E isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 3.81 (s, 3H, CH3), 6.88 (d, J = 8.3 Hz, 1H, H7), 7.07 (d, J = 8.7 Hz, 2H), 7.43–7.66 (m, 5H), 7.76 (d, J = 8.3 Hz, 2H), 7.91 (d, J = 7.2 Hz, 2H), 8.24 (s, 1H, CH=), 10.18 (br.s, 1H, NH), 10.60 (br.s, 1H, NH).
- Selected peaks of Z isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 3.82 (s, 3H, CH3), 6.82 (d, J = 8.4 Hz, 1H, H7), 7.03 (d, J = 8.6 Hz, 2H), 7.86 (d, J = 8.6 Hz, 1H, Hind), 8.00 (d, J = 7.1 Hz, 2H), 8.09 (s, 1H, CH=), 8.51 (d, J = 8.7 Hz, 2H, H2′,H6′), 9.85 (br.s, 1H, NH), 10.22 (br.s, 1H, NH).
- 13C NMR (100.6 MHz, DMSO-d6, ppm): 55.40, 109.72, 113.80, 114.38, 115.63, 121.12, 121.85, 122.59, 124.28, 125.29, 125.54, 126.53, 126.92, 127.59, 127.64, 128.36, 128.42, 131.41, 131.47, 131.79, 131.85, 132.84, 132.88, 134.59, 135.12, 136.24, 136.85, 136.88, 138.94, 160.61, 161.29, 165.24, 165.36, 167.62, 169.24.
- (E)-3-(4-Ethoxybenzylidene)-2-oxindole 22
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.115 g (0.8 mmol) of 4-ethoxybenzaldehyde and 60 µL (0.7 mmol) of piperidine with ethanol as solvent the yellow powder (0.146 g, 73%) was obtained as a single E isomer.
- m.p. = 170–171 °C (m.p.lit. 169–171 [35])
- 1H NMR (400.13 MHz, DMSO-d6, ppm): 1.35 (t, J = 7.0 Hz, 3H, CH3), 4.07–4.14 (m, 2H, CH2), 6.83–6.89 (m, 2H, H7,Hind), 7.06 (d, J = 8.7 Hz, 2H, H3′,H5′), 7.21 (td, J1 = 7.0 Hz, J2 = 0.7 Hz, 1H, Hind), 7.56 (s, 1H, CH=), 7.65 (d, J = 7.8 Hz, 1H, H4), 7.69 (d, J = 8.7 Hz, 2H, H2′,H6′), 10.55 (br.s, 1H, NH).
- (E,Z)-3-(3,4,5-Trimethoxybenzylidene)-2-oxindole 23
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.143 g (0.8 mmol) of 3,4,5-trimethoxybenzaldehyde and 60 µL (0.7 mmol) of piperidine with ethanol as solvent the yellow powder (0.187 g, 84%) was obtained as a mixture of two isomers. According to 1H NMR stereoisomer ratio is 1.66:1. [36]
- Major isomer 1H NMR (400.13 MHz, DMSO-d6, ppm): 3.89 (s, 6H, CH3), 3.95 (s, 3H, CH3), 6.86–6.95 (m, 2H, Hind), 7.24 (t, J = 7.6 Hz, 1H, Hind), 7.27 (s, 2H, H2′,H6′), 7.79–7.83 (m, 2H, Hind,CH=), 8.13 (br.s, 1H, NH).
- Selected peaks of minor isomer 1H NMR (400.13 MHz, DMSO-d6, ppm): 3.98 (s, 9H, CH3), 6.88 (d, J = 7.7 Hz, 1H, H7) 7.06 (t, J = 7.4 Hz, 1H, Hind), 7.49 (s, 1H, CH=), 7.54 (d, J = 7.5 Hz, 1H, H4), 8.02 (br.s, 1H, NH)
- (E,Z)-3-(3,5-Dimethoxy-4-hydroxybenzylidene)-5-benzoylamino-2-oxindole 26
- From 0.100 g (0.4 mmol) of 5-benzoylamino-oxindole, 0.089 g (0.4 mmol) of 3,5-dimethoxy-4-hydroxybenzaldehyde and 30 µL (0.35 mmol) of piperidine with ethanol as solvent the black powder (0.133 g, 79%) was obtained as a mixture of two isomers. According to 1H NMR stereoisomer ratio is 1.66:1.
- Major isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 3.78 (s, 6H, CH3), 5.04 (br.s, 1H, OH), 6.83 (d, J = 8.3 Hz, 1H, H7), 7.05 (s, 2H, H2′,H6′), 7.37–7.59 (m, 4H), 7.87 (d, J = 7.2 Hz, 2H, HAr), 8.06 (s, 1H, CH=), 8.52 (br.s, 1H, H4), 9.51 (br.s, 1H, NH), 10.15 (br.s, 1H, NH);
- Selected signals of minor isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 3.72 (s, 6H, CH3), 6.78 (d, J = 8.3 Hz, 1H, H7), 7.01 (s, 2H, H2′,H6′), 7.91 (s, 1H, CH=), 7.96 (d, J = 7.0 Hz, 2H, HAr), 8.02 (s, 1H, H4), 10.13 (br.s, 1H, NH);
- 13C NMR (100.6 MHz, DMSO-d6, ppm): 56.08, 56.15, 108.11, 108.45, 109.93, 111.80, 114.39, 122.00, 122.08, 122.93, 123.81, 127.91, 128.76, 128.79, 131.77, 133.27, 135.39, 135.49, 138.22, 139.03, 148.57, 165.66, 169.89.
- (E,Z)-3-(4-Dimethylaminobenzylidene)-2-oxindole 30
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.12 g (0.8 mmol) of 4-(dimethylamino)benzaldehyde and 60 µL (0.7 mmol) of piperidine with ethanol as solvent the reddish-brown powder (0.107 g, 54%) was obtained as a mixture of two isomers. According to 1H NMR E/Z stereoisomer ratio is 1:1 respectively [30].
- 1H NMR (400.13 MHz, DMSO-d6, ppm): 3.01 (s, 6H, CH3), 3.02 (s, 6H, CH3), 6.70–6.95 (m, 8H), 7.10 (t, J = 7.7 Hz, 1H), 7.17 (t, J = 7.8 Hz, 1H), 7.51 (s, 1H, CH=), 7.57–7.67 (m, 4H), 7.78 (d, J = 7.5 Hz, 1H, H4), 8.44 (d, J = 8.7 Hz, 2H, H2′,H6′)*, 10.54 (br. s, 2H, NH).
- *– Z isomer.
- (E,Z)-3-(4-Fluorobenzylidene)-2-oxindole 33
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.95 g (0.8 mmol) of 4-fluorobenzaldehyde and 60 µL (0.7 mmol) of piperidine with ethanol as solvent yellow powder (0.122 g, 68%) was obtained as a mixture of two isomers. According to 1H NMR E/Z stereoisomer ratio is 2:1, respectively [37].
- E isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 6.78–6.89 (m, 2H, H7,Hind), 7.17–7.25 (m, 1H, Hind), 7.27–7.38 (m, 2H, H3′,H5′), 7.49 (d, J = 7.6 Hz, 1H, H4), 7.58 (s, 1H CH=), 7.73–7.75 (m, 2H, H2′,H6′), 10.62 (br. s 1H, NH).
- Selected peaks of Z isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 6.98 (t, J = 7.5 Hz, 1H, Hind), 7.69 (d, J = 7.5 Hz, 1H, H4), 7.8 (s, 1H, CH=), 8.45–8.5 (m, 2H, H2′, H6′), 10.66 (br.s, 1H, NH).
- (E,Z)-3-(1-[2-(Methoxycarbonyl)ethyl]-1H-pyrazol-4-ylmethylidene)-2-oxindole 45
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.142 g (0.8 mmol) methyl 3-(4-formyl-1H-pyrazol-1-yl)propanoate and 60 µL (0.7 mmol) of piperidine with ethanol as solvent the yellow powder (0.187 g, 84 %) was obtained as a mixture of two isomers. According to 1H NMR E/Z stereoisomer ratio is 1:3.5, respectively.
- Z isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 2.87–2.97 (m, 2H, CH2), 3.59 (s, 3H, CH3), 4.39–4.49 (m, 2H, CH2), 6.81 (d, J = 7.6 Hz, 1H, H7), 6.91–7.00 (m, 1H, Hind), 7.14 (t, J = 7.4 Hz, 1H, Hind), 7.57 (d, J = 7.3 Hz, 1H, H4), 7.67 (s, 1H, CH=), 8.24 (s, 1H, CHpyr), 8.83 (s, 1H, NHpyr), 10.54 (br. s, 1H, NHind).
- Selected peaks of E-isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 2.87–2.97 (m, 2H, CH2), 3.59 (s, 3H, CH3), 4.39–4.49 (m, 2H, CH2), 6.86 (d, J = 7.6 Hz, 1H, H7), 7.21 (t, J = 7.6 Hz, 1H, Hind), 7.45 (s, 1H, CH=), 7.82 (d, J = 7.4 Hz, 1H), 8.00 (s, 1H), 8.42 (s, 1H), 10.5 (br. s, 1H, NHind).
- 13C NMR (100.6 MHz, DMSO-d6, ppm): 33.87, 47.31, 51.64, 109.28, 116.98, 118.89, 120.87, 121.77, 124.81, 126.30, 127.82, 134.70, 139.98, 143.53, 167.65, 169.30, 171.10.
- HRMS (ESI), m/z: (M+H) found 298.1178, C16H16N3O3 requires 298.1192, (M+Na) 320.0095 C16H15N3O3Na requires 320.1011.
3.1.2. Influence of solvent on E/Z Isomer Ratio
- (E)-3-(2-Pyridinylmethylidene)-2-oxindole (E)-1
- From 0.200 g (1.5 mmol) of 2-oxindole, 0.16 mL (1.5 mmol) of 2-pyridinecarboxaldehyde and 120 µL (1.5 mmol) of piperidine with ethanol as solvent the brown powder (0.109 g, 61%) was obtained as a single E isomer [28].
- 1H NMR (400.13 MHz, DMSO-d6, ppm): 6.84 (d, J = 7.7 Hz, 1H, H7), 6.95 (td, J1 = 7.7 Hz, J2 = 0.9 Hz, 1H, H5), 7.25 (td, J1 = 7.5 Hz, J2 = 1.3 Hz, H6), 7.41 (ddd, J1 = 7.5 Hz, J2 = 4.8 Hz, J3 = 1.1 Hz, 1H, H4′), 7.54 (s, 1H, CH=), 7.82 (d, J = 7.5 Hz, 1H, H6′), 7.90 (td, J1 = 7.7 Hz, J2 = 1.8 Hz, 1H, H5′), 8.4 (d, J = 3.9 Hz, 1H, H3′), 8.98 (d, J = 7.3 Hz, 1H, H4), 10.61 (br.s, 1H, NH).
- (E,Z)-3-(2-Pyridinylmethylidene)-2-oxindole (E,Z)-1
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.08 mL (0.8 mmol) of 2-pyridinecarboxaldehyde and 60 µL (0.7 mmol) of piperidine with 1,4-dioxane as solvent the brown oil (0.145 g, 81%) was obtained as a mixture of two isomers. According to 1H NMR E/Z stereoisomer ratio is 1.3:1 respectively. 1H NMR (DMSO-d6) for E isomer is identical to described above.
- Selected signals of Z-isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 7.06 (d, J = 7.9 Hz, 1H, H4), 7.63 (s, 1H, CH=).
- 3-(Hydroxy(pyridin-2-yl)methyl)-2-oxindole 1a
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.08 mL (0.8 mmol) of 2-pyridinecarboxaldehyde and 60 µL (0.7 mmol) of piperidine with ethyl acetate as solvent the beige powder (0.088 g, 49%) was obtained. According to 1H NMR the ratio of two diastereoisomers of 1a and the final condensation product 1 is 6.92:4.17:1 respectively.
- Major isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 4.08 (d, J = 2.4 Hz, 1H), 5.33 (dd, J1 = 4.7 Hz, J2 = 1.9 Hz, 1H), 6.62 (t, J = 7.6 Hz, 1H), 6.77 (d, J = 7.7 Hz, 1H), 7.02–7.10 (m, 1H), 7.34 (dd, J1 = 7.2 Hz, J2 = 2.2 Hz, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.85 (td, J1 = 7.7 Hz, J2 = 1.6 Hz, 1H), 8.62 (d, J = 4.2 Hz, 1H), 10.39 (br.s, 1H)
- Selected peaks of minor isomer 1H NMR (400.13 MHz, DMSO-d6, ppm): 3.96 (d, J = 3.0 Hz, 1H), 5.27 (t, J = 3.5 Hz, 1H), 6.67 (d, J = 7.7 Hz, 1H), 6.83 (t, J = 7.5 Hz, 1H), 7.13 (dd, J1 = 7.1 Hz, J2 = 1.8 Hz, 1H), 7.25 (d, J = 7.3 Hz, 1H), 7.42 (d, J = 8.0 Hz, 1H), 7.66 (td, J1 = 7.8 Hz, J2 = 1.7 Hz, 1H), 8.35 (d, J = 4.1 Hz, 1H), 10.17 (br.s, 1H).
- 1H spectrum also contains signals of 1.
- 13C NMR (100.6 MHz, DMSO-d6, ppm): 57.07, 57.29, 77.74, 78.66, 113.84, 114.22, 125.66, 125.79, 125.91, 127.01, 127.45, 129.06, 129.43, 131.52, 132.66, 133.04, 141.01, 141.75, 148.43, 148.94, 152.96, 153.87, 166.51, 167.34, 181.07, 182.69.
- HRMS (ESI), m/z: (M+H) found 241.0979, C14H13N2O2 requires 241.0977; (M+Na) found 263.0797, C14H12N2O2Na requires 263.0796.
- (E,Z)-3-(4-Hydroxybenzylidene)-2-oxindole (E)-14
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.092 g (0.8 mmol) of 4-hydroxybenzaldehyde and 60 µL (0.7 mmol) of piperidine with ethanol as solvent the yellow powder (0.136 g, 76%) was obtained as a mixture of two isomers. According to 1H NMR E/Z stereoisomeric ratio is 19:1, respectively [29].
- 1H NMR (400.13 MHz, DMSO-d6, ppm): 6.82–6.93 (m, 4H), 7.19 (t, J = 7.6 Hz, 1H, Hind), 7.53 (s, 1H, CH=), 7.61 (d, J = 8.5 Hz, 2H, HAr), 7.69 (d, J = 7.6 Hz, 1H, H4), 10.14 (br.s, 1H, OH), 10.52 (br. s, 1H, NH).
- (E,Z)-3-(4-Hydroxybenzylidene)-2-oxindole (E,Z)-14
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.092 g (0.8 mmol) of 4-hydroxybenzaldehyde and 60 µL (0.7 mmol) of piperidine in 1,4-dioxane or ethyl acetate as solvent the yellow powder was obtained as a mixture of two isomers. Yields: 0.150 g, 84% (1,4-dioxane); 0.109 g, 61% (ethyl acetate). According to 1H NMR E/Z stereoisomeric ratio is 1.75:1, respectively. 1H NMR (400.13 MHz, DMSO-d6, ppm) for E isomer was identical to 14.
- Selected peaks of Z isomer, 1H NMR (400.13 MHz, DMSO-d6, ppm): 6.79 (d, J = 7.6 Hz, 1H, H7), 6.94 (td, J1 = 7.5 Hz, J2 = 0.6 Hz, 1H, Hind), 7.13 (td, J1 = 7.7 Hz, J2 = 0.6 Hz, 1H, Hind), 7.64 (s, 1H, CH=), 8.39 (d, J = 8.7 Hz, 2H, H2′, H6′)
- (E,Z)-3-((5-Ethoxycarbonyl-1H-pyrazol-4-yl)methylidene)-2-oxindole (E,Z)-39
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.134 g (0.8 mmol) of ethyl 4-formyl-1H-pyrazole-5-carboxylate and 60 µL (0.7 mmol) of piperidine with ethanol, 1,4-dioxane or ethyl acetate as solvent the yellow powder was obtained as a mixture of two isomers. Yields: 0.136 g, 76% (ethanol); 0.154 g, 86% (1,4-dioxane); 0.120 g, 67% (ethyl acetate). According to 1H NMR major to minor stereoisomer ratio is 1.6:1 in all solvents [29].
- Major isomer 1H NMR (400.13 MHz, DMSO-d6, ppm): 1.35 (t, J = 7.1 Hz, 3H, CH3), 4.33–4.40 (m, 2H, CH2), 6.5 (m, 1H, H7), 6.99 (td, J1 = 7.5 Hz, J2 = 0.7 Hz, 1H, Hind), 7.17–7.24 (m, 1H, Hind), 7.47 (d, J = 7.5 Hz, 1H, H4), 8.23 (s, 1H, CH=), 9,28 (br.s, 1H, Hpyrazol), 10.64 (s., 1H, NHind).
- Selected peaks of minor isomer 1H NMR (400.13 MHz, DMSO-d6, ppm): 1.27 (t, J = 7.1 Hz, 3H, CH3), 4.25–4.33 (m, 2H, CH2), 6.90 (td, J1 = 7.6 Hz, J2 = 0.9 Hz, 1H), 7.62 (d, J = 7.6 Hz, 1H, H4), 7.82 (s, 1H, CH=), 8.42 (br.s, 1H, Hpyrazol), 10.54 (s, 1H, NHind).
- 3-((3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)methylidene)-2-oxindole 44
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.160 g (0.8 mmol) of 3,5-dimethyl-1-phenyl-1H-pyrazole-4-carbaldehyde and 60 µL (0.7 mmol) of piperidine with ethanol, 1,4-dioxane or ethyl acetate as solvent yellow powder was obtained as single isomer. Yields: 0.159 g, 63% (ethanol); 0.157 g, 62% (1,4-dioxane); incomplete conversion of started oxindole (76% conversion) in ethyl acetate. According to 1H NMR a single isomer was obtained in all solvents. [29]
- 1H NMR (400.13 MHz, DMSO-d6, ppm): 2.15–2.22 (m, 6H, CH3), 6.87 (d, J = 7.6 Hz, 1H, H7), 6.92 (td. J1 = 7.6 Hz, J2 = 0.7 Hz, 1H, Hind), 7.07 (d, J = 7.7 Hz, 1H, H4), 7.20 (td, J1 = 7.6 Hz, J2 = 0.8 Hz, 1H, Hind), 7.4–7.48 (m, 2H, Har, CH=), 7.53 (t, J = 7.5 Hz, 2H, Har), 7.57–7.62 (m, 2H, Har), 10.60 (br.s, 1H).
3.1.3. General Procedure for Synthesis of 3-arylidene-2-oxindoles using MW Activation
- (E,Z)-3-(2-Pyridinylmethylidene)-2-oxindole (E,Z)-1
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.08 mL (0.8 mmol) of 2-pyridinecarboxaldehyde and 60 µL (0.67 mmol) of piperidine the brown oil (0.149 g, 83%) was obtained as a mixture of isomers. According to 1H NMR E:Z stereoisomer ratio is 3.7:1, respectively. 1H NMR signals were identical to described in Section 3.1.2. for 1.
- (E,Z)-3-(4-Hydroxybenzylidene)-2-oxindole (E,Z)-14
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.092 g (0.8 mmol) of 4-hydroxybenzaldehyde and 60 µL (0.67 mmol) of piperidine the yellow powder (0.159 g, 89%) was obtained as a mixture of two isomers. According to 1H NMR E/Z stereoisomer ratio is 1.75:1, respectively. 1H NMR signals were identical to described in Section 3.1.2. for 14.
- (E,Z)-3-((5-Ethoxycarbonyl-1H-pyrazol-4-yl)methylidene)-2-oxindole (E,Z)-39
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.134 g (0.8 mmol) of ethyl 4-formyl-1H-pyrazole-5-carboxylate and 60 µL (0.67 mmol) of piperidine the yellow powder (0.089 g, 50%) was obtained as a mixture of two isomers. According to NMR 1H major to minor stereoisomer ratio is 1.6:1. 1H NMR signals were identical to described in Section 3.1.2. for (E,Z)-39.
- 3-((3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)methylidene)-2-oxindole 44
- From 0.100 g (0.8 mmol) of 2-oxindole, 0.160 g (0.8 mmol) of 3,5-dimethyl-1-phenyl-1H-pyrazole-4-carbaldehyde and 60 µL (0.67 mmol) of piperidine the yellow powder was obtained as single isomer 1H NMR signals were identical to described in Section 3.1.2 for 44. According to 1H NMR conversion is 68%.
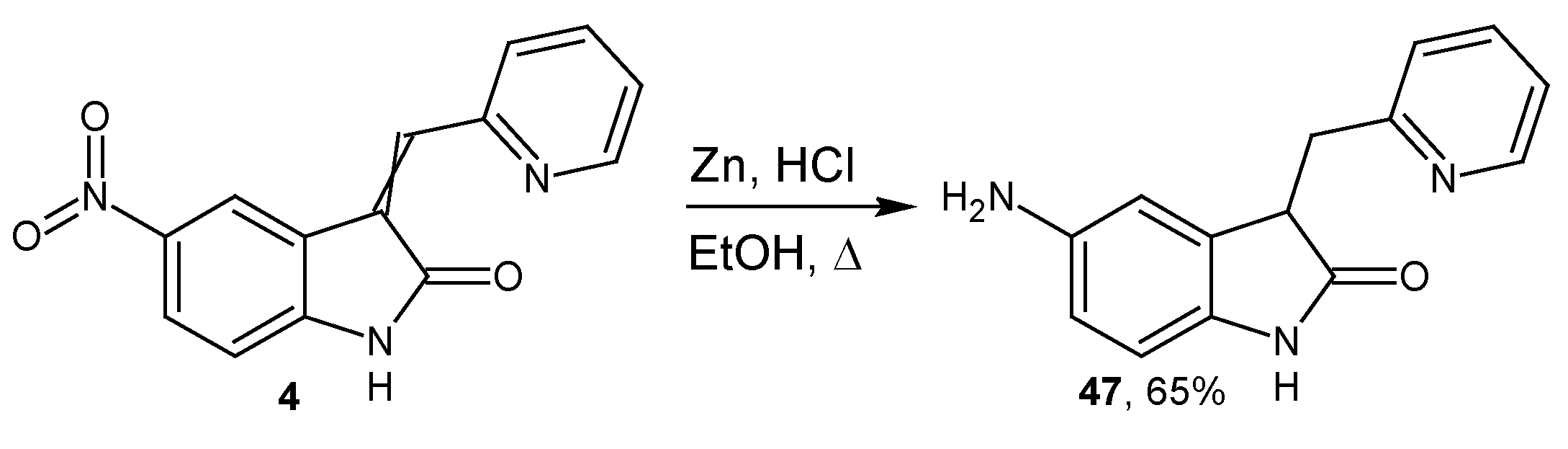
3.1.4. Synthesis of 3-(pyridin-2-ylmethyl)-5-amino-2-oxindole 47
- To the suspension of 0.146 g (0.6 mmol) 3-(2-pyridinylmethylidene)-5-nitro-2-oxindole and 0.350 g (5.6 mmol) zinc powder in 5 mL of MeOH the 0.6 mL of HCl conc. was rapidly added with vigorous stirring. After 30 min, the reaction was terminated by addition of NaHCO3 and pH was adjusted to 8. The reaction mixture was extracted with EtOAc, organic fraction was dried with Na2SO4 and the solvent was removed under reduced pressure. The compound (0.086 g, 65%) was obtained as brown powder.
- 1H NMR (400.13 MHz, DMSO-d6, ppm): 2.88–3.0 (m, 1H, CH2), 3.28–3.36 (m, 1H, CH2), 3.83–3.90 (m, 1H, CH), 4.51 (br.s, 2H, NH2), 5.98 (s, 1H, H4), 6.33 (dd, J1 = 7.6 Hz, J2 = 1.8 Hz, 1H, H6), 6.49 (d, J = 8.1 Hz, 1H, H7), 7.2–7.26 (m, 2H, Hpy), 7.65–7.72 (td, J1 = 7.7 Hz, J2 = 1.8 Hz, 1H, Hpy), 8.495 (d, J = 4.1 Hz, 1H, H3′), 9.99 (br. s, 1H, NH).
- 13C NMR (100.6 MHz, DMSO-d6, ppm): 38.15, 45.04, 109.46, 111.31, 112.63, 121.76, 123.60, 130.41, 132.34, 136.42, 143.20, 149.03, 158.41, 178.07.
- HRMS (ESI), m/z: (M+H) found 240.1128, C14H14N3O requires 240.1137, (M+Na) found 262.0939, C14H13N3ONa requires 262.0956.
3.2. Biology
3.2.1. NQO2 Assay
3.2.2. Cell Culture
3.2.3. Antiproliferative Assay
3.3. In Silico Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rashid, M.H.; Babu, D.; Siraki, A.G. Interactions of the Antioxidant Enzymes NAD(P)H: Quinone Oxidoreductase 1 (NQO1) and NRH: Quinone Oxidoreductase 2 (NQO2) with Pharmacological Agents, Endogenous Biochemicals and Environmental Contaminants. Chem. Interact. 2021, 345, 109574. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.P.; Björklund, M. NQO2 Is a Reactive Oxygen Species Generating Off-Target for Acetaminophen. Mol. Pharm. 2014, 11, 4395–4404. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Nakai, M. Increased Hippocampal Quinone Reductase 2 in Alzheimer’s Disease. Neurosci. Lett. 2011, 502, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, X.; Liu, X.; Liu, X.; Jiang, X.; Du, J.; Wang, Q.; Liang, Y.; Ma, W. NQO2 Inhibition Relieves Reactive Oxygen Species Effects on Mouse Oocyte Meiotic Maturation and Embryo Development. Biol. Reprod. 2017, 97, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Janda, E.; Nepveu, F.; Calamini, B.; Ferry, G.; Boutin, J.A. Molecular Pharmacology of NRH:Quinone Oxidoreductase 2: A Detoxifying Enzyme Acting as an Undercover Toxifying Enzyme. Mol. Pharmacol. 2020, 98, 620–633. [Google Scholar] [CrossRef]
- Cassagnes, L.E.; Chhour, M.; Pério, P.; Sudor, J.; Gayon, R.; Ferry, G.; Boutin, J.A.; Nepveu, F.; Reybier, K. Oxidative Stress and Neurodegeneration: The Possible Contribution of Quinone Reductase 2. Free Radic Biol. Med. 2018, 120, 56–61. [Google Scholar] [CrossRef]
- Benoit, C.E.; Bastianetto, S.; Brouillette, J.; Tse, Y.C.; Boutin, J.A.; Delagrange, P.; Wong, T.P.; Sarret, P.; Quirion, R. Loss of Quinone Reductase 2 Function Selectively Facilitates Learning Behaviors. J. Neurosci. 2010, 30, 12690–12700. [Google Scholar] [CrossRef]
- Kadnikov, I.A.; Voronkov, D.N.; Voronin, M.V.; Seredenin, S.B. Analysis of Quinone Reductase 2 Implication in Mechanism of Antiparkinsonian Action of Afobazole. Neurochem. J. 2020, 14, 227–234. [Google Scholar] [CrossRef]
- Voronin, M.V.; Kadnikov, I.A.; Zainullina, L.F.; Logvinov, I.O.; Verbovaya, E.R.; Antipova, T.A.; Vakhitova, Y.V.; Seredenin, S.B. Neuroprotective Properties of Quinone Reductase 2 Inhibitor M-11, a 2-Mercaptobenzimidazole Derivative. Int. J. Mol. Sci. 2021, 22, 13061. [Google Scholar] [CrossRef]
- Chhour, M.; Aubouy, A.; Bourgeade-Delmas, S.; Pério, P.; Ternet-Fontebasso, H.; Haidara, M.; Ferry, G.; Nepveu, F.; Boutin, J.A.; Reybier, K. Antimalarial Properties of Dunnione Derivatives as NQO2 Substrates. Molecules 2019, 24, 3697. [Google Scholar] [CrossRef]
- Chesnokova, N.B.; Beznos, O.V.; Lozinskaya, N.A.; Volkova, M.S.; Zaryanova, E.V.; Zefirov, N.S.; Grigoryev, A.V. Novel Agonists of Melatonin Receptors as Promising Hypotensive and Neuroprotective Agents for Therapy of Glaucoma. Biochem. (Moscow) Suppl. Ser. B Biomed. Chem. 2017, 11, 272–278. [Google Scholar] [CrossRef]
- Mamoor, S. NQO2 Is Differentially Expressed in the Blood of Patients with Coronavirus Co-Infections. OSF Prepr. 2020. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Li, N.; Liu, W.T.; Liang, J.Z.; Sun, Y.; Zhang, W.X.; Fang, R.D.; Huang, S.L.; Sun, Z.H.; et al. Curcumol Overcomes TRAIL Resistance of Non-Small Cell Lung Cancer by Targeting NRH:Quinone Oxidoreductase 2 (NQO2). Adv. Sci. 2020, 7, 2002306. [Google Scholar] [CrossRef]
- Dorai, T.; Shah, A.; Summers, F.; Mathew, R.; Huang, J.; Hsieh, T.; Wu, J.M. NRH:Quinone Oxidoreductase 2 (NQO2) and Glutaminase (GLS) Both Play a Role in Large Extracellular Vesicles (LEV) Formation in Preclinical LNCaP-C4-2B Prostate Cancer Model of Progressive Metastasis. Prostate 2018, 78, 1181–1195. [Google Scholar] [CrossRef]
- Kwang, S.A.; Gong, X.; Sethi, G.; Chaturvedi, M.M.; Jaiswal, A.K.; Aggarwal, B.B. Deficiency of NRH:Quinone Oxidoreductase 2 Differentially Regulates TNF Signaling in Keratinocytes: Up-Regulation of Apoptosis Correlates with down-Regulation of Cell Survival Kinases. Cancer Res. 2007, 67, 10004–10011. [Google Scholar] [CrossRef]
- Hussein, B.; Ikhmais, B.; Kadirvel, M.; Magwaza, R.N.; Halbert, G.; Bryce, R.A.; Stratford, I.J.; Freeman, S. Discovery of Potent 4-Aminoquinoline Hydrazone Inhibitors of NRH:Quinoneoxidoreductase-2 (NQO2). Eur. J. Med. Chem. 2019, 182, 111649. [Google Scholar] [CrossRef]
- Alnabulsi, S.; Santina, E.; Russo, I.; Hussein, B.; Kadirvel, M.; Chadwick, A.; Bichenkova, E.V.; Bryce, R.A.; Nolan, K.; Demonacos, C.; et al. Non-Symmetrical Furan-Amidines as Novel Leads for the Treatment of Cancer and Malaria. Eur. J. Med. Chem. 2016, 111, 33–45. [Google Scholar] [CrossRef]
- Yerer, M.B.; Demirpolat, E.; Cumaoğlu, A.; Torçuk, C.; İmamoğlu, N.N.; Koşar, M. Comparison of Natural NQO2 Inhibitors as a New Target for Cancer Treatment in Different Cell Lines. Turk. J. Biochem. 2015, 40, 224–233. [Google Scholar] [CrossRef]
- Knox, R.J.; Jenkins, T.C.; Hobbs, S.M.; Chen, S.; Melton, R.G.; Burke, P.J. Bioactivation of 5-(Aziridin-1-Yl)-2,4-Dinitrobenzamide (CB 1954) by Human NAD(P)H Quinone Oxidoreductase 2: A Novel Co-Substrate-Mediated Antitumor Prodrug Therapy. Cancer Res. 2000, 60, 4179–4186. [Google Scholar]
- Gong, X.; Kole, L.; Iskander, K.; Jaiswal, A.K. NRH:Quinone Oxidoreductase 2 and NAD(P)H:Quinone Oxidoreductase 1 Protect Tumor Suppressor P53 against 20S Proteasomal Degradation Leading to Stabilization and Activation of P53. Cancer Res. 2007, 67, 5380–5388. [Google Scholar] [CrossRef]
- Gaikwad, N.W.; Yang, L.; Rogan, E.G.; Cavalieri, E.L. Evidence for NQO2-Mediated Reduction of the Carcinogenic Estrogen Ortho-Quinones. Free Radic. Biol. Med. 2009, 46, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A.; Ferry, G. Is There Sufficient Evidence That the Melatonin Binding Site MT3 Is Quinone Reductase 2? J. Pharmacol. Exp. Ther. 2019, 368, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A. Quinone Reductase 2 as a Promising Target of Melatonin Therapeutic Actions. Expert Opin. Ther. Targets 2016, 20, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.N.; Jensen, K.C.; Mesecar, A.D.; Fanwick, P.E.; Cushman, M. Design, Synthesis, and Biological Evaluation of Potent Quinoline and Pyrroloquinoline Ammosamide Analogues as Inhibitors of Quinone Reductase 2. J. Med. Chem. 2012, 55, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, M.S.; Barnes, J.; Humphries, M.; Whitehead, R.C.; Bryce, R.A.; Leys, D.; Stratford, I.J.; Nolan, K.A. Novel Inhibitors of NRH:Quinone Oxidoreductase 2 (NQO2): Crystal Structures, Biochemical Activity, and Intracellular Effects of Imidazoacridin-6-Ones. J. Med. Chem. 2011, 54, 6597–6611. [Google Scholar] [CrossRef]
- Alnabulsi, S.; Hussein, B.; Santina, E.; Alsalahat, I.; Kadirvel, M.; Magwaza, R.N.; Bryce, R.A.; Schwalbe, C.H.; Baldwin, A.G.; Russo, I.; et al. Evaluation of Analogues of Furan-Amidines as Inhibitors of NQO2. Bioorg. Med. Chem. Lett. 2018, 28, 1292–1297. [Google Scholar] [CrossRef]
- Volkova, M.S.; Jensen, K.C.; Lozinskaya, N.A.; Sosonyuk, S.E.; Proskurnina, M.V.; Mesecar, A.D.; Zefirov, N.S. Synthesis of Novel MT3 Receptor Ligands via an Unusual Knoevenagel Condensation. Bioorg. Med. Chem. Lett. 2012, 22, 7578–7581. [Google Scholar] [CrossRef]
- Lozinskaya, N.A.; Babkov, D.A.; Zaryanova, E.V.; Bezsonova, E.N.; Efremov, A.M.; Tsymlyakov, M.D.; Anikina, L.V.; Zakharyascheva, O.Y.; Borisov, A.V.; Perfilova, V.N.; et al. Synthesis and Biological Evaluation of 3-Substituted 2-Oxindole Derivatives as New Glycogen Synthase Kinase 3β Inhibitors. Bioorg. Med. Chem. 2019, 27, 1804–1817. [Google Scholar] [CrossRef]
- Klochkov, V.G.; Bezsonova, E.N.; Dubar, M.; Melekhina, D.D.; Temnov, V.V.; Zaryanova, E.V.; Lozinskaya, N.A.; Babkov, D.A.; Spasov, A.A. Towards Multi-Target Antidiabetic Agents: In Vitro and in Vivo Evaluation of 3,5-Disubstituted Indolin-2-One Derivatives as Novel α-Glucosidase Inhibitors. Bioorg Med. Chem. Lett. 2022, 55, 128449. [Google Scholar] [CrossRef]
- Sun, L.; Tran, N.; Tang, F.; App, H.; Hirth, P.; Mcmahon, G.; Tang, C. Synthesis and Biological Evaluations of 3-Substituted Indolin-2-Ones: A Novel Class of Tyrosine Kinase Inhibitors That Exhibit Selectivity toward Particular Receptor Tyrosine Kinases. J. Med. Chem. 1998, 41, 2588–2603. [Google Scholar] [CrossRef]
- Buryanovskyy, L.; Fu, Y.; Boyd, M.; Ma, Y.; Hsieh, T.C.; Wu, J.M.; Zhang, Z. Crystal Structure of Quinone Reductase 2 in Complex with Resveratrol. Biochemistry 2004, 43, 11417–11426. [Google Scholar] [CrossRef]
- Wu, K.; Knox, R.; Sun, X.Z.; Joseph, P.; Jaiswal, A.K.; Zhang, D.; Deng, P.S.K.; Chen, S. Catalytic Properties of NAD(P)H:Quinone Oxidoreductase-2 (NQO2), a Dihydronicotinamide Riboside Dependent Oxidoreductase. Arch. Biochem. Biophys. 1997, 347, 221–228. [Google Scholar] [CrossRef]
- Pegan, S.D.; Sturdy, M.; Ferry, G.; Delagrange, P.; Boutin, J.A.; Mesecar, A.D. X-Ray Structural Studies of Quinone Reductase 2 Nanomolar Range Inhibitors. Protein Sci. 2011, 20, 1182–1195. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, C.; Lei, L.; Lin, K.; Yu, C. Synthesis of 2-Oxindoles from Substituted Indoles by Hypervalent-Iodine Oxidation. Eur. J. Org. Chem. 2018, 2018, 1437–1442. [Google Scholar] [CrossRef]
- Delong, W.; Lanying, W.; Yongling, W.; Shuang, S.; Juntao, F.; Xing, Z. Natural α-Methylenelactam Analogues: Design, Synthesis and Evaluation of α-Alkenyl-γ and δ-Lactams as Potential Antifungal Agents against Colletotrichum Orbiculare. Eur. J. Med. Chem. 2017, 130, 286–307. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, P. Rationally Designed Molecules for Resurgence of Cyanide Mitigated Cytochrome c Oxidase Activity. Bioorg. Chem. 2019, 82, 229–240. [Google Scholar] [CrossRef]
- Kniess, T.; Bergmann, R.; Kuchar, M.; Steinbach, J.; Wuest, F. Synthesis and Radiopharmacological Investigation of 3-[4′-[18F]Fluorobenzylidene]Indolin-2-One as Possible Tyrosine Kinase Inhibitor. Bioorg. Med. Chem. 2009, 17, 7732–7742. [Google Scholar] [CrossRef]
- Bazanov, D.R.; Pervushin, N.V.; Savin, E.V.; Tsymliakov, M.D.; Maksutova, A.I.; Savitskaya, V.Y.; Sosonyuk, S.E.; Gracheva, Y.A.; Seliverstov, M.Y.; Lozinskaya, N.A.; et al. Synthetic Design and Biological Evaluation of New P53-MDM2 Interaction Inhibitors Based on Imidazoline Core. Pharmaceuticals 2022, 15, 444. [Google Scholar] [CrossRef]

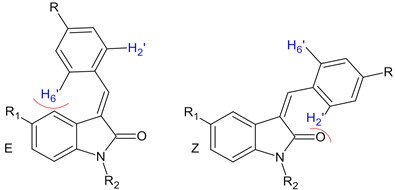 | ||||||
|---|---|---|---|---|---|---|
| № | R | R1 | R2 | Chemical Shift of H2′ H6′ (ppm) | E:Z Ratio | |
| E | Z | |||||
| 14 | -OH | H | H | 7.47 | 8.24 | 19:1 |
| 15 | -OH | H | Me | 7.48 | - | 1:0 |
| 16 | -OH | NHC(O)CH3 | H | 7.60 | 8.38 | 2.6:1 |
| 17 | -OH | NHC(O)OCH3 | H | 7.63 | 8.41 | 25:1 |
| 18 | -OH | NH(2-furoyl) | H | 7.66 | 8.40 | 2:1 |
| 19 | -OH | NHBz | H | 7.67 | 8.42 | 2.3:1 |
| 20 | -OMe | H | H | 7.70 | 8.47 | 5:1 |
| 21 | -OMe | NHBz | H | 7.76 | 8.51 | 2:1 |
| 22 | -OEt | H | H | 7.69 | - | 1:0 |
| 28 | -NO2 | H | H | 7.94 | 8.27 | 10:1 |
| 29 | -NO2 | NHC(O)OCH3 | H | 7.93 | 8.26 | 3:1 |
| 30 | -NO2 | NHBz | H | 7.85 | 8.25 | 2:1 |
| 31 | -N(Me)2 | H | H | 7.57–7.67 1 | 8.44 | 1:1 |
| 32 | -Br | Br | H | 7.73 | 8.31 | 1.6:1 |
| 33 | -Br | NHC(O)OCH3 | H | 7.67 | 8.31 | 12.5:1 |
| 34 | -F | H | H | 7.73–7.75 1 | 8.45–8.50 1 | 2:1 |
 | |||||
|---|---|---|---|---|---|
| № | R1 | R2 | E-Isomer | E:Z Ratio | |
| Chemical Shift of H4 (ppm) | Chemical Shift of H3′ (ppm) | ||||
| 1 | H | H | 8.98 | 8.40 | 1:0 |
| 2 | H | CH3 | 8.85–8.91 1 | 1:0 | |
| 3 | Br | H | 9.27 | 8.87 | 1:0 |
| 4 | NO2 | H | 10.11 | 8.85 | 1:0 |
| 52 | AcNH | H | 9.31 | 8.89 | 7.7:1 |
| 62 | BzNH | H | 9.46 | 8.88 | 5:1 |
| Activation Method | Thermal Activation (reflux) | MW | |||
|---|---|---|---|---|---|
| Aldehyde | Solvent | Ethanol | 1,4-dioxane | Ethyl Acetate | 1,4-dioxane |
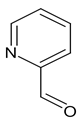 | E:Zproducts ratio for compound 1 | 1:0 | 1a, then 1.3:1 1,2 | 1a as product 1 | 3.7:1 |
| Reaction time, min | 120 | 90 | 10 | 11.5 | |
| Yield, % | 63 | 81 | 49 | 83 | |
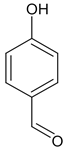 | E:Zproducts ratio for compound 14 | 19:1 | 2.3:1 | 2.3:1 | 2.3:1 |
| Reaction time, min | 60 | 60 | 30 | 11.5 | |
| Yield, % | 76 | 84 | 61 | 89 | |
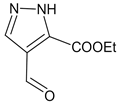 | E:Zproducts ratio for compound 39 | 1.6:1 | 1.6:1 | 1.6:1 | 1.6:1 |
| Reaction time, min | 120 | 120 | 20 | 11.5 | |
| Yield, % | 61 | 86 | 67 | 50 | |
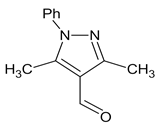 | E:Zproducts ratio for compound 44 | 1:0 | 1:0 | 1:0 | 1:0 |
| Reaction time, min | 150 | 120 | 120 | 11.5 | |
| Yield, % | 63 | 62 | Conversion 76% | Conversion 68% | |
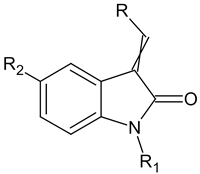 | NQO2 | A549, CC50 (μM) | ||||
|---|---|---|---|---|---|---|
| № | R | R1 | R2 | Inhibition 1, % | IC50 ± SEM (μM) | |
| 1 | 2-pyridyl | H | H | 19.56 | >>50 | 47.97 ± 4.42 |
| 2 | 2-pyridyl | Me | H | 64.43 | 16.78 ± 0.09 | - 2 |
| 3 | 2-pyridyl | H | Br | 18.63 | >>50 | 90.56 ± 1.50 |
| 4 | 2-pyridyl | H | NO2 | 1.85 | >>50 | n.a. 3 |
| 5 | 2-pyridyl | H | AcNH | 46.35 | 41.13 ± 2.44 | 135.9 |
| 6 | 2-pyridyl | H | BzNH | 43.13 | n.d 2. | 17.43 ± 1.80 |
| 7 | 3-pyridyl | H | H | 59.17 | n.d. | 67.45 ± 7.58 |
| 8 | 3-pyridyl | H | Br | 44.24 | n.d. | 22.30 ± 1.42 |
| 9 | 3-pyridyl | H | NO2 | 4.25 | >>50 | 428.59 ± 23.97 |
| 10 | 4-pyridyl | H | H | 27.29 | >>50 | 80.27 ± 9.87 |
| 11 | 4-pyridyl | H | Br | 41.92 | n.d. | 22.30 ± 1.42 |
| 12 | 4-pyridyl | H | NO2 | 19.23 | >>50 | n.a. |
| 13 | 3-OH-C6H4 | H | AcNH | 89.07 | 0.64 ± 0.04 | 175.83 ± 4.76 |
| 14 | 4-OH-C6H4 | H | H | 31.68 | >>50 | 80.85 ± 12.22 |
| 15 | 4-OH-C6H4 | Me | H | 98.87 | 0.62 ± 0.04 | n.a. |
| 16 | 4-OH-C6H4 | H | AcNH | 70.41 | 0.99 ± 0.03 | n.a. |
| 17 | 4-OH-C6H4 | H | MeOC(O)NH | 93.63 | 0.44 ± 0.02 | 44.43 ± 0.29 |
| 18 | 4-OH-C6H4 | H | 2-furoylNH | 96.4 | 0.37 ± 0.02 | 146.25 ± 11.31 |
| 19 | 4-OH-C6H4 | H | BzNH | 62.89 | n.d. | 412.17 ± 3.98 |
| 20 | 4-OMe-C6H4 | H | H | 40.82 | n.d. | 54.76 |
| 21 | 4-OMe-C6H4 | H | BzNH | 30.89 | >>50 | 42.33 ± 8.30 |
| 22 | 4-OEt-C6H4 | H | H | 25.41 | >>50 | - |
| 23 | 3,4,5-triOMe-C6H2 | H | H | 85.71 | 1.6 ± 0.14 | 25.72 |
| 24 | 3,4,5-triOMe-C6H2 | H | AcNH | 92.78 | 0.37 ± 0.01 | 148.99 ± 2.63 |
| 25 | 3,4,5-triOMe-C6H2 | H | 2-furoylNH | 90.98 | 0.66 ± 0.02 | 200.90 ± 22.65 |
| 26 | 3,4-diOMe-4-OH-C6H2 | H | BzNH | 40.47 | n.d. | n.a. |
| 27 | 4-NO2-C6H4 | H | H | 4.66 | >>50 | - |
| 28 | 4-NO2-C6H4 | H | MeOC(O)NH | 74.0 | n.d. | n.a. |
| 29 | 4-NO2-C6H4 | H | BzNH | 21.79 | >>50 | - |
| 30 | 4-NMe2-C6H4 | H | H | 27.30 | >>50 | n.a. |
| 31 | 4-Br-C6H4 | H | Br | 68.63 | n.d. | 241.71 ± 3.19 |
| 32 | 4-Br-C6H4 | H | MeOC(O)NH | 95.46 | 0.61 ± 0.03 | n.a. |
| 33 | 4-F-C6H4 | H | H | 19.46 | >>50 | 38.56 ± 10.45 |
| 34 | 2-furyl | H | H | 59.91 | n.d. | 594.74 ± 25.37 |
| 35 | 2-furyl | H | Br | −15.2 | >>50 | 449.07 ± 18.72 |
| 36 | 2-furyl | H | NO2 | 59.55 | n.d. | 328.55 ± 17.00 |
| 37 | 2-thienyl | H | H | 38.1 | n.d. | 491.01 ± 19.85 |
| 38 | 2-thienyl | H | Br | 29.9 | >>50 | 426.53 ± 6.20 |
| 39 | 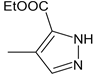 | H | H | 47.32 | 0.34 ± 0.02 | 231.3 |
| 40 | 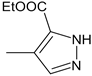 | H | NO2 | 92.3 | 1.88 ± 0.08 | - |
| 41 | 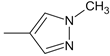 | H | H | 59.76 | 9.40 ± 0.12 | - |
| 42 |  | H | H | 54.35 | n.d. | - |
| 43 | 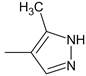 | H | H | 77.81 | 7.61 ± 0.19 | 218.9 |
| 44 |  | H | H | 43.58 | n.d. | n.a. |
| 45 | 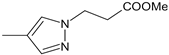 | H | H | 28.97 | >>50 | n.a. |
 | ||||||
| № | R | R1 | R2 | |||
| 46 | 4-NH2-C6H4 | H | H | 19.05 | >>50 | - |
| 47 | 2-pyridyl | H | NH2 | 26.93 | >>50 | n.a. |
 | ||||||
| № | R | R1 | R2 | |||
| 48 | 4-Me-C6H4 | H | OMe | −50.28 | >>50 | n.a. |
| 49 | 4-Me-C6H4 | H | Br | −85.22 | >>50 | n.a. |
| 50 | 4-MeO-C6H4 | H | Br | 78.82 | n.d. | 473.55 ± 18.21 |
| 51 | 4-Me-C6H4 | H | 2-furoyl-NH | 25.63 | >>50 | n.a. |
| 52 | 4-Me-C6H4 | H | H | −92.90 | >>50 | 170.63 ± 13.41 |
| Melatonin | 63.5 ± 2.7 | |||||
| Quercetin | 98 | 0.08 ± 0.02 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozinskaya, N.A.; Bezsonova, E.N.; Dubar, M.; Melekhina, D.D.; Bazanov, D.R.; Bunev, A.S.; Grigor’eva, O.B.; Klochkov, V.G.; Sokolova, E.V.; Babkov, D.A.; et al. 3-Arylidene-2-oxindoles as Potent NRH:Quinone Oxidoreductase 2 Inhibitors. Molecules 2023, 28, 1174. https://doi.org/10.3390/molecules28031174
Lozinskaya NA, Bezsonova EN, Dubar M, Melekhina DD, Bazanov DR, Bunev AS, Grigor’eva OB, Klochkov VG, Sokolova EV, Babkov DA, et al. 3-Arylidene-2-oxindoles as Potent NRH:Quinone Oxidoreductase 2 Inhibitors. Molecules. 2023; 28(3):1174. https://doi.org/10.3390/molecules28031174
Chicago/Turabian StyleLozinskaya, Natalia A., Elena N. Bezsonova, Meriam Dubar, Daria D. Melekhina, Daniil R. Bazanov, Alexander S. Bunev, Olga B. Grigor’eva, Vladlen G. Klochkov, Elena V. Sokolova, Denis A. Babkov, and et al. 2023. "3-Arylidene-2-oxindoles as Potent NRH:Quinone Oxidoreductase 2 Inhibitors" Molecules 28, no. 3: 1174. https://doi.org/10.3390/molecules28031174
APA StyleLozinskaya, N. A., Bezsonova, E. N., Dubar, M., Melekhina, D. D., Bazanov, D. R., Bunev, A. S., Grigor’eva, O. B., Klochkov, V. G., Sokolova, E. V., Babkov, D. A., Spasov, A. A., & Sosonyuk, S. E. (2023). 3-Arylidene-2-oxindoles as Potent NRH:Quinone Oxidoreductase 2 Inhibitors. Molecules, 28(3), 1174. https://doi.org/10.3390/molecules28031174











